Abstract
Hepatitis B virus (HBV) infections are highly prevalent globally, representing a serious public health problem. The diverse modes of transmission and the burden of the chronic carrier population pose challenges to the effective management of HBV. Vaccination is the most effective preventive measure available in the current scenario. Still, HBV is one of the significant health issues in various parts of the globe due to non-response to vaccines, the high number of concealed carriers, and the lack of access and awareness. Universal vaccination programs must be scaled up in neonates, especially in the developing parts of the world, to prevent new HBV infections. Novel treatments like combinational therapy, gene silencing, and new antivirals must be available for effective management. The prolonged infection of HBV, direct and indirect, can promote the growth of hepatocellular carcinoma (HCC). The present review emphasizes the problems and probable solutions for better managing HBV infections, causal risk factors of HCC, and mechanisms of HCC.
Keywords: Chronic hepatitis B, Hepatitis B virus, Hepatocellular carcinoma, Hepatitis B vaccine, Viral hepatitis
Introduction
Viral hepatitis is a severe liver infection affecting large segments of the world population. The hepatitis B virus (HBV) is one of several hepatitis viruses {hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), and hepatitis E virus (HEV)} which are significant contributors to viral hepatitis[1, 2]. All these viruses produce similar symptoms and clinically cannot be differentiated. Laboratory diagnostic assays are required for the correct and assured diagnosis of viral hepatitis [3]. HBV infection affects the normal functions of the liver and causes inflammation, known as hepatitis. Liver cancer or hepatocellular carcinoma (HCC) is the second most prevalent human cancer after tobacco-related cancer. Viral hepatitis is accountable for up to 80% of cases of HCC worldwide, and most cases are of untreated HBV or HCV infection [1, 2, 4]. HBV infections can be broadly divided into acute and chronic categories of hepatitis infection. Clinical symptoms due to HBV infections have a broad range and vary from asymptomatic or mild to severe life-threatening [5]. Acute HBV infections are generally self-limiting, and only a few cases have acute inflammation or hepatocellular necrosis, with 0.5–1% of case fatality [5, 6]. Chronic hepatitis B (CHB) is less common than the acute form and does not lead to significant liver sickness in most cases. CHB may cause advanced liver fibrosis, cirrhosis, and HCC in some patients [7]. HBV is a ubiquitous virus. About one-third (> 2 billion) of the global human population has a history of HBV infection in their lifetime[8–10]. The majority of these HBV patients are recovering without any noticeable damage. About 350 million people are chronically HBV-infected and act as the carrier by spreading the infection to a vulnerable population [2]. HBV is a dangerous virus accountable for 4 million acute clinical cases annually. HBV is a reason for about one million deaths due to chronic active hepatitis, cirrhosis, or liver cancer [2]. World Health Organization (WHO) estimates that cirrhosis and HCC caused by HBV were responsible for more than 820,000 deaths globally in 2019 [2]. The present review emphasizes the problems and probable solutions for better managing HBV infections, causal risk factors of HCC, and mechanisms of HCC.
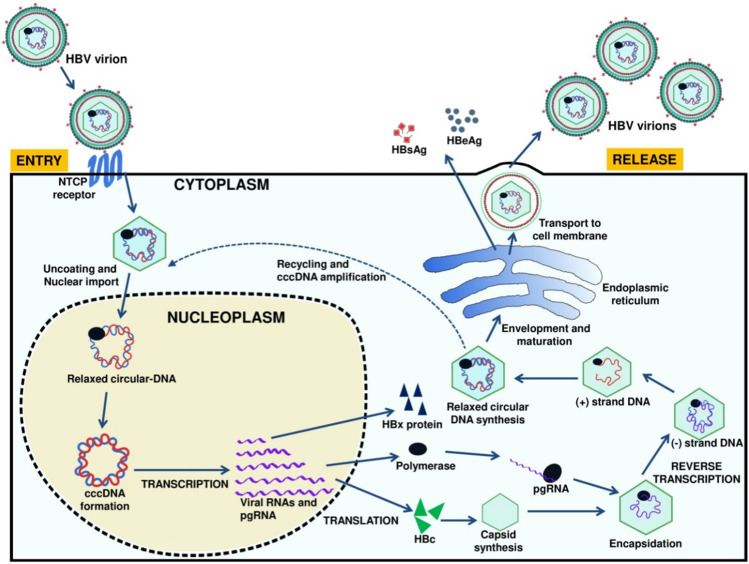
The virus and its life cycle
HBV belongs to the family Hepadnaviridae and contains a partially double-stranded DNA genome of about 3.2 kb, covalently linked to the viral polymerase. HBV is an enveloped virus of 42 nm in diameter. It has an inner nucleocapsid region (27 nm in diameter) composed of hepatitis B core antigen (HBcAg) and hepatitis B e antigen (HBeAg). The host-driven lipid envelope of HBV contains surface antigen (HBsAg), which helps in the binding of HBV to receptors of hepatocytes [5, 11]. HBV binding and entry into hepatocytes have not been clearly understood, but experiments showed that it might be through endocytosis [12–14]. Various hepatocyte receptors proteins like transferrin, asialoglycoprotein, immunoglobulin A receptor, and human liver endonexin receptor were proposed as binding receptors for HBV [12–16]. Past studies showed sodium taurocholate co-transporting polypeptide (NTCP) as the potential receptor for HBV, which also acts as a significant species determinant specificity [17]. Only the nucleocapsid part of HBV enters the cytoplasm, which reaches into the nucleus via a nuclear pore and delivers viral DNA into the nucleoplasm (Fig. 1) [13]. HBV has partially double-stranded DNA, converted into a covalently closed circular (ccc) super-coiled DNA molecule inside the host nucleus [11, 18]. This cccDNA of HBV is transcribed into four important viral RNAs of different genomic lengths; a 3.5-kb RNA coding for polymerase and pre-core protein, 2.4- and 2.1-kb RNA coding for surface protein, and 0.7-kb RNA coding for regulatory X protein. HBV produces polyadenylated RNA, which is transported from the nucleus to the cytoplasm of the hepatocyte through the nuclear pore before translation. The 3.5-kb RNA, termed pre-genomic RNA (pgRNA), codes for core proteins and reverse transcriptase (RT) and also acts as a template for viral DNA synthesis [19, 20]. The nucleocapsids are synthesized in the cytoplasm and matured into DNA-containing nucleocapsids, which are imported either into the nucleus for cccDNA amplification or into the endoplasmic reticulum (ER) and released as progeny viruses [20]. Inside the endoplasmic reticulum lumen, mainly amino and carboxy-termini are trimmed, and the resultant proteins are secreted as the hepatitis B pre-core antigen (HBeAg) and surface antigen (HBsAg) (Fig. 1). The X protein determines the efficiency of HBV replication by interacting with various transcription factors and can stimulate cell proliferation and cell death [21]. Viral polymerase enzyme is multifunctional and involved in multiple viral activities like priming, polymerase and nuclease activity, and packaging of RNA pre-genome into nucleocapsids [22, 23]. Nuclear localization signals on polymerase mediate covalently linked HBV genome transport through the nuclear pore [23, 24].
Fig. 1.
Life cycle of hepatitis B virus inside a hepatocyte
Transmission
HBV can be transmitted through three transmission modes; parenteral/percutaneous, sexual, and perinatal/mother-to-child [8, 25–27]. The parenteral/percutaneous mode of transmission is the most common in which HBV spread through contaminated blood and other body fluids like saliva, semen, and menstrual or vaginal fluid [28, 29]. HBV can be transmitted from the accidental exchange of contaminated blood or other body fluids during medical, surgical, and dental procedures. Injection drug abuse remains a significant transmission mode in the USA and Western Europe [30–32]. Parenteral/percutaneous transmission can also occur during acupuncture, body piercing, scarification, and tattooing [33, 34]. HBV is categorized as a sexually transmitted disease (STD) and spreads from an infected person to an unvaccinated or susceptible person during homosexual or heterosexual activities. In countries with low and intermediate prevalence, sexual contact is the primary cause of HBV infection [35, 36]. HBV infection can be transmitted through the perinatal mode from HBeAg seropositive mother to their neonate without prophylaxis. The comparative risk of perinatal HBV infection is more if the mother has become acute HBV positive in the later stage of pregnancy than in the early stage. The infection rate increases if the mother is positive for both HBeAg and HBsAg. HBV infection spreads during delivery or after birth due to placental tears and hemorrhages [37]. There is no experimental evidence that HBV transmits through breast milk. Perinatal spread of HBV is a standard route in China and South-East Asian countries. In neonates and children (less than 1 year old) infected through perinatal mode, the risk of CHB infection is 90% [38, 39].
Prevalence
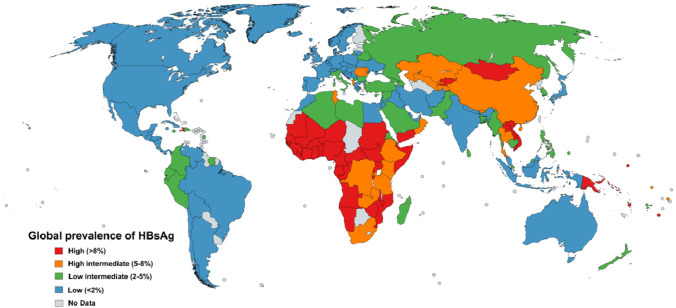
On a global scale, the HBV attack is not uniform. The population comes under three broad categories based on the prevalence of disease, viz., high (> 8%), higher intermediate (5–8%), lower intermediate (2–5%), and low (< 2%) [28, 36]. Figure 2 shows the HBV virus’s global prevalence based on the HBsAg distribution. The regions with high endemicity include countries of Asia–Pacific and sub-Saharan Africa. Most African countries have high or intermediate endemicity [40]. About 70–90% of the adult human population from these areas is HBsAg seropositive, out of which about 8–20% serve as HBV carriers [41, 42]. In a higher intermediate endemic country like the Republic of China, the occurrence of chronic hepatitis B is very high in newborns (90%) and young children (30%) infected with HBV [43]. The parts of the world, including Southern Europe, the Middle East, South Asia, Russia, Japan, and South America, are categorized into the region of low intermediate (2–5%) to low (< 2%) endemicity. About 10–60% of the population in these areas is infected, of which 2–7% serve as carriers[44, 45]. In India, about 2–8% of the population have HBV infection, and about 3% act as a carrier [46]. Developed parts of the world, like North America, Australia, New Zealand, and some portions of Europe and South America, have a low prevalence of HBV [40, 47]. The prolonged infection of HBV, direct and indirect, can promote the growth of HCC, a major reason for global mortality.
Fig. 2.
Global prevalence of hepatitis B virus (adapted from Schweitzer et al. 2015)
Risk groups for HBV and HCC
Susceptibility to HBV infection depends upon many personal factors like age, nutritional standard, and immunization status of patients. Risk groups for HBV infection include injection drug users, healthcare persons, patients undergoing surgery, hemodialysis or blood transfusions, people having homosexual activities or heterosexual activities with multiple sex partners, and neonates born to chronic carrier mothers or mothers having acute HBV infection during pregnancy [33, 48, 49]. CHB is a persistent problem in neonates delivered by HBeAg-positive mothers, and the severity of infection decreases in them with age (20–60% under 1–5 years and < 5% in adults) [50, 51]. Immunity against a specific disease can be developed through effective vaccination or infection recovery. Immunocompromised or immunosuppressed people are at more risk of HBV infections [52]. A patient’s age is an essential factor in determining the severity and outcome of HBV infection. CHB is highly prevalent in neonates up to six months of age [38, 39]. Although the liver has the highest regeneration power, the age and nutritional status of the patient also play an essential role in recovery from viral hepatitis infections. Various factors responsible for HCC due to chronic viral hepatitis include gender (hepatocellular carcinoma is much more common in men than women), race/ethnicity, cirrhosis, inherited metabolic diseases, heavy alcohol use, tobacco use, and obesity.
HBV and liver cirrhosis
Liver cirrhosis is a progressive stage of liver infection that may occur due to various reasons, including the conditions of HBV or HCV infection, alcoholism, and autoimmune diseases. The presence of more than one factor may cause more dangerous liver cirrhosis [8, 53]. During HBV infection, hepatocytes may gradually be damaged and lead to scar formation. The initial stage of liver damage is called fibrosis, which leads to a severe condition known as liver cirrhosis. Unfortunately, most patients do not experience any unusual symptoms until full-blown cirrhosis or end-stage liver disease. Therefore, they do not get early and effective treatment, which worsens the situation [54]. Liver transplantation is the only solution available for end-stage cirrhosis patients. Cirrhosis and hepatocellular carcinoma (HCC) are significant reasons for premature death in 25% of HBV patients who get infected in childhood, while 15% die from these diseases who got HBV infection after childhood [6, 55].
Detection of HCC and HBV
In addition, early detection of HCC is a significant barrier. In recent times, the early identification of HCC has centered on surveillance through ultrasonography (US) [56], and alpha-fetoprotein serological tests (AFP) [57]. However, the specificity and sensitivity of the US and AFP are not adequate for the diagnosis of HCC. Also, Recent technological advances offer hope for HCC detection at an early stage. The tests used to diagnose HCC generally involve cross-sectional diagnostic imaging, serological diagnosis, and histological diagnosis. Cross-sectional diagnostic imaging, such as ultrasound (US) [56], computed tomography (CT), and magnetic resonance imaging (MRI), is indispensable not only for the diagnosis of hepatocellular carcinoma (HCC) but also for the assessment of tumor staging and therapeutic response [58]. In serological diagnosis, various types of biomarkers are used for detection. They may be proteinous and mRNA-type biomarkers in nature. Some of them are like, alpha-fetoprotein (AFP) has emerged as a most promising and well-studied biomarker candidate. Dysregulated levels of AFP in the plasma strongly correlate with HCC malignancy. Some mRNA and protein biomarkers are alpha-fetoprotein (AFP), AFP-L3 [59], Protein induced by vitamin K absence II (PIVKA II) [60], Glypican-3 (GPC3) [60], Golgi protein-73 (GP73) [61], Osteopontin (OPN) [62], Dickkopf-1 (DKK-1) [63], lncRNAs, etc. while various are in progress.
In recent times, the concept of “liquid biopsy” has attracted a significant deal of interest and revolutionized the area of tumor diagnostics. Circulating DNA (ctDNA) and circulating tumor cells are important markers for measuring liquid biopsies [64]. Although other biomarkers are being developed. To effectively screen, diagnose, and subsequently treat individuals who are diseased with HBV, it is vital to know the current diagnostic tests. HBV infection is indicated by the presence of HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc IgM and IgG antibodies [65]. The development of serological markers permits the identification of patients infected with HBV. Many serological methods, including radioimmunoassay, enzyme immunoassay, Electrochemiluminescence immunoassay, chemiluminescence immunoassay, micro-particle enzyme immunoassay, and chemiluminescence immunoassay might be used [66]. As in the technique of molecular detection, HBV DNA provides a direct assessment of viral load, revealing the multiplication activity of the virus. It may be detected at an initial stage of infection. The detection of HBV DNA is a great marker of replication activity, and larger titers of HBV DNA are associated with a more rapid course of the illness and an increased risk of HCC. Reverse hybridization, genotype-specific PCR tests, real-time PCR, restriction fragment-length polymorphism, Loop-mediated isothermal amplification assay, sequence analysis, microarray (DNA Chip), and fluorescence polarization assay may be used to validate HBV genotyping [67–69]. Also, the Identification of HBV genotype, HBV mutations, and other prognostic markers enables tailored therapies and risk-surveillance methods, such as hepatocellular carcinoma screening. In the future, these characteristics may allow classification not just of treatment options, but also of individuals at increased risk of recurrence when current medications are terminated. In the majority of the country, Hepatitis viral infection is diagnosed through biochemical assay specifically liver functional test. Most of the cases recover without much significant damage to their liver, some cases convert into liver cirrhosis or HCC. Liver cirrhosis and HCC detect in the later stage when their treatment is very difficult.
Mechanism of HCC due to HBV
Some patients with persistent infection of chronic HBV may develop hepatocellular carcinoma (HCC) or liver cancer. CHB is the primary factor responsible for HCC in the HBV-endemic regions of the world [70–73]. Worldwide, liver cancer is annually accountable for more than one million deaths. HCC is a dangerous and drastic type of disease, the seventh and ninth most prevalent respective cancer in male and female patients. Comparatively, male patients with CHB are at more risk of developing HCC than female patients due to their male hormones [73–75]. Geography, race, age, gender, obesity, alcoholism, and family history are important variables associated with the HCC [76, 77]. The geographic distribution pattern of HCC is related to the frequency of persistent HBV infection. In developing parts of the world, like Africa and East Asia, HBV is the major contributor (60–80%) of liver cancer. In contrast, about 20% of the HBV cases in developed countries contribute to HCC [78, 79]. The diagnosis of HCC can be made by specific cancer markers like serum alfa-fetoprotein (AFP) and by diagnostic imaging of the liver using ultrasound imaging, computed tomography (CT) scan, or magnetic resonance imaging (MRI) [80].
In HBsAg carrier patients, regular check-ups of their liver should be done by these methods at least once every six months. Treatments like surgery, hepatic irradiation, and anticancer drugs are available to treat liver cirrhosis and cancer patients [81]. Hepatitis B virus infection is one of the most significant risk factors for hepatocellular cancer (HCC). Currently, it is considered that HBV-induced HCC is the result of a complicated interplay between viral components and various host factors. Several pathways are believed to be involved in the pathogenesis of HBV-induced HCC, such as HBV–DNA burden, HBV-DNA integration into host genetic machinery, immune-mediated inflammation, HBx protein, etc. [82, 83].
Chronic illness due to HBV results from its presence in the host cells for a long time via various mechanisms that include infection of immune defense control centers, viral inhibition of antigen presentation, selective immune suppression, down-regulation of viral gene expression, and viral mutations. It functionally incapacitates virus-specific T cells from recognizing HBV antigen, and such cells ultimately survive the virus for a long period [84]. People with HBV infection are more likely to get HCC depending on several factors, but HBV–DNA levels are the most significant. When there were 10,000 copies of HBV-DNA per milliliter, the chance of getting HCC went up a lot. People whose HBV–DNA load was below the lower limit of detection (LOD) (300 copies/ml) were half as likely to get HCC as people with viral loads of 10,000–100,000 copies/ml and people with viral loads of > 100,000 copies/ml were six times as likely to get HCC [85, 86]. HBV DNA gets integrated with the human genome, which leads to changes in several genes and ultimately affects cell growth, division, and survival. C-terminal truncated HBx, which is a result of HBV integration, has been emphasized for its role in the development of HCC [87]. Apart from being involved in many intracellular signaling pathways linked to cell growth and death. HBx is a major factor in the development of HCC through direct as well as indirect mechanisms, even though it is not directly linked to cancer progression.
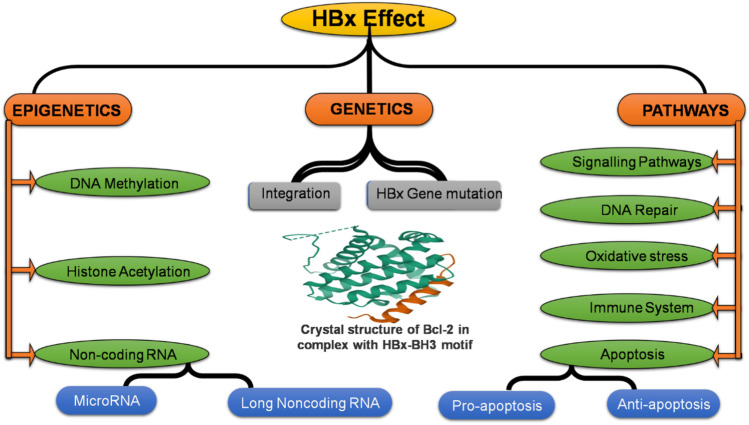
All mammalian hepadnaviruses have a very similar phylogeny, which suggests that the HBx gene, which codes for the HBx protein, is very important to the virus’s life cycle. This gene has 452 nucleotides while the encoded HBx protein is 17 kDa in size and 154 aa in length. HBx protein has many different roles, (as shown in Fig. 3) in the host cell. It can modulate various cellular processes, including cell cycle progress, Apoptosis (effect on pro-apoptosis, and anti-apoptosis), DNA repair (impact on transcription factor IIH, base, human 8-oxoguanine DNA glycosylase 1, DNA glycosylase A and excision repair pathway through both p53-dependent and independent mechanisms), mutation (effect on C-terminal truncation point mutation), immune system (evasion chronic infection), epigenetics (methylation, and acetylation), signalling pathways (effect on notch pathway, PI3K/mTOR, Wnt/beta-catenin signaling pathway), transcriptional pathways like noncoding RNA (microRNA, and long noncoding RNA), cell proliferation, oxidative stress (effect on reactive oxygen species, and NAD(P)H: quinone oxidoreductase 1, Forkhead box class O 4), and genetic stability by interacting with different host factors [88, 89].
Fig. 3.
Various mechanism of HCC by HBV (HBx)
Immune and inflammatory responses induce cytokines and chemokines to be released, which leads to oxidative stress. This, in turn, leads to the constant activation of genes that cause cirrhosis, such as TERT, MLL4, RAR, CCNE1, Cyclin A2, FN1, ROCK1, SENP5, ANGPT1, PDGF receptor, calcium signaling-related genes, ribosomal protein genes, epidermal growth factor receptor (EGFR), and mevalonate kinase carboxypeptidase, which promote HCC proliferation and angiogenesis [90]. HBV products like proteins and mRNAs are involved in many other signaling pathways in hepatocytes. This affects the expression and function of certain genes and can lead to liver disorders. The majority of these alterations are attributable to HCC, and more research is ongoing.
Treatment and prophylaxis
HBV infections can be acute or chronic, and most acute infections are generally self-limiting and do not require specific treatments. There are no specific antivirals or medications for acute HBV; only supportive therapy can be given [91–93] Antiviral treatment is given to patients with chronic hepatitis B to prevent the progression of the disease. Steroid therapy is given to symptomatic patients of chronic HBV with severe histologic lesions in their liver biopsies [94]. Interferon therapy can be provided in CHB infections and acts as an immunomodulator but is generally associated with side effects. The efficacy of interferon therapy is higher in young patients than in aged ones [95]. Specific antiviral drugs such as adefovir, lamivudine, tenofovir, and telbivudine are available for CHB patients. However, antiviral therapy is associated with the risk of developing resistance after prolonged use [96]. Nutritional standards and effective antiviral medicines for HBV-positive patients determine the effective recovery of CHB patients.HBV vaccines are used mainly as the primary prevention to reduce the risk of HBV infection. The first plasma-derived HBV vaccine developed from HBsAg antigen inactivated with the help of urea, pepsin, formaldehyde, and the heat was introduced in 1982 [97]. Other HBV vaccine from recombinant yeast sources was also introduced until the mid-1980s [98]. Presently, recombinant DNA vaccines are being used in national vaccination programs and proving to be highly safe and effective in controlling HBV infections. Universal infant vaccination programs have been carried out worldwide [99]. HBV vaccines are most effective when given early, and antibody response rates decline gradually after 40 years of age [99, 100]. Previous studies proved that the protective effects of HBV vaccination might continue for at least 10 to 15 years or even beyond 15 years in some cases[101–103].
Challenges of HBV and HCC
HBV can be transmitted through various routes like parenteral, perinatal, and sexual, which increase the chances of the virus spreading and pose challenges in controlling the outbreak. Vaccination is available against HBV and is effective in 95% of HBV cases. However, the remaining 5% of vaccinated individuals failed to develop immunity, and the mechanism of this non-response to the HBV vaccine is not fully understood yet [104–106]. Underlying medical conditions such as CHB, HIV infection, hemodialysis, immature neonates, age, and immunosuppression are some factors that seem to be associated with non-response to the HBV vaccine [10]. Most CHB patients remain asymptomatic and are not diagnosed with HBV, but they still threaten infection transmission. All HBsAg-positive carriers serve as the potential source of HBV infection. Large segments of the world population are HBV-positive and act as the carrier, making controlling the disease more challenging [6, 8, 107]. Mother-to-child transmission of HBV is still a common mode of infection in developing parts of the world. About 10% of the children born to infected mothers still have CHB infection despite vaccines [108, 109]. HBV can be diagnosed by rapid commercial, immunological, and modern molecular assays. A good array of diagnostic assays are available, but their distribution is not equal [110–112]. Developing regions of the world still require rapid, sensitive, specific, and cost-effective diagnostic assays to effectively screen HBV-infected populations. More than 75% of the world population lives in hyper-endemic areas of HBV, which provide an easy chance of HBV transmission between infected and susceptible persons [113–115]. HBV carrier people are clinically asymptomatic and may spread the infection for a long time without knowing. Occult HBV infection is another problem in which screening tests based on HBsAg failed to detect the infection due to a very low copy of the virus. Occult HBV infections can be categorized into seropositive and seronegative infections based on the detection of antibodies against HBcAg and HBsAg. About 20% of occult HBV infections are seronegative and are challenging to diagnose [116]. PCR-based detection of HBV DNA is the only reliable method for detecting these infections. Many occult HBV infections go undetected, further spreading infection in the healthy population [115].
Opportunities
Vaccination is considered the major prophylactic measure to reduce the mortality caused by HBV infection in healthy patients. The effectiveness of HBV vaccination can be increased by changing the route of administration. The conventional intramuscular (IM) route of the HBV vaccine, when replaced with intradermal (ID) administration after every two weeks, induces 94% anti-HBV response in previously nonresponder individuals [117]. Recombinant vaccines observed seroconversion in the healthcare worker group at a rate of 95.5% via ID and 85% via IM routes [118]. The intradermal route of vaccination has shown better responses to HBV than conventional intramuscular route vaccination in certain special groups like healthcare workers, dialysis patients, immunocompromised (HIV-infected) patients, and in patients with celiac disease [118–124]. There is a positive impact of hepatitis B recombinant vaccine on immune response in more than 90% of the normal population [125]. On the other hand, despite the staggering response of commercial antivirals, the long-term medication and secluded persistence of the virus in hepatocytes remain a big challenge. Researchers have devised various strategies to tackle these problems, such as identifying novel drug targets, employing the concept of trained immunity, genome editing, and gene therapy. Immense research on novel drug targets for HBV therapy includes nucleoside inhibitors, immune stimulants, and viral entry inhibitors. Immune stimulant molecules are screened that elicit a potent innate immune response [126]. Identified dynamic targets of the immune system mainly consist of pattern recognition receptors and Toll-like receptors [127, 128] 2, 4-Diaminoquinazolines is one such potent Toll-like receptor agonist that shows promising activity in vivo that produces an optimum cytokine profile [129]. Another target for antiviral development is a viral entry inhibitor, which helps reduce the intrahepatic spread of infection. These molecules target NTCP, a leading receptor for viral entry [130, 131]. Myrcludex B is a synthetic N-acylated lipopeptide that showed promising activity in blocking receptor activity, both in vitro and in vivo[132]. Advanced studies on universal antiviral therapy for HBV are based on using ex vivo techniques to engineer the genome of viruses. Modern techniques like CRISPR/Cas9 system enable cleavage by targeting the specific genes of the Virus [133]. A research group proposed targeting the covalently closed circular DNA of the virus using the CRISPR/Cas9 system [134]. Medicinal plants are an excellent alternative to combat the HBV burden as they pose very natural and economical means with few side effects and require low technical expertise. Available HBV therapies have feeble efficiencies in long-term usage with many adverse side effects and limitations in the form of resistance in the case of long-term treatments [135]. Therefore a sensitive, safe, and cost-effective anti-HBV therapy is needed. Worldwide, herbal medicine formulations have a long history of their capability to cure liver infections [136, 137]. Numerous medicinal plants have anti-HBV potential, which must undergo in vivo clinical trials before being used as therapeutics. Oenanthe javanica, Curcumin, and Phyllanthus species are well-documented medicinal plants having anti-HBV properties [138, 139]. Thus, the efficacy of these and other similar medicinal plants against HBV is explored by modern research to replace conventional synthetic and harmful drugs. Acanthus ilicifolius L. can reduce HBV-induced liver damage by suppressing the activity of enzyme transaminase [140]. Phyllanthus extracts show a reduction in HBV DNA synthesis and secretion of HBsAg and HBcAg in infected hepatocytes [141]. This may be due to the induction of β-interferon, cyclooxygenase-2, and interleukin-6 through the activation of signal-regulated kinases and c-jun N-terminal kinases [142]. Phytochemicals like glucopyranosides and flavones obtained from Alternanthera philoxeroides exhibited significant anti-HBV activities in the cell culture model [143]. Active components (oxymatrine, artemisinin, artesunate, and wogonin) from traditional Chinese medicinal plants have potent anti-HBV activities [144]. There is enormous information in the Indian Ayurvedic system, Chinese traditional medicines, and modern medical science that claims various medicinal plants’ effectiveness against many viruses.
Conclusion
HBV is a major pathogen responsible for viral hepatitis and is associated with considerable morbidity and mortality worldwide. Although HBV is much improved in developed parts of the world, the population living in endemic developing countries is still the major reservoir of the virus. CHB and HCC are also serious problems related to HBV, which can be controlled effectively by using universal vaccination programs. Better management of HBV infections can be achieved through increased vaccination among risk groups, awareness campaigns, effective surveillance of the carrier population, and the development of cost-effective diagnoses and treatments. More effective HBV screening and surveillance measures should be devised, especially for CHB infections. More emphasis is needed on research that improves the impact and coverage of the current vaccination program. More inputs are required in gene silencing technology development of antiviral and novel therapeutic Phyto-compounds to eradicate the HBV problem.
Author contribution
Sanjit Boora, Vikrant Sharma, and Samander Kaushik have performed the conception or design of the study. Samander Kaushik, Sulochana Kaushik, Ajoy Varma Bhupatiraju, and Sandeep Singh in editing, acquisition, analysis, or interpretation of the data.
Data availability
Not applicable.
Declarations
All data relevant to the study is included in the article.
Ethical approval
NA.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO (2019) Hepatitis. https://www.who.int/news-room/questions-and-answers/item/hepatitis. Accessed 10 Oct 2022
- 2.WHO (2022) Hepatitis-b. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b Accessed 10 Oct 2022
- 3.Hoofnagle JH, Di Bisceglie AM (1991) Serologic diagnosis of acute and chronic viral hepatitis. In: Seminars in liver disease, vol 11, no. 02. Thieme Medical Publishers, Inc, pp 73–83 [DOI] [PubMed]
- 4.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49(S5):S13–S21. doi: 10.1002/hep.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspinall EJ, Hawkins G, Fraser A, et al. Hepatitis B prevention, diagnosis, treatment and care: a review. Occup Med. 2011;61(8):531–540. doi: 10.1093/occmed/kqr136. [DOI] [PubMed] [Google Scholar]
- 7.Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol. 2017;9(5):227. doi: 10.4254/wjh.v9.i5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 9.Trépo C, Chan HL, Lok A. Hepatitis B virus infection. The Lancet. 2014;384(9959):2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 10.Meireles LC, Marinho RT, Van Damme P. Three decades of hepatitis B control with vaccination. World J Hepatol. 2015;7(18):2127. doi: 10.4254/wjh.v7.i18.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Huang H, Liu Y, Chen R et al (2020) HBV genome and life cycle. In: Tang H (eds) Hepatitis B Virus Infection. Advances in experimental medicine and biology, vol 1179. Springer, Singapore, pp 17–37. 10.1007/978-981-13-9151-4_2 [DOI] [PubMed]
- 12.Herrscher C, Roingeard P, Blanchard E. Hepatitis B virus entry into cells. Cells. 2020;9(6):1486. doi: 10.3390/cells9061486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes CN, Zhang Y, Makokha GN, et al. Early events in hepatitis B virus infection: from the cell surface to the nucleus. J Gastroenterol Hepatol. 2016;31(2):302–309. doi: 10.1111/jgh.13175. [DOI] [PubMed] [Google Scholar]
- 14.Huang HC, Chen CC, Chang WC, et al. Entry of hepatitis B virus into immortalized human primary hepatocytes by clathrin-dependent endocytosis. Virol J. 2012;86(17):9443–9453. doi: 10.1128/JVI.00873-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W. The hepatitis B virus receptor. Annu Rev Cell Dev Biol. 2015;31:125–147. doi: 10.1146/annurev-cellbio-100814-125241. [DOI] [PubMed] [Google Scholar]
- 16.Yan H, Zhong G, Xu G et al (2012) Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 1:e00049 [DOI] [PMC free article] [PubMed]
- 17.Salhab A, Amer J, Safadi R (2018) Sodium taurocholate co-transporting polypeptide (NTCP) as novel checkpoint in NK cells activity in liver fibrosis. In: Hepatology, vol 68, pp 647A-648A. Wiley, Hoboken, pp 07030–5774
- 18.Bowden S, Jackson K, Littlejohn M, Locarnini S (2004) Quantification of HBV covalently closed circular DNA from liver tissue by Real-Time PCR. In: Hamatake RK, Lau JYN (eds) Hepatitis B and D Protocols. Methods in Molecular Medicine, vol 95. Humana Press, pp 41–50. 10.1385/1-59259-669-X:41 [DOI] [PubMed]
- 19.Tang H, McLachlan A. Transcriptional regulation of hepatitis B virus by nuclear hormone receptors is a critical determinant of viral tropism. PNAS. 2001;98(4):1841–1846. doi: 10.1073/pnas.98.4.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urban S, Schulze A, Dandri M, Petersen J. The replication cycle of hepatitis B virus. J Hepatol. 2010;52(2):282–284. doi: 10.1016/j.jhep.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 21.Lucifora J, Arzberger S, Durantel D, et al. Hepatitis B virus X protein is essential to initiate and maintain virus replication after infection. J Hepatol. 2011;55(5):996–1003. doi: 10.1016/j.jhep.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Nassal M. Hepatitis B viruses: reverse transcription a different way. Virus Res. 2008;134(1–2):235–249. doi: 10.1016/j.virusres.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Jones SA, Hu J. Hepatitis B virus reverse transcriptase: diverse functions as classical and emerging targets for antiviral intervention. Emerg Microbes Infect. 2013;2(1):1–11. doi: 10.1038/emi.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dandri M, Petersen J (2016) Mechanism of hepatitis B virus persistence in hepatocytes and its carcinogenic potential. Clin Infect Dis 62(suppl_4):S281–S288 [DOI] [PMC free article] [PubMed]
- 25.Inoue T, Tanaka Y. Hepatitis B virus and its sexually transmitted infection-an update. Microbial Cell. 2016;3(9):420. doi: 10.15698/mic2016.09.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mariano A, Mele A, Tosti ME, et al. Role of beauty treatment in the spread of parenterally transmitted hepatitis viruses in Italy. J Med Virol. 2004;74(2):216–220. doi: 10.1002/jmv.20182. [DOI] [PubMed] [Google Scholar]
- 27.Hui PW, Ng C, Cheung KW, Lai CL. Acceptance of antiviral treatment and enhanced service model for pregnant patients carrying hepatitis B. Hong Kong Med J. 2020;26(4):318. doi: 10.12809/hkmj208451. [DOI] [PubMed] [Google Scholar]
- 28.Scott RM, Snitbhan R, Bancroft WH, et al. Experimental transmission of hepatitis B virus by semen and saliva. J Infect Dis. 1980;142(1):67–71. doi: 10.1093/infdis/142.1.67. [DOI] [PubMed] [Google Scholar]
- 29.Karayiannis P, Novick DM, Lok AS, et al. Hepatitis B virus DNA in saliva, urine, and seminal fluid of carriers of hepatitis B e antigen. Br Med J (Clin Res Ed) 1985;290(6485):1853–1855. doi: 10.1136/bmj.290.6485.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hebo HJ, Gemeda DH, Abdusemed KA. Hepatitis B and C viral infection: prevalence, knowledge, attitude, practice, and occupational exposure among healthcare workers of Jimma University Medical Center, southwest Ethiopia. Sci World J. 2019 doi: 10.1155/2019/9482607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roien R, Mousavi SH, Ozaki A, et al. Assessment of knowledge, attitude, and practice of health-care workers towards hepatitis B virus prevention in Kabul, Afghanistan. JMDH. 2021;14:3177. doi: 10.2147/JMDH.S334438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coppola N, De Pascalis S, Onorato L, et al. Hepatitis B virus and hepatitis C virus infection in healthcare workers. World J Hepatol. 2016;8(5):273. doi: 10.4254/wjh.v8.i5.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou J, Liu Z, Gu F. Epidemiology and prevention of hepatitis B virus infection. Int J Med Sci. 2005;2(1):50. doi: 10.7150/ijms.2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aljarbou AN. The Emergent Concern of Hepatitis B globally with special attention to Kingdom of Saudi Arabia. Int J Health Sci. 2013;7(3):333. doi: 10.12816/0006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russi JC, Serra M, Viñoles J, et al. Sexual transmission of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus type 1 infections among male transvestite comercial sex workers in Montevideo, Uruguay. Am J Trop Med. 2003;68(6):716–720. doi: 10.4269/ajtmh.2003.68.716. [DOI] [PubMed] [Google Scholar]
- 36.Van Houdt R, Bruisten SM, Geskus RB, et al. Ongoing transmission of a single hepatitis B virus strain among men having sex with men in Amsterdam. J Viral Hepat. 2010;17(2):108–114. doi: 10.1111/j.1365-2893.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 37.Chilaka VN, Konje JC. Viral hepatitis in pregnancy. Eur J Obstet Gynecol. 2021;256:287–296. doi: 10.1016/j.ejogrb.2020.11.052. [DOI] [PubMed] [Google Scholar]
- 38.Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol. 1977;105(2):94–98. doi: 10.1093/oxfordjournals.aje.a112370. [DOI] [PubMed] [Google Scholar]
- 39.Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis. 1995;20(4):992–1000. doi: 10.1093/clinids/20.4.992. [DOI] [PubMed] [Google Scholar]
- 40.Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. The Lancet. 2015;386(10003):1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 41.Custer B, Sullivan SD, Hazlet TK et al (2004) Global epidemiology of hepatitis B. virus J Clin Gastroenterol 38(10):S158–S168 [DOI] [PubMed]
- 42.Shepard CW, Simard EP, Finelli L, et al. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28(1):112–125. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- 43.Yan YP, Su HX, Ji Z, et al. Epidemiology of hepatitis B virus infection in China: current status and challenges. J Clin Transl Hepatol. 2014;2(1):15. doi: 10.14218/JCTH.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Razavi-Shearer D, Gamkrelidze I, Nguyen MH, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 45.Mahoney FJ. Update on diagnosis, management, and prevention of hepatitis B virus infection. Clin Microbiol Rev. 1999;12(2):351–366. doi: 10.1128/CMR.12.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puri P. Tackling the hepatitis B disease burden in India. JCEH. 2014;4(4):312–319. doi: 10.1016/j.jceh.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ott JJ, Stevens GA, Groeger J, Wiersma Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 48.Khosravani A, Sarkari B, Negahban H, et al. Hepatitis B Infection among high risk population: a seroepidemiological survey in Southwest of Iran. BMC infec dis. 2012;12(1):1–4. doi: 10.1186/1471-2334-12-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozer A, Yakupogullari Y, Beytur A, et al. Risk factors of hepatitis B virus infection in Turkey: a population-based, case-control study: risk factors for HBV infection. Hepat Mon. 2011;11(4):263. [PMC free article] [PubMed] [Google Scholar]
- 50.Chu CM, Liaw YF (2016) Natural history of hepatitis B virus infection. In: Hepatitis B virus in human diseases. Humana Press, Cham, pp 217–247
- 51.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology-Baltimore then Orlando- 2007;45(2):507. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 52.Mandalà M, Fagiuoli S, Francisci D, et al. Hepatitis B in immunosuppressed cancer patients: pathogenesis, incidence and prophylaxis. Crit Rev Oncol. 2013;87(1):12–27. doi: 10.1016/j.critrevonc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Nishikawa H, Osaki Y (2015) Liver cirrhosis: evaluation, nutritional status, and prognosis. Mediators Inflamm 2015. 10.1155/2015/872152 [DOI] [PMC free article] [PubMed]
- 54.Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol. 2012;56(5):1171–1180. doi: 10.1016/j.jhep.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 55.Fattovich G. Natural history of hepatitis B. J Hepatol. 2003;39:50–58. doi: 10.1016/S0168-8278(03)00139-9. [DOI] [PubMed] [Google Scholar]
- 56.Wu J, Ge L, Lu L. Letter to the editor on “Perfluorobutane contrast-enhanced ultrasonography for the diagnosis of HCC: a systematic review and meta-analysis”. Abdom Radiol. 2022;1:1–2. doi: 10.1007/s00261-021-03338-8. [DOI] [PubMed] [Google Scholar]
- 57.Pan YX, Sun XQ, Hu ZL, et al. Prognostic values of alpha-fetoprotein and des-gamma-carboxyprothrombin in hepatocellular carcinoma in China: An analysis of 4792 patients. J Hepatocell. 2021;25:657–670. doi: 10.2147/JHC.S316223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King MJ, Laothamatas I, Reddy A, Wax R, Lewis S. Cross-sectional imaging findings of atypical liver malignancies and diagnostic pitfalls: emphasis on computed tomography, and magnetic resonance imaging. Radiologic Clinics. 2022;60(5):775–794. doi: 10.1016/j.rcl.2022.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Liu R, Li Y, Wu A, et al. Identification of plasma hsa_circ_0005397 and combined with serum AFP, AFP-L3 as potential biomarkers for hepatocellular carcinoma. Front pharmacol. 2021;12:639963. doi: 10.3389/fphar.2021.639963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caviglia GP, Ciruolo M, Abate ML, et al. Alpha-fetoprotein, protein induced by vitamin K absence or antagonist II and glypican-3 for the detection and prediction of hepatocellular carcinoma in patients with cirrhosis of viral etiology. Cancers. 2020;12(11):3218. doi: 10.3390/cancers12113218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali OM, El Amin HA, Sharkawy YL, et al. Golgi protein 73 versus alpha-fetoprotein as a new biomarker in early diagnosis of hepatocellular carcinoma. Int J Gen Med. 2020;18:193–200. doi: 10.2147/IJGM.S253622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bruha R, Vitek L, Smid V. Osteopontin–A potential biomarker of advanced liver disease. Ann Hepatol. 2020;19(4):344–352. doi: 10.1016/j.aohep.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Suda T, Yamashita T, Sunagozaka H, et al. (2022) Dickkopf-1 promotes angiogenesis and is a biomarker for hepatic stem cell-like hepatocellular carcinoma. Int J Biol Sci. 2022;23(5):2801. doi: 10.3390/ijms23052801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu X, Li J, Gassa A, et al. Circulating tumor DNA as an emerging liquid biopsy biomarker for early diagnosis and therapeutic monitoring in hepatocellular carcinoma. Int J Biol Sci. 2020;16(9):1551. doi: 10.7150/ijbs.44024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Osasona OG, Oguntoye T, Eromon P, et al. Atypical serologic profiles of hepatitis B virus infection across clinical cohorts of patients in Southwestern Nigeria. J Immunoass Immunochem. 2023;44(2):176–191. doi: 10.1080/15321819.2023.2168556. [DOI] [PubMed] [Google Scholar]
- 66.Zangiabadian M, Zamani A, Nasiri MJ, Behzadi E, Fooladi AA. Diagnostic accuracy and validity of serological and molecular tests for hepatitis B and C. Curr Pharm Biotechnol. 2022;23(6):803–817. doi: 10.2174/1389201022666210719162802. [DOI] [PubMed] [Google Scholar]
- 67.Pas SD, Tran N, de Man RA, Burghoorn-Maas C, Vernet G, Niesters HG. Comparison of reverse hybridization, microarray, and sequence analysis for genotyping hepatitis B virus. J Clin Microbiol. 2008;46(4):1268–1273. doi: 10.1128/JCM.01519-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang J, Liang X, Ma H, Nie L, Tian Y, Chen G, Wang Y. Detection of hepatitis B virus M204V mutation quantitatively via real-time PCR. J Clin Transl Hepatol. 2021;9(2):143. doi: 10.14218/JCTH.2020.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen X, Wang S, Tan Y, Huang J, Yang X, Li S. Nanoparticle-based lateral flow biosensors integrated with loop-mediated isothermal amplification for the rapid and visual diagnosis of hepatitis B virus in clinical application. Front Bioeng Biotechnol. 2021;9:731415. doi: 10.3389/fbioe.2021.731415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takano S, Yokosuka O, Imazeki F, et al. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatol. 1995;21(3):650–655. doi: 10.1002/hep.1840210308. [DOI] [PubMed] [Google Scholar]
- 71.Chu CM. Natural history of chronic hepatitis B virus infection in adults with emphasis on the occurrence of cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2000;15:E25–E30. doi: 10.1046/j.1440-1746.2000.02097.x. [DOI] [PubMed] [Google Scholar]
- 72.Liu F, Wang XW, Chen L, et al. Systematic review with meta-analysis: development of hepatocellular carcinoma in chronic hepatitis B patients with hepatitis B surface antigen seroclearance. Aliment Pharmacol Ther. 2016;43(12):1253–1261. doi: 10.1111/apt.13634. [DOI] [PubMed] [Google Scholar]
- 73.Ozakyol A. Global epidemiology of hepatocellular carcinoma (HCC epidemiology) JGO. 2017;48(3):238–240. doi: 10.1007/s12029-017-9959-0. [DOI] [PubMed] [Google Scholar]
- 74.Yip TCF, Wong GLH, Chan HLY, et al. Elevated testosterone increases risk of hepatocellular carcinoma in men with chronic hepatitis B and diabetes mellitus. J Gastroenterol Hepatol. 2020;35(12):2210–2219. doi: 10.1111/jgh.15079. [DOI] [PubMed] [Google Scholar]
- 75.Bashir Hamidu R, Chalikonda DM, Hann HW. Gender disparity in host responses to hepatitis B-related hepatocellular carcinoma: a case series. Vaccines. 2021;9(8):838. doi: 10.3390/vaccines9080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blonski W, Kotlyar DS, Forde KA. Non-viral causes of hepatocellular carcinoma. World J Gastroenterol. 2010;16(29):3603. doi: 10.3748/wjg.v16.i29.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McGlynn KA, London WT. Epidemiology and natural history of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2005;19(1):3–23. doi: 10.1016/j.bpg.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 78.Yang JD, Hainaut P, Gores GJ, Amadou A, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 80.Kale A (2021) Hepatocellular carcinoma: diagnosis and surveillance. Hepatocellular Carcinoma - Challenges and Opportunities of a Multidisciplinary Approach. IntechOpen. 10.5772/intechopen.99839
- 81.Maor Y, Malnick S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int J Hepatol. 2013 doi: 10.1155/2013/815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tarocchi M, Polvani S, Marroncini G, Galli A. Molecular mechanism of hepatitis B virus-induced hepatocarcinogenesis. World J Gastroenterol. 2014;20(33):11630. doi: 10.3748/wjg.v20.i33.11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang M, Xi D, Ning Q. Virus-induced hepatocellular carcinoma with special emphasis on HBV. Hepatol Int. 2017;11(2):171–180. doi: 10.1007/s12072-016-9779-5. [DOI] [PubMed] [Google Scholar]
- 84.Ortega-Prieto AM, Dorner M. Immune evasion strategies during chronic hepatitis B and C virus infection. Vaccines. 2017;5(3):24. doi: 10.3390/vaccines5030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen CJ, Yang HI, Su JU, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 86.Burns GS, Thompson AJ. Viral hepatitis B: clinical and epidemiological characteristics. Cold Spring Harb Perspect Med. 2014;4(12):a024935. doi: 10.1101/cshperspect.a024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu Q, Gu S, Liang J, Lin Z, et al. The biological function of hepatitis B virus X protein in hepatocellular carcinoma. Oncol Res. 2019;27(4):509. doi: 10.3727/096504018X15278771272963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ali A, Abdel-Hafiz H, Suhail M, Al-Mars A, et al. Hepatitis B virus, HBx mutants and their role in hepatocellular carcinoma. World J Gastroenterol. 2014;20(30):10238. doi: 10.3748/wjg.v20.i30.10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu S, Koh SS, Lee CG. Hepatitis B virus X protein and hepatocarcinogenesis. Int J Mol Sci. 2016;17(6):940. doi: 10.3390/ijms17060940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ogunwobi OO, Harricharran T, Huaman, et al. Mechanisms of hepatocellular carcinoma progression. World J Gastroenterol. 2019;25(19):2279. doi: 10.3748/wjg.v25.i19.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mahoney, FJ, Kane M (1999) Hepatitis B vaccine in: vaccines. Plotkin, SA, Orenstein, WA
- 92.Durantel D, Zoulim F. New antiviral targets for innovative treatment concepts for hepatitis B virus and hepatitis delta virus. J Hepatol. 2016;64(1):S117–S131. doi: 10.1016/j.jhep.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 93.Gane EJ. Future anti-HBV strategies. Liver Int. 2017;37:40–44. doi: 10.1111/liv.13304. [DOI] [PubMed] [Google Scholar]
- 94.Fujiwara K, Yokosuka O, Kojima H, et al. Importance of adequate immunosuppressive therapy for the recovery of patients with “life-threatening” severe exacerbation of chronic hepatitis B. World J Gastroenterol. 2005;11(8):1109–1114. doi: 10.3748/wjg.v11.i8.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rijckborst V, Janssen HL. The role of interferon in hepatitis B therapy. Curr Hepat Rep. 2010;9(4):231–238. doi: 10.1007/s11901-010-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clercq ED, Férir G, Kaptein S, Neyts J. Antiviral treatment of chronic hepatitis B virus (HBV) infections. Viruses. 2010;2(6):1279–1305. doi: 10.3390/v2061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Szmuness W, Stevens CE, Zang EA, et al. A controlled clinical trial of the efficacy of the hepatitis B vaccine (Heptavax B): a final report. Hepatology. 1981;1(5):377–385. doi: 10.1002/hep.1840010502. [DOI] [PubMed] [Google Scholar]
- 98.McAleer WJ, Buynak EB, Maigetter RZ et al (1992) Human hepatitis B vaccine from recombinant yeast. 1984. Biotechnology (Reading, Mass.) 24:500–502 [PubMed]
- 99.Vitaliti G, Praticò AD, Cimino C, et al. Hepatitis B vaccine in celiac disease: yesterday, today and tomorrow. World J Gastroenterol. 2013;19(6):838. doi: 10.3748/wjg.v19.i6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Averhoff F, Mahoney F, Coleman P, et al. Immunogenicity of hepatitis B vaccines: implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med. 1998;15(1):1–8. doi: 10.1016/S0749-3797(98)00003-8. [DOI] [PubMed] [Google Scholar]
- 101.Geier MR, Geier DA, Zahalsky AC. A review of hepatitis B vaccination. Expert Opin Drug Saf. 2003;2(2):113–122. doi: 10.1517/14740338.2.2.113. [DOI] [PubMed] [Google Scholar]
- 102.Zanetti AR, Mariano A, Romanò L, et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005;366(9494):1379–1384. doi: 10.1016/S0140-6736(05)67568-X. [DOI] [PubMed] [Google Scholar]
- 103.Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011;53(1):68–75. doi: 10.1093/cid/cir270. [DOI] [PubMed] [Google Scholar]
- 104.Coates T, Wilson R, Patrick G, et al. Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther. 2001;23(3):392–403. doi: 10.1016/S0149-2918(01)80044-8. [DOI] [PubMed] [Google Scholar]
- 105.Walayat S, Ahmed Z, Martin D, Puli S, et al. Recent advances in vaccination of non-responders to standard dose hepatitis B virus vaccine. World J Hepatol. 2015;7(24):2503. doi: 10.4254/wjh.v7.i24.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roukens AH, Visser LG. Hepatitis B vaccination strategy in vaccine low and non-responders: a matter of quantity of quality? Hum Vaccin. 2011;7(6):654–657. doi: 10.4161/hv.7.6.14986. [DOI] [PubMed] [Google Scholar]
- 107.Tseng TC, Liu CJ, Chang CT, et al. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis. J Hepatol. 2020;72(6):1105–1111. doi: 10.1016/j.jhep.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 108.Chen HL, Lin LH, Hu FC, et al. Effects of maternal screening and universal immunization to prevent mother-to-infant transmission of HBV. Gastroenterology. 2012;142(4):773–781. doi: 10.1053/j.gastro.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 109.World Health Organization (2020) Prevention of mother-to-child transmission of hepatitis B virus (HBV): Guidelines on antiviral prophylaxis in pregnancy. World Health Organization [PubMed]
- 110.Zangiabadian M, Zamani A, Nasiri MJ, et al. Diagnostic accuracy and validity of serological and molecular tests for hepatitis B and C. Curr Pharm Biotechnol. 2022;23(6):803–817. doi: 10.2174/1389201022666210719162802. [DOI] [PubMed] [Google Scholar]
- 111.Datta S, Chatterjee S, Veer V. Recent advances in molecular diagnostics of hepatitis B virus. World J Gastroenterol. 2014;20(40):14615. doi: 10.3748/wjg.v20.i40.14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sharma V, Kumar D, Dhull D, et al. Molecular detection of hepatitis B viruses (HBV) Int Blood Res Rev. 2017;7(1):1–6. doi: 10.9734/IBRR/2017/31171. [DOI] [Google Scholar]
- 113.Forbi JC, Obagu JO, Gyar SD, et al. Application of dried blood spot in the sero-diagnosis of hepatitis B infection (HBV) in an HBV hyper-endemic nation. Ann Afr Med. 2010;9(1):44. doi: 10.4103/1596-3519.62625. [DOI] [PubMed] [Google Scholar]
- 114.Tesfaye T (2020) A mathematical model analysis on the spread and control of chronic and hyper toxic forms of hepatitis b virus in Ethiopia (Doctoral dissertation)
- 115.Villar LM, Cruz HM, Barbosa JR, et al. Update on hepatitis B and C virus diagnosis. World J Virol. 2015;4(4):323. doi: 10.5501/wjv.v4.i4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2(8):479–486. doi: 10.1016/S1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 117.Playford EG, Hogan PG, Bansal AS, et al. Intradermal recombinant hepatitis B vaccine for healthcare workers who fail to respond to intramuscular vaccine. Infect Control Hosp Epidemiol. 2002;23(2):87–90. doi: 10.1086/502012. [DOI] [PubMed] [Google Scholar]
- 118.Leonardi S, Praticò AD, Lionetti E, et al. Intramuscular vs intradermal route for hepatitis B booster vaccine in celiac children. WJG. 2012;18(40):5729. doi: 10.3748/wjg.v18.i40.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levitz RE, Cooper BW, Regan HC. Immunization with high-dose intradermal recombinant hepatitis B vaccinein healthcare workers who failed to respond to intramuscular vaccination. Infect Control Hosp Epidemiol. 1995;16(2):88–91. doi: 10.2307/30140948. [DOI] [PubMed] [Google Scholar]
- 120.Chanchairujira T, Chantaphakul N, Thanwandee T, Ong-Ajyooth L. Efficacy of intradermal hepatitis B vaccination compared to intramuscular vaccination in hemodialysis patients. J Med Assoc Thai. 2006;89(Suppl 2):S33–S40. [PubMed] [Google Scholar]
- 121.Ghebrehewet S, Baxter D, Falconer M, Paver K. Intradermal recombinant hepatitis B vaccination (IDRV) for non-responsive healthcare workers (HCWs) Hum Vaccin. 2008;4(4):280–285. doi: 10.4161/hv.4.4.5687. [DOI] [PubMed] [Google Scholar]
- 122.Barraclough KA, Wiggins KJ, Hawley CM, et al. Intradermal versus intramuscular hepatitis B vaccination in hemodialysis patients: a prospective open-label randomized controlled trial in nonresponders to primary vaccination. AJKD. 2009;54(1):95–103. doi: 10.1053/j.ajkd.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 123.Fabrizi F, Dixit V, Messa P, Martin P. Intradermal vs intramuscular vaccine against hepatitis B infection in dialysis patients: a meta-analysis of randomized trials. J Viral Hepat. 2011;18(10):730–737. doi: 10.1111/j.1365-2893.2010.01354.x. [DOI] [PubMed] [Google Scholar]
- 124.Whitaker JA, Rouphael NG, Edupuganti S, et al. Strategies to increase responsiveness to hepatitis B vaccination in adults with HIV-1. Lancet Infect Dis. 2012;12(12):966–976. doi: 10.1016/S1473-3099(12)70243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Filippelli M, Lionetti E, Gennaro A, et al. Hepatitis B vaccine by intradermal route in non responder patients: an update. WJG. 2014;20(30):10383. doi: 10.3748/wjg.v20.i30.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut. 2012;61(12):1754–1764. doi: 10.1136/gutjnl-2011-301073. [DOI] [PubMed] [Google Scholar]
- 127.Guo H, Jiang D, Ma D, et al. Activation of pattern recognition receptor-mediated innate immunity inhibits the replication of hepatitis B virus in human hepatocyte-derived cells. Virol J. 2009;83(2):847–858. doi: 10.1128/JVI.02008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang RN, Pan Q, Zhang Z et al (2015) Saturated fatty acid inhibits viral replication in chronic hepatitis B virus infection with nonalcoholic Fatty liver disease by toll-like receptor 4-mediated innate immune response. Hepat Mon 15(5):e27909. 10.5812/hepatmon.32636 [DOI] [PMC free article] [PubMed]
- 129.Embrechts W, Herschke F, Pauwels F, Stoops, et al. 2, 4-Diaminoquinazolines as dual toll-like receptor (TLR) 7/8 modulators for the treatment of hepatitis B virus. J Med Chem. 2018;61(14):6236–6246. doi: 10.1021/acs.jmedchem.8b00643. [DOI] [PubMed] [Google Scholar]
- 130.Colpitts CC, Verrier ER, Baumert TF. Targeting viral entry for treatment of hepatitis B and C virus infections. ACS Infect Dis. 2015;1(9):420–427. doi: 10.1021/acsinfecdis.5b00039. [DOI] [PubMed] [Google Scholar]
- 131.Verrier ER, Colpitts CC, Sureau C, et al. Hepatitis B virus receptors and molecular drug targets. Hepatol Int. 2016;10(4):567–573. doi: 10.1007/s12072-016-9718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Urban S, Bartenschlager R, Kubitz R, et al. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147(1):48–64. doi: 10.1053/j.gastro.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 133.Bhardwaj P, Kant R, Behera SP, et al. Next-generation diagnostic with CRISPR/Cas: beyond nucleic acid detection. Int J Mol Sci. 2022;23(11):6052. doi: 10.3390/ijms23116052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kumar N, Sharma S, Kumar R, et al. Host-directed antiviral therapy. Clin Microbiol Rev. 2020;33(3):e00168–e219. doi: 10.1128/CMR.00168-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Karayiannis P. Hepatitis B virus: old, new and future approaches to antiviral treatment. J Antimicrob Chemother. 2003;51(4):761–785. doi: 10.1093/jac/dkg163. [DOI] [PubMed] [Google Scholar]
- 136.Samani ZN, Kopaei MR (2018) Effective medicinal plants in treating hepatitis B. Int J Pharm Sci 9(9):3589–3596. 10.13040/IJPSR.0975-8232.9(9).3589-96
- 137.Siddiqui MH, Alamri SA, Al-Whaibi MH, et al. A mini-review of anti-hepatitis B virus activity of medicinal plants. Biotechnol Biotechnol Equip. 2017;31(1):9–15. doi: 10.1080/13102818.2016.1240593. [DOI] [Google Scholar]
- 138.Xia J, Inagaki Y, Song P, et al. Advance in studies on traditional Chinese medicines to treat infection with the hepatitis B virus and hepatitis C virus. Biosci Trends. 2016;10(5):327–336. doi: 10.5582/bst.2016.01110. [DOI] [PubMed] [Google Scholar]
- 139.Wei ZQ, Zhang YH, Ke CZ, et al. Curcumin inhibits hepatitis B virus infection by down-regulating cccDNA-bound histone acetylation. World J Gastroenterol. 2017;23(34):6252. doi: 10.3748/wjg.v23.i34.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wei PH, Wu SZ, Mu XM, et al. Effect of alcohol extract of Acanthus ilicifolius L. on anti-duck hepatitis B virus and protection of liver. J Ethnopharmacol. 2015;160:1–5. doi: 10.1016/j.jep.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 141.Mehrotra R, Rawat S, Kulshreshtha DK, et al. In vitro effect of Phyllanthus amarus on hepatitis B virus. IJMR. 1991;93:71–73. [PubMed] [Google Scholar]
- 142.Kristófi R, Eriksson JW. Metformin as an anti-inflammatory agent: a short review. Int J Endocrinol. 2021;251(2):R11–R22. doi: 10.1530/JOE-21-0194. [DOI] [PubMed] [Google Scholar]
- 143.Li B, Guo QL, et al. New anti-HBV C-boivinopyranosyl flavones from Alternanthera philoxeroides. Molecules. 2016;21(3):336. doi: 10.3390/molecules21030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cui X, Wang Y, Kokudo N, et al. Traditional Chinese medicine and related active compounds against hepatitis B virus infection. Biosci Trends. 2010;4(2):39–47. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.