Abstract
Objective
The drug‐refractory epilepsy (DRE) in children is commonly observed but the underlying mechanisms remain elusive. We examined whether fatty acids (FAs) and lipids are potentially associated with the pharmacoresistance to valproic acid (VPA) therapy.
Methods
This single‐center, retrospective cohort study was conducted using data from pediatric patients collected between May 2019 and December 2019 at the Children's Hospital of Nanjing Medical University. Ninety plasma samples from 53 responders with VPA monotherapy (RE group) and 37 non‐responders with VPA polytherapy (NR group) were collected. Non‐targeted metabolomics and lipidomics analysis for those plasma samples were performed to compare the potential differences of small metabolites and lipids between the two groups. Plasma metabolites and lipids passing the threshold of variable importance in projection value >1, fold change >1.2 or <0.8, and p‐value <0.05 were regarded as statistically different substances.
Results
A total of 204 small metabolites and 433 lipids comprising 16 different lipid subclasses were identified. The well‐established partial least squares‐discriminant analysis (PLS‐DA) revealed a good separation of the RE from the NR group. The FAs and glycerophospholipids status were significantly decreased in the NR group, but their triglycerides (TG) levels were significantly increased. The trend of TG levels in routine laboratory tests was in line with the lipidomics analysis. Meanwhile, cases from the NR group were characterized by a decreased level of citric acid and L‐thyroxine, but with an increased level of glucose and 2‐oxoglutarate. The top two enriched metabolic pathways involved in the DRE condition were biosynthesis of unsaturated FAs and linoleic acid metabolism.
Significance
The results of this study suggested an association between metabolism of FAs and the medically intractable epilepsy. Such novel findings might propose a potential mechanism linked to the energy metabolism. Ketogenic acid and FAs supplementation might therefore be high‐priority strategies for DRE management.
Keywords: children, drug‐refractory epilepsy, fatty acids, lipidomics, metabolomics
Key Points.
Disentangling attributable determinants for drug‐refractory epilepsy (DRE) risk remains a core challenge in epilepsy research field.
A decrease in plasma fatty acids (FAs) levels but an increase in triglycerides were revealed for DRE cases by integrating metabolomics and lipidomics.
This study brings out the differences in lipid subtypes between two groups which has not been explicitly discussed in previous studies.
Novel findings in this study might propose a potential mechanism linked to energy metabolism.
1. INTRODUCTION
Epilepsy is a progressive, chronic neurological disorder that affects around 65 million people worldwide, with an estimated lifetime prevalence of 6.4‰. 1 Anti‐seizure medications (ASMs) treatment is currently the mainstay of epilepsy therapeutics, however, approximately one‐third of cases develop drug‐refractory epilepsy (DRE). 2 The sobering reality is that a relative proportion of the medically intractable population still remains unaltered over many decades, despite dozens of new drugs and old ones like valproic acid (VPA) are available for the clinical use. Obviously, it bears a significant educational, social, cognitive, and economic burden. Therefore, disentangling the attributable determinants of individual risks for DRE remains a core challenge in epilepsy research and therapeutics.
Interestingly, ketogenic diet, formulated a century ago, was observed anecdotally to control seizures but now becomes the signature metabolic therapy for DRE. 3 Indeed, research on the use of the classic ketogenic diet and its alternative forms like medium‐chain triglycerides (MCT) has provided relevant insights into the roles of fatty acids (FAs) in seizure control. 4 As one of the proposed indirect mechanisms, ketogenic diet induces prominent ketonemia through enhanced FA oxidation, which elevates blood levels of the principle ketone bodies like β‐hydroxybutyrate and acetoacetate, restores the impaired bioenergetics and mitochondrial function, modulates the tricarboxylic acid (TCA) cycle and respiratory chain activity, and improves redox regulation and antioxidant capacity. 5
This metabolic mechanism is likely to trigger an increasing interest to monitor the plasma FAs levels in DRE cases and in responders to ASM treatments. Indeed, the capacity to reduce the seizure burden in children 6 , 7 and adults 8 with DRE and underlying mechanisms 9 , 10 , 11 , 12 have received extensive attention in recent years. For example, increasing evidence indicates that imbalances in metabolism and FA levels drive the initiation and progression of epilepsy. 9 , 13 In the meantime, blood FAs' abnormalities have been reported in individuals with central nervous system disorders. 14 However, one intriguing question that has emerged is whether the altered FAs in DRE cases are a common occurrence, as yet understudied, that may be used as a biomarker for drug sensitivity.
Lipidomics is an emerging field involving the systematic study of lipids in biological systems. 15 , 16 Advances in mass‐spectrometry (MS) make detailed lipid composition characterization of a given biological sample possible, which includes different FAs classes and other lipid categories. 17 , 18 , 19 , 20 Interestingly, recent increasing studies have revealed the metabolic or lipid profiles between responders and non‐responders to ASM treatments. 21 , 22 , 23 Unfortunately, although data from clinical trials have confirmed FAs supplementation resulted in beneficial effects on seizure frequency in adults and children with epilepsy, 24 published reports are scarce on the FAs status in drug‐responsive as well as in non‐responsive patients. The true role of FAs metabolism in drug resistance remains largely elusive.
The hypothesis of the current study was that plasma FAs and other lipids levels in children with epilepsy who were responsive to VPA monotherapy would be very different to those cases were resistant to various VPA polytherapy, by using MS‐based approaches followed by statistical analysis.
2. MATERIALS AND METHODS
2.1. Study subjects
This single‐center, retrospective cohort study was performed using data from pediatric patients between May 2019 and December 2019 at the Children's Hospital of Nanjing Medical University. All of those children were diagnosed with epilepsy and treated with VPA monotherapy or polytherapy with various ASMs. Routine therapeutic drug monitoring (TDM) of VPA for those cases was performed in our laboratory.
The exclusion criteria were: (1) the age of epilepsy onset was more than 6 years old; (2) the diagnosis of epilepsy did not match or contained high‐risk secondary factors (such as viral meningitis, traumatic brain injury); (3) lost to follow‐up; (4) patients were responsive with two types of ASMs; (5) hemolyzed samples; (6) patients receiving therapy with ketogenic diet; (7) patients with other diseases (like high lipidemia and leukemia). Comprehensive demographic details were collected from the hospital information system (HIS) included basic demographics, laboratory examinations, procedures, medication records, and body weight (kg).
According to the clinical outcomes, patients were divided into the responsive group (RE, those who receiving VPA monotherapy and are seizure‐free for a minimum of three times the longest preintervention interseizure interval or 12 months, whichever is longer) and the non‐responsive group (NR, those defined as failure after adequate trials of VPA polytherapy with other appropriately chosen ASMs schedules to achieve sustained seizure freedom). 25 The study protocol complied with the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of Children's Hospital of Nanjing Medical University (Protocol number 201902055–1). Informed consent was obtained from the parents of each patient before enrollment in this study.
2.2. Plasma sample collection
For this study, left‐over plasma specimens were tested after completing the routine TDM of VPA test. Briefly, ASMs treatment was maintained for more than 5 half‐lives to ensure that blood sampling was performed under steady status. Peripheral venous blood samples (2 mL) were collected 30 min before the next scheduled dose. Blood was collected into tubes containing the anticoagulant EDTA, and plasma was separated by centrifugation at 4000 rpm for 8 min. After the plasma trough concentration of VPA was determined, the plasma was collected immediately and stored at −80°C until omics analysis. 26
2.3. Preparation of plasma metabolites and lipids
For plasma metabolomic analysis, 100 μL of plasma samples were precipitated by adding 400 μL of cold methanol: acetonitrile (1:1, v/v), then vortexed for 3 min and sonicated for another 10 min in an ice bath. After centrifugation at 12000 g for 15 min at 4°C, the supernatant was dried using a vacuum freeze dryer. The dried samples were reconstituted in 100 μL of 50% acetonitrile and centrifuged for liquid chromatography (LC)‐MS/MS analysis. 27
For plasma lipidomic analysis, 25 μL of plasma samples were aliquoted into EP tubes and diluted with 100 μL of isopropanol, and then vortexed for 3 min. To improve precipitation efficiency, samples were kept for 2 h at 4 °C and then centrifuged at 12000 g for 15 min at 4°C. Extracts were transferred to inserts and placed in vials for LC–MS/MS analysis. 28
Pooled quality control (QC) samples that consisted of 10 μL of small metabolites or lipids extracts from each sample were used for monitoring the stability of the instrument during sequence running of the samples. The QC samples were inserted into the analysis sequence after running 1 blank sample and 9 real samples when the studied plasma samples were randomly analyzed.
2.4. Metabolomic and lipidomic analysis
Metabolomic and lipidomic analysis of plasma samples were performed using a SCIEX ExionLC system coupled with SCIEX Triple TOF 5600 plus System (SCIEX). Polar metabolite profiling was analyzed by hydrophilic interaction liquid chromatography (HILIC)‐LC–MS/MS methods with positive electrospray ionization (ESI) mode and negative ESI mode, namely Method 1 and Method 2, respectively. Lipids analysis by reverse‐phase (RP)‐LC–MS methods with positive ESI mode and negative ESI mode, named Method 3 and Method 4, respectively.
Chromatographic separation in Methods 1 and 2 was performed on a Acquity UPLC BEH Amide column (100 × 2.1 mm, 1.7 μm) with the temperature set at 40 °C and with a flow rate of 0.30 mL/min. The mobile phase A (MPA) was water with 25 mM NH4OAc and 25 mM NH4OH, the mobile phase B (MPB) was 100% acetonitrile. Methods 3 and 4 was performed on a Kinetex C18 (2.1 mm × 100 mm; 2.6 μm) column with the temperature set at 55°C and a flow rate of 0.30 mL/min. MPA and MPB were composed of acetonitrile: methanol: water (1:1:1, v/v/v) with 5 mM ammonium acetate and 100% isopropanol with 5 mM ammonium acetate, respectively. The gradient elution programs for metabolomic and lipidomic analysis are shown in Tables S1 ans S2, respectively. A 2‐μL resultant solution was injected for LC–MS/MS analysis.
MS/MS analysis was performed using the following parameters: ion spray voltage, 5.5 kV (+) and 4.5 kV (−); curtain gas, 40 psi; declustering potential, 80 V (+/−); and interface heater temperature, 550°C. Collision energy was set at 35 ± 15 V for both positive and negative modes, along with dynamic background subtraction. The experiments were run with 200 ms accumulation time for TOF MS m/z 60–1000 for small metabolites and m/z 200–1250 for lipids, and 50 ms accumulation time for TOF MS/MS m/z 30–1000 for small metabolites and m/z 100–1250 for lipids combined with information dependent acquisition mode.
2.5. Raw data extraction and compounds identification
For plasma metabolomics, the original data were converted to .xzmal format using Analysis Base File Converter software. Then the XCMS package (Version 6.0) was used to extract peak abundances and retention time alignment for those potentially small metabolites. Metabolite identification was first performed through matching the accurate precursor mass (MS1) and MS2 spectrum (MS/MS) with those from the standard spectral library in Masterview (Version 1.2). Mass error less than 5 ppm and library hit score more than 70 of the metabolites were regarded as acceptable for further investigation. Meanwhile, we uploaded the MS1 peak table and MS2 spectra in a .mgf format to MetDNA (Version 2, http://metdna.zhulab.cn/) to identify metabolites. 29 The “HILIC”, “Sciex TripleTOF”, and “35 ± 15” were selected for liquid chromatography, instrument, and collision energy, respectively. We performed Masterview and MetDNA annotation in both positive and negative modes, separately.
For plasma lipidomics, MS‐DIAL (Version 4.8) was used to filter and identify candidate lipids based on exact mass, retention time, and MS/MS patterns. PeakView workstation (Version 2.2; SCIEX) was used to check lipids MS/MS information. MultiQuant software (Version 3.0; SCIEX) was employed to quantify peak area.
Relative standard deviation (RSD) of semi‐quantified metabolomics and lipidomics data was calculated in QC samples to evaluate the quality of the data. Small metabolites or lipids with an RSD > 30% in QCs were excluded from the dataset.
2.6. Statistical analysis
The log‐transformed original data were treated by auto scaling (e.g., mean‐centered and divided by the standard deviation of each variable). Partial least squares discriminate analysis (PLS‐DA) was performed using MetaboAnalyst software (Version 5.0, https://www.metaboanalyst.ca/). Plasma small metabolites and lipids passing the thresholds of variable importance in projection (VIP) value >1, fold change >1.2 or <0.8, and p‐value <0.05 were regarded as statistically different substances between the RE and NR groups. Those resultant substances were further used for pathway and network analysis by KEGG with the MetaboAnalyst software.
3. RESULTS
3.1. Clinical characteristics of patients
Detailed clinical characteristics of the study patients are summarized in Table 1. A total of 37 children in the NR group and 53 children in the RE group, with epilepsy onset ages younger than 6 years old, were enrolled in this study. The median age in the NR group was significantly higher than that in the RE group (5.25 vs 3.17 years). Patients with DRE received VPA and other 1 to 3 ASMs, including oxcarbazepine, topiramate, levetiracetam, clonazepam, lamotrigine, vigabatrin, lacosamide, and perampanel. There were no statistical differences in sex and plasma trough concentrations (C 0) of VPA between the two groups.
TABLE 1.
Clinical and demographic characteristics of the study participants.
| NR (n = 37) | RE (n = 53) | p value | |
|---|---|---|---|
| Age (year) | 5.25 (2.79–6.59) | 3.17 (1.75–5.54) | |
| Sex (Male/Female) | 28/9 | 27/26 | |
| C 0 (μg/mL) | 66.65 (56.60–86.98) | 62.20 (52.40–71.50) | 0.12 |
| Number of ASMs a | |||
| 1 | 0 | 53 | |
| 2 | 10 | 0 | |
| 3 | 22 | 0 | |
| 4 | 5 | 0 | |
| ALT (U/L) | 10.00 (8.00–13.75) | 12.00 (9.00–13.50) | 0.12 |
| AST (U/L) | 23.00 (21.00–27.75) | 28.00 (25.50–32.00) | <0.01 |
| Urea (mmol/L) | 4.74 (3.89–6.05) | 4.40 (3.87–5.00) | 0.19 |
| Scr (μmol/L) | 29.71 ± 9.06 | 26.65 ± 6.59 | 0.07 |
| Cys C (mg/L) | 0.89 (0.72–1.01) | 0.77 (0.71–0.89) | 0.16 |
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferases; Cys C, cystatin C; Scr, serum creatinine.
Patients in RE group took VPA monotherapy, while cases in NR group were treated with various ASMs. ASMs include VPA, oxcarbazepine, topiramate, levetiracetam, clonazepam, lamotrigine, vigabatrin, lacosamide, perampanel.
The laboratory tests results, especially those for monitoring liver and kidney function, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen, creatinine, and cystatin C, were within normal ranges.
3.2. Changes in serum biochemical indicators of lipids
The results of the total cholesterol (TC), TG, and high‐density lipoproteins (HDL) levels for those children in the RE and NR groups are shown in Table 2. Notably, TG and TC were higher in the NR group than those in the RE group, with a statistical difference of TC (p < 0.01) and an increase in trend of TG (p = 0.06), respectively.
TABLE 2.
Changes in blood biochemical indictors of lipid profile.
| NR (n = 37) | RE (n = 53) | p value | |
|---|---|---|---|
| TC (mmol/L) | 4.48 ± 1.00 | 3.93 ± 0.65 | <0.01 |
| TG (mmol/L) | 0.84 (0.67–1.21) | 0.71 (0.61–1.02) | 0.06 |
| HDL (mmol/L) | 1.58 ± 0.42 | 1.48 ± 0.35 | 0.22 |
Abbreviations: HDL, high‐density lipoprotein; TC, total cholesterol; TG, triglycerides.
3.3. Plasma metabolomics and lipidomics profiles
For plasma metabolomics, 169 and 285 metabolites were identified with Methods 1 and 2, respectively. After removing the duplicate compounds and compounds with RSD more than 30%, we ultimately identified 204 metabolites (Tables S3). According to the Human Metabolome Database (https://hmdb.ca/), these small molecule metabolites could be classified as amino acids, organic acids, FAs, lipids, and bile acids. For the plasma lipidomics, lipids profiles were obtained with the Methods 3 and 4. According to the exact mass, retention time, and MS/MS patterns, a total of 433 lipids (comprising 16 different lipid subclasses), in positive and negative ion modes, were identified (Tables S4 ans S5).
All small metabolites and lipids in this set were subjected to the PLS‐DA model after the data treated by log transformation and auto scaling. The PLS‐DA analysis revealed a good separation of RE from NR group (Figure 1A). Such finding clearly demonstrated the difference in plasma metabolites and lipids levels that existed in the RE and NR cases. Metabolites and lipids with VIPplsda>2 are shown in Figure 1B. The top 50 heatmap plots of those significant differential metabolites/lipids in the NR and RE groups are shown in Figure 1C, with TG and FAs as the most prominent classes.
FIGURE 1.
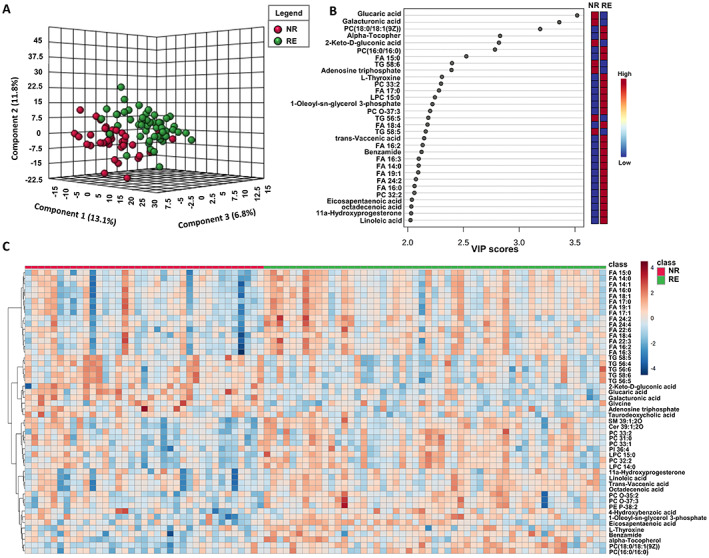
Plasma metabolomic and lipidomic profiles. (A) PLS‐DA model constructed from metabolomic and lipidomic profiling separated NR and RE group. (B) Metabolites and lipids with VIPplsda > 2. (C) Heatmap showing significant TOP 50 features found in the NR and RE group.
3.4. Identifying plasma substance changes and some related pathways associated with the DRE
Pathway analysis (Figure 2A) showed the top five enriched metabolic pathways in medically intractable epilepsy including: (1) biosynthesis of unsaturated FAs, (2) linoleic acid (LA) metabolism, (3) primary bile acid biosynthesis, (4) citrate cycle and (5) alanine, aspartate, and glutamate metabolism. The correlations between these metabolites are shown in Figure 2B. Nodes in the interaction‐based network represent metabolic pathways and two nodes are connected by edges if they are significantly correlated (p < 0.05). The pathways of urea cycle, transfer of acetyl groups into mitochondria, glutamate metabolism, citric acid cycle, and ammonia recycling were found to be obviously associated within themselves and with other metabolic pathways.
FIGURE 2.
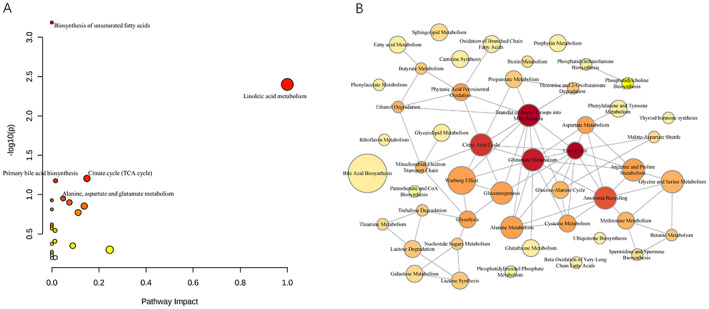
The related pathways associated with the DRE. (A) All enrolled pathways by MetaboAnalyst 5.0 analysis. (B) The co‐occurrence network showed correlation among various small metabolites and lipids with statistical difference.
Statistical analysis identified 23 significantly upregulated and 72 downregulated plasma metabolites or lipids that were associated with the NR cases (Tables S6). The normalized FAs levels in the NR group were obviously decreased as compared to the RE group (Figure 3A). For example, the FA 22:6 like docosahexaenoicacid (DHA) and FA 22:5 like docosapentaenoic acid (DPA) were significantly decreased in the NR group. Unlike the FAs changes observed, the normalized TG levels in the RE group were significantly lower than those in the NR group (Figure 3B), which was in line with our laboratory tests findings for TG monitoring (Table 2). Widespread decreases of glycerophospholipids (GP), such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylinositol (PI) were observed in the NR group (Figure 3C). Lower citric acid and L‐thyroxine levels while higher glucose and 2‐oxoglutarate levels were found in the NR group (Figure 3D).
FIGURE 3.
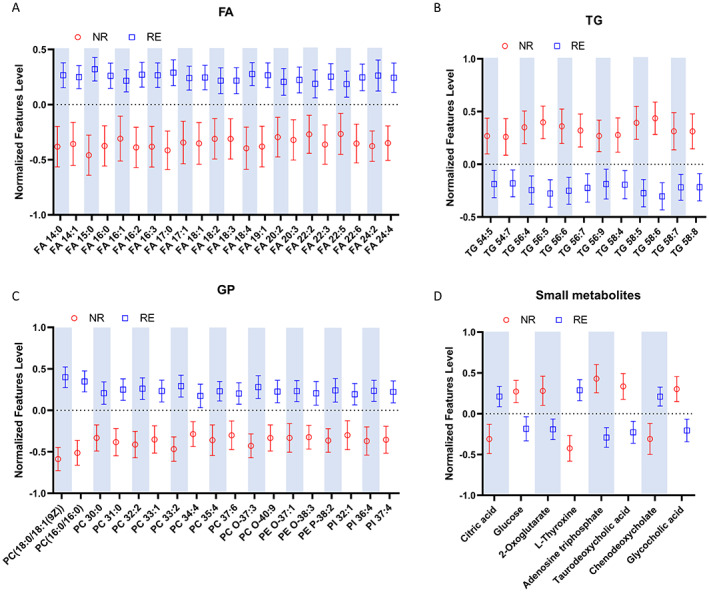
Plasma relative levels of the defined differential metabolites and lipids. (A) Relative FAs levels. (B) Relative TG levels. (C) Relative GP levels. (D) Changes of other small metabolites.
4. DISCUSSION
Indeed, recent studies reveal that epilepsy might be induced by a metabolic etiology, 30 , 31 and epileptic seizures also contribute to changes of small endogenous metabolites and lipids. 32 Here our data unravel differences in plasma lipids profiles between samples obtained from non‐responsive children with epilepsy to multidrug treatments including VPA and responsive controls who received VPA monotherapy by integrating metabolomics and lipidomics followed by specific statistical analysis. The underpinning mechanisms might involve some cellular energy metabolism pathways (Figure 4). Arguably, decrease in FAs levels but an increase in TG levels measured in the plasma samples from the medically intractable cases was a novel finding with relevant clinical value for better understanding the potential determinants of DRE conditions.
FIGURE 4.
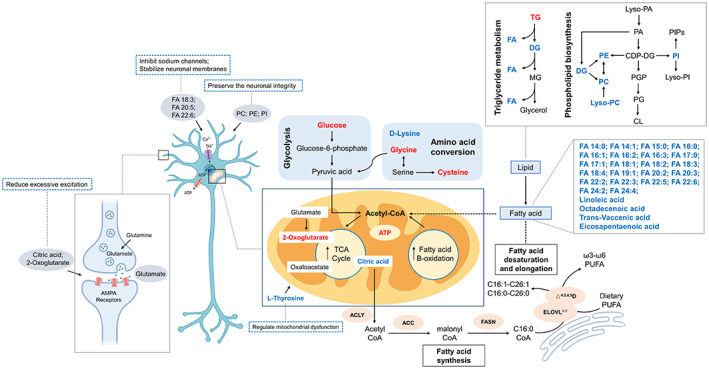
DRE‐associated metabolites and lipids in cellular metabolic pathways. Upregulated metabolites or lipids were colored in red and downregulated metabolites were colored in blue.
The first important finding in this study was that children from the NR group experienced a significant decrease in several types of FAs, including octadecenoic acid, trans‐vaccenic acid, and polyunsaturated fatty acids (PUFAs) like LA and DHA based on the metabolomic analysis, and further revealed a total of 22 FAs after completing lipidomic analysis. As FAs play essential roles in energy supply, which in line with previous study, 32 great energy consumption could be thought in the NR group evidenced by the lower level of FAs.
Interestingly, it has been proved that PUFAs, especially for n‐3 isoforms, have anti‐seizure activities. 33 , 34 For instance, α‐LA (ALA, FA 18:3) and DHA (FA 22:6) were shown to suppress voltage‐gated Na+ and Ca2+ channels and to stabilize neuronal membranes, thereby resulting in an increase in the action potential firing threshold and an overall reduction in the hippocampus burst activity and neuronal excitability. 35 , 36 Similarly, n‐3 PUFAs increase the voltage‐gated K+ channel 7.1 to increase the action potential firing threshold. 37 Some PUFAs also bind to peroxisome proliferator‐activated receptor (PPAR)α and was thought to induce a coordinated upregulation of some energy transcripts, which are responsible for the enhanced energy reserves and limited hyperexcitability. 33 On the other hand, PUFAs such as DPA and DHA can inhibit several inflammatory mediators such as interleukin (IL)‐1 β, IL‐6, and tumor necrosis factor (TNF)‐α, 34 which play critical roles in epileptic seizures. 38 Additionally, n‐3 PUFAs could reduce formation of lesions caused by status epilepticus and exert a neuroprotective effect in an epilepsy animal model. 39 Therefore, alterations in FAs may be involved in the pathological process of the medically intractable epilepsy, which could be a relevant metabolic mechanism.
In line with our finding, previous research reported by Abuknesha et al. 40 support this hypothesis. Compared with healthy controls, Sudanese DRE patients experienced significantly higher saturated and monounsaturated FAs but lower n‐6 and n‐3 PUFAs in plasma. Intriguingly, some saturated FAs such as myristic (FA 14:0) and palmitic acids (FA 16:0) can covalently modify several proteins involved in cell signaling. 41 Particularly, the covalent attachment of palmitate (FA 16:0) to a cysteine residue on proteins, called palmitoylation, is one of the most important lipid modifications. 42
Palmitoylation of target proteins has been shown to be associated with epileptic seizures by controlling the strength of excitatory synaptic transmission and plasticity. 43 , 44 Of note, a significantly decreased level of palmitic acids (FA 16:0) in the NR group was observed in our study, which was thought to change the palmitoylation profiles in neurons and contribute to the occurrence of drug‐resistance. Interestingly, in a clinical study, Elzbieta et al. 45 found that patients with uncontrolled epilepsy tended to have significantly lower palmitic acid levels than those seizure‐free patients. It was reported that palmitic acid is the precursor of N‐palmitoylethanolamide, an endocannabinoid with presynaptic cannabinoid CB1 receptors activation properties, which exerts anti‐inflammatory activities by binding to PPARα 46 and thereby playing anticonvulsant activity in rat epilepsy models. 47
Coincidentally, an earlier study revealed the anticonvulsant effect of VPA, a branched short chain FA and conjugated to CoA for processing, was mediated by interfering with palmitoylation of A‐kinase anchoring protein (AKAP79/150) owing to the high CoA occupancy. 48 Based on this hypothesis, Hoshi proposed that the ketogenic diet like MCT would dominate cytosolic acyl‐CoA pool, reduce palmitoyl‐CoA, and have a similar outcome achieved by VPA. Therefore, producing shorter FAs, rather than creating a ketosis, could be considered as a prototypical mechanism for linking lipid metabolism and anti‐seizure action. 49
Another relevant finding of our study was that a significantly increased TG level was observed in the NR group of patients, compared with those controls in the RE group. Impressively, such finding from non‐targeted lipidomic study was consistent with the routine laboratory tests for monitoring serum biochemistry (Table 2). TG, as a reservoir of FAs, can be mobilized and degraded in mitochondria through a series of lipases. 50 The increased TG levels in the NR group was thought partially to explain the decreased concentrations of free FAs, which potentially resulted in dysfunction of FA‐related signaling pathways, such as unsaturated FAs biosynthesis and LA metabolism (Figure 2A).
Obviously, the cause‐effect relationship between medically intractable epilepsy and lipids profile is a very interesting research area. Accumulating evidence has revealed that those epileptic patients have a high risk of obesity and metabolic syndromes, 51 , 52 which could be partially caused by the potential effect of epilepsy on hypothalamic neuroendocrine control of energy homeostasis. 53 In addition, long‐term use of some ASMs, especially enzyme‐inducing ASMs (EIASMs), can lead to elevated serum lipids. 54 In this study, children in the NR group were co‐administered with some EIASMs like oxcarbazepine, and non‐EIASMs such as levetiracetam, lamotrigine, perampanel, topiramate or lacosamide. But their effects on the lipid profiles were far from reaching consistent conclusion in previous studies. 55 , 56 Indeed, in our study, patients took VPA as monotherapy or polytherapy in the RE and the NR group, respectively. Interestingly, there was no statistical difference in C 0 of plasma VPA between the two groups, suggesting that VPA treatment might play a minor role in the elevated TG and TC levels. Therefore, whether changes in lipids like TG and TC are really involved in the DRE or only represent epiphenomena needs more in‐depth research.
A further important finding of our study is the downregulation of GP (including PC, PE, and PI). It is well recognized that GP preserve the neuronal integrity via supplying the brain's energetic needs and regulating ion channel function, which consequently is essential to control neuronal excitation. 57 Recent lipidomic researches revealed the elevated levels of GP in hippocampus both in animal seizure models and in patients with temporal lobe epilepsy. 58 , 59 , 60 But their results appeared to be out of line with our study, which might be owing to the tested sample matrix was plasma instead of hippocampus. Therefore, the alteration of GP levels, whether lower or higher, might lead to a disruption of lipids balance that modulates seizure by affecting membrane proteins that are sensitive to lipid environment. 58
One more observation needs to be further highlighted. In our study, lower citric acid levels while higher glucose and 2‐oxoglutarate were found to be associated with drug‐resistance, which are the essential metabolites of tricarboxylic acid (TCA) cycle or glucose metabolism. These results provided additional evidence to support the hypothesis that dysfunction in glucose metabolism and TCA cycle pathways within brain might contribute to the initiation and progression of seizures. 61 For example, excessive excitation of neurons decreased the size of the TCA cycle intermediate pool, including citric acid and 2‐oxoglutarate in epilepsy. 62
More interestingly, a decreased level of L‐thyroxine was found in the NR group. Thyroid hormones (THs) have a positive effect on aspects of epilepsy, from development to healing and recreation via regulating mitochondrial dysfunction, oxidative stress, and deregulation of GABAergic system. 63 Furthermore, THs were shown to increase significantly the rate of adenosine triphosphate (ATP) hydrolysis. 63 Therefore, a higher ATP level observed in the NR group in our study (Figure 3D), which could partly attribute to the decreased L‐thyroxine level. Indeed, this also may be a result of the requirement for increased ATP production to meet the energy demands of seizure activity. 64
One of the key strengths of this study is that it brings out the differences in lipid subtypes between RE's and NR's which has not been explicitly discussed in previous studies. The question of whether and how the lipidomic profiles contribute to ASM responsiveness is yet to be determined. Of note, we ultimately identified 204 small metabolites and 433 lipids, which could facilitate us to delve into the association between the energy metabolism homeostasis and the DRE (Figure 4).
Glucose, the main source of energy of the brain, is taken up increasingly by neurons and astrocytes during epileptic seizures. Meanwhile, more pyruvate participates in the TCA with glycolytic flux increasing to produce more ATP. 65 Once the glucose is limited, lactate, ketone bodies, and medium‐chain FAs can be used as the alternative fuels. 66 As an intermediate in the TCA, the citric acid plays an indispensable role in the energy metabolism. Amino acids can also be used as substrates to participate in the energy metabolism, such as the conversion from glycine to pyruvate. 67 FA β‐oxidation (FAO) is an important process in cellular energy production, which contributes up to 20% of the total brain energy requirement. 14 Increasing evidence indicates that glycolipids and phospholipids in oligodendrocytes control the formation of myelin, which is important to remyelination that following pathological demyelination in diverse central nervous system disorders. 68 FA synthesis not only has implication on promoting neurogenesis, but also on controlling the neuronal dendrite expansion. 69 Collectively, based on the above discussion, the metabolites and lipids profiles of those cases from the NR and the RE group revealed a potential mechanism underlying the FAs metabolism and pharmacoresistance in epilepsy management.
However, there are limitations in our study owing to its retrospective nature. Firstly, this is the first report to investigate the underlying mechanism of childhood medically intractable epilepsy from the perspective of energy metabolism based on lipidomics, but the sample size of children enrolled in the final study was relatively small, with 37 patients in the NR group and 53 controls in the RE group. And the small sample size might increase the bias, such as there was a skewed sex ratio in the NR group. Secondly, our study was a non‐targeted metabolic and lipidomic research only providing us comprehensive metabolic profiles, semi‐quantitatively instead of quantitatively. Fortunately, the routine blood biochemical tests for TG monitoring in those patients partially confirmed the lipid change observed. However, targeted analysis for above lipids like FAs and TG, as well as metabolites like citric acid, should be performed to further reveal their roles in the DRE disease. Thirdly, because of patients in the RE and NR groups receiving different medication regimen, the potential contribution of ASMs to the changes in FAs, TG, and other small metabolites might not be completely ruled out based on those previous inconclusive reports. Similarly, the types of epilepsy and underlying etiology/associated comorbidities observed somehow in those patients remain largely unknown, which also potentially change the lipid profiles. Additionally, the limitation because of randomization and eligibility criteria of our retrospective study should also be concerned. This posterior analysis was fixed at a certain timepoint after a certain period of ASMs therapy, and could not track the real‐time changes in the process from the epilepsy onset to the final DRE diagnosis. Therefore, the time‐related lipid profiles such as FAs and TG, which we are concerned with, cannot be monitored yet.
5. CONCLUSIONS
In conclusion, this retrospective study integrated metabolomic and lipidomic techniques and revealed a decrease in FAs and an increase in TG, as well as changes of other small metabolites like citric acid, were associated with the response to ASMs therapy, evidenced by the clinical outcomes of children from the RE and the NR groups. Such novel findings proposed a potential mechanism related to the energy metabolism, especially of FAs metabolism, for better understanding the childhood DRE disease. Owing to the nature of energy metabolism, ketogenic diet like MCT and FAs supplementation may therefore be alternative strategies for the childhood DRE management. Arguably, to clarify the roles of FAs and TG, as well as the energy metabolism pathways, in the DRE condition, multicenter clinical trials and basic researches are warranted in the future.
AUTHORS’ CONTRIBUTIONS
H.L. Guo and F. Chen contributed to the conception and design of the study; N. Dong, Y.T. Zhao, R.H. Dai, and Y.H. Hu contributed to the acquisition and analysis of data; H.L. Guo and W.J. Wang, performed statistical analyses; H.L. Guo, W.J. Wang and F. Chen contributed to the first draft. All authors contributed to the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflicts of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publications and affirm that this report is in consistent with those guidelines.
Supporting information
TableS1‐S6
ACKNOWLEDGMENTS
This research was supported by the Specially Appointed Medical Expert Project of Jiangsu Commission of Health (2019), Jiangsu Research Hospital Association for Precision Medication (JY202106), Nanjing Postdoctoral Research Funding Program (284077), and by the Medical Science and Technique Foundation of Nanjing Health Commission (YKK20119, YKK20124 and YKK22160). We thank Dr. Zhi‐Min Long and Hai‐Hong Zha from SCIEX, Analytical Instrument Trading Co., Ltd, Shanghai, China, for their kind help during the data analysis.
Guo H‐L, Wang W‐J, Dong N, Zhao Y‐T, Dai H‐R, Hu Y‐H, et al. Integrating metabolomics and lipidomics revealed a decrease in plasma fatty acids but an increase in triglycerides in children with drug‐refractory epilepsy. Epilepsia Open. 2023;8:466–478. 10.1002/epi4.12712
Hong‐Li Guo and Wei‐Jun Wang contributed equally to this work.
Wei‐Jun Wang, Na Dong, Yue‐Tao Zhao, Hao‐Ran Dai visiting graduate students from China Pharmaceutical University.
Contributor Information
Hong‐Li Guo, Email: guomomohl@163.com.
Feng Chen, Email: cy.chen508@gmail.com.
REFFERENCES
- 1. Gozzelino L, Kochlamazashvili G, Baldassari S, Mackintosh AI, Licchetta L, Iovino E, et al. Defective lipid signalling caused by mutations in PIK3C2B underlies focal epilepsy. Brain. 2022;145(7):2313–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feng L, Wang J, Li X, Hu Y, Hong S, Jiang L. Prospective control study of efficacy and influencing factors of a ketogenic diet on refractory epilepsy in children. Transl Pediatr. 2022;11(1):138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zarnowska IM. Therapeutic use of the ketogenic diet in refractory epilepsy: what we know and what still needs to Be learned. Nutrients. 2020;12(9):2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Augustin K, Khabbush A, Williams S, Eaton S, Orford M, Cross JH, et al. Mechanisms of action for the medium‐chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018;17(1):84–93. [DOI] [PubMed] [Google Scholar]
- 5. Rho JM, Boison D. The metabolic basis of epilepsy. Nat Rev Neurol. 2022;18(6):333–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7(6):500–6. [DOI] [PubMed] [Google Scholar]
- 7. Sondhi V, Agarwala A, Pandey RM, Chakrabarty B, Jauhari P, Lodha R, et al. Efficacy of ketogenic diet, modified Atkins diet, and low glycemic index therapy diet among children with drug‐resistant epilepsy: a randomized clinical trial. JAMA Pediatr. 2020;174(10):944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neves GS, Lunardi MS, Lin K, Rieger DK, Ribeiro LC, Moreira JD. Ketogenic diet, seizure control, and cardiometabolic risk in adult patients with pharmacoresistant epilepsy: a review. Nutr Rev. 2021;79(8):931–44. [DOI] [PubMed] [Google Scholar]
- 9. Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak JA, et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain. 2016;139(Pt 2):431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lutas A, Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013;36(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pedersen S, Kverneland M, Nakken KO, Rudi K, Iversen PO, Gervin K, et al. Genome‐wide decrease in DNA methylation in adults with epilepsy treated with modified ketogenic diet: a prospective study. Epilepsia. 2022;63(9):2413–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dahlin M, Singleton SS, David JA, Basuchoudhary A, Wickström R, Mazumder R, et al. Higher levels of Bifidobacteria and tumor necrosis factor in children with drug‐resistant epilepsy are associated with anti‐seizure response to the ketogenic diet. EBioMedicine. 2022;80:104061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang P, Terbach N, Plant N, Chen PE, Walker MC, Williams RS. Seizure control by ketogenic diet‐associated medium chain fatty acids. Neuropharmacology. 2013;69:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bogie JFJ, Haidar M, Kooij G, Hendriks JJA. Fatty acid metabolism in the progression and resolution of CNS disorders. Adv Drug Deliv Rev. 2020;159:198–213. [DOI] [PubMed] [Google Scholar]
- 15. Giles C, Takechi R, Lam V, Dhaliwal SS, Mamo JCL. Contemporary lipidomic analytics: opportunities and pitfalls. Prog Lipid Res. 2018;71:86–100. [DOI] [PubMed] [Google Scholar]
- 16. Han X. The emerging role of lipidomics in prediction of diseases. Nat Rev Endocrinol. 2022;18(6):335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wenk MR. The emerging field of lipidomics. Nat Rev Drug Discov. 2005;4(7):594–610. [DOI] [PubMed] [Google Scholar]
- 18. Griffiths WJ, Wang Y. Mass spectrometry: from proteomics to metabolomics and lipidomics. Chem Soc Rev. 2009;38(7):1882–96. [DOI] [PubMed] [Google Scholar]
- 19. Shevchenko A, Simons K. Lipidomics: coming to grips with lipid diversity. Nat Rev Mol Cell Biol. 2010;11(8):593–8. [DOI] [PubMed] [Google Scholar]
- 20. Brugger B. Lipidomics: analysis of the lipid composition of cells and subcellular organelles by electrospray ionization mass spectrometry. Annu Rev Biochem. 2014;83:79–98. [DOI] [PubMed] [Google Scholar]
- 21. Al Zweiri M, Sills GJ, Leach JP, et al. Response to drug treatment in newly diagnosed epilepsy: a pilot study of (1)H NMR‐ and MS‐based metabonomic analysis. Epilepsy Res. 2010;88(2–3):189–95. [DOI] [PubMed] [Google Scholar]
- 22. Murgia F, Muroni A, Puligheddu M, Polizzi L, Barberini L, Orofino G, et al. Metabolomics As a tool for the characterization of drug‐resistant epilepsy. Front Neurol. 2017;8:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boguszewicz L, Jamroz E, Ciszek M, Emich‐Widera E, Kijonka M, Banasik T, et al. NMR‐based metabolomics in pediatric drug resistant epilepsy ‐ preliminary results. Sci Rep. 2019;9(1):15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sohouli MH, Razmpoosh E, Zarrati M, Jaberzadeh S. The effect of omega‐3 fatty acid supplementation on seizure frequency in individuals with epilepsy: a systematic review and meta‐analysis. Nutr Neurosci. 2021;25(11):1–10. [DOI] [PubMed] [Google Scholar]
- 25. Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010;51(6):1069–77. [DOI] [PubMed] [Google Scholar]
- 26. Xu ZY, Guo HL, Li L, et al. Genetic and non‐genetic factors contributing to the significant variation in the plasma trough concentration‐to‐dose ratio of Valproic acid in children with epilepsy. Front Pediatr. 2020;8:599044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou Y, Xu Y, Zhang X, Zhao P, Gong X, He M, et al. Plasma metabolites in treatment‐requiring retinopathy of prematurity: potential biomarkers identified by metabolomics. Exp Eye Res. 2020;199:108198. [DOI] [PubMed] [Google Scholar]
- 28. Sarafian MH, Gaudin M, Lewis MR, Martin FP, Holmes E, Nicholson JK, et al. Objective set of criteria for optimization of sample preparation procedures for ultra‐high throughput untargeted blood plasma lipid profiling by ultra performance liquid chromatography‐mass spectrometry. Anal Chem. 2014;86(12):5766–74. [DOI] [PubMed] [Google Scholar]
- 29. Shen X, Wang R, Xiong X, Yin Y, Cai Y, Ma Z, et al. Metabolic reaction network‐based recursive metabolite annotation for untargeted metabolomics. Nat Commun. 2019;10(1):1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Almannai M, Al Mahmoud RA, Mekki M, El‐Hattab AW. Metabolic Seizures. Front Neurol. 2021;12:640371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang D, Wang X, Kong J, Wu J, Lai M. GC–MS‐based metabolomics discovers a shared serum metabolic characteristic among three types of epileptic seizures. Epilepsy Res. 2016;126:83–9. [DOI] [PubMed] [Google Scholar]
- 33. Nathan J, Bailur S, Datay K, Sharma S, Khedekar KD. A switch to polyunsaturated fatty acid based ketogenic diet improves seizure control in patients with drug‐resistant epilepsy on the mixed fat ketogenic diet: a retrospective open label trial. Cureus. 2019;11(12):e6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taha AY, Burnham WM, Auvin S. Polyunsaturated fatty acids and epilepsy. Epilepsia. 2010;51(8):1348–58. [DOI] [PubMed] [Google Scholar]
- 35. Boland LM, Drzewiecki MM. Polyunsaturated fatty acid modulation of voltage‐gated ion channels. Cell Biochem Biophys. 2008;52(2):59–84. [DOI] [PubMed] [Google Scholar]
- 36. Yazdi S, Stein M, Elinder F, Andersson M, Lindahl E. The molecular basis of polyunsaturated fatty acid interactions with the shaker voltage‐gated Potassium Channel. PLoS Comput Biol. 2016;12(1):e1004704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valenzuela C. M‐channels and n‐3 polyunsaturated fatty acids: role in pain and epilepsy. Acta Physiol (Oxf). 2016;218(1):7–9. [DOI] [PubMed] [Google Scholar]
- 38. Costagliola G, Depietri G, Michev A, Riva A, Foiadelli T, Savasta S, et al. Targeting inflammatory mediators in epilepsy: a systematic review of its molecular basis and clinical applications. Front Neurol. 2022;13:741244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pessoa DT, da Silva ELA, Costa EVL, Nogueira RA. Effect of diet with omega‐3 in basal brain electrical activity and during status epilepticus in rats. Epilepsy Res. 2017;137:33–8. [DOI] [PubMed] [Google Scholar]
- 40. Abuknesha NR, Ibrahim F, Mohamed IN, Salih MAM, Daak AA, Elbashir MI, et al. Plasma fatty acid abnormality in Sudanese drug‐resistant epileptic patients. Prostaglandins Leukot Essent Fatty Acids. 2021;167:102271. [DOI] [PubMed] [Google Scholar]
- 41. Calder PC. Functional roles of fatty acids and their effects on human health. JPEN J Parenter Enteral Nutr. 2015;39(1 Suppl):18 S–32 S. [DOI] [PubMed] [Google Scholar]
- 42. Sohn H, Park M. Palmitoylation‐mediated synaptic regulation of AMPA receptor trafficking and function. Arch Pharm Res. 2019;42(5):426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shimell JJ, Shah BS, Cain SM, Thouta S, Kuhlmann N, Tatarnikov I, et al. The X‐linked intellectual disability gene Zdhhc9 is essential for dendrite outgrowth and inhibitory synapse formation. Cell Rep. 2019;29(8):2422–2437.e2428. [DOI] [PubMed] [Google Scholar]
- 44. Itoh M, Yamashita M, Kaneko M, Okuno H, Abe M, Yamazaki M, et al. Deficiency of AMPAR‐Palmitoylation aggravates seizure susceptibility. J Neurosci. 2018;38(47):10220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Plonka‐Poltorak E, Zagrodzki P, Kryczyk‐Koziol J, et al. Does valproate therapy in epileptic patients contribute to changing atherosclerosis risk factors? The role of lipids and free fatty acids. Pharmacol Rep. 2016;68(6):1339–44. [DOI] [PubMed] [Google Scholar]
- 46. Carta G, Murru E, Lisai S, Sirigu A, Piras A, Collu M, et al. Dietary triacylglycerols with palmitic acid in the sn‐2 position modulate levels of N‐acylethanolamides in rat tissues. PLoS One. 2015;10(3):e0120424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Citraro R, Russo E, Scicchitano F, van Rijn C, Cosco D, Avagliano C, et al. Antiepileptic action of N‐palmitoylethanolamine through CB1 and PPAR‐alpha receptor activation in a genetic model of absence epilepsy. Neuropharmacology. 2013;69:115–26. [DOI] [PubMed] [Google Scholar]
- 48. Kay HY, Greene DL, Kang S, Kosenko A, Hoshi N. M‐current preservation contributes to anticonvulsant effects of valproic acid. J Clin Invest. 2015;125(10):3904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hoshi N. M‐current suppression, seizures and lipid metabolism: a potential link between neuronal Kv7 channel regulation and dietary therapies for epilepsy. Front Physiol. 2020;11:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chitraju C, Mejhert N, Haas JT, Diaz‐Ramirez LG, Grueter CA, Imbriglio JE, et al. Triglyceride synthesis by DGAT1 protects adipocytes from lipid‐induced ER stress during lipolysis. Cell Metab. 2017;26(2):407–18. e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Daniels ZS, Nick TG, Liu C, Cassedy A, Glauser TA. Obesity is a common comorbidity for pediatric patients with untreated, newly diagnosed epilepsy. Neurology. 2009;73(9):658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vooturi S, Jayalakshmi S. Metabolic syndrome in people with epilepsy. Epilepsy Behav. 2020;106:106992. [DOI] [PubMed] [Google Scholar]
- 53. Janousek J, Barber A, Goldman L, Klein P. Obesity in adults with epilepsy. Epilepsy Behav. 2013;28(3):391–4. [DOI] [PubMed] [Google Scholar]
- 54. Okada S, Nishina M, Koizumi K, Katayama M, Inoue S, Suga S. Impact of enzyme‐inducing anti‐epilepsy drugs on lipid levels in elderly patients with epilepsy. Epilepsy Res. 2020;166:106428. [DOI] [PubMed] [Google Scholar]
- 55. Mintzer S, Trinka E, Kraemer G, Chervoneva I, Werhahn KJ. Impact of carbamazepine, lamotrigine, and levetiracetam on vascular risk markers and lipid‐lowering agents in the elderly. Epilepsia. 2018;59(10):1899–907. [DOI] [PubMed] [Google Scholar]
- 56. Mintzer S, Dimova S, Zhang Y, Steiniger‐Brach B, de Backer M, Chellun D, et al. Effects of lacosamide and carbamazepine on lipids in a randomized trial. Epilepsia. 2020;61(12):2696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dabke P, Brogden G, Naim HY, Das AM. Ketogenic diet: impact on cellular lipids in hippocampal murine neurons. Nutrients. 2020;12(12):3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Qiu X, Zhang L, Kinoshita M, Lai W, Zheng W, Peng A, et al. Integrative analysis of non‐targeted lipidomic data and brain structural imaging identifies phosphatidylethanolamine associated with epileptogenesis. Metabolomics. 2020;16(10):110. [DOI] [PubMed] [Google Scholar]
- 59. Zhang H, Ren P, Huang Y, Zeng W, Zhong K, Gao H, et al. Untargeted lipidomic analysis of human hippocampus for temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res. 2020;161:106299. [DOI] [PubMed] [Google Scholar]
- 60. Johnson A, Grove RA, Madhavan D, Boone CHT, Braga C, Kyllo H, et al. Changes in lipid profiles of epileptic mouse model. Metabolomics. 2020;16(10):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McDonald TS, Carrasco‐Pozo C, Hodson MP, Borges K. Alterations in cytosolic and mitochondrial [U‐(13)C]glucose metabolism in a chronic epilepsy mouse model. eNeuro. 2017;4(1):e0431‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Akiyama T, Saigusa D, Hyodo Y, Umeda K, Saijo R, Koshiba S, et al. Metabolic profiling of the cerebrospinal fluid in pediatric epilepsy. Acta Med Okayama. 2020;74(1):65–72. [DOI] [PubMed] [Google Scholar]
- 63. Tamijani SM, Karimi B, Amini E, et al. Thyroid hormones: possible roles in epilepsy pathology. Seizure. 2015;31:155–64. [DOI] [PubMed] [Google Scholar]
- 64. Wasterlain CG, Thompson KW, Suchomelova L, Niquet J. Brain energy metabolism during experimental neonatal seizures. Neurochem Res. 2010;35(12):2193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boison D, Steinhauser C. Epilepsy and astrocyte energy metabolism. Glia. 2018;66(6):1235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thevenet J, De Marchi U, Domingo JS, et al. Medium‐chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte‐neuron lactate and ketone body shuttle systems. FASEB J. 2016;30(5):1913–26. [DOI] [PubMed] [Google Scholar]
- 67. Nissen JD, Pajecka K, Stridh MH, Skytt DM, Waagepetersen HS. Dysfunctional TCA‐cycle metabolism in glutamate dehydrogenase deficient astrocytes. Glia. 2015;63(12):2313–26. [DOI] [PubMed] [Google Scholar]
- 68. Dimas P, Montani L, Pereira JA, Moreno D, Trötzmüller M, Gerber J, et al. CNS myelination and remyelination depend on fatty acid synthesis by oligodendrocytes. Elife. 2019;8:e44702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ziegler AB, Thiele C, Tenedini F, Richard M, Leyendecker P, Hoermann A, et al. Cell‐autonomous control of neuronal dendrite expansion via the fatty acid synthesis regulator SREBP. Cell Rep. 2017;21(12):3346–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TableS1‐S6


