Abstract
Objective
Cannabidiol (CBD) is approved for treatment of Dravet syndrome (DS), Lennox‐Gastaut syndrome (LGS), and tuberous sclerosis complex (TSC). Several studies suggest antiseizure effects also beyond these three epilepsy syndromes.
Methods
In a retrospective multicenter study, we analyzed the efficacy and tolerability of CBD in patients with epilepsy at 16 epilepsy centers.
Results
The study cohort comprised 311 patients with epilepsy with a median age of 11.3 (0‐72) years (235 children and adolescents, 76 adults). Therapy with CBD was off‐label in 91.3% of cases due to age, epilepsy subtype, lack of adjunct therapy with clobazam, and/or higher dose applied. CBD titration regimens were slower than recommended, with good tolerability of higher doses particularly in children. Of all patients, 36.9% experienced a reduction in seizure frequency of >50%, independent of their epilepsy subtype or clobazam co‐medication. The median observation period was 15.8 months. About one third of all patients discontinued therapy within the observation period due to adverse effects or lack of efficacy. Adverse effects were reported frequently (46.9%).
Significance
Our study highlights that CBD has an antiseizure effect comparable to other antiseizure medications with a positive safety profile independent of the epilepsy subtype. Comedication with clobazam was not associated with a better outcome. Higher doses to achieve seizure frequency reduction were safe, particularly in children. These findings call for further trials for an extended approval of CBD for other epilepsy subtypes and for children <2 years of age.
Keywords: cannabidiol, clobazam, drug‐resistant seizures, DS, epilepsy, LGS, TSC
Key points.
CBD has an antiseizure effect comparable to other antiseizure medications with a positive safety profile independent of the epilepsy subtype.
Comedication to CBD with clobazam is not associated with a better outcome.
Higher CBD doses than recommended in studies leading to approval to achieve seizure frequency reduction were safe, particularly in children.
1. INTRODUCTION
The interest in the medical use of plant‐based cannabidiol (CBD), a nonpsychotropic phytocannabinoid, is high given its multiple reported effects. 1 , 2 , 3 , 4 Several studies have highlighted its antiseizure effect in children with drug‐resistant epilepsy (DRE). 5 , 6 , 7 , 8 , 9 Phase III studies of CBD reported promising effects on seizure frequency and associated problems in Lennox‐Gastaut syndrome (LGS), Dravet syndrome (DS) and tuberous sclerosis complex (TSC). 10 , 11 , 12 , 13 , 14 , 15 , 16 The latter findings led to an approval for these indications.
Epidyolex is approved by EMA up to a dose of 20 mg/kg/d for individuals >2 years with LGS or DS, and with a higher maximum dose of 25 mg/kg/d in those with TSC. 10 , 11 , 12 , 13 , 14 , 15 , 16 Due to FDA Epidyolex is approved for those epilepsy subtypes over an age of 1 year and above. In Europe, the approval for LGS and DS requires an adjunct treatment with clobazam, while the adjunctive treatment with clobazam is not mandatory in other countries such as the USA, since an independent benefit of CBD has been proven. 17 , 18 , 19 , 20 Overall, CBD seems to have a good safety profile. 3 , 5 , 21
Given the need for novel treatment options particularly in DRE, we hypothesized that CBD could be a promising ASM and had been offered “off label” in individuals with epilepsy subtypes beyond LGS, DS, and TSC. Here, we aimed to collect real‐world data on the application, dosing, adverse effect profile, and efficacy of CBD in more than 300 children and adults with various epilepsy subtypes.
2. PATIENTS AND METHODS
We conducted a retrospective, multicenter study at 16 epilepsy centers based on medical records of 311 patients with epilepsy who were treated with CBD as antiseizure medication in Germany between December 2015 and December 2021. Epidyolex® was used in 220 (70.7%) patients, and the CBD formulation standardized in Germany (Neues Rezeptur Formularium 22.10, NRF) 22 containing 100 mg/mL CBD dissolved in medium chain triglycerides in 91 (29.3%) subjects.
NRF 22.10 is available in Germany as a nonprescription drug available in pharmacies and was used synonymously with Epidyolex prior to approval. We have decided to include both NRF 22.10 and Epidyolex as they contain the same active ingredient concentration of highly purified CBD derived from Cannabis sativa plant and almost the same composition of additives (medium‐chain triglycerides, with an additional low ethanol content in Epidyolex).
We included all patients with epilepsy who received CBD as an ASM and could be identified in patient records of the participating study centers. Six patients, who were treated with drugs containing CBD other than oral Epidyolex® or NRF, were excluded from the analysis. The retrospective analysis of epilepsy patient data was approved by the local ethic committee (approval no. EA2/084/18). The need for informed consent was waived.
We extracted seizure‐ and treatment‐specific data from electronic and paper‐based medical records using a standardized data collection sheet. Patient information and information on epilepsy as well as treatment and outcome were documented in the clinical information system by specialists at the respective epilepsy centers. Seizure outcome was assessed by the specialist treating the patients and was based on patient history, seizure diaries, and/or parental declaration in minors. Seizure outcome was assessed at the last follow up and divided into the categories: seizure freedom, seizure reduction >50%, seizure reduction <50%, and no effect/worsening. Missing data were marked in the dataset, but core data were available in all patients, so no patient had to be excluded.
Data were stored in REDCap (https://www.project‐redcap.org). Statistical analysis was conducted with IBM SPSS Statistics Version 25 program and specific tests are mentioned in the result section. Test results with P < 0.05 were considered statistically significant. Figures were generated with the help of GraphPad Prism version 9.3.1 (GraphPad Software, San Diego, CA, USA).
3. RESULTS
Our cohort comprised 311 patients (235 children, 76 adults) with epilepsy who were treated with CBD (Table 1). In our cohort, the majority of patients (66.2%) had epilepsy syndromes or etiologies other than LGS (n = 65, 20.9%), DS (n = 28, 9.0%), and TSC (n = 12, 3.9%). The etiology of epilepsy was predominantly genetic (n = 111, 35.7%; eg, DS, LGS, Angelman syndrome, Rett syndrome) or structural (n = 105, 33.8%; eg, hypoxic encephalopathy, structural defects secondary to infection, FCD, gyration disorder), followed by an unknown (n = 58, 18.6%) or genetic & structural (n = 37, 11.9%; eg, TSC, lissencephaly) etiology (Table 1, Tables S1 and S2). Prior to the introduction of CBD, 19 patients had undergone epilepsy surgery including hemispherotomy (n = 8), selective amygdalohippocampectomy (n = 3), temporoparietal lobe disconnection (n = 2), and lobectomy (n = 2).
TABLE 1.
Overview of cohort and CBD treatment specification.
| Children (n = 235) | Adults (n = 76) | Total (n = 311) | |
|---|---|---|---|
| Sex | |||
| Female | 103 (44.6%) | 35 (46.1%) | 138 (44.4%) |
|
Male Unknown |
128 (55.4%) 4 (1.7%) |
41 (53.9%) 0 |
169 (54.3%) 4 (1.3%) |
| Age years, median ± SD (range) | 9 ± 4.5 (0.2‐17.9) | 29.4 ± 10.8 (18‐72) | 11.3 ± 11.3 (0.2‐72) |
| Etiology | |||
| Structural | 77 (32.8%) | 28 (36.8%) | 105 (33.8%) |
| Genetic | 90 (38.3%) | 21 (27.6%) | 111 (35.7%) |
| Structural + genetic | 34 (14.5%) | 3 (3.9%) | 37 (11.9%) |
| Unknown | 34 (14.5%) | 24 (31.6%) | 58 (18.6%) |
| Approved indications | |||
| Lennox‐Gastaut syndrome | 44 (18.7%) | 21 (27.6%) | 65 (20.9%) |
| Dravet syndrome | 20 (8.5%) | 8 (10.5%) | 28 (9.0%) |
| Tuberous sclerosis | 12 (3.9%) | 0 | 12 (3.9%) |
| Seizure type | |||
| Focal onset | 153 (65.1%) | 61 (80.2%) | 214 (68.8%) |
| Motor | 133 (56.6%) | 47 (61.8%) | 180 (57.9%) |
| Nonmotor | 70 (29.8%) | 34 (44.7%) | 104 (33.4%) |
| Focal to bilateral tonic‐clonic | 41 (17.4%) | 29 (38.2%) | 70 (22.5%) |
| Generalized onset | 183 (77.8%) | 51 (67.1%) | 234(59.2%) |
| Motor | 168 (71,5%) | 50 (65.8%) | 218 (70.0%) |
| Nonmotor | 70 (29.8%) | 19 (25%) | 89 (28.6%) |
| Unknown onset | 11 (4.7%) | 0 (0%) | 11 (3.5%) |
| No. of medications | |||
| Previous ASM | 5 ± 3.4 (0‐16) | 8 ± 2.7 (1‐13) | 6 ± 3.4 (0‐16) |
| Comedication ASM | 3 ± 1.1 (0‐6) | 3 ± 1.1 (0‐5) | 3 ± 1.1 (0‐6) |
| Lifetime ASM | 8 ± 3.9 (1‐19) | 10 ± 4.2 (1‐17) | 8 ± 4.0 (1‐19) |
| CBD dosage in mg/kg/d, median ± SD (range) | |||
| start dose ± SD | 3.3 ± 2.2 (0.08‐20) | 2.8 ± 1.5 (0.77‐5.7) | 3.2 ± 2.0 (0.08‐20.0) |
| Titration per week | 2.8 ± 1.6 (0.08‐10.6) | 2.66 ± 1.2 (0.62‐5.7) | 2.8 ± 1.6 (0.08‐10.6) |
| End dose± SD | 19.0 ± 7.8 (2.56‐45) | 13.3 ± 6.15 (2.5‐32) | 17.8 ± 7.7 (2.5‐45) |
| Follow‐up | |||
|
No. of treatments ended Duration |
68 (28.9%) 5.5 ± 8.4 m (7 d to 41.7 m) |
27 (35.5%) 7.0 ± 6.4 m (3 d to 24.1 m) |
95 (30.5%) 6.2 ± 7.8 m (3 d to 41.7 m) |
| Reason to end | |||
| No effect | 42 (17.9%) | 19 (25%) | 61 (19.6%) |
| Side effects | 21 (8.9%) | 8 (10.5%) | 29 (9.3%) |
| Other | 11 (4.6%) | 4 (5.3%) | 15 (4.8%) |
|
No. of ongoing treatments Duration |
167 (71.1%) 21.7 ± 15.7 m (17 d to 5.9 y) |
49 (64.5%) 20.3 ± 16.2 m (8 d to 5.59 y) |
216 (69.5%) 21.4 ± 15.8 m (8 d to 5.9 y) |
| Effect on seizures | |||
| Seizure freedom | 17 (7.2%) | 3 (3.9%) | 20 (6.4%) |
| Reduction >50% | 73 (31.1%) | 22 (27.9%) | 95 (30.5%) |
| Reduction <50% | 72 (30.6%) | 25 (32.9%) | 97 (31.2%) |
| No change | 56 (23.8%) | 16 (21.1%) | 72 (23.2%) |
| Increased frequency | 17 (7.2%) | 10 (13.2%) | 27 (8.7%) |
| Effect reduction >3 months | |||
| Yes | 39 (16.6%) | 6 (7.9%) | 45 (14.5%) |
| No | 63 (26.8%) | 24 (31.6%) | 87 (28.0%) |
| n.a. a | 133 (56.6%) | 46 (60.5%) | 179 (57.6%) |
| Side effects | 107 (45.5%) | 39 (51.3%) | 146 (46.9%) |
| Sleepiness | 51 (21.7%) | 24 (31.6%) | 75 (24.1%) |
| Gastrointestinal symptoms | 59 (25.1%) | 19 (25.0%) | 78 (25.1%) |
| Psychiatric symptoms | 15 (6.4%) | 4 (5.3%) | 19 (6.1%) |
| Liver enzyme increase | 3 (1.3%) | 2 (2.6%) | 5 (1.6%) |
| Muscle weakness | 5 (2.1%) | 0 (0.0%) | 5 (1.6%) |
| Other effects | 3 c (1.3%) | 3 b (3.9%) | 6 (1.9%) |
| Positive effects | |||
| Improved mood | 76 (32.3%) | 20 (26.3%) | 96 (30.9%) |
| Improved night sleep | 63 (26.8%) | 12 (15.8%) | 75 (24.1%) |
| Improved development | 37 (15.7%) | 2 (2.6%) | 39 (12.5%) |
| Increase in appetite | 32 (13.6%) | 3 (3.9%) | 35 (11.3%) |
| Reduction of spasticity | 22/58 (37.9%) | 2/11 (18.2%) | 24/69 (34.8%) |
Patients with treatment less than 3 month, no prior effect on seizures, assessment not done.
1x dizziness, 1x trembling, 1x increased bronchial secretion.
1x dizziness, 1x trembling, 1x respiratory insufficiency, 1x urine retention.
3.1. Concomitant antiseizure medication
Before CBD was added, patients had been treated with a median total number of five ASM (range 0‐16), and 74.9% (n = 233) of all patients were drug resistant (Tables 1 and 2). Drug resistance was defined as failure of seizure control through two tolerated, appropriately chosen and dosed ASM. 23 The median number of ASM given concomitantly with CBD was 3 (range 0‐6). Eleven patients (3.5%) received CBD as monotherapy. 46.3% of the patients received clobazam. Further frequent comedications included valproate (43.1%), lamotrigine (25.4%), levetiracetam (15.8%), brivaracetam (14.8%), and lacosamide (14.5%).
TABLE 2.
Rates of seizure freedom dependent on successive ASM regimens including CBD.
| No. of ASMs tried including CBD | Total no. (%) of patients (n = 311) | Seizure freedom (n = 20) | Seizure reduction >50%, (n = 95) | Seizure freedom + Seizure reduction >50% (n = 115) | |||
|---|---|---|---|---|---|---|---|
| No. (%) of pat. Within group | % of pat. Of total cohort | No. (%) of pat. Within group | % of pat. Of total cohort | No. (%) of pat. Within group | % of pat. Of total cohort | ||
| First | 2 (0.6%) | 1 (5.0%) | 0.6% | 0 | 0 | 1 (0.7%) | 0.3% |
| Second | 26 (8.4%) | 3 (15.0%) | 1% | 4 (4.2%) | 1.3% | 7 (6.1%) | 2.3% |
| Third | 22 (7.1%) | 2 (10.0%) | 0.6% | 6 (6.3%) | 1.9% | 8 (7.0%) | 2.6% |
| Fourth | 32 (10.3%) | 1 (5.0%) | 0.3% | 14 (14.8%) | 4.5% | 15 (13.0%) | 4.8% |
| Fifth | 25 (8.0%) | 2 (10.0%) | 0.6% | 7 (7.4%) | 2.3% | 9 (7.8%) | 2.9% |
| Sixth | 20 (6.4%) | 2 (10.0%) | 0.6% | 5 (5.3%) | 1.6% | 7 (6.1%) | 2.3% |
| Seventh | 35 (11.3%) | 1 (5.0%) | 0.3% | 10 (10.5%) | 3.2% | 11 (9.6%) | 3.5% |
| Eighth | 24 (7.7%) | 0 | ‐ | 7 (7.4%) | 2.3% | 7 (6.1%) | 2.3% |
| Ninth | 21 (8.8%) | 3 (15.0%) | 1% | 6 (6.3%) | 1.9% | 9 (7.8%) | 2.8% |
| Tenth | 18 (5.8%) | 3 (15.0%) | 0.6% | 5 (5.3%) | 1.6% | 8 (7.0%) | 2.6% |
| Eleventh | 17 (5.5%) | 1 (5.0%) | 0.3% | 7 (7.4%) | 2.3% | 8 (7.0%) | 2.6% |
| Twelfth | 9 (2.9%) | 0 | ‐ | 2 (2.1%) | 0.6% | 2 (1.7%) | 0.6% |
| Thirteenth | 7 (2.3%) | 0 | ‐ | 2 (2.1%) | 0.6% | 2 (1.7%) | 0.6% |
| Fourteenth | 5 (1.6%) | 0 | ‐ | 3 (3.2%) | 1.0% | 3 (2.6%) | 0.9% |
| Fifteenth | 3 (0.6%) | 0 | ‐ | 1 (1.1%) | 0.3% | 1 (0.9%) | 0.3% |
| Sixteenth | 1(0.3%) | 0 | ‐ | 0 | ‐ | 0 | ‐ |
| Seventeenth | 1 (0.3%) | 0 | ‐ | 0 | ‐ | 0 | ‐ |
| Unknown | 43 (13.8%) | 1 (5.0%) | 0.3% | 16 (16.8%) | 5.1% | 17 (14.8%) | 5.5% |
3.2. CBD titration
CBD was started at a median age of 11.3 years (range 2 months to 72 years, IQR 7.25‐17.83). The median starting dose was 3.2 mg/kg/d (range 0.08‐20 mg/kg/d, IQR 2.1‐5.0) and did not differ significantly between children (median 3.3 mg/kg/d, range 0.08‐20 mg/d) and adults (median 2.8 mg/kg/d, range 0.77‐5.7 mg/d) (Figure 1A, Table 1). Up‐titration was performed with a median of 2.8 mg/kg per week (range 0.08‐10.6, IQR 1.8‐4, Figure 1A, Table 1). The median end dose was 17.8 mg/kg/d (range 2.5‐45, IQR 11.7‐21.93) and was significantly higher in children (median 19.0 mg/kg/d, max. 45.0, Kruskal‐Wallis test, P < 0.0001) compared to adults (13.3 mg/kg/d, max. 32.0; Figure 1A, Table 1).
FIGURE 1.
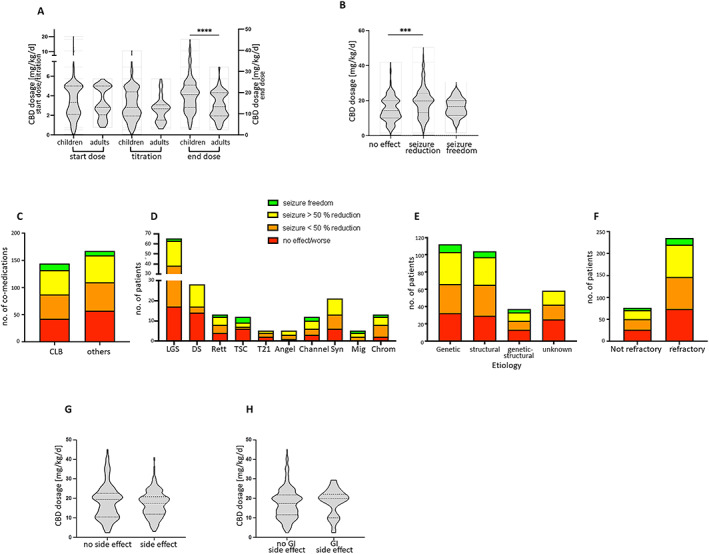
A. Violine plot of CBD dosage [mg/kg/d] showing start, titration, and end dose for children and adults. ****Children received a significant (Mann‐Whitney test, start dose: P = 0.5235 titration: P = 0.0941, end dose: P < 0.0001) higher end dose than adults. (A, C, H, I) Lines showing median and quartiles. B. Violine plot of CBD dosage in [mg/kg/d| shown for patients with no effect of CBD on seizures, seizure reduction <50% and >50%, and seizure freedom. Lines are showing median and quartiles. ***Significant higher doses were applied in patients with seizure reduction compared to patient with no CBD effect (Kruskal‐Wallis test, multiple comparison, P = 0.0001, no effect/worse: median: 14.25, IQR: 10‐0‐20.0; <50% reduction: median: 18.0, IQR: 11.95‐21.85; >50% reduction: median 20.0, IQR: 15.0‐25.0; seizure freedom: median 16.7, IQR 11.58‐20.0). C. Boxplot of number of patients treated with a concomitant CLB or other co‐medication. (C‐F): Colors showing outcome of the effect of CBD on seizures: red = no effect/ more seizures, orange = seizure reduction <50%, yellow = seizure reduction >50%, green = seizure freedom. D. Boxplot of number of patients sorted by genetic syndromes and causes of epilepsies and outcome due to CBD treatment (Chi square test, P = 0.15). LGS = Lennox‐Gastaut Syndrome, DS = Dravet Syndrome, Rett = Rett syndrome, TSC = Tuberous Sklerosis Complex, T21 = trisomy 21, Angel = Angelman syndrome, Channel = channelopathies, Syn = Synapsis, Mig = migration disorders, Chrom = chromosomal abnormalities. E. Boxplot of number of patients sorted by etiology of their epilepsies and outcome due to CBD treatment (Chi square test, P = 0.36). F. Boxplot of number of patients sorted by refractory or nonrefractory epilepsy epilepsies and outcome due to CBD treatment (Chi square test, P = 0.93). G. In our cohort CBD dosage [mg/kg/d| had no effect on the presence of side effects in general (Mann‐Whitney test, P = 0.4466) or H. gastrointestinal (GI) side effects (Mann‐Whitney test, P = 0.5097).
3.3. Effects of CBD treatment
The median follow‐up of all patients was 15.8 months (3 days to 5.9 years, IQR 6.9 month to 2.2 years; Table 1). Approximately one‐third (n = 95, 30.5%) of the patients terminated therapy within the observation period. The median duration of treatment in this group was 6.2 months (range 3 days to 41.7 months). Reasons for discontinuation were lack of sufficient therapeutic efficacy (n = 61, 19.6%), adverse effects (n = 29, 9.3%), and other causes such as nonadherence, lack of reimbursement by the health insurance, pregnancy, and nontherapy‐related death. 18 patients of 311 patients discontinued the treatment before 3 months of treatment due to lack of effect or increased seizure frequency (n = 6), to side effects (n = 10) (tiredness (n = 5), gastrointestinal side effects (n = 4), psychiatric symptoms (n = 1)), incompliance (n = 1), and not therapy‐related death (n = 1).
The median follow‐up of the other patients (n = 216, 69.5%) who did not end the treatment was 21.4 months (8 days to 5.9 years; Table 1). In the entire cohort after a median treatment duration of 15.8 months (range 3 days to 5.9 years, IQR 6.9 month to 2.2 years) following CBD introduction seizure frequency was reduced in 192 of 311 patients (61.7%), thereof seizure reduction of >50% was obtained in 95 of 311 patients (30.5%), and seizure‐freedom was obtained in a further 20 patients (6.4%). Looking specifically only at the 20 patients who were seizure‐free, the observation period was median 21 months (range 3.1 months to 5.7 years). In 23.2% of all patients (n = 72), no change of seizure frequency occurred, and 8.7% (n = 27) showed an increased seizure frequency. Previous treatments of those 20 patients included ASM treatment with levetiracetam (n = 14), oxcarbazepine (n = 10), sultiame (n = 10), topiramate (n = 10), valproic acid (n = 7), lamotrigine (n = 6), ethosuximide (n = 5), lacosamide (n = 5), zonisamide (n = 4), rufinamide (n = 4), vigabatrin (n = 3), phenobarbital (n = 3), carbamazepine (n = 2), clobazam (n = 2), perampanel (n = 2), phenytoin (n = 2), brivaracetam (n = 1). One patient had undergone hemispherotomy before CBD treatment.
The outcome for patients with LGS, DS, and TSC did not significantly differ from other epilepsy subtypes. Seizure freedom was achieved in 7% in LGS, 5% in DS, and 1.3% in TSC (Table S3).
Significant higher doses (Kruskal‐Wallis test, multiple comparison, P = 0.0003) were applied in patients with seizure reduction compared to patient with no CBD effect. The CBD effect correlated with the CBD end dose (Kruskal‐Wallis test, P = 0.00014) (Figure 1B). The CBD effect did not correlate with age at CBD initiation (Kruskal‐Wallis test, P = 0.3904). Patients receiving clobazam as comedication had no significantly improved effect on seizure frequency reduction compared to patients without clobazam (Chi‐square test, P = 0.5084; Figure 1C).
To address a potential honeymoon effect of CBD, we assessed a possible reduction of the effect of CBD on seizure frequencies after the first 3 months of CBD treatment (Table 1). Excluding patients without a positive effect on seizures in the first 3 months of treatment and those with a treatment duration under 3 months (n = 102), the initial positive effect decreased in 21.5% (45/209) and remained stable in 41.6% (87/209) of patients. In 36.8% (77/209) these data were not available.
The effect of CBD on seizures did not differ significantly between individuals with the epilepsy subtypes approved for CBD treatment (LGS, DS, TSC) and those with other epilepsy subtypes (Figure 1D). None of the patients with DS became seizure‐free. The outcome of the various patient groups depending on the etiology and drug resistance of their epilepsy subtype is illustrated in Figure 1D‐F.
3.4. Adverse effects of CBD treatment
Adverse effects were reported in almost half of the patients (n = 146, 46.9%) and overall did not correlate with the CBD dosage (Table 1; Figure 1G). These included particularly reduced vigilance and tiredness in 75 patients (24.1%) as well as nondose‐dependent gastrointestinal adverse effects in 78 patients (25.1%) (Figure 1H), including diarrhea in 38 cases. Moreover, 19 patients (6.1%) reported psychiatric symptoms such as aggressiveness, panic attacks, and psychotic symptoms. In five cases increased liver enzymes were reported. Four of these received valproate as comedication out of a total of 130 patients receiving valproate comedication (88 with available liver values). Adverse effects resulting in treatment interruption occurred in 29 of all 311 patients (9.3%) and included gastrointestinal adverse effects such as diarrhea (n = 12, 3.9%), sleepiness (n = 6, 1.9%), psychiatric symptoms (n = 6, 1.9%), muscle weakness (n = 2, 0.6%), and dizziness (n = 1, 0.3%).
3.5. Positive side effects
Perceived positive effects of the CBD therapy were improved mood (n = 96, 30.9%), improved night sleep (n = 75, 24.1%), improved development and concentration (n = 39, 12.5%), and increase in appetite (n = 35, 11.3%). Spasticity was reduced in 24 of 69 patients (34.8%) (Table 1).
4. DISCUSSION
We report the dosing, efficacy, and tolerability of CBD treatment in a large cohort of 311 children and adolescents with various epilepsy syndromes and etiologies in a retrospective multicenter study. We provide real‐world data including off‐label applications. Our cohort included 75.6% patients with DRE. We provide information on the real‐world CBD dosing regimen. Moreover, we show that the efficacy of treatment with CBD in epilepsy does not differ between individuals with LGS, DS, and TSC and those with other epilepsy subtypes. Due to the results of our study CBD seems not to offer a disease targeted effect relating to seizure generation or pathology of DS, LGS, or TSC. Therapy with CBD was off‐label in most cases (91.3%) due to age under 2 years (n = 28), epilepsy subtype (n = 206), lack of adjunct therapy with clobazam (n = 167), and/or higher dose applied than approved (n = 93).
The EMA dosing recommendation for CBD specifies a starting dose of 5 mg/kg/d in two doses with a weekly up‐titration by 5 mg/kg/d to a maximum dose of 20 mg/kg/d for LGS and DS and to 25 mg/kg/d for TSC. The starting dose of CBD in the present cohort was lower with a median 3.2 mg/kg/d, and up‐titration was also performed less rapid with steps of a median of 2.8 mg/kg per week. While the median end dose applied to individuals in our study was as recommended by the producer (median 19.0 mg/kg/d in children and 13.3 mg/kg/d in adults), it was significantly lower in adults. The maximum dose applied was higher in children (max. 45 mg/kg/d) than in adults (max. 32 mg/kg/d). The dosing in children <2 years of age was similarly performed.
Treatment of all individuals with various epilepsy subtypes resulted in seizure freedom in 6.4%, seizure reduction >50% in 30.5%, and seizure reduction <50% in 31.2%. The effect was independent of the coadministration of clobazam, supporting that an introduction of clobazam to meet in‐label treatment restraints 24 may not be maintained.
The use of CBD as the first administered ASM appears to be less effective compared to other ASMs, we observed a lower rate of seizure freedom in our cohort than published by Chen et al, although the number of first‐line therapies in our cohort is little 25 (Table 2). The efficacy as a third or fourth drug corresponds to that from previous studies. 25 , 26 , 27 The fact that CBD, as a relatively new ASM, has already been used in non‐DRE (78 patients, 25.1%) in our cohort might reflect the high expectations regarding its efficacy.
Seizure freedom or seizure frequency reduction >50% was seen in 40.8% of patients with LGS, DS, or TSC and in 36.9% of those with non‐LGS, non‐DS, and non‐TSC epilepsy. No patients with DS attained seizure freedom. Our retrospective data show similar effectiveness of CBD in in‐label epilepsy types compared to the effectiveness shown in randomized and controlled studies, leading to approval in LGS, DS, and TSC (Table S3). 10 , 11 , 12 , 13 , 16 , 28 This did not differ between those individuals with LGS, DS, and TSC receiving CLB compared to those not receiving that comedication. This intriguing finding could be due to selection bias with highly DRE patients included in the present study versus the strict selection criteria of the previous studies, which makes reproducibility in real‐word data studies challenging to achieve. 10 , 13 , 14 Thus, we cannot confirm a unique seizure reduction for LGS, DS, and TSC in our study, but rather support the effectiveness in a larger spectrum of epilepsy subtypes. 29 , 30
A honeymoon effect is known for many ASM, 25 , 31 , 32 and we here demonstrate that this is also the case for CBD. One‐fifth of patients who had reported seizure freedom or reduced seizure frequency (> or <50%) in the first 3 months showed reduced efficacy after 3 months, indicating a honeymoon effect in CBD. 33 Another recently published open‐label study reported that about one‐third of children and adults with epilepsy subtypes showed some tolerance to CBD. 20 In a study by Thiele et al. 14 that led to the approval of CBD, no evidence for the tolerance of CBD could be found; a possible honeymoon effect was first assessed after one year of treatment with CBD. Further studies are needed to address a potential honeymoon effect.
In addition to the effect on seizures, we observed effects on soft signs in 42.1% of all patients similar to findings in previous studies. 4 , 8 These effects included improved mood, night sleep, development, and appetite as well as reduction of preexisting spasticity. We feel this to be a potent strength of CBD, although not jet sufficiently defined, and suggest focusing on those soft signs on shorter time intervals in further investigations. Furthermore, the relatively high placebo effect, which was already evident in previous studies, must be mentioned. 20 , 34 In addition to a reduction in seizure frequency with placebo use in the RCTs, there were also positive effects on soft markers. 11 , 14 , 17 , 21
Overall, the drug tolerability was good with known adverse effects in 146 patients (46.9%) that were, however, not severe and well‐controllable. 10 , 11 , 15 , 16 , 29 , 35 The profile of adverse effects did not differ between in‐label and off‐label treatment (Table S3). The most prominent adverse effects that occurred in about a quarter of the patients were gastrointestinal adverse effects and reduced vigilance. Increased sleepiness was observed particularly in those cases concomitantly treated with clobazam (32.6%, 47/144) versus those without clobazam (16.7%, 28/167). Less frequently, psychiatric symptoms or elevation of liver enzymes occurred. Given the low number of cases with liver toxicity when CBD was added to valproic acid in our study, we cannot infer valproic acid as a clear risk factor but suggest close monitoring as part of the protocol. 10 , 11 , 13 , 28 Overall, adverse effects led to a discontinuation of the therapy in only 9.3% (n = 29). The most frequent cause was diarrhea, which has been previously reported and linked in some cases to sesame oil allergy. 36 Several patients or caretakers rated a softer stool as positive in individuals with frequent constipation. Neither diarrhea nor the other adverse effects in our study were dependent on the CBD dose, in contrast to previous studies. 10 , 11 , 12 Our results support the overall good safety profile with tolerable adverse effects of CBD in a real‐world data study, in line with previous randomized controlled trials. 11 , 13 , 36 , 37
However, limitations of our study were a retrospective design with missing data, heterozygous documentation between the centers, possible confounders such as prior medication, diets, epilepsy surgery. Additionally, the cohort was heterogeneous in age, seizure type, and CBD titration plan.
In summary, we highlight an overall seizure freedom rate of CBD comparable to that of many other ASMs in DRE 25 with a positive safety profile. We show that comedication with clobazam was not associated with a better outcome and that higher end doses were safely applied, particularly in children, to achieve reduction of seizure frequency. This applies to children and adults with various epilepsy subtypes. Our data, therefore, call for further trials to aim for an extended approval of CBD for other epilepsy subtypes and for children <2 years of age.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHOR CONTRIBUTIONS
AMK, FK, CP, KAK contributed to the conception and design of the study. FK, LLB; TB, AB, IB,CMB, JF, CH, NAH, MH, MH, CJ, GJK, GK, HL, KLM, FP, MP, SSB, JS, ASB, DS, BJS, AS, SS, HDV, CW, JW, BW, CP, KAK, AMK contributed to acquisition of data. FK organized the database, FK and LLB analyzed the data. FK wrote the first draft of the manuscript, FK and LLB created tables and figures. All authors discussed the results, revised the first draft, and contributed to the final manuscript.
CONFLICT OF INTEREST STATEMENT
The current study was financially supported by GW Pharma to fund the position of a physician to gather the necessary patient data. The study was supported by the Einstein Stiftung Fellowship through the Günter Endres Fond. AMK has served on advisory boards, received institutional funding from GW Pharma, Biogen, Avexis, PTC, Ethipharm, Nutricia, and Desitin outside the submitted work. KAK received honoraria for lectures and advice from EISAI; GW pharmaceuticals, and Zogenix. AB reports grants from UCB Pharma GmbH and honoraria for speaking engagements and advisory boards from Biogen GmbH, Desitin Arzneimittel GmbH, Eisai GmbH, GW Pharma GmbH, Neuraxpharm GmbH, Shire/Takeda GmbH, UCB Pharma GmbH, and ViroPharma GmbH outside the submitted work. MH reports personal fees as speaker and consultant within the last 3 years from Angelini/Arvelle, Bial, Desitin, Eisai, GW Pharma, Neuraxpharma, UCB and Zogenix. BW reports honoraria as speaker and consultant within the last five years from Takeda, AbbVie, Ipsen, Neuraxpharma. MP reports fees as speaker and consultant for Zogenix. TB has received personal fees as speaker and consultant from Bial, Desitin Arzneimittel GmbH, Eisai, GW Pharmaceuticals, Neuraxpharm, Novartis, Nutricia, Shire, Takeda, UCB Pharma, and Zogenix. BJS has served on advisory boards and received honoraria for lectures from Al.Jaziri, Angelinie, Desitin, Eisai, GW Pharma, UCB, and Zogenix. CH has received personal fees as speaker and consultant from Desitin, Eisai, GW Pharmaceuticals, Novartis, Nutricia, Shire/Takeda GmbH, AS reports personal fees and grants from Angelini Pharma, Desitin Arzneimittel, Eisai, GW Pharmaceuticals companies, Marinus Pharma, Precisis, UCB Pharma, UNEEG medical, and Zogenix. HL received honoraria for speaking or advisory boards from Arvelle, Bial, Esai, UCB, and Zogenix, and research support from Bial and Boehringer Ingelheim. SSB reports personal fees and grants from Desitin Arzneimittel, Eisai, GW Pharmaceutical companies, Marinus Pharma, UCB Pharma, and Zogenix. ASB has received research support from BIAL, PRECISIS and UNEEG, and personal honoraria for advice or lectures from Angelini, BIAL,GW, and UCB. MH reports honoraria for speaking engagements and advisory boards from UCB Pharma GmbH and Eisai GmbH outside the submitted work. All other authors have indicated they have no potential conflicts of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Table S1
Table S2
Table S3
Kühne F, Becker L‐L, Bast T, Bertsche A, Borggraefe I, Boßelmann CM, et al. Real‐world data on cannabidiol treatment of various epilepsy subtypes: A retrospective, multicenter study. Epilepsia Open. 2023;8:360–370. 10.1002/epi4.12699
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Devinsky O, Cilio MR, Cross H, Fernandez‐Ruiz J, French J, Hill C, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borges R, Batista J, Viana R, Baetas A, Orestes E, Andrade M, et al. Understanding the molecular aspects of tetrahydrocannabinol and Cannabidiol as antioxidants. Molecules. 2013;18(10):12663–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burstein S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem. 2015;23(7):1377–85. [DOI] [PubMed] [Google Scholar]
- 4. Batalla A, Bos J, Postma A, Bossong MG. The impact of Cannabidiol on human brain function: a systematic review. Front Pharmacol. 2021;11:618184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones NA, Hill AJ, Smith I, Bevan SA, Williams CM, Whalley BJ, et al. Cannabidiol displays Antiepileptiform and Antiseizure properties In vitro and In vivo. J Pharmacol Exp Ther. 2010;332(2):569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Szaflarski JP, Bebin EM, Cutter G, DeWolfe J, Dure LS, Gaston TE, et al. Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open‐label add‐on prospective study. Epilepsy Behav. 2018;87:131–6. [DOI] [PubMed] [Google Scholar]
- 7. Tzadok M, Uliel‐Siboni S, Linder I, Kramer U, Epstein O, Menascu S, et al. CBD‐enriched medical cannabis for intractable pediatric epilepsy. Seizure. 2016;35:41–4. [DOI] [PubMed] [Google Scholar]
- 8. Rosenberg EC, Louik J, Conway E, Devinsky O, Friedman D. Quality of life in childhood epilepsy in pediatric patients enrolled in a prospective, open‐label clinical study with cannabidiol. Epilepsia. 2017;58(8):e96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Press CA, Knupp KG, Chapman KE. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav. 2015;45:49–52. [DOI] [PubMed] [Google Scholar]
- 10. Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of Cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N Engl J Med. 2018;378(20):1888–97. [DOI] [PubMed] [Google Scholar]
- 11. Thiele EA, Marsh ED, French JA, Mazurkiewicz‐Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2018;391(10125):1085–96. [DOI] [PubMed] [Google Scholar]
- 12. Devinsky O, Nabbout R, Miller I, Laux L, Zolnowska M, Wright S, et al. Long‐term cannabidiol treatment in patients with Dravet syndrome: an open‐label extension trial. Epilepsia. 2019;60(2):294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of Cannabidiol for drug‐resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011–20. [DOI] [PubMed] [Google Scholar]
- 14. Thiele EA, Bebin EM, Bhathal H, Jansen FE, Kotulska K, Lawson JA, et al. Add‐on Cannabidiol treatment for drug‐resistant seizures in tuberous sclerosis complex: a placebo‐controlled randomized clinical trial. JAMA Neurol. 2021;78(3):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thiele EA, Bebin EM, Filloux F, Kwan P, Loftus R, Sahebkar F, et al. Long‐term cannabidiol treatment for seizures in patients with tuberous sclerosis complex: an open‐label extension trial. Epilepsia. 2022;63(2):426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scheffer IE, Halford JJ, Miller I, Nabbout R, Sanchez‐Carpintero R, Shiloh‐Malawsky Y, et al. Add‐on cannabidiol in patients with Dravet syndrome: results of a long‐term open‐label extension trial. Epilepsia. 2021;62(10):2505–17. [DOI] [PubMed] [Google Scholar]
- 17. Gunning B, Mazurkiewicz‐Bełdzińska M, Chin RFM, Bhathal H, Nortvedt C, Dunayevich E, et al. Cannabidiol in conjunction with clobazam: analysis of four randomized controlled trials. Acta Neurol Scand. 2021;143(2):154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug‐drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56(8):1246–51. [DOI] [PubMed] [Google Scholar]
- 19. Greenwich Biosciences . Epidiolex, full prescribing information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
- 20. Lattanzi S, Trinka E, Striano P, Zaccara G, Del Giovane C, Nardone R, et al. Cannabidiol efficacy and clobazam status: a systematic review and meta‐analysis. Epilepsia. 2020;61(6):1090–8. [DOI] [PubMed] [Google Scholar]
- 21. Chesney E, Oliver D, Green A, Sovi S, Wilson J, Englund A, et al. Adverse effects of cannabidiol: a systematic review and meta‐analysis of randomized clinical trials. Neuropsychopharmacology. 2020;45(11):1799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Avoxa – Mediengruppe Deutscher Apotheker GmbH . Ölige Cannabidiol‐Lösung (NRF 22.10.) 2022. https://be.fagron.com/sites/default/files/product/document/dac_‐_cbd_oplossing.pdf
- 23. Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies: definition of drug resistant epilepsy. Epilepsia. 2009;51(6):1069–77. [DOI] [PubMed] [Google Scholar]
- 24. Savage TE, Sourbron J, Bruno PL, Skirvin LA, Wolper ES, Anagnos CJ, et al. Efficacy of cannabidiol in subjects with refractory epilepsy relative to concomitant use of clobazam. Epilepsy Res. 2020;160:106263. [DOI] [PubMed] [Google Scholar]
- 25. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30‐year longitudinal cohort study. JAMA Neurol. 2018;75(3):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kwan P, Brodie MJ. Clinical trials of antiepileptic medications in newly diagnosed patients with epilepsy. Neurology. 2003;60(Issue 11, Supplement 4):S2–12. [DOI] [PubMed] [Google Scholar]
- 27. Perucca E, Brodie MJ, Kwan P, Tomson T. 30 years of second‐generation antiseizure medications: impact and future perspectives. Lancet Neurol. 2020;19(6):544–56. [DOI] [PubMed] [Google Scholar]
- 28. McCoy B, Wang L, Zak M, Al‐Mehmadi S, Kabir N, Alhadid K, et al. A prospective open‐label trial of a cbd/thc cannabis oil in dravet syndrome. Ann Clin Transl Neurol. 2018;5(9):1077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herlopian A, Hess EJ, Barnett J, Geffrey AL, Pollack SF, Skirvin L, et al. Cannabidiol in treatment of refractory epileptic spasms: an open‐label study. Epilepsy Behav. 2020;106:106988. [DOI] [PubMed] [Google Scholar]
- 30. Devinsky O, Verducci C, Thiele EA, Laux LC, Patel AD, Filloux F, et al. Open‐label use of highly purified CBD (Epidiolex®) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav. 2018;86:131–7. [DOI] [PubMed] [Google Scholar]
- 31. Loscher W, Schmidt D. Experimental and clinical evidence for loss of effect (tolerance) during prolonged treatment with antiepileptic drugs. Epilepsia. 2006;47(8):1253–84. [DOI] [PubMed] [Google Scholar]
- 32. Avanzini G. Is tolerance to antiepileptic drugs clinically relevant? Epilepsia. 2006;47(8):1285–7. [DOI] [PubMed] [Google Scholar]
- 33. Uliel‐Sibony S, Hausman‐Kedem M, Fattal‐Valevski A, Kramer U. Cannabidiol‐enriched oil in children and adults with treatment‐resistant epilepsy‐does tolerance exist? Brain Develop. 2021;43(1):89–96. [DOI] [PubMed] [Google Scholar]
- 34. Zaccara G, Giovannelli F, Schmidt D. Placebo and nocebo responses in drug trials of epilepsy. Epilepsy Behav. 2015;43:128–34. [DOI] [PubMed] [Google Scholar]
- 35. Sands TT, Rahdari S, Oldham MS, Caminha Nunes E, Tilton N, Cilio MR. Long‐term safety, tolerability, and efficacy of Cannabidiol in children with refractory epilepsy: results from an expanded access program in the US. CNS Drugs. 2019;33(1):47–60. [DOI] [PubMed] [Google Scholar]
- 36. Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, et al. Cannabidiol in patients with treatment‐resistant epilepsy: an open‐label interventional trial. Lancet Neurol. 2016;15(3):270–8. [DOI] [PubMed] [Google Scholar]
- 37. Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, et al. Chronic Administration of Cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21(3):175–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


