Abstract
Preeclampsia is a hypertensive disorder in pregnancy characterized by placental malperfusion and subsequent multi-organ injury. It accounts for approximately 14% of maternal deaths and 10–25% of perinatal deaths globally. In addition, preeclampsia has been attracting attentions for its association with risks for developing chronic diseases in later life for both mother and child. This mini-review discusses on latest knowledge on prediction, prevention, management, and long-term outcomes of preeclampsia and also touches on association between COVID-19 and preeclampsia.
HTN hypertension, HDP hypertensive disorders of pregnancy, PE preeclampsia, BP blood pressure, cfDNA cell-free DNA, ST2 human suppression of tumorigenesis 2, sFlt-1 soluble fms-like tyrosine kinase-1, PIGF placental growth factor, VEGF vascular endothelial growth factor, VEGFR VEGF receptor, TGFβ transforming growth factor β, ENG endoglin, sENG soluble ENG, PRES posterior reversible encephalopathy syndrome, AKI acute kidney injury, CVD cardiovascular disease, ESKD end-stage kidney disease, ACE angiotensinogen converting enzyme, Ang angiotensin.
Keywords: Soluble fms-like tyrosine kinase-1, Placental growth factor, Low dose aspirin, COVID-19
HTN hypertension, HDP hypertensive disorders of pregnancy, PE preeclampsia, BP blood pressure, cfDNA cell-free DNA, ST2 human suppression of tumorigenesis 2, sFlt-1 soluble fms-like tyrosine kinase-1, PIGF placental growth factor, VEGF vascular endothelial growth factor, VEGFR VEGF receptor, TGFβ transforming growth factor β, ENG endoglin, sENG soluble ENG, PRES posterior reversible encephalopathy syndrome, AKI acute kidney injury, CVD cardiovascular disease, ESKD end-stage kidney disease, ACE angiotensinogen converting enzyme, Ang angiotensin.
Introduction
Hypertensive disorders of pregnancy (HDP) affect approximately 10% of all pregnancies globally. HDP, particularly preeclampsia (PE), accounts for as high as 14% of maternal mortality and results in 10–25% of perinatal deaths. The 2019 Maternal Mortality update from the WHO report noted the major contribution of PE and eclampsia to worldwide maternal deaths. Since once it develops, termination is the only given way to ameliorate the symptoms of PE, trials and cohort studies regarding PE, give insight into the development of diagnostic and prognostic tools and preventive care.
Prediction
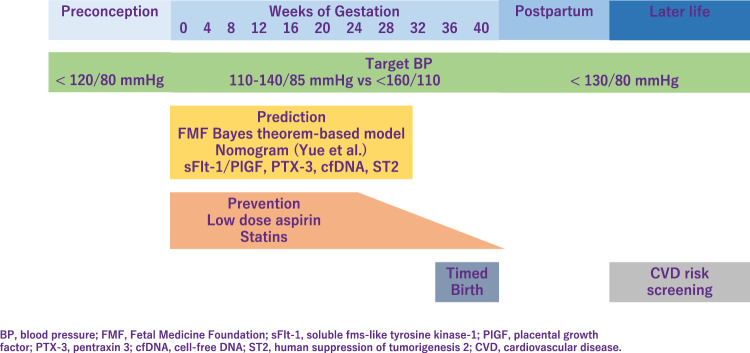
It is important to provide early screening and identify pregnant women at high risk who might benefit from prophylactic agents [1]. The Fetal Medicine Foundation proposed a Bayes theorem-based model to predict preterm PE using a combination of maternal characteristics, medical history, mean arterial pressure, uterine artery pulsatility index, and serum placental growth factor (PlGF). This model can predict ~90% of early PE cases, with delivery at <32 weeks of gestation and 75% of preterm PE cases, with delivery at <37 weeks of gestation [2, 3]. Recently, Yue et al. have developed and validated a new nomogram for the early prediction of PE in pregnant Chinese women [4]. This nomogram included body mass index (BMI), blood pressure (BP), uterine artery ultrasound parameters, and serological indicators and can be easily utilized to facilitate the individualized prediction of PE.
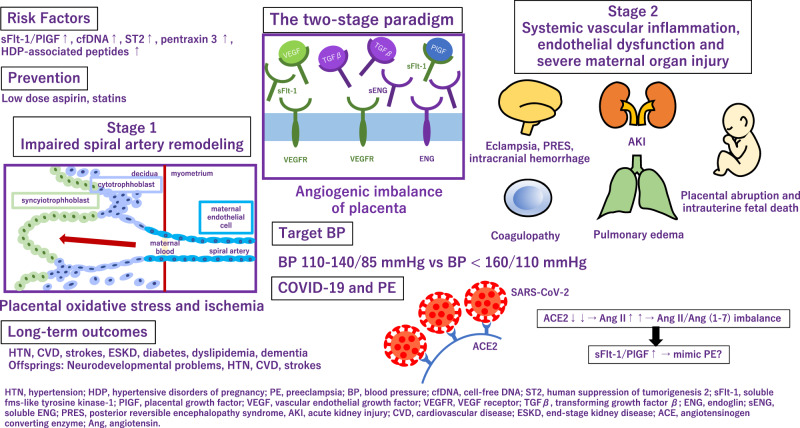
Application of angiogenic and antiangiogenic biomarkers into clinical practice will help reduce the considerable burden of morbidity and mortality associated with adverse pregnancy outcomes as a consequence of PE. PE is associated with alteration of angiogenic and antiangiogenic factors such as soluble fms-like tyrosine 1 (sFlt-1), soluble endoglin (sENG) and PlGF [5–9] (Fig. 1). Quantification of the sFlt-1/PlGF ratio, has been shown to be a useful biomarker test for aiding the diagnosis and short-term prediction of PE [10]. PRediction of short-term Outcomes in preGNant wOmen with Suspected PE Study (PROGNOSIS) proposed and validated sFlt-1/PIGF ratio cutoff of 38 to predict the development of PE in women with clinical suspicion [11–13]. Furthermore, the sFlt-1/PlGF ratio test is likely to lessen the avoidable hospitalization of women at low risk of developing PE in the short term while identifying high-risk individuals requiring appropriate management [14].
Fig. 1.
Alteration of angiogenic and antiangiogenic factors in preeclampsia
Besides sFlt-1 and PlGF, several biomarkers have been reported as candidate markers for predicting the development of PE. The first trimester pregnancy-associated plasma protein A can predict PE and superimposed PE in the third trimester [15]. Plasma cell-free DNA and human suppression of tumorigenesis served as diagnostic biomarkers for gestational hypertension (GH) and PE [16]. Pentraxin 3, an acute-phase protein which is produced and released in response to inflammatory stimuli, has been proposed as a novel biomarker predicting placental failure [17] and was also associated with PE [18]. Using an effective peptidomic analysis, Wakabayashi et al. identified seven circulating HDP-associated peptides (P-2081, P-2091, P-2127, P-2209, P-2378, P-2858, and P-3156) and proposed them as biomarkers for the diagnosis of HDP [19]. Interestingly, they have also investigated that these peptides are possible biomarkers for discriminating cardiovascular risk even in general population [20].
Prevention
Low-dose aspirin is highly promising for the prevention of PE and is extensively studied. The American College of Obstetricians and Gynecologists (ACOG), International Society for the Study of Hypertension in Pregnancy (ISSHP), National Institute for Health, and Care Excellence, Japan Society for the Study of Hypertension in Pregnancy (JSSHP) recommend initiation of low-dose aspirin in women with high risk factor to reduce the risk of PE [21–23]. A systematic review established benefits of low-dose aspirin taken during pregnancy [24]. Aspirin use was significantly associated with lower risk of PE, perinatal mortality, preterm birth, and intrauterine growth restriction. According to the guideline proposed by the ACOG in 2018 [23], chronic hypertension (CH) is one of the risk factors for the development of PE, and aspirin is recommended for this group of patients. However, incidence of superimposed PE was not significantly different in the pre-ACOG group and post ACOG group, indicating that aspirin did not reduce the incidence of superimposed PE in patients with CH. Aspirin decreases the risk of PE, but its effectiveness in women with CH remains controversial [25]. Aspirin is preferably started before 16 weeks of gestation and continued until delivery. However, initiation of aspirin may complicate peripartum bleeding, which could be mitigated by discontinuing aspirin earlier. Recent multicenter, randomized trial showed that aspirin discontinuation at 24 to 28 weeks of gestation is noninferior to aspirin continuation for preventing preterm PE in individuals at high risk of PE and a normal sFlt-1/PlGF ratio between 24 weeks 0 days and 27 weeks 6 days of gestation.
The properties and mechanisms of action of statins make them candidates for the prevention of PE. In a meta-analysis, which included 10 studies describing 1391 women with uteroplacental insufficiency disorders: 703 treated with pravastatin and 688 not treated with statins, pravastatin prolonged pregnancy duration and improved associated obstetrical outcomes in pregnancies complicated with uteroplacental insufficiency disorders [26]. In contrast, 1120 women with singleton pregnancies at high risk of term PE were randomly assigned to receive pravastatin at a dose of 20 mg/d or placebo from 35 to 37 weeks of gestation until delivery or 41 weeks. Pravastatin in women at high risk of term PE did not reduce the incidence of delivery with PE [27].
Management
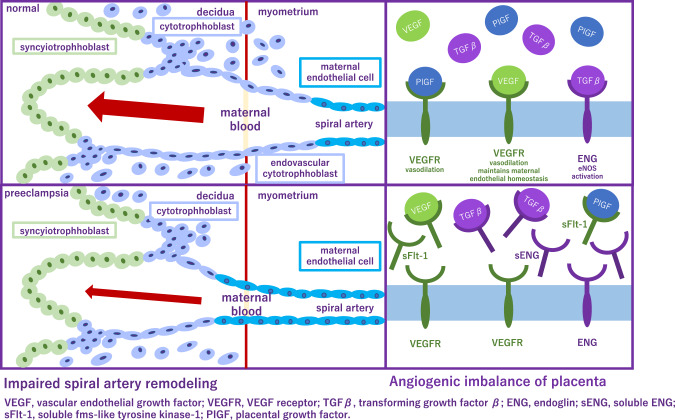
In 2017, the American College of Cardiology/American Heart Association hypertension (ACC/AHA) treatment guidelines identified hypertension as BP ≥ 130/80 mmHg. However, the reference BP for hypertension during pregnancy as specified in international guidelines [eg. ISSHP [22], ACOG [28, 29]], is ≥140/90 mmHg (Fig. 2). In Japan, guidelines of Japanese Society of Hypertension and JSSHP, both define hypertension as BP ≥ 140/90 mmHg whether the patient is pregnant or not [21, 30, 31]. Respecting BP control, from a preventive point of view, understanding the importance of preconception BP is of particular interest. It is relatively easy to intervene before pregnancy, and furthermore, this may have a greater impact on gestational outcome. In previous study, preconception BP and its change into early pregnancy was evaluated as risk markers for the development of HDP. Among 586 women with a pregnancy >20 weeks’ gestation, preconception BP levels were higher for preterm PE, term PE, and GH as compared with no HDP [32]. In large cohort study, which examined whether high BP in the preconception period was associated with GH and PE, when participants with normal BP were used as the reference, the adjusted ORs for GH were 1.48, 1.70, and 1.29, and for PE, the adjusted ORs were 1.55, 1.95, and 1.99 for the participants with prehypertension (SBP 120–139 mmHg or DBP 80–89 mmHg), stage 1 hypertension (SBP 140–159 mmHg or DBP 90–99 mmHg), and stage 2 hypertension (SBP ≥ 160 mmHg or DBP ≥ 100 mmHg), respectively [33]. These results support an association between hypertension and also prehypertension prior to pregnancy and an increased risk of GH and PE.
Fig. 2.
Management of preeclampsia
Target BP during pregnancy differs between guidelines. ISSHP recommends that BP ≥ 140/90 mmHg should be treated with a goal BP 110–140/85 mmHg, while ACOG recommends antihypertensive medications when BP ≥ 160/110 mmHg with goal BP below this threshold. JSSHP recommends to initiate antihypertensive medications when BP ≥ 140/90 mmHg for CH and BP ≥ 160/90 mmHg (depending on the situation, ≥140/90 mmHg) for other categories of HDP and to set target BP depending on the conditions of each case [21]. Studies reported that women in a low-risk cohort with stage 1 hypertension defined as 130–139 mmHg/80–89 mmHg, according to the ACC/AHA, are more likely to develop PE than women with normotensive in the early gestation. Based on the randomized controlled trial in China, the authors investigated whether PE was more likely to occur in stage 1 hypertensive women compared to the normotensive pregnant women between gestational age 12–20 weeks, in a high-risk cohort [34]. This subanalysis have revealed that stage 1 hypertension might be an additional risk factor for PE in high-risk pregnant women, and aspirin intervention might be useful in preventing PE. A meta-analysis established that the category of elevated BP had a risk ratio of 2.0 (95% prediction interval, 0.8–4.8), the stage 1 hypertension category had a risk ratio of 3.0 (95% prediction interval, 1.1–8.5), and the stage 2 hypertension category had a risk ratio of 7.9 (95% prediction interval, 1.8–35.1) [35]. However, none of the systolic BP measurements of <120 mmHg, <130 mmHg, or <140 mmHg were useful to rule out the development of PE. Another meta-analysis investigated that BP ≥ 120/80 mmHg, particularly ≥130/80 mmHg, at <20 weeks of gestation, is associated with increased maternal and perinatal risks and the authors proposed new BP categories in pregnancy as normal (<120/80), high normal (120–129/ < 80), and elevated (130–139/80–89) [36]. One retrospective study has shown that systolic BP < 130 mmHg within 14 weeks of gestation reduced the risk of developing early-onset superimposed PE in women with CH [37]. The benefits and safety of the treatment of mild hypertension (BP, <160/100 mm Hg) during pregnancy are still uncertain. Data are needed on whether a strategy of targeting a BP of less than 140/90 mmHg reduces the incidence of adverse pregnancy outcomes without compromising fetal growth.
Nifedipine, labetalol, and hydralazine alone or in combination are presently recommended by ACOG for the acute lowering of severe BP (≥160 mm Hg systolic and/or ≥110 mm Hg diastolic) in pregnancy [38]. A randomized controlled trial demonstrated that oral antihypertensives, methyldopa, nifedipine, and labetalol, all reduced BP in severe range to the reference range in most women [39]. As single drugs, nifedipine retard use resulted in a greater frequency of primary outcome [BP control (defined as 120–150 mm Hg SBP and 70–100 mm Hg DBP) within 6 h with no adverse outcomes.] attainment. A meta-analysis demonstrated that all commonly prescribed oral antihypertensives (labetalol, other β-blockers, methyldopa, calcium channel blockers, and mixed/multi-drug therapy) versus placebo/no therapy reduced the risk of severe hypertension by 30 to 70% in nonsevere pregnancy hypertension [40]. In addition, labetalol decreased proteinuria/PE and fetal/newborn death compared with placebo/no therapy, and proteinuria/PE compared with methyldopa and calcium channel blockers.
Currently, magnesium sulfate (MgSO4) is the primary treatment option and it is administered prophylactically to women with severe PE who are at risk of developing eclampsia. While MgSO4 is effective in preventing seizures, it is not as effective in reducing hypertension or other maternal organ injuries such as proteinuria in PE patients. Therefore, finding a therapeutic agent that improves multiple PE symptoms is urgent. In rat model, cyclosporin A (CsA) effectively attenuated PE manifestation and eclampsia-like seizure severity. In addition, CsA treatment significantly reduced the inflammatory cytokine levels and improved pregnancy outcomes following eclampsia-like seizures. The decreased inflammatory cytokines in PE are coincident with attenuated PE manifestation, suggesting that CsA treatment might decrease the PE severity through decreasing systemic inflammation [41]. Crocin, a hydrophilic carotenoid pigment, is a major compound with pharmacological activities found in Crocus sativus L. (saffron). Crocin alleviated inflammatory and oxidative stress in placental tissues, thereby protecting against GH, one of the major phenotypes of PE, and activated the Nrf-2/HO-1 pathway [42].
In women with a PE at term, immediate delivery reduces the risk of adverse maternal outcomes or progression to severe disease without affecting neonatal outcomes. However, in women with a PE diagnosed before term, benefits of delivery for the mother need to be weighed against the adverse consequences of iatrogenic preterm birth for the infant. In women with late preterm PE, the optimal time for termination is unclear because limitation of maternal disease progression needs to be balanced against infant complications. A randomized controlled study has shown that incidence of maternal death and severe hypertension was significantly lower in the planned delivery group compared with the expectant management group. However, planned delivery led to more neonatal unit admissions for the infant, principally for a listed indication of prematurity and without an excess of respiratory or other morbidity, intensity of care, or length of stay. This trade-off should be circumspectly discussed with women with late preterm PE to decide the optimal timing of delivery [43].
Long-term outcomes
Women with history of PE have increased cardiovascular disease (CVD) risk. Pregnancy has been labeled as a stress test which reveals women with cardiovascular dysfunction or poor reserve [44, 45]. A rise of 10-year maternal CVD risk is associated with PE, while those with sustained hypertension after delivery have a two-fold increase in the risk of developing CVD in the next 10–30 years. Adverse pregnancy outcomes (APO) including PE, occur in 10 to 20% of all pregnancies and are also associated with a 1.8- to 4.0-fold risk of future CVD [46, 47]. Women with a history of HDP are reported to have stiffer arteries and have a 2–5 times higher risk of hypertension in later life, compared to normotensive gestations [48, 49]. Association between HDP and later hypertension was reported to be stronger in younger women and in obese women in the 30–70 age group [50]. Additionally, PE is considered a risk factor for chronic diseases such as hypothyroidism, diabetes mellitus and dyslipidemia, each of which independently increases the incidence of cardiovascular morbidity [45]. PE was included as a “risk-enhancer” in the updated 2018 cholesterol guideline [51] and in the 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease [52]. The ACOG recommends that women with APO undergo cardiovascular risk screening within 3 months postpartum [53].
Data from the Stroke Prevention in Young Women Study showed that women with a history of PE are 60% more likely to suffer from ischemic stroke after multivariable adjustment [54]. Studies from the World Health Organization also showed an increased risk of hemorrhagic stroke [55] and venous thromboembolism [56] in women with history of hypertension in pregnancy. Prior PE is also associated with an increased risk for the development of end-stage kidney disease. A meta-analysis of 2,309,946 women(among whom 103,308 women with PE) demonstrated that history of PE also increases the risk of vascular dementia [57].
The mechanisms responsible for these associations remain unclear. One possible mechanism maybe the presence of remaining angiotensin II type 1 receptor agonistic autoantibody (AT1-AA) postpartum. AT1-AAs are elevated in women with PE. AT1-AA binds to angiotensin II type 1 receptor (AT1R) and increases AT1R activity, intracellular calcium levels, and activation of intracellular mitogen activated protein kinase/extracellular signal regulated kinases (MAPK/ERK) pathways. Previous study reported that −18% of postpartum PE women have elevated circulating AT1-AAs 1 year after delivery [58]. These women with elevated AT1-AAs had increased sFlt-1, decreased free VEGF, and higher insulin resistance compared with autoantibody negative women and these correlations may suggest a mechanism by which women with PE have increased risks of severe complications later in life. JSSHP recommends to explain to women with a history of HDP that they have a higher risk of developing subsequent lifestyle diseases and cerebrovascular/cardiovascular diseases and that regular follow-up and lifestyle intervention guidance can reduce the incidence [21].
Preterm delivery is associated with long-term neurodevelopmental problems in the offspring. Zwertbroek et al. found that early delivery in women with late preterm HDP is associated with poorer neurodevelopmental outcomes in their children at 2 years of age [59]. These findings indicate an increased risk of developmental delay after early delivery compared to expectant monitoring. The infant from a PE pregnancy also appears at increased risk for CVD [60]. Infants of mothers with PE have higher BP during young adulthood and an increased risk for stroke in later life [61]. Other differences have also been shown, including increased BMI [62] and hormonal changes.
COVID-19
Pregnancy could potentially affect the susceptibility to and the severity of COVID-19 and pregnant women are at an increased risk of mortality and morbidity due to COVID-19. In addition, what we need to be aware of is that although pregnant women are less likely to complain of the symptoms of COVID-19, they are more than twice as likely to require critical care or mechanical ventilation than nonpregnant women [63]. Kalafat et al. have proposed that the mini-model which includes the maternal age, BMI, and pregnancy trimester can be used to estimate the risk of developing critical COVID-19 before disease onset. The addition of inflammatory markers to maternal BMI at the time of diagnosis can accurately predict critical COVID-19, PE, and the progression time from diagnosis to clinical deterioration [64].
Although few cases of intrauterine transmission of SARS-CoV-2 have been documented, it appears to be rare [65]. It is possibly related to low levels of SARS-CoV-2 viremia and the decreased coexpression of angiotensin-converting enzyme (ACE) 2 and transmembrane serine protease 2 which is needed for SARS-CoV-2 entry into cells in the placenta. However, evidence is accumulating that SARS-CoV-2 infection is associated with a number of adverse pregnancy outcomes including PE, preterm birth, and stillbirth [66]. This tendency is reported to be observed especially among pregnant women with severe COVID-19 disease, but one large, longitudinal, prospective, observational study assessing the effect of COVID-19 during pregnancy on mothers and neonates have shown that COVID-19 severity does not seem to be a factor in this association [67]. Additionally, besides the direct impact of COVID-19 on pregnancy outcomes, there is evidence that the pandemic and its effects on healthcare systems have had detrimental effects on pregnancy outcomes even among pregnant women not infected with SARS-CoV-2 [68].
Also, some severe cases of COVID-19, patients present with PE-like symptoms (Fig. 3). PE mimicry by COVID-19 was confirmed following the alleviation of PE symptoms without delivery of placenta [69]. In COVID-19, ACE 2 function decreases and subsequently Ang (angiotensin) II activity increases [70]. Although COVID-19 shows an increase in the sFlt-1/PlGF ratio due to pathologic Ang II/Ang (1–7) imbalance like PE [71], sFlt1/PlGF ratio did not correlate with the severity [72]. Most experts believe that SARS-Cov-2 is likely to become endemic, the continued collection of data on the effects of COVID-19 during pregnancy are needed.
Fig. 3.
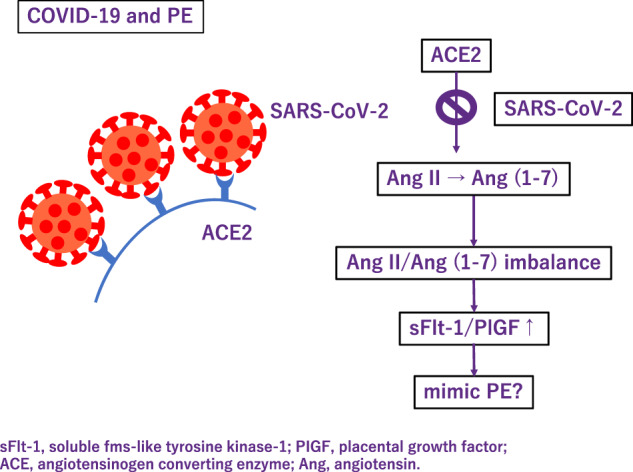
Association between COVID-19 and preeclampsia
Future perspectives
Pregnancy period is said to be a window where we can catch a glimpse of woman’s future. Though the symptoms of PE manifest typically in late pregnancy, fundamental alteration that underlies exists earlier in pregnancy or even preconceptionally and lasts throughout life. Earlier prediction, prevention and longer follow up is necessary for comprehensive management of PE. There is much to be done to decrease PE related maternal and fetal deaths and also to reduce maternal risks for chronic diseases in later life.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–22. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 2.Chaemsaithong P, Pooh RK, Zheng M, Ma R, Chaiyasit N, Tokunaka M, et al. Prospective evaluation of screening performance of first-trimester prediction models for preterm preeclampsia in an Asian population. Am J Obstet Gynecol. 2019;221:650.e1–650.e16. doi: 10.1016/j.ajog.2019.09.041. [DOI] [PubMed] [Google Scholar]
- 3.O’Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, Poon LC, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am J Obstet Gynecol. 2016;214:103.e1–103.e12. doi: 10.1016/j.ajog.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Yue C, Gao J, Zhang C, Ni Y, Ying C. Development and validation of a nomogram for the early prediction of preeclampsia in pregnant Chinese women. Hypertens Res. 2021;44:417–25. doi: 10.1038/s41440-020-00558-1. [DOI] [PubMed] [Google Scholar]
- 5.Duhig KE, Myers J, Seed PT, Sparkes J, Lowe J, Hunter RM, et al. Placental growth factor testing to assess women with suspected pre-eclampsia: a multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet. 2019;393:1807–18. doi: 10.1016/S0140-6736(18)33212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1–8. doi: 10.1016/j.ajog.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 7.Verlohren S, Perschel FH, Thilaganathan B, Dröge LA, Henrich W, Busjahn A, et al. Angiogenic markers and cardiovascular indices in the prediction of hypertensive disorders of pregnancy. Hypertension. 2017;69:1192–7. doi: 10.1161/HYPERTENSIONAHA.117.09256. [DOI] [PubMed] [Google Scholar]
- 8.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 9.Levine RJ, Maynard SE, Qian C, Lim K-H, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 10.Cerdeira AS, O’Sullivan J, Ohuma EO, Harrington D, Szafranski P, Black R, et al. Randomized interventional study on prediction of preeclampsia/eclampsia in women with suspected preeclampsia: INSPIRE. Hypertension. 2019;74:983–90. doi: 10.1161/HYPERTENSIONAHA.119.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisler H, Llurba E, Chantraine FJ, Vatish M, Staff AC, Sennström M, et al. Soluble fms-like tyrosine kinase-1 to placental growth factor ratio: ruling out pre-eclampsia for up to 4 weeks and value of retesting. Ultrasound Obstet Gynecol J Int Soc Ultrasound Obstet Gynecol. 2019;53:367–75. doi: 10.1002/uog.19178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hund M, Allegranza D, Schoedl M, Dilba P, Verhagen-Kamerbeek W, Stepan H. Multicenter prospective clinical study to evaluate the prediction of short-term outcome in pregnant women with suspected preeclampsia (PROGNOSIS): study protocol. BMC Pregnancy Childbirth. 2014;14:324. doi: 10.1186/1471-2393-14-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 14.Ohkuchi A, Masuyama H, Yamamoto T, Kikuchi T, Taguchi N, Wolf C, et al. Economic evaluation of the sFlt-1/PlGF ratio for the short-term prediction of preeclampsia in a Japanese cohort of the PROGNOSIS Asia study. Hypertens Res J Jpn Soc Hypertens. 2021;44:822–9. doi: 10.1038/s41440-021-00624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Wang X, Hu W, Chen Y, Ning W, Lu S, et al. A risk model that combines MAP, PlGF, and PAPP-A in the first trimester of pregnancy to predict hypertensive disorders of pregnancy. J Hum Hypertens. 2022;36:184–91. doi: 10.1038/s41371-021-00488-6. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Li H, Wang N, Song X, Zhao K, Zhang C. Assessment of plasma cell-free DNA and ST2 as parameters in gestational hypertension and preeclampsia. Hypertens Res. 2021;44:996–1001. doi: 10.1038/s41440-021-00650-0. [DOI] [PubMed] [Google Scholar]
- 17.Zhou P, Luo X, Qi H-B, Zong W-J, Zhang H, Liu D-D, et al. The expression of pentraxin 3 and tumor necrosis factor-alpha is increased in preeclamptic placental tissue and maternal serum. Inflamm Res. 2012;61:1005–12. doi: 10.1007/s00011-012-0507-x. [DOI] [PubMed] [Google Scholar]
- 18.Colmenares-Mejía CC, Quintero-Lesmes DC, Bautista-Niño PK, Guio Mahecha E, Beltrán Avendaño M, Díaz Martínez LA, et al. Pentraxin-3 is a candidate biomarker on the spectrum of severity from pre-eclampsia to HELLP syndrome: GenPE study. Hypertens Res. 2020;43:884–91. doi: 10.1038/s41440-020-0434-0. [DOI] [PubMed] [Google Scholar]
- 19.Araki Y, Yanagida M. Hypertensive disorders of pregnancy: strategy to develop clinical peptide biomarkers for more accurate evaluation of the pathophysiological status of this syndrome. Adv Clin Chem. 2020;94:1–30. doi: 10.1016/bs.acc.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Wakabayashi I, Yanagida M, Araki Y. Associations of cardiovascular risk with circulating peptides related to hypertensive disorders of pregnancy. Hypertens Res. 2021;44:1641–51. doi: 10.1038/s41440-021-00747-6. [DOI] [PubMed] [Google Scholar]
- 21.Takagi K, Nakamoto O, Watanabe K, Tanaka K, Matsubara K, Kawabata I, et al. A review of best practice guide 2021 for diagnosis and management of hypertensive disorders of pregnancy (HDP) - misc. - researchmap. Hypertens Res Pregnancy. 2022;10:57–73. doi: 10.14390/jsshp.10.57. [DOI] [Google Scholar]
- 22.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 23.Espinoza J, Vidaeff A, Pettker CM, Simhan H. ACOG Committee Opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44–e52. [DOI] [PubMed]
- 24.Henderson JT, Vesco KK, Senger CA, Thomas RG, Redmond N. Aspirin use to prevent preeclampsia and related morbidity and mortality: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:1192–206. doi: 10.1001/jama.2021.8551. [DOI] [PubMed] [Google Scholar]
- 25.Magee LA, Khalil A, Kametas N, von Dadelszen P. Toward personalized management of chronic hypertension in pregnancy. Am J Obstet Gynecol. 2022;226:S1196–210. doi: 10.1016/j.ajog.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch A, Rotem R, Ternovsky N, Hirsh Raccah B. Pravastatin and placental insufficiency associated disorders: a systematic review and meta-analysis. Front Pharm. 2022;13:1021548. doi: 10.3389/fphar.2022.1021548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Döbert M, Varouxaki AN, Mu AC, Syngelaki A, Ciobanu A, Akolekar R, et al. Pravastatin versus placebo in pregnancies at high risk of term preeclampsia. Circulation. 2021;144:670–9. doi: 10.1161/CIRCULATIONAHA.121.053963. [DOI] [PubMed] [Google Scholar]
- 28.Espinoza J, Vidaeff A, Pettker CM, Simhan H. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135:e237–e260. [DOI] [PubMed]
- 29.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26–e50. doi: 10.1097/AOG.0000000000003020. [DOI] [PubMed] [Google Scholar]
- 30.Metoki H, Iwama N, Hamada H, Satoh M, Murakami T, Ishikuro M, et al. Hypertensive disorders of pregnancy: definition, management, and out-of-office blood pressure measurement. Hypertens Res. 2022;45:1298–309. doi: 10.1038/s41440-022-00965-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019) Hypertens Res. 2019;42:1235–481. doi: 10.1038/s41440-019-0284-9. [DOI] [PubMed] [Google Scholar]
- 32.Nobles CJ, Mendola P, Mumford SL, Silver RM, Kim K, Andriessen VC, et al. Preconception blood pressure and its change into early pregnancy: early risk factors for preeclampsia and gestational. Hypertension. 2020;76:922–9. doi: 10.1161/HYPERTENSIONAHA.120.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li N, An H, Li Z, Ye R, Zhang L, Li H, et al. Preconception blood pressure and risk of gestational hypertension and preeclampsia: a large cohort study in China. Hypertens Res. 2020;43:956–62. doi: 10.1038/s41440-020-0438-9. [DOI] [PubMed] [Google Scholar]
- 34.Huai J, Lin L, Juan J, Chen J, Li B, Zhu Y, et al. Preventive effect of aspirin on preeclampsia in high‐risk pregnant women with stage 1 hypertension. J Clin Hypertens. 2021;23:1060–7. doi: 10.1111/jch.14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slade LJ, Mistry HD, Bone JN, Wilson M, Blackman M, Syeda N, et al. American College of Cardiology and American Heart Association blood pressure categories—a systematic review of the relationship with adverse pregnancy outcomes in the first half of pregnancy. Am J Obstet Gynecol. 2023;228:418–29.e34. [DOI] [PMC free article] [PubMed]
- 36.Suzuki H, Takagi K, Matsubara K, Mito A, Kawasaki K, Nanjo S, et al. Maternal and perinatal outcomes according to blood pressure levels for prehypertension: a review and meta-analysis. Hypertens Res Pregnancy. 2022;10:29–39.
- 37.Ueda A, Hasegawa M, Matsumura N, Sato H, Kosaka K, Abiko K, et al. Lower systolic blood pressure levels in early pregnancy are associated with a decreased risk of early-onset superimposed preeclampsia in women with chronic hypertension: a multicenter retrospective study. Hypertens Res. 2022;45:135–45. doi: 10.1038/s41440-021-00763-6. [DOI] [PubMed] [Google Scholar]
- 38.Committee on Obstetric Practice. Committee Opinion No. 692: emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;129:e90–5. doi: 10.1097/AOG.0000000000002019. [DOI] [PubMed] [Google Scholar]
- 39.Easterling T, Mundle S, Bracken H, Parvekar S, Mool S, Magee LA, et al. Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: an open-label, randomised controlled trial. Lancet. 2019;394:1011–21. doi: 10.1016/S0140-6736(19)31282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bone JN, Sandhu A, Abalos ED, Khalil A, Singer J, Prasad S, et al. Oral antihypertensives for nonsevere pregnancy hypertension: systematic review, network meta- and trial sequential analyses. Hypertension. 2022;79:614–28. doi: 10.1161/HYPERTENSIONAHA.121.18415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Q, Hu B, Han X, Yang J, Di X, Bao J, et al. Cyclosporin A ameliorates eclampsia seizure through reducing systemic inflammation in an eclampsia-like rat model. Hypertens Res. 2020;43:263–70. doi: 10.1038/s41440-019-0387-3. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Huang J, Lv Y, Chen Y, Rao J. Crocin exhibits an antihypertensive effect in a rat model of gestational hypertension and activates the Nrf-2/HO-1 signaling pathway. Hypertens Res. 2021;44:642–50. doi: 10.1038/s41440-020-00609-7. [DOI] [PubMed] [Google Scholar]
- 43.Chappell LC, Brocklehurst P, Green ME, Hunter R, Hardy P, Juszczak E, et al. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet. 2019;394:1181–90. doi: 10.1016/S0140-6736(19)31963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thilaganathan B, Kalafat E. Cardiovascular system in preeclampsia and beyond. Hypertension. 2019;73:522–31. doi: 10.1161/HYPERTENSIONAHA.118.11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vakhtangadze T, Gakhokidze N, Khutsishvili M, Mosidze S. The link between hypertension and preeclampsia/eclampsia-life-long cardiovascular risk for women. Vessel. 2019;3:19. [Google Scholar]
- 46.Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minissian MB, Kilpatrick S, Eastwood J-A, Robbins WA, Accortt EE, Wei J, et al. Association of spontaneous preterm delivery and future maternal cardiovascular disease. Circulation. 2018;137:865–71. doi: 10.1161/CIRCULATIONAHA.117.031403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leon LJ, McCarthy FP, Direk K, Gonzalez-Izquierdo A, Prieto-Merino D, Casas JP, et al. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records. Circulation. 2019;140:1050–60. doi: 10.1161/CIRCULATIONAHA.118.038080. [DOI] [PubMed] [Google Scholar]
- 49.Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott NS, et al. Long-term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol. 2019;74:2743–54. doi: 10.1016/j.jacc.2019.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagata M, Kogure M, Nakaya N, Tsuchiya N, Nakamura T, Hirata T, et al. Hypertensive disorders of pregnancy, obesity, and hypertension in later life by age group: a cross-sectional analysis. Hypertens Res. 2020;43:1277–83. doi: 10.1038/s41440-020-0463-8. [DOI] [PubMed] [Google Scholar]
- 51.Wilson PWF, Polonsky TS, Miedema MD, Khera A, Kosinski AS, Kuvin JT. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3210–27. doi: 10.1016/j.jacc.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:1376–414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.American College of Obstetricians and Gynecologists’ Presidential Task Force on Pregnancy and Heart Disease and Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 212: pregnancy and heart disease. Obstet Gynecol. 2019;133:e320–56. doi: 10.1097/AOG.0000000000003243. [DOI] [PubMed] [Google Scholar]
- 54.Brown DW, Dueker N, Jamieson DJ, Cole JW, Wozniak MA, Stern BJ, et al. Preeclampsia and the risk of ischemic stroke among young women. Stroke. 2006;37:1055–9. doi: 10.1161/01.STR.0000206284.96739.ee. [DOI] [PubMed] [Google Scholar]
- 55.Poulter NR, Chang CL, Farley TMM, Meirik O, Marmot MG. Haemorrhagic stroke, overall stroke risk, and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. 1996;348:505–10. [PubMed]
- 56.World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contarception. Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case-control study. Lancet. 1995;346:1575–82. [PubMed]
- 57.Samara AA, Liampas I, Dadouli K, Siokas V, Zintzaras E, Stefanidis I, et al. Preeclampsia, gestational hypertension and incident dementia: a systematic review and meta-analysis of published evidence. Pregnancy Hypertens. 2022;30:192–7. doi: 10.1016/j.preghy.2022.10.008. [DOI] [PubMed] [Google Scholar]
- 58.Hubel CA, Wallukat G, Wolf M, Herse F, Rajakumar A, Roberts JM, et al. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension. 2007;49:612–7. doi: 10.1161/01.HYP.0000256565.20983.d4. [DOI] [PubMed] [Google Scholar]
- 59.Zwertbroek EF, Franssen MTM, Broekhuijsen K, Langenveld J, Bremer H, Ganzevoort W, et al. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring in mild hypertensive disorders of pregnancy: 2-year outcomes of the HYPITAT-II trial. Am J Obstet Gynecol. 2019;221:154.e1–154.e11. doi: 10.1016/j.ajog.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 60.Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. doi: 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- 61.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–61. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 62.Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, Merrill DC, et al. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39:337–42. doi: 10.1161/hy02t2.102904. [DOI] [PubMed] [Google Scholar]
- 63.Allotey J, Fernandez S, Bonet M, Stallings E, Yap M, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed]
- 64.Kalafat E, Prasad S, Birol P, Tekin AB, Kunt A, Di Fabrizio C, et al. An internally validated prediction model for critical COVID-19 infection and intensive care unit admission in symptomatic pregnant women. Am J Obstet Gynecol. 2022;226:403.e1–403.e13. doi: 10.1016/j.ajog.2021.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. Can Med Assoc J. 2021;193:E540–E548. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Papageorghiou AT, Deruelle P, Gunier RB, Rauch S, García-May PK, Mhatre M, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225:289.e1–289.e17. doi: 10.1016/j.ajog.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol-Urganci I, et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e759–e772. doi: 10.1016/S2214-109X(21)00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mendoza M, Garcia-Ruiz I, Maiz N, Rodo C, Garcia-Manau P, Serrano B, et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG Int J Obstet Gynaecol. 2020;127:1374–80. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu J, Deng W, Li S, Yang X. Advances in research on ACE2 as a receptor for 2019-nCoV. Cell Mol Life Sci CMLS. 2021;78:531–44. doi: 10.1007/s00018-020-03611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giardini V, Carrer A, Casati M, Contro E, Vergani P, Gambacorti-Passerini C. Increased sFLT-1/PlGF ratio in COVID-19: a novel link to angiotensin II-mediated endothelial dysfunction. Am J Hematol. 2020;95:E188–91. doi: 10.1002/ajh.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soldavini CM, Di Martino D, Sabattini E, Ornaghi S, Sterpi V, Erra R, et al. sFlt-1/PlGF ratio in hypertensive disorders of pregnancy in patients affected by COVID-19. Pregnancy Hypertens. 2022;27:103–9. doi: 10.1016/j.preghy.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]