Abstract
Background
Impaired muscle function has been identified as a risk factor for declining cognitive function and cardiovascular health, both of which are risk factors for late‐life dementia (after 80 years of age). We examined whether hand grip strength and timed‐up‐and‐go (TUG) performance, including their change over 5 years, were associated with late‐life dementia events in older women and whether any associations provided independent information to Apolipoprotein E ℇ4 (APOE ℇ4) genotype.
Methods
Grip strength and TUG were assessed in community‐dwelling older women (mean ± SD; age 75.0 ± 2.6 years) at baseline (n = 1225) and 5 years (n = 1052). Incident 14.5‐year late‐life dementia events (dementia‐related hospitalization/death) were obtained from linked health records. Cardiovascular risk factors (Framingham Risk Score), APOE genotyping, prevalent atherosclerotic vascular disease and cardiovascular‐related medications were evaluated at baseline. These were included in multivariable‐adjusted Cox‐proportional hazards models assessing the relationship between muscle function measures and late‐life‐dementia events.
Results
Over follow‐up, 207 (16.9%) women had a late‐life dementia event. Compared with women with the highest grip strength (Quartile [Q] 4, 25.8 kg), those with the lowest grip strength (Q1, 16.0 kg) had greater hazard for a late‐life dementia event (HR 2.27 95% CI 1.54–3.35, P < 0.001). For TUG, the slowest women (Q4, 12.4 vs. Q1, 7.4 s) also recorded a greater hazard for a late‐life dementia event (HR 2.10 95% CI 1.42–3.10, P = 002). Weak hand grip (<22 kg) or slow TUG (>10.2 s) provided independent information to the presence of an APOE ℇ4 allele (n = 280, 22.9%). Compared with women with no weakness and no APOE ℇ4 allele, those with weakness and APOE ℇ4 allele had a greater hazard (HR 3.19 95% CI 2.09–4.88, P < 0.001) for a late‐life dementia event. Women presenting with slowness and the APOE ℇ4 allele also recorded a greater hazard for a late‐life dementia event (HR 2.59 95% CI 1.64–4.09, P < 0.001). For 5‐year muscle function changes, compared with women with the lowest performance decrement (Q1), those with the largest decrement (Q4) had higher hazards for a late‐life dementia event (grip strength HR 1.94 95% CI 1.22–3.08, P = 0.006; TUG HR 2.52 95% CI 1.59–3.98, P < 0.001) over the next 9.5 years.
Conclusions
Weaker grip strength and slower TUG, and a greater decline over 5 years, were significant risk factors for a late‐life‐dementia event in community‐dwelling older women, independent of lifestyle and genetic risk factors. Incorporating muscle function measures as part of dementia screening appears useful to identify high‐risk individuals who might benefit from primary prevention programmes.
Keywords: Grip strength, Timed‐up‐and‐go, Cognitive function, Alzheimer's disease
Introduction
Dementia is a global public health concern with increasing social and economic impacts. In 2018, dementia was estimated to cost $US1 trillion to the global economy, and this is projected to reach $US2 trillion by 2030. An estimated 50 million people live with dementia globally with this number projected to triple by 2050. 1 An extended asymptomatic prodromal period of up to 20 years, characterized by gradual accumulation of neuropathological disease processes, is a common manifestation of dementia, in particular Alzheimer's disease (AD) including its related dementias. 2 Late‐life dementias are those that occur after the age of 80 years, often characterized by a set of pathological processes that affect the size of the cortex and hippocampus (e.g. tauopathy, inflammation, synucleinopathy, amyloid aggregation and strokes). This form of dementia can be influenced by positive or negative consequences of environmental exposures (e.g. physical activity or obesity). 3 As such, it is essential that populations at high risk for developing late‐life dementia are identified during this asymptomatic prodromal period for early inclusion into primary prevention strategies.
Drug treatments for dementia typically serve to control symptoms, as oppose to alter its progression, 4 especially at the early stage. 5 Clearly, there is a need to focus on environmental/lifestyle/behavioural factors that can slow the progression and/or prevent dementia. Indeed, the 2020 Lancet Commission on dementia prevention, intervention and care highlighted physical inactivity as a major modifiable risk factor for dementia. 6 Recent work also suggests that compromised muscle function, including reduced muscle strength and poorer physical function, are linked with compromised cognitive function and its decline. 7 , 8 A scoping review (15 studies) including individuals over 60 years reported that weaker hand grip strength was associated with decline in cognition and incident dementia over time (ranging from 1 to 7 years). 7 It has also been suggested that a decline in cognitive function may precede loss of strength. 7 However, findings from 190 406 middle‐aged to older adults from the UK Biobank cohort, utilizing neuroimaging measures, indicated that observed associations between hand grip strength and cognitive, neuroimaging and dementia outcomes were not due to reverse causation. 9 Because the UK Biobank sample was still relatively young, with a mean age of 56.5 years, it is less informative about dementia outcomes that occur later in life. Accelerated decline of gait speed is another feature of neurodegenerative diseases including dementia. 8 Mobility, as measured by the timed‐up‐and‐go (TUG) test, has been associated with cognitive performance, 10 although it may have limited predictive value when cognitive function remains stable over time. 11 In terms of dementia incidence, impaired TUG performance at age 66 years has been associated with up to 34% and 65% greater relative hazards for total dementia and vascular dementia over 3.8 years, respectively. 12
Collectively, the aforementioned findings may be pertinent to late‐life dementia due to its strong links with vascular and non‐vascular risk factors. 3 Perhaps, the proposed link between impaired muscle strength with incidence of dementia may in part be mediated by vascular‐related mechanisms (and environment/lifestyle), rather than primarily genetic variants (e.g. Apolipoprotein E ℇ4; APOE ℇ4) that are commonly associated with AD, 9 a greater cause of death for women than men after 80 years. 13 Specifically, APOE ℇ4 has been implicated in the deposition of amyloid β, hyperphosphorylated tau tangles (neurofibrillary tangles) in the brain, lipid metabolism and vascular integrity/function that can severely disrupt brain blood flow. 14 Previous work reporting that impaired muscle function is related to dementia has typically adopted younger cohorts (e.g. 56–66 years), 9 , 12 not considered genetic risk (e.g. APOE ℇ4), 15 nor the concurrent change in strength and physical function over time. To our knowledge, no study has examined the relationship between muscle function and late‐life dementia. As such, the aim of this study was to determine the relationship of hand grip strength and TUG performance (and their changes over 5‐years) with long‐term risk for a late‐life dementia event (comprising any dementia‐related hospitalization or death) in community‐dwelling older women (>70 years) and whether any associations provided independent information to APOE ℇ4 genotype.
Methods
Study population
The population included individuals from the Perth Longitudinal Study of Aging in Women (PLSAW). Ambulant community‐dwelling older Australian women (≥70 years) were recruited in 1998 to a 5‐year, double‐blind, randomized placebo‐controlled trial of daily calcium supplementation to prevent fracture, the Calcium Intake Fracture Outcome Study (CAIFOS). 16 Participants were included based on an expected survival beyond 5 years. Exclusion criterion was the use of any medications (including hormone replacement therapy) that could interfere with bone metabolism. 16 Women from this study had a comparable medication and disease burden to that of the general population and had a Mini‐Mental State Examination (MMSE) score ≥25 upon study entry. However, socio‐economic status was higher than the general population. 16 All women received 1.2 g of elemental calcium (calcium carbonate) or a matching placebo each day for 5 years. After the completion of CAIFOS, participants were subsequently enrolled in a further 10 years of observational follow‐up: the PLSAW (http://www.lsaw.com.au).
From the 1460 women recruited, cardiovascular risk factors used to compute the General Framingham Risk Score (FRS) and APOE genotypes were assessed in 1260 women. After excluding women with missing data (n = 20) and those who experienced a dementia event (including hospitalization or death) before the age of 80 years (n = 15), 1225 women were included in the study (Figure 1 ). For all study participants, written informed consent was obtained, including follow‐up from electronic linked health records. Ethics approval was granted by the Human Ethics Committee of the University of Western Australia.
Figure 1.
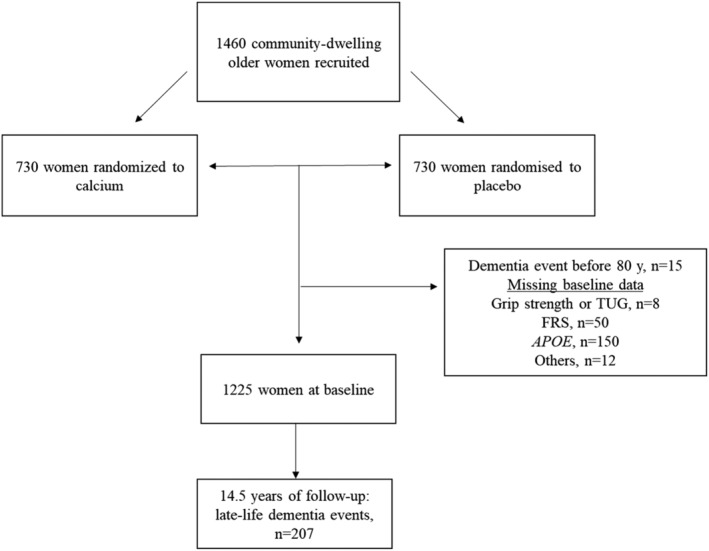
Participant flow chart. APOE, apolipoprotein E; FRS, General Framingham Risk Sore. Grip strength and timed‐up‐and‐go (TUG) was assessed in 1052 women at 5 years.
Baseline risk factor assessment
Previous medical history and current use of medications were verified by each participants primary healthcare provider, where possible. The International Classification of Primary Care–Plus (ICPC‐Plus) method[S1] was adopted, enabling aggregation of different terms for similar pathologic entities as defined by the ICD‐9 coding system. Information was used to determine prevalent diabetes (T89001‐90009) and atherosclerotic vascular disease (ASVD). Prevalent ASVD included coronary heart disease (ICD‐9‐CM codes 410‐414); heart failure (ICD‐9‐CM code 428); cerebrovascular disease excluding haemorrhage (ICD‐9‐CM codes 433‐438); and peripheral arterial disease (ICD‐9‐CM codes 440‐444).[S2] Cardiovascular medications included antihypertensives, statins and low‐dose aspirin. Physical activity and smoking history were captured using questionnaires. Being a former/current smoker was defined as smoking >1 cigarette/day for >3 months at any time during the participants' life. Participants were asked about participation in sport, recreation and/or regular physical activities undertaken in the 3 months prior to their baseline visit, as described previously.[S3] Briefly, the level of activity, expressed in expended kilojoules per day, was calculated from the questionnaire using a validated method applying the type of activity, time engaged in the activity and the participant's body weight. Weight (in kg) was assessed using digital scales. Height (in cm) was assessed using a stadiometer, and the body mass index (BMI) was calculated as kg/m2. Blood pressure was measured (average of three measurements) on the right arm with a mercury column manometer with participants seated upright and rested for 5 min. Genotyping for APOE was performed by polymerase chain reaction amplification with oligonucleotide primers.[S4, S5] Alcohol intake was obtained from a questionnaire (none, <10 standard drinks per week or ≥10 standard drinks per week). The 10‐year estimated General Framingham Cardiovascular Risk Score (FRS) was calculated using BMI and included age, sex, previous diabetes, smoking status and systolic blood pressure.[S6]
Muscle strength and physical function assessment
Measures of muscle function including hand grip strength and TUG were assessed at baseline (1998) and 5 years later (2003), as described previously. 17 Briefly, a hand‐held dynamometer (Jamar Hand Dynamometer, Lafayette Instrument Company, USA) was used to assess grip strength (in kg). For TUG, this was the time taken (to the nearest tenth of a second) for an individual to rise from a chair, walk 3 m, turn around and to return to sit on the chair. Interobserver coefficient of variation error was 7% and 6%, respectively for grip strength and TUG in our laboratory, as assessed on a random sample of 30 women.
Late‐life dementia outcomes
Dementia outcomes over 14.5 years were tracked through the Western Australian Data Linkage System (Department of Health Western Australia) and retrieved from the Western Australia Hospital Morbidity Data Collection (HMDC). Records were obtained for each participant from 1998 until 2013. Codes were identified using the International Classification of Diseases, Injuries and Causes of Death Clinical Modification (ICD‐9‐CM) or the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD‐10‐AM). 18 , 19 The primary outcome of the study was late‐life (after the age of 80 years) dementia events (hospitalization and/or death). This was undertaken as the inclusion of death data has been reported to improve identification of those with dementia.[S7] Dementia codes included AD (ICD‐9‐CM 331.0, ICD‐10‐AM F00, G30), vascular dementias (ICD‐9‐CM 290.4, ICD‐10‐AM F01) and unspecified dementias (F03).[S8] These were also considered individually as secondary outcomes. The principal or additional discharge diagnosis codes from hospital morbidity data collection was used to define events. To increase identification of dementia cases we used the linked coded multiple causes of death data (dementia codes as above) or parts 1 and 2 of the death certificate where coded cause of death data was not yet available. This methodology has been used to investigate the aetiology and consequences of dementia.[S9]
Statistical analysis
The statistical analysis was performed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA), Stata software, version 14 (StataCorp LLC, College Station, Texas, USA) and R software (version 3.4.2, R Foundation for Statistical Computing, Vienna, Austria).[S10] Baseline characteristic comparisons for women who did not present versus presented with a late‐life dementia event were obtained using one‐way ANOVA, Mann–Whitney U test or Pearson's chi‐square where appropriate. Cox proportional hazards modelling were used to investigate the relationship between grip strength and TUG (as separate exposures) and late‐life dementia outcomes including (i) events (hospitalization and/or death), (ii) hospitalizations and (iii) deaths. Global tests (estat phtest) indicated proportional hazards assumptions were not violated when considering the relationships between grip strength or TUG and all late‐life dementia outcomes. These relationships are presented graphically using the ‘effects’ R package.[S11] Hazard ratios (HRs) and 95% confidence intervals (CIs) were obtained from the model with grip strength or TUG fitted as a continuous variable through a restricted cubic spline using the ‘rms’ R package.[S12] HR estimates were graphed and calculated relative to a reference value being the median of Quartile 4 (Q) for grip strength and Q1 for TUG whilst being plotted against the outcome variable, with 95% CIs provided. P‐values for HRs were obtained using Wald tests. The x‐axis was truncated at 3 SD above the mean for visual simplicity only for all graphs. Cox proportional hazards modelling was also used to examine the influence of binary exposures (weak grip strength [<22 kg] and slow TUG [>10.2 s]) with all late‐life dementia outcomes. Cut‐points for weak grip strength (<22 kg) and slow TUG were (>10.2 s) were selected due to their potential association with adverse health outcomes (e.g. weakness, risk of falling, fractures).[S13, S14] For all regression analysis, we considered two models (i) unadjusted and (ii) multivariable adjusted: treatment code (calcium or placebo), FRS, cardiovascular medications (statins and low‐dose aspirin), prevalent ASVD, APOE genotypes (ℇ2/3, ℇ2/4, ℇ3/3, ℇ3/4, ℇ4/4), physical activity and alcohol intake.
Additional analysis
We undertook analysis replacing FRS [estimate of cardiovascular disease (CVD) risk] with the individual variables used to derive the computed score (age, BMI, previous diabetes, smoking history and systolic blood pressure) to the multivariable‐adjusted model. Considering the advanced age of our cohort, competing risks analyses based on Fine and Gray's proportional subhazards model[S15] to account for the competing risk of non‐dementia mortality was also undertaken. We also undertook analysis where both grip strength and TUG were simultaneously included (as continuous variables) as part of multivariable‐adjusted analysis with late‐life dementia events. Carrying the APOE ℇ4 allele is known to be associated with increased risk of dementia. 3 As such, the relationship between grip strength, TUG and late‐life dementia events in carriers and non‐carriers of the APOE ℇ4 allele was investigated separately. We also dichotomized grip strength or TUG and APOE ℇ4 allele into four groups. For grip strength, these included (i) no weakness [grip strength >22 kg] and no APOE ℇ4 allele; (ii) no weakness and an APOE ℇ4 allele; (iii) weakness [grip strength ≤22 kg] and no APOE ℇ4 allele; and (iv) weakness and APOE ℇ4 allele. For TUG, these four groups included (i) no slowness [TUG ≤10.2 s] and no APOE ℇ4 allele; (ii) no slowness and an APOE ℇ4 allele; (iii) slowness [TUG >10.2 s] and no APOE ℇ4 allele; and (iv) slowness and APOE ℇ4 allele. Finally, in women who also undertook muscle strength and physical function tests at year 5 (n = 1052), we explored the relationship between change in grip strength and TUG (continuously and by Q) over 5 years (1998–2003) with late‐life dementia outcomes over the next 9.5 years. Here, based on the 5‐year change in grip strength and TUG, these women then categorized into Q based on the decrement in performance for each test; Q1, smallest change; Q2, some change; Q3, moderate change; and Q4, largest change. These analyses were limited to women who did not present with a late‐life dementia event between 1998 and 2003.
Results
Participant flowchart is presented in Figure 1 . Baseline characteristics of participants are displayed in Table 1 , whilst comparison with those who were excluded is presented in Table S1 . At baseline, women who experienced a late‐life dementia event tended to be slightly older, have a lower BMI, were a smoker and had a higher FRS, weaker hand grip strength and slower TUG. A higher proportion of these women also had a history of smoking and presented with the APOE ℇ4 allele (n = 280, 22.9%).
Table 1.
Baseline characteristics of the study population stratified by development of late‐life dementia
| Whole cohort | No late‐life dementia | Late‐life dementia | P‐value | |
|---|---|---|---|---|
| Number (%) | 1225 | 1018 (83.1) | 207 (18.5) | |
| Age, years | 75.1 ± 2.7 | 75.0 ± 2.6 | 76.1 ± 2.8 | <0.001 |
| Body mass index, kg/m2 | 27.2 ± 4.6 | 27.4 ± 4.6 | 26.4 ± 4.5 | 0.006 |
| Ever smoked, yes (%) | 440 (35.9) | 352 (34.6) | 88 (42.5) | 0.029 |
| Systolic blood pressure, mmHg | 138 ± 18 | 138 ± 18 | 138 ± 21 | 0.699 |
| Antihypertensive medication, yes (%) | 523 (42.7) | 436 (42.8) | 87 (42.0) | 0.832 |
| Diabetes, yes (%) | 73 (6.0) | 53 (5.2) | 20 (9.7) | 0.014 |
| Estimated CVD risk (Framingham), % | 22.1 ± 11.0 | 21.8 ± 10.6 | 23.5 ± 12.6 | 0.044 |
| Statins medication, yes (%) | 234 (19.1) | 196 (19.3) | 38 (18.4) | 0.765 |
| Low‐dose aspirin, yes (%) | 245 (22.0) | 195 (19.2) | 50 (24.2) | 0.101 |
| Previous ASVD, yes (%) | 140 (11.4) | 115 (11.3) | 25 (12.1) | 0.748 |
| Physical activity, kcal | 113 (36–204) | 113 (36–202) | 108 (42–208) | 0.920 |
| Alcohol, n (%) | 0.416 | |||
| None | 233 (19.0) | 187 (18.4) | 46 (22.2) | |
| <10 standard drinks p/w | 792 (64.7) | 662 (65.0) | 130 (62.8) | |
| ≥10 standard drinks p/w | 200 (16.3) | 169 (16.6) | 31 (15.0) | |
| Randomization, calcium (%) | 615 (50.2) | 516 (50.7) | 99 (47.8) | 0.453 |
| APOE genotypes, yes (%) | <0.001 | |||
| APOE ℇ 2/3 | 193 (15.8) | 173 (17.0) | 20 (9.7) | |
| APOE ℇ 2/4 | 27 (2.2) | 23 (2.3) | 4 (1.9) | |
| APOE ℇ 3/3 | 752 (61.4) | 633 (62.2) | 119 (57.5) | |
| APOE ℇ 3/4 | 235 (19.2) | 179 (17.6) | 56 (27.1) | |
| APOE ℇ 4/4 | 18 (1.5) | 10 (1.0) | 8 (3.9) | |
| Grip strength (kg) | 20.6 ± 4.6 | 20.9 ± 4.5 | 19.1 ± 4.8 | <0.001 |
| Timed‐up‐and‐go (s) | 9.3 (8.1–11.0) | 9.2 (8.0–10.9) | 9.8 (8.4–11.3) | 0.002 |
Data expressed as mean ± SD, median (IQR) or number (%).
APOE, apolipoprotein E; ASVD, atherosclerotic vascular disease; mmHg, millimetres mercury.
Muscle function and late‐life dementia over 14.5 years
For a late‐life dementia hospitalization (15 155 total person years) and/or death (15 568 total person years), the mean ± SD follow‐up was 12.4 ± 3.3 and 12.7 ± 3.1 years, respectively. A diagrammatic representation for the near‐linear relationship (p for nonlinearity = 0.599) between hand grip strength and late‐life dementia events (P < 0.001) is presented in Figure 2 A . In the multivariable‐adjusted analysis, compared with women with the highest grip strength (Q4, 25.8 kg), those with the lowest grip strength (Q1, 16.0 kg) had higher hazards for a late‐life dementia event (2.27 times), hospitalization (2.26 times) or death (2.45 times) (Table 2 ). Similarly, women with weak grip strength (<22 kg, n = 744, 60.7%) presented with higher relative hazards for a late‐life dementia event (1.71 times), hospitalization (1.76 times) or death (1.62 times) (Table S2 ) compared with women with higher grip strength (≥22 kg), in the multivariable‐adjusted analysis.
Figure 2.
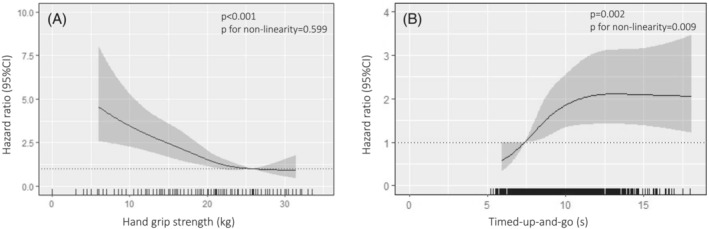
Restricted cubic splines based on multivariable‐adjusted Cox proportional hazards analysis highlighting the relative hazard between (A) hand grip strength and (B) timed‐up‐and‐go with any late‐life dementia event (hospitalization/death) over 14.5 years. Shaded represent 95% confidence intervals. Rug plot on the x‐axis represents an observation. The reference value represents the median hand grip strength (25.8 kg) and timed‐up‐and‐go (7.4 s) for the strongest (Quartile 4) and fastest women (Quartile 1), respectively.
Table 2.
Hazard ratios (95% CI) for any late‐life dementia events, hospitalizations and deaths over 14.5 years by quartiles of hand grip strength
| Quartiles for hand grip strength a | ||||||
|---|---|---|---|---|---|---|
| Events | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Late‐life dementia events | 207 (16.9) | Unadjusted | 2.34 (1.59–3.42) | 1.68 (1.23–2.30) | 1.28 (1.00–1.64) | 1.00 (ref) |
| Adjusted | 2.27 (1.54–3.35) | 1.63 (1.19–2.24) | 1.24 (0.96–1.58) | 1.00 (ref) | ||
| Late‐life dementia hospitalizations | 183 (14.9) | Unadjusted | 2.30 (1.54–3.45) | 1.63 (1.18–2.26) | 1.23 (0.95–1.59) | 1.00 (ref) |
| Adjusted | 2.26 (1.50–3.41) | 1.59 (1.14–2.21) | 1.18 (0.92–1.52) | 1.00 (ref) | ||
| Late‐life dementia deaths | 83 (6.8) | Unadjusted | 2.60 (1.40–4.85) | 1.84 (1.09–3.10) | 1.40 (0.90–2.17) | 1.00 (ref) |
| Adjusted | 2.45 (1.31–4.60) | 1.73 (1.02–2.94) | 1.34 (0.86–2.09) | 1.00 (ref) | ||
Estimated hazard ratios and 95% CI from Cox proportional hazards analysis comparing the median hand grip strength from each quartile compared to Quartile 4. Median hand grip strength for Quartiles 1–4 was 16, 19.5, 22 and 25.8 kg, respectively. Bolded indicates P ≤ 0.05 compared to Quartile 4. Multivariable‐adjusted model includes general Framingham Risk Score, treatment code (calcium or placebo), alcohol intake, prevalent atherosclerotic vascular disease, prescription of statin medications, use of low‐dose aspirin, physical activity and apolipoprotein E genotype.
The multivariable‐adjusted non‐linear relationship (P for nonlinearity = 0.009) between TUG and late‐life dementia events (P = 0.002) is presented in Figure 2 B . Compared with women with the fastest TUG (Q1, 7.4 s), those with the slowest TUG (Q4, 12.4 s) had higher relative hazards for a late‐life dementia event (2.10 times), hospitalization (2.13 times) and/or death (2.98 times), respectively (Table 2 ). Similarly, women with slow TUG (>10.2 s) had higher relative hazards for a late‐life dementia event (1.54 times), hospitalization (1.54 times) and/or death (1.91 times). When considering women with poor overall muscle function (weak grip strength plus slow TUG, n = 317, 25.9%), these individuals recorded higher relative hazards for a late‐life dementia event (1.72 times), hospitalization (1.71 times) and/or deaths (2.03 times) (Table S2). For the different types of late‐life dementias, 97 recorded AD, and 148 women recorded an unspecified late‐life dementia event. When considering AD events, compared with women with higher grip strength, those with weak grip strength recorded higher hazards (HR 1.79 95% CI 1.14–2.82, P = 0.011). This was not observed for slow TUG (HR 1.28 95% CI 0.84–1.95, P = 0.257). However, for unspecified dementia events, women with weak grip strength (HR 1.80 95% CI 1.25–2.60, P = 0.002) and slow TUG (HR 1.75 95% CI 1.26–2.45, P = 0.001) recorded higher relative hazards. Low numbers of women recorded vascular (n = 18) and other causes of late‐life dementia (n = 8); hence, no further analysis for these outcomes were performed.
Additional analyses
For analysis where the individual measures (age, BMI, prevalent diabetes, SBP) used to compute the FRS were added to the multivariable‐adjusted model, the results (Table S3 ) were comparable to the primary analysis (Table 2 ). Noteworthy, FRS was independently associated with late‐life dementia events (P < 0.05) in all of the primary analysis considering muscle function measures presented in Tables 2 and 3 . Specifically, for late‐life dementia events, compared with women with the highest grip strength (Q4), individuals with the lowest grip strength (Q1) had a 1.95 times higher relative hazard. For TUG, the slowest women (Q4) had 2.17 times higher relative hazard for a late‐life dementia event. Competing risks analyses for non‐dementia deaths did not substantially affect the point estimates for quartiles of grip strength and TUG (Table S4 ). When both grip strength and TUG were included simultaneously as part of multivariable‐adjusted analysis, grip strength (per kg decrease, HR 1.08 95% CI 1.05–1.11, P < 0.001) but not TUG was associated with lower risk for a late‐life dementia event. In women who carried an APOE ℇ4 allele (n = 280), those with the lowest grip strength (Q1) had 2.2 times higher hazard for a late‐life dementia event (Table S5 ), compared with women with the highest grip strength (Q4). For TUG, women with slower TUG (Q2, Q3, Q4) had 1.69–1.99 times greater hazards for a late‐life dementia event. Similarly, for women who did not carry the APOE ℇ4 allele (n = 945), those with the lower grip strength (Q1, Q2, Q3) compared with Q4 recorded between 1.39 and 2.47 times lower hazards for a late‐life dementia event. For TUG, slower women (Q3, Q4) recorded between 1.81 and 2.12 times higher relative hazard for a late‐life dementia event.
Table 3.
Hazard ratios (95% CI) for any late‐life dementia events, hospitalizations and deaths over 14.5 years by quartiles of timed‐up‐and‐go time.
| Quartiles for timed‐up‐and‐go performance a | |||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Late‐life dementia events | Unadjusted | 1.00 (ref) | 1.48 (1.10–1.99) | 1.87 (1.36–2.58) | 2.17 (1.48–3.17) |
| Adjusted | 1.00 (ref) | 1.50 (1.11–2.01) | 1.86 (1.35–2.58) | 2.10 (1.42–3.10) | |
| Late‐life dementia hospitalizations | Unadjusted | 1.00 (ref) | 1.47 (1.07–2.01) | 1.88 (1.34–2.65) | 2.18 (1.45–3.27) |
| Adjusted | 1.00 (ref) | 1.49 (1.09–2.04) | 1.88 (1.33–2.65) | 2.13 (1.41–3.22) | |
| Late‐life dementia deaths | Unadjusted | 1.00 (ref) | 2.08 (1.15–3.77) | 2.69 (1.44–5.04) | 3.08 (1.57–6.05) |
| Adjusted | 1.00 (ref) | 2.09 (1.15–3.79) | 2.66 (1.42–5.01) | 2.98 (1.50–5.90) | |
Estimated hazard ratios and 95% CI from Cox proportional hazards analysis comparing the median timed‐up‐and‐go (TUG) time from each quartile compared to Quartile 1. Median TUG for Quartiles 1–4 was 7.4, 8.7, 10.1 and 12.4 s, respectively. Bolded indicates P ≤ 0.05 compared to Quartile 1. Multivariable‐adjusted model includes general Framingham Risk Score, treatment code (calcium or placebo), alcohol intake, prevalent atherosclerotic vascular disease, prescription of statin medications, use of low‐dose aspirin, physical activity and apolipoprotein E genotype.
In analysis where the entire cohort was dichotomized based on grip strength and APOE ℇ4 allele into four groups (Figure 3 ), compared with women with no APOE ℇ4 and higher grip strength (n = 379), women with weak grip strength and APOE ℇ4 (n = 179) had 3.19 times higher hazard for a late‐life dementia event. Women with higher grip strength with the APOE ℇ4 (n = 101) and those with weak grip strength but no APOE ℇ4 (n = 566) recorded 2.26 and 1.83 times higher relative hazards, respectively (Figure 3 A ). Women with slow TUG and APOE ℇ4 (n = 97) recorded 2.59 times higher relative hazards for a late‐life dementia event compared with individuals with faster TUG and no APOE ℇ4 (n = 615) (Figure 3 B ). Women with faster TUG with the APOE ℇ4 (n = 183) and those with slow TUG but no APOE ℇ4 (n = 330) recorded 2.09 and 1.66 times higher relative hazards, respectively.
Figure 3.
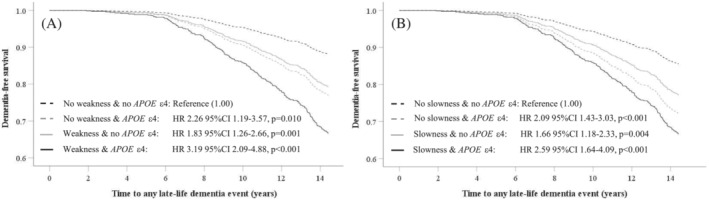
Multivariable‐adjusted Cox proportional hazards analysis for late‐life dementia events (hospitalization and/or death) dichotomized by weak hand grip strength (A) or slow timed‐up‐up‐and‐go (B) in‐conjunction with/without the presence of the apolipoprotein E ℇ4 (APOE ε4).
Change in muscle function over 5 years
A total of 1052 women were included in all analysis considering 5‐year change in muscle function. Mean change (95% CI) in grip strength and TUG over 5 years was −3.1 (−3.4, −2.9) kg and 1.6 (1.4, 1.8) s, respectively. Each kg decrease in grip strength or second increase in TUG was associated with between 7% and 9% greater relative hazard for a late‐life dementia event, hospitalization or death (Table S6 ). Compared with women with the lowest decrement (Q1) in grip strength, those with the largest decrement (Q4) had higher hazards for a late‐life dementia event and hospitalization (1.92 and 2.32 times, respectively) (Table 4 ). For late‐life dementia deaths, statistical significance was slightly attenuated for Q4 (P = 0.054) but not Q3 (P = 0.036). Baseline grip strength (per kg) remained significantly associated (all P < 0.001) with late‐life dementia events (HR 1.11 95% CI 1.07–1.15), hospitalizations (HR 1.12 95% CI 1.07–1.16) and deaths (HR 1.13 95% CI 1.06–1.20) as part of these analyses. For TUG, compared with women with the least change in TUG over 5 years (Q1), those with the largest increase in TUG (Q4) had 2.52, 2.67 and 4.11 times higher hazards for a late‐life dementia event, hospitalization and death, respectively (Table 4 ). Baseline TUG (per sec) remained significantly associated (all P ≤ 0.001) with late‐life dementia events (HR 1.07 95% CI 1.03–1.10), hospitalizations (HR 1.07 95% CI 1.03–1.11) and deaths (HR 1.09 95% CI 1.03–1.14) as part of these analyses.
Table 4.
Multivariable‐adjusted hazard ratios (95% CI) for late‐life dementia outcomes over 9.5 years presented by quartiles of change in grip strength or timed‐up‐and‐go (TUG) over 5 years (1998–2003) in 1052 women
| Quartiles of change in grip strength a or timed‐up‐and‐go b | ||||||
|---|---|---|---|---|---|---|
| Events over 9.5 years | 5‐year change | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| Late‐life dementia events | 168 (15.9) | Grip strength | 1.00 (ref) | 1.28 (0.84–1.97) | 1.19 (0.68–1.74) | 1.92 (1.21–3.05) |
| TUG | 1.00 (ref) | 1.16 (0.70–1.93) | 1.60 (0.99–2.60) | 2.52 (1.59–3.98) | ||
| Late‐life dementia hospitalisations | 149 (14.1) | Grip strength | 1.00 (ref) | 1.48 (0.93–2.35) | 1.18 (0.70–2.1.96) | 2.32 (1.41–3.82) |
| TUG | 1.00 (ref) | 1.33 (0.77–2.29) | 1.75 (1.03–2.95) | 2.67 (1.62–4.40) | ||
| Late‐life dementia deaths | 66 (6.3) | Grip strength | 1.00 (ref) | 1.15 (0.54–2.45) | 2.16 (1.06–4.40) | 2.16 (0.99–4.72) |
| TUG | 1.00 (ref) | 1.41 (0.58–3.43) | 2.22 (0.97–5.10) | 4.11 (1.90–8.89) | ||
Range for Quartiles 1–4 for grip strength was < −0.8 kg, −0.8 to < −3.0 kg, −3.0 to < −5.5 kg and ≥ − 5.5 kg, respectively.
Range for Quartiles 1–4 for TUG was <0.02 s, 0.02 to <1.33 s, 1.33 s to <2.75 s, and ≥2.75 s, respectively. Multivariable‐adjusted model includes Framingham risk score, treatment code (calcium or placebo), alcohol intake, prevalent ASVD, prescription of statin medications, use of low‐dose aspirin, physical activity, apolipoprotein E genotype and baseline grip strength or TUG (where appropriate).
Discussion
Our results indicate that lower hand grip strength and/or slower TUG (including their decline over 5 years) identified women with the greatest late‐life dementia risk, independent of and complementary to genetic (e.g. APOE ℇ4) as well as cardiovascular risk factors (e.g. FRS and ASVD). Consequently, physical assessment could be considered for use to assist clinicians screen individuals at higher risk of late‐life dementia for direction towards dementia‐related primary and secondary prevention strategies. 20
Independent of lifestyle factors (e.g. smoking, physical activity, alcohol intake), we observed a 2.3–2.5 times higher hazard for a late‐life dementia event, hospitalization or death, in women with the lowest compared with the highest grip strength (Q1, 16.0 kg vs. Q4, 25.8 kg). These findings complement a study of 466 788 younger individuals (median age 56.5 years, 54.5% women) from the UK Biobank where those with the lowest hand grip strength had 72% and 87% higher risk of dementia incidence and mortality over ~9 years, respectively, compared with the strongest individuals. 15 Unlike our study, genetic risk (e.g. APOE) was not considered. Another study including 2288 participants over the age of 65 years from the Adult Changes in Thought study also observed an association between higher hand grip strength (per kg) and lower risk of dementia (HR 0.87 95% CI 0.77; 0.99) almost 6 years later. 21 When considering change in grip strength over time, a study in community‐dwelling Japanese men and women (n = 1055, mean age 68 years without dementia) reported those with the greatest decline in grip strength (≥15%) over 15 years had 51% higher risk for any dementia compared with those with unchanged grip strength. 22 Collectively, such findings are comparable to our results where women with the largest 5‐year decline in grip strength (Q1) recorded between 1.9 and 2.4 times greater risk for a late‐life dementia event or hospitalization over the next 9.5 years. Here, it is important to highlight that our study is the first to focus on late‐life dementia in community‐dwelling older women (mean age ~75 years). Furthermore, the aforementioned findings are supported by a scoping review (15 studies with participants over the age of 60 years), 7 which found that grip strength may be used as a surrogate marker to monitor changes in cognitive function over time, a major risk factor for dementia. Possibly due to a range of underlying shared pathologies, grip strength may present as a surrogate measure of CVD, inflammation and frailty, 23 which are known risk factors for dementia. 6 Grip strength could be a discriminating measure of neurological function and brain health due to overlapping neural basis of cognitive and motor decline. 24 Data from over 40 000 people aged 40–70 years from the UK Biobank 25 point to a potential role of brain regions vulnerable to neuropathology in AD, 26 with volume of subcortical, hippocampal regions and temporal cortices associated with grip strength. In the context of CVD, a major contributor to vascular damage to white matter in the brain, 27 the presence (and severity) of carotid plaques 28 and calcification of the abdominal aorta 29 has also been associated with poorer grip strength, the latter reported as a risk factor for late‐life dementia. 30
We also observed that women with the slowest TUG (Q4, 12.4 s) had twofold to threefold higher relative hazard for a late‐life dementia‐related event, hospitalization and/or death, compared with those with the fastest TUG performance (Q1, 7.4 s). Most importantly, we report that women with the greatest decrement in TUG performance over 5 years recorded between 2.5 and 4.1 times greater risk for a late‐life dementia event or death. Likewise, in 3663 older individuals (mean age 73.5 years) who were free from dementia at baseline and followed over 9 years, slower gait speed was associated with 59% increased hazard of dementia. 31 Of importance, the gait assessments obtained 4 and 7 years prior to dementia onset were also associated with an increased risk (46% and 30%, respectively). Noteworthy, in comparison to gait speed, TUG involves a more complex test incorporating manoeuvres typically used in daily life such as balance, walking and transferring (e.g. sitting and standing from a chair). 32 More complex functional tasks may have greater potential to identify individuals at risk for dementia because cognitive and motor function share common neural substrates. 8 , 33 It is possible that the added complexity of the task translates to slow TUG time which may serve as a symptom of predementia (e.g. declining cognitive function). Unsurprisingly, a dual decline in both gait speed and cognition (specifically memory) has also been reported to have the strongest association with dementia risk in 16 855 individuals (mean age 75 years, 56% women). 34 The aforementioned findings are supported by a scoping review including 39 studies (n = 57 456) advocating the evaluation of physical function via gait speed as beneficial in the clinical environment for dementia risk assessment. 8 When considering a combination of muscle weakness and poor physical function, these measures are likely to improve risk stratification, as indicated by a higher relative hazard (between 1.6 and 2.0 times) for late‐life dementia outcomes.
Concurrent decline to cognitive and physical function is considered to be a result of dysregulation across multiple cellular processes such as genetic alterations, nutrient and lipid metabolism and elevated pro‐inflammatory proteins. 35 For post‐menopausal women, lower circulating oestrogen levels may be exacerbated in vascular cognitive impairment and dementia pathogenesis. 36 Whilst we identified the poorest physical function (including 5‐year decline) was associated with the greatest dementia risk in women, further research is needed to examine the role of events specific to women (pregnancy, menopause and sex hormones) and sex‐specific risk profiles for vascular disorders 37 in the pathways leading to cognitive and physical decline. In regard to potential mechanisms, components of the TUG test (e.g. slow gait speed) may capture a range of unhealthy behaviours (e.g. poor diet and physical activity levels, smoking), as well as CVD, 38 which increase the risk of dementia. Risk factors for compromised vasculature have also been suggested to increase vascular lesions of the brain (e.g. stroke and white matter lesions) that are capable of compromising motor control due to disrupted neuronal circuits. 31 Noteworthy, higher grip strength has also been associated with increased white matter hyperintensity volume, 9 which may be influenced by the vasculature, thus having strong implications for late‐life dementia. Unsurprisingly, endothelial dysfunction is a common mechanism implicated in vascular dementia and AD 39 as well as poor muscle function and related phenotypes including sarcopenia and frailty. 40 Collectively, a greater decline in grip strength and/or TUG, such as those observed over 5 years in our study, is likely to reflect a systematic exacerbation of the aforementioned risk factors that together increases dementia risk.
From a feasibility perspective, both grip strength and TUG are inexpensive and simple screening tools that can be used to identify individuals at a community level with impaired function. This information in conjunction with medical history can be considered by clinicians to determine if further assessment of dementia risk is required (e.g. genetics, cognitive function). Future work should seek to determine if the addition of such tests to current clinical dementia assessment tools substantially improves risk stratification. Under clinical guidance, early screening would also offer patients awareness around primary prevention strategies aiming to eliminate, reverse or attenuate dementia‐related risk factors. To this end, muscle function tests may be considered as an informative risk factor for a range of negative health outcomes including dementia.
This study has several strengths, including a large representative sample of community‐dwelling older women from the PLSAW cohort. For example, the prevalence of APOE ℇ4 allele in our cohort was 22.9%, which is comparable to the 22–28% reported in other Australian populations. 41 , 42 Furthermore, we demonstrate that grip strength and TUG provided independent information to genetic risk (e.g. APOE ℇ4) when considering late‐life dementia. Of importance, in the subgroup analysis where we stratify women by APOE ℇ4 and function, results demonstrate the importance of considering weakness and slowness irrespective of genetic risk. However, it is important to highlight the nature of this analysis is exploratory and hypothesis generating, requiring further investigation in large prospective studies. We also considered a range of covariates, including CVD risk factors, medications and physical activity. To reduce the risk of selection and misclassification bias, we included high‐quality objective administrative data from two sources including an 18‐year retrospective period (e.g. prevalent ASVD from ICD codes) and 14.5 years of prospective follow‐up. The ICD coding for dementia categorization also reduces potential bias in dementia diagnosis. Muscle function was also assessed using standardized assessments for hand grip strength and TUG. Nevertheless, limitations of this study must be acknowledged.
The observational nature of the study does not allow causality to be established. Furthermore, our results may not be generalized to other populations including younger women or men; thus, replication in other cohorts is essential. Although linked hospital discharge administrative data are reported to have high accuracy (96.7%), it has low sensitivity (21.2%) compared with chart review, therefore increasing dementia ascertainment compared with death certificates alone. 43 Noteworthily, ICD‐10 dementia diagnoses from hospital records has substantial agreement (k = 0.71) with chart review including a sensitivity of 67% and a positive predictive value of 76%.[S16] The incidence of dementia in the current study (18.5%) is similar to other studies in Australian women aged over 70 years followed over 16 years (20.4%) 44 using multiple administrative data (e.g. aged care assessments data, hospital admissions, pharmaceutical data, death records and self‐reported survey data). Of importance, our results would have been biased towards the null if dementia diagnosis was overlooked. Given that women are at greater risk of developing late‐life dementia, 13 it is important to elucidate the sex‐specific contribution of dementia risk factors, which could encompass a decline in physical function. However, as men and women have a different pattern of risk factors associated with grip strength decline, 45 our findings may be less applicable to men. Additionally, as we adopted a Cox proportional hazards model, our hazard estimates are based on the first late‐life dementia event; it does not account for any further risk in women who may have presented with multiple dementia‐related hospitalizations. Further, severity was unable to be assessed, although dementia‐related hospitalizations are likely to be of a very serious nature as it warranted hospitalization. Low appendicular lean mass in conjunction with compromised strength/function are components of sarcopenia, a potential risk factor of interest. However, DXA‐derived lean mass was only obtained at year 1 in a small subset (~22.6%) of women. Due to compromised statistical power, we were unable to explore this further. Finally, we had limited power to assess the relationship between muscle function and dementia sub‐types. Vascular mechanisms are implicated in this association, 9 but we did not have sufficient vascular dementia events to include in our analyses. Larger studies are needed to examine this relationship in women given that slow TUG was associated with unspecified late‐life dementia, but not AD events. However, the smaller AD sample may have contributed to this null finding.
In conclusion, our data indicate that muscle weakness and slower physical function, including their decline over 5 years, should be considered a risk factor for late‐life dementia in community‐dwelling older women. Despite current data indicating that functional assessment is not common in clinical practice, 46 we provide further evidence for the importance of hand grip strength and TUG as potential clinical assessments to support early identification of community‐dwelling older women at risk of late‐life dementia. These findings are especially relevant to clinicians to direct high‐risk individuals during the asymptomatic prodromal period towards dementia‐related primary prevention programmes focusing on modifiable lifestyle factors (e.g. diet, exercise, smoking) to promote healthy ageing.
Conflict of interest
All authors declare no conflicts of interest.
Ethical approval
All participants provided written informed consent. Ethics approval was granted by the Human Ethics Committee of the University of Western Australia. CAIFOS and PLSAW studies were retrospectively registered on the Australian New Zealand Clinical Trials Registry (CAIFOS trial registration number #ACTRN12615000750583 and PLSAW trial registration number #ACTRN12617000640303) and complied with the Declaration of Helsinki. Human ethics approval for the use of linked data was provided by the Human Research Ethics Committee of the Western Australian Department of Health (project number #2009/24).
Supporting information
Table S1. Baseline characteristics of included and excluded participants.
Table S2. Hazard ratios (95% CI) for any late‐life dementia events, hospitalizations and deaths over 14.5 years by weak hand grip strength and slow timed‐up‐and‐go.
Table S3. Multivariable‐adjusted hazard ratios (95% CI) including the individual variables that were used to compute the General Framingham Risk Score (age, body mass index, prevalent diabetes, systolic blood pressure) for late‐life dementia outcomes over 14.5 years by quartiles of hand grip strength and timed‐up‐and‐go (TUG).
Table S4. Competing risk (non‐dementia mortality) for late‐life dementia events by quartiles of hand grip strength and timed‐up‐and‐go (TUG).
Table S5. Multivariable‐adjusted hazard ratios (95% CI) for late‐life dementia events over 14.5 years by quartiles of hand grip strength and timed‐up‐and‐go (TUG) examined in women with or without the Apolipoprotein E ℇ4 (APOE ℇ4) genotype.
Table S6. Multivariable‐adjusted hazard ratios (95% CI) for any late‐life dementia event, hospitalizations and deaths over 9.5 years for the change in grip strength or timed‐up‐and‐go over 5 years (1998–2003) in 1052 women.
Acknowledgements
The authors wish to thank the staff at the Western Australia Data Linkage Branch, Hospital Morbidity Data Collection, the Australian Co‐ordinating Registry, the State Registries of Births, Deaths and Marriages, the Coroners, the National Coronial Information system and the Victorian Department of Justice and Community Safety for providing the cause of death unit record file data. The study was supported by project grants 254627, 303169 and 572604 from the National Health and Medical Research Council of Australia (NHMRC). The salary of M.S. is supported by a Royal Perth Hospital Research Foundation Fellowship (RPHRF CAF) and an Emerging Leader Fellowship from the Western Australian Future Health Research and Innovation Fund. The salary of J.M.H. is supported by a NHMRC of Australia Senior Research Fellowship (1116973). The salary of J.R.L. is supported by a National Heart Foundation Future Leader Fellowship (102817). The salary of H.M. is supported by a NHMRC‐ARC Dementia Development Fellowship (1097696). The salary of D.S. is supported by an Australian NHMRC Investigator Grant (GNT1174886). None of the funding agencies had any role in the conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle. 47
Radavelli‐Bagatini S., Macpherson H., Scott D., Daly R. M., Hodgson J. M., Laws S. M., et al (2023) Impaired muscle function, including its decline, is related to greater long‐term late‐life dementia risk in older women, Journal of Cachexia, Sarcopenia and Muscle, 14, 1508–1519, 10.1002/jcsm.13227
Joshua R. Lewis and Marc Sim are joint last authors.
References
- 1. Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M. World Alzheimer Report 2015: The global impact of dementia: an analysis of prevalence, incidence, cost and trends. Alzheimer's Disease Int, London, UK.
- 2. Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol 2013;12:357–367. [DOI] [PubMed] [Google Scholar]
- 3. Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late‐life dementia. Nat Rev Neurol 2009;5:649–658. [DOI] [PubMed] [Google Scholar]
- 4. Briggs R, Kennelly SP, O'Neill D. Drug treatments in Alzheimer's disease. Clin Med 2016;16:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer's disease. New Eng J Med 2023;388(1):9–21. [DOI] [PubMed] [Google Scholar]
- 6. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet 2020;396:413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fritz NE, McCarthy CJ, Adamo DE. Handgrip strength as a means of monitoring progression of cognitive decline–a scoping review. Ageing Res Rev 2017;35:112–123. [DOI] [PubMed] [Google Scholar]
- 8. Grande G, Triolo F, Nuara A, Welmer A‐K, Fratiglioni L, Vetrano DL. Measuring gait speed to better identify prodromal dementia. Exp Gerontol 2019;124:110625. [DOI] [PubMed] [Google Scholar]
- 9. Duchowny KA, Ackley SF, Brenowitz WD, Wang J, Zimmerman SC, Caunca MR, et al. Associations between handgrip strength and dementia risk, cognition, and neuroimaging outcomes in the UK Biobank Cohort Study. JAMA Netw Open 2022;5:e2218314‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Patten R, Lee EE, Graham SA, Depp CA, Kim H‐C, Jeste DV, et al. The utility of the timed up‐and‐go test in predicting cognitive performance: a cross‐sectional study of independent living adults in a retirement community. J Appl Gerontol 2020;39:1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Donoghue O, Feeney J, O'Leary N, Kenny RA. Baseline mobility is not associated with decline in cognitive function in healthy community‐dwelling older adults: findings from the Irish longitudinal study on ageing (TILDA). Am J Geriatr Psychiatry 2018;26:438–448. [DOI] [PubMed] [Google Scholar]
- 12. Lee JE, Shin DW, Jeong S‐M, Son KY, Cho B, Yoon JL, et al. Association between timed up and go test and future dementia onset. J Gerontol: Series A 2018;73:1238–1243. [DOI] [PubMed] [Google Scholar]
- 13. Buckley RF, Waller M, Masters CL, Dobson A. To what extent does age at death account for sex differences in rates of mortality from Alzheimer disease? Am J Epidemiol 2019;188:1213–1223. [DOI] [PubMed] [Google Scholar]
- 14. Safieh M, Korczyn AD, Michaelson DM. ApoE4: an emerging therapeutic target for Alzheimer's disease. BMC Med 2019;17:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esteban‐Cornejo I, Ho FK, Petermann‐Rocha F, Lyall DM, Martinez‐Gomez D, Cabanas‐Sánchez V, et al. Handgrip strength and all‐cause dementia incidence and mortality: findings from the UK Biobank prospective cohort study. J Cachexia Sarcopenia Muscle 2022;13:1514–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5‐year, double‐blind, placebo‐controlled trial in elderly women. Arch Intern Med 2006;166:869–875. [DOI] [PubMed] [Google Scholar]
- 17. Sim M, Lewis JR, Blekkenhorst LC, Bondonno CP, Devine A, Zhu K, et al. Dietary nitrate intake is associated with muscle function in older women. J Cachexia Sarcopenia Muscle 2019;10:601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization . Manual of the international statistical classification of diseases, injuries, and causes of death: based on the recommendations of the ninth revision conference, 1975, and adopted by the Twenty‐ninth World Health Assembly, 1975 revision ed. Geneva: World Health Organization; 1977. [Google Scholar]
- 19. World Health Organization . ICD‐10: international statistical classification of diseases and related health problems: tenth revision, 2nd ed. Geneva: World Health Organization; 2004. [Google Scholar]
- 20. Sabbagh MN, Perez A, Holland TM, Boustani M, Peabody SR, Yaffe K, et al. Primary prevention recommendations to reduce the risk of cognitive decline. Alzheimers Dement 2022;18:1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang L, Larson EB, Bowen JD, van Belle G. Performance‐based physical function and future dementia in older people. Arch Intern Med 2006;166:1115–1120. [DOI] [PubMed] [Google Scholar]
- 22. Hatabe Y, Shibata M, Ohara T, Oishi E, Yoshida D, Honda T, et al. Decline in handgrip strength from midlife to late‐life is associated with dementia in a Japanese community: The Hisayama Study. J Epidemiol 2020;30:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bohannon RW. Grip strength: an indispensable biomarker for older adults. Clin Interven Ageing 2019;14:1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carson RG. Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging 2018;71:189–222. [DOI] [PubMed] [Google Scholar]
- 25. Jiang R, Westwater ML, Noble S, Rosenblatt M, Dai W, Qi S, et al. Associations between grip strength, brain structure, and mental health in >40,000 participants from the UK Biobank. BMD Med 2022;20:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pini L, Pievani M, Bocchetta M, Altomare D, Bosco P, Cavedo E, et al. Brain atrophy in Alzheimer's disease and aging. Ageing Res Rev 2016;30:25–48. [DOI] [PubMed] [Google Scholar]
- 27. Moroni F, Ammirati E, Rocca MA, Filippi M, Magnoni M, Camici PG. Cardiovascular disease and brain health: focus on white matter hyperintensities. Int J Cardiol: Heart Vasculature 2018;19:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gebre AK, Sim M, Dalla Via J, Rodríguez AJ, Hodgson JM, Bondonno CP, et al. Measures of carotid atherosclerosis and fall‐related hospitalization risk: The Perth Longitudinal Study of Ageing Women. Nutr Metab Cardiovasc Dis 2022;33:95–104. [DOI] [PubMed] [Google Scholar]
- 29. Rodríguez AJ, Lewis JR, Scott DS, Kiel DP, Schousboe JT, Ebeling PR, et al. Aortic calcification is associated with five‐year decline in handgrip strength in older women. Calcif Tissue Int 2018;103:589–598. [DOI] [PubMed] [Google Scholar]
- 30. Porter T, Sim M, Prince RL, Schousboe JT, Bondonno C, Lim WH, et al. Abdominal aortic calcification on lateral spine images captured during bone density testing and late‐life dementia risk in older women: a prospective cohort study. The Lancet Reg Health‐Western Pacific 2022;26:100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dumurgier J, Artaud F, Touraine C, Rouaud O, Tavernier B, Dufouil C, et al. Gait speed and decline in gait speed as predictors of incident dementia. J Gerontol: Series A 2017;72:655–661. [DOI] [PubMed] [Google Scholar]
- 32. Podsiadlo D, Richardson S. The timed ‘up & go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 33. Hanakawa T, Goldfine AM, Hallett M. A common function of basal ganglia‐cortical circuits subserving speed in both motor and cognitive domains. Eneuro 2017;4:ENEU17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Collyer TA, Murray AM, Woods RL, Storey E, Chong TT‐J, Ryan J, et al. Association of dual decline in cognition and gait speed with risk of dementia in older adults. JAMA Netw Open 2022;5:e2214647‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sargent L, Nalls M, Amella EJ, Slattum PW, Mueller M, Bandinelli S, et al. Shared mechanisms for cognitive impairment and physical frailty: a model for complex systems. Alzheimer's Dementia: Trans Res Clin Interven 2020;6:e12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nguyen DH, Cunningham JT, Sumien N. Estrogen receptor involvement in vascular cognitive impairment and vascular dementia pathogenesis and treatment. Geroscience 2021;43:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu D, Montagne A, Zhao Z. Alzheimer's pathogenic mechanisms and underlying sex difference. Cell Mol Life Sci 2021;78:4907–4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Veronese N, Stubbs B, Volpato S, Zuliani G, Maggi S, Cesari M, et al. Association between gait speed with mortality, cardiovascular disease and cancer: a systematic review and meta‐analysis of prospective cohort studies. J Am Dir Assoc 2018;19:981–988, e7. [DOI] [PubMed] [Google Scholar]
- 39. Martins‐Filho RK, Zotin MC, Rodrigues G, Pontes‐Neto O. Biomarkers related to endothelial dysfunction and vascular cognitive impairment: a systematic review. Dementia Geriatric Cog Disorders 2020;49:365–374. [DOI] [PubMed] [Google Scholar]
- 40. Amarasekera AT, Chang D, Schwarz P, Tan TC. Does vascular endothelial dysfunction play a role in physical frailty and sarcopenia? A systematic review. Age Ageing 2021;50:725–732. [DOI] [PubMed] [Google Scholar]
- 41. Martins RN, Clarnette R, Fisher C, Broe GA, Brooks WS, Montgomery P, et al. ApoE genotypes in Australia: roles in early and late onset Alzheimer's disease and Down's syndrome. Neuroreport 1995;6:1513–1516. [PubMed] [Google Scholar]
- 42. Fowler C, Rainey‐Smith SR, Bird S, Bomke J, Bourgeat P, Brown BM, et al. Fifteen years of the Australian imaging, biomarkers and lifestyle (AIBL) study: progress and observations from 2,359 older adults spanning the spectrum from cognitive normality to Alzheimer's disease. J Alzheimer's Disease Rep 2021;5:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zilkens RR, Spilsbury K, Bruce DG, Semmens JB. Linkage of hospital and death records increased identification of dementia cases and death rate estimates. Neuroepidemiology 2009;32:61–69. [DOI] [PubMed] [Google Scholar]
- 44. Waller M, Mishra GD, Dobson AJ. Estimating the prevalence of dementia using multiple linked administrative health records and capture–recapture methodology. Emerg Themes Emidemiol 2017;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sternäng O, Reynolds CA, Finkel D, Ernsth‐Bravell M, Pedersen NL, Dahl Aslan AK. Factors associated with grip strength decline in older adults. Age Ageing 2015;44:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ling CH, Taekema D, De Craen AJ, Gussekloo J, Westendorp RG, Maier AB. Handgrip strength and mortality in the oldest old population: the Leiden 85‐plus study. Can Med Assoc J 2010;182:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. Wiley Online Library; 2017. p 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of included and excluded participants.
Table S2. Hazard ratios (95% CI) for any late‐life dementia events, hospitalizations and deaths over 14.5 years by weak hand grip strength and slow timed‐up‐and‐go.
Table S3. Multivariable‐adjusted hazard ratios (95% CI) including the individual variables that were used to compute the General Framingham Risk Score (age, body mass index, prevalent diabetes, systolic blood pressure) for late‐life dementia outcomes over 14.5 years by quartiles of hand grip strength and timed‐up‐and‐go (TUG).
Table S4. Competing risk (non‐dementia mortality) for late‐life dementia events by quartiles of hand grip strength and timed‐up‐and‐go (TUG).
Table S5. Multivariable‐adjusted hazard ratios (95% CI) for late‐life dementia events over 14.5 years by quartiles of hand grip strength and timed‐up‐and‐go (TUG) examined in women with or without the Apolipoprotein E ℇ4 (APOE ℇ4) genotype.
Table S6. Multivariable‐adjusted hazard ratios (95% CI) for any late‐life dementia event, hospitalizations and deaths over 9.5 years for the change in grip strength or timed‐up‐and‐go over 5 years (1998–2003) in 1052 women.


