Abstract
The decrease of physical abilities and functional decline that can be caused by musculoskeletal conditions such as sarcopenia, can lead to higher levels of dependency and disability. Therefore, it may influence patient reported outcome measures (PROM), such as the health‐related quality of life (HRQoL). The purpose of this systematic review and meta‐analysis is to provide a comprehensive overview of the relationship between sarcopenia and HRQoL. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) were followed throughout the whole process of this work. A protocol was previously published on PROSPERO. The electronic databases MEDLINE, Scopus, Allied and Complementary Medicine (AMED), EMB Review – ACP Journal Club, EBM Review ‐ Cochrane Central of Register of Controlled Trials and APA PsychInfo were searched until October 2022 for observational studies reporting a HRQoL assessment in both sarcopenic and non‐sarcopenic individuals. Study selection and data extraction were carried out by two independent researchers. Meta‐analysis was performed using a random effect model, reporting an overall standardized mean difference (SMD) and its 95% confidence interval (CI) between sarcopenic and non‐sarcopenic individuals. Study quality was measured using the Newcastle‐Ottawa Scale and the strength of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool. The search strategy identified 3725 references from which 43 observational studies were eligible and included in this meta‐synthesis study. A significantly lower HRQoL was observed for sarcopenic individuals compared with non‐sarcopenic ones (SMD −0.76; 95% CI −0.95; −0.57). Significant heterogeneity was associated with the model (I 2 = 93%, Q test P‐value <0.01). Subgroup analysis showed a higher effect size when using the specific questionnaire SarQoL compared with generic questionnaires (SMD −1.09; 95% CI −1.44; −0.74 with the SarQoL versus −0.49; 95% CI −0.63; −0.36 with generic tools; P‐value for interaction <0.01). A greater difference of HRQoL between sarcopenic and non‐sarcopenic was found for individuals residing in care homes compared with community‐dwelling individuals (P‐value for interaction <0.001). No differences were found between age groups, diagnostic techniques, and continents/regions. The level of evidence was rated as moderate using the GRADE assessment. This systematic review and meta‐analysis combining 43 observational studies shows that HRQoL is significantly reduced in sarcopenic patients. The use of disease‐specific HRQoL instruments may better discriminate sarcopenic patients with respect to their quality of life.
Keywords: Sarcopenia, Quality of life, HRQoL, Older people
1. Introduction
The normal aging process is accompanied by a progressive degradation of musculoskeletal functions. Indeed, from the age of 60, a decrease in muscle mass (−1% per year) and muscle strength (−2.5 to 3% per year) can be observed. 1 Sarcopenia is not part of the normal aging process, and has recently been defined by the European Work Group on Sarcopenia in Older people (EWGSOP) as ‘a progressive and generalized skeletal muscle disorder that is associated with an increased likelihood of adverse outcomes including falls, fractures, physical disability and mortality’. 2 This condition affects older people and is associated with higher mortality and morbidity. 3 Current evidence suggests an impact of sarcopenia on health‐related quality of life (HRQoL). 4 , 5
The assessment of QoL as a health parameter has been gradually introduced in the measurement of the impact of pathologies and more specifically of chronic diseases. 6 Indeed, with the constant increase in life expectancy, the improvement of medical technologies and better prevention, pathologies tend to become chronic and their assessment cannot be limited to mortality or morbidity. 7 The measurement of patient reported outcomes (PROM), and more specifically HRQoL, has become an important indicator increasingly used in epidemiological studies, particularly encouraged by the numerous validations and adaptations of existing tools. In addition, HRQoL measures have been shown to be significant predictors of hard clinical outcomes, such as hospitalization or mortality, reinforcing the importance of their assessment. 8 , 9 , 10 , 11 , 12 The reduction in physical capacity and functional decline that can be caused by musculoskeletal disorders such as sarcopenia, can lead to a higher levels of dependency and disability and therefore influence the HRQoL. 13 , 14 Measurement of this specific PROM is recommended in interventional trials, and HRQoL should be used as co‐primary endpoint to evaluate the effectiveness of interventions in sarcopenia. 15 HRQoL tools exist under the form of generic or specific tools. Generic tools can be applied to any population suffering from any disease and offer the possibility to obtain comparisons between populations whereas specific tools are specifically designed for a particular population and offer the advantage of being more sensitive to change. To date, there are only two HRQoL specific questionnaires for sarcopenic patients, the Sarcopenia and Quality of Life (SarQoL) questionnaire 16 , 17 , 18 and the Age‐Related Muscle Loss Questionnaire (ARMQL), 19 although the latter is not fully validated.
In 2016, Woo and colleagues published a systematic review on the relationship between biomarkers of sarcopenia (i.e., muscle mass, muscle strength, and physical performance) and HRQoL. 4 The authors searched the literature up to December 2015 and included 20 articles. However, only one study used a consensus definition of sarcopenia. In 2019, the widely used definitions of sarcopenia established by the European Work Group on Sarcopenia in Older people and the Asian Working Group for Sarcopenia were revised. 2 , 20 These revisions followed the assignment, in 2016, of an International Statistical Classification of Diseases and Related Health Problems – Clinical Modification code (ICD‐10‐CM) to diagnose sarcopenia. 21 As this is a major advance, a significant number of studies have been published using one of the established diagnostic criteria, making it worthwhile to revisit the question of the association between sarcopenia and HRQoL.
The aim of this systematic review and meta‐analysis is therefore to summarize the evidence on the association between primary sarcopenia and HRQoL. More specifically, this meta‐research work aims to evaluate whether primary sarcopenia affects HRQoL by comparing HRQoL reported by sarcopenic participants with that reported by non‐sarcopenic participants.
2. Methods
The proposed systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta‐analysis (PRISMA2020). 22 The completed PRISMA checklist is available in Appendix S1. A protocol has been developed and published in PROSPERO (CRD42020215377). The current article reports results coming from observational studies. Because the researchers' team changed during the realization of the project, an amendment to the protocol was published in October 2022.
The research project can be summarized with the following PICOs format: P (Population): Older people with sarcopenia; I (Intervention/Predictors): NA; C (Comparator): Older people without sarcopenia; O (Outcome): a measure of quality of life; S (Study design): Observational studies (i.e., cross‐sectional and longitudinal studies).
2.1. Literature search
MEDLINE, Allied and Complementary Medicine (AMED), EMB Review – ACP Journal Club, EBM Review ‐ Cochrane Central of Register of Controlled Trials, APA PsychInfo (via OVID platform for all the mentioned bibliographic databases) and Scopus were searched in October 2022 for any observational studies reporting a measure of HRQoL for sarcopenic individuals in comparison with non‐sarcopenic individuals. For convenience of translation, the search was limited to English and French studies. 23 A combination of terms of Medical Subject Headings (MeSH) and keywords was used in the search strategy (the complete search strategies for Ovid and Scopus are available in Appendix S2).
Additionally, bibliographies of all included studies were manually checked for other potentially relevant publications. Moreover, references retrieved from previous systematic reviews and review articles performed on the same or similar topic were hand searched and included to search for additional references matching our selection criteria. We also contacted experts in the field to obtain their opinions about our search strategy and the included papers. They were also proposed to provide us any missing studies or grey literature they knew about.
The search results from the electronic sources and hand searching were imported in Covidence software for data management, as recommended by the Cochrane collaboration.
2.2. Study selection
Inclusion criteria (Table 1) guided the first step of references selection based on title and abstract. Three reviewers (C. B., C. D. and M. L.) performed this screening independently to exclude irrelevant articles with every single reference screened by two different reviewers. In the second step, the reviewers read the full texts of each non‐excluded articles to determine eligibility for inclusion in this systematic review. Disagreements during both stages were resolved by consensus between the two reviewers.
Table 1.
Inclusion criteria
| Inclusion criteria | |
|---|---|
| Design | Observational studies (i.e., cross sectional studies or longitudinal studies) |
| Language | English |
| Participants |
Community‐dwelling or residents in assisted living facilities, ≥60 years of age or the mean or median age of the sample is ≥60 years. A diagnosis of sarcopenia should be performed (based on at least 2 biomarkers) and participants should be divided in two groups according to the presence of sarcopenia. |
| Outcome |
Quality of life in a quantitative format (i.e., a scale), measured with a validated instrument specifically designed to measure quality of life. Quality of life measurement should be reported for sarcopenic and non‐sarcopenic participants. |
Both cross‐sectional studies and longitudinal studies were accepted if those studies provided a HRQoL assessment for both a sarcopenic and a non‐sarcopenic group. To be consistent with the objectives of the present systematic review and meta‐analysis, only cross‐sectional data from longitudinal studies were used (i.e., HRQoL for sarcopenic and non‐sarcopenic individuals at a certain time; longitudinal changes in HRQoL for both populations were not used).
Studies were excluded if they included individuals with acute sarcopenia (i.e., development of sarcopenia within a short amount of time after a stressor event such as hospitalization or illness 2 ); sarcopenia was diagnosed on the basis of a single biomarker (e.g., muscle mass only); only a screening tool (e.g., the SARC‐F) was applied without further diagnosing the condition; hospitalized, pre‐/post‐operative or disease‐specific participants were recruited; only sarcopenic obesity was diagnosed in the study; HRQoL was examined using qualitative research methods; and/or no original data was reported (i.e., exclusion of commentaries, editorials, and letters to the editor).
2.3. Data extraction
Data were independently extracted by two reviewers and encoded in a standardized Excel file, pre‐tested on a sample of 5 studies. The following information were extracted: article information (e.g., title and year of publication), population characteristics (e.g., description of the population and sarcopenia diagnosis), outcomes (e.g., HRQoL instrument and results), funding, conflict of interest and conclusion.
Disagreements were resolved by consensus or with the help of an additional reviewer (O. B.). When full text was not available or data were missing, authors were contacted.
2.4. Quality appraisal
Quality assessment of studies was performed with Newcastle‐Ottawa Quality Assessment Scale (NOS). Initially, the NOS has been developed for longitudinal studies, but we used an adapted version for cross‐sectional studies (accessed online on August 2022: https://www.kcgg.ugent.be/pdf/NEWCASTLE‐OTTAWA_QUALITY_ASSESSMENT_SCALE.pdf). This adapted version has already been used by several other studies that have felt the need to adapt the NOS scale to appropriately assess the quality of cross‐sectional studies. 24 This scale consists of three items: selection, comparability and outcome. According to different criteria, a maximum number of stars can be attributed for each item with a maximum total number of 7 stars for cross‐sectional studies. Concerning the item ‘comparability’, we assigned a score of 0 when a significant difference in age of sarcopenic and non‐sarcopenic participants was identified without being integrated in a multivariate analysis.
Each study was evaluated independently by the two reviewers. Disagreements were resolved by consensus or with the help of a third reviewer.
2.5. Grading the evidence
We used the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) method 25 to rate the certainty of the evidence and to summarize the overall quality of the evidence from the pooled studies. The evidence score started with a high‐quality evidence and was downgraded by one or two levels if any of the following pre‐specified criteria was present: (1) risk of bias (RoB) (i.e., high risk of bias in more than 75% of the included studies; (2) inconsistency (i.e., unexplained substantial heterogeneity I 2 > 50%); (3) indirectness (i.e., presence of factors that limit the generalizability of the results); (4) imprecision (i.e., large 95% confidence interval (CI) recommendation altered if 95% CI represents the true effect); (5) publication bias (i.e., small study effect P > 0.05 and significant impact on the estimator). Each meta‐analysis outcome assessed was determined to be of very low, low, moderate or high certainty.
2.6. Data synthesis
A random effect model was chosen given the expected heterogeneity of protocols and sarcopenia diagnosis across individual studies. To provide a comparison between outcomes reported by the different studies, effect size as standardized mean difference with 95% CIs were measured for each outcome. We extracted mean and standard deviations (SDs) HRQoL values of both groups (i.e., sarcopenic and non‐sarcopenic) in each individual study. When data were not available in the right format or incomplete, we first contacted authors of individual studies to obtain missing values. If the missing data could not be obtained from the authors, we used different strategies to obtain the missing information, or an estimation of the missing information, to be sure to include the study in our analyses and maintain an exhaustivity to our research. We used the following techniques: (1) We referred to the methods described in section 7.7.3 of the Cochrane Handbook for Systematic Review to obtain missing SDs from P‐values or 95% confidence intervals; (2) when only median and interquartile ranges were available, we used the formula proposed by Hozo et al. 26 to convert them into mean and SDs.
When a study reported multiple results for HRQoL according to different sarcopenia diagnosis, we used preferentially first the revised version of the EWGSOP criteria (EWGSOP2 criteria). 2 When different scales were used to measure HRQoL within the same study, we extracted, preferentially first, the results of specific HRQoL questionnaire (e.g., the SarQoL), then the SF‐36 Physical Component Scale, then the SF‐36 Physical function, then the EQ‐5D and then any other scales/subscales for measuring HRQoL.
Subgroup analyses were performed according on the HRQoL instrument used (individual tool or generic vs. specific), on the diagnostic criteria for sarcopenia (EWGSOP1 vs. EWGSOP2 vs. Asian Working Group on Sarcopenia (AWGS)) and on age of participants (>75 years or <75 years). Meta‐regression was also performed on age of participants, treated as a continuous variable and on quality of study (number of stars obtained to the NOS scale).
Results were examined for heterogeneity using Cochran's Q statistic and the I 2 statistic. Potential publication bias was explored by means of a contour‐enhanced funnel plot. We used the Egger's regression asymmetry test to detect publication bias. In case of significant publication bias, the Trim and Fill method was applied to assess the impact of potential missing studies on the pooled effect size.
We also conducted one‐way sensitivity analyses to evaluate the stability of our results when one study is removed at a time. Because it was feasible for all studies using the same HRQoL questionnaire (i.e., the SarQoL), we also performed a sensitivity analysis by changing the effect size within this meta‐analysis. Therefore, for all studies measuring HRQoL using the SarQoL, the difference between sarcopenic and non‐sarcopenic participants was also measured using the Mean Difference and its 95% CI.
For all results, a two‐sided P‐value of 0.05 or less were considered as significant. All analyses were performed using R Software (R‐4.2.1) and appropriate packages (meta, metafor, tidyverse, devtools, esc, mathjax, and dmetar).
3. Results
A total of 3725 references were identified using the search strategies applied on to the databases in October 2022. After removing duplicates, 2293 references were assessed for eligibility based on their title/abstract and 188 of these were further assessed based on their full text. From these 188 studies, 39 met our inclusion criteria and four additional studies were further identified through a manual search. The list of excluded studies and their respective reasons of exclusion is available on our Open Science Framework deposit (https://osf.io/rqhvy/). Therefore, a total of 43 observational studies were included in this systematic review, 42 cross‐sectional studies 5 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 and one prospective study 68 for which baseline values of HRQoL for the sarcopenic and non‐sarcopenic groups were used (Figure 1).
Figure 1.
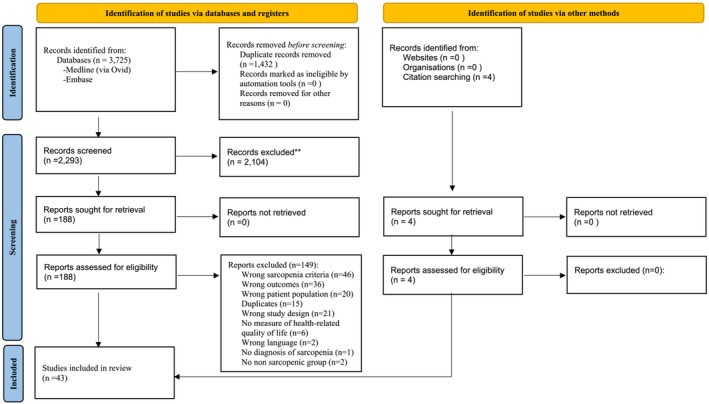
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA 2020) flowchart of study selection.
3.1. Cross‐sectional studies
The total of the 43 studies combined data from 30 322 participants, 4108 sarcopenic and 26 214 non‐sarcopenic. The characteristics of the included studies are shown in Table 2. The EWGSOP criteria were used in 34 (79.1%) of the studies (EWGSOP1: n = 19; EWGSOP2: n = 15) whereas the AWGS criteria were used in the remaining 9 studies (20.9%). About 46.5% of the studies used the specific HRQoL questionnaire SarQoL (n = 20) and the others used generic questionnaires (i.e., SF‐36 n = 11, EQ 5D n = 8, CASP n = 2, OHIP‐14 n = 1, OPQoL n = 1, WHOQoL n = 1). The calculated median age of the participants was 74.6 years. Regarding the quality of the studies, out of a total of 7 points on the NOS scale, 3 studies received 3 points, 14 studies received 4 points, 18 studies received 5 points, 7 studies received 6 points and only one study received the maximum value of 7 points. The quality assessment of each study is shown in Table 3.
Table 2.
Characteristics of included studies
| First author's name, year | Country | Participants (type of population, sample size, age, sex ratio) | Sarcopenia ratio | Definition of sarcopenia | Tool used to access sarcopenia | Tool to assess HRQoL |
|---|---|---|---|---|---|---|
| Alekna, 2019 53 | Lithuania | Community dwelling older adults, Sample size: 176, Age: 78.2 (74.1–82.6), Women: 59.7% | 58 (32.9%) | EWGSOP2 | Muscle mass: DXA Muscle strength: handgrip dynamometer Physical performance: SPPB | SarQoL |
| Beaudart, 2015 36 | Belgium | Community dwelling older adults, Sample Size: 534, Age: 73.5 ± 6.16, women: 60.3% | 73 (13.7%) | EWGSOP1 | Muscle mass: DXA Muscle strength: handgrip dynamometer Physical performance: SPPB |
SF‐36 EQ‐5D |
| Beaudart, 2017 38 | Belgium | Community dwelling older adults, Sample size: 296, Age: 73.3 (68.9–78.6), Women: 57.1% | 43 (14.5%) | EWGSOP1 | Muscle mass: DXA Muscle strength: handgrip dynamometer Physical performance: SPPB | SarQoL |
| Beaudart, 2017 51 | UK | Community dwelling participants of the Hertfordshire Cohort Study, Sample size: 297, Age: 79.5 ± 2.62, Women: 46.1% | 14 (4.7%) | EWGSOP1 | Muscle mass: DXA Muscle strength: handgrip dynamometer Physical performance: gait speed | SarQoL |
| Beaudart, 2018 34 | Belgium | Community dwelling older adults, Sample size: 387, Age: 74.02 ± 5.99, Women: 58.5% | 50 (12.9%) 48 (12.4%) 17 (4.4%) 38 (9.8%) |
EWGSOP1 IWGS SSCWD FNIH |
Muscle mass: DXA Muscle strength: handgrip dynamometer Physical performance: gait speed | SarQoL |
| Chew, 2020 54 | Singapore | Community‐dwelling older adults, Sample size: 200, Age: 67.9 ± 7.86, Women: 68.5% | 31 (15.5%) | EWGSOP2 | Muscle mass: DXA Muscle strength: handgrip dynamometer Physical performance: gait speed | EQ‐5D |
| De Souza Orlandi, 2018 60 | Brazil | Community dwelling older adults, Sample size: 226, Age: 69.97 ± 6.82 | 43 (19%) | EWGSOP1 | NR |
EQ‐5D SF‐36 |
| De Souza Orlandi, 2022 24 | Brazil | Community dwelling older adults, Sample size: 221, Women: 68.3% | 55 (36.4%) | EWGSOP2 | Muscle mass: DXA Muscle strength: handgrip dynamometer Physical performance: gait speed | SarQoL |
| Erdogan, 2019 41 | Turkey | Community dwelling older adults, Sample size: 100, Age: 74.7 ± 6.1, Women: 71.0% | 27 (27.0%) | EWGSOP2 | Muscle mass: BIA Muscle strength: handgrip dynamometer Physical performance: gait speed | SarQoL |
| Fábrega‐Cuadros, 2020 33 | Spain | Community dwelling older adults, Sample size: 252, Age: 74.00 (70.00–78.00), Women: 82.54% | 66 (26.2%) | EWGSOP2 | Muscle mass: BIA Muscle strength: handgrip dynamometer | SarQoL |
| Fábrega‐Cuadros, 2021 43 | Spain | Community dwelling older adults, Sample size: 304, Age: 72.04 ± 7.88, Women: 83.88% | 72 (28.23%) | EWGSOP2 | Muscle mass: BIA Muscle strength: handgrip dynamometer Physical performance: gait speed | SF‐36 |
| Gasparik, 2017 22 | Romania | Community dwelling older adults recruited in hospital, Sample size: 100, Age: 72 (67–79), Women: 69% | 13 (13.0%) | EWGSOP1 | Muscle mass: Lee equation Muscle strength: handgrip dynamometer Physical performance: gait speed | SarQoL |
| Geerinck, 2018 31 | Belgium | Community dwelling older adults, Sample size: 92, Age: 82 (73–85), Women: 43.5% | 30 (32.6%) | EWGSOP1 | Muscle mass: BIA Muscle strength: handgrip dynamometer Physical performance: gait speed | SarQoL |
| Geerinck, 2020 50 | Belgium | Community dwelling older adults, Sample size: 296, Age: 73.3 (68.9–78.6) | 13 (4.4%) | EWGSOP2 | Muscle mass: DXA Muscle strength: handgrip dynamometer | SarQoL |
| Geerinck, 2021 28 | Belgium | Community dwelling older adults, Sample size:214, Age: 76 (73–81), Women: 63.1% | 21 (9.8%) | EWGSOP2 | Muscle mass: DXA Muscle strength: handgrip dynamometer | SF‐36 SarQoL |
| Guillamon‐Escudero, 2022 42 | Spain | Community dwelling older adults, Sample size: 202, Age: 73 ± 7, Women: 164 (81.2%) | 54 (26.7%) | EWGSOP2 | Muscle mass: BIA Muscle strength: handgrip dynamometer Physical performance: gait speed | SarQoL |
| Ilhan, 2019 5 | Turkey | Community dwelling older adults, Sample size: 408, Age: 77.1 ± 6.8, Women: 69.0% | 11 (2.7%) | EWGSOP1 | Muscle mass: BIA Muscle strength: handgrip dynamometer Physical performance: gait speed | EQ‐5D |
| Imai, 2022 40 | Japan | Community dwelling older adults, Sample size: 113, Age: 76.3 ± 5.6 | 22 (19.5%) | AWGS | Muscle mass: BIA Muscle strength: handgrip dynamometer Physical performance: gait speed | EQ‐5D |
| Kitamura, 2022 29 | Japan | Community older people covered by long term care insurance attending rehabilitation in a one‐day care centre, Sample size: 64, Age: 79.3 ± 8.8, Women: 67.3% | 24 (55.8%) | AWGS | Muscle mass: BIA Muscle strength: handgrip dynamometer | EQ 5D‐3L |
| Konstantynowicz, 2018 52 | Poland | Community dwelling older adults, Sample size: 106, Age: 73.3 ± 5.94, Women: 65.1% | 60 (56.6%) | EWGSOP1 | Muscle mass: DXA Muscle strength: handgrip dynamometer Physical performance: gait speed | SarQoL |
| Le, 2021 49 | China | Community‐dwelling older adults and outpatient department of geriatrics, Sample size: 159, Women: 46.5% | 51 (32.01%) | AWGS | Muscle mass: Lee equation Muscle strength: handgrip dynamometer Physical performance: gait speed | SarQoL |
| Lee, 2022 61 | Taïwan | Community dwelling older adults, Sample size: 100, Age: >65 years, Women: 28% | 50 (50.0%) | AWGS | Muscle mass: BIA Muscle strength: handgrip dynamometer Physical performance: gait speed or SPBB | SarQoL |
| Losa‐Reyna, 2020 45 | Spain | Community dwelling older adults, Sample size: 1189, Age: 75.8 ± 5.9, Women: 53.7% | 97 (8.16%) | EWGSOP2 |
Muscle mass: DXA Muscle strength: handgrip dynamometer Physical performance: gait speed |
EQ‐5D |
| Mahmoodi, 2022 23 | Iran | Community dwelling older adults, Sample size: 128, Women: 41.4% | 88 (68.5%) | AWGS | Muscle mass: BIA Muscle strength: handgrip dynamometer Physical performance: gait speed | SarQoL |
| Manrique‐Espinoza, 2017 59 | Mexico | Community dwelling older adults, Sample size: 543, Age: 76.1 ± 3.1, Women: 52.7% | 198 (36.5%) | EWGSOP1 | Muscle mass: Calf circumference Muscle strength: handgrip dynamometer Physical performance: gait speed | SF‐36 |
| Marques, 2018 37 | Brazil | Community dwelling older adults, Sample size: 604, Women: 65.2% | 37 (6.1%) | EWGSOP1 | Muscle mass: DXA Muscle strength: handgrip dynamometer | CASP‐16 |
| Matijević, 2020 48 | Serbia | Community dwelling older adults, Sample size: 699, Age: 70 (67–74), Women: 72.7% | 12 (1.7%) | EWGSOP2 | Muscle mass: DXA Muscle strength: handgrip dynamometer Physical performance: gait speed | SarQoL |
| Mijnarends, 2016 56 | Netherlands | Community dwelling older adults, Sample size: 227, Age: 74.9 ± 7.2 | 53 (23.3%) | EWGSOP1 | Muscle mass: BIA Muscle strength: handgrip dynamometer Physical performance: gait speed |
EQ‐5D EQVAS |
| Montero‐Errasquín, 2022 46 | Spain | Community dwelling older adults, Sample size: 86, Age: 77.6 ± 5.3, Women: 80.2% | 16 (18.6%) 13 (15.1%) |
EWGSOP1 FNIH |
Muscle mass: DXA Muscle strength: handgrip dynamometer | SarQoL |
| Mori, 2019 57 | Japan | Community‐dwelling adults aged ≥60 years, Sample size: 331, Age: 71.5 ± 5.1, Women: 71.9% | 19 (5.7%) | AWGS | Muscle mass: BIA Muscle strength: handgrip dynamometer Physical performance: gait speed | SF‐36 |
| Öztürk, 2018 39 | Turkey | Community dwelling geriatric population referred to outpatient clinic, Sample size: 423, Age: 71.8 ± 6.01, Women: 56.7% | 61 (14.4%) | EWGSOP1 | Muscle mass: BIA Muscle strength: handgrip dynamometer Physical performance: gait speed | SF‐36 |
| Patel, 2013 27 | United‐Kingdom | Community dwelling older adults, Sample size: 103, Age: 72.4 ± 4.4, Women: 0.00%, 100% of men | 7 (6.8%) | EWGSOP1 | Muscle mass: DXA Muscle strength: handgrip dynamometer Physical performance: gait speed | SF‐36 |
| SilvaNeto, 2016 32 | Brazil | Community dwelling older adults, Sample size: 70, Age: 65.58 ± 6.67, Women: 55.7% | 7 (10%) | EWGSOP1 | Muscle mass: DXA Muscle strength: handgrip dynamometer Physical performance: gait speed | SF‐36 |
| Simsek, 2022 44 | Turkey | Nursing home residents, Sample size: 172, Age: 81.78 ± 7.03, Women: 57.6% | 88 (51.2%) | EWGSOP2 | Muscle mass: BIA Muscle strength: handgrip dynamometer | EQ‐5D |
| Singhal, 2019 26 | India | Community dwelling people, outpatients of the Department of Geriatric Medicine, Sample size: 100, Age: 72.5 ± 6.4, Women: 31% | 53 (53%) | AWGS | Muscle mass: DXA Muscle strength: handgrip dynamometer Physical performance: gait speed | OPQoL |
| Smith, 2022 62 | Multicentre | Community dwelling older adults, Sample size: 14 585, Age: 72.6 ± 11.5, Women: 55.0% | 1750 (12.0%) | EWGSOP2 | Muscle mass: Lee equationMuscle strength: handgrip dynamometer Physical performance: gait speed | WHOQoL |
| Takahashi, 2018 58 | Japan | Community dwelling older adults, dental clinic outpatients, Sample size: 279, Age: 76 ± 7.5, Women: 62.0% | 86 (30.8%) | AWGS | Muscle mass: Calf circumference Muscle strength: handgrip dynamometer Physical performance: gait speed | OHIP‐14 |
| Tsekoura, 2020 30 | Greece | Community dwelling older adults, Sample size: 176, Age: 71.19 ± 7.95, Women: 77.27% | 50 (28.4%) | EWGSOP1 | Muscle mass: BIA Muscle strength: handgrip dynamometer Physical performance: gait speed | SarQoL |
| Umegaki, 2022 63 | Japan |
Community dwelling older adults attending a memory clinic, Sample size: 23, Age: NR 1‐year follow‐up |
23 (100%) | AWGS | Muscle mass: BIAMuscle strength: handgrip dynamometer Physical performance: gait speed | EQ‐5D |
| Veronese, 2022 47 | Italy | Community dwelling older adults, Sample size: 4044, Age: 70.7 ± 7.6, Women: 55.1% | 375 (9.3%) | EWGSOP2 | Muscle mass: Lee equation Muscle strength: handgrip dynamometer | CASP‐19 |
| Woo, 2018 25 | Australia | Community dwelling older adults, Sample size: 727, Age: 73.9 ± 6.2 for men, 73.2 ± 6 for women, Women: 50.9% | 71 (9.77%) | EWGSOP1 | Muscle mass: DXA Muscle strength: handgrip dynamometer | SF‐36 |
| Yalcin, 2017 35 | Turkey | Residents of a Turkish nursing home, N = 241, 83.27 ± 5.65, women: 123 (52.1%) | 93 (38.6%) | EWGSOP1 | Muscle mass: BIA Muscle strength: handgrip dynamometer Physical performance: gait speed | SF‐36 |
| Yoo, 2020 55 | Korea | Community dwelling older adults, Sample size: 450, Age: 73.9 ± 6.6, Women: 87.7% | 53 (11.8%) | EWGSOP2 | Muscle mass: BIA Muscle strength: handgrip dynamometer | SarQoL |
AWGS, Asian Working Group on Sarcopenia; BIA, bioelectrical impendance analysis; CASP‐19 (or 16), Quality of Life Scale; DXA, dual energy X‐ray absorptiometry; EQ‐5D, EuroQol five‐dimensions; EWGSOP, European Working Group on Sarcopenia in Older People; FNIH, Foundation for the National Institutes of Health Biomarkers Consortium Sarcopenia Project; HRQoL, health‐related quality of life; NR, not reported; OHIP‐14, Oral Health Impact Profile‐14; SarQoL, Sarcopenia and Quality of life questionnaire; SF‐36, Short‐Form 36; SPPB, Short Physical Performance Battery; WHOQoL, World Health Organization Quality of Life questionnaire.
Table 3.
Quality appraisal of included studies
| First author's name, year | Selection | Comparability | Outcome | Total score* |
|---|---|---|---|---|
| Alekna, 2019 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Beaudart, 2015 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Beaudart, 2017 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Beaudart, 2017 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Beaudart, 2018 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Chew, 2020 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| De Souza Orlandi, 2018 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| De Souza Orlandi, 2022 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Erdogan, 2019 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Fábrega‐Cuadros, 2020 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Fábrega‐Cuadros, 2021 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Gasparik, 2017 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Geerinck, 2018 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Geerinck, 2020 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Geerinck, 2021 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Guillamon‐Escudero, 2022 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Ilhan, 2019 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Imai, 2022 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Kitamura, 2022 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Konstantynowicz, 2018 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Le, 2021 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Lee, 2022 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Losa‐reyna, 2020 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Mahmoodi, 2022 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Manrique‐Espinoza, 2017 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Marques, 2018 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Matijević, 2020 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Mijnarends, 2016 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Montero‐Errasquín, 2022 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Mori, 2019 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Öztürk, 2018 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Patel, | ★★★ | ★★ | ★★ | ★★★★★★★ |
| SilvaNeto, 2016 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Simsek, 2022 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Singhal, 2019 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Smith, 2022 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Takahashi, 2018 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Tsekoura, 2020 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Umegaki, 2022 a | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Veronese, 2022 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Woo, 2018 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Yalcin, 2017 | ★★★ | ★★ | ★★ | ★★★★★★★ |
| Yoo, 2020 | ★★★ | ★★ | ★★ | ★★★★★★★ |
Total score is on 7 points for cross‐sectional studies (adapted NOS scale for cross‐sectional studies)
Umegaki et al. 63 is a longitudinal study. However, for the present paper, only baseline values of the sarcopenic and non‐sarcopenic groups were used in analyses. This study was therefore used as a cross‐sectional one. As a matter of consistence between studies, we decided to apply the same NOS scale than the other cross‐sectional studies.
Authors of 14 papers were contacted because the data were not usable in the format presented in the paper and 11 authors responded to provide the data in the correct format. Therefore, imputations (i.e., transformation of 95% CI into SD and transformation of median and interquartile range into mean and SD) were only necessary for 3 out of the 43 studies (6.98%). 32 , 39 , 60
A general forest plot including the 43 observational studies is shown in Figure 2 and highlights a significantly important reduced HRQoL for sarcopenic participants compared with non‐sarcopenic ones (SMD −0.76; 95% CI −0.95; −0.57). The model was associated with significant heterogeneity (I 2 = 93%, Q test P‐value <0.01). The leave one analysis removing some outliers (e.g., Alekna, 2019, Le, 2021, Mahmoodi, 2022 or Yoo, 2020) did not affect the global results (SMD −0.71 [−0.87; −0.54] when removing Mahmoodi, 2022; SMD −0.73 [−0.92; −0.54] when removing any of the three at that time).
Figure 2.
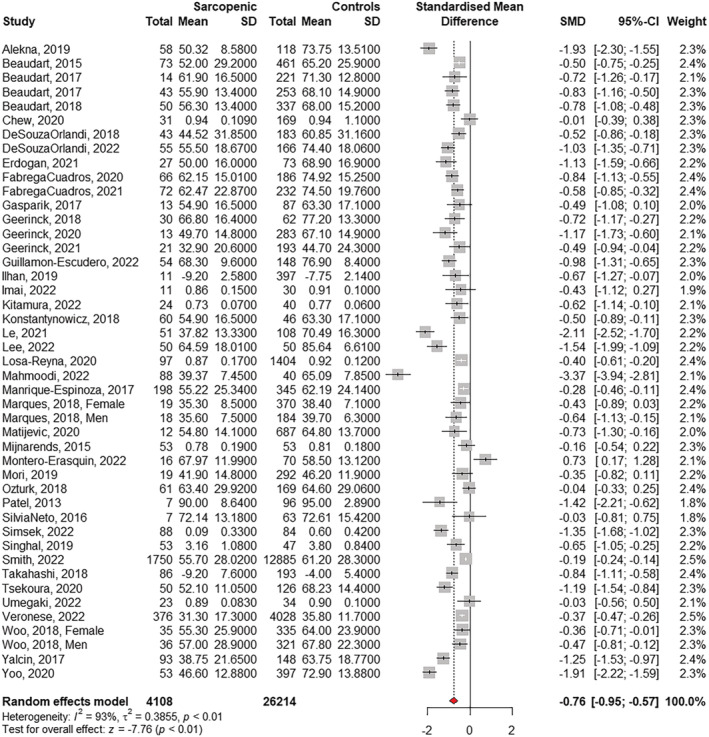
Quality of life in sarcopenia – Forest plot including 43 observational studies published until October 2022. CI, confidence interval; SD, standard deviation; SMD, standardized mean difference.
Asymmetry was observed in the funnel plot (Figure 3), which was further confirmed by the Egger's test (t = −4.56, df = 43, P‐value<0.01), indicating significant publication bias in the model. The Trim and Fill method applied to all studies identified 18 potentially missing studies. After applying the Trim and Fill method to the data, the HRQoL of sarcopenic participants was still reduced compared with non‐sarcopenic participants but in a moderate manner (SMD recalculated with 18 imputed studies: −0.31, 95% CI −0.55; −0.07).
Figure 3.
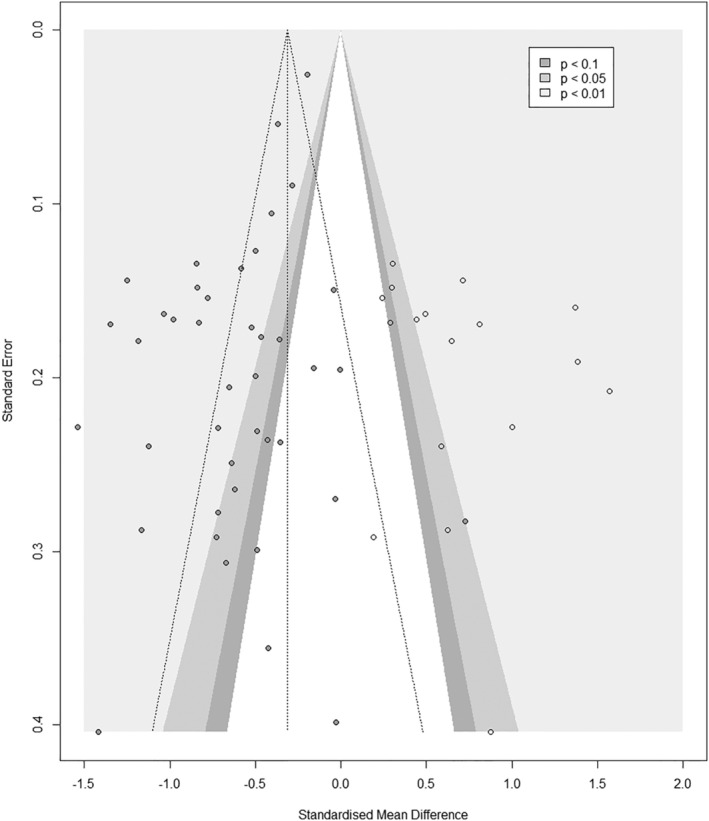
Contour Enhanced funnel plot for cross‐sectional studies on sarcopenia and quality of life – Trim and Fill method (the 18 imputed studies are represented by circles that have no filled colour).
The results of the subgroups analyses are shown in Table 4. A significant difference in HRQoL found in sarcopenic populations was found when using a specific HRQoL questionnaire compared with a generic one. The specific HRQoL questionnaire SarQoL better discriminated sarcopenia in terms of HRQoL (SMD of −1.09 [−1.44; −0.74] using the SarQoL versus −0.49 [−0.63; −0.36] using generic tools [P‐value for interaction <0.01]). Because all studies using a specific HRQoL used the same tool, the SarQoL questionnaire (n = 20), it was also possible to perform a post‐hoc sensitivity analysis (not specified in the protocol) by changing the SMD estimate with a Mean Difference (MD) estimate. A MD of −15.01 points/100 (95% CI of −19.00; −11.01) on the SarQoL questionnaire was found for sarcopenic compared with non‐sarcopenic participants.
Table 4.
Subgroup analyses
| No. studies | No. patients | SMD (95% CI) | I 2 | P for heterogeneity | P for interaction | |
|---|---|---|---|---|---|---|
| HRQoL scale (n = 44) a | <0.01 | |||||
| Generic | 24 | 26 143 | −0.49 (−0.63; −0.36) | 83% | <0.01 | |
| Specific b | 20 | 4475 | −1.09 (−1.44; −0.74) | 91% | <0.01 | |
| Age of participants (n = 42) c | 0.48 | |||||
| <75 years | 27 | 25 463 | −0.78 (−1.02; −0.53) | 93% | <0.01 | |
| >75 years | 15 | 4268 | −0.76 (−1.11; −0.40) | 92% | <0.01 | |
| Sarcopenia diagnosis (n = 45) d | 0.16 | |||||
| EWGSOP2 | 15 | 23 826 | −0.86 (−1.16; −0.57) | 95% | <0.01 | |
| EWGSOP1 | 19 | 5257 | −0.54 (−0.72; −0.36) | 79% | <0.01 | |
| AWGS | 9 | 1239 | −1.11 (−1.79; −0.42) | 94% | <0.01 | |
| FNIH | 2 | 473 | −0.73 (−1.65; 0.19) | 88% | <0.01 | |
| EWGSOP diagnosis vs. others (n = 45) | 0.15 | |||||
| EWGSOP | 34 | 29 083 | −0.68 (−0.85; −0.51) | 92% | <0.01 | |
| Others | 11 | 1712 | −1.03 (−1.61; −0.46) | 93% | <0.01 | |
| Settings (n = 43) | <0.01 | |||||
| Community dwelling | 41 | 29 909 | −0.73 (−0.93; −0.54) | 93% | <0.01 | |
| Care homes | 2 | 413 | −1.29 (−1.51; −1.08) | 0% | 0.66 | |
| Continent (n = 42) e | 0.08 | |||||
| Europe | 20 | 10 269 | −0.70 (−0.91; −0.48) | 85% | <0.01 | |
| America | 5 | 1651 | −0.53 (−0.79; −0.26) | 72% | <0.01 | |
| Asia | 16 | 3040 | −1.02 (−1.46; −0.58) | 94% | <0.01 | |
| Australia | 1 | 727 | −0.41 (−0.66; −0.17) | NA | NA | |
| Europe region (n = 20) | 0.26 | |||||
| Northern Europa | 11 | 2545 | −0.81 (−1.11; −0.52) | 83% | <0.01 | |
| Southern Europa | 9 | 7724 | −0.56 (−0.89; −0.23 | 85% | <0.01 |
For the general Forest Plot, when a study presented results for multiple HRQoL scale, the specific scale was used for analyses. One out of the 43 included studies presented results for both generic and specific scale. Therefore, it was possible to add an additional study in the subgroup of generic scale (n = 44).
Because all the 20 studies assessing HRQoL using a specific HRQoL questionnaire used the same HRQoL questionnaire (i.e., the SarQoL), a post‐hoc sensitivity analysis was performed changing the SMD estimate with a MD estimate. A MD of −15.01 (95% CI −19.00; −11.01), I 2 92%, P < 0.01 between sarcopenic and non‐sarcopenic participants was found.
Age was missing in one study, therefore subgroup on age of participants included only 42 out of the 43 observational studies (n = 42).
For the general Forest Plot, when a study presented results for multiple definition of sarcopenia, the EWGSOP2 definition was used for analyses. Two out of the 43 included studies presented results for different diagnosis criteria. Therefore, it was possible to add a subgroup of FNIH definition (n = 45). Given the data obtained we also developed a subgroup analysis to compare EWGSOP definitions (version 1 or 2 combined) versus others (n = 45).
The study of Smith et al. was removed from the analyses per continent (n = 42) as this study is composed with participants from different countries and different continents. Authors did not provide separate analyses per country.
AWGS, Asian Working Group on Sarcopenia; CI, confidence interval; EWGSOP, European Working Group on Sarcopenia in Older People; FNIH, Foundation for the National Institutes of Health Biomarkers Consortium Sarcopenia Project; HRQoL, health‐related quality of life; SMD, standardized mean difference.
A subgroup interaction was also found regarding clinical setting. A larger difference of HRQoL between sarcopenic individuals and non‐sarcopenic ones was found among those living in care homes (n = 2, SMD of −1.29, 95% CI −1.51; −1.08) compared with those living in the community (n = 41, SMD of −0.73, 95% CI −0.93; −0.54).
No other differences were found in subgroups defined by diagnostic techniques, age, and continents or regions.
Results of meta‐regressions performed on age and RoB are shown in Table 5. No significant effect of age and RoB of individual studies on the association between HRQoL and sarcopenia was observed.
Table 5.
Meta‐regressions model between HRQoL and sarcopenia and age and risk of bias in univariate analysis
| Covariates | Level | β‐coefficient | Std.err. (β) | Z | P‐value |
|---|---|---|---|---|---|
| Mean age | Years | −0.0475 | 0.0286 | −1.6603 | 0.097 |
| Risk of bias | Points | 0.1826 | 0.1147 | 1.5925 | 0.111 |
Using GRADE assessment, the meta‐analysis which included 43 observational studies with 30 322 participants, was rated as moderate level of evidence. No serious risk of bias, no serious indirectness and no serious imprecision were observed for the association. We did not downgrade the publication bias item because, even if publication bias appears to be significant, the Trim and Fill method showed that the impact on this publication bias on the results is moderate. The level of evidence was downgraded only because inconsistency of results (unexplained heterogeneity I 2 > 50%) was observed.
4. Discussion
The aim of this study was to qualitatively and quantitatively summarize all data on the relationship between sarcopenia and HRQoL in order to provide a clear assessment of the impact of sarcopenia on this health parameter. Understanding the impact of sarcopenia on HRQoL is important for healthcare providers and regulators and may guide the development of care strategies for sarcopenic patients.
Forty‐three observational studies evaluating the association between sarcopenia and HRQoL were identified in the literature. The results showed a significant decrease in HRQoL in sarcopenic compared with non‐sarcopenic elderly. It is not surprising to observe a reduced HRQoL in sarcopenic patients as sarcopenia has already been shown to be responsible for many adverse health outcomes such as mobility decline, disability, falls, fractures, hospitalization and death. 3 , 69 , 70 , 71
Regarding the magnitude of the effect size, an even larger SMD was found when the analyses focused on the studies using a specific HRQoL questionnaire compared with a generic one. These results suggest that a specific HRQoL may better discriminate sarcopenic participants in terms of their HRQoL and thus may be more appropriate to accurately assess the impact of sarcopenia on HRQoL. Of the 20 studies that used a specific HRQoL questionnaire, all used the SarQoL. This is not surprising because the SarQoL is currently the only validated specific HRQoL questionnaire for sarcopenia. This questionnaire is available in more than 35 languages and has already been validated in multiple populations. In the SarQoL questionnaire, as in all disease‐specific questionnaires, the vast majority of items are directly related to the disease. In the case of sarcopenia, the items included in SarQoL are therefore muscle oriented. 16 The use of such a questionnaire may more accurately reflect the added value of a targeted intervention for sarcopenia, as all items may be affected. Generic tools may therefore not be able to detect subtle effects of a specific condition on HRQoL, in contrast to specific instruments. In regards to this, a recent publication on the SarQoL questionnaire revealed that this questionnaire has a higher responsiveness than common generic tools such as the SF‐36 or the EQ 5D. 72 Therefore, the use of this specific questionnaire in clinical trials evaluating treatments for the management of sarcopenia should be recommended, as patient‐related outcomes are encouraged to be included as co‐primary endpoints in such trials. 73
The results also revealed a larger difference of HRQoL between sarcopenic and non‐saropenic for individuals living in care facilities compared with those living in the community. While these results may indicate that individuals in institutions may have a more severe status of sarcopenia and therefore a greater impact on HRQoL, they should nevertheless be taken with caution as only two studies (n = 413) reported results on individuals in care facilities compared with 41 studies (n = 29 909) reporting results on community‐dwelling individuals.
The results did not highlight any difference in regards of the strength of association between sarcopenia and HRQoL for different age groups, for different sarcopenia diagnoses, or for different regions/countries/continents. Regarding the ethnicity of participants, although the difference between groups was not significant, we still observed nevertheless a larger SMD for studies conducted in Asia or using the AWGS criteria for sarcopenia diagnoss. However, this association may be biased by the results of some outliers, such as Mahmoodi et al., 28 Lee et al., 66 Le et al. 54 and Yoo et al. 60 who reported larger SMD compared with other studies. Sensitivity analyses revealed that these individual studies did not impact the global estimated effect size. Unfortunately, the association between gender of sarcopenic participants and HRQoL could not be measured in the present analyses. In fact, most of the individual studies were composed of a sample of men and women together (women/men ratio ranging from 28% to 87.7% of women), and separate analyses for gender were not performed.
4.1. Strength and limitations
This is the very first time that a meta‐analysis has been performed to measure the association between sarcopenia and HRQoL. We were able to include a large amount of evidence in this systematic review and all of the available studies were also included in the meta‐analysis, which also ensures the exhaustivity of the statistical synthesis. Of course, our study also contains some methodological limitations. First, it is important to highlight an important heterogeneity observed in the forest plot, which downgrades the certainty of evidence (GRADE of evidence is ‘Moderate’). We investigated this heterogeneity by performing additional subgroup analyses and meta‐regressions, but were unable to explain the remaining heterogeneity. Because sarcopenia is a multifactorial disease that may be associated with various comorbidities, studies performed on sarcopenia are always complex to interpret. Moreover, even if we tried to standardize the diagnostic criteria, the device used to measure the biomarkers of sarcopenia and the cut‐offs used for the diagnosis may have introduced an important heterogeneity in the condition of interest, as previously reported. 74 , 75 Therefore, the characteristics of sarcopenic participants may vary from one study to another, which could have led to some variations in the results of HRQoL. It is important to raise that all but one study (97.7%) agreed on the fact that HRQoL was reduced in sarcopenic participants compared with non‐sarcopenic. There was no inconsistency in the direction of the estimates but only in their magnitude. Second, only cross‐sectional data were included, which does not allow to investigate the causal relationship between sarcopenic and HRQoL. The present systematic review allowed the inclusion of both cross‐sectional studies and prospective studies as long as these studies included two groups that could be compared in terms of their HRQoL. Surprisingly, only one prospective study was identified, which means that data on the evolution of HRQoL in sarcopenic individuals are almost inexistant. Prospective studies, who would allow to investigate deeply the causal relationship between sarcopenia and HRQoL, are therefore needed. Finally, as a last limitation, we regret not being able to run sex‐specific analyses. Indeed, given the different body composition profile between men and women, it would have been relevant to provide analyses of the impact of sarcopenia on HRQoL stratified by sex. However, published evidence with sex‐stratified analyses was so limited that it was simply not possible to perform subgroup analyses in our meta‐analysis. Authors of further studies are encouraged to provide separate analyses by gender.
5. Conclusion
This systematic review and meta‐analysis of observational studies highlighted a large decrease in HRQoL in sarcopenic compared with non‐sarcopenic older adults. The results also revealed that using disease‐specific HRQoL instruments may better discriminate sarcopenic patients with regard to their quality of life. Although a large amount of evidence was included in the meta‐analytic model, the final association was rated as ‘moderate level of evidence’ according to the GRADE assessment because important unexplained heterogeneity was observed in the results. As poor quality of life in older people has been shown to be associated with several negative health outcomes such as falls, hospitalizations and mortality, these findings allowed us to suggest that diagnosis of sarcopenia in community‐dwelling and institutionalized older people should be considered as a priority in clinical practice. The earlier sarcopenia is detected, the earlier programs for the prevention and treatment of this condition can be initiated to prevent the important impact that sarcopenia can have on HRQoL.
Conflict of interest
C.B., O.B. and J.‐Y.R. are shareholder of SARQOL SRL, a spin‐off of the University of Liege. The other co‐authors have no conflicts of interest to declare. This is a systematic review. No ethical approval, consent to participate or consent to publish is required. 76
Supporting information
Appendix S1. PRISMA checklist
Appendix S2. Search strategies (Medline via Ovid and Scopus)
Beaudart C., Demonceau C., Reginster J.‐Y., Locquet M., Cesari M., Cruz Jentoft A. J., et al (2023) Sarcopenia and health‐related quality of life: A systematic review and meta‐analysis, Journal of Cachexia, Sarcopenia and Muscle, 14, 1228–1243, 10.1002/jcsm.13243
References
- 1. Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment‐facts and numbers. J Cachexia Sarcopenia Muscle 2020;11:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beaudart C, Zaaria M, Pasleau F, Reginster J‐Y, Bruyère O, Stenroth L. Health Outcomes of Sarcopenia: A Systematic Review and Meta‐Analysis. PLoS ONE 2017;12:e0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woo T, Yu S, Visvanathan R. Systematic literature review on the relationship between biomarkers of sarcopenia and quality of life in older people. J Frailty Aging 2016;5:88–99. [DOI] [PubMed] [Google Scholar]
- 5. Beaudart C, Locquet M, Reginster JYJ‐Y, Delandsheere L, Petermans J, Bruyère O. Quality of life in sarcopenia measured with the SarQoL®: impact of the use of different diagnosis definitions. Aging Clin Exp Res 2018;30:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaplan RM, Ries AL. Quality of life: concept and definition. COPD 2007;4:263–271. [DOI] [PubMed] [Google Scholar]
- 7. Megari K. Quality of Life in Chronic Disease Patients. Health Psychol Res 2013;1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown DS, Thompson WW, Zack MM, Arnold SE, Barile JP. Associations Between Health‐Related Quality of Life and Mortality in Older Adults. Prev Sci 2015;16:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsai SY, Chi LY, Lee CH, Chou P. Health‐related quality of life as a predictor of mortality among community‐dwelling older persons. Eur J Epidemiol 2007;22:19–26. [DOI] [PubMed] [Google Scholar]
- 10. Cavrini G, Broccoli S, Puccini A, Zoli M. EQ‐5D as a predictor of mortality and hospitalization in elderly people. Qual Life Res 2012;21:269–280. [DOI] [PubMed] [Google Scholar]
- 11. Sargent‐Cox KA, Anstey KJ, Luszcz MA. The choice of self‐rated health measures matter when predicting mortality: evidence from 10 years follow‐up of the Australian longitudinal study of ageing. BMC Geriatr 2010;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phyo AZZ, Freak‐Poli R, Craig H, Gasevic D, Stocks NP, Gonzalez‐Chica DA, et al. Quality of life and mortality in the general population: a systematic review and meta‐analysis. BMC Public Health 2020;20:1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsekoura M, Kastrinis A, Katsoulaki M, Billis E, Gliatis J. Sarcopenia and Its Impact on Quality of Life. Adv Exp Med Biol 2017;987:213–218. [DOI] [PubMed] [Google Scholar]
- 14. Beaudart C, Reginster J‐Y, Bruyère O, Geerinck A. Quality of Life and Sarcopenia. In Cruz‐Jentoft AJ, Morley JE, eds. Sarcopenia, Second ed; 2021. [Google Scholar]
- 15. Reginster JY, Beaudart C, Al‐Daghri N, Avouac B, Bauer J, Bere N, et al. Update on the ESCEO recommendation for the conduct of clinical trials for drugs aiming at the treatment of sarcopenia in older adults. Aging Clin Exp Res 2021;33:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beaudart C, Biver E, Reginster J‐YJ‐Y, Rizzoli R, Rolland Y, Bautmans I, et al. Development of a self‐administrated quality of life questionnaire for sarcopenia in elderly subjects: the SarQoL. Age Ageing 2015;44:960–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beaudart C, Biver E, Reginster J‐Y, Rizzoli R, Rolland Y, Bautmans I, et al. Validation of SarQoL®, a specific health‐related quality of life questionnaire for sarcopenia. J Cachexia Sarcopenia Muscle 2018;8:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beaudart C, Reginster J‐Y, Geerinck A, Locquet M, Bruyère O. Current review of the SarQoL®: a health‐related quality of life questionnaire specific to sarcopenia. Expert Rev Pharmacoecon Outcomes Res 2017;17:335–341. [DOI] [PubMed] [Google Scholar]
- 19. Evans CJ, Chiou C‐F, Fitzgerald KA, Evans WJ, Ferrell BR, Dale W, et al. Development of a new patient‐reported outcome measure in sarcopenia. J Am Med Dir Assoc 2011;12:226–233. [DOI] [PubMed] [Google Scholar]
- 20. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:300–307.e2. [DOI] [PubMed] [Google Scholar]
- 21. Anker SD, Morley JE, von Haehling S. Welcome to the ICD‐10 code for sarcopenia. J Cachexia Sarcopenia Muscle 2016;7:512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Phys Ther 2009;89:1006–1012. [PMC free article] [PubMed] [Google Scholar]
- 23. Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of english‐language restriction on systematic review‐based meta‐analyses: A systematic review of empirical studies. Int J Technol Assess Health Care 2012;28:138–144. [DOI] [PubMed] [Google Scholar]
- 24. Patra J, Bhatia M, Suraweera W, Morris SK, Patra C, Gupta PC, et al. Exposure to second‐hand smoke and the risk of tuberculosis in children and adults: a systematic review and meta‐analysis of 18 observational studies. PLoS Med 2015;12:e1001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–394. [DOI] [PubMed] [Google Scholar]
- 26. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ildiko GA, Gabriela M, Charlotte B, Olivier B, Raluca‐Monica P, Jean‐Yves R, et al. Psychometric performance of the Romanian version of the SarQoL®, a health‐related quality of life questionnaire for sarcopenia. Arch Osteoporos 2017;12:103. [DOI] [PubMed] [Google Scholar]
- 28. Mahmoodi M, Hejazi N, Bagheri Z, Nasimi N, Clark C. Validation of the Persian version of the Sarcopenia‐specific Quality of life questionnaire (SarQoL®‐IR). Aging Clin Exp Res 2022;35:137–145. [DOI] [PubMed] [Google Scholar]
- 29. de Souza Orlandi FI, Duarte Nunes JI, Gabriela Mendes dos Santos D III, Cristina Martins Gratão AI, Silvana Zazzetta MV. Cross‐cultural adaptation and validation of Sarcopenia and Quality of Life (SarQoL) in Brazil. Sao Paulo Med J 2022;141:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woo T, Yu S, Adams R, Visvanathan R. The Association Between Sarcopenia and Quality of Life is Different in Community Dwelling Older Australian Men and Women. Geriatr Med Care 2018;2:1–6. [Google Scholar]
- 31. Singhal S, Dewangan GC, Bansal R, Upadhyay AD, Dwivedi SN, Das CJ, et al. Sarcopenia and its Association with Geriatric Syndromes and Quality of Life in Older Indian Outpatients – A Cross‐sectional Pilot Observational Study. J Indian Acad Geriatr 2019;15:66–74. [Google Scholar]
- 32. Patel HP, Syddall HE, Jameson K, Robinson S, Denison H, Roberts HC, et al. Prevalence of sarcopenia in community‐dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: Findings from the Hertfordshire Cohort Study (HCS). Age Ageing 2013;42:378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Geerinck A, Beaudart C, Reginster JY, Locquet M, Monseur C, Gillain S, et al. Development and validation of a short version of the Sarcopenia Quality of Life questionnaire: the SF‐SarQoL. Qual Life Res 2021;30:2349–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kitamura M, Izawa KP, Ishihara K, Brubaker PH, Matsuda H, Okamura S, et al. Differences in Health‐Related Quality of Life in Older People with and without Sarcopenia Covered by Long‐Term Care Insurance. Eur J Investig Health Psychol Educ 2022;12:536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsekoura M, Billis E, Gliatis J, Tsepis E, Matzaroglou C, Sakkas GK, et al. Cross cultural adaptation of the Greek sarcopenia quality of life (SarQoL) questionnaire. Disabil Rehabil 2020;42:1006–1012. [DOI] [PubMed] [Google Scholar]
- 36. Geerinck A, Scheppers A, Beaudart C, Bruyère O, Vandenbussche W, Bautmans R, et al. Translation and validation of the Dutch SarQoL®, a quality of life questionnaire specific to sarcopenia. J Musculoskelet Neuronal Interact 2018;18:463–472. [PMC free article] [PubMed] [Google Scholar]
- 37. Silva Neto LS, Karnikowski MGO, Osório NB, Pereira LC, Mendes MB, Galato D, et al. Association between sarcopenia and quality of life in quilombola elderly in Brazil. Int J Gen Med 2016;9:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fábrega‐Cuadros R, Martínez‐Amat A, Cruz‐Díaz D, Aibar‐Almazán A, Hita‐Contreras F. Psychometric Properties of the Spanish Version of the Sarcopenia and Quality of Life, a Quality of Life Questionnaire Specific for Sarcopenia. Calcif Tissue Int 2020;106:274–282. [DOI] [PubMed] [Google Scholar]
- 39. Silay K, Yalcin A. Sarcopenia and health‐related quality of life in turkish nursing home residents: A cross‐sectional study. Asian J Gerontol Geriatr 2017;12:42–46. [Google Scholar]
- 40. Beaudart C, Reginster JY, Petermans J, Gillain S, Quabron A, Locquet M, et al. Quality of life and physical components linked to sarcopenia: The SarcoPhAge study. Exp Gerontol 2015;69:103–110. [DOI] [PubMed] [Google Scholar]
- 41. Marques LP, Confortin SC, Ono LM, Barbosa AR, d'Orsi E. Quality of life associated with handgrip strength and sarcopenia: EpiFloripa Aging Study. Arch Gerontol Geriatr 2019;81:234–239. [DOI] [PubMed] [Google Scholar]
- 42. Beaudart C, Biver E, Reginster JY, Rizzoli R, Rolland Y, Bautmans I, et al. Validation of the SarQoL®, a specific health‐related quality of life questionnaire for Sarcopenia. J Cachexia Sarcopenia Muscle 2017;8:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. İlhan B, Bahat G, Erdoğan T, Kiliç C, Karan MA. Anorexia is Independently Associated with Decreased Muscle Mass and Strength in Community Dwelling Older Adults. J Nutr Health Aging 2019;23:202–206. [DOI] [PubMed] [Google Scholar]
- 44. Öztürk ZA, Türkbeyler İH, Abiyev A, Kul S, Edizer B, Yakaryılmaz FD, et al. Health‐related quality of life and fall risk associated with age‐related body composition changes; sarcopenia, obesity and sarcopenic obesity. Intern Med J 2018;48:973–981. [DOI] [PubMed] [Google Scholar]
- 45. Imai R, Imaoka M, Nakao H, Hida M, Tazaki F, Inoue T, et al. Association between chronic pain with presarcopenia and central sensitization in Japanese community‐dwelling older adults: A cross‐sectional study. Medicine (United States) 2022;101:E29998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Erdogan T, Eris S, Avci S, Oren MM, Kucukdagli P, Kilic C, et al. Sarcopenia quality‐of‐life questionnaire (SarQoL)®: translation, cross‐cultural adaptation and validation in Turkish. Aging Clin Exp Res 2021;33:2979–2988. [DOI] [PubMed] [Google Scholar]
- 47. Guillamón‐Escudero C, Diago‐Galmés A, Zuazua Rico D, Maestro‐González A, Tenías‐Burillo JM, Soriano JM, et al. SarQoL Questionnaire in Community‐Dwelling Older Adults under EWGSOP2 Sarcopenia Diagnosis Algorithm: A New Screening Method? Int J Environ Res Public Health 2022;19:8473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fábrega‐Cuadros R, Hita‐Contreras F, Martínez‐Amat A, Jiménez‐García JD, Achalandabaso‐Ochoa A, Lavilla‐Lerma L, et al. Associations between the severity of sarcopenia and health‐related quality of life in community‐dwelling middle‐aged and older adults. Int J Environ Res Public Health 2021;18:8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Şimşek H, Uçar A. Nutritional status and quality of life are associated with risk of sarcopenia in nursing home residents: a cross‐sectional study. Nutr Res 2022;101:14–22. [DOI] [PubMed] [Google Scholar]
- 50. Losa‐Reyna J, Alcazar J, Rodríguez‐Gómez I, Alfaro‐Acha A, Alegre LM, Rodríguez‐Mañas L, et al. Low relative mechanical power in older adults: An operational definition and algorithm for its application in the clinical setting. Exp Gerontol 2020;142:111141. [DOI] [PubMed] [Google Scholar]
- 51. Montero‐Errasquín B, Vaquero‐Pinto N, Sánchez‐Cadenas V, Geerinck A, Sánchez‐García E, Mateos‐Nozal J, et al. Spanish translation, cultural adaptation and validation of the SarQoL®: a specific health‐related quality of life questionnaire for sarcopenia. BMC Musculoskelet Disord 2022;23:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Veronese N, Koyanagi A, Cereda E, Maggi S, Barbagallo M, Dominguez LJ, et al. Sarcopenia reduces quality of life in the long‐term: longitudinal analyses from the English longitudinal study of ageing. Eur Geriatr Med 2022;13:633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matijević R, Hrnjaković O, Đurđević A, Geerinck A, Beaudart C, Bruyère O, et al. Translation and psychometric performance of the Serbian version of the sarcopenia quality of life (SarQoL®) questionnaire. Srp Arh Celok Lek 2020;148:742–748. [Google Scholar]
- 54. Le X, Wei Y, Hao D, Shan L, Li X, Shi Q, et al. Psychometric Properties of the Chinese Version of the Sarcopenia and Quality of Life, a Quality of Life Questionnaire Specific for Sarcopenia. Calcif Tissue Int 2021;109:415–422. [DOI] [PubMed] [Google Scholar]
- 55. Geerinck A, Locquet M, Reginster JY, Bruyère O, Beaudart C. Discriminative Power of the Sarcopenia Quality of Life (SarQoL®) Questionnaire with the EWGSOP2 Criteria. J Frailty Aging 2021;10:193–194. [DOI] [PubMed] [Google Scholar]
- 56. Beaudart C, Edwards M, Moss C, Reginster JY, Moon R, Parsons C, et al. English translation and validation of the SarQoL®, a quality of life questionnaire specific for sarcopenia. Age Ageing 2017;46:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Konstantynowicz J, Abramowicz P, Glinkowski W, Taranta E, Marcinowicz L, Dymitrowicz M, et al. Polish validation of the sarQol®, a quality of life questionnaire specific to sarcopenia. J Clin Med 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alekna V, Kilaite J, Tamulaitiene M, Geerinck A, Mastaviciute A, Bruyère O, et al. Validation of the Lithuanian version of sarcopenia‐specific quality of life questionnaire (SarQoL®). Eur Geriatr Med 2019;10:761–767. [DOI] [PubMed] [Google Scholar]
- 59. Chew J, Yeo A, Yew S, Lim JP, Tay L, Ding YY, et al. Muscle Strength Definitions Matter: Prevalence of Sarcopenia and Predictive Validity for Adverse Outcomes Using the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) Criteria. J Nutr Health Aging 2020;24:614–618. [DOI] [PubMed] [Google Scholar]
- 60. Yoo J, Ha YC, Kim M, Seo SH, Kim MJ, Lee GY, et al. Translation and validation of the Korean version of the Sarcopenia Quality of Life (SarQoL‐K®) questionnaire and applicability with the SARC‐F screening tool. Qual Life Res 2021;30:603–611. [DOI] [PubMed] [Google Scholar]
- 61. Mijnarends DM, Schols JMGA, Halfens RJG, Meijers JMM, Luiking YC, Verlaan S, et al. Burden‐of‐illness of Dutch community‐dwelling older adults with sarcopenia: Health related outcomes and costs. Eur Geriatr Med 2016;7:276–284. [Google Scholar]
- 62. Mori H, Tokuda Y. Differences and overlap between sarcopenia and physical frailty in older community‐dwelling Japanese. Asia Pac J Clin Nutr 2018;28:157–165. [DOI] [PubMed] [Google Scholar]
- 63. Takahashi M, Maeda K, Wakabayashi H. Prevalence of sarcopenia and association with oral health‐related quality of life and oral health status in older dental clinic outpatients. Geriatr Gerontol Int 2018;18:915–921. [DOI] [PubMed] [Google Scholar]
- 64. Manrique‐Espinoza B, Salinas‐Rodríguez A, Rosas‐Carrasco O, Gutiérrez‐Robledo LM, Avila‐Funes JA. Sarcopenia Is Associated With Physical and Mental Components of Health‐Related Quality of Life in Older Adults. J Am Med Dir Assoc 2017;18:636.e1–636.e5. [DOI] [PubMed] [Google Scholar]
- 65. de Souza OF, Brochine Lanzotti R, Gomes Duarte J, Novais Mansur H, Zazzetta MS, Iost Pavarini SC, et al. Translation, adaptation and validation of rapid geriatric assessment to the Brazilian context. J Nutr Health Aging 2018;22:1115–1121. [DOI] [PubMed] [Google Scholar]
- 66. Lee S‐C, Chang C‐F, Wang J‐Y, Liang P‐J. Translation and validation of the Taiwanese SarQoL, a quality of life questionnaire specific to sarcopenia. J Formos Med Assoc 2022;122:249–257. [DOI] [PubMed] [Google Scholar]
- 67. Smith L, Sánchez GFL, Veronese N, Soysal P, Kostev K, Jacob L, et al. Association between sarcopenia and quality of life among adults aged ≥ 65 years from low‐ and middle‐income countries. Aging Clin Exp Res 2022;34:2779–2787. [DOI] [PubMed] [Google Scholar]
- 68. Umegaki H, Suzuki Y, Komiya H, Watanabe K, Nagae M, Yamada Y. Impact of Sarcopenia on Decline in Quality of Life in Older People with Mild Cognitive Impairment. J Alzheimers Dis 2022;88:23–27. [DOI] [PubMed] [Google Scholar]
- 69. Veronese N, Demurtas J, Soysal P, Smith L, Torbahn G, Schoene D, et al. Sarcopenia and health‐related outcomes: an umbrella review of observational studies. Eur Geriatr Med 2019;10:853–862. [DOI] [PubMed] [Google Scholar]
- 70. Fernandes LV, Paiva AEG, Silva ACB, de Castro IC, Santiago AF, de Oliveira EP, et al. Prevalence of sarcopenia according to EWGSOP1 and EWGSOP2 in older adults and their associations with unfavorable health outcomes: a systematic review. Aging Clin Exp Res 2022;34:505–514. [DOI] [PubMed] [Google Scholar]
- 71. Zhao Y, Zhang Y, Hao Q, Ge M, Dong B. Sarcopenia and hospital‐related outcomes in the old people: a systematic review and meta‐analysis. Aging Clin Exp Res 2019;31:5–14. [DOI] [PubMed] [Google Scholar]
- 72. Geerinck A, Bruyere O, Locquet M, Reginster J‐Y, Beaudart C. Evaluation of the Responsiveness of the SarQoL((R)) Questionnaire, a Patient‐Reported Outcome Measure Specific to Sarcopenia. Adv Ther 2018;35:1842–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reginster J‐Y, Cooper C, Rizzoli R, Kanis JA, Appelboom G, Bautmans I, et al. Recommendations for the conduct of clinical trials for drugs to treat or prevent sarcopenia. Aging Clin Exp Res 2016;28:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Beaudart C, Reginster JY, Slomian J, Buckinx F, Dardenne N, Quabron A, et al. Estimation of sarcopenia prevalence using various assessment tools. Exp Gerontol 2015;61:31–37. [DOI] [PubMed] [Google Scholar]
- 75. Beaudart C, Reginster J‐YJ‐YY, Slomian J, Buckinx F, Locquet M, Bruyère O. Prevalence of sarcopenia: the impact of different diagnostic cut‐off limits. J Musculoskelet Neuronal Interact 2014;14:425–431. [PubMed] [Google Scholar]
- 76. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. PRISMA checklist
Appendix S2. Search strategies (Medline via Ovid and Scopus)


