Abstract
People living with human immunodeficiency virus (HIV) (PLWH) appear to be at an increased risk of sarcopenia, which can have a devastating effect on their life due to consequences such as physical disability, poor quality of life, and finally death. This systematic review examined sarcopenia prevalence and its associated factors in PLWH. A systematic search was conducted using the keywords in the online databases including Scopus, PubMed, Web of Science, Embase and Cochrane databases from the dates of inception up to May 2022. The retrieved articles underwent a two‐step title/abstract and full‐text review process, and the eligible papers were selected and included in the qualitative synthesis. Data relating to the study population, purpose of study, gender, age, race, body mass index, medical history, paraclinical results and antiretroviral therapy as associated factors of sarcopenia were extracted. In addition, the prevalence of sarcopenia in PLWH and its promoting and reducing factors were also extracted. We reviewed the 14 related studies for identifying of sarcopenia prevalence and its associated factors in PLWH. The total number of PLWH in all the reviewed studies was 2592. There was no criterion for the minimum number of people with HIV and the lowest number of PLWH was 27, and the highest number was 860. Some studies reported a significantly higher prevalence of sarcopenia in HIV‐infected individuals compared with HIV‐negative controls as follows: 24.2–6.7%, 15–4% and 10–6%, respectively. We showed that, age (30–50 years), being female, >5 years post‐HIV diagnosis, multiple vertebral fractures, cocaine/heroin use and lower gamma‐glutamyl transferase level were the main promoting factors of sarcopenia. Higher educational level, employment, physical exercise, calf circumference >31 cm, and gait speed >0.8 m/s were also factors to reduce sarcopenia. Sarcopenia prevalence in PLWH is higher than HIV‐negative population. Given the importance and prevalence of sarcopenia among PLWH and its associated consequences (i.e., mortality and disability), determining its risk factors is of great importance.
Keywords: human immunodeficiency virus, acquired immunodeficiency syndrome, sarcopenia, wasting syndrome
Introduction
Sarcopenia is a condition characterized by progressive and generalized reduction of skeletal muscle strength and quality as well as impaired physical performance. Adverse consequences of sarcopenia include physical disability, poor quality of life and increased risk of death. 1 The cause of sarcopenia is sometimes identified. However, there are scenarios when no evident cause can be isolated. This muscle‐wasting condition is divided into two categories: primary and secondary sarcopenia. Primary sarcopenia is age‐related sarcopenia that has no other evident cause except aging. 2 In contrast, secondary sarcopenia is when this condition is caused by factors such as physical activity, disease, and nutrition. Physical activity‐related sarcopenia results from prolonged bed rest, a sedentary lifestyle, reconditioning or zero‐gravity conditions. As for disease‐related sarcopenia, this is associated with advanced organ failure (brain, lung, heart, kidney and liver), inflammatory disease, HIV, malignancy or endocrine disease. Finally, nutrition‐related sarcopenia results from an inadequate dietary intake of energy and/or protein, which might be caused by malabsorption, gastrointestinal disorders or the use of medications that lead to anorexia. Sarcopenia leads to adverse clinical consequences, including increased hospitalization, frailty and falls, and premature death in different clinical studies. Furthermore, sarcopenia places a significant economic burden on the society and health care system. 3
For people living with human immunodeficiency virus (PLWH) the prevalence of sarcopenia is estimated to be 24.1% in those aged 35 to 60 years. 4 Sarcopenia has negative effects on the self‐reliance and ability to perform activities of daily living in PLWH, 5 which increases their risk of institutionalization. The etiology of sarcopenia for PLWH appears to be multifactorial with both HIV‐specific complications (e.g., persistent chronic immune activation and inflammation) and lifestyle (e.g., smoking, drug use, alcohol consumption, and malnutrition) being contributing factors. 5 , 6 , 7 , 8 To counteract sarcopenia, exercise is considered an excellent treatment option to the low risk of side effects and minimal cost. Specifically, resistance training in the early stages of sarcopenia among older adults is shown to produce meaningful improvements in the diagnostic measures of sarcopenia. 9 For PLWH, resistance training alone and combined with aerobic training has produced favourable health effects to counteract the effects of sarcopenia. 10 , 11 However, the most effective exercise intervention to target the consequences of sarcopenia in PLWH is presently unknown. Such an intervention would enhance muscle strength, increase mobility and function, decrease fatigue, decrease the risk of metabolic disorders and decrease the risk of falls and skeletal fracture. Although, developing an optimal exercise intervention for PLWH likely requires further examination of factors that contribute to sarcopenia in this population to guide the exercise prescription and complementary practices (e.g., protein supplementation).
The purpose of this systematic review was to examine the prevalence of sarcopenia and its associated increasing or reducing factors among PLWH. The findings may assist practitioners with the management of PLWH, and guide future studies investigating the effectiveness of different interventions in management of HIV‐associated sarcopenia.
Methodology
Study design and search strategy
To investigate this topic, a systematic literature review of current evidence was conducted. The preferred Reporting Items for Systematic Reviews and the PRISMA checklist was employed to ensure the reliability and validity of reported results. A search from dates of inception up to May 2022 was conducted using the following electronic databases: PubMed, Scopus, Web of Science and Embase.
The search strategy employed combined the terms (‘HIV’[mesh] OR ‘Acquired Immunodeficiency Syndrome’[mesh] OR Acquired Immunodeficiency Syndrome*[tiab] OR Acquired Immune Deficiency Syndrome Virus [tiab] OR Acquired Immunodeficiency Syndrome Virus[tiab] OR Acquired Immunologic Deficiency Syndrome*[tiab] OR Acquired Immune Deficiency Syndrome*[tiab] OR Acquired Immuno‐Deficiency Syndrome*[tiab] OR Acquired Immunodeficiency[tiab] OR HIV[tiab] OR HTLV‐III[tiab] OR Human T Lymphotropic Virus Type III[tiab] OR Human T‐Lymphotropic Virus Type III[tiab] OR Human Immunodeficiency Virus*[tiab] OR Human Immuno deficiency Virus*[tiab] OR Human T Cell Lymphotropic Virus Type III[tiab] OR Human T‐Cell Leukemia Virus Type III[tiab] OR LAV‐HTLV‐III[tiab] OR Lymphadenopathy‐Associated Virus*[tiab] OR Lymphadenopathy Associated Virus[tiab] OR Lymphadenopathy Associated Retrovirus[tiab] OR LAV[tiab] OR LAV (AIDS)[tiab] OR AIDS Virus*[tiab] OR AIDS[tiab] OR AIDS associated lentivirus[tiab] OR AIDS associated retrovirus[tiab] OR AIDS associated virus[tiab] OR AIDS related virus[tiab] OR Immunodeficiency associated virus[tiab]) AND (‘Sarcopenia’[mesh] OR ‘HIV Wasting Syndrome’[mesh] OR ‘Wasting Syndrome’[mesh] OR Sarcopenia*[tiab] OR HIV Wasting Syndrome[tiab] OR Wasting Syndrome*[tiab] OR Wasting Disease*[tiab] OR HIV Wasting Disease[tiab] OR Slim Disease[tiab] OR AIDS Wasting Syndrome[tiab] OR AIDS associated weight loss[tiab] OR Immunodeficiency associated weight loss[tiab]).
Inclusion and exclusion criteria
At first, after the initial search, the original English articles that reported the prevalence, and associated factors of sarcopenia among PLWH were included, and the following articles were excluded:
Abstracts/conference abstracts or articles with unavailable full texts
Ongoing unpublished clinical trials
Review articles, meta‐analyses, protocols, or any other non‐original study
Randomized clinical trials (RCTs) protocols
Articles that had no available original data or values.
Selection of studies and data screening
We utilized EndNote X9 software to organize the extracted articles. The main search results from various aforementioned databases were gathered in a single EndNote library and duplicates were removed. Two independent authors screened the extracted articles in two steps. First, they screened the title and abstract of the retrieved records and then excluded ineligible articles. Then, the full texts of the remaining articles were reviewed according to the inclusion and exclusion criteria and the eligible articles were included in the final results.
Data extraction
The following data were extracted by four independent researchers: first author, country, type of study, study population, purpose of the study, gender percentage, age, race, BMI, medical history, paraclinical results, antiretroviral therapy (ART), the prevalence or rate of sarcopenia and the possible associated promoting or reducing factors. Finally, the extracted findings were organized into a table and for qualitative synthesis.
Quality assessment
The Newcastle–Ottawa Scale (NOS) was used to assess the quality and risk of bias among included studies, and the data are available in Figure 1 and Table 1 . For the cohort studies, there was a maximum score of 9 and for the cross‐sectional studies there was a maximum score of 10, based on three categories that include: selection, comparability and exposure/outcome. 17 Studies with poor‐quality assessment scores of four or less were excluded from this systematic review.
Figure 1.
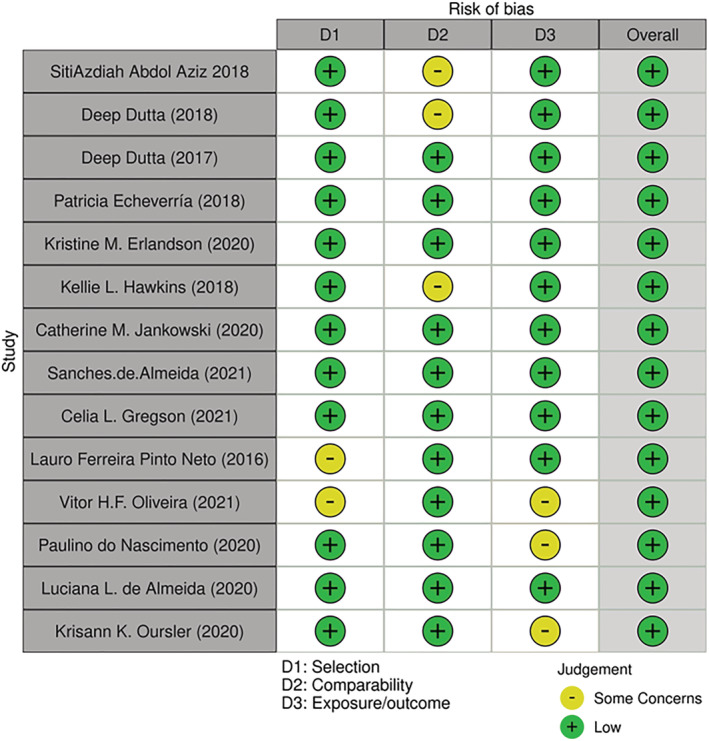
Risk of bias according to Newcastle–Ottawa Scale NOS.
Table 1.
Assessment of study quality using the Newcastle–Ottawa Scale (NOS)
| Cohort studies | ||||
|---|---|---|---|---|
| The first author (reference) | Selection (out of 4) | Comparability (out of 2) | Exposure/outcome (out of 3) | Total score (out of 9) |
| SitiAzdiahAbdol Aziz 12 | *** | * | ** | 7 |
| Deep Dutta 13 | *** | * | *** | 7 |
| Deep Dutta 13 | **** | ** | ** | 8 |
| Patricia Echeverría 14 | *** | ** | *** | 8 |
| Kristine M. Erlandson 15 | *** | ** | ** | 7 |
| Kellie L. Hawkins 16 | *** | * | *** | 7 |
| Catherine M. Jankowski 17 | *** | ** | *** | 8 |
| Cross‐sectional studies | ||||
|---|---|---|---|---|
| The first author (reference) | Selection (out of 5) | Comparability (out of 2) | Exposure/outcome (out of 3) | Total score (out of 10) |
| Sanches.de. Almeida 18 | **** | ** | *** | 9 |
| Celia L. Gregson 19 | **** | ** | *** | 9 |
| Lauro Ferreira Pinto Neto 20 | *** | ** | *** | 8 |
| Vitor H. F. Oliveira 5 | *** | ** | ** | 7 |
| Luciana Caroline Paulino do Nascimento 21 | **** | ** | ** | 8 |
| Luciana L. de Almeida 22 | **** | ** | *** | 9 |
| Krisann K. Oursler 23 | ***** | ** | ** | 9 |
Results
Literature search and study selection
The initial search identified 4875 references from which 2655 duplicate articles were eliminated. The remaining 2220 records were screened based on their title/abstract. Using the inclusion and exclusion criteria, 2206 articles were excluded, and finally, the full texts of 14 articles were reviewed. The PRISMA 2020 flow diagram of article selection process is shown in Figure 2 . A total of 14 articles met the inclusion criteria; hence, they were used in the final qualitative analysis.
Figure 2.

PRISMA 2020 flow diagram of article selection process.
Description of the included studies
The included studies were from United States (n = 5), Brazil (n = 5), India (n = 2), Malaysia (n = 1) and North‐eastern South Africa (n = 1). The studies were cohort studies (n = 7) and cross‐sectional (n = 7) studies. In total, 6 sarcopenia‐defining criteria were mentioned by the included studies, and 13 of 14 studies specified their criteria. The most common utilized criteria were the European Working Group on Sarcopenia in Older People (EWGSOP), which was used by seven articles. 18 , 19 , 20 , 21 , 22 , 23 , 24 The next common method was the measurement of appendicular skeletal muscle mass index (ASMI), which was used by three papers. 14 , 16 , 17 Finally, the Asian Working Group for Sarcopenia (AWGS), percentage skeletal muscle mass (PSMM) and Sarcopenia Definitions and Outcomes Consortium (SDOC) methods were each used by one included study. 12 , 13 , 15
Table 2 provides the descriptive information from the studies that were included in the review. All studies included participants >35 years old with the race of participants being White, Black, Indian, African, Malay, Chinese, Hispanic and non‐Hispanic adults.
Table 2.
Characteristics, sarcopenia prevalence and associated factors in people living with HIV
| ID | Author/Country | Type of study | Study Population | Purpose of the study | Sarcopenia defining criteria | Gender (%) | Associated factors in Sarcopenia | Other findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (mean±SD) | Race (%) | BMI (kg/m2) | Medical History (%) | Paraclinical results | Antiretroviral therapy (%) | prevalence/Rate of sarcopenia | Associated factors | |||||||||
| Promoting | Reducing | |||||||||||||||
| 1 |
SitiAzdiahAbdol Aziz 12 Malaysia |
Cohort | 153 HIV + infected and 153 HIV‐uninfected | Risk factors and health‐related outcomes associated with sarcopenia among treated HIV‐infected individuals, compared with matched uninfected‐ individuals | the Asian Working Group for Sarcopenia (AWGS) |
HIV+: male 83, female 17 HIV‐: male 73, female 27 |
HIV+: 43 HIV‐: 41 |
HIV + and HIV‐:Malay: 19, 26 Chinese: 72, 67 Indian: 9, 7 |
Underweight HIV+: 10% HIV‐: 5% Normal HIV+: 60% HIV‐: 49% Overweight HIV+: 24% HIV‐: 31% Obese HIV+: 6% HIV‐: 15% |
Alcohol drinker HIV+: 45 HIV‐: 41 Smoker HIV+: 29 HIV‐: 14 |
CD4+: 550 cells/ml | 6 years |
HIV+: 10% HIV‐: 6% |
Higher BMI b Higher baseline CD4 + T‐cell count b Longer exposure to D‐drugs b Lower GGT level b |
Higher education level b Being employed b |
No significant difference in S between the two groups. In older individuals (>50 years) prevalence of S in HIV‐infected was significantly higher than HIV‐uninfected. S was associated with an increased mortality risk score and functional disability. |
| 2 |
Sanches.de. Almeida 18 Brazil |
Cross‐sectional |
44 HIV + infected patients (S 11, NS 33) |
Factors related to sarcopenia, correlating their anthropometric and clinical markers in hospitalized PLWH. | EWGSOP |
S: Male: 63 Female:37 NS: Male: 67 Female: 33 |
41.65 ± 12.18 |
Black:(68) White: (31) S: B:64 W:36 NS: B:70 W: 30 |
S: 18.80 ± 3.23 NS: 23.35 ± 4.08 b |
Alcohol drinker S:72 NS:55 Smoker S: 64 NS:39 Illicit drugs S:27 NS:21 Comorbidities S:46 NS:27 a |
CD4+: S:160 b NS:178 |
Proteaseinhibitor S:46 NS:40 ART (years): S:0 NS:0.33 |
25% | N/A |
Calf circumference greater than 31 cm/by 60% b Gait speed greater than 0.8 m/s/by 98% b |
The presence of sarcopenia had a significant correlation with the CD4 cell count. |
| 3 |
Deep Dutta 13 India |
Cohort |
103 HIV+ S:18 NS:85 |
Occurrence, predictors of osteoporosis in premenopausal women with HIV |
Percentage skeletal muscle mass (PSMM) (total LM/weight × 100) of <2 SD below mean in healthy control group was defined as Sarcopenia |
Female 100 |
S:39 NS:35 |
Indian |
22.54 ± 4.04 |
Tuberculosis: S: (48) NS:(35.3) Viral infection: (1) |
CD4+: S:225 NS:181 |
HAART S: (94.5) NS: (90.6) |
17.47% | N/A | N/A | Patients with sarcopenia had significantly lower BMD (lumbar spine and femur), fat mass, bone mineral content, and gynoid fat. |
| 4 |
Deep Dutta 13 India |
Cohort | 115 HIV+ | Evaluated osteoporosis in men with HIV | N/A | Male 100 | 40 | Indian | 21.61 |
Tuberculosis: (35.6) Opportunistic fungal infection: (0.9) Viral infection: (1.74) IRIS:(51.3) |
CD4+: 143 |
HAART: 90.4 |
40% | Osteoporosis b | N/A | N/A |
| 5 |
Patricia Echeverría 14 USA |
Cohort |
860 HIV+ |
Evaluated sarcopenia in HIV patients |
Skeletal muscle mass index (SMI) of <5.5 kg/m2 in women and <7.26 kg/m2 in men |
Male 76 Female 24 |
52 | N/A | 23 | N/A |
CD4+: 552 |
8 years | 25.7% |
Age(30–50 year) b Being female b Time since diagnosis of HIV infection >5 years b Total cholesterol <140 mg/dL b |
Body mass index >20 b | N/A |
| 6 |
Kristine M. Erlandson 15 USA |
Cohort |
HIV+:362 HIV‐:247 |
Evaluation of the performance of novel muscle weakness metrics and association with slowness and falls in older persons with or at risk for HIV infection | Sarcopenia Definitions and Outcomes Consortium (SDOC) |
HIV+: Male 55.3 Female 44.7 HIV‐: Male 65.6 Female 34.4 |
HIV infected Male: 59 ± 5 Female: 50 ± 5 Uninfected: Male: 60 ± 5 Female: 49 ± 6 |
Black: HIV+: 44.6 HIV‐: 38.4 White: HIV+: 47.2 HIV‐: 70 Hispanic ethnicity: HIV+: 12 HIV‐: 11 |
Male HIV+:25.9 ± 4.2 Male HIV‐: 26.6 ± 4.7 Female HIV+: 29.5 ± 6 Female HIV_: 30.7 ± 6.1 |
Smoker: HIV+: 27.3 HIV‐: 19.4 Diabetes mellitus: HIV+: 40.6 HIV‐: 32.8 Hypertension: HIV+: 59.1 HIV‐: 58.3 |
CD4 + T cells < 500 cells/μ in 30.4% of HIV+ |
N/A |
muscle weakness men:16–66% women:0–47% |
N/A | N/A | N/A |
| 7 |
Celia L. Gregson northeastern South Africa |
Cross‐sectional |
HIV+: 163 HIV‐: 642 |
Prevalence of age‐related osteoporosis and sarcopenia, and association between HIV, bone mineral density (BMD), muscle strength and lean mass, and gait speed. |
EWGSOP‐2 |
HIV+: Male 20.8 Female 79.2 HIV‐: Male 34.9 Female 65.1 |
HIV+: Male 46.2 ± 14.3 Female 44.1 ± 11.8 HIV‐: Male 43.8 ± 16.3 Female 45.0 ± 14.9 |
African |
Underweight: HIV+: 4.9% HIV‐: 2.2% Normal: HIV+: 39.2% HIV‐: 32.4 Overweight: HIV+: 30% HIV‐: 27% Obese: HIV+: 26.3% HIV‐: 38.5% |
Smoker: HIV+: 2.4 HIV‐: 3.1 Alcohol: HIV+: 9.8 HIV‐: 8 |
N/A | 88.3% |
HIV+: 4.3% HIV‐: 7.3% |
Osteoporosis b | N/A | N/A |
| 8 |
Kellie L. HAWKINS USA |
Cohort |
HIV+: 199 HIV‐: 200 |
Associations between frailty and measures of body composition among adult men with HIV and without HIV |
ASMI ≤ 7.26 kg/m2 |
Male 100 |
HIV+: 60.1 HIV‐: 60.0 |
White: HIV+: 69 HIV: 79 |
HIV+: 25.3 HIV‐: 25.3 |
Alcohol: HIV+: 8 HIV: 8 Injection drug use: HIV+: 16 HIV‐: 7 Tobacco use: HIV+: 19 HIV‐: 17 Depression (CES‐D ≥ 16): HIV+: 25 HIV‐: 19 HBV or HCV infection: HIV+: 13 HIV‐: 4 Diabetes: HIV+: 17 HIV‐: 10 Kidney disease: HIV+: 30 HIV: 9 Hypertension: HIV+: 62 HIV‐: 52 |
CD4 +: 641 | 12.5 years |
HIV+: 41% HIV‐: 36% |
N/A | N/A | N/A |
| 9 |
Catherine M. Jankowski 13 USA |
Cohort |
HIV+: 27 HIV‐: 28 |
Effects of prescribed exercise on body composition in older PLWH and uninfected controls. |
ASMI/m2 < 7.26 for men and < 5.45 for women |
HIV+: Male 93 Female 7 HIV‐: Male 93 Female 93 |
HIV+: 56 HIV‐: 61 |
White: HIV+: 70 HIV‐: 89 Non‐Hispanic: HIV+: 85 HIV‐: 86 |
HIV+: 26.9 HIV‐: 29.6 |
Smoker: HIV+:15 HIV‐:11 |
CD4 +: 546 | 17 years |
HIV+: 15% HIV‐: 4% |
N/A | N/A | Rate of sarcopenia in HIV + was significantly higher than in HIV‐. |
| 10 |
Lauro Ferreira da Silva Pinto Neto 20 Brazil |
Cross‐sectional |
33 HIV+ 60 HIV‐ |
Evaluation of pre‐sarcopenia and sarcopenia in HIV‐infected individuals compared with healthy elderly adults | EWGSOP |
HIV+: female 42.4%, male 57.6% HIV‐: female 71.7%, male 28.3% |
HIV+: 59 ± 7 b HIV‐: 70 ± 7 b |
N/A |
HIV+: 25 ± 6 HIV‐: 28 ± 6 |
N/A | Undetectable viral load b | Regular use of ART b |
HIV+ PS:12% S:24.2% HIV‐ PS:6.7% S:6.7% |
N/A | N/A | HIV + individuals had a 4.9 times higher risk for sarcopenia compared with HIV‐. In HIV‐ persons only age was significantly related. |
| 11 |
Vitor H.F. Oliveira 24 Brazil |
Cross‐sectional | 302 HIV+ | Compare the prevalence of sarcopenia using two operational definition in PLHIV | EWGSOP‐1 and EWGSOP‐2 |
HIV+: Female 50% Male 50% |
HIV+: 51.7 ± 9.0 |
White 43.5 Multiracial: 35.2 Black: 18.9 Indihenous: 2.3 |
26.4 ± 5.5 | N/A |
CD4+: 173 Viral load: undetectable (75.1%), detectable (24.9%) |
9.5 ± 6.7 | 4.3% | Low Muscle strength b | N/A | N/A |
| 12 |
Luciana Caroline Paulino do Nascimento 21 Brazil |
Cross‐sectional | 99 HIV+ | Sarcopenia prevalence in PLHIV receiving ART | EWGSOP |
Female: 32.3 Male 67.7 |
41 ± 11 | N/A | Undernutrition (8.2%), Eutrophy (43.4%), Overweight (47.5%) | Anaemia (17.2), hypertension (11), DM (6.1) b , CKD (5.1), smoker (19.2), alcoholic (46.9%) |
Viral load: undetectable (57%), detectable (43%) CD4+: ≤350 (19%), >350 (61%) |
48 months |
PS:16% S:18.2% |
Higher CD4+ b | N/A | Sarcopenia significantly mostly observed in elderly population, in patients with DM, and malnourished patients. |
| 13 |
Luciana L. de Almeida 22 Brazil |
Cross‐sectional | 101 HIV+ | Association of sarcopenia with vertebral fractures in PLHIV | EWGSOP |
Female 42.4 Male 57.6 |
57 ± 6 | N/A | 26 ± 2 |
Morphometric vertebral fractures (66.7%), smoking (9%), Alcoholic (9%) |
N/A | 65.3 |
PS: 16.9% S: 12% |
Higher BMI b Lower hip BMD b Elderly ages b Multiple vertebral fractures b |
N/A | N/A |
| 14 |
Krisann K. Oursler 23 USA |
Cross‐sectional | 31 | Assess the association of osteoporosis with low muscle mass in older PLHIV | EWGSOP |
Female (7) Male (93) |
62.1 ± 6.6 | African American (67), Caucasian (32) | 27.6 | N/A | CD4+: 683.9 | Smoking (74), Cocaine‐heroin use (72%), alcoholic (11%), hypertension (46), DM (34), HCV (21) | 13% | Cocaine/heroine use b | N/A | Sarcopenia was significantly higher in African American b |
Abbreviations: BMD, bone mineral density; GGT, gamma‐glutamyl transferase; HAART, highly active antiretroviral therapy; NS, non‐sarcopenia; PS, pre‐sarcopenia; S, sarcopenia.
Systemic arterial hypertension, heart disease, kidney disease, diabetes mellitus, cancer, chronic obstructive pulmonary disease, bronchial asthma and pulmonary tuberculosis.
Indicating positive association with pre‐sarcopenia and sarcopenia.
Sarcopenia prevalence and associated factors
Some studies reported a significantly higher prevalence of sarcopenia in HIV‐infected individuals compared with HIV‐negative controls as follows: 24.2–6.7%, 20 15–4% 17 and 10–6%, 12 respectively. Prevalence of sarcopenia in a cohort study of 153 HIV‐infected individuals and 153 HIV‐negative controls was 10% and 6%, respectively. 12 In a cohort study conducted between 362 HIV‐infected individuals including 53.3% men and 44.7% women; and 247 HIV‐negative controls, including 65.5% men and 34.4% women, the prevalence of sarcopenia in men was higher than women (16–66% vs. 0–47%). 15 The prevalence of sarcopenia in 199 HIV‐infected individuals was slightly higher than 200 HIV‐negative controls, 41% vs 36%. 16 One other study noted a greater discrepancy of approximately 17.5%, among 33 HIV‐infected individuals and 60 HIV‐negative controls (24.2% vs. 6.7%). 20 The difference between the prevalence of sarcopenia between 27 HIV‐infected individuals and 28 HIV‐negative controls was approximately 11% (15% vs. 4%). 17 In a cross‐sectional study, unlike previous studies, the sarcopenia prevalence was higher in HIV‐negative controls (7.3%) compared with HIV‐infected individuals (4.3%). 19
A cohort study conducted among 153 HIV‐positive and 153 HIV‐negative subjects reported higher Body Mass Index (BMI) as a promoting factor in sarcopenia, those who had higher BMI were significantly prone to having sarcopenia. 12 Also, higher baseline cluster of differentiation 4 (CD4+) T‐cell count, longer exposure to D‐drugs and lower gamma‐glutamyl transferase (GGT) level were sarcopenic‐promoting factors; however, they stated that those who have an occupation and higher education were significantly less likely to experience sarcopenia. 12 Another study among 44 HIV‐infected adults reported that a calf circumference >31 cm and a gait speed >0.8 m/s was associated with a lower incidence of sarcopenia by 60% and 98%, respectively. 18 Two studies reported that osteoporosis was associated with sarcopenia among PLWH. Female gender, age between 30 and 50 years, more than 5 years since diagnosis of HIV infection, and total cholesterol <140 mg/dL, and older than 50 years could be promoting factors for sarcopenia in HIV‐infected individual. 14 Furthermore, a cross‐sectional study of 44 HIV‐infected subjects with sarcopenia incidence of 25% showed that the presence of sarcopenia had a significant correlation with the CD4 cell count. 18 A similar finding was reported in a cross‐sectional study conducted among 99 HIV+ individuals with a mean age of 41 ± 11 years and sarcopenia and pre‐sarcopenia incidence of 18.2% and 16%, respectively. 21
Viral load of 57% of HIV infected individuals was undetectable and they were receiving ART for 48 months. Also, sarcopenia was significantly higher in those who were ˃65 years old, had diabetes mellitus and were malnourished. In fact, elderly patients diagnosed with type 2 diabetes had a 30% greater 3‐year decline in muscle strength. Additionally, a higher baseline CD4 + T‐cell count was associated with a greater incidence of sarcopenia. 21 To support this, a study indicated that weight and BMI were significantly lower when there were lower levels of CD4 + T cells and the greatest weight loss occurred when CD4 + T cells counts were lower than 600 cells per millilitre. Low muscle strength was reported as a significant factor of sarcopenia in a study which was conducted among 302 HIV+ individuals with a sarcopenia incidence rate of 4.3%, mean CD4 + count of 173, and 75.1% undetectable viral load. 24 A cross‐sectional study conducted among 101 HIV + individuals with a mean age of 57 ± 6 years and sarcopenia and pre‐sarcopenia rates of 12% and 16.9%, respectively, reported high BMI, lower hip bone mineral density, elderly ages and multiple vertebral fractures as promoting factors for sarcopenia. 22 Cocaine or heroin use was reported as a promoting factors of sarcopenia in a study with 31 HIV + African Americans and Caucasians with a 13% prevalence of sarcopenia and mean CD4 + count of 683.9 cells per millilitre. Also, sarcopenia incidence was significantly higher in African American individuals. 23 Another cross‐sectional study of 33 HIV‐infected persons with 24.2% and 12% prevalence of sarcopenia and pre‐sarcopenia, respectively, reported a strong positive association between the regular use of ART and undetectable viral load with sarcopenia and pre‐sarcopenia. 20
Discussion
This systematic review aimed to ascertain the prevalence of sarcopenia in PLWH as well as factors associated with its promotion and reduction. The introduction of ART has transformed HIV, once perceived as a dreadful infection, into a chronic disease. 25 As ART prolongs the lifespan of an HIV‐infected person, its chronicity may present with a significant decrease in muscle quantity or quality, muscle strength and physical performance, which is otherwise known as sarcopenia. 26 There are limited data concerning the prevalence of sarcopenia in PLWH; even though similar to other chronic diseases, this syndrome seems to occur more frequently and at an earlier age than in the general population. The findings of studies included in the present review indicated a significant prevalence of sarcopenia among HIV‐positive individuals. The regular use of ART, osteoporosis, high BMI, high baseline CD4 + T‐cell count and being female between 30 to 50 years were positively linked to the likelihood of developing sarcopenia in an HIV‐infected patient. Reducing factors associated with sarcopenia in PLWH include a calf circumference >31 cm and being highly educated with good employment status. From an exercise prescription perspective, interventions that improve body composition (reduced fat mass and increased lean body mass), increase muscle strength and decrease systematic inflammation appear to optimize the skeletal muscle quality and general health of PLWH.
Sarcopenia prevalence and promoting factors
The variability of sarcopenia in the general population is well acknowledged; and it is understandable why determining the exact prevalence of sarcopenia in PLWH remains challenging. The variability of sarcopenia prevalence in older adults is attributed to factors such as age, underlying medical conditions, social status and even gender. 26 , 27 , 28 , 29 However, findings from the present review indicate a substantial prevalence of sarcopenia of at least 10% in HIV patients compared with HIV‐negative patients. 12 , 17 , 20 Furthermore, PLWH are 2.2 times more likely to develop fractures than those without HIV. 30 Several studies have shown that HIV‐infected patients may experience significant bone loss throughout the disease; as such, substantial efforts have recently been directed towards preventing and managing bone diseases in PLWH. 31 One underlying reason for the reduced bone health in PLWH might be vitamin D deficiency, which is proven to be more frequent in PLWH due to immune activation, ART exposure and the increased probability of metabolic diseases. 32 Hence, one of the factors associated with sarcopenia is osteoporosis as supported by some studies in this systematic review. 13 , 15 , 17 , 19
Other promoting factors worth discussing include age and female gender. It has been shown that the females who test positive for HIV, compared with males, have an increased risk of developing sarcopenia. This risk has a positive correlation with age and BMI. 12 Cruz‐Jentoft et al. and Pinto Neto et al. further hypothesized that HIV patients with at least 5 years post‐diagnosis of this infectious disease are more prone to developing sarcopenia. 20 , 28 Findings from the Fat Redistribution and Metabolic Change in HIV Infection (FRAM) study indicated that HIV positive men had less skeletal muscle mass compared with individuals without HIV, but women with HIV had a slightly greater skeletal muscle mass than those without HIV. Although, over time, the skeletal muscle loss (i.e., age‐related) was similar irrespective of being HIV positive. 33 Many cross‐sectional studies on the general population have shown that men have larger muscle mass and cross‐sectional area values than women; and the largest muscle cross‐sectional area age‐related changes occurred in men. This liable gender difference in muscle mass loss due to aging may be due to declines in testosterone, growth hormone and insulin‐like growth factor. 34 Further studies are needed to establish a direct gender‐risk relationship with sarcopenia in HIV patients. Nevertheless, HIV patients with a total cholesterol <140 mg/dL, higher baseline CD4 + T‐cell count, and lower GGT levels seem to be more likely to develop sarcopenia. 12 , 14 , 18 , 35 Further, lower GGT levels may not directly be implicated in medical conditions, but its occurrence together with other sarcopenic‐promoting factors may put PLWH at an increased risk of progressing to a sarcopenic state. Hence, higher BMI, CD4 levels, and the duration of ART usage are positively linked to sarcopenia in these patients.
Sarcopenia reducing factors
The level of immune system activation in PLWH is high. Additionally, the incidence of a sedentary lifestyle and high‐risk behaviors is greater in PLWH compared with the general population. Hence, they are more prone to develop sarcopenia at an earlier age compared with the general population. 36 There is no definitive cure for sarcopenia, however, accumulating evidence supports that regular physical activity, in combination with appropriate nutritional support, is the gold standard for improving sarcopenia and preventing disability. 37 A review by Naseeb and Volp indicated that high protein intake (dietary or supplemental), would increase muscle mass, reduce muscle loss, trigger protein synthesis and improve physical performance. 38 Consuming 1.3‐ to 1.4‐g/kg protein per day in healthy older adults is considered effective and safe for improving lean body mass.
In regards to exercise ad physical activities, the WHO 2020 guidelines highlight exercise to be safe and effective for PLWH, but the exercise recommendations for this clinical population are vague and appear to be aligned with the targeted exercise prescription for healthy adults. 39 A systematic review concluded that either aerobic exercise or a combination of aerobic and resistance exercise is safe and can produce positive health and fitness effects in PLWH. Specifically, three sessions per week over a minimum of 5 weeks can lead to improvements in cardiorespiratory fitness, strength, body composition and quality of life in this population. 40 Also, it has been shown that long‐term aerobic exercise lessens muscle strength reduction due to aging. 38 However, due to its anabolic nature progressive resistance training is the most effective form of exercise to simulate muscle growth and is a potent stimulus for augmenting muscle strength. 41 , 42 A more recent meta‐analysis found that combined aerobic and resistance training was the most effective exercise intervention to improve cardiorespiratory fitness and health‐related quality of life. 43 In addition, Ghayomzadeh et al. found that combined training in PLWH increased muscle mass, reduced circulatory levels of pro‐inflammatory cytokines and improve muscle function. 3 Additionally, there were a number of participants with pre‐sarcopenic conditions in the combined training group where this condition was reversed while no change occurred in the control group. Regarding inflammatory biomarkers one meta‐analysis Ibeneme et al. reported that aerobic, resistance or combination exercise two to five times per week can improve cardiopulmonary function; however, they did not find any associated changes in inflammatory biomarkers (IL‐6 and IL‐1β). 44 Some studies have found resistance training to be a better exercise option for muscle mass and strength enhancement; however, careful manipulation of exercise intensity, volume and progression is required to ensure the programme is effective and safe. Details concerning resistance training prescription (e.g., loads, repetitions, sets, number of exercises, etc.) to prevent and manage sarcopenia is comprehensively covered in the review by Law et al. and may be applied cautiously to PLWH. 45 In addition, exercise can be effective for the improvement of some metabolic parameters, especially blood glucose and HDL in PLWH. One systematic review of RCTs stated that combined aerobic and resistance exercise have shown improvements in blood glucose levels, high‐density lipoprotein (HDL), triglyceride (TG) and total cholesterol (TC), while aerobic exercise only reported improvements in HDL and the resistance exercise improved TG, TC, HDL and low‐density lipoprotein (LDL). 46 However, due to methodological issues, small number of studies and differences in exercise protocols, these findings should be interpreted with caution. One other study concluded that PLWH under ART showed elevated levels of advanced glycation end product (a risk factor for cardiovascular diseases, diabetes and other chronic diseases development) when comparing with healthy subjects and physically active HIV patients and found that short‐term moderate‐intensity aerobic exercise counteracts this condition. 47
Anabolic‐androgenic steroids (AAS) use is another plausible treatment that could be used in PLWH because these drugs can increase physical function and muscle mass. 48 In healthy adults, AAS have been shown to produce small increases in muscle strength and moderate increase in lean mass, 49 while in older men with low testosterone similar positive effects have been shown following testosterone replacement therapy. 50 Although, a major obstacle to AAS use in a sarcopenic population are the side effects and general safety. 49 Due to the increase risk of vitamin D deficiency in PLWH, supplementation with vitamin D is a safe and effective therapeutic agent to improve muscle strength in those people with sarcopenia. 51
Our systematic review found several reducing factors for sarcopenia in PLWH. Half of these factors were related to physical activity, both directly and indirectly. A sedentary lifestyle and absence of physical activity are known causes of muscle mass loss, which leads to a further reduction in physical activity. 52 In addition to immunologic factors contributing to the development of sarcopenia in HIV‐positive patients, these individuals are believed to have a more sedentary lifestyle compared with the general population due to the high frequency of risk behaviors. 36 Sarcopenia‐associated alterations in calf circumference and gait speed could be partly explained by the level of activity of the individuals. Additionally, many previous studies have noted that improved muscular function because of regular activity improves body composition and inflammatory state in HIV patients. 4
Our results showed that higher education and employment are among factors associated with a lower incidence of sarcopenia. Previous studies have demonstrated that higher sociodemographic characteristics of patients reduce the risk of developing sarcopenia in the general population. 53 Although this association has not been explained previously, these factors are the primary determinants of an individual's lifestyle (diet, physical activity, medication compliance, etc.). We found that a higher BMI is associated with both increased and decreased risk of sarcopenia development. These mixed results could be due to several reasons, including, different definitions of BMI proposed in the included studies as well as BMI not being are liable index for measuring body composition (i.e., amount of fat relative to muscle mass). It should also be considered that many PLWH suffer from lipoatrophy to different extents caused by ART treatment and the disease itself, which may influence how BMI scores are interpreted.
Due to the inability of BMI to appropriately unveil fat and muscle mass in patients, in clinical practice, body composition is being used to better estimate sarcopenia. An explanation for body composition compared with BMI being a better predictor of sarcopenia is partly explained by the anti‐inflammatory and pro‐inflammatory roles of muscle and fat tissue, respectively. There are various methods to evaluate body composition which rely on using different imaging modalities. Commonly used imaging modalities for body composition analysis include whole‐body dual‐energy X‐ray absorptiometry (dual‐X‐ray absorptiometry [DXA or DEXA]), computed tomography and magnetic resonance imaging. These modalities result in indices representative of whole‐body muscle mass and fat mass. One of the commonly used indices is appendicular lean muscle mass and its derivatives, which is the sum of lean muscle mass of both legs and arms. Although, assessing body composition and its correlation with muscle strength has remained an issue in the prediction of sarcopenia, due to myosteatosis, 54 , 55 however it provides better prognosticating values than BMI.
The European Working Group on Sarcopenia in Older People (EWGSOP) use low muscle strength as the probable identification of sarcopenia (grip strength of <27 kg for males and <16 kg for females), low muscle mass or quality as the confirmatory criterion (ASM/height2 of <7.0 kg/m2 for males and <5.5 kg/m2 for females), and decreased physical function (gait speed of ≤0.8 m/s both for males and females) as an indicator for defining severe sarcopenia. 26 The Asian Working Group for Sarcopenia (AWGS) describe it as low muscle mass (7.0 kg/m2 for men and 5.4 kg/m2 for women via the DXA, and 7.0 kg/m2 for men and 5.7 kg/m2 for women via bio‐impedance analysis), plus low muscle strength (<26 kg for men and <18 kg for women), and/or low physical performance (gait speed of ≤0.8 m/s). 56 The assessment criteria as per the Japan Society of Hepatology (JSH) is low handgrip strength (<26 kg for males and <18 kg for females) and low muscle mass (<7.0 kg/m2 for males and <5.7 kg/m2 for females), respectively. 57 Currently, the criteria used to diagnose sarcopenia are based on older adults, and as such, developing a diagnostic criterion for sarcopenia in PLWH should be considered.
Limitations and general recommendations
This systematic review described the common factors associated with decreased muscle mass in both PLWH and the general population, as well as some factors that appear to be specific to PLWH. Our review provides a substantial global representation from the studies that were analyzed. However, caution is warranted when interpreting the findings from this review due to the low number of studies included and lack of consistency with the type of outcomes reported. The prevalence of sarcopenia reported in the included studies appears to be influenced by various factors such as gender, BMI and the use of ART. It is challenging to analyse and provide an exact prevalence of sarcopenia in PLWH. There is a pressing need for further research on this topic to better understand the prevalence and associated promoting and reducing factors for sarcopenia in PLWH, which may shed light on potential interventions to improve clinical outcomes. Additionally, it is recommended that the sarcopenia be assessed by the operational definition of Baumgartner and the EWGSOP according to gender in future studies. Although, it would be ideal if work is commenced on the development of a diagnostic criteria of sarcopenia for PLWH.
Conclusions
Given the importance and prevalence of sarcopenia among PLWH and its associated consequences (i.e., mortality and disability) determining its risk factors is of great importance. Our study found that age (30–50 years), female gender, >5 years post HIV diagnosis, a total cholesterol level of <140 mg/dL, high BMI, higher baseline CD4 + T‐cell count, longer exposure to drugs and lower GGT level may be promoting factors for sarcopenia in PLWH. Meanwhile; higher education level, employment, calf circumference >31 cm, gait speed >0.8 m/s, and BMI > 20 appeared to be reducing factors for sarcopenia in this population. Combined training (aerobic and resistance exercise) is a safe and effective treatment for sarcopenia in PLWH. In addition, special attention should be given to factors associated with sarcopenia in PLWH that have been identified in the present review.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Acknowledgements
The present study was conducted in collaboration with Khalkhal University of Medical Sciences, Iranian Research Center for HIV/AIDS, Iranian Institute for Reduction of High‐Risk Behaviors, Tehran University of Medical Sciences.
SeyedAlinaghi S. A., Ghayomzadeh M., Mirzapour P., Maroufi S. F., Pashaei Z., Ali Z., et al (2023) A systematic review of sarcopenia prevalence and associated factors in people living with human immunodeficiency virus, Journal of Cachexia, Sarcopenia and Muscle, 14, 1168–1182, 10.1002/jcsm.13212
References
- 1. Iannuzzi‐Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci 2002;57:M772–M777. [DOI] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghayomzadeh M, Hackett D, SeyedAlinaghi S, Gholami M, Hosseini Rouzbahani N, Azevedo VF. Combined training improves the diagnostic measures of sarcopenia and decreases the inflammation in HIV‐infected individuals. J Cachexia Sarcopenia Muscle 2022;13:1024–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonato M, Turrini F, Galli L, Banfi G, Cinque P. The role of physical activity for the management of sarcopenia in people living with HIV. Int J Environ Res Public Health 2020;17:1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oliveira VHF, Borsari AL, Webel AR, Erlandson KM, Deminice R. Sarcopenia in people living with the human immunodeficiency virus: a systematic review and meta‐analysis. Eur J Clin Nutr 2020;74:1009–1021. [DOI] [PubMed] [Google Scholar]
- 6. Talar K, Hernández‐Belmonte A, Vetrovsky T, Steffl M, Kałamacka E, Courel‐Ibáñez J. Benefits of resistance training in early and late stages of frailty and sarcopenia: a systematic review and meta‐analysis of randomized controlled studies. J Clin Med 2021;10:1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guimarães NS, Raposo MA, Greco D, Tupinambás U, Premaor MO. People living with HIV, lean mass, and sarcopenia: a systematic review and meta‐analysis. J Clin Densitom 2021;25:113–123. [DOI] [PubMed] [Google Scholar]
- 8. Mehraeen E, Safdari R, Seyedalinaghi SA, Mohammadzadeh N, Arji G. Identifying and validating requirements of a mobile‐based self‐management system for people living with HIV. Stud Health Technol Inform 2018;248:140–147. [PubMed] [Google Scholar]
- 9. Zanetti HR, Lopes LTP, Gonçalves A, Soares VL, Soares WF, Hernandez AV, et al. Effects of resistance training on muscle strength, body composition and immune‐inflammatory markers in people living with HIV: a systematic review and meta‐analysis of randomized controlled trials. HIV Res Clin Pract 2021;22:119–127. [PubMed] [Google Scholar]
- 10. Soares VL, Soares WF, Zanetti HR, Neves FF, Silva‐Vergara ML, Mendes EL. Daily undulating periodization is more effective than nonperiodized training on maximal strength, aerobic capacity, and TCD4+ cell count in people living with HIV. J Strength Cond Res 2022;36:1738–1748. [DOI] [PubMed] [Google Scholar]
- 11. Mehraeen E, Safdari R, Mohammadzadeh N, Seyedalinaghi SA, Forootan S, Mohraz M. Mobile‐based applications and functionalities for self‐management of people living with HIV. Stud Health Technol Inform 2018;248:172–179. [PubMed] [Google Scholar]
- 12. Abdul Aziz SA, McStea M, Ahmad Bashah NS, Chong ML, Ponnampalavanar S, Syed Omar SF, et al. Assessment of sarcopenia in virally suppressed HIV‐infected Asians receiving treatment. AIDS 2018;32:1025–1034. [DOI] [PubMed] [Google Scholar]
- 13. Dutta D, Garga UC, Gadpayle AK, Bansal R, Anand A, Gaurav K, et al. Occurrence & predictors of osteoporosis & impact of body composition alterations on bone mineral health in asymptomatic pre‐menopausal women with HIV infection. Indian J Med Res 2018;147:484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Echeverría P, Bonjoch A, Puig J, Estany C, Ornelas A, Clotet B, et al. High prevalence of sarcopenia in HIV‐infected individuals. Biomed Res Int 2018;2018:5074923–5074925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Erlandson KM, Travison TG, Zhu H, Magaziner J, Correa‐De‐Araujo R, Cawthon PM, et al. Application of selected muscle strength and body mass cut points for the diagnosis of sarcopenia in men and women with or at risk for HIV infection. J Gerontol Ser A Biol Sci Med Sci 2020;75:1338–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hawkins KL, Zhang L, Ng DK, Althoff KN, Palella FJ Jr, Kingsley LA, et al. Abdominal obesity, sarcopenia, and osteoporosis are associated with frailty in men living with and without HIV. AIDS 2018;32:1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jankowski CM, Mawhinney S, Wilson MP, Campbell TB, Kohrt WM, Schwartz RS, et al. Body composition changes in response to moderate‐ or high‐intensity exercise among older adults with or without HIV infection. J Acquir Immune Defic Syndr 2020;85:340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Almeida TS, Cortez AF, Cruz MR, Almeida VP. Predictors of sarcopenia in young hospitalized patients living with HIV. Braz J Infect Dis 2021;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gregson CL, Madanhire T, Rehman A, Ferrand RA, Cappola AR, Tollman S, et al. Osteoporosis, rather than sarcopenia, is the predominant musculoskeletal disease in a rural South African community where human immunodeficiency virus prevalence is high: a cross‐sectional study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2021;37:244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pinto LFS, Sales MC, Scaramussa ES, da Paz CJC, Morelato RL. Human immunodeficiency virus infection and its association with sarcopenia. Braz J Infect Dis 2016;20:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nascimento LCP, Santos EM, Gomes Silva LL, Sabino Pinho CP. Sarcopenia and consumptive syndrome in HIV‐infected patients receiving antiretroviral therapy in a public hospital in Northeast Brazil. Rev Chil Nutr 2020;47:430–442. [Google Scholar]
- 22. de Almeida LL, Ilha TA, de Carvalho JA, Stein C, Caeran G, Comim FV, et al. Sarcopenia and its association with vertebral fractures in people living with HIV. Calcif Tissue Int 2020;107:249–256. [DOI] [PubMed] [Google Scholar]
- 23. Oursler KK, Iranmanesh A, Jain C, Birkett KL, Briggs BC, Garner DC, et al. Low muscle mass is associated with osteoporosis in older adults living with HIV. AIDS Res Hum Retroviruses 2020;36:300–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oliveira VH, Borsari AL, Cárdenas JDG, Junior CMA, Castro NF, Marinello PC, et al. Low agreement between initial and revised European consensus on definition and diagnosis of sarcopenia applied to people living with HIV. JAIDS J Acquir Immune Defic Syndr 2021;86:e106–e113. [DOI] [PubMed] [Google Scholar]
- 25. Makuku R, Seyedmirzaei H, Tantuoyir MM, Rodríguez‐Román E, Albahash A, Mohamed K, et al. Exploring the application of immunotherapy against HIV infection in the setting of malignancy: a detailed review article. Int Immunopharmacol 2022;105:108580. [DOI] [PubMed] [Google Scholar]
- 26. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu P, Hao Q, Hai S, Wang H, Cao L, Dong B. Sarcopenia as a predictor of all‐cause mortality among community‐dwelling older people: a systematic review and meta‐analysis. Maturitas 2017;103:16–22. [DOI] [PubMed] [Google Scholar]
- 28. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papadopoulou SK. Sarcopenia: a contemporary health problem among older adult populations. Nutrients 2020;12:1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Premaor MO, Compston JE. The hidden burden of fractures in people living with HIV. JBMR Plus 2018;2:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McComsey GA, Tebas P, Shane E, Yin MT, Overton ET, Huang JS, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis 2010;51:937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsieh E, Yin MT. Continued interest and controversy: vitamin D in HIV. Curr HIV/AIDS Rep 2018;15:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yarasheski KE, Scherzer R, Kotler DP, Dobs AS, Tien PC, Lewis CE, et al. Age‐related skeletal muscle decline is similar in HIV‐infected and uninfected individuals. J Gerontol A Biol Sci Med Sci 2011;66:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol (1985) 2003;95:1717–1727. [DOI] [PubMed] [Google Scholar]
- 35. Guimarães NS, Raposo MA, Greco D, Tupinambás U, Premaor MO. People living with HIV, lean mass, and sarcopenia: a systematic review and meta‐analysis. J Clin Densitom 2022;25:113–123. [DOI] [PubMed] [Google Scholar]
- 36. Onen NF, Overton ET, Seyfried W, Stumm ER, Snell M, Mondy K, et al. Aging and HIV infection: a comparison between older HIV‐infected persons and the general population. HIV Clin Trials 2010;11:100–109. [DOI] [PubMed] [Google Scholar]
- 37. Calvani R, Miccheli A, Landi F, Bossola M, Cesari M, Leeuwenburgh C, et al. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J Frailty Aging 2013;2:38–53. [PMC free article] [PubMed] [Google Scholar]
- 38. Naseeb MA, Volpe SL. Protein and exercise in the prevention of sarcopenia and aging. Nutr Res 2017;40:1–20. [DOI] [PubMed] [Google Scholar]
- 39. Bull FC, Al‐Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Brien KK, Tynan AM, Nixon SA, Glazier RH. Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta‐analysis using the Cochrane Collaboration protocol. BMC Infect Dis 2016;16:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grgic J, McLlvenna LC, Fyfe JJ, Sabol F, Bishop DJ, Schoenfeld BJ, et al. Does aerobic training promote the same skeletal muscle hypertrophy as resistance training? A systematic review and meta‐analysis. Sports Med (Auckland, NZ) 2019;49:233–254. [DOI] [PubMed] [Google Scholar]
- 42. McLeod JC, Stokes T, Phillips SM. Resistance exercise training as a primary countermeasure to age‐related chronic disease. Front Physiol 2019;10:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gomes‐Neto M, Saquetto MB, Alves IG, Martinez BP, Vieira JPB, Brites C. Effects of exercise interventions on aerobic capacity and health‐related quality of life in people living with HIV/AIDS: systematic review and network meta‐analysis. Phys Ther 2021;101. [DOI] [PubMed] [Google Scholar]
- 44. Ibeneme SC, Omeje C, Myezwa H, Ezeofor SN, Anieto EM, Irem F, et al. Effects of physical exercises on inflammatory biomarkers and cardiopulmonary function in patients living with HIV: a systematic review with meta‐analysis. BMC Infect Dis 2019;19:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Law TD, Clark LA, Clark BC. Resistance exercise to prevent and manage sarcopenia and dynapenia. Annu Rev Gerontol Geriatr 2016;36:205–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Quiles NN, Piao L, Ortiz A. The effects of exercise on lipid profile and blood glucose levels in people living with HIV: a systematic review of randomized controlled trials. AIDS Care 2020;32:882–889. [DOI] [PubMed] [Google Scholar]
- 47. Rodrigues KL, Borges JP, Lopes GO, Pereira E, Mediano MFF, Farinatti P, et al. Influence of physical exercise on advanced glycation end products levels in patients living with the human immunodeficiency virus. Front Physiol 2018;9:1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Falqueto H, Dos Santos MR, Manfredi LH. Anabolic‐androgenic steroids and exercise training: breaking the myths and dealing with better outcome in sarcopenia. Front Physiol 2022;13:838526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Andrews MA, Magee CD, Combest TM, Allard RJ, Douglas KM. Physical effects of anabolic‐androgenic steroids in healthy exercising adults: a systematic review and meta‐analysis. Curr Sports Med Rep 2018;17:232–241. [DOI] [PubMed] [Google Scholar]
- 50. Parahiba SM, Ribeiro ÉCT, Corrêa C, Bieger P, Perry IS, Souza GC. Effect of testosterone supplementation on sarcopenic components in middle‐aged and elderly men: a systematic review and meta‐analysis. Exp Gerontol 2020;142:111106. [DOI] [PubMed] [Google Scholar]
- 51. Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc 2010;11:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Landi F, Marzetti E, Martone AM, Bernabei R, Onder G. Exercise as a remedy for sarcopenia. Curr Opin Clin Nutr Metab Care 2014;17:25–31. [DOI] [PubMed] [Google Scholar]
- 53. Foley RN, Wang C, Ishani A, Collins AJ, Murray AM. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol 2007;27:279–286. [DOI] [PubMed] [Google Scholar]
- 54. Roh E, Choi KM. Health Consequences of Sarcopenic Obesity: A Narrative Review, Vol. 11; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Borga M, West J, Bell JD, Harvey NC, Romu T, Heymsfield SB, et al. Advanced body composition assessment: from body mass index to body composition profiling. J Invest Med 2018;66:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 57. Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 2016;46:951–963. [DOI] [PubMed] [Google Scholar]


