Abstract
Mast cells play pivotal roles in innate host defenses against venom. Activated mast cells release large amounts of prostaglandin D2 (PGD2). However, the role of PGD2 in such host defense remains unclear. We found that c-kit-dependent and c-kit-independent mast cell–specific hematopoietic prostaglandin D synthase (H-pgds) deficiency significantly exacerbated honey bee venom (BV)–induced hypothermia and increased mortality rates in mice. BV absorption via postcapillary venules in the skin was accelerated upon endothelial barrier disruption resulting in increased plasma venom concentrations. These results suggest that mast cell–derived PGD2 may enhance host defense against BV and save lives by inhibiting BV absorption into circulation.
Keywords: lipid mediator, bee venom, biological defense, vasculature, barrier
Humans and other mammals are often at risk of attack by venomous animals and insects. Honey bee venom (BV) contains several toxic and antigenic components, including melittin and phospholipase A2, that induce mast cell activation. In humans, BV-induced anaphylaxis is often reported after second BV injection, whereas in mice, BV-induced hypothermia occurs after first BV injection experimentally (1, 2).
Mast cells are required for innate defense against venom. Mast cell deficiency impairs resistance to BV and snake venom (1). BV-activated mast cells release heparin, which can alleviate BV-induced decrease in mouse survival rates (2). Mast cell protease 4 and carboxypeptidase A gene deficiency leads to the inhibition of venom component degradation (1, 3).
Activated mast cells also release de novo synthesized mediators, particularly prostaglandin D2 (PGD2). PGD2 synthesized by hematopoietic PGD synthase (H-PGDS) exerts its pathophysiological effects via D prostanoid receptor (DP) 1 and/or chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2). Mast cell–derived PGD2 attenuates vascular hyperpermeability and systemic anaphylactic reactions via DP1 (4). PGD2-DP1 signaling enhances vascular barrier function in vitro (5). These studies suggest that mast cell–derived PGD2 negatively regulates BV-induced anaphylactic reactions. However, the role of PGD2 in the innate defense system against venom remains unclear, which we investigated here using BV and mice.
Results
Subcutaneous BV administration (160 μg × 4 areas) led to a greater significant temperature decline in c-kit mutant mast cell–deficient mice (KitW-sh/W-sh) than in wild-type (WT) mice (Fig. 1A). Almost all the KitW-sh/W-sh mice (4/5) died after 24 h of BV injection, whereas all WT mice survived (Fig. 1E, Dataset 1).
Fig. 1.
Mast cell–derived PGD2 retains bee venom in the skin. (A) Change in body temperature upon subcutaneous injection of BV in WT, KitW-sh/W-sh, KitW-sh/W-sh reconstituted BMMC generated from WT (KitW-sh/W-sh +BMMCWT) or H-pgds−/− (KitW-sh/W-sh +BMMCHpgds−/−) (N = 4 to 6). (B) Change in body temperature upon subcutaneous injection of BV in control (H-pgdsWT) and mast cell–specific H-pgds-deficient mice (H-pgdsΔmast) (N = 7 each). (C) PGD2 levels in ear skin of H-pgdsWT or H-pgdsΔmast mice after 30 min of BV injection (N = 5 to 6). (D) Change in body temperature upon intravenous injection of BV in H-pgdsWT and H-pgdsΔmast mice (N = 4 to 5). (E) The differences in mouse survival rates at 24 h after BV injection were analyzed using Fisher’s exact test (*P < 0.05 vs. WT or H-pgdsWT, †P < 0.05 vs. KitW-sh/W-sh, ‡P < 0.05 vs. KitW-sh/W-sh +BMMCWT). (F–H) Representative pictures (F), change in mean fluorescent intensity (ΔMFI) (N = 5 to 7) (G) and FITC-BV concentration (N = 4 each, at 30 min after injection) (H) in the ear skin of H-pgdsWT and H-pgdsΔmast mice after intradermal injection of FITC-BV. (Scale bar, 100 μm.) (I) Change in ΔMFI in the skin of H-pgdsWT and H-pgdsΔmast mice, which were intradermally injected FITC (N = 3 each). (J) Number of mast cells in mucosa and submucosa of CAE-stained glandular stomach (N = 5) of naive mice. (K and L) Mean density of PECAM-1-positive blood vessels (K) and LYVE1-positive lymph vessels (L) in the ear skin of H-pgdsWT and H-pgdsΔmast naive mice (N = 4 to 6). *P < 0.05. ***P < 0.001 (Tukey’s multiple-comparisons test for A, B, and D; two-tailed unpaired t test for C and G–L).
Mast cell reconstitution using WT BMMCs led to almost complete recovery from hypothermia and improved survival rates in KitW-sh/W-sh mice (Fig. 1 A and E, Dataset 1). In contrast, mice subjected to reconstitution using H-pgds−/− BMMCs showed almost the same hypothermia levels and survival rates as KitW-sh/W-sh mice did.
KitW-sh/W-sh mice exhibit abnormalities such as neutrophilia (6, 7); therefore, we used c-kit-independent mast cell–specific H-pgds-deficient mice (Mcpt5Cre+ H-pgdsfl/fl; hereinafter referred to as H-pgdsΔmast mice). Subcutaneous BV administration (160 to 200 μg × 4 areas) decreased body temperature in the control mice (Mcpt5Cre- H-pgdsfl/fl; hereinafter referred to as H-pgdsWT mice), and mast cell–specific H-pgds deficiency significantly exacerbated the BV-induced temperature decline (Fig. 1B). Almost all the H-pgdsWT mice (6/7) survived after 24 h of BV injection, but many H-pgdsΔmast mice (5/7) died at this time point (Fig. 1E, Dataset 2). BV induced PGD2 production in the skin of H-pgdsWT mice, which was significantly lower in H-pgdsΔmast mice (Fig. 1C). Intravenous administration of BV (80 μg; sublethal dose) resulted in the same hypothermia levels and survival rates in both mouse lines (Fig. 1 D and E, Dataset 3).
BV is absorbed from the skin into the blood and lymph. FITC-BV intensity gradually faded over 30 min in H-pgdsWT mouse skin (Fig. 1 F and G). FITC-positive phagocytes were observed 30 min after FITC-BV injection. In contrast, fluorescence rapidly disappeared within 15 min, and very few FITC-positive phagocytes were observed in H-pgdsΔmast mouse skin. Consistent with these findings, H-pgdsΔmast mouse ear tissue exhibited lower FITC levels than H-pgdsWT levels (Fig. 1H). The intensity of intradermally injected unconjugated FITC did not change after 30 min in mouse ear tissue in both the groups (Fig. 1I). Notably, the number of mast cells in the ear skin of H-pgdsWT and H-pgdsΔmast mice is comparable (4). Here, we confirmed that the number of mast cells in mucosa and submucosa of glandular stomach (Fig. 1J), the densities of platelet endothelial cell adhesion molecule (PECAM)-1-positive blood vessels (Fig. 1K), and lymphatic vessel endothelial hyaluronan receptor (LYVE)-1-positive lymph vessels were comparable between both mouse lines (Fig. 1L).
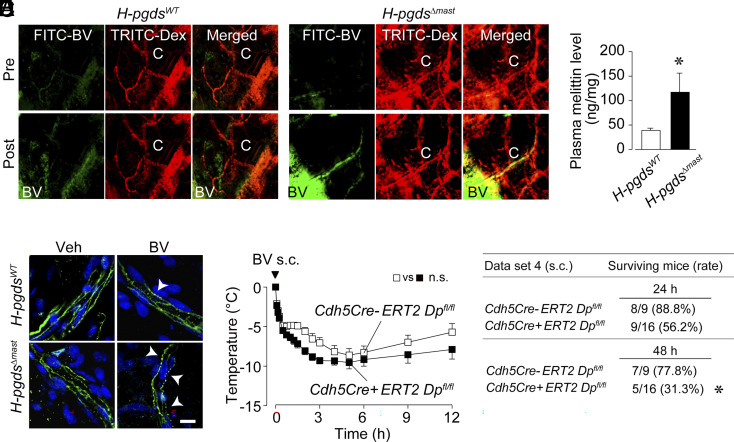
Furthermore, we found that FITC-BV was absorbed into the capillary vessels only in H-pgdsΔmast mice (Fig. 2A, “C” in right lower panels). Consistent with these data, H-pgdsΔmast mice had higher plasma melittin levels than H-pgdsWT mice (Fig. 2B). BV injection disrupted the vascular endothelial (VE)-cadherin line labeled green in both mouse lines (Fig. 2C, arrowheads). This barrier disruption was much more severe in H-pgdsΔmast than in H-pgdsWT mice, suggesting that mast cell–derived PGD2 deficiency enhanced endothelial barrier disruption. Compared with control mice (Cdh5Cre-ERT2 Dpfl/fl), endothelial cell-specific DP1-deficient mice (Cdh5Cre+ERT2 Dpfl/fl) showed a slight increase in body temperature decline (not significant) but significantly low survival rates after subcutaneous BV administration (Fig. 2 D and E, Dataset 4).
Fig. 2.
Mast cell–derived PGD2 reduces BV absorption into blood via postcapillary vessels. (A) Images of ear skin of H-pgdsWT and H-pgdsΔmast mice after 5 min of intradermal injection of FITC-BV. (B) Plasma concentration of melittin in H-pgdsWT and HpgdsΔmast mice (N = 9 to 12) after 30 min of BV injection. (C) Images of VE-cadherin staining (green) of the ear skin of H-pgdsWT and H-pgdsΔmast mice at 30 min after BV injection. Arrowheads mean broken areas of VE-cadherin. Blue represents DAPI. (Scale bar, 10 μm.) (D) Change in body temperature after subcutaneous injection of BV in control (Cdh5Cre-ERT2 Dpfl/fl) and endothelial cell-specific DP1-deficient mice (Cdh5Cre+ERT2 Dpfl/fl) (N = 9 to 16). *P < 0.05. (Tukey’s multiple-comparisons test for D; two-tailed unpaired t test for B). (E) The differences in mouse survival rates at 24 h and 48 h after BV injection were analyzed using Fisher’s exact test.
Discussion
Mast cells release heparin and proteases that contribute to the detoxification of BV (8). Our results suggested that mast cell–derived PGD2 promoted endothelial barrier function via the endothelial DP1 receptor and facilitated the retention of BV in the skin by inhibiting its absorption into circulation. Thus, PGD2 assists in BV digestion mediated by preformed mediators by retaining BV at the local injection site. PGD2 production onset in mast cells has been shown to be delayed by approximately 30 s relative to preformed mediator release (9). This delay seems appropriate for rapid venom digestion and preventing BV absorption into the circulation by the defense system. If humans possess these protective pathways, pharmacological DP1 stimulation may be applicable to bee sting treatment.
IgE-mediated adaptive immunity also protects against lethal BV doses (10). PGD2-CRTH2 signaling enhances BV-specific IgE production and inhibits severe anaphylactic reactions (11). This finding suggests that PGD2 is crucial in establishing effective innate and adaptive immune systems against BV. Consistent with previous findings (2), many FITC-positive phagocytes were observed after FITC-BV injection under PGD2-sufficient conditions (Fig. 1F). The DP1 receptor has been found to inhibit the departure of epidermal dendritic cells from draining lymph nodes (12). Thus, mast cell–derived PGD2 may contribute to effective antigen-specific IgE production and venom component detoxification by retaining BV at the injection site. We speculate that the time lag in the release of mast cell–derived preformed detoxifiers and de novo synthesized restrainers may contribute to dividing the venom component into “toxic” and “antigenic” subcomponents.
Materials and Methods
Detailed procedures are described in SI Appendix. All mice were female on the C57BL/6J background. BV was subcutaneously injected. Rectal temperature and survival rate were measured for several days. In some experiments, BV was intravenously injected. The ears were dissected and subjected to whole-mount staining. PGD2 and melittin concentrations were measured using LC-MS/MS. To visualize mouse ear vessels under a microscope, TRITC-dextran was intravenously injected, and FITC-BV solution was intradermally injected into the mouse ear and was monitored.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This study was supported by the Japan Society for the Promotion of Science and Asahi Group Foundation, Terumo Life Science Foundation, Research Support Program, and Sekisui Chemical Co., Ltd.
Author contributions
Y.F., T.N., and T. Murata designed research; Y.F., T.N., T. Maehara, and A.H. performed research; K.A. and T. Murata contributed new reagents/analytic tools; Y.F., T.N., T. Maehara, and A.H. analyzed data; and Y.F., T.N., and T. Murata wrote the paper.
Competing interests
The authors declare no competing interest.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Metz M., et al. , Mast cells can enhance resistance to snake and honeybee venoms. Science 313, 526–530 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Higginbotham R. D., Karnella S., The significance of the mast cell response to bee venom. J. Immunol. 106, 233–240 (1971). [PubMed] [Google Scholar]
- 3.Akahoshi M., et al. , Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J. Clin. Invest. 121, 4180–4191 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura T., et al. , Mast cell–derived prostaglandin D2 attenuates anaphylactic reactions in mice. J. Allergy Clin. Immunol. 140, 630–632.e9 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi K., et al. , Prostaglandin D2-DP signaling promotes endothelial barrier function via the cAMP/PKA/Tiam1/Rac1 pathway. Arterioscler. Thromb. Vasc. Biol. 33, 565–571 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Grimbaldeston M. A., et al. , Mast cell-deficient W-sash c-kit mutant kitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 167, 835–848 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reber L. L., et al. , Selective ablation of mast cells or basophils reduces peanut-induced anaphylaxis in mice. J. Allergy Clin. Immunol. 132, 881–888.e11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starkl P., et al. , IgE antibodies increase honeybee venom responsiveness and detoxification efficacy of mast cells. Allergy 77, 499–512 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis R. A., et al. , Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J. Immunol. 129, 1627–1631 (1982). [PubMed] [Google Scholar]
- 10.Marichal T., et al. , A beneficial role for immunoglobulin E in host defense against honeybee venom. Immunity 39, 963–975 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kida M., Nakamura T., Fujiwara Y., Nakamura M., Murata T., PGD2/CRTH2 signaling promotes acquired immunity against bee venom by enhancing IgE production. FASEB J. 35, e21616 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Angeli V., et al. , Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J. Exp. Med. 193, 1135–1147 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.