Significance
Inattentional blindness, the inability to notice unexpected objects if attention is focused on a task, is one of the most striking phenomena in cognitive psychology. It is particularly surprising, in light of the research on attentional capture and motion perception, that human observers suffer from this effect even when the unexpected object is moving. Inattentional blindness is commonly interpreted as an inevitable cognitive deficit—the flip side of task focusing. We show that this interpretation is incomplete, as observers can balance the need to focus on task demands with the need to hedge for unexpected but potentially important objects by redeploying attention in response to fast motion. This finding is consistent with the perspective of a fundamentally competent agent who effectively operates in an uncertain world.
Keywords: inattentional blindness, selective attention, endogenous attention, exogenous attention, visual motion
Abstract
It is widely believed that observers can fail to notice clearly visible unattended objects, even if they are moving. Here, we created parametric tasks to test this belief and report the results of three high-powered experiments (total n = 4,493) indicating that this effect is strongly modulated by the speed of the unattended object. Specifically, fast—but not slow—objects are readily noticeable, whether they are attended or not. These results suggest that fast motion serves as a potent exogenous cue that overrides task-focused attention, showing that fast speeds, not long exposure duration or physical salience, strongly diminish inattentional blindness effects.
When human observers are engaged in a task, they tend to not perceive unattended but otherwise salient visual stimuli, a phenomenon dubbed “inattentional blindness” (1, 2). Attention—focusing processing resources on a location, feature, or object in the environment—is a central characteristic of cognition (3). Focusing on relevant information is critical, as dividing attention has deleterious effects on task performance (4–6) outside of detecting basic visual features and textures (7). Hence, inattentional blindness has been interpreted as an inevitable byproduct of task-focused attention, based on the presumption that unattended information remains largely unprocessed. Inattentional blindness has been demonstrated in a large variety of settings, including static (8) and real-world (9) protocols. It has been reported that even unexpected moving stimuli remain largely unnoticed by observers engaged in a demanding task (10–12). This view, that dynamic unexpected stimuli do not override the deployment of task-focused attention, has become widely accepted (13–15).
Contrary to this view, moving stimuli (16) as well as abrupt stimulus onsets (17) and motion onsets (18) are effective in capturing attention. The allocation of attention is not always voluntary. Exogenous attention is involuntary, occurring rapidly and transiently (~80 to 120 ms) in response to sudden stimulus onsets, whereas endogenous attention is voluntary, takes longer to be deployed (~300 ms), and can be sustained in a goal-driven fashion (19–22). The phenomenon of attentional capture is consistent with the well-established view that the primate visual system is highly sensitive to changes over space (23, 24) and time (25, 26), that diverting endogenous attention away from a stimulus merely attenuates, but does not eliminate, sensory processing of that stimulus (27–29) and that goal-directed endogenous and stimulus-driven exogenous attention may compete in dynamic environments (30). Thus, one could expect moving objects to be readily noticed, as they enter and traverse a scene.
Here, we attempt to reconcile the ostensibly conflicting results and interpretations between the literature on inattentional blindness and that on attentional capture, by probing the effect of relative speed on inattentional blindness. We suspect that the dynamic attributes of stimuli used by inattentional blindness researchers were not strong enough to override the endogenous attention deployed to the primary task (10). One way to strengthen the motion signal is to increase the relative speed of the unexpected object. Hence, we hypothesize that fast speeds will override endogenous attention and diminish the strength of inattentional blindness. Note that this hypothesis pertains to fast speeds specifically, not the physical salience of a feature more generally as has been previously suggested.
Results
Study 1: Fast-Moving Gorillas.
In this study, we aimed to test the hypothesis that fast-moving stimuli diminish the strength of inattentional blindness, using an ecologically valid approach (31) that mimics the protocol of Simons & Chabris (10) but also includes faster speeds of the unexpected object.
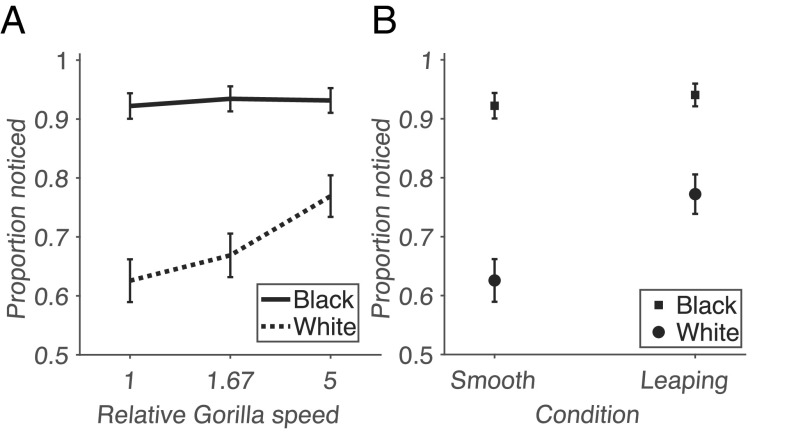
We found that participants who were instructed to count the passes made by the black team were much more likely to detect the unexpected gorilla that entered the scene than those counting the passes made by the white team, replicating the findings of Simons & Chabris (10). There was evidence of a strong effect of deploying endogenous selective attention to passes by the black vs. white team: χ2 (1, N = 1,231) = 104.59, P = 1.50e-24; Cramer’s V = 0.29. To quantify the effect of object speed on detectability, we performed a comparison of the slope of object detection as a function of speed, using a bootstrap analysis. This slope is positive in conditions where participants counted the passes of the white team (P = 0.0024) but indistinguishable from zero in conditions where participants counted the passes of the black team (P = 0.37), see Fig. 1A.
Fig. 1.
Proportion of participants reporting a relevant unexpected event. (A) x-Axis: Relative Gorilla speed in traversing the screen, relative to the longest duration. y-Axis: Proportion reported. Solid line: Participants reporting passes by the Black team. Dashed line: Participants reporting passes by the White team. Error bars indicate SEM. (B) As in (A), but comparing the performance of participants in the smoothly walking vs. leaping gorilla (otherwise time matched—spending 10 s on the screen) condition.
These results indicate that the speed of unexpected objects affects their detection probability, but only for the participants paying attention to the passes of the white team, as the performance of those paying attention to the passes of the black team is high across the board, potentially indicating a ceiling effect.
In addition, we wondered whether the way in which the gorilla moves across the screen affects detection rates. In the original video (10), the gorilla not only moved slowly (in terms of translational motion) but also walked smoothly and upright, like a person. We were wondering whether a gorilla that spends the same overall time on the screen as in the original video (10 s), but moves by leaping, would be more noticeable. We found that this was the case for participants that reported pass counts by the white team (Fig. 1B): χ2 (1, N = 337) = 8.48, P = 0.0036; Cramer’s V = 0.16. Again, there was no evidence for a difference between the two conditions when participants counted the passes made by the black team: χ2 (1, N = 305) = 0.40, P = 0.53; Cramer’s V = 0.036. See Table 1 for a summary of these results.
Table 1.
Results from study 1
| Video Condition | Task Condition | Time on Screen (s) | proportion detected UMO | n detected UMO | Sample size |
|---|---|---|---|---|---|
| Original Gorilla (Simons et al.) | Black | 10 | 0.92 | 142 | 154 |
| NYU Gorilla 1 (Leaping) | Black | 10 | 0.94 | 142 | 151 |
| NYU Gorilla 2 (1.67× speed) | Black | 6 | 0.93 | 128 | 137 |
| NYU Gorilla 3 (5× speed) | Black | 2 | 0.93 | 136 | 146 |
| Original Gorilla (Simons et al.) | White | 10 | 0.63 | 112 | 179 |
| NYU Gorilla (Leaping) | White | 10 | 0.77 | 122 | 158 |
| NYU Gorilla 2 (1.67× speed) | White | 6 | 0.67 | 109 | 163 |
| NYU Gorilla 3 (5× speed) | White | 2 | 0.77 | 110 | 143 |
A plausible account that integrates all of these observations is that it is fast object speeds—be they local or global—that trigger attentional capture.
However, this study has several limitations. First, due to the overwhelming popularity of the original results, it is now hard to recruit truly naive participants. The original paper reported detection rates of 8% (for the white team condition) and 67% (for the black team condition), in contrast to around 60% (for the white team condition) and over 90% (for the black team condition) when we reused the original video.
Moreover, experimental control is hard to maintain in such a naturalistic task. There are many differences between the videos other than the speed of the gorilla and the color of the shirt of the players that might make a difference, e.g., specific passing patterns, visual clutter in the background, and other differences that are hard to match. However, it speaks to the robustness of inattentional blindness effects that we were able to replicate the original findings by Simons & Chabris (10) online, using similar videos.
Study 2: Parametrically Varying the Speed of Unexpected Objects.
The purpose of this study is to address the limitations mentioned above. To do so, we created a parametric version of the task that allows for experimental control of potential confounds. Specifically, we asked participants to count how many randomly moving dots of a given color were crossing a central line during the trial while an unexpected object was traversing the screen at various speeds (see the Materials and Methods section for details).
As in study 1, there was an effect of endogenous attention on the detection performance of unexpected objects. We conceptually replicated Simons & Chabris (10) and Most et al. (11)—participants were much more likely to detect an unexpected moving object (UMO) when counting line crossings of black dots than when counting line crossings of white dots: χ2 (1, N = 1242) = 195.05, P = 2.51e-44; Cramer’s V = 0.40.
The probability that the unexpected object was noticed depended on speed, with faster objects being more likely to be noticed (Fig. 2B). Dividing the data by speed conditions shows that there is an effect of speed on noticeability of the UMO when participants were counting the line crossings of white dots: χ2 (11, N = 632) = 69.38, P = 1.60e-10; Cramer’s V = 0.33, but not when counting the line crossings of black dots: χ2 (11, N = 610) = 18.97, P = 0.062; Cramer’s V = 0.18. As a chi-squared test can only reveal that there is an overall difference as a function of speed grouping, but not if this effect is directional, we performed a bootstrap analysis of the slopes presented in Fig. 2B. We determined that both slopes are significantly positive, as a function of relative speed (black: 0.019, P = 0.0018; white: 0.067, P < 1e-8) and that the slope for the noticeability of UMOs was significantly higher when counting white dot line crossings than when counting the line crossings of black dot crossings (P = 3.72e-5). See Table 2 for a summary of these results.
Fig. 2.
(A) Schematic of task and stimulus display. (B) Proportion of participants reporting a relevant unexpected object, by experimental condition. x-Axis: Speed of object relative to baseline. y-Axis: Proportion reporting noticing the unexpected object. Solid line: Participants reporting dot crossings by black dots. Dashed line: Participants reporting dot crossings by white dots. Error bars indicate SEM.
Table 2.
Results from study 2
| Count Task Color | UMO Relative Speed |
UMO Speed (deg/s) |
UMO on Screen (s) |
Detected UMO (%) |
Detected UMO (n) |
Sample Size |
|---|---|---|---|---|---|---|
| Black | 1 | 1.5 | 6.0 | 81.03 | 47 | 58 |
| Black | 1.75 | 2.63 | 3.43 | 77.50 | 31 | 40 |
| Black | 2 | 3.0 | 3.0 | 82.93 | 34 | 41 |
| Black | 2.42 | 3.63 | 2.48 | 83.33 | 45 | 54 |
| Black | 3 | 4.5 | 2.0 | 86.11 | 31 | 36 |
| Black | 3.5 | 5.25 | 1.71 | 91.89 | 34 | 37 |
| Black | 4 | 6.0 | 1.5 | 94.38 | 84 | 89 |
| Black | 4.58 | 6.87 | 1.31 | 91.11 | 41 | 45 |
| Black | 5.25 | 7.88 | 1.14 | 97.67 | 42 | 43 |
| Black | 6 | 9.0 | 1.0 | 89.66 | 52 | 58 |
| Black | 6.88 | 10.32 | 0.87 | 89.36 | 42 | 47 |
| Black | 8 | 12.0 | 0.75 | 91.94 | 57 | 62 |
| White | 1 | 1.5 | 6.0 | 21.43 | 15 | 70 |
| White | 1.75 | 2.63 | 3.43 | 38.78 | 19 | 49 |
| White | 2 | 3.0 | 3.0 | 29.17 | 14 | 48 |
| White | 2.42 | 3.63 | 2.48 | 44.83 | 26 | 58 |
| White | 3 | 4.5 | 2.0 | 57.69 | 30 | 52 |
| White | 3.5 | 5.25 | 1.71 | 60.53 | 23 | 38 |
| White | 4 | 6.0 | 1.5 | 56.34 | 40 | 71 |
| White | 4.58 | 6.87 | 1.31 | 60.00 | 27 | 45 |
| White | 5.25 | 7.88 | 1.14 | 56.25 | 27 | 48 |
| White | 6 | 9.0 | 1.0 | 60.61 | 20 | 33 |
| White | 6.88 | 10.32 | 0.87 | 72.73 | 40 | 55 |
| White | 8 | 12.0 | 0.75 | 75.38 | 49 | 65 |
Thus, we found that only 21% of participants noticed the UMO of the unattended color if it moved slowly—at the same speed as the dots—but that detection rate of the UMO was strongly modulated by speed such that it was more noticeable at faster speeds.
This is surprising, as the exposure duration of the UMO in the fastest condition is only 1/8 that of the slowest condition—the unexpected object is on the screen for a much shorter duration. Prior reports (32) had suggested that it is longer exposure duration, not faster speeds that increase the probability that a UMO is noticed. However, we attribute this seeming disagreement to technical differences in the experimental design. Specifically, whereas the absolute speed difference in Kreitz et al. (32) was large, the relative speed difference between the task dots and the unexpected object was only 1.6×, due to the fact that their task dots were briskly moving. As speed is represented logarithmically (33), this difference is relatively subtle and indeed consistent with our results—the effect of increased speed seems to be negligible below ~2×. Similarly, a previous report suggests that speed is unimportant in determining the detectability of unexpected objects (34) but a closer inspection of the methods employed reveals that this study only compared “slow” and “slower” conditions and did not have the necessary statistical power to discern a potential effect of speed. Thus, we believe that both of these prior reports are compatible with our findings, as they were confined to a narrow speed regimen of relative—and slow—speeds. We predict that using those protocols with a wide relative speed range that includes high speeds would also yield higher detection probabilities of unexpected objects.
A fundamental limitation of our study consists in the fact that we confounded increased speed with increased dissimilarity. We also did not systematically vary color, so it is possible that this effect might be inherent to black UMOs.
Study 3: Controlling for Effects of Stimulus Similarity.
It is well known that the similarity between targets and distracters plays a critical role in the detection of unexpected objects (11). The faster-moving UMOs in study 2 were less similar to the attended objects. Thus, to establish that the probability of UMO detection is speed dependent, we have to rule out similarity as a parsimonious explanation of the effects in study 2.
In this experiment, we used UMOs that moved both slower and faster than the attended dots. In addition, we addressed the question as to whether the speed dependence is particular to black UMOs by varying the color of the UMO.
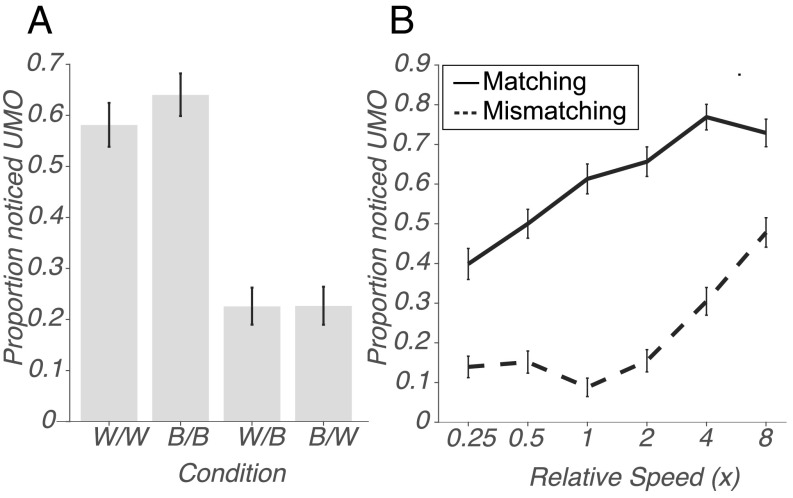
We found that the color of the UMO by itself did not affect its noticeability. Detection rates for white UMOs and black UMOs were statistically indistinguishable for both matching conditions: χ2 (1, N = 1,018) = 3.72, P = 0.54; Cramer’s V = 0.06 and mismatching: χ2 (1, N = 1,002) = 0.0011, P = 0.97; Cramer’s V = 0.010. Thus, we collapsed these 4 conditions into two conditions—matching and mismatching conditions for further analysis. If the color of the unexpected object matched the attended color, detection rates were considerably higher, see Fig. 3A, a statistically significant difference: χ2 (1, N = 2020) = 306.33, P = 1.37e-68; Cramer’s V = 0.39. See Table 3 for a summary of these results.
Fig. 3.
(A) The proportion of participants reporting a relevant unexpected object, by condition. x-Axis: Attention/UMO color conditions—W = White, B = Black. The first letter in each combination represents the UMO color and the second the color of the dots participants were instructed to count. y-Axis: Proportion of participants reporting an unexpected object. (B) The proportion of participants reporting a relevant unexpected object, as a function of speed. x-Axis: Speed of UMO relative to speed of counted dots. y-Axis: Proportion of participants reporting the unexpected object. Solid line: Participants presented with a UMO matching the color of the dots they were instructed to count (i.e., Black/Black and White/White). Dashed line: Participants presented with a UMO of a different color than the dots they were instructed to count (White/Black and Black/White). Error bars indicate 95% CIs in (A) and the SEM in (B).
Table 3.
Results from study 3
| Task Condition | UMO Relative Speed |
UMO Speed (deg/s) |
UMO on Screen (s) |
Detected UMO (%) |
Detected UMO (n) |
Sample size |
|---|---|---|---|---|---|---|
| Matching | 0.25 | 0.45 | 13.33 | 39.87 | 63 | 158 |
| Matching | 0.5 | 0.9 | 6.67 | 50.00 | 95 | 190 |
| Matching | 1 | 1.8 | 3.33 | 61.31 | 103 | 168 |
| Matching | 2 | 3.6 | 1.67 | 65.64 | 107 | 163 |
| Matching | 4 | 7.2 | 0.83 | 76.88 | 133 | 173 |
| Matching | 8 | 14.4 | 0.42 | 72.89 | 121 | 166 |
| Different | 0.25 | 0.45 | 13.33 | 13.94 | 23 | 165 |
| Different | 0.5 | 0.9 | 6.67 | 15.15 | 25 | 165 |
| Different | 1 | 1.8 | 3.33 | 8.78 | 13 | 148 |
| Different | 2 | 3.6 | 1.67 | 15.48 | 26 | 168 |
| Different | 4 | 7.2 | 0.83 | 30.46 | 53 | 174 |
| Different | 8 | 14.4 | 0.42 | 47.80 | 87 | 182 |
Speed, as in studies 1 and 2, was a major determinant of detectability in mismatching conditions (Fig. 3B). Increased speed enhanced the detectability of UMOs in matching as well as mismatching conditions, although the rate at which speed impacted detectability differed between matching versus mismatching conditions. In the matching color condition, detection rates ranged from 40% for ¼ speed through 61% for matching speed to 73% for 8× speed. In the mismatching color condition, detection rates ranged from 14% for ¼ speed through 9% for matching speed to 48% for 8× speed. One interesting difference pertains to the effects of speed in the matching conditions. In study 2, performance in the matching condition was close to ceiling, whereas this task was much harder. Thus, we are able to show an effect of slower speeds on detection, if participants are expecting objects in that color, i.e., in matching conditions. Specifically, slower UMOs are noticed less often. In the mismatching conditions, only high speeds have an effect on detection. Plausibly, attention has to be captured first, if the unexpected object color does not match the feature people are attending to, which happens at faster speeds.
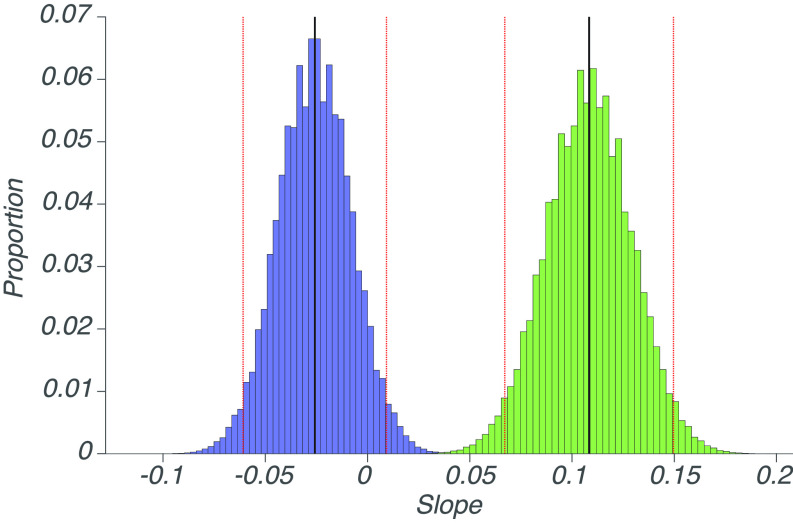
Slower speeds of mismatching UMOs were slightly more noticeable than conditions in which the UMO speed was the same as that of the dots. However, the slope from ¼ to 1 (relative speed) was much shallower than that of from 1 to 4 (relative speed): The exact p value given by a permutation test is 1.65e-5. Beyond mere significance tests, the high statistical power of our experiment also enables us to reliably estimate the respective slopes. Going from equal speed to faster speed, each doubling of the speed increases noticeability rates by 0.108 (±0.041), whereas going from slower speeds to equal speed, each doubling of the speed decreases noticeability rates by 0.026 (±0.035); the uncertainty bound reflects the 95% CI, see Fig. 4.
Fig. 4.
Bootstrap analysis of slopes. Histograms of bootstrapped slopes for slow to similar (blue) and similar to fast (green) conditions. The solid black lines indicate the empirical slopes, whereas the dashed red lines denote the respective 95% CIs.
Precise estimates of these slopes are possible only with a sufficiently large sample. A typical inattentional blindness study uses on the order of 200 participants (35). We subsampled our data, by randomly and repeatedly picking a subset of 200 datapoints and found that the median 95% CI was ±0.125 for fast slopes and ±0.107 for slow slopes, respectively. Thus, estimating slopes with such sample sizes would be far too unreliable to be useful; the CIs are so large that there would have been no evidence for a difference between fast and slow speeds.
We conclude that the speed dependence of UMO detection generalizes to other colors and rule out a confound of study 2, namely the similarity of the UMO to the attended dots at slower speeds. The effect of speed is particularly pronounced in the mismatched condition. At high UMO speeds, a majority of participants notice the unexpected object, a higher proportion than that for the matching condition at slow speeds.
UMO detection rates in study 3 are somewhat lower than in study 2, which we attribute to the fact that the task was harder, as we increased the speed of the dots by 50% and the trial duration was longer. Also, it has been suggested that the quality of the mTurk pool has degraded (36), although this is controversial (37).
UMO detection rates were lowest when the speed of the UMO matched that of the dots, as would be expected from a similarity-based account. However, simple probability summation (38) could underlie this effect, and it is hard to tease apart a similarity from a probability summation account of our data. Simply put, in the slowest condition, the UMO is on the screen four times as long as in the condition where the UMO speed matches that of the background dots, so participants could be expected to be more likely to notice it. Yet, we found strong support for the notion that faster relative speeds enhance detectability much more than slower relative speeds. It is remarkable that this is the case, given how briefly the fastest UMOs are on the screen (1/32 of the time as the slowest UMO – less than half a second). Given the strong effect of speed and the counteracting but weaker effect of exposure duration (32), we predict that a fast-moving UMO that is presented for the same duration as a slow-moving UMO (e.g., by going back and forth) would fully close the detectability gap between matching and mismatching conditions. Thus, if we matched exposure duration across all conditions, extremely fast-moving UMOs would be equally noticeable whether they are attended to or not, in which case speed would completely override the effect of goal-directed attention. Another way in which this gap could be closed might be by using extremely fast UMOs. Alas, this is not practical, given current technology. Most of our participants are running the experiment from LCD screens, with refresh rates in the 50 to 60 Hz range. Extrapolating from our data, a stimulus could be expected to close that gap around a relative speed of 16×. Given the base speed of about 1.8 deg/s and given the spatial frequency composition of the stimuli, the temporal frequencies needed to achieve 16× relative speed would exceed Nyquist frequency (39), given typical refresh rates. In other words, the sampling rate—here the refresh rate of a monitor—has to be more than twice the fastest frequency in the stimulus—here temporal frequency—to avoid distortions of the signal. Consequently, the stimuli might be temporally aliased, and we would not know if any given mTurk participant was seeing flicker or the actual stimulus. Thus, we recommend follow-up studies aimed at determining whether fast relative speeds can fully overcome the effects of attentional set to be performed in a laboratory setting using displays with sufficiently high refresh rates, around 120 Hz or higher (40). The primate motion detection system extends well into this speed range—well suited to detect stimuli moving at 32 deg/s, and beyond (33).
Discussion
It has been suggested that unexpected objects are hard to notice if attention is otherwise engaged, even if they are moving (10, 11). This has become the prevalent view in the field and attributed as a key factor in “looked but failed to see” events in sports (41) and traffic accidents (42). Our results indicate that unexpected moving objects (UMOs) are indeed not readily noticeable if attention is focused on a task. However, this effect strongly depends on the speed of the UMO: Faster UMOs strongly increase the probability that the UMO is seen.
This is interesting for several reasons. First, prior research suggested that top–down factors (attentional set) determine inattentional blindness effects (12). This is a report of a bottom–up factor that strongly influences the strength of inattentional blindness without obvious confounds. There have been other investigations on the impact of physical salience on inattentional blindness, specifically flicker (43), which predicted that faster speeds would be less noticeable, but the lack of statistical power makes these findings hard to interpret. Second, it has been claimed that exposure duration, not speed, matters for the detectability of unexpected dynamic events (32). We attribute this discrepancy to the fact that Kreitz et al. used only a rather narrow range of relative speeds, diminishing the apparent effect of speed. In addition, our results dovetail nicely with recent studies suggesting that motion onsets, not probability summation drive detectability of UMOs (44). Third, consistent with our findings, motion onsets have been noted as potent cues to capture attention (45). However, much of the existing literature on attentional capture relies on the “irrelevant feature search task” (17), which utilizes characters or digits, many trials, without a competing primary task, in contrast to the tasks used in inattentional blindness research, making a direct comparison of these findings difficult. Our research links these research traditions, juxtaposing endogenous and exogenous attention in an inattentional blindness task, which has been previously attempted for a change blindness task (46). Importantly, our findings also contribute to the ongoing debate on the impact of physical salience on inattentional blindness (47), suggesting that it is fast speeds specifically, not the physical salience of a feature more generally, that captures attention, as has been previously suggested (48).
The most important implication of our findings pertains to the theoretical interpretation of inattentional blindness. A typical ecological situation demands much of the attentional deployment systems of the organism (49). On the one hand, it behooves the organism to stay focused on a task, as research on selective attention overwhelmingly shows the benefits of concentrating processing resources in an undivided fashion. In other words, it is important that the focus of attention is maintained in the face of many potential diversions, including dynamic distractions (50). On the other hand, such intense focus would lead to the virtual exclusion of unattended stimuli from processing, commonly dubbed inattentional blindness. It has been suggested that inattentional blindness is an inevitable byproduct of focusing attention on a task, rendering the appearance of unexpected objects unnoticed, even if they are relevant or salient (51). As a consequence, unexpected novel objects would go unnoticed, even if they are more relevant. Thus, such a system would leave the observer in a vulnerable position. The conventionally offered resolution to this conundrum—that such situations are just not very common (52)—is not compelling, as even rare events can have a disproportionate impact (53). Rapid dynamic events are highly likely to have such an impact. For instance, a single missed predator could bring about the demise of the organism. An agent needs to focus on the task without falling prey to distractions, but at the same time do this task without falling prey to unexpected predators.
We propose an alternative, and experimentally testable, hypothesis that any stimulus quality that is both potentially relevant to the survival of a particular organism and that strongly violates the expectations from the stimulus statistics of the environment will lead to an override of endogenous attention. It has been suggested that a priority map determines the order in which attention is deployed to process the most relevant sensory inputs (54). But organisms face a fundamentally uncertain environment, so it is not a priori obvious which information is most relevant at any given moment. It is not implausible that motion signals may contribute to reordering priority maps (55). The primate brain contains dedicated pathways for processing moving stimuli and for leveraging this information to detect and interpret dynamic changes in the environment (56, 57). For instance, motion is effective in breaking camouflage (58–61), motion enhances conspicuity (62), motion can be a cue for animacy (63), and organisms—their actions and intentions—can be recognized from their motion signature alone (64, 65). It makes good evolutionary sense to update priority maps when moving objects are detected, as they could indicate the presence of another organism—predator, prey, or conspecific—that has to be reckoned with, assigning them a higher priority. Slow speeds are much more common in the environment than fast speeds (66), so—to prevent false positives—this reprioritization and override of task-focused attention should mostly happen for fast, but not slow, motion. A plausible computational mechanism for detecting fast speeds is that high-speed camouflage-breaking motion generates a motion streak signal, whereas low-speed motion does not (67). Based on this hypothesis, we predict that inattentional blindness is preserved in other species for slow—but not fast—moving objects. Indeed, any sensory stimulus that strongly violates biologically significant environmental statistics experienced by an organism—such as stimuli that resemble ecological threats (68, 69) will tip the balance between exogenous and endogenous attention. Note that not any generic threat display will do (70).
Taken together, our findings reconcile and rebalance multiple perspectives on attention. Most task performance is enhanced by focusing on task-relevant information. However, this leads to inattentional blindness: To maintain attentional focus, many events in the environment go unnoticed. Irrelevant stimuli should not break this focus, but without the possibility of an override, an observer might be missing out on more relevant information than the task they are currently engaged in. Focusing attention while allowing for its redeployment in response to fast motion represents an elegant solution to this challenge, as it achieves both at the same time, allowing observers to maintain situational awareness in an uncertain environment.
Materials and Methods
Participants.
We recruited participants on the Amazon Mechanical Turk platform. All research participants were randomly assigned to experimental conditions. We excluded participants that reported suspecting that the study was about inattentional blindness, those who reported being already familiar with inattentional blindness tasks, those who reported technical problems during stimulus presentation as well as those who reported having ADHD, those who reported taking medication to treat ADHD, and those who reported not doing the task.
In addition, we excluded data from participants that exhibited a task performance that was implausibly low for participants who engaged with the task in good faith. The cutoff for acceptable task performance was determined by visual inspection of the distribution of count errors. We reasoned that count errors in the long tail of the distribution reflect nonengagement in the counting task, so we placed the cutoff where that long tail begins. The specific cutoff is ultimately arbitrary, but we reanalyzed the data with a broad range of such cutoffs and the results support the same conclusions. In general, we aimed for a tradeoff between retaining as much data as possible and only excluding people who were extremely unlikely to have been engaged in the task. Retaining as much as data as possible is important to avoid statistical artifacts in this task in particular (71). Using these criteria, we retained 1,231 out of 1,624 participants for further analysis in study 1, 1,242 out of 1,487 participants in study 2, and 2,020 out of 2,800 participants in study 3 (for details, see Table 4). None of our conclusions depend on whether we included all participants or adopt even more stringent criteria (e.g., only keeping data from participants with perfect pass counts).
Table 4.
Exclusion criteria and outcomes across all studies
| Exclusion rule | Experiment 1 (n, %) | Experiment 2 (n, %) | Experiment 3 (n, %) | Totals (n, %) |
|---|---|---|---|---|
| Reported low video quality or other technical problems | 8 (0.53%) | 313 (11.7%) | 10 (0.6%) | 331 (5.6%) |
| Reported to be already familiar with the task | 133 (8.94%) | 281 (10.03%) | 149 (9.17%) | 563 (9.52%) |
| Reported having ADD or taking ADD medication | 83 (6%) | 578 (20.63%) | 165 (10%) | 826 (13.97%) |
| Inadequate count performance | 50 (3.36%) | 453 (16.17%) | 88 (6.67%) | 591 (10.0%) |
| Reported not paying attention | 3 (0.20%) | 31 (10.03%) | 17 (1%) | 51 (0.86%) |
| Missing responses or videos | N/A | N/A | 55 (3.4%) | 55 (0.65%) |
| Total recruited | 1,487 | 2,800 | 1,624 | 5,911 |
| Total retained | 1,242 (84%) | 2,020 (72%) | 1,231 (76%) | 4,493 (76%) |
| Total excluded | 245 (16%) | 780 (28%) | 393 (24%) | 1,562 (24%) |
All research participants provided informed consent prior to the beginning of the experiment, in accordance with the principles expressed in the Declaration of Helsinki. All procedures were approved by the University Committee on Activities Involving Human Subjects (UCAIHS) at NYU.
Stimuli and Task.
For study 1, we used 4 videos as stimuli. One was the original “gorilla” video used by Simons & Chabris (10). In this video, two teams of 3 “basketball players”, one wearing white shirts (“the white team) and one wearing black shirts (“the black team”), move about in front of elevators and pass basketballs to each other. At some time around the middle of the video, someone wearing a gorilla suit enters the screen from the right then stops in the middle before leaving the screen on the left, 10 s after entering. In the other three videos, we replicated this setup in front of elevators at NYU. In two of those videos, the gorilla takes 6 and 2 s to traverse the scene. In the remaining video, the gorilla takes 10 s to traverse the screen—as in the original video—but instead of walking, the gorilla moves in a more biologically appropriate fashion, i.e., in leaps. Participants were instructed to count either the number of passes made by the team wearing black shirts (“black team”) or the passes made by the team wearing white shirts (“white team”). This yields eight experimental conditions (four videos × two task conditions), to which participants were assigned randomly. Importantly, each participant performed only a single trial, in a “one shot” protocol. The rationale for this is that once someone saw the unexpected stimulus, it is no longer an unexpected stimulus, so the nature of the task would be transformed to a visual search task in subsequent trials. The videos themselves were hosted on Youtube, so as to ensure smoothness, but with disabled video controls (i.e., participants could not rewind or pause the video).
For study 2, we asked participants to provide screen parameters (specifically screen size and aspect ratio) to ensure that the stimulus appeared in a similar fashion on a wide variety of screens, prior to starting the task. In addition, we asked participants to maintain a viewing distance of 2 feet from the screen, consistent with the preferred (72) and recommended (73) ergonomic viewing distance. Assuming this viewing distance, we created a stimulus display where circular dots (each subtending 0.03 degrees of visual angle) moved linearly in a random direction at a speed of 1.2 degrees of visual angle per second on a gray background. The direction of motion of each dot was idiosyncratic to that dot. At any given time, 10 of these dots (five black and five white) were present on the screen. When a dot exited the square “play area” in the center of screen, it was immediately replaced with one of the same color starting at a random position, moving in a random direction within the play area. The screen was divided in the middle by a vertical green line. This stimulus was displayed for 28 s. Halfway through the stimulus display, an “unexpected moving object” (UMO)—a black (same hue as the black dots) isosceles triangle that covered roughly the same area as an individual moving dot—entered the play area on the right edge and traversed it in the center (bisecting the vertical green line in the middle) before vanishing on the left side (Fig. 1A). The UMO moved at a speed that was randomly chosen from the following 12 speed conditions: [1.0, 1.75, 2.0, 2.42, 3.0, 3.5, 4.0, 4.58, 5.25, 6.0, 6.88, 8.0]× relative to a baseline speed of 1.5 deg/s, so the UMO moved minimally at 1.5 deg/s and maximally at 12 deg/s. This log spacing of conditions is inspired by the logarithmic nature of speed perception in the primate visual system [Nover et al., (33)]. For details of this stimulus display, see Table 5. This stimulus display was created using MGL (Gardner Laboratory, Neuroscience Institute, Stanford University, 2014) with MATLAB (MATLAB R2013a, The Mathworks Inc., 2013). MGL is a MATLAB library designed for displaying full screen psychophysical stimuli. As MGL is not executable online, we created a library of functions—JGL—that consists of a JavaScript version of the corresponding MGL functions, allowing for online deployment of MGL code. Participants were instructed to count the number of times the dots crossed the green vertical line. Each participant was assigned to one of two color conditions (counting crossings by either white or black dots only) and one of the 12 UMO speed conditions detailed above, yielding 24 (12 × 2) experimental conditions. As in study 1, each participant performed only a single trial where an unexpected object entered the display. Before this trial, participants were exposed to a trial with a duration of 20 s, where they were also asked to count the number of crossings, but no UMO entered the screen. The purpose of this practice run was to familiarize participants with the task and to put them at ease; nothing unexpected happened during this trial.
Table 5.
Stimulus parameters for studies 2 and 3
| Stimulus Parameter | Experiment 2 | Experiment 3 |
|---|---|---|
| Dot speed (deg/s) | 1.2 | 1.8 |
| Dot area (deg2) | 0.03 | 0.05 |
| UMO area (deg2) | 0.05 | 0.05 |
| Dot play area (deg2) | 16 | 16 |
| UMO path length (deg) | 9 | 6 |
For study 3, we modified the stimulus display as detailed above. Importantly, we extended the color conditions to both black and white UMOs, fully crossed with instructions on whether to count black or white dots. Participants were assigned to one of these 4 conditions, where they would be asked to count the dots of a target color (black or white) and could encounter one of two UMOs (black or white). In addition, we used a relative speed range that included slower as well as faster speeds. Specifically, there were six speed conditions: UMO moving at [¼ ½ 1 2 4 8]× relative to the speed of the dots. The six speed conditions were fully crossed with the UMO color and attention conditions, yielding 2 × 2 × 6 = 24 experimental conditions. To accommodate the slower speed conditions, allowing the UMO to fully traverse the screen in a reasonable time, we had to increase the speed of the dots to 1.8 degrees of visual angle per second and we extended the overall stimulus duration (how long the dots moved on the screen) to 33 s.
Measures and Analysis
After the stimulus display, participants were asked to report how many passes they counted/how many times the dots crossed the central green line, for study 1 and studies 2/3, respectively.
We then asked whether they noticed anything unexpected and if so, what. Responses to the latter question were independently coded by two raters. These raters were blind as to the experimental conditions that the participants were assigned to. Raters judged whether the response of the participant indicated that the participant noticed the unexpected moving object (a “gorilla” in study 1 and a “spaceship” in studies 2 and 3) or not (the interrater agreement was 0.99). The data were recorded in a sql-lite database via Psiturk (74).
We used chi-squared tests to assess the statistical significance of detection rates between different experimental conditions. To assess the statistical reliability of slope differences, we employed bootstrap resampling, using 100 million runs each (75). To correct for multiple comparisons, we adopted a more conservative significance threshold of 0.005 (76) throughout. All data in this study were analyzed using MATLAB (Mathworks, Inc.).
Acknowledgments
We thank Tuvia Lerea for creating the JGL environment to run the experiment online. We thank Marisa Carrasco for comments on the manuscript. We thank Dan Grossman for coding participant responses and are grateful to the NSF for supporting this project with grant DGE 1342536 to W.E.M.
Author contributions
P.W., W.E.M., and D.J.H. designed research; P.W. and M.W.K. performed research; P.W. and M.W.K. analyzed data; and P.W., W.E.M., M.W.K., and D.J.H. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: J.M.W., Brigham & Women’s Hospital/Harvard Medical School; F.P., The University of Texas at Austin; and M.R., Barnard College Department of Psychology.
Contributor Information
Pascal Wallisch, Email: pascal.wallisch@nyu.edu.
David J. Heeger, Email: david.heeger@nyu.edu.
Data, Materials, and Software Availability
Human behavioral data have been deposited in [OSFHome] (DOI 10.17605/OSF.IO/GPY6J) (77).
References
- 1.Rock I., Linnett C. M., Grant P., Mack A., Perception without attention: Results of a new method. Cogn. Psychol. 24, 502–534 (1992). [DOI] [PubMed] [Google Scholar]
- 2.Mack A., Rock I., Inattentional Blindness, MIT Press/Bradford Books Series in Cognitive Psychology (MIT Press, Cambridge, MA, 1998), p. 273. [Google Scholar]
- 3.James W., The Principles of Psychology (Henry Holt and Co., Inc, New York, NY, 1890), vol. I. [Google Scholar]
- 4.Pashler H., Attentional limitations in doing two tasks at the same time. Curr. Dir. Psychol. Sci. 1, 44–48 (1992). [Google Scholar]
- 5.Pashler H., Dual-task interference in simple tasks: Data and theory. Psychol. Bull 116, 220–244 (1994). [DOI] [PubMed] [Google Scholar]
- 6.Strayer D. L., Drews F. A., Crouch D. J., A comparison of the cell phone driver and the drunk driver. Hum. Factors 48, 381–391 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Jigo M., Heeger D. J., Carrasco M., An image-computable model of how endogenous and exogenous attention differentially alter visual perception. Proc. Natl. Acad. Sci. U.S.A. 118, e2106436118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koivisto M., Hyona J., Revonsuo A., The effects of eye movements, spatial attention, and stimulus features on inattentional blindness. Vision Res. 44, 3211–3221 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Chabris C. F., Weinberger A., Fontaine M., Simons D. J., You do not talk about Fight Club if you do not notice Fight Club: Inattentional blindness for a simulated real-world assault. Iperception 2, 150–153 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons D. J., Chabris C. F., Gorillas in our midst: Sustained inattentional blindness for dynamic events. Perception 28, 1059–1074 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Most S. B., et al. , How not to be seen: The contribution of similarity and selective ignoring to sustained inattentional blindness. Psychol. Sci. 12, 9–17 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Most S. B., Scholl B. J., Clifford E. R., Simons D. J., What you see is what you set: Sustained inattentional blindness and the capture of awareness. Psychol. Rev. 112, 217–242 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Galotti K. M., Cognitive Psychology In and Out of the Laboratory (SAGE Publications, Thousand Oaks, CA, ed. 5, 2014), p. 475. [Google Scholar]
- 14.Goldstein E. B., Brockmole J. R., Sensation and Perception (Cengage Learning, Australia, Boston, MA, ed. 10, 2017), p. 460. [Google Scholar]
- 15.Wolfe J. M., Kosovicheva A., Wolfe B., Normal blindness: When we Look But Fail To See. Trends Cogn. Sci. 26, 809–819 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franconeri S. L., Simons D. J., Moving and looming stimuli capture attention. Percept. Psychophys. 65, 999–1010 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Yantis S., Jonides J., Abrupt visual onsets and selective attention: Evidence from visual search. J. Exp. Psychol. Hum. Percept. Perform. 10, 601–621 (1984). [DOI] [PubMed] [Google Scholar]
- 18.Hillstrom A. P., Yantis S., Visual motion and attentional capture. Percept. Psychophys. 55, 399–411 (1994). [DOI] [PubMed] [Google Scholar]
- 19.Ling S., Carrasco M., Sustained and transient covert attention enhance the signal via different contrast response functions. Vision Res. 46, 1210–1220 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller H. J., Rabbitt P. M., Reflexive and voluntary orienting of visual attention: Time course of activation and resistance to interruption. J. Exp. Psychol. Hum. Percept. Perform. 15, 315–330 (1989). [DOI] [PubMed] [Google Scholar]
- 21.Nakayama K., Mackeben M., Sustained and transient components of focal visual attention. Vision Res. 29, 1631–1647 (1989). [DOI] [PubMed] [Google Scholar]
- 22.Carrasco M., Visual attention: The past 25 years. Vision Res. 51, 1484–1525 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barlow H. B., Summation and inhibition in the frog’s retina. J. Physiol. 119, 69–88 (1953). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enroth-Cugell C., Robson J. G., The contrast sensitivity of retinal ganglion cells of the cat. J. Physiol. 187, 517–552 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller J. R., Metha A. B., Krauskopf J., Lennie P., Rapid adaptation in visual cortex to the structure of images. Science 285, 1405–1408 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Holcombe A. O., Seeing slow and seeing fast: Two limits on perception. Trends Cogn. Sci. 13, 216–221 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Herrmann K., Montaser-Kouhsari L., Carrasco M., Heeger D. J., When size matters: Attention affects performance by contrast or response gain. Nat. Neurosci. 13, 1554–1559 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pestilli F., Carrasco M., Heeger D. J., Gardner J. L., Attentional enhancement via selection and pooling of early sensory responses in human visual cortex. Neuron 72, 832–846 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maunsell J. H. R., Neuronal mechanisms of visual attention. Annu. Rev. Vis. Sci. 1, 373–391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grubb M. A., White A. L., Heeger D. J., Carrasco M., Interactions between voluntary and involuntary attention modulate the quality and temporal dynamics of visual processing. Psychon. Bull. Rev. 22, 437–444 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redlich D., Memmert D., Kreitz C., A systematic overview of methods, their limitations, and their opportunities to investigate inattentional blindness. Appl. Cogn. Psychol. 35, 136–147 (2021). [Google Scholar]
- 32.Kreitz C., Furley P., Memmert D., Inattentional blindness is influenced by exposure time not motion speed. Q. J. Exp. Psychol. 69, 495–505 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Nover H., Anderson C. H., DeAngelis G. C., A logarithmic, scale-invariant representation of speed in macaque middle temporal area accounts for speed discrimination performance. J. Neurosci. 25, 10049–10060 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beanland V., Pammer K., Looking without seeing or seeing without looking? Eye movements in sustained inattentional blindness. Vision Res. 50, 977–988 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Matias J., Belletier C., Izaute M., Lutz M., Silvert L., The role of perceptual and cognitive load on inattentional blindness: A systematic review and three meta-analyses. Q. J. Exp. Psychol. 75, 1844–1875 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Kennedy R., et al. , The shape of and solutions to the MTurk quality crisis. Polit. Sci. Res. Methods 8, 614–629 (2020). [Google Scholar]
- 37.Johnson D., Ryan J. B., Amazon mechanical turk workers can provide consistent and economically meaningful data. South. Econ. J. 87, 369–385 (2020). [Google Scholar]
- 38.Verghese P., Watamaniuk S. N., McKee S. P., Grzywacz N. M., Local motion detectors cannot account for the detectability of an extended trajectory in noise. Vision Res. 39, 19–30 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Nyquist H., Certain topics in telegraph transmission theory. Trans. Am. Inst. Electr. Eng. 47, 617–644 (1928). [Google Scholar]
- 40.Poth C. H., et al. , Ultrahigh temporal resolution of visual presentation using gaming monitors and G-Sync. Behav. Res. Methods 50, 26–38 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Memmert D., Furley P., “I spy with my little eye!”: Breadth of attention, inattentional blindness, and tactical decision making in team sports. J. Sport Exerc. Psychol. 29, 365–381 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Pammer K., Sabadas S., Lentern S., Allocating attention to detect motorcycles: The role of inattentional blindness. Hum. Factors 60, 5–19 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Palmer D. B., Yamani Y., Bobrow T. L., Karpinsky N. D., Krusienski D. J., Transient signals and inattentional blindness in a multi-object tracking task. Iperception 9, 2041669518754595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood K., Simons D. J., Now or never: Noticing occurs early in sustained inattentional blindness. R. Soc. Open Sci. 6, 191333 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abrams R. A., Christ S. E., Motion onset captures attention. Psychol. Sci. 14, 427–432 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Smith T. J., Lamont P., Henderson J. M., Change blindness in a dynamic scene due to endogenous override of exogenous attentional cues. Perception 42, 884–886 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Anderson B. A., et al. , The past, present, and future of selection history. Neurosci. Biobehav. Rev. 130, 326–350 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Theeuwes J., Stimulus-driven capture and attentional set: Selective search for color and visual abrupt onsets. J. Exp. Psychol. Hum. Percept. Perform. 20, 799–806 (1994). [DOI] [PubMed] [Google Scholar]
- 49.Sunquist M., Sunquist F., Gittleman J., “Carnivore behavior, ecology, and evolution” in Ecological Constraints on Predation by Large Felids (1989), 283–301. [Google Scholar]
- 50.Peters R. A., Hemmi J. M., Zeil J., Signaling against the wind: Modifying motion-signal structure in response to increased noise. Curr. Biol. 17, 1231–1234 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Simons D. J., Attentional capture and inattentional blindness. Trends Cogn. Sci. 4, 147–155 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Chabris C. F., Simons D. J., The Invisible Gorilla: And Other Ways Our Intuitions Deceive Us (Crown, New York, NY, ed. 1, 2010), p. 306. [Google Scholar]
- 53.Taleb N. N., The Black Swan: The Impact of the Highly Improbable (Random House, New York, NY, ed. 1, 2007), p. 366. [Google Scholar]
- 54.Fecteau J. H., Munoz D. P., Salience, relevance, and firing: A priority map for target selection. Trends Cogn. Sci. 10, 382–390 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Yantis S., Johnson D. N., Mechanisms of attentional priority. J. Exp. Psychol. Hum. Percept. Perform. 16, 812 (1990). [DOI] [PubMed] [Google Scholar]
- 56.Grossman E., et al. , Brain areas involved in perception of biological motion. J. Cogn. Neurosci. 12, 711–720 (2000). [DOI] [PubMed] [Google Scholar]
- 57.Wallisch P., Movshon J. A., Structure and function come unglued in the visual cortex. Neuron 60, 195–197 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Regan D., Beverley K. I., Figure-ground segregation by motion contrast and by luminance contrast. J. Opt. Soc. Am. A 1, 433–442 (1984). [DOI] [PubMed] [Google Scholar]
- 59.Hall J. R., Cuthill I. C., Baddeley R., Shohet A. J., Scott-Samuel N. E., Camouflage, detection and identification of moving targets. Proc. Biol. Sci. 280, 20130064 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuthill I. C., Matchette S. R., Scott-Samuel N. E., Camouflage in a dynamic world. Curr. Opin. Behav. Sci. 30, 109–115 (2019). [Google Scholar]
- 61.Pembury Smith M. Q. R., Ruxton G. D., Camouflage in predators. Biol. Rev. Camb. Philos. Soc. 95, 1325–1340 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Hancock P. A., On the dynamics of conspicuity. Hum. Factors 61, 857–865 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Pratt J., Radulescu P. V., Guo R. M., Abrams R. A., It’s alive! Animate motion captures visual attention Psychol. Sci. 21, 1724–1730 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Neri P., Morrone M. C., Burr D. C., Seeing biological motion. Nature 395, 894–896 (1998). [DOI] [PubMed] [Google Scholar]
- 65.Blake R., Shiffrar M., Perception of human motion. Annu. Rev. Psychol. 58, 47–73 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Stocker A. A., Simoncelli E. P., Noise characteristics and prior expectations in human visual speed perception. Nat. Neurosci. 9, 578–585 (2006). [DOI] [PubMed] [Google Scholar]
- 67.Yin J., et al. , Breaking cover: Neural responses to slow and fast camouflage-breaking motion. Proc. Biol. Sci. 282, 20151182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LeDoux J. E., The Emotional Brain: The Mysterious Underpinnings of Emotional Life (Simon & Schuster, New York, NY, 1996), p. 384p. [Google Scholar]
- 69.Pessoa L., Adolphs R., Emotion processing and the amygdala: From a “low road” to “many roads” of evaluating biological significance. Nat. Rev. Neurosci. 11, 773–783 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang H., Wang J., Liu Y., Yan C., Ye X., Threat-relevant stimuli cannot be better detected by preschoolers in an inattentional blindness task. Psychol. Res. 86, 823–830 (2022). [DOI] [PubMed] [Google Scholar]
- 71.White R. C., Davies M., Aimola Davies A. M., Inattentional blindness on the full-attention trial: Are we throwing out the baby with the bathwater? Conscious Cogn. 59, 64–77 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Jaschinski W., The proximity-fixation-disparity curve and the preferred viewing distance at a visual display as an indicator of near vision fatigue. Optom. Vis. Sci. 79, 158–169 (2002). [DOI] [PubMed] [Google Scholar]
- 73.Rempel D., Willms K., Anshel J., Jaschinski W., Sheedy J., The effects of visual display distance on eye accommodation, head posture, and vision and neck symptoms. Hum. Factors 49, 830–838 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Gureckis T. M., et al. , psiTurk: An open-source framework for conducting replicable behavioral experiments online. Behav. Res. Methods 48, 829–842 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Efron B., Tibshirani R., An Introduction to the Bootstrap, Monographs on Statistics and Applied Probability (Chapman & Hall, New York, NY, 1993), p. 436. [Google Scholar]
- 76.Benjamin D. J., et al. , Redefine statistical significance. Nat. Hum. Behav. 2, 6–10 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Wallisch P., Mackey W. E., Karlovich M. W., Heeger D. J., The Visible Gorilla: Fast motion captures visual attention [Data set]. OSF Storage. https://osf.io/gpy6j/. Deposited 29 October 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Human behavioral data have been deposited in [OSFHome] (DOI 10.17605/OSF.IO/GPY6J) (77).