Abstract
Aim
The importance of endothelial cell (EC) autophagy to vascular homeostasis in the context of health and disease is evolving. Earlier, we reported that intact EC autophagy is requisite to maintain shear-stress-induced nitric oxide (NO) generation via glycolysis-dependent purinergic signalling to endothelial NO synthase (eNOS). Here, we illustrate the translational and functional significance of these findings.
Methods and results
First, we assessed translational relevance using older male humans and mice that exhibit blunted EC autophagy and impaired arterial function vs. adult controls. Active hyperaemia evoked by rhythmic handgrip exercise-elevated radial artery shear-rate similarly from baseline in adult and older subjects for 60 min. Compared with baseline, indexes of autophagy initiation, p-eNOSS1177 activation, and NO generation, occurred in radial artery ECs obtained from adult but not older volunteers. Regarding mice, indexes of autophagy and p-eNOSS1177 activation were robust in ECs from adult but not older animals that completed 60-min treadmill-running. Furthermore, 20 dyne • cm2 laminar shear stress × 45-min increased autophagic flux, glycolysis, ATP production, and p-eNOSS1177 in primary arterial ECs obtained from adult but not older mice. Concerning functional relevance, we next questioned whether the inability to initiate EC autophagy, glycolysis, and p-eNOSS1177 in vitro precipitates arterial dysfunction ex vivo. Compromised intraluminal flow-mediated vasodilation displayed by arteries from older vs. adult mice was recapitulated in vessels from adult mice by (i) NO synthase inhibition; (ii) acute autophagy impairment using 3-methyladenine (3-MA); (iii) EC Atg3 depletion (iecAtg3KO mice); (iv) purinergic 2Y1-receptor (P2Y1-R) blockade; and (v) germline depletion of P2Y1-Rs. Importantly, P2Y1-R activation using 2-methylthio-ADP (2-Me-ADP) improved vasodilatory capacity in arteries from (i) adult mice treated with 3-MA; (ii) adult iecAtg3KO mice; and (iii) older animals with repressed EC autophagy.
Conclusions
Arterial dysfunction concurrent with pharmacological, genetic, and age-associated EC autophagy compromise is improved by activating P2Y1-Rs.
Keywords: Aging, Endothelial cells, Nitric oxide, Vascular function
Graphical Abstract
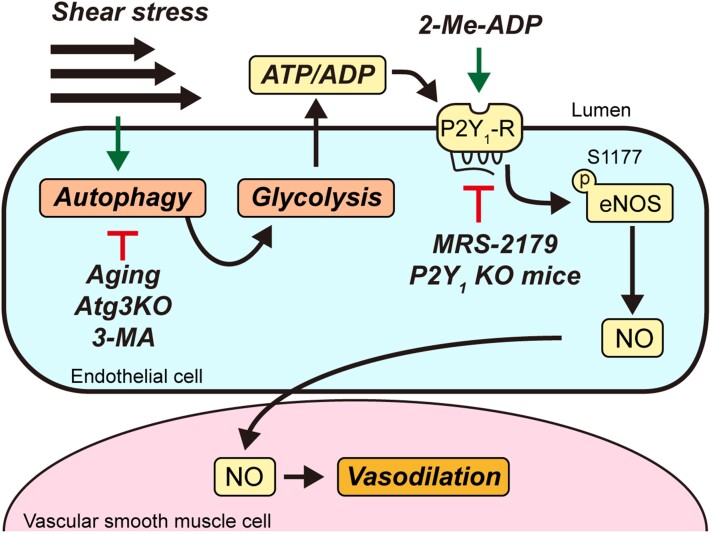
Graphical Abstract.
1. Introduction
Autophagy is critical for maintaining quality control of the cell1 and integrity of the cardiovascular system.2 Aging compromises the process of autophagy in a number of cell types.3,4 With specific regard to endothelial cells (ECs), one report indicates a protein responsible for autophagy initiation was lower (i.e. beclin-1), and a marker of undegraded autophagy substrates was higher (i.e. p62/sequestosome-1), in primary brachial artery (BA) ECs from older vs. adult subjects under basal conditions.5 Because older participants in that study displayed BA nitric oxide (NO)-dependent endothelial dysfunction, the authors suggested a link exists between defective EC autophagy and compromised EC NO generation.
Since the circulating milieu and EC phenotype associated with aging is complex, we used a reductionist approach to determine whether EC autophagy suppression per se is sufficient to impair shear-stress-induced NO generation in bovine aortic ECs (BAECs). Inhibiting EC autophagy via pharmacological and genetic approaches prevented shear stress-induced activating phosphorylation of endothelial NO synthase at serine 1177 (p-eNOSS1177) and NO generation, and heightened shear stress-evoked reactive oxygen species (ROS) production and pro-inflammatory gene expression.6 We concluded that EC autophagy plays a critical role in maintaining NO bioavailability, and contributes importantly to oxidant/antioxidant and inflammatory/anti-inflammatory balance in ECs. After substantiating these findings in human arterial endothelial cells (HAECs), we revealed that genetic repression of autophagy impairs EC glycolysis and subsequent autocrine signalling via the purinergic 2Y1-receptor (P2Y1-R) to eNOS, to an extent that NO generation is compromised.7 By manipulating P2Y1-R signalling this phenotype was recapitulated in ECs with intact autophagy, and rescued in ECs with genetic repression of autophagy.7 Here, we sought to determine the translational and functional relevance of these findings by addressing three unanswered questions. First, is autophagy and NO generation impaired in arterial ECs from older vs. adult humans and mice in response to elevated shear-rate evoked by active hyperaemia? Second, does repressed shear-stress-induced NO generation after EC autophagy diminution in vitro translate to impaired intraluminal flow-mediated arterial vasodilation examined ex vivo? If so (i.e. third), can limited intraluminal flow-mediated arterial vasodilation in the context of pharmacological, genetic, and aging-associated EC autophagy compromise in mice be rejuvenated by targeting P2Y1-Rs?
Here, we report that elevated arterial shear-rate associated with active hyperaemia evoked by RHE initiates autophagy, p-eNOSS1177 activation, and NO generation in primary arterial ECs obtained via j-wire from the radial artery of adult but not older male volunteers. In mice, robust activation of arterial EC autophagy and NO generation in response to an acute bout of treadmill-running was observed in adult but not older animals. Furthermore, physiological shear-stress increased autophagic flux, the extracellular acidification rate (ECAR, an estimate of glycolysis), ATP generation, and p-eNOSS1177, in primary arterial ECs obtained from adult but not older mice. These results indicate our earlier findings that shear-stress-induced NO generation is blunted by pharmacological and genetic autophagy repression in BAECs and HAECs can be translated to primary arterial ECs from older vs. adult humans and mice. Next, we demonstrated that P2Y1-R activation rejuvenates intraluminal flow-mediated vasodilation that is otherwise attenuated in femoral arteries from (i) adult mice (7-month) after pharmacological autophagy compromise; (ii) adult mice (4-month) with inducible depletion of Atg3 specifically in ECs; and older mice (24-month) that display indexes of repressed arterial EC autophagy and EC glycolysis in response to treadmill-running and shear-stress, respectively. Collectively, solid evidence is provided that targeting purinergic signalling to endothelial NO synthase in the context of compromised EC autophagy mitigates arterial dysfunction.
2. Methods
Antibodies, primers, and other materials are shown in Supplementary material online, Tables S1–S4.
2.1. Human studies
Human subjects volunteered for our study. Protocol approval and written informed consent were obtained according to the Declaration of Helsinki and was approved by the University of Utah (UU) and the Department of Veterans Affairs Salt Lake City Health Care System Institutional Review Boards. Adult and older male subjects visited the laboratory twice within 7 days. On Day 1, a flow-mediated dilation (FMD) test was completed and a maximal handgrip workload evaluation was performed.8 On Day 2, cells were obtained using a j-wire before and after rhythmic handgrip exercise (RHE) that elevated arterial shear-rate for 60 min.8 On the day of collection, one set of cells was stained without fixation for NO and superoxide anion (O2•–) generation using 4-Amino-5-Methylamino-2′,7′-Difluorofluorescein Diacetate (DAF-FM Diacetate) and dihydroethidium (DHE), respectively.8 A second set of cells from the same subject was fixed with 4% paraformaldehyde and frozen at −80°C until staining for protein expression using immunofluorescent antibodies that recognize p-eNOSS1177, eNOS, Beclin1, autophagy-related gene 3 (Atg3), microtubule-associated proteins 1A/1B light chain 3B (LC3B), p62, and lysosomal-associated membrane protein 1 (LAMP1). Both sets of cells were co-stained with VE-cadherin and 4′,6-diamidino-2-phenylindole (DAPI) to identify ECs and nuclei, respectively, using confocal microscopy.8–10
2.2. Cell studies
HAECs: HAECs (Lonza Inc.) were maintained in endothelial basal medium-2 (Lonza Inc.) containing supplements (EGM-2 SingleQuots, Lonza Inc.) in a 5% CO2 atmosphere at 37°C.7 The Atg3 gene was deleted using CRISPR/Cas9. HAECs were transfected with single-guide RNA (sgAtg3) for the Atg3 gene (sequence: 5′-ACAAACGTGGCGAATAT-3′; Synthego) or a non-targeting sgRNA [wild type (WT)] by electroporation using the following conditions: 1400 V, 20 ms, 2 pulses. HAECs were treated with (i) 1 μmol/L 3-MA (to inhibit autophagy initiation)7; (ii) 100 μmol/L 2-Me-ADP (to activate P2Y1-Rs)7; (iii) 5 μmol/L MRS2179 (to block P2Y1-Rs)7; or (iv) 50 μmol/L ADP (to activate P2Y1-Rs).7 In addition, sgAtg3 or WT HAECs were exposed to (v) 0 or 20 dyne • cm2 shear stress for 45 min and prepared to assess NO generation, protein expression, and cell viability.7
Primary arterial ECs from mice (MAECs). MAECs from adult (7 months) and older (24 months) mice were obtained as we describe here and in the online supplement.7 MAECs were passaged at 70–80% confluency and Passages 4–6 were used to (i) measure autophagy indexes and mTOR signalling ±100 μmol/L 2-Me-ADP; (ii) assess autophagic flux using 10 nmol/L bafilomycin-A1 (Baf)11–13; or (iii) perform a glycolysis stress test.7
2.3. Animal studies
Procedures involving mice were approved by the Institutional Animal Care and Use Committee at the University of Utah. All procedures were performed according to the NIH Guide for the Care and Use of Laboratory Animals.
Older vs. adult mice. Male C57BL/6 mice were obtained from the Jackson Laboratories (C67BL/6J) at 5–6 months of age, or the National Institute on Aging (C67BL/6C) rodent colony at 21 months of age, and housed in AALAC-accredited facilities at the University of Utah. Animals were housed 4 per cage, maintained on a 12:12 h light: dark cycle in a temperature-controlled environment (22–23°C), and were provided standard rodent chow and water ad libitum. Mice were anaesthetized using 2–5% inhaled isoflurane (1-chloro-2,2,2-trifluoroethyl difluoromethyl ether) combined with 100% oxygen. Upon attaining an appropriate plane of anaesthesia, the heart was removed. Separate cohorts of adult (7 months) and old (24 months) mice were used to assess the following: (i) EC and media + adventitia (M+A) mRNA expression via qRT-PCR (carotid and iliac arteries); (ii) arterial admixture protein expression via immunoblotting (aorta, iliac, femoral arteries); (iii) arterial EC protein expression via en face immunofluorescent staining7,14–18 (aorta); (iv) vasoreactivity ex vivo via myography14–16 (femoral artery); and (v) vascular morphology by Masson’s Trichrome staining19 (aorta).
Inducible endothelial cell-specific Atg3 knockout (iecAtg3KO) mice. Atg3 mediates lipidation of Atg8/LC3-I to form mature LC3-II and is required for the formation of autophagosomes.1 Flox-Atg3 mice were developed as described.7,20 Atg3flox/flox mice were crossed with Cdh5-CreERT2 mice.21 Atg3flox/flox and Cdh5-CreERT2 mice were on a C57BL/6 background. Atg3flox/flox/Cdh5-CreERT2 and Atg3flox/flox littermates (WT) were used in this study. Floxed Atg3 and Cdh5-Cre was confirmed by PCR analysis of genomic DNA. To activate Cdh5-Cre in 4-month-old mice, 4 mg tamoxifen was administered via oral gavage to iecAtg3KO and WT mice for 4 consecutive days. Separate cohorts of WT and iecAtg3KO mice were used for mRNA expression, protein expression, function, and morphology as described.22
P2Y1-R knockout (KO) mice. Femoral arteries from 2-month-old P2Y1-R KO mice and their WT littermates23 were obtained to measure vascular function ex vivo via isobaric myography.14–16
2.4. Statistics
Data are presented as mean ± standard deviation of the mean. Significance was accepted when P<0.05. Normality of distribution for each data set was performed by GraphPad Prism software version 9. For comparison between two groups, unpaired or paired t-tests were performed. For comparison among three or more mean values, if the data were distributed normally, a one-way ANOVA was performed. If significance was obtained, a Tukey’s post hoc test was used to identify the location of the differences. If the data were not distributed normally, a Kruskall–Wallis ANOVA was completed. If significance was obtained, a Dunn’s post hoc test was used to identify the location. Two-way ANOVA was used to determine significance concerning the following: (i) RHE-Pre and RHE-Post between adult and old subjects and (ii) intraluminal flow-mediated vasodilation in arteries. Two-way repeated measured ANOVA was used to determine significance concerning haemodynamic variables during the RHE protocol. Information concerning statistical tests are included in each legend.
3. Results
3.1. Elevated arterial shear-rate heightens autophagy and NO generation in ECs from adult but not older males
Elevated arterial shear-rate associated with active hyperaemia evoked by RHE increases autophagy initiation, and NO and O2•– production, in radial artery ECs obtained from healthy adult males.8 Furthermore, pharmacologic and genetic repression of EC autophagy prevents shear-stress-induced NO generation in HAECs and BAECs.6,7 These findings prompted us to test the hypothesis that shear-induced autophagy initiation and NO generation are blunted in ECs from older vs. adult volunteers. Subject characteristics are presented in Table 1.
Table 1.
Characteristics of adult and old human subjects
| Adult | Old | |
|---|---|---|
| Age, years | 23±1 | 68±2* |
| Height, cm | 181±3 | 180±2 |
| Weight, kg | 75±2 | 91±9 |
| Body mass index, kg/m2 | 23±1 | 28±2* |
| Cholesterol, mg/dL | 137±16 | 149±22 |
| Triglycerides, mg/dL | 50±5 | 111±31 |
| HDL, mg/dL | 45±3 | 41±6 |
| LDL, mg/dL | 83±13 | 94±21 |
| Glucose, mg/dL | 81±2 | 83±7 |
| RBC, M/µL | 5±0 | 5±0 |
| Hb, g/dL | 15±0 | 15±0 |
| Hct, % | 42±1 | 44±1 |
| Maximal workload, kg | 25±2 | 19±1 |
Values are mean scores ± SE (n=6 per group).
HDLs, high-density lipoproteins; LDLs, low-density lipoproteins; RBCs, red blood cells; Hb, haemoglobin; Hct, haematocrit.
P<0.05 vs. adult using an unpaired t-test.
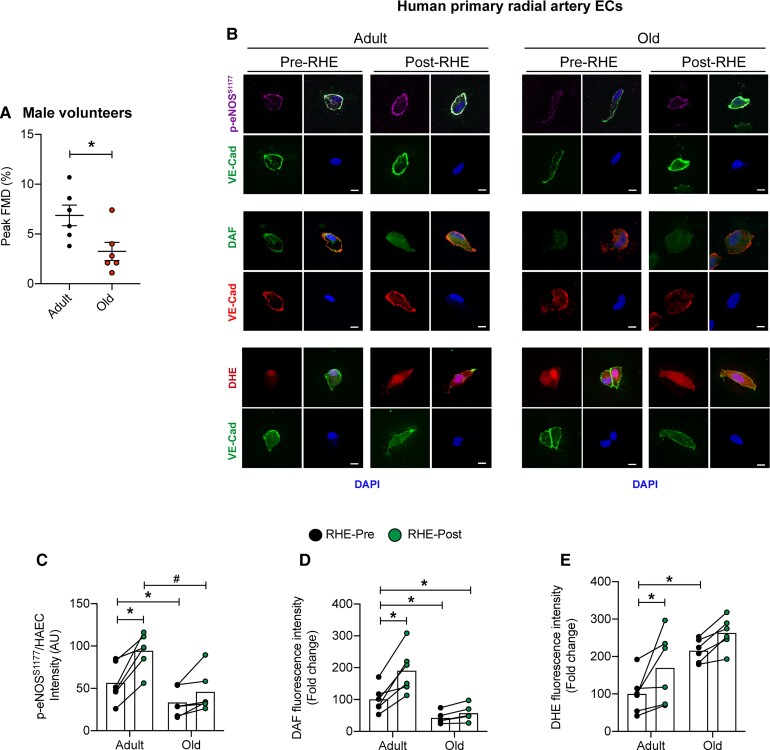
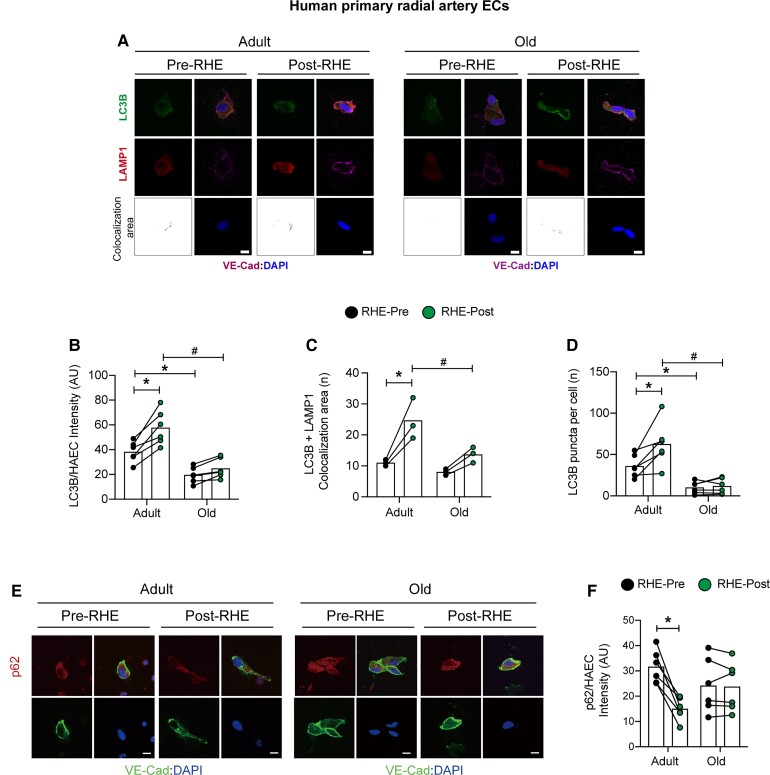
FMD peak was lower in older vs. adult male subjects (Figure 1A). Comparing RHE-Pre between groups, i.e. the influence of aging, p-eNOSS1177 and NO generation were lower (Figure 1B–D) and O2•- was higher (Figure 1B and E) in ECs from older vs. adult participants, whereas no differences existed between groups for total eNOS protein expression (Supplementary material online, Figure S1). Regarding autophagy, while LC3B and LC3B-bound puncta were lower in ECs from older vs. adult participants under baseline conditions (i.e. RHE-Pre; Figure 2A–D), no differences existed between groups regarding LC3B colocalization with LAMP1, Beclin1, Atg3, or p62 (Figure 2C and F, Supplementary material online, Figure S2).
Figure 1.
ECs from older males with impaired arterial function are resistant to elevated radial artery shear-rate concerning nitric oxide generation and ROS. (A) FMD (%) peak was lower in older vs. adult male subjects. Representative images (B) and mean immunofluorescence staining intensity (C–E) is shown. Comparing RHE-Pre with RHE-Pre between groups, i.e. the influence of aging, p-eNOSS1177 and DAF are lower in ECs from older vs. adult participants, while DHE staining is elevated in ECs from older vs. adult subjects (i.e. Bar 1 vs. 3). Comparing RHE-Pre with RHE-Post between groups, i.e. the influence of elevated arterial shear rate, p-eNOSS1177, DAF, and DHE are elevated in ECs from adult (Bar 1 vs. 2) but not older (Bar 3 vs. 4) subjects. For C–E, ∼50 ECs from each time point and each subject were measure. Data are from the same 6 subjects in adult and per group as shown in A. *P<0.05 vs. RHE-Pre adult; #P<0.05 vs. RHE-Post adult. Scale bar: 10 μm. Magnification: 60×. Significance was assessed via unpaired t-test (A) and a two-way ANOVA (C–E).
Figure 2.
ECs from older males are resistant to elevated radial artery shear-rate concerning autophagosome formation. Representative images (A and E) and mean immunofluorescence staining intensity (B–D and F) is shown. Comparing RHE-Pre with RHE-Pre between groups, LC3B expression and LC3B-bound puncta are lower in ECs from older vs. adult subjects. Comparing RHE-Pre with RHE-Post between groups LC3B, LC3B-bound puncta, LC3B colocalization with LAMP1, and degradation of p62, increased in ECs from adult (Bar 1 vs. 2) but not older (Bar 3 vs. 4) subjects. For B–D and F, ∼50 ECs from each time point and each subject were measured. For B, D, and F, 6 subjects per group. For C, n=3 per group. *P<0.05 vs. RHE-Pre adult; #P<0.05 vs. RHE-Post adult. Scale bar: 10 μm. Magnification: 60×. Statistical significance was assessed via two-way ANOVA (B–D and F).
Relative to measures obtained at baseline, i.e. RHE-Pre, exercise elevated BA blood flow velocity and arterial shear-rate ∼3-fold for 60 min to values that were not different between adult and older subjects (Supplementary material online, Figure S3), and systemic haemodynamics were unaltered by this stimulus in both groups (Supplementary material online, Table S5). When compared with values obtained at RHE-Pre, RHE-Post ECs displayed increased p-eNOSS1177, NO generation, and O2•- production, in adult but not older subjects (Figure 1). Regarding autophagy, compared with RHE-Pre, increased expression of Beclin-1, Atg3, LC3B, LC3B+LAMP1 colocalization, LC3B-bound puncta, and decreased expression (i.e. increased degradation) of p62, was displayed by ECs from adult but not older participants at RHE-Post (Figure 2, Supplementary material online, Figure S2). These findings provide translational relevance of our in vitro observations that pharmacologic and genetic repression of EC autophagy limits shear-stress-induced autophagy initiation and NO generation.6,7
3.2. Physiological shear-stress increases autophagic flux, indexes of glycolysis, ATP production, and eNOS activation in primary ECs from adult but not older mice
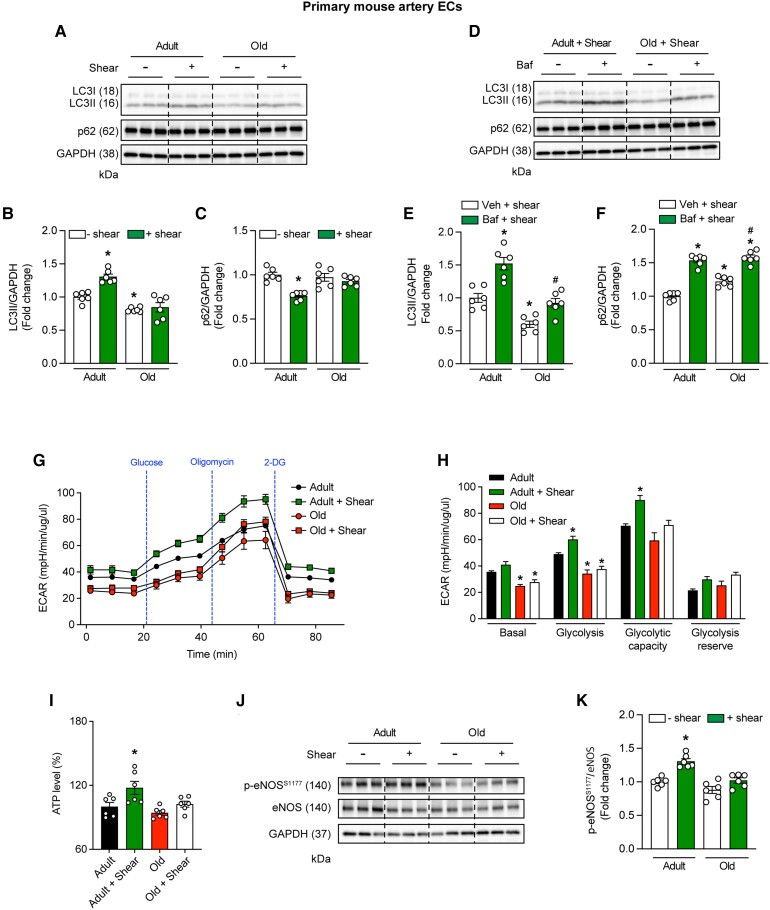
Next, we tested the hypothesis that shear-induced autophagy initiation and NO generation are blunted in ECs from older vs. adult mice. This was requisite because interventions completed later involve murine arteries. LC3-II:GAPDH accrual and p62:GAPDH degradation in response to 20 vs. 0 dyne • cm2 shear stress was robust in ECs from adult vs. older mice (Figure 3A–C).
Figure 3.
ECs from older mice are resistant to shear stress-induced activation of autophagic flux, glycolysis, ATP production, and p-eNOSS1177. MAECs were exposed to 0 (–shear) or 20 dyne • cm2 shear stress (+shear) for 45-min. Representative images (A) and mean data indicate shear stress increases LC3-II/GAPDH accrual (B) and p62/GAPDH degradation (C) in MAECs from adult but not older mice. Regarding autophagic flux, representative images (D) and mean data indicate that, compared with shear stress + vehicle treatment, shear stress + BAF caused greater accumulation of LC3-II/GAPDH (E) and p62/GAPDH (F) in MAECs from adult vs. old mice. For B,C,E,F, n=6 wells of a six-well plate; differences among groups concerning mean densitometry were identified via one-way ANOVA; *P<0.05 vs. Adult-Shear; *P<0.05 vs. Adult+Veh+Shear; #P<0.05 vs. Old+Veh+Shear. Regarding ECAR, treatment of MAECs with: 0 mmol/L glucose, 5 mmol/L glucose, 1 μmol/L oligomycin, and 50 mmol/L 2-deoxy D glucose (2-DG) indicate basal and shear-induced glycolysis is repressed in old vs. adult mice (G and H). For G and H, n=5 wells × 50 000 cells per well of a 24-well plate. For (H), statistical significance was assessed via one-way ANOVA; *P<0.05 vs. adult. (I) Shear stress increased ATP production to a greater extent in MAECs from adult vs. older mice. Representative image (J) and mean densitometry (K) indicate shear stress increases p-eNOSS1177/eNOS protein expression to a greater extent in MAECs from adult vs. older animals. For I and K, n=6 wells of a 6 well plate per treatment. Statistical significance was assessed via one-way ANOVA; *P<0.05 vs. static-adult.
Because the process of autophagy is dynamic, we sought to distinguish trafficking of the autophagosome to the lysosome from lysosomal degradation of the autophagosome.11 Adult and old MAECs exposed to shear stress were treated with vehicle or the V-ATPase inhibitor bafilomycin (Baf). Baf prevents degradation of autophagolysosome contents. As such, increased accumulation of LC3-II:GAPDH and p62:GAPDH in the presence vs. the absence of Baf indicates heightened autophagosome formation i.e. autophagic flux.11–13,22 Compared with the respective vehicle treatment, LC3-II:GAPDH increased to a greater extent (P<0.05) in adult (52%) vs. old (32%) MAECs upon treatment with Baf (Figure 3D and E). Likewise, compared with the respective vehicle treatment, p62:GAPDH increased 54% in adult MAECs vs. 37% in old MAECs upon treatment with Baf (Figure 3D and F). On balance, these findings are similar to those we observe in primary arterial ECs from older humans (Figures 1 and 2).
ECs produce ATP primarily via glycolysis in response to shear stress.24,25 Earlier, we showed that EC glycolysis is repressed in BAECs under basal conditions and in response to shear-stress upon siRNA-mediated Atg3 knockdown.7 Here, we determined whether primary ECs from older mice exhibit compromised glycolysis vs. results obtained from adult animals. The ECAR was assessed in primary ECs from adult and old mice at 0 and 20 dyne • cm2 shear stress × 45 min under the following conditions: 0 mmol/L glucose; 5 mmol/L glucose (to stimulate glycolysis, lactate production, and increase ECAR); 1 µmol/L oligomycin (to inhibit mitochondrial ATP production); and 50 mmol/L 2-deoxyglucose (2-DG, to inhibit glycolysis).26 Shear-stress increased ECAR to a greater extent in primary ECs from adult but not older mice (Figure 3G and H). As would be predicted, shear-stress-induced ATP production (Figure 3I) and p-eNOSS1177 (Figure 3J and K) also were more robust in ECs from adult vs. older mice.
3.3. Repressed EC autophagy in older vs. adult mice is associated with compromised intraluminal FMD
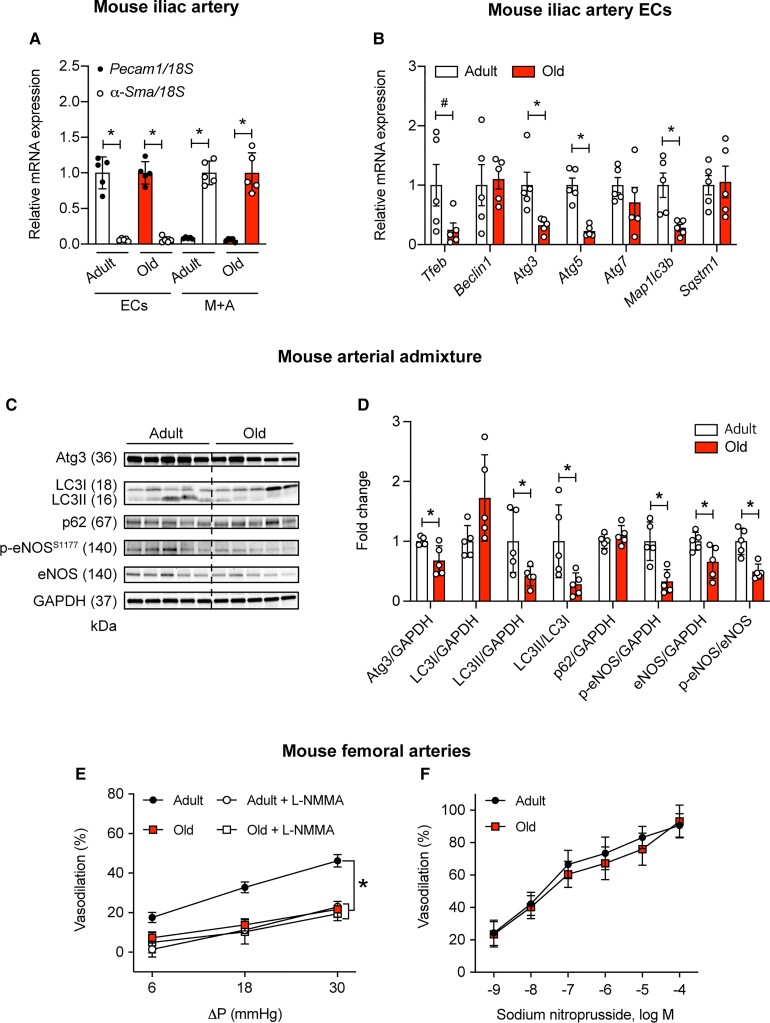
Next, we tested whether the age-associated reduction in shear-induced EC autophagy initiation and eNOS activation is translated to impaired intraluminal FMD. mRNA expression was assessed in ECs and media + adventitia (M+A) obtained from iliac arteries of adult and older mice. Purity of the iliac EC and M+A fraction was verified by quantifying mRNA expression of platelet/endothelial cell adhesion molecule 1 (Pecam1) and alpha-smooth muscle actin (α-Sma), respectively. Pecam1 mRNA was highly expressed in iliac ECs, but not in M+A, whereas α-Sma mRNA was highly expressed in M+A, but not in the EC fraction (Figure 4A). In the EC fraction, we demonstrate that mRNA expression of transcriptional factor EB (Tfeb), Atg3, Atg5, and Map1lc3b is lower in older vs. adult mice (Figure 4B). A similar pattern of findings was obtained in carotid arteries from the same mice (Supplementary material online, Figure S4).
Figure 4.
Repressed EC autophagy associates with compromised intraluminal flow-mediated vasodilation in arteries from old vs. adult mice. Pecam1 mRNA expression is robust whereas α-Sma is minimal in the EC fraction from iliac (A) arteries of adult and old mice. α-Sma mRNA expression is robust whereas Pecam1 is minimal in the media + adventitia (M + A) fraction from iliac (A) arteries of adult and old mice. Iliac artery ECs (B) of older mice display repressed mRNA expression of autophagy indices vs. adult mice. For A and B values are normalized by 18S; n=5 mice per group × 2 iliac arteries per mouse. For A, *P<0.05 vs. Pecam1. For B and D, *P<0.05 vs. adult. Representative images (C) and mean densitometry (D) from arterial admixtures indicate autophagy indexes (normalized to GAPDH) and p-eNOSS1177 (normalized to eNOS) are compromised in older vs. adult mice. For C and D, n=5 per group; *P<0.05 vs. Adult. (E) Intraluminal flow-mediated vasodilation (%) was (i) greater in femoral arteries from adult vs. older mice and (ii) sensitive to L-NMMA in femoral arteries from adult but not older mice. Robust intraluminal flow-mediated vasodilation is displayed by arteries from adult but not older mice in the presence of vehicle (DMSO, F). After a 30-min incubation with (i) 5 mmol/L 3-MA, intraluminal flow-mediated vasodilation is repressed in arteries from adult but not older mice (F). For E and F, n=6 mice per group × 1 artery per mouse. *P<0.05 vs. adult. Statistical significance was assessed by unpaired t-test (A–D) and a two-way repeated measures ANOVA (E and F).
Admixtures of aorta, iliac, and femoral artery from older mice exhibited lower protein expression of Atg3:GAPDH, LC3-II:GAPDH, LC3-II:LC3-I, and p-eNOSS1177:eNOS, whereas expression of p62 trended higher (P=0.09), vs. lysates from adult mice (Figure 4C and D). These findings support that indexes of autophagy-related mRNA and protein expression are lower in ECs and arterial lysates from older vs. adult mice.
Next, we tested whether impaired NO-mediated vasodilation exists in femoral arteries from old mice with repressed EC autophagy vs. adult mice with intact EC autophagy. Animal and vessel characteristics are shown in Supplementary material online, Table S6 and Figure S5. First, we confirmed that (i) pressure gradients (ΔP) of 6, 18, and 30 mmHg evoke similar flow rates when separated by 30 min; (ii) ΔPs of 6, 18, and 30 mmHg cause vasodilatory responses that are not different when separated by 30 min; and (iii) phenylephrine (PE)-induced precontraction can be sustained for a duration that is sufficient to complete an intervention (Supplementary material online, Figure S6). Second, we showed that (i) blunted FMD exists in arteries from older vs. adult mice (Figure 4E); (ii) intraluminal incubation with 10−3 mol/L NG-monomethyl-L-arginine (L-NMMA) represses FMD in arteries from adult but not older mice (Figure 4E); and (iii) vasodilation in response to sodium nitroprusside is not different between groups (Figure 4F). These findings reveal that aging impairs intraluminal FMD in an NO-dependent manner in arteries wherein repressed EC and vascular autophagy have been documented.
3.4. ECs from adult but not older mice display autophagy initiation and NO generation in response to active hyperaemia
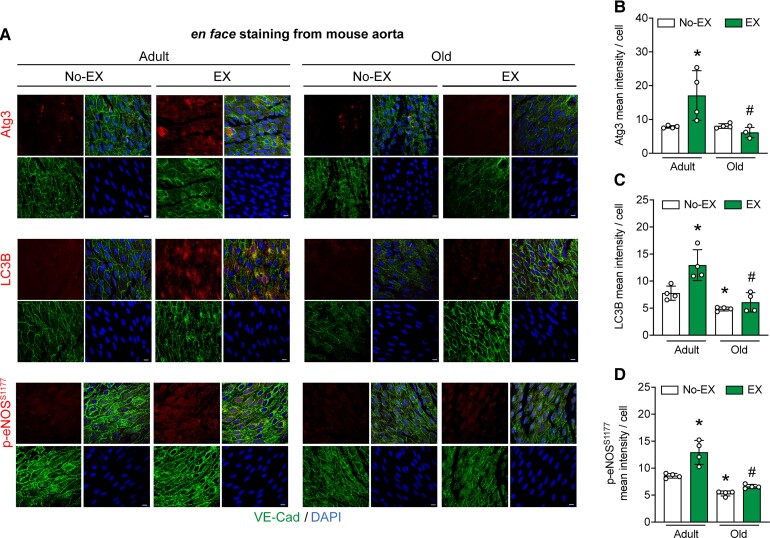
Data shown in Figures 1, 2, and Supplementary material online, Figure S2 indicate autophagy initiation and NO generation are blunted in ECs from older vs. adult males that complete 60-min RHE. Results displayed in Figures 2B, D, 4B, and Supplementary material online, Figure S4 indicate an age-associated repression of arterial EC autophagy exists under basal conditions and in response to physiological shear-stress in vitro. Here, we determined whether autophagy initiation and eNOS activation are impaired in arterial ECs from older vs. adult mice in response to active hyperaemia evoked by 60-min treadmill-running. The mode, intensity, and duration of exercise was chosen based on its ability to heighten arterial p-eNOSS1177 and eNOS enzyme activity in mice.17 Supporting our hypothesis, treadmill-running increased expression of Atg3, LC3B, and p-eNOSS1177 in aortic ECs from adult but not older mice (Figure 5A–D). These results are congruent with those observed in ECs from older vs. adult humans challenged with RHE (Figures 1 and 2) and ECs from older vs. adult mice exposed to shear-stress in vitro (Figure 3).
Figure 5.
ECs from adult but not older mice display autophagy initiation and NO generation in response to 60-min treadmill-running. Representative images (A) and mean immunofluorescence data (B–D) from aortic ECs from mice that did not (No-EX) or did (EX) complete 60-min treadmill-running are shown. The four quadrants of each staining protocol represent VE-cadherin (bottom left); DAPI (blue, bottom right); protein of interest (top left), and merge (top right). Atg3, LC3B, and p-eNOSS1177 (B–D) increased in EX vs. No-EX-adult (Bar 1 vs. 2) but not older (Bar 3 vs. 4) mice. LC3B (C) and p-eNOSS1177 (D) were lower in older vs. adult mice, whereas Atg3 was not different between groups (i.e. Bar 1 vs. 3). Four mice/aorta per group × 5 images per aorta × 20 ECs per image were quantified. For A–D, *P<0.05 vs. No-EX-Adult; #P<0.05 vs. EX-Adult. Scale bar: 10 μm. Magnification: 60×. Statistical significance was assessed via one-way ANOVA.
3.5. Autophagy inhibition mimics an aging vascular phenotype concerning intraluminal FMD
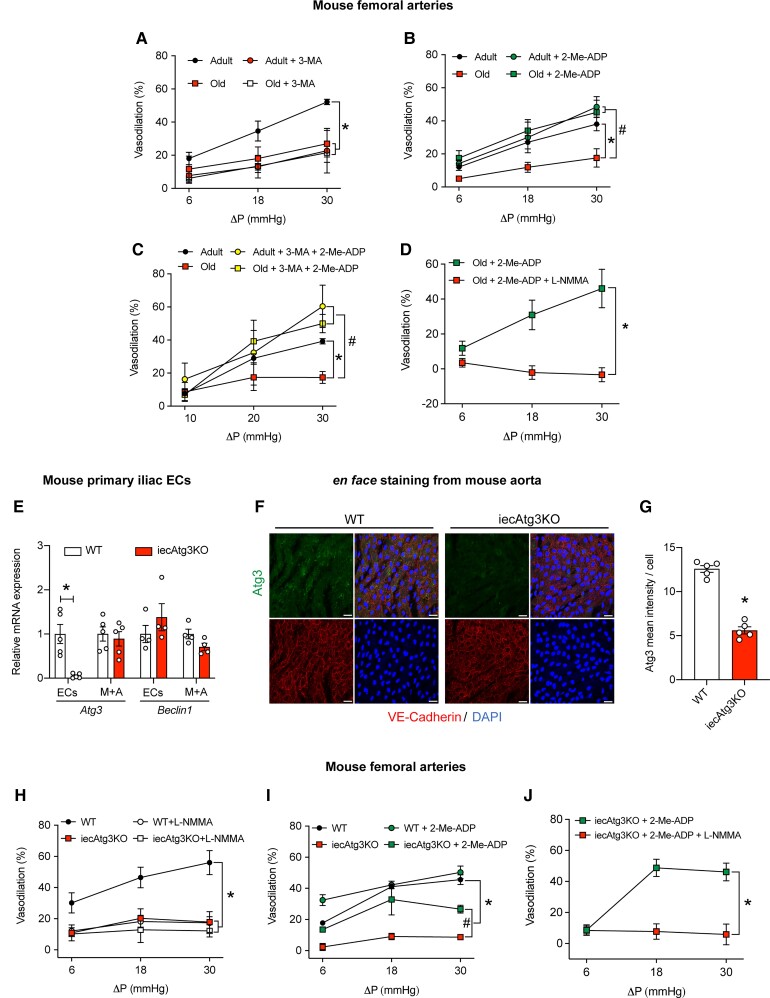
To this point our data indicate that the age-related suppression of EC autophagy (Figures 4B and 5A–D, and Supplementary material online, Figure S4) associates with (i) impaired glycolysis in ECs exposed to shear-stress (Figure 4G–I) and (ii) blunted intraluminal FMD in arteries (Figure 5E). However, the circulating environment accompanying aging contains myriad factors other than compromised autophagy that might limit intraluminal FMD. Here we assessed the contribution from repressed autophagy per se to arterial function. To do so we used the class III PI3K inhibitor 3-methyladenine (3-MA) that impairs autophagy initiation.7,27,28 Demonstrating efficacy, shear-stress-induced Atg3:GAPDH and LC3-II:GAPDH expression, and p62:GAPDH degradation, were prevented by 3-MA in HAECs (Supplementary material online, Figure S7A–D). Concurrent with these observations, shear-stress-induced p-eNOSS1177:eNOS and NO generation increased in the absence but not the presence of 3-MA (Supplementary material online, Figure S8). Importantly, neither cell death nor apoptosis was increased by 3-MA vs. vehicle treatment (Supplementary material online, Figure S7E–G).
Next, we tested the hypothesis that arteries from adult mice treated with 3-MA exhibit an aging phenotype. Supporting this, intraluminal FMD displayed by femoral arteries from adult mice was blunted after autophagy inhibition using 3-MA, to an extent that was not different from responses exhibited by arteries from old mice in the absence (i.e. first response) or presence (i.e. second response, 30-min later) of 3-MA (Figure 6A). Of note, shear-stress increased glycolysis in the absence but not the presence of 3-MA in MAECs (data not shown), substantiating results displayed in Figure 3G–I, and supporting the overall hypothesis that EC autophagy repression limits EC glycolysis to an extent that associates with compromised intraluminal FMD.
Figure 6.
Impaired flow-mediated vasodilation evoked by pharmacological, physiological, or genetic autophagy repression is rejuvenated by 2-Me-ADP. Robust intraluminal flow-mediated vasodilation is displayed by arteries from adult but not older mice in the presence of vehicle (DMSO; A–C). After a 30-min incubation with (i) 3-MA, intraluminal flow-mediated vasodilation is repressed in arteries from adult mice (A); (ii) 100 μmol/L 2-Me-ADP, intraluminal flow-mediated vasodilation is re-established in arteries from older mice (B); (iii) 3-MA + 2-Me-ADP, intraluminal flow-mediated vasodilation is improved in arteries from adult and older mice (C); (iv) 2-Me-ADP + L-NMMA, intraluminal flow-mediated vasodilation is prevented in arteries from older mice. For A–D, n=3–4 mice per group; one or two arteries per mouse was used. For A–C, *P<0.05 vs. adult; #P<0.05 vs. old. For (D), *P<0.05 vs. old + 2-Me-ADP + L-NMMA. Iliac EC Atg3 mRNA expression is minimal in iecAtg3KO vs. WT mice, whereas iliac M+A Atg3 mRNA expression is similar between groups (E). Beclin1 mRNA expression is not different between groups in iliac ECs or M+A. Data shown in E were normalized by 18S; n=5 mice per group × 2 iliac arteries per mouse. Representative en face images (F) and mean immunofluorescent staining intensity (normalized by the number of cells; G) of ECs from aorta of WT and iecAtg3KO mice. ECs were identified by co-staining for DAPI and VE-cadherin. For each quadrant (F): bottom right DAPI; bottom left, VE-cadherin; top left: Atg3; top right, merge. Atg3 was lower in ECs from iecAtg3KO vs. WT mice (G). For F and G, n=4 mice × 3 segments of aorta per mouse per group. For E, scale bar: 10 μm, magnification=60×. Robust intraluminal flow-mediated vasodilation is displayed by femoral arteries from WT but not iecAtg3KO mice (G and H). After a 30-min incubation with (i) 10−3 mol/L L-NMMA, intraluminal flow-mediated vasodilation is repressed in arteries from WT but not iecAtg3KO mice (H); (ii) 100 μmol/L 2-Me-ADP, intraluminal flow-mediated vasodilation is improved in arteries from iecAtg3KO mice (I); (iii) 2-Me-ADP + L-NMMA, intraluminal flow-mediated vasodilation is prevented in arteries from iecAtg3KO mice (J). For H and I, n=5 mice per group × 1 artery per mouse. *P<0.05 vs. WT; #P<0.05 vs. iecAtg3KO. For J, n=3 mice per group × 2 arteries per mouse. *P<0.05 vs. 2-Me-ADP in iecAtg3KO mice. Statistical significance was assessed via two-way repeated measures ANOVA (A–D, H–J) and an unpaired t-test (E and G).
3.6. 2-Me-ADP restores intraluminal FMD in arteries with physiological and pharmacological autophagy repression
Shear-stress stimulates the release of ATP in ECs and its hydrolysis product ADP is an important ligand of the P2Y1-R which mediates eNOS phosphorylation and NO generation.25 Earlier, we reported that genetic repression of autophagy impairs EC glycolysis, ATP/ADP production, and subsequent autocrine signalling via the P2Y1-R to eNOS, to an extent that NO generation is compromised.7 Importantly, shear-induced p-eNOSS1177 and NO generation was restored in autophagy-deficient BAECs by concurrent treatment with the P2Y1-R agonist 2-Me-ADP. Findings shown in Supplementary material online, Figure S8 illustrate that 3-MA impairs shear-stress-induced autophagy and NO generation in HAECs. In agreement with our previous findings in BAECs,7 repressed shear-induced p-eNOSS1177 and NO generation after 3-MA was normalized in HAECs by 2-Me-ADP (Supplementary material online, Figure S8).
Because 2-Me-ADP restored shear-stress-induced p-eNOSS1177 and NO generation in HAECs after inhibiting autophagy initiation using 3-MA (Supplementary material online, Figure S8), we tested whether a similar pattern of results is translated to arteries that display an aging-associated reduction in vascular autophagy. Intraluminal FMD was impaired in arteries from old vs. adult mice, but 2-Me-ADP rejuvenated vasodilatory responses in arteries from aged animals to values that were not different from adult mice treated with 2-Me-ADP (Figure 6B). Importantly, 2-Me-ADP impacted neither EC autophagy, mTOR signalling (Supplementary material online, Figure S9), cell death, nor apoptosis (Supplementary material online, Figure S10).
Next, we determined whether P2Y1-R activation reestablishes arterial function in a manner that is independent from defective autophagy. Regarding pharmacological repression of autophagy, depressed intraluminal FMD observed in arteries from adult mice treated with 3-MA (Figure 6A) was recovered by incubation with 3-MA + 2-Me-ADP (Figure 6C). Concerning physiological i.e. age-associated impairment of EC autophagy, blunted vasodilatory capacity in arteries from old mice was rejuvenated by concurrent treatment with 2-Me-ADP, even in the presence of 3-MA (Figure 6C). Neither 3-MA, 2-Me-ADP, nor their combination, influenced responses to sodium nitroprusside (Supplementary material online, Figure S11A–C), indicating vascular smooth muscle function was not affected by the respective treatments.
Next, we substantiated that 2-Me-ADP restores FMD in the context of repressed autophagy by reactivating purinergic signalling to eNOS and subsequent NO generation. In this regard, we show that L-NMMA negates 2-Me-ADP-induced FMD improvements in arteries from older mice (Figure 6D). Together, our findings indicate purinergic reactivation of autocrine signalling using 2-Me-ADP improves intraluminal FMD that is otherwise impaired in arteries with physiological (i.e. aging) or pharmacological (i.e. 3-MA) autophagy disruption. Animal and vessel characteristics are shown in Supplementary material online, Table S7.
3.7. 2-Me-ADP restores intraluminal FMD in arteries from mice with inducible disruption of Atg3 specifically in ECs
Incorporating a genetic loss of function approach, we knocked down Atg3 in HAECs using CRISPR–Cas9. Twenty dyne • cm2 shear-stress increased Atg3:GAPDH and LC3-II:GAPDH expression, and p62:GAPDH degradation, in WT but not sgAtg3KO HAECs (Supplementary material online, Figure S12A–D). As expected, genetic diminution of Atg3 in HAECs prevented shear-induced eNOS activation and NO generation, but both endpoints were rejuvenated by concurrent treatment with 2-Me-ADP which had no impact on autophagy (Supplementary material online, Figure S12E–G). No treatments influenced cell death or apoptosis (Supplementary material online, Figure S13).
To determine translational relevance, we generated mice with tamoxifen-inducible depletion of Atg3 specifically in ECs i.e. iecAtg3KO mice. Genotyping results and the tamoxifen regimen to induce depletion are shown in Supplementary material online, Figure S14A and B. en face immunofluorescent staining of abdominal aorta segments indicate Atg3 and p-eNOSS1177 protein expression are lower in ECs from iecAtg3KO vs. WT mice (Figure 6F and G, Supplementary material online, Figure S14C and D). As a complementary procedure, mRNA expression was assessed in ECs and M+A obtained from iliac arteries of both genotypes. Reduced Atg3 gene expression was observed in iliac artery ECs (Supplementary material online, Figure S14E) obtained from iecAtg3KO vs. WT mice. Substantiating specificity of knockdown, mRNA expression of Beclin1 was similar in iliac ECs and M+A regardless of genotype (Figure 6E).
Next, we determined whether our results in HAECs with genetic autophagy compromise concerning shear-induced eNOS activation and NO generation can be translated to arteries from iecAtg3KO mice regarding intraluminal FMD. Animal and vessel characteristics are shown in Supplementary material online, Table S8 and Figure S15. Blunted vasodilation in response to three different flow rates was observed in arteries from iecAtg3KO vs. WT mice. Moreover, intraluminal incubation with 10−3 mol/L L-NMMA repressed FMD in arteries from WT but not iecAtg3KO mice, suggesting strongly that EC-specific autophagy repression impairs NO-dependent vasodilation (Figure 6H). Importantly, incubation with 2-Me-ADP improved vasodilatory capacity in arteries from iecAtg3KO mice via NO generation (Figure 6I and J). Vascular smooth muscle responses were similar in arteries from both genotypes (Supplementary material online, Figure S11D). These findings illustrate that purinergic activation of autocrine signalling using 2-Me-ADP improves FMD that is otherwise impaired in arteries from mice with EC-specific autophagy depletion.
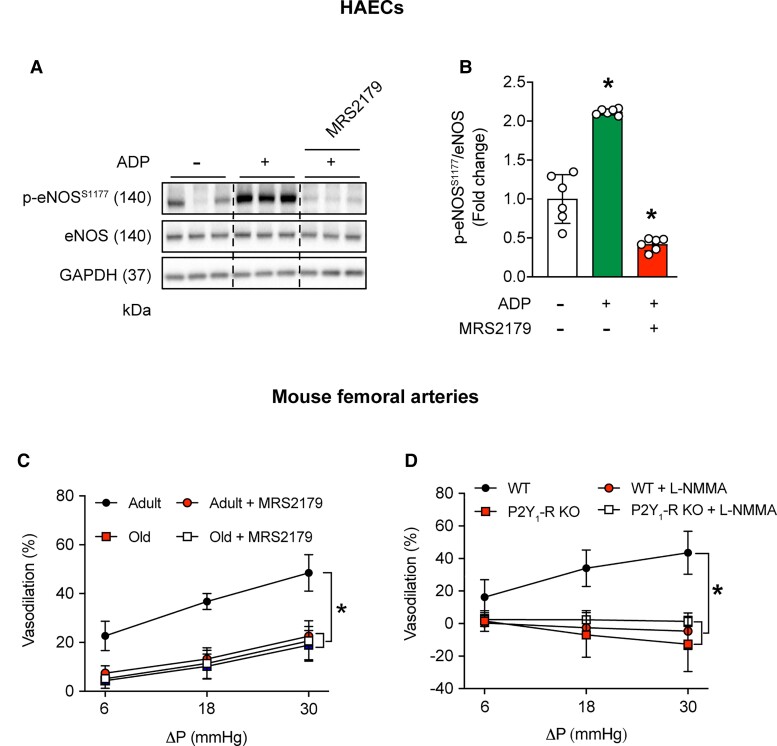
3.8. P2Y1-R inhibition in adult mice mimics an aging vascular phenotype
Earlier, we documented that shear-stress-induced p-eNOSS1177 and NO generation is prevented in BAECs treated with the P2Y1-R blocker MRS2179.7 Using this loss of function approach, we sought to determine functional relevance. First, we validated in HAECs that ADP-induced p-eNOSS1177 activation is negated by concurrent treatment with MRS2179 (Figure 7A and B) in the absence of cell death or apoptosis (Supplementary material online, Figure S16). Supporting our hypothesis, intraluminal flow-mediated vasodilation displayed by arteries from adult mice in the presence of vehicle was markedly depressed during a second flow-response curve in the presence of MRS2179 (Figure 7C). Vascular smooth muscle function was not impacted (Supplementary material online, Figure S11E). Of interest, limited vasodilatory capacity displayed by arteries from adult mice treated with MRS2179 was similar to responses exhibited by vessels from old mice treated with vehicle or MRS2179.
Figure 7.
P2Y1-R inhibition in adult mice mimics an aging phenotype. Representative images (A) and mean densitometry (B) are shown. Adenosine diphosphate (ADP; 50 μmol/L) increased p-eNOSS1177/eNOS protein expression in vehicle (PBS)-treated HAECs but not HAECs treated with MRS2179 (5 μmol/L, 30 min). For B, n=6 wells of a six-well plate; differences among groups concerning mean densitometry were identified via one-way ANOVA; *P<0.05 vs. control (-ADP and -MRS2179). (C) Robust intraluminal flow-mediated vasodilation displayed by femoral arteries from adult mice is impaired by 30-min intraluminal incubation with 5 μmol/L MRS2179. Blunted vasodilatory capacity exhibited by arteries from older vs. adult mice is not repressed further by MRS2179. For C, n=3 mice per group × 1 artery per mouse. *P<0.05 vs. adult. (D) Intact intraluminal flow-mediated vasodilation displayed by arteries from WT but not P2Y1-R KO mice is nullified by 30-min incubation with L-NMMA. For D, n=4 mice per group × 1 artery per mouse. *P<0.05 vs. WT. Statistical significance was assessed via one-way ANOVA (B) and two-way repeated measures ANOVA (C and D).
Previously we reported that shear-stress-induced p-eNOS1177 and NO generation are prevented in BAECs transfected with P2Y1-R vs. scrambled siRNA.7 To determine functional relevance, we assessed intraluminal FMD in arteries from 2-month-old male P2Y1-R KO mice.23 Robust intraluminal FMD observed in femoral arteries from WT mice was absent in arteries from P2Y1-R KO animals, whereas vascular smooth muscle function was similar between groups (Supplementary material online, Figure S11F). Notably, intraluminal FMD displayed by arteries from WT mice was sensitive to NOS inhibition using L-NMMA, whereas vessels from P2Y1-R KO mice were resistant to this intervention. Animal and vessel characteristics are shown in Supplementary material online, Table S9.
Our findings provide solid evidence that physiological (aging), pharmacological (3-MA), and genetic (sgAtg3; iecAtg3KO mice) autophagy repression specifically in ECs compromises intraluminal FMD. Importantly, targeted activation of P2Y1-Rs via 2-Me-ADP in each context is sufficient to improve shear-stress-induced eNOS activation, NO generation, and/or arterial vasodilatory capacity.
4. Discussion
The need is urgent to reveal new therapeutic targets for intervention in the context of age-associated vascular disease. Here we report for the first time that (i) older males with impaired vascular function display repressed EC autophagy initiation and NO generation in response to active hyperaemia (Figures 1 and 2); (ii) shear-stress-induced autophagic flux, glycolysis, ATP generation, and eNOS activation is compromised in primary arterial ECs from older mice (Figure 3); (iii) older mice with limited intraluminal flow-mediated arterial vasodilation exhibit blunted EC autophagy initiation and eNOS activation in response to active hyperaemia (Figure 4); (iv) inhibiting autophagy or disrupting P2Y1-Rs in the vasculature of adult mice phenocopies age-related vascular dysfunction (Figures 6 and 7); and (v) P2Y1-R activation rejuvenates intraluminal flow-mediated vasodilation that is otherwise attenuated in arteries from mice after pharmacological, genetic, and aging-associated EC autophagy compromise (Figure 6). These results provide solid evidence that age-associated arterial dysfunction concurrent with repressed EC autophagy can be re-established by activating purinergic autocrine signalling.
4.1. Autophagy and NO generation is impaired in arterial ECs from older vs. adult humans and mice in response to elevated shear-rate
While dysregulated autophagic activity is associated with age-related pathologies including neurodegeneration, cancer, and immunosuppression, knowledge concerning the contribution from this process to aging-related cardiovascular complications in general29–31 and EC dysfunction in particular,2,6–8,32 is evolving. From our search of the available literature, the influence of aging on arterial EC autophagy has been reported once in humans under basal conditions.5 In that study, lower protein expression of Beclin-1 together with p62 accumulation was observed in ECs obtained via BA j-wire from older (61–71 years) vs. adult (20–31 years) subjects.5 In contrast, we observed similar Beclin-1, Atg3, LC3B + LAMP1 colocalization, and p62 protein expression in radial artery ECs at baseline (i.e. RHE-Pre) in older (68±2 years) vs. adult (23±1 years) participants. The discrepant findings concerning basal autophagy are curious because EC biopsy procedures and immunofluorescent staining protocols were similar between reports. Of greater importance is our novel finding that ECs from older subjects are unresponsive to physiological elevations of arterial shear-rate concerning autophagy initiation and eNOS activation. Specifically, in addition to a lack of shear-induced p-eNOSS1177 and NO generation, ECs from older vs. adult volunteers were refractory to phagophore initiation (Beclin-1), autophagosome formation and maturation (Atg3 and LC3B puncta), autophagosome fusion with the lysosome (LC3B + LAMP1 colocalization), and lysosomal degradation of autophagosome contents (p62), in response to active hyperaemia evoked by 60-min RHE (Figures 1, 2, and Supplementary material online, Figure S2). It is important to note that these results in ECs were obtained despite BA blood flow velocity, absolute shear-rate, and the fold-increase in shear-rate above baseline being similar between adult and older subjects. Based on these data, the lack of eNOS activation and NO generation we observed previously in BAECs and HAECs exposed to shear stress after genetic and pharmacological autophagy compromise6,7 appears to translate to primary arterial ECs from older humans with physiological repression of EC autophagy.
Substantiating our earlier findings from HAECs after genetic autophagy repression, primary ECs from mice with age-associated autophagy compromise displayed blunted autophagic flux, glycolysis, ATP production, and eNOS activation in response to physiological shear stress in vitro. Next, we determined that responses to shear stress in vitro could be recapitulated in ECs exposed to shear stress evoked by active hyperaemia. We report for the first time that aging mitigates the ability of arterial ECs to respond to active hyperaemia concerning EC autophagy upregulation. Specifically, 60 min of submaximal treadmill-running elevated Atg3, LC3B, and p-eNOSS1177 in aortic ECs from adult but not older mice (Figure 5).
4.2. Repressed shear-stress-induced NO generation after EC autophagy diminution in vitro translates to impaired intraluminal flow-mediated arterial vasodilation examined ex vivo
We reasoned that a failure of shear-stress/active hyperaemia-induced EC autophagy, glycolysis, and ATP production might limit eNOS activation and precipitate impaired intraluminal flow-mediated vasodilation displayed by arteries from old vs. adult mice. In support of the contribution from NO to vasodilation, NOS inhibition abrogated vasodilatory capacity displayed by femoral arteries from adult mice with intact EC autophagy, but not older mice with compromised EC autophagy. However, because the circulating milieu associated with aging is complex, and factors other than depressed EC autophagy certainly contribute to endothelial dysfunction in this context, we tested and demonstrated that vascular autophagy inhibition per se (Figure 6A), and EC-specific autophagy depletion (Figure 6H), are sufficient to phenocopy age-associated endothelial dysfunction. These data are the first to demonstrate that EC autophagy depletion is sufficient to attenuate endothelial function. As such, age-associated reductions in arterial EC autophagy contribute importantly to limiting vasodilatory capacity exhibited by older rodents and humans.
4.3. Impaired intraluminal flow-mediated vasodilation after pharmacological, genetic, or aging-associated EC autophagy compromise is rejuvenated by targeting P2Y1-Rs
In a previous study, we determined a molecular pathway that links repressed EC autophagy to impaired NO generation in vitro. Specifically, ECs with genetic depletion of autophagy exhibit diminished EC glycolysis and attenuated intra and extracellular ATP accumulation when exposed to shear-stress.7 Since studies using cultured ECs24,25 and isolated cerebral arteries33 documented that ATP degradation products (e.g. ADP) activate P2Y1-Rs and trigger NO generation, we evaluated this pathway further. Gain of function maneuvers that restored purinergic signalling (e.g. GLUT1 overexpression; 2-Me-ADP) rescued shear-induced p-eNOSS1177 and NO production in BAECs with impaired autophagy.7 Here we determined whether key results from BAECs studied in vitro after genetic autophagy disruption can be translated to primary arterial ECs and arteries from older mice with physiological autophagy compromise. First, we confirmed that 2-Me-ADP rescues shear stress-induced p-eNOSS1177 and NO generation in HAECs that is otherwise prevented by pharmacological (Supplementary material online, Figure S8) and genetic (Supplementary material online, Figure S12) autophagy disruption. Demonstrating functional relevance, 2-Me-ADP improved intraluminal flow-mediated vasodilation that was otherwise attenuated in (i) femoral arteries from aged mice with diminished basal EC autophagy and blunted hyperaemia-induced EC autophagy (Figure 6) and (ii) adult mice with EC-specific depletion of autophagy (i.e. iecAtg3KO mice; Figure 6). If P2Y1-R activation preserves arterial function in a manner that is independent from defective autophagy, we predict that 2-Me-ADP should improve vasodilatory capacity even in the presence of pharmacological, genetic, and physiological autophagy compromise. In support of this, otherwise blunted intraluminal flow-mediated vasodilation displayed by arteries from (i) older mice treated with 3-MA; (ii) adult mice treated with 3-MA; and (iii) adult iecAtg3KO mice, is improved by concurrent treatment with 2-Me-ADP (Figure 6).
Loss of function approaches also were used in our previous study to confirm whether purinergic signalling serves as a link between repressed EC autophagy and impaired NO generation in vitro. For example, inhibiting glucose-transport via GLUT1 siRNA, blocking purinergic signalling via ectonucleotidase-mediated ATP/ADP degradation (e.g. apyrase), or inhibiting P2Y1-Rs using pharmacological (e.g. MRS2179) or genetic (e.g. P2Y1-R siRNA) procedures, prevented shear-induced p-eNOSS1177 and NO generation in ECs with intact autophagy.7 We sought to translate these results from ECs studied in vitro to arteries evaluated ex vivo. For example, if purinergic signalling to eNOS is an important component of intraluminal flow-mediated vasodilation in arteries from adult mice with intact autophagy, then disrupting P2Y1-R activation should precipitate a defect in the response. After validating that ADP-induced p-eNOSS1177 is negated by concurrent treatment with MRS2179 in HAECs, we demonstrated that robust intraluminal flow-mediated vasodilation displayed by arteries from adult mice could be attenuated by luminal incubation with this P2Y1-R blocker, to values not different from those exhibited by arteries from older mice (Figure 7). These results were substantiated using arteries from mice with germline P2Y1-R deficiency.
4.4. Experimental considerations
Several considerations should be addressed when integrating our findings into what is currently known. First, because a relatively low yield of ECs/protein is obtained via j-wire biopsy of the radial artery endothelium of male subjects, we used immunofluorescence to quantify protein expression vs. immunoblotting.8–10,34 Nevertheless, in an attempt to maximize accuracy of our measurements, 3–4 j-wires were used per time point per subject, and fluorescence intensity from ∼50 ECs per endpoint per subject were averaged. At present, procedures using ECs obtained via j-wire (healthy volunteers) and/or arterial catheter sheath (patients with suspected or confirmed cardiovascular disease) are being optimized for single-cell RNAseq in an attempt to enhance translational relevance of our future investigations. Second, we confirmed compromised EC autophagy (carotid arteries/iliac arteries) in older vs. adult mice in a perfusion territory different from where function was assessed (i.e. femoral arteries). However, several lines of evidence give us confidence that a cause and effect relationship does exist between repressed EC autophagy and impaired intraluminal flow-mediated vasodilation. For example, the pattern of age-related autophagy compromise is similar between the iliac artery (Figure 4) and the carotid artery (Supplementary material online, Figure S4), two perfusion territories that are relatively distant from one another. As such, it is not unreasonable to speculate that two vessel segments that are adjacent i.e. the iliac and femoral arteries, would be similar with regard to EC autophagy status. Third, because our experiments were completed using male humans and mice, sex differences could not be investigated. This important issue will be addressed.
5. Conclusions
Evidence from our laboratory and others indicate aging is associated with repressed vascular autophagy and impaired NO-mediated arterial function, but a confirmed link has not been established. eNOS is regulated at multiple levels,35 including via nucleotide activation of purinergic receptors.25,36,37 Our earlier findings in vitro indicate that genetic repression of autophagy precipitates defects in purinergic signalling to eNOS that can be rescued. Here we provide solid evidence that dysfunction displayed by aged arteries with repressed EC autophagy can be re-established by pharmacological promotion of purinergic autocrine signalling. Further research concerning the G protein-coupled P2Y family of receptors is warranted in an effort to explore new therapeutic treatment options for aging-related endothelial dysfunction that is secondary to or associated with defective EC autophagy.
Supplementary Material
Contributor Information
Jae Min Cho, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA; Division of Endocrinology, Metabolism and Diabetes, Program in Molecular Medicine University of Utah School of Medicine, Salt Lake City, UT, USA.
Seul-Ki Park, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA; Division of Endocrinology, Metabolism and Diabetes, Program in Molecular Medicine University of Utah School of Medicine, Salt Lake City, UT, USA.
Oh Sung Kwon, Department of Kinesiology, University of Connecticut, Storrs, CT, USA; Department of Orthopedic Surgery & Center on Aging, University of Connecticut School of Medicine, Storrs, CT, USA.
David Taylor La Salle, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA; Geriatric Research, Education, and Clinical Center, George E. Whalen Veterans Affairs Medical Center, Salt Lake City, UT, USA.
James Cerbie, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA; Geriatric Research, Education, and Clinical Center, George E. Whalen Veterans Affairs Medical Center, Salt Lake City, UT, USA.
Caitlin C Fermoyle, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA; Geriatric Research, Education, and Clinical Center, George E. Whalen Veterans Affairs Medical Center, Salt Lake City, UT, USA.
David Morgan, Department of Anesthesiology, University of Utah, Salt Lake City, UT, USA.
Ashley Nelson, Geriatric Research, Education, and Clinical Center, George E. Whalen Veterans Affairs Medical Center, Salt Lake City, UT, USA.
Amber Bledsoe, Department of Anesthesiology, University of Utah, Salt Lake City, UT, USA.
Leena P Bharath, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA; Division of Endocrinology, Metabolism and Diabetes, Program in Molecular Medicine University of Utah School of Medicine, Salt Lake City, UT, USA.
Megan Tandar, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA.
Satya P Kunapuli, Sol Sherry Thrombosis Research Center, Lewis Katz School of Medicine, Temple University, Philadelphia, PA, USA.
Russell S Richardson, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA; Geriatric Research, Education, and Clinical Center, George E. Whalen Veterans Affairs Medical Center, Salt Lake City, UT, USA; Department of Internal Medicine, Division of Geriatrics, University of Utah, Salt Lake City, UT, USA.
Pon Velayutham Anandh Babu, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA.
Sohom Mookherjee, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA; Division of Endocrinology, Metabolism and Diabetes, Program in Molecular Medicine University of Utah School of Medicine, Salt Lake City, UT, USA.
Bellamkonda K Kishore, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA; Nephrology Research, George E. Whalen VA Medical Center, Salt Lake City, UT, USA; Department of Internal Medicine, Division of Nephrology, University of Utah, Salt Lake City, UT, USA.
Fei Wang, Nephrology Research, George E. Whalen VA Medical Center, Salt Lake City, UT, USA; Department of Internal Medicine, Division of Nephrology, University of Utah, Salt Lake City, UT, USA.
Tianxin Yang, Nephrology Research, George E. Whalen VA Medical Center, Salt Lake City, UT, USA; Department of Internal Medicine, Division of Nephrology, University of Utah, Salt Lake City, UT, USA.
Sihem Boudina, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA; Division of Endocrinology, Metabolism and Diabetes, Program in Molecular Medicine University of Utah School of Medicine, Salt Lake City, UT, USA.
Joel D Trinity, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA; Geriatric Research, Education, and Clinical Center, George E. Whalen Veterans Affairs Medical Center, Salt Lake City, UT, USA; Department of Internal Medicine, Division of Geriatrics, University of Utah, Salt Lake City, UT, USA.
John David Symons, Department of Nutrition and Integrative Physiology, University of Utah, Salt Lake City, UT, USA; Division of Endocrinology, Metabolism and Diabetes, Program in Molecular Medicine University of Utah School of Medicine, Salt Lake City, UT, USA.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Author contributions
J.M.C., S.K.P., J.D.T., L.P.B., S.B., and J.D.S. contributed to the conception and design of the study. J.M.C., S.K.P., O.S.K., D.T.L.S., F.W., T.Y., J.C., M.T., J.D.T., and J.D.S. performed experiments and data acquisition. D.M., A.N., and A.B. collected endothelial cells from human subjects. S.P.K. contributed reagents. J.M.C., S.K.P., and J.D.S. wrote the manuscript. All authors provided important intellectual contributions.
Funding
Support provided by the following: American Heart Association (20PRE35110066 to J.M.C., 17POST33670663 to S.K.P., and AHA16GRNT31050004 to J.D.S.); National Institute of Food and Agriculture ( 2019-67017-29253 to P.V.A.B.), U.S. Department of Veterans Affairs (1BX000596-09 to B.K.K. and I01CX001999 to J.D.T.), and National Institutes of Health (R01AT010247 to P.V.A.B., R01HL149870-01A1 to S.B., R01HL142603 to J.D.T., and RO3AGO52848 and RO1HL141540 to J.D.S.).
Data availability
Data are available in the article and the online supplementary material.
References
- 1. Kuma A, Mizushima N. Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Semin Cell Dev Biol 2010;21:683–690. [DOI] [PubMed] [Google Scholar]
- 2. Nussenzweig SC, Verma S, Finkel T. The role of autophagy in vascular biology. Circ Res 2015;116:480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell 2011;146:682–695. [DOI] [PubMed] [Google Scholar]
- 4. Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med 2013;368:651–662. [DOI] [PubMed] [Google Scholar]
- 5. Larocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol 2012;July 15:3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, Goodrich R, Mills T, Deeter L, Sargsyan A, Anandh Babu PV, Graham TE, Symons JD. Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can J Physiol Pharmacol 2014;92:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bharath LP, Cho JM, Park S-K, Ruan T, Li Y, Mueller R, Bean T, Reese V, Richardson RS, Cai J, Sargsyan A, Pires K, Anandh Babu PV, Boudina S, Graham TE, Symons JD. Endothelial cell autophagy maintains shear stress-induced nitric oxide generation via glycolysis-dependent purinergic signaling to endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol 2017;37:1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park SK, La Salle DT, Cerbie J, Cho JM, Bledsoe A, Nelson A, Morgan DE, Richardson RS, Shiu YT, Boudina S, Trinity JD, Symons JD. Elevated arterial shear rate increases indexes of endothelial cell autophagy and nitric oxide synthase activation in humans. Am J Physiol Heart Circ Physiol 2019;316:H106–H112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casey DP, Ueda K, Wegman-Points L, Pierce GL. Muscle contraction induced arterial shear stress increases endothelial nitric oxide synthase phosphorylation in humans. Am J Physiol Heart Circ Physiol 2017;313:H854–H859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fetterman JL, Holbrook M, Flint N, Feng B, Bretomicronn-Romero R, Linder EA, Berk BD, Duess MA, Farb MG, Gokce N, Shirihai OS, Hamburg NM, Vita JA. Restoration of autophagy in endothelial cells from patients with diabetes mellitus improves nitric oxide signaling. Atherosclerosis 2016;247:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang XJ, Chen S, Huang KX, Le WD. Why should autophagic flux be assessed? Acta Pharmacol Sin 2013;34:595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy 2007;3:542–545. [DOI] [PubMed] [Google Scholar]
- 13. Gottlieb RA, Andres AM, Sin J, Taylor DP. Untangling autophagy measurements: all fluxed up. Circ Res 2015;116:504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bharath LP, Ruan T, Li Y, Ravindran A, Wan X, Nhan JK, Walker ML, Deeter L, Goodrich R, Johnson E, Munday D, Mueller R, Kunz D, Jones D, Reese V, Summers SA, Babu PVA, Holland WL, Zhang Q-J, Abel ED, Symons JD. Ceramide-initiated protein phosphatase 2A activation contributes to arterial dysfunction in vivo. Diabetes 2015;64:3914–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res 2009;104:1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Q-J, Holland WL, Wilson L, Tanner JM, Kearns D, Cahoon JM, Pettey D, Losee J, Duncan B, Gale D, Kowalski CA, Deeter N, Nichols A, Deesing M, Arrant C, Ruan T, Boehme C, McCamey DR, Rou J, Ambal K, Narra KK, Summers SA, Abel ED, Symons JD. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes 2012;61:1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang Q-J, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J Physiol 2009;587:3911–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vion AC, Kheloufi M, Hammoutene A, Poisson J, Lasselin J, Devue C, Pic I, Dupont N, Busse J, Stark K, Lafaurie-Janvore J, Barakat AI, Loyer X, Souyri M, Viollet B, Julia P, Tedgui A, Codogno P, Boulanger CM, Rautou PE. Autophagy is required for endothelial cell alignment and atheroprotection under physiological blood flow. Proc Natl Acad Sci USA 2017;114:E8675–E8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, Harrison DG. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res 2014;114:616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cai J, Pires KM, Ferhat M, Chaurasia B, Buffolo MA, Smalling R, Sargsyan A, Atkinson DL, Summers SA, Graham TE, Boudina S. Autophagy ablation in adipocytes induces insulin resistance and reveals roles for lipid peroxide and Nrf2 signaling in adipose-liver crosstalk. Cell Rep 2018;25:1708–1717. e1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 2009;137:1124–1135. [DOI] [PubMed] [Google Scholar]
- 22. Cho JM, Park SK, Ghosh R, Ly K, Ramous C, Thompson L, Hansen M, Mattera M, Pires KM, Ferhat M, Mookherjee S, Whitehead KJ, Carter K, Buffolo M, Boudina S, Symons JD. Late-in-life treadmill training rejuvenates autophagy, protein aggregate clearance, and function in mouse hearts. Aging Cell 2021;20:e13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liverani E, Rico MC, Tsygankov AY, Kilpatrick LE, Kunapuli SP. P2Y12 receptor modulates sepsis-induced inflammation. Arterioscler Thromb Vasc Biol 2016;36:961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat 1999;194(Pt 3):335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. da Silva CG, Specht A, Wegiel B, Ferran C, Kaczmarek E. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation 2009;119:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, Redin C, Boudina S, Gygi SP, Brivet M, Thummel CS, Rutter J. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 2012;337:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caro LH, Plomp PJ, Wolvetang EJ, Kerkhof C, Meijer AJ. 3-Methyladenine, an inhibitor of autophagy, has multiple effects on metabolism. Eur J Biochem 1988;175:325–329. [DOI] [PubMed] [Google Scholar]
- 28. Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 2010;285:10850–10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. J Clin Invest 2015;125:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghosh R, Vinod V, Symons JD, Boudina S. Protein and mitochondria quality control mechanisms and cardiac aging. Cells 2020;9:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Torisu K, Singh KK, Torisu T, Lovren F, Liu J, Pan Y, Quan A, Ramadan A, Al-Omran M, Pankova N, Boyd SR, Verma S, Finkel T. Intact endothelial autophagy is required to maintain vascular lipid homeostasis. Aging Cell 2016;15:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang F. Autophagy in vascular endothelial cells. Clin Exp Pharmacol Physiol 2016;43:1021–1028. [DOI] [PubMed] [Google Scholar]
- 33. You J, Johnson TD, Childres WF, Bryan RM, Jr. Endothelial-mediated dilations of rat middle cerebral arteries by ATP and ADP. Am J Physiol 1997;273:H1472–1477. [DOI] [PubMed] [Google Scholar]
- 34. Hughes WE, Chabowski DS, Ait-Aissa K, Fetterman JL, Hockenberry J, Beyer AM, Gutterman DD. Critical interaction between telomerase and autophagy in mediating flow-induced human arteriolar vasodilation. Arterioscler Thromb Vasc Biol 2021;41:446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res 2007;75:247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andrews AM, Jaron D, Buerk DG, Barbee KA. Shear stress-induced NO production is dependent on ATP autocrine signaling and capacitative calcium entry. Cell Mol Bioeng 2014;7:510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peti-Peterdi J, Kishore BK, Pluznick JL. Regulation of vascular and renal function by metabolite receptors. Annu Rev Physiol 2016;78:391–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the article and the online supplementary material.