Abstract
Heat stress tolerance in plants is a complex trait controlled by multiple genes of minor effect which are influenced by the environment and this makes breeding and selection complicated. Emmer wheat (Triticum dicoccon Schrank) carries valuable diversity that can be used to improve the heat tolerance of modern bread wheat. A diverse set of emmer-based genotypes was developed by crossing emmer wheat with hexaploid wheat. These materials, along with their hexaploid recurrent parents and commercial cultivars, were evaluated at optimum (E1) and heat stressed (E2) sowing times in the field for three consecutive years (2014-2016). The material was genotyped using the Infinium iSelect SNP 90K SNP Assay. The phenotypic data were combined across years within each sowing time and best linear unbiased estimators calculated for each genotype in each environment. These estimates were used for GWAS analysis. Significant phenotypic and genotypic variation was observed for all traits. A total of 125 and 142 marker-trait associations (MTAs) were identified in E1 and E2, respectively. The highest number of MTAs were observed on the A genome (106), followed by the B (105) and D (56) genomes. MTAs with pleiotropic effects within and across the environments were observed. Many of the MTAs found were reported previously for various traits, and a few significant MTAs under heat stress were new and linked to emmer genome. Genomic regions identified on chromosomes 2B and 3A had a significant positive impact on grain yield under stress with a 7% allelic effect. Genomic regions on chromosomes 1A and 4B contributed 11% and 9% of the variation for thousand kernel weight (TKW) under heat stress respectively. Following fine mapping, these regions could be used for marker-assisted selection to improve heat tolerance in wheat.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11032-021-01222-3.
Keywords: GWAS, Heat tolerance, Emmer wheat, Genetic variation
Introduction
Heat stress tolerance is a complex trait controlled by many genes of minor effect that are highly influenced by the environment and this makes breeding and selection complicated (Howarth 2005; Bohnert et al. 2006). In field crops, primary gene pool relatives can be used to create new genetic variation for the improvement of traits controlling heat tolerance (Nevo and Chen 2010). Emmer wheat (Triticum dicoccon Schrank) has potentially valuable genetic diversity that can be used to improve heat tolerance of modern wheat cultivars (Zaharieva et al. 2010; Nevo 2014). Introgression of favorable alleles from close relatives of wheat into modern cultivars can improve their abiotic stress tolerance (Ullah et al. 2018; Ullah et al. 2021). Gradual addition of wild alleles into the modern wheat genome can enhance the tolerance of crops to environmental stresses without losing agronomically important traits (Trethowan 2014). While conventional breeding methods have led to improvements in heat tolerance, the rate of progress has been relatively slow. Identification of quantitative trait loci (QTL) linked to heat tolerance would improve the effectiveness of breeding and selection by advancing the rate of genetic progress (Huang and Han 2014; Mwadzingeni et al. 2017).
Genome sequences are available for a number of crop species and effective single nucleotide polymorphism (SNP) genotyping assays are available in wheat (Varshney et al. 2011; Bazakos et al. 2017). Genome-wide association studies (GWAS) or linkage disequilibrium (LD) mapping has been used to identify relationships between target traits and genetic markers (Gupta et al. 2014; Bazakos et al. 2017). This procedure requires a large set of diverse genotypes with accompanying genotypic and phenotypic data (Mackay and Powell 2007; Mitchell-Olds 2010). Initially, genotyping was expensive, which limited the adoption of GWAS; however, this has changed with recent advances in low-cost high-throughput sequencing technologies and data processing tools (Provart et al. 2016). High-density genome-wide SNP arrays such as Infinium iSelect SNP 90K are regularly used to identify QTLs using GWAS in wheat (Cook et al. 2012; Wang et al. 2014).
GWAS has been used to identify QTLs linked to simply inherited traits in wheat (Rasheed et al. 2014; Ogbonnaya et al. 2017). However, for more complex traits such as abiotic stress tolerance, GWAS is still limited by effective, accurate, and relevant phenotyping methods that can be applied to large populations (Chen et al. 2017). As the genes controlling abiotic stress traits are greatly influenced by the environment, multi-environment trials (METs) permit the estimation of genotype-by-environment interaction and therefore provide evidence of the stability of key traits (Ma’arup et al. 2020). Nevertheless, continued testing is required to improve the accuracy of broad-scale field screening (Trethowan 2014; Ogbonnaya et al. 2017).
GWAS studies have been used extensively to identify allelic variation in hexaploid and tetraploid wheat under different climatic conditions for abiotic stress tolerance traits (Wang et al. 2014; Sukumaran et al. 2018). However, limited information is available on allelic diversity in wild relatives that could be used to improve abiotic stress tolerance of modern wheat (Sehgal et al. 2015; Gupta et al. 2019). Nevertheless, what information is available suggests that unique chromosomal regions in emmer wheat do influence abiotic stress tolerance when transferred to bread wheat (Ma’arup 2016; Chandrasekhar et al. 2017). Some studies have documented the direct introgression of traits from emmer wheat into bread wheat including rust resistance (Liu et al. 2017), grain protein content (Liu et al. 2019; Ullah et al. 2020), water use efficiency (Ma’arup et al. 2020), and improved heat tolerance (Ullah et al. 2018, 2021).
It is important that the phenotypes used for GWAS are relevant to the target environment. For this reason, field-based screening provides the best estimation of phenotype if environmental variation can be limited or assessed. However, there are very few reports of heat tolerance assessed in the field on large populations for physiological, phenological, quality, and yield traits. This study used multi-year, multi-environment phenotypic data assessed on a large and diverse population of hexaploid emmer-based wheat materials to conduct a GWAS to identify marker-trait associations (MTAs) linked to yield and agro-physiological traits in north-western NSW.
Materials and methods
Plant material and environment
The experimental materials comprised 542 lines, including emmer-based hexaploid wheat genotypes, their bread wheat parents and commercial check cultivars (Supplementary Table 1). The details of the emmer-based lines and cross combinations used to develop these materials are given in Ullah et al. (2018).
Field experiments were established at the IA Watson Grains Research Centre, The University of Sydney, Narrabri, NSW, during the cropping seasons of 2014, 2015, and 2016. Each year, experiment 1 (E1) was sown at the optimal sowing date for the region in mid-May and experiment 2 (E2) was sown 8 weeks later. E1 and E2 experiments were sown adjacent to each other. Experiments were arranged in randomized complete block designs with two replications arranged in a regular grid for each time of sowing. Plots were trimmed to 8 m2 prior to harvest to reduce border effects. Irrigation was used as required to limit drought stress. Further details of the experiments and environmental data are provided in Ullah et al. (2019).
Phenotyping data assessment
The phenotypic data was assessed following the protocols described by Pask et al. (2012). Data recorded included days to flowering and maturity, normalized difference vegetative index (NDVI) at anthesis (Z61) and milk stage (Z73), grain filling period, plant height (cm), thousand kernel weight (g), screenings (%), and grain yield (t ha−1) (Table 1). The methods of collection are given in Ullah et al. (2019).
Table 1.
Traits assessed in each year and used in GWAS
| Year | |||
|---|---|---|---|
| 1Traits | 2014 | 2015 | 2016 |
| NDA | ✓ | ✓ | ✓ |
| NDM | ✓ | ✓ | ✓ |
| DTF (days) | ✓ | ✓ | ✓ |
| DTM (days) | ✓ | ✓ | ✓ |
| PH (cm) | ✓ | ✓ | ✓ |
| GFP (days) | ✓ | ✓ | ✓ |
| TKW (g) | ✓ | ✓ | ✓ |
| SCR (%) | ✓ | ✓ | ✓ |
| GY (t ha−1) | ✓ | ✓ | ✓ |
NDA NDVI at anthesis, NDM NDVI at milk, NDVI normalized difference vegetation index, DTF days to flowering, DTM days to maturity, PH plant height, GFP grain filling period, TKW thousand kernel weight, Scr screenings, GY grain yield
Statistical analysis of phenotypic data
To compute the Wald statistics, a combined analysis of genotypes across the 3 years, was conducted using the linear mixed model component of the residual maximum likelihood (REML) function in GenStat version 16.0 (Payne et al. 2011). Genotypes, environments, and their interaction were considered fixed terms and ranges/rows within environments random terms in the model. Spatial correction of field trial data and the generation of best linear unbiased estimators (BLUEs) were performed for individual experiments and sowing time combined across years using autocorrelation models (Fikere et al. 2020a; Fikere et al. 2020b). Broad sense heritability (H) was estimated as described in Fikere et al. (2018) using restricted maximum likelihood (REML) in ASReml version 3 (Gilmour et al. 2009). Specifically, BLUEs were fitted as phenotypes and lines were fitted as independent random effects for H. Correlation among variables was estimated on phenotypic data using R (R Core Team 2018).
SNP genotyping and data analysis
DNA of all genotypes was extracted in the molecular laboratory of The Plant Breeding Institute, Cobbitty, following the CTAB method given by Doyle and Doyle (1990). The material was genotyped using the Infinium iSelect 90K SNP Assay (Wang et al. 2014; Cavanagh et al. 2013) by AgriBio, La Trobe University, Victoria, Australia, following the protocol prescribed by the manufacturer. The phenotypic data, as described above, were combined across all 3 years within each sowing time and BLUEs calculated for each genotype in E1and E2. These estimates were used for subsequent GWAS analyses. A total of 41,666 quality controlled SNPs were used initially. The data was then formatted and filtered using the PLINK software http://pngu.mgh.harvard.edu/~purcell/plink/ to identify and maintain SNPs with call rates greater than 40% (Purcell et al. 2007; Anderson et al. 2010). SNPs without a map position were included in the analyses and SNPs with a minor allele frequency (MAF) <0.01 were excluded. Therefore, the SNP set changed from year-to-year depending on the number of lines in the phenotypic dataset. Following filtering, 35,266 SNP markers were generated. Once the genotype data was curated, the phenotype and SNP map files were produced for GWAS analyses. This analysis was performed using the genome-wide complex trait analysis (GCTA) software (Yang et al. 2011). The model fitted the overall mean (mu) and fixed SNP effects along with the genomic relationship matrix (GRM) to account for population structure in the samples, as given below:
Where y represents population structure in the sample (n1 vector of phenotypes with n being the sample size), mu denotes the overall mean, SNP is the fixed SNP effect, and GRM the genomic relationship matrix.
Following the linkage disequilibrium analysis, those marker-trait associations with a −log10 P value >3 were considered significant (P˂0.001). The significance of association was further tested using false discovery rate (FDR<0.001). The SNP markers used in this study segregated only within the population studied and not all markers are unique to one position. The physical map positions in centimorgan (cM) were determined where possible based on anchoring and ordering of next generation sequencing contig assemblies by population sequencing (PopSeq) (Mascher et al. 2013). Positions in cM were available only for markers that (i) had a physical position, (ii) were uniquely mapped within a chromosome, and (iii) were segregating in the population. The markers selected therefore had −log10 P>3, either a positive or negative effect on the trait and where relevant a pleiotropic effect.
Results
Climate and phenotypic data
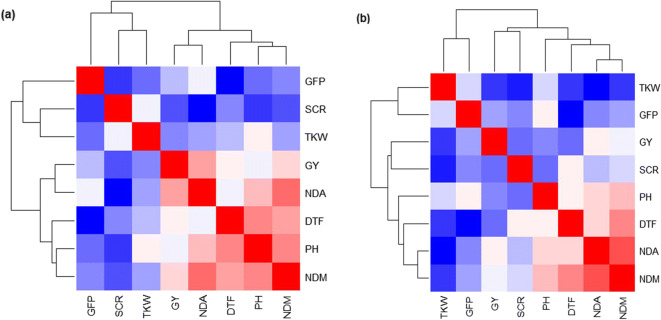
The late sown experiments experienced greater heat stress, mainly at the reproductive stage, as discussed previously (Ullah et al. 2019). The combined analysis of fixed effects (2014-2016) depicted significant differences for genotype and environment main effects and their interactions (P<0.05). Trait means were higher in E1 than E2 (Table 2). The highest heritability was observed for TKW, plant height, and days to flowering and the lowest for screenings percentage (Table 2). Higher temperature reduced grain yield (40%), kernel weight (25%), plant height (17%), and grain filling period (33%) and increased screenings (67%). Grain yield was positively associated with NDVI grain fill (anthesis and milk stage) and negatively correlated with screenings percentage across environments. Days to flowering were positively associated with grain yield in E1; however, a negative relationship was observed in E2. Plant height was positively associated with NDVI grain fill across environments (Fig. 1).
Table 2.
Means of various traits and Wald Statistics (2014-2016) in two contrasting environments (E1, optimal sowing; E2, delayed sowing—heat stressed)
| Environment | NDA | NDM | DTF (days) |
GFP (days) |
PH (cm) |
SCR (%) |
TKW (g) |
GY (t ha−1) |
|
|---|---|---|---|---|---|---|---|---|---|
| Wald Statistics | G | 1543.73*** | 1705.93*** | 54921.91*** | 2275.50*** | 10880.52*** | 10865.71*** | 16976.33*** | 1645.51*** |
| E | 5.42* | 5.12* | 298.73*** | 21.71*** | 20.31*** | 14.80*** | 11.73*** | 18.61*** | |
| G×E | 622.78*** | 684.14*** | 2424.81*** | 1292.31*** | 1255.22*** | 7737.21*** | 1654.43*** | 1123.52*** | |
| E1 | Max | 0.79 | 0.63 | 118.37 | 50.19 | 111.51 | 7.04 | 52.96 | 5.36 |
| Min | 0.78 | 0.58 | 93.93 | 44.79 | 77.74 | 4.17 | 36.43 | 4.78 | |
| Av. | 0.78 | 0.61 | 112.21 | 47.25 | 101.97 | 5.17 | 44.92 | 5.09 | |
| Std. | 0.01 | 0.01 | 2.64 | 0.71 | 3.97 | 0.45 | 2.62 | 0.07 | |
| E2 | Max | 0.71 | 0.53 | 88.58 | 33.79 | 92.49 | 19.21 | 39.64 | 3.24 |
| Min | 0.68 | 0.45 | 76.25 | 33.68 | 58.49 | 15.46 | 27.39 | 3.03 | |
| Av. | 0.70 | 0.49 | 80.41 | 33.74 | 83.96 | 17.01 | 32.49 | 3.17 | |
| Std. | 0.01 | 0.01 | 1.88 | 0.01 | 4.21 | 0.46 | 2.42 | 0.03 | |
| Reduction % | 10 | 16 | 25 | 33 | 17 | 67 | 25 | 40 | |
| Heritability | 0.36 | 0.48 | 0.71 | 0.11 | 0.79 | 0.05 | 0.81 | 0.15 |
NDA NDVI at anthesis, NDM NDVI at milk, DTF days to flowering, PH plant height, GFP grain filling period, TKW thousand kernel weight, Scr screenings, GY grain yield
Fig. 1.
Correlation coefficients of key traits in two environments. (a) Optimal (E1) and (b) heat stress (E2), 2014-2016 combined. Greater intensity of red and blue color indicates increasingly positive and negative correlations, respectively. Abbreviations: NDA, NDVI at anthesis; NDM, NDVI at milk; DTF, days to flowering; PH, plant height; GFP, grain filling period; TKW, thousand kernel weight; Scr, screenings; GY, grain yield
Population structure and association analysis
The genetic relatedness among genotypes using SNP markers is represented as a heat map of the G matrix (Fig. 2a). A small to high range of relatedness was observed in the material as expected because some individuals within and across families shared the parents. Principle component (PC) analyses explained 89% of the variation and divided genotypes into 10 sub-groups (Fig. 2b).
Fig. 2.
Population structure analysis, (a) heat map of kinship matrix with the tree shown on the top and left, where yellow represents more and red represents less related individuals, (b) plot of the first two principal components in the selected populations
A total of 125 and 142 MTAs were identified at –log10 P>3 in E1 and E2, respectively (Supplementary Table 3-5). The highest number of MTAs were observed on the A genome (106), followed by the B (105) and D (56) genomes (Supplementary Table 3-5). The MTAs for each trait varied with the highest number observed for plant height (52) followed by TKW (51), DTF (37), NDVI anthesis (31), NDVI milk stage (24), GFP (23), DTM (23), grain yield (13), and screenings (13). The highest number of MTAs were detected on chromosomes 1B (27), 7A (22), 1A (20), and 3B (19) and the smallest on chromosomes 4D (3) and 7D (4). The significant MTAs were visualized in Manhattan plots in both E1 and E2 environments (Fig. 3).
Fig. 3.
Manhattan plots showing genome-wide significant MTAs for various traits and their chromosomal locations under optimum (E1) and heat stressed (E2) environments. Abbreviations: NDA, NDVI at anthesis; NDM, NDVI at milk; DTF, days to flowering; PH, plant height; GFP, grain filling period; TKW, thousand kernel weight; Scr, screenings; GY, grain yield
Agronomical traits
Thirteen MTAs were detected for grain yield in both environments. Seven MTAs were unique to E2 and one MTA on chromosome 7B (~25.79-31.06 cM) was consistent across both environments and had a pleiotropic effect on other traits across environments. Several regions on chromosomes 1A, 1D, 2A, 2B, 3A, 3D, 4B, 7A, and 7B were associated with grain yield and the highest number of MTAs for yield found on chromosome 3D (2) across environments. Chromosomes 1A, 1D, 2B, 3A, 3D, 7A, and 7B contained MTAs for grain yield in E2. However, 2 MTAs (SNP order 10229, 18412) located on chromosomes 2B (~108.31 cM) and 3A (~237.88 cM) respectively, had a significant positive impact on grain yield under stress with a 7% allelic effect (Fig. 4, Supplementary Table 3-5).
Fig. 4.
Heatmap of significant markers with positive and negative associations of traits in two environments (E1, optimum and E2, heat stressed). Red indicates a positive association (i.e., the presence of the marker associated with higher mean of phenotypic value) and blue indicates negative associations (i.e., the presence of the marker associated with lower mean of phenotypic value). Abbreviations: NDA, NDVI at anthesis; NDM, NDVI at milk; DTF, days to flowering; PH, plant height; GFP, grain filling period; TKW, thousand kernel weight; Scr, screenings; GY, grain yield
A total of 51 MTAs were detected for TKW, of these 15 were common to both environments. Five MTAs had a pleiotropic effect across both environments. Fourteen MTAs were linked to TKW in E2 only. The MTAs for TKW were scattered across the whole wheat genome in both environments. Chromosomes 7A (5), 6A (4), 5B (4), 3B (4), 3A (3), 2B (3), and 1A (3) contained the most loci controlling kernel weight under both normal and stressed environments. MTAs on chromosome 1A (~128.88 cM) and 4B (~225.27 cM) contributed 11% and 9% of the variation for this trait under heat stress respectively.
A total of 52 MTAs were observed for plant height and of these 15 were consistent across both environments (Supplementary Table 3-5). Thirteen MTAs were pleiotropic across environments and 4 within a specific environment. Six MTAs were unique to E1 and 17 to E2. A greater number of MTAs for plant height was observed on chromosomes 1B and 7A and no MTAs were found on chromosomes 4A, 7B, and 7D. The greatest variance (14%) was explained by an MTA (SNP order 37390) on 7A chromosome (~235 cM) under heat stress.
Grain quality traits
Wheat grain quality under heat stress was impacted by screenings. A total of 13 MTAs were identified for screenings (Fig. 4). An MTA located on 5B (~133.47-137.79 cM) chromosome with lower screenings under heat stress was linked to greater TKW under both environments. Seven MTAs were unique to E2. Two MTAs (SNP order 16529, 28359) located on chromosomes 2D (uncharacterized) and 5B (~133.47-137.79 cM) were associated with low screenings, and contributed 8% of the allelic variation under stress. Six MTAs were specific to E1. Of these, 4 MTAs were associated with reduced screenings and three were located on chromosomes 6B, 6D, and 7A (Supplementary Table 3-5).
Phenological traits
A total of 37 MTAs were detected across environments for days to flowering (Fig. 4). Sixteen MTAs were detected in E1 and 7 in E2, exclusively. MTAs were observed on all chromosomes except 4D, 5D, and 6D. Chromosomes 1B (3) carried the highest number of MTAs for days to flowering in E1 and 2A (3) in E2.
A total of 23 MTAs were detected across the two environments and only one MTA on 5A chromosome (~49.22-455.48 cM) was common to both E1 and E2 for grain filling period. Ten MTAs were unique to E1 and 11 to E2. Five MTAs had a positive impact on grain filling period in E1 (located on chromosomes 1B, 2A, 3B, 4A, and 4B) and 2 in E2 (located on chromosome 5B). Greater numbers of MTAs were associated with chromosome 1B (2), 3B (2), 4A (2), and 5A (2) in E1 and 1A (2) and 5B (2) in E2.
Physiological traits
Seventeen MTAs for NDVI at anthesis were detected in E2 only. Most of the MTAs for NDVI at anthesis were located on chromosomes 1A, 1B, 2D, and 3B across environments. An MTA (SNP order 277) on chromosome 1A (~82.57-135.18 cM) contributed up to 9% of variation for the trait in heat stressed environment. A total of 24 MTAs for NDVI at milk stage (grain filling period) were detected across both environments. Overall, 6 and 12 MTAs were specific to E1 and E2, respectively. All chromosomes except 3A, 4B, and 5B contained MTAs for NDVI at milk stage.
Co-located MTAs
Several MTAs were associated with two or more traits, both across (Table 3) and within (Table 4) environments. Markers with pleiotropic effects were located on all chromosomes except 3A, 4A, 4B, 4D, and 7D. The highest number of pleiotropic effects was observed on chromosomes 3D, 7A, and 5B.
Table 3.
Significant MTAs (−log10 P>3) with pleiotropic effects on traits across the environments (E1, optimal sowing; E2, delayed sowing—heat stressed)
| 1Trait | Chr | SNP | Cumulative bp | Probe ID | Position cM | Environment |
|---|---|---|---|---|---|---|
| DTF, DTM, PH | 2A | 8928 | 7473398 | 11194_2A | 138.31-154.39 | E1, E2 |
| DTM, NDA, PH | 3D | 21970 | 11570881 | 34976_3D | 130.33 | E1, E2 |
| DTM, NDA, PH | 2D | 16467 | 509980 | 668_2D | Uncharacterized | E1, E2 |
| NDA, GY | 2B | 10229 | 519653 | 65439_2B | 108.31 | E1, E2 |
| DTF, GFP, TKW, GY | 7B | 38685 | 938870 | 70085_7B | 27.15 | E1, E2 |
| NDM, TKW | 1B | 4785 | 2300607 | 28242_1B | 232.97-292.81 | E1, E2 |
| PH, TKW | 1A | 333 | 2433212 | 36084_1A | 128.75 | E1, E2 |
| DTF, GFP, PH, TKW | 7A | 37549 | 3426422 | 45179_7A | 266.81 | E1, E2 |
NDA NDVI at anthesis, NDM NDVI at milk, DTF days to flowering, DTM days to maturity, PH plant height, GFP grain filling period, TKW thousand kernel weight, GY grain yield
Table 4.
Significant MTAs (−log10 P>3) with pleiotropic effects on traits within a specific environment (E1, optimal sowing; E2, delayed sowing—heat stressed)
| 1Trait | Chr | SNP | Cumulative bp | Probe ID | Position cM | Environment |
|---|---|---|---|---|---|---|
| DTF, GFP | 5B | 27743 | 7613 | 56932_5B | 91.29-99.67 | E2 |
| NDM, SCR | 6D | 35520 | 19348 | 60100_6D | 0-36.55 | E1 |
| NDA, NDM, TKW | 1B | 2526 | 105517 | 9851_1B | 86.66-194.31 | E2 |
| DTF, DTM | 2D | 16462 | 341033 | 58635_2D | 157.28-160.11 | E1 |
| DTF, GY | 7B | 38648 | 378485 | 27367_7B | 3.26 | E2 |
| NDM, TKW | 6A | 31996 | 4478405 | 65922_6A | 159.37-160.12 | E2 |
| GFP, TKW | 5B | 28997 | 5447965 | 78397_5B | 179.61 | E2 |
| NDA, PH | 5D | 30767 | 6859445 | 22168_5D | 128.68 | E2 |
| NDA, TKW, DTM, GY | 1A | 1049 | 12898830 | 25487_1A | 102.81 | E1 |
| DTM, NDA, GY | 1A | 361 | 18542971 | 63445_1A | 0-175.47 | E1 |
| NDA, GY | 3D | 21765 | 580383 | 45954_3D | Uncharacterized | E1 |
| DTF, PH | 2D | 16593 | 1261056 | 4432_2D | 157.28 | E2 |
| DTM, PH | 7A | 37803 | 1268906 | 25307_7A | 306.24 | E1 |
NDA NDVI at anthesis, NDM NDVI at milk, DTF days to flowering, DTM days to maturity, PH plant height, GFP grain filling period, TKW thousand kernel weight, Scr screenings, GY grain yield
The most significant SNP, 37549, located on chromosome 7A (~267 cM) had significant effects on days to flowering, grain filling period and yield in E2 and plant height and TKW in E1 explaining 10% of the variation (Table 3). The SNP 38688, located on 7B (~25.79-31.06 cM), had pleiotropic effects on days to flowering, grain filling period and yield in E2 and TKW and yield in E1, accounting for 6% allelic variation. A high frequency of pleiotropic associations were detected for NDVI at anthesis and plant height on chromosomes 1D, 3D, 5A, 5D, and 7A for both E1 and E2. Pleiotropic regions for increased plant height and greater TKW across environments were observed on chromosome 5B, 6B, 7A. Similarly, one marker (SNP 4785) was linked to a pleiotropic region on 1B (~232.97-292.81 cM) controlling higher NDVI (milk stage) and higher TKW in both E1 and E2 and contributed around 8% of the variation. Similarly, chromosome 3D (SNP order 21970, ~130.33 cM) had pleiotropic region for days to maturity, NDVI anthesis, plant height and yield in E1 and days to maturity, NDVI anthesis and milk, plant height, screenings, TKW and yield in E2 (accounting for 9% of the variation).
Many of the observed MTAs were pleiotropic in one environment only (Table 4). An MTA (SNP order 361) on chromosome 1A (~0-175.47 cM) for yield, NDVI at anthesis and days to maturity were co-located in E1. An MTA (SNP order 28997) increased grain filling period and TKW, located on 5B (~179.63 cM), was pleiotropic in E2. Similarly, TKW, NDVI at anthesis, and milk had pleiotropic effects in E2 located on chromosomes 1D, 1B, and 6B. Pleiotropic effects for days to flowering, days to maturity and grain filling period were frequently observed on various chromosomes in both E1 and E2. An MTA (SNP order 8928) on 2A (~138.31-154.39 cM) also positively influenced both NDVI anthesis, plant height, and yield in E2 and explained 9% of the phenotypic variation.
Discussion
Multi-trait GWAS was effective in identifying genomic regions linked to heat stress tolerance. Overall, greater numbers of MTAs were discovered on the A and B genomes as compared to the D genome and this is consistent with earlier findings (Quarrie et al. 2005; Ogbonnaya et al. 2017). The AB genome contribution is clearly greater but to a large extent reflects the poorer marker coverage of the D-genome (Ogbonnaya et al. 2017). Grain yield reduction is the cumulative outcome of heat stress on wheat. While some of the observed MTAs in this study were “yield” related in that they appeared in both environments, others such as SNP 10229 and 18412 located on 2B and 3A, respectively, were unique to heat stressed conditions and can be combined with the “yield” only MTAs to enhance wheat yield stability. Unfortunately, it has not been possible to confirm unique emmer alleles in these materials. The cluster positions contributed by emmer (a tetraploid) need to be reconciled with the 90K SNP cluster positions of the same SNPs in bread wheat (hexaploid). Nevertheless, the positive contributions of emmer wheat to yield are suggested by our previous work as many of the emmer-derived lines showed significantly greater yield than their recurrent bread wheat parents (Ullah et al. 2018; Ullah et al. 2021) and comparable yield to the commercial cultivar Suntop (Ullah et al. 2019). The presence of MTAs for yield in both environments on chromosomes 1A, 2A, 3A, 2B, 1D, 3D, 4B, 7A, and 7B validated previous findings (Lopes et al. 2015; Acuña-Galindo et al. 2015; Ogbonnaya et al. 2017).
MTAs associated with TKW were scattered across the whole genome in the current study making selection difficult. Nevertheless, there was a highly significant effect on 7A that influenced TKW in both environments that could be targeted in selection. Similar chromosomal locations of significant QTLs influencing TKW in emmer-derived material were reported under drought stress (Ma’arup 2016). Peng et al. (2003) reported significant QTL for grain size in emmer wheat located on chromosomes 6B and 7B. The region of 7B was also observed in the current study suggesting an influence of the emmer genome. These QTL regions on 7B chromosome were further validated by studies with genome analysis using emmer-derived lines under different environments for traits including grain yield, TKW, and days to flowering (Ma’arup 2016; Liu et al. 2019). The two MTAs related to low screenings (SNP order, 16529, 28359) under stress in this study provide good targets for breeding and selection if combined with the large QTL for higher TKW located on 6A. The positions of these two MTAs for screenings on chromosomes 2D and 5B are similar to those reported previously in modern wheat (Mason et al. 2013; Rasheed et al. 2014).
Some of the MTAs related to NDVI (milk, Z61) in the current study also validate previous findings (Pinto et al. 2010; Li et al. 2015; Gao et al. 2016). A number of MTAs for NDVI at anthesis located on chromosomes 2A, 3B, 5D, and 6B also partially validate earlier work. Senescence rates under heat stress were published by others (Vijayalakshmi et al. 2010; Bennett et al. 2012b; Shirdelmoghanloo et al. 2016) and these effects were located in similar positions on chromosomes 2A, 4A, 5A, 7A, 3B, 6B, 4D, and 5D to the current study. Limited genetic information is available on the emmer wheat contribution to heat tolerance in the materials derived. However, comparison with the recurrent hexaploid parent suggests that various unique alleles, attributable to emmer wheat, likely control the heat response.
There was a high degree of similarity observed between MTAs for days to flowering, grain filling duration, and maturity as expected. The same chromosomal locations observed on 1A, 1B, 3A, 3B, 4A, 5A, 5B, and 7A were also reported by others to control phenology under heat stress (Wang et al. 2009; Reynolds and Rebetzke 2011; Cossani and Reynolds 2012). It appears that photoperiod and vernalization influenced phenology to some degree as significant MTAs on 2A, 2B, and 2D, equivalent to the photoperiod genes Ppd-A1, Ppd-B1, and Ppd-D1 and 5A and 5B, equivalent to vernalization genes Vrn-A1 and Vrn-B2, were observed (Takenaka and Kawahara 2012; Lopes et al. 2015). The QTLs for late flowering on 2B and early flowering on 3A were reported in wild emmer wheat (Zhou et al. 2016). Most MTAs for plant height under stress observed in the current study were associated with chromosomes 2A, 2B, 3A, 3B, 5D, 6B, and 7A and this correlated with other studies (Marza et al. 2006; Maccaferri et al. 2008; Bennett et al. 2012a; Acuña-Galindo et al. 2015). The lack of strong associations on 4B and 4D suggest that the semi-dwarfing genes Rht-B1b and Rht-D1b were not influential (Ellis et al. 2002; Lopes et al. 2015). This was not surprising as all the materials evaluated were semi-dwarf in stature. MTAs for NDVI anthesis and milk stage were closely correlated in the current study. Those genotypes able to maintain greenness under both conditions generally produced higher yield (Ullah et al. 2019). The maintenance of greenness had a positive impact on grain filling duration and yield and it is likely that delayed senescence under heat stress was responsible (Vijayalakshmi et al. 2010).
A strong association among agronomic traits was observed in both environments indicating a degree of pleiotropy, as found by others in tetraploid wheat (Wang et al. 2019). Some MTAs (SNP order 8928) on chromosome 2A (~138.31-154.39 cM) had pleiotropic effects on increased plant height and longer days to flowering and maturity under both conditions. These observations indicate that plant biomass depends upon the length of the growth cycle as concluded in our previous study (Ullah et al. 2019). Some QTL (SNP order 38688) on chromosome 7B (~3.27 cM) with pleiotropic effects for reduced days to flowering and reduced grain yield under stress indicate that short duration genotypes have lower yield under heat stress in the current study. This is not always observed with many authors linking heat escape to earlier flowering (Mondal et al. 2013; Mondal et al. 2015). The frequently observed co-location of MTAs for NDVI at anthesis and plant height in the current study suggests that greenness at anthesis results in taller plants that yield more under heat stress. It may simply indicate higher photosynthetic reserves at anthesis that are later translocated to the developing grain. A number of studies associate physiological traits with plant stature and phenology (Bennett et al. 2012b; Rebetzke et al. 2013; Edae et al. 2014; Gao et al. 2016). One marker identified on chromosome 7B (~27.15 cM) had a pleiotropic effect on days to flowering, grain filling period, TKW, and yield. Similar results were obtained by Ogbonnaya et al. (2017) who found MTAs on the same chromosome associated with pleiotropic effects for days to flowering and yield components. However, other studies identified regions on chromosome 7D as pleiotropic for grain yield, days to flowering and TKW (Hanocq et al. 2004; Hai et al. 2008; Mason et al. 2013).
MTAs with pleiotropic effect on yield and NDVI anthesis in both environments were observed on chromosomes 2A, 2B, and 3D, whereas others reported an association between grain yield and greenness on 4D under heat stress (Bennett et al. 2012b). It may be that a mild photoperiod response linked to Ppd-A1 and Ppd-B1 (located on 2A and 2B) has contributed to greater biomass and hence higher NDVI at anthesis in these materials. The MTAs with pleiotropic effects on plant height under both conditions and greater TKW under stress located on chromosome 1A could be contributed by emmer wheat, although unconfirmed. Similar results were obtained from field phenotyping, where taller genotypes under stress were associated with greater TKW. Similarly, chromosome 7A had a pleiotropic region detected using SNP marker 37549 (~267 cM) controlling days to flowering, grain filling period, plant height TKW, and grain yield. Previous studies reported pleiotropic effects for various traits including plant height and TKW on 7A from GWAS in a Chinese wheat population (Gao et al. 2016). Others reported pleiotropic regions for height and TKW on chromosome 4B in hexaploid wheat (Huang et al. 2004; McCartney et al. 2005). Clearly, some chromosomes were more related to certain traits than others. Chromosomes 1B had more MTAs for plant height, and 6A had more MTAs for TKW under both conditions. The chromosomal regions on 6A were previously associated with grain quality in emmer-derived genotypes (Peleg et al. 2009; Xu et al. 2012).
In this study, a number of MTAs for agronomic, physiological, phenological, and quality traits were identified in both optimum and heat stress conditions in emmer-derived material. Some MTAs were specific to heat stress only and could be targeted in breeding to improve the heat stress tolerance of wheat. These regions can also be combined with those that contribute to yield in both environments to increase yield and yield stability. Chromosomal regions that control multiple traits were also identified and these should also be selection targets. This study also suggests that the basis of heat tolerance differs among genotypes and that the new AB genome diversity introduced from emmer wheat, may have contributed to these differential responses to high temperature. Nevertheless, the MTAs identified in this study must be validated in other materials in different environments before they can be used extensively in marker assisted selection and breeding.
Supplementary information
(DOCX 56 kb)
Acknowledgements
The writer is grateful to The University of Sydney, International Postgraduate Research Scholarship (IPRS) Scheme and the Australian Government International Research Training Program for the scholarship.
Author contributions
SU designed the experiments, collected the data, analyzed the results, and wrote this article. IR facilitated in analyzing the genetics results. RT aided in the conception and design of the study, analysis and interpretation of data, obtaining of funding, and final approval of the version to be submitted.
Funding
This research was provided by the Grains Research and Development Corporation (US00057, US00059, US00080, and US00081) and the Generation Challenge Program, heat.
Declarations
Ethics approval and consent to participate
All of the authors have read and have abided by the statement of ethical standards for manuscripts submitted to Molecular Breeding.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acuña-Galindo MA, Mason RE, Subramanian NK, Hays DB. Meta-analysis of wheat QTL regions associated with adaptation to drought and heat stress. Crop Sci. 2015;55(2):477–492. doi: 10.2135/cropsci2013.11.0793. [DOI] [Google Scholar]
- Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, Zondervan KT. Data quality control in genetic case-control association studies. Nat Protoc. 2010;5(9):1564–1573. doi: 10.1038/nprot.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazakos C, Hanemian M, Trontin C, Jiménez-Gómez JM, Loudet O. New strategies and tools in quantitative genetics: how to go from the phenotype to the genotype. Annu Rev Plant Biol. 2017;68:435–455. doi: 10.1146/annurev-arplant-042916-040820. [DOI] [PubMed] [Google Scholar]
- Bennett D, Izanloo A, Reynolds M, Kuchel H, Langridge P, Schnurbusch T. Genetic dissection of grain yield and physical grain quality in bread wheat (Triticum aestivum L.) under water-limited environments. Theor Appl Genet. 2012;125(2):255–271. doi: 10.1007/s00122-012-1831-9. [DOI] [PubMed] [Google Scholar]
- Bennett D, Reynolds M, Mullan D, Izanloo A, Kuchel H, Langridge P, Schnurbusch T. Detection of two major grain yield QTL in bread wheat (Triticum aestivum L.) under heat, drought and high yield potential environments. Theor Appl Genet. 2012;125(7):1473–1485. doi: 10.1007/s00122-012-1927-2. [DOI] [PubMed] [Google Scholar]
- Bohnert HJ, Gong Q, Li P, Ma S. Unraveling abiotic stress tolerance mechanisms–getting genomics going. Curr Opin Plant Biol. 2006;9(2):180–188. doi: 10.1016/j.pbi.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Cavanagh CR, Chao S, Wang S, Huang BE, Stephen S, Kiani S, Forrest K, Saintenac C, Brown-Guedira GL, Akhunova A. Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc Natl Acad Sci. 2013;110(20):8057–8062. doi: 10.1073/pnas.1217133110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar K, Nashef K, Ben-David R. Agronomic and genetic characterization of wild emmer wheat (Triticum turgidum subsp. dicoccoides) introgression lines in a bread wheat genetic background. Genet Resour Crop Evol. 2017;64(8):1917–1926. doi: 10.1007/s10722-016-0481-1. [DOI] [Google Scholar]
- Chen J, Chopra R, Hayes C, Morris G, Marla S, Burke J, Xin Z, Burow G (2017) Genome-wide association study of developing leaves’ heat tolerance during vegetative growth stages in a sorghum association panel. Plant Genome 10. 10.3835/plantgenome2016.09.0091 [DOI] [PubMed]
- Cook JP, McMullen MD, Holland JB, Tian F, Bradbury P, Ross-Ibarra J, Buckler ES, Flint-Garcia SA. Genetic architecture of maize kernel composition in the nested association mapping and inbred association panels. Plant Physiol. 2012;158(2):824–834. doi: 10.1104/pp.111.185033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossani CM, Reynolds MP. Physiological traits for improving heat tolerance in wheat. Plant Physiol. 2012;160(4):1710–1718. doi: 10.1104/pp.112.207753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LL, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Edae EA, Byrne PF, Haley SD, Lopes MS, Reynolds MP. Genome-wide association mapping of yield and yield components of spring wheat under contrasting moisture regimes. Theor Appl Genet. 2014;127(4):791–807. doi: 10.1007/s00122-013-2257-8. [DOI] [PubMed] [Google Scholar]
- Ellis M, Spielmeyer W, Gale K, Rebetzke G, Richards R. “Perfect” markers for the Rht-B1b and Rht-D1b dwarfing genes in wheat. TAG Theor Appl Genet. 2002;105(6):1038–1042. doi: 10.1007/s00122-002-1048-4. [DOI] [PubMed] [Google Scholar]
- Fikere M, Barbulescu DM, Malmberg MM, Shi F, Koh JC, Slater AT, MacLeod IM, Bowman PJ, Salisbury PA, Spangenberg GC. Genomic prediction using prior quantitative trait loci information reveals a large reservoir of underutilised blackleg resistance in diverse canola (Brassica napus L.) lines. Plant Genome. 2018;11(2):1–16. doi: 10.3835/plantgenome2017.11.0100. [DOI] [PubMed] [Google Scholar]
- Fikere M, Barbulescu D, Malmberg MM, Spangenberg GC, Cogan NO, Daetwyler HD. Meta-analysis of GWAS in canola blackleg (Leptosphaeria maculans) disease traits demonstrates increased power from imputed whole-genome sequence. Sci Rep. 2020;10(1):1–15. doi: 10.1038/s41598-020-71274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikere M, Barbulescu DM, Malmberg MM, Maharjan P, Salisbury PA, Kant S, Panozzo J, Norton S, Spangenberg GC, Cogan NO. Genomic prediction and genetic correlation of agronomic, blackleg disease, and seed quality traits in canola (Brassica napus L.) Plants. 2020;9(6):719. doi: 10.3390/plants9060719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Liu J, Yang L, Wu X, Xiao Y, Xia X, He Z. Genome-wide linkage mapping of QTL for physiological traits in a Chinese wheat population using the 90K SNP array. Euphytica. 2016;209(3):789–804. doi: 10.1007/s10681-016-1682-6. [DOI] [Google Scholar]
- Gilmour A, Gogel B, Cullis B, Thompson R (2009) ASReml user guide release 3.0 VSN International Ltd. Hemel Hempstead, Hp1 1ES, UK
- Gupta PK, Kulwal PL, Jaiswal V (2014) Association mapping in crop plants: opportunities and challenges. In Advances in genetics, Vol. 85, 109-147: Elsevier [DOI] [PubMed]
- Gupta OP, Pandey V, Gopalareddy K, Sharma P, Singh GP (2019) Genomic intervention in wheat improvement. In Plant Biotechnology: Progress in Genomic Era, 77-90: Springer
- Hai L, Guo H, Wagner C, Xiao S, Friedt W. Genomic regions for yield and yield parameters in Chinese winter wheat (Triticum aestivum L.) genotypes tested under varying environments correspond to QTL in widely different wheat materials. Plant Sci. 2008;175(3):226–232. doi: 10.1016/j.plantsci.2008.03.006. [DOI] [Google Scholar]
- Hanocq E, Niarquin M, Heumez E, Rousset M, Le Gouis J. Detection and mapping of QTL for earliness components in a bread wheat recombinant inbred lines population. Theor Appl Genet. 2004;110(1):106–115. doi: 10.1007/s00122-004-1799-1. [DOI] [PubMed] [Google Scholar]
- Howarth C. Abiotic stresses: plant resistance through breeding and molecular approaches. New York: Howarth Press Inc.; 2005. Genetic improvements of tolerance to high temperature. [Google Scholar]
- Huang X, Han B. Natural variations and genome-wide association studies in crop plants. Annu Rev Plant Biol. 2014;65:531–551. doi: 10.1146/annurev-arplant-050213-035715. [DOI] [PubMed] [Google Scholar]
- Huang X, Kempf H, Ganal M, Röder M. Advanced backcross QTL analysis in progenies derived from a cross between a German elite winter wheat variety and a synthetic wheat (Triticum aestivum L.) Theor Appl Genet. 2004;109(5):933–943. doi: 10.1007/s00122-004-1708-7. [DOI] [PubMed] [Google Scholar]
- Li X-M, He Z-H, Xiao Y-G, Xia X-C, Trethowan R, Wang H-J, Chen X-M. QTL mapping for leaf senescence-related traits in common wheat under limited and full irrigation. Euphytica. 2015;203(3):569–582. doi: 10.1007/s10681-014-1272-4. [DOI] [Google Scholar]
- Liu W, Maccaferri M, Chen X, Laghetti G, Pignone D, Pumphrey M, Tuberosa R. Genome-wide association mapping reveals a rich genetic architecture of stripe rust resistance loci in emmer wheat (Triticum turgidum ssp. dicoccum) Theor Appl Genet. 2017;130(11):2249–2270. doi: 10.1007/s00122-017-2957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Huang L, Wang C, Liu Y, Yan Z, Wang Z, Xiang L, Zhong X, Gong F, Zheng Y, Liu D, Wu B (2019) Genome-wide association study reveals novel genomic regions associated with high grain protein content in wheat lines derived from wild emmer wheat. Front Plant Sci 10:464. 10.3389/fpls.2019.00464 [DOI] [PMC free article] [PubMed]
- Lopes M, Dreisigacker S, Peña R, Sukumaran S, Reynolds MP. Genetic characterization of the wheat association mapping initiative (WAMI) panel for dissection of complex traits in spring wheat. Theor Appl Genet. 2015;128(3):453–464. doi: 10.1007/s00122-014-2444-2. [DOI] [PubMed] [Google Scholar]
- Ma’arup R (2016) The use of genetic diversity from emmer wheat to improve bread wheat. (PhD dessertation), The University of Sydney, NSW, Australia
- Ma’arup R, Trethowan RM, Ahmed NU, Bramley H, Sharp PJ (2020) Emmer wheat (Triticum dicoccon Schrank) improves water use efficiency and yield of hexaploid bread wheat. Plant Sci 295:110212 [DOI] [PubMed]
- Maccaferri M, Sanguineti MC, Corneti S, Ortega JLA, Salem MB, Bort J, DeAmbrogio E, del Moral LFG, Demontis A, El-Ahmed A. Quantitative trait loci for grain yield and adaptation of durum wheat (Triticum durum Desf.) across a wide range of water availability. Genetics. 2008;178(1):489–511. doi: 10.1534/genetics.107.077297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay I, Powell W. Methods for linkage disequilibrium mapping in crops. Trends Plant Sci. 2007;12(2):57–63. doi: 10.1016/j.tplants.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Marza F, Bai G-H, Carver B, Zhou W-C. Quantitative trait loci for yield and related traits in the wheat population Ning7840× Clark. Theor Appl Genet. 2006;112(4):688–698. doi: 10.1007/s00122-005-0172-3. [DOI] [PubMed] [Google Scholar]
- Mascher M, Muehlbauer GJ, Rokhsar DS, Chapman J, Schmutz J, Barry K, Muñoz-Amatriaín M, Close TJ, Wise RP, Schulman AH. Anchoring and ordering NGS contig assemblies by population sequencing (POPSEQ) Plant J. 2013;76(4):718–727. doi: 10.1111/tpj.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RE, Hays DB, Mondal S, Ibrahim AM, Basnet BR. QTL for yield, yield components and canopy temperature depression in wheat under late sown field conditions. Euphytica. 2013;194(2):243–259. doi: 10.1007/s10681-013-0951-x. [DOI] [Google Scholar]
- McCartney C, Somers D, Humphreys D, Lukow O, Ames N, Noll J, Cloutier S, McCallum B. Mapping quantitative trait loci controlling agronomic traits in the spring wheat cross RL4452×'AC Domain'. Genome. 2005;48(5):870–883. doi: 10.1139/g05-055. [DOI] [PubMed] [Google Scholar]
- Mitchell-Olds T. Complex-trait analysis in plants. Genome Biol. 2010;11(4):113. doi: 10.1186/gb-2010-11-4-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S, Singh R, Crossa J, Huerta-Espino J, Sharma I, Chatrath R, Singh G, Sohu V, Mavi G, Sukuru V. Earliness in wheat: a key to adaptation under terminal and continual high temperature stress in South Asia. Field Crop Res. 2013;151:19–26. doi: 10.1016/j.fcr.2013.06.015. [DOI] [Google Scholar]
- Mondal S, Joshi AK, Huerta-Espino J, Singh RP (2015) Early maturity in wheat for adaptation to high temperature stress. In Advances in Wheat Genetics: From Genome to Field, 239-245: Springer
- Mwadzingeni L, Shimelis H, Rees DJG, Tsilo TJ. Genome-wide association analysis of agronomic traits in wheat under drought-stressed and non-stressed conditions. PLoS One. 2017;12(2):e0171692. doi: 10.1371/journal.pone.0171692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo E. Evolution of wild emmer wheat and crop improvement. J Syst Evol. 2014;52(6):673–696. doi: 10.1111/jse.12124. [DOI] [Google Scholar]
- Nevo E, Chen G. Drought and salt tolerances in wild relatives for wheat and barley improvement. Plant Cell Environ. 2010;33(4):670–685. doi: 10.1111/j.1365-3040.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- Ogbonnaya FC, Rasheed A, Okechukwu EC, Jighly A, Makdis F, Wuletaw T, Hagras A, Uguru MI, Agbo CU. Genome-wide association study for agronomic and physiological traits in spring wheat evaluated in a range of heat prone environments. Theor Appl Genet. 2017;130(9):1819–1835. doi: 10.1007/s00122-017-2927-z. [DOI] [PubMed] [Google Scholar]
- Pask A, Pietragalla J, Mullan D, Reynolds M (2012) Physiological breeding II: a field guide to wheat phenotyping. Cimmyt
- Payne R, Murray D, Harding S, Baird D, Soutar D (2011) An introduction to GENSTAT for Windows., 14th edn (VSN International: Hemel Hempstead, UK)
- Peleg Z, Cakmak I, Ozturk L, Yazici A, Jun Y, Budak H, Korol AB, Fahima T, Saranga Y. Quantitative trait loci conferring grain mineral nutrient concentrations in durum wheat × wild emmer wheat RIL population. Theor Appl Genet. 2009;119(2):353–369. doi: 10.1007/s00122-009-1044-z. [DOI] [PubMed] [Google Scholar]
- Peng J, Ronin Y, Fahima T, Röder MS, Li Y, Nevo E, Korol A. Domestication quantitative trait loci in Triticum dicoccoides, the progenitor of wheat. Proc Natl Acad Sci. 2003;100(5):2489–2494. doi: 10.1073/pnas.252763199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto RS, Reynolds MP, Mathews KL, McIntyre CL, Olivares-Villegas J-J, Chapman SC. Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor Appl Genet. 2010;121(6):1001–1021. doi: 10.1007/s00122-010-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provart NJ, Alonso J, Assmann SM, Bergmann D, Brady SM, Brkljacic J, Browse J, Chapple C, Colot V, Cutler S. 50 years of Arabidopsis research: highlights and future directions. New Phytol. 2016;209(3):921–944. doi: 10.1111/nph.13687. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81(3):559–575 [DOI] [PMC free article] [PubMed]
- Quarrie S, Steed A, Calestani C, Semikhodskii A, Lebreton C, Chinoy C, Steele N, Pljevljakusić D, Waterman E, Weyen J. A high-density genetic map of hexaploid wheat (Triticum aestivum L.) from the cross Chinese Spring× SQ1 and its use to compare QTLs for grain yield across a range of environments. Theor Appl Genet. 2005;110(5):865–880. doi: 10.1007/s00122-004-1902-7. [DOI] [PubMed] [Google Scholar]
- R Core Team (2018) R: A language and environment for statistical computing; 2015
- Rasheed A, Xia X, Ogbonnaya F, Mahmood T, Zhang Z, Mujeeb-Kazi A, He Z. Genome-wide association for grain morphology in synthetic hexaploid wheats using digital imaging analysis. BMC Plant Biol. 2014;14(1):128. doi: 10.1186/1471-2229-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebetzke GJ, Rattey AR, Farquhar GD, Richards RA, Condon ATG. Genomic regions for canopy temperature and their genetic association with stomatal conductance and grain yield in wheat. Funct Plant Biol. 2013;40(1):14–33. doi: 10.1071/FP12184. [DOI] [PubMed] [Google Scholar]
- Reynolds M, Rebetzke G (2011) Application of plant physiology in wheat breeding. The World Wheat Book: A History of Wheat Breeding 2: 877-906
- Sehgal D, Vikram P, Sansaloni CP, Ortiz C, Saint Pierre C, Payne T, Ellis M, Amri A, Petroli CD, Wenzl P. Exploring and mobilizing the gene bank biodiversity for wheat improvement. PLoS One. 2015;10(7):e0132112. doi: 10.1371/journal.pone.0132112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirdelmoghanloo H, Taylor JD, Lohraseb I, Rabie H, Brien C, Timmins A, Martin P, Mather DE, Emebiri L, Collins NC. A QTL on the short arm of wheat (Triticum aestivum L.) chromosome 3B affects the stability of grain weight in plants exposed to a brief heat shock early in grain filling. BMC Plant Biol. 2016;16(1):1. doi: 10.1186/s12870-016-0784-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran S, Reynolds MP, Sansaloni C. Genome-wide association analyses identify QTL hotspots for yield and component traits in durum wheat grown under yield potential, drought, and heat stress environments. Front Plant Sci. 2018;9:81. doi: 10.3389/fpls.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka S, Kawahara T. Evolution and dispersal of emmer wheat (Triticum sp.) from novel haplotypes of Ppd-1 (photoperiod response) genes and their surrounding DNA sequences. Theor Appl Genet. 2012;125(5):999–1014. doi: 10.1007/s00122-012-1890-y. [DOI] [PubMed] [Google Scholar]
- Trethowan R. Delivering drought tolerance to those who need it: from genetic resource to cultivar. Crop Pasture Sci. 2014;65(7):645–654. doi: 10.1071/CP13401. [DOI] [Google Scholar]
- Ullah S, Bramley H, Daetwyler H, He S, Mahmood T, Thistlethwaite R, Trethowan R (2018) Genetic contribution of emmer wheat (Triticum dicoccon Schrank) to heat tolerance of bread wheat. Front Plant Sci 9:1529. 10.3389/fpls.2018.01529 [DOI] [PMC free article] [PubMed]
- Ullah S, Bramley H, Mahmood T, Trethowan R. A strategy of ideotype development for heat-tolerant wheat. J Agron Crop Sci. 2019;00(2019):1–13. [Google Scholar]
- Ullah S, Bramley H, Mahmood T, Trethowan R. The impact of emmer genetic diversity on grain protein content and test weight of hexaploid wheat under high temperature stress. J Cereal Sci. 2020;95:103052. doi: 10.1016/j.jcs.2020.103052. [DOI] [Google Scholar]
- Ullah S, Bramley H, Mahmood T, Trethowan R (2021) Implications of emmer (Triticum dicoccon Schrank) introgression on bread wheat response to heat stress. Plant Sci 304:110738 [DOI] [PubMed]
- Varshney RK, Bansal KC, Aggarwal PK, Datta SK, Craufurd PQ. Agricultural biotechnology for crop improvement in a variable climate: hope or hype? Trends Plant Sci. 2011;16(7):363–371. doi: 10.1016/j.tplants.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Vijayalakshmi K, Fritz AK, Paulsen GM, Bai G, Pandravada S, Gill BS. Modeling and mapping QTL for senescence-related traits in winter wheat under high temperature. Mol Breed. 2010;26(2):163–175. doi: 10.1007/s11032-009-9366-8. [DOI] [Google Scholar]
- Wang R, Hai L, Zhang X, You G, Yan C, Xiao S. QTL mapping for grain filling rate and yield-related traits in RILs of the Chinese winter wheat population Heshangmai× Yu8679. Theor Appl Genet. 2009;118(2):313–325. doi: 10.1007/s00122-008-0901-5. [DOI] [PubMed] [Google Scholar]
- Wang S, Wong D, Forrest K, Allen A, Chao S, Huang BE, Maccaferri M, Salvi S, Milner SG, Cattivelli L. Characterization of polyploid wheat genomic diversity using a high-density 90 000 single nucleotide polymorphism array. Plant Biotechnol J. 2014;12(6):787–796. doi: 10.1111/pbi.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Xu S, Chao S, Sun Q, Liu S, Xia G (2019) A genome-wide association study of highly heritable agronomic traits in durum wheat. Front Plant Sci 10:919. 10.3389/fpls.2019.00919 [DOI] [PMC free article] [PubMed]
- Xu Y, An D, Liu D, Zhang A, Xu H, Li B. Molecular mapping of QTLs for grain zinc, iron and protein concentration of wheat across two environments. Field Crop Res. 2012;138:57–62. doi: 10.1016/j.fcr.2012.09.017. [DOI] [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharieva M, Ayana NG, Al Hakimi A, Misra SC, Monneveux P. Cultivated emmer wheat (Triticum dicoccon Schrank), an old crop with promising future: a review. Genet Resour Crop Evol. 2010;57(6):937–962. doi: 10.1007/s10722-010-9572-6. [DOI] [Google Scholar]
- Zhou W, Wu S, Ding M, Li J, Shi Z, Wei W, Guo J, Zhang H, Jiang Y, Rong J. Mapping of Ppd-B1, a major candidate gene for late heading on wild emmer chromosome arm 2BS and assessment of its interactions with early heading QTLs on 3AL. PLoS One. 2016;11(2):e0147377. doi: 10.1371/journal.pone.0147377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 56 kb)