Abstract
Mucosa-associated lymphoid tissue (MALT) lymphoma involving meningeal tissue is rare condition, easily mistaken for meningiomas upon imaging. In this report, a case of primary left temporal lobe MALT lymphoma that was initially misdiagnosed as temporal meningioma is presented, with subsequent investigation into the mechanism and treatments. Clinically, MALT lymphomas can be easily confused with meningiomas based solely on imaging and clinical manifestations. MALT lymphomas are indolent, localized lesions that can be cured through surgical resection and radiotherapy. Currently, radiotherapy is the most commonly used treatment; however, the patient in the present report did not receive any chemotherapy or radiotherapy after surgery, and recent related examinations revealed a recurrence of lymphomas that had metastasized throughout the body. As a result, future patients may benefit from chemotherapy or radiotherapy, and clinicians should be more meticulous regarding patient follow-up.
Introduction
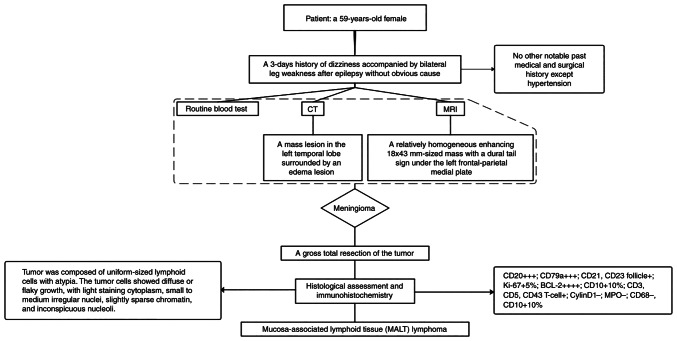
Primary central nervous system lymphoma (PCNSL) is an extranodal non-Hodgkin lymphoma that primarily affects the brain parenchyma, eyes, cranial nerve and meninges. It is an extremely rare occurrence, accounting for <3% of intracranial tumors in the US (1,2). PCNSL is primarily composed of diffuse large B-cell lymphomas of the activated B-cell subtype (3,4), with a small percentage of these lymphomas being marginal zone B-cell lymphoma (MZBL). MZBL also includes extranodal MZL of mucosa-associated lymphoid tissue (MALT) lymphoma, nodal MZL and splenic MZL (5). MALT lymphoma was initially thought to arise from gastrointestinal lymphoma, which is the most common site; however, it can also occur in other sites, such as the lungs, head and neck, skin, thyroid and breast (5,6). Meningioma, a common tumor of the central nervous system, is easy to diagnose because of its unique imaging features, such as the dural tail sign (7). MALT lymphoma involving meningeal tissue is uncommon and can be easily confused with meningiomas clinically. In the present report, the patient had similar imaging manifestations with meningioma, but was finally diagnosed with MALT lymphoma based on pathologic findings (Fig. 1). MALT lymphoma is rare in clinical practice, therefore, the mechanism and treatments were investigated.
Figure 1.
A flow diagram outlining the process of diagnostic examination.
Case report
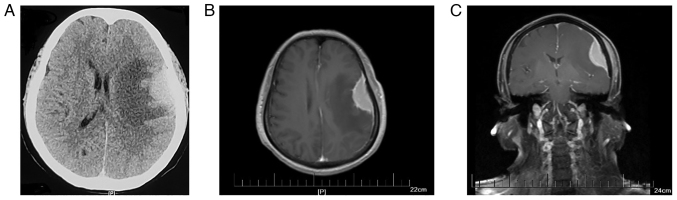
A 59-year-old female with a history of hypertension, but no other significant medical and surgical history, presented to The Affiliated Huai'an No. 1 People's Hospital of Nanjing Medical University (Huai'an, China) with a 3-day history of dizziness accompanied by bilateral leg weakness after an epileptic seizure with no apparent cause in November 2015. No abnormalities were found after neurological examination or routine laboratory tests. Cranial computerized tomography (CT) revealed a mass lesion in the left temporal lobe surrounded by an edema lesion (Fig. 2A). Enhanced magnetic resonance imaging (MRI) showed a relatively homogeneous enhancing mass measuring 18×43 mm with a dural tail sign under the left frontal-parietal medial plate, which is a typical imaging manifestation of meningioma (Fig. 2B and C). Based on this information, a diagnosis of meningioma was made on November 11, 2015. The patient subsequently underwent a gross total resection of the tumor. Intraoperatively, a dark red-white 4×5×3 cm-sized tumor based on the dura mater was observed, along with invasion and adhesion of adjacent brain tissue.
Figure 2.
MRI and CT scans of subdural mass before craniotomy. (A) Cranial CT showing a mass lesion in the left temporal lobe with an edema lesion surrounded. (B) Axialand (C) coronal MRI showing a relatively homogeneous enhancing 18×43 mm-sized mass exhibiting dural tail sign under the left frontal parietal medial plate and thickening of the adjacent cranial plate.
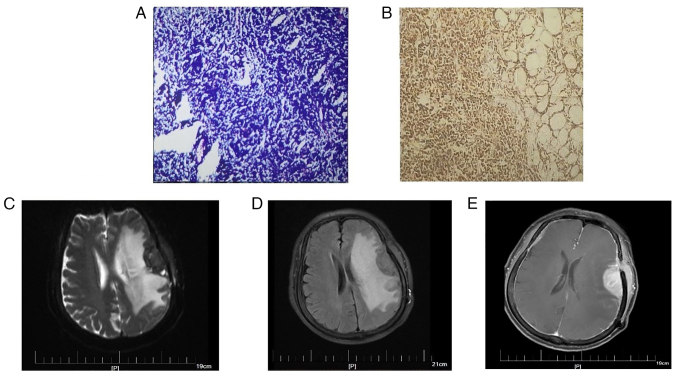
For H&E staining, surgical specimens were fixed in 10% neutral formalin at room temperature for 24–48 h, and paraffin-embedded sections were produced (4 µm), stained with H&E at room temperature for 5 min and analyzed under a light microscope. Histological assessment of the tumor revealed a diffuse infiltration containing uniformly sized lymphocytes with atypia (Fig. 3A), consistent with a lymphoma diagnosis, as opposed to a meningioma diagnosis. Generally, most meningiomas are benign, and atypia is rare. The meningothelial meningioma, the most common subtype, is composed of polygonal, ill-defined, arachnoid epithelial cells variable in size, with abundant cytoplasm and large nuclei. The characteristic structure of meningiomas is the arrangement of cells in concentric circles of different sizes, with small blood vessels; the vessel walls can exhibit hyalinization, calcification or psammoma bodies.
Figure 3.
Pathological findings of tumors and the MRI scans of tumor recurrence. (A) H&E staining of the tumor revealed that the tumor was composed of uniform-sized lymphoid cells with atypia. The tumor cells showed diffuse or flaky growth, with light staining of the cytoplasm, small to medium irregular nuclei, slightly sparse chromatin, and inconspicuous nucleoli. (B) Immunohistochemical findings for CD20: The tumors cells highly expressed CD20. (C) T2 phase, (D) T2FLAIR and (E) enhanced MRI scans. Axial MRI showed abnormal enhancement of left frontotemporal lobe with surrounding edema and multiple enhancement of intracranial meninges as well as left frontotemporal subcutaneous and left buccal lesions.
Subsequently, H&E staining and immunohistochemistry was performed on the patient's tumor tissues. The primary antibodies used included anti-CD2, anti-CD3, anti-CD5, anti-CD10, anti-CD20, anti-CD21, anti-CD23, anti-CD43, anti-CD68, anti-CD79a, anti-Bcl-2, anti-Bcl-6, anti-MPO, anti-Cyclin D1 and anti-Ki-67 Immunohistochemical findings were as follows: CD20+++; CD79a+++; CD21+, CD23 follicle+; Ki-67+ 5%; BCL-2++++; CD10+10%; CD3+, CD5+, CD43 T-cell+; Cyclin D1−; MPO−; CD68−; and CD10+10% (Fig. 3B shows representative staining for CD20). The characteristics of small B-cell malignant lymphoma were consistent with extranodal MZBL of the MALT type.
Based on the immunohistochemical findings and histological assessment, it was recommended the patient receive chemotherapy or radiation therapy; however, the patients' economic situation led to her decision to be discharged from hospital. Patient follow-up after discharge from hospital continued via telephone and the internet (messaging) for 2 years, during which time the patient experienced no discomfort such as dizziness, headache and weakness. After this 2-year period, the patient began experiencing these symptoms; however, no abnormalities were found upon examination. Unfortunately, a cranial enhancing CT performed in November 2021 revealed a mass lesion in the left temporal lobe, along with left frontotemporal lobe and basal ganglia edema, which could not rule out postoperative recurrence; however, the patient opted out of treatment. The patient developed slurred speech accompanied by intermittent nausea, vomiting, headache and dizziness after 2 months, prompting her to re-evaluate treatment. Given the possibility of lymphoma recurrence, an MRI in January 2022 was performed and subsequently revealed abnormal enhancement of the left frontotemporal lobe along with surrounding edema and multiple enhancements of the intracranial meninges (Fig. 3C-E). Furthermore, a PET-CT scan showed multiple metastases throughout the patient's body. The patient was transferred to the oncology department for antitumor therapy after discharge.
Discussion
Primary central nervous system lymphomas are predominantly aggressive diffuse high-grade B-cell lymphomas of the large B-cell type. MALT lymphomas arise from B-cells in the MALT marginal area and are also known as extranodal marginal area B-cell lymphoma. The majority of MALT lymphomas occur in middle-aged women, and symptoms include epilepsy, headache and visual disturbance (8). The cytologic composition can vary, including small lymphocytic, plasmacytoid and marginal zone type cells; these may have reactive follicles, numerous transformed lymphocytes, plasma cells and other inflammatory cells (9).
Currently, there are a few widely accepted mechanisms for the formation of MALT lymphomas. In embryology, meningothelial cells are concentrated in the arachnoid membrane and dural venous sinuses, similar to epithelial cells in other sites where MALT lymphoma develops (4,10) In addition, dural-based MALT may be caused by the implantation metastasis of undiagnosed or disappearing MALT lymphoma at the meninges (11). Furthermore, the role of chronic inflammatory disease, including hepatitis C (12) and Helicobacter pylori-associated gastritis cannot be ruled out (8,9,12,13). Additionally, autoimmune diseases have been reported to be associated with MALT lymphoma, such as Grave's disease (14), Sjögren syndrome (8,14), scleroderma (14) and Hashimoto thyroiditis (9,13). Furthermore, IgG4 expression has been linked to primary intracranial MZBLs (15,16); Venkataraman et al (15) demonstrated this association through a series of retrospective analyses. A number of cases from the literature have been collated to further understand the characteristics of MALT lymphomas (Table I). Historically, the majority of cases occur in middle-aged women who primarily present with headaches and seizures, yet other manifestations can include hearing impairment, numbness, visual impairment and dysphasia, depending on the location of the tumor.
Table I.
Summary of patient characteristics with intracranial extranodal marginal zone B-cell lymphomas.
| First author/s, year | Case | Age | Sex | Location | Symptoms | Treatment | Remission/outcome | Immunohistochemistry | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|
| Rottnek et al, 2004 | 1 | 47 | M | Left tentorial | Seizure, visual field defects and memory loss | Subtotal excision and radiation | NED at 8 months | CD20+, CD79a+, CD43+ and kappa LCR | (9) |
| Kambham et al, 1998 | 2 | 39 | F | Left CP angle (dura) | Hearing loss and facial pain/weakness | Subtotal excision | AWD after 4 years | CD20+, CD79a+, CD21+ germinal centers and kappa LCR | (17) |
| 3 | 62 | F | Left parietal-occipital area | Headaches | Radiation | AWD after 6 months | CD20+ and CD79a+ | ||
| Kumar et al, 1997 | 4 | 40 | F | Right cavernous sinus | Numbness and visual field defects | Radiation | NED at 63 months | CD20+, CD3+ reactive T cells and lambda LCR | (10) |
| 5 | 62 | F | Biparietal dural | Seizures | Fludarabine | NED at 22 months | CD20+, CD3+ reactive T cells and lambda LCR | ||
| 6 | 52 | F | Left frontal dural | Seizures and numbness | Radiation/chemotherapy | NED at 9 months | CD20+, CD3+ reactive T cells and kappa LCR | ||
| 7 | 43 | F | Left tentorial | Dizziness, headaches, blurred vision and numbness | Radiation | NED at 7 months | CD20+, CD3+ reactive T cells, CD43+ and lambda LCR | ||
| 8 | 57 | F | Left anterior falx cerebri | Seizures | Radiation | NED at 14 months | CD20+, CD3+ reactive T cells, CD43+ and CD23− | ||
| Itoh et al, 2001 | 9 | 28 | F | CP angle | Tinnitus, nausea, headache and bilateral papilledema | Excision | NED at 2 years | CD20+, CD10+ (follicular center cells), BCL2+ in some follicular centers, CD43+ and CD3+ (50% cells) | (18) |
| Goetz et al, 2002 | 10 | 64 | F | Right frontoparietal dura | Left hemiparesis and headache | Excision and radiation | NED at 3 months | IgD+/CD20+ small lymphocytes, IgD−/CD20+ lymphoplasmacytoid cells, CD20−/CD138+ plasma cells and kappa LCR | (13) |
| Ferguson et al, 2010 | 11 | 29 | F | Right frontal dural | Exophthalmos and visual loss | Decompression of the optic nerve, subtotal resection and 30 Gy radiation | NED at 3 years | CD20+, BCL-6−, kappa LCR and CD5− | (14) |
| Jesionek-Kupnicka et al, 2013 | 12 | 60 | F | Left parietooccipital dural | Headache, periodic cramp of the right face and numbness of the | Excision and radiotherapy (WS3D 6MV photons) | NED | CD20+, CD79a+, BCL-2+ (reactive follicles with germinal centers), CD3−, CD5−, CD23−, CD10−, BCL-6−, Cyclin D1−, Ki-67+ (10%), | (4) |
| Kamoshima et al, 2011 | 13 | 55 | F | Left frontal dural | Seizures | Subtotal excision and 40 Gy radiation | NED at 36 months | CD20+, CD5−, CD23−, CD10−, Cyclin D1−, CD3+ (some lymphocytes) | (8) |
| Shaia et al, 2010 | 14 | 61 | F | Dura of the right posterior fossa | Nausea, vomiting and pain over the top of scalp | Excision and 30 Gy radiation | NED at 6 months | CD20+, CD79a+, CD5−, CD10−, CD23−, CD43− and kappa LCR | (19) |
| Tu et al, 2005 | 15 | 49 | M | Frontal | Seizures | Chemotherapy (MTX and fludarabine) | NED at 7.6 years | Not available | (20) |
| 16 | 48 | F | Dura, tentorium and falx | Headache and ear pain | Chemotherapy (MTX and leucovorin) and radiation | NED at 20 months | Not available | ||
| Lehman et al, 2002 | 17 | 63 | F | Supratentorial and infratentorial dural | Seizure | Excision and 36 Gy radiation | NED | CD20+, CD45+, CD3+ (small population) and CD138+ (small population) | (21) |
| Venkataraman et al, 2011 | 18 | 62 | F | Bilateral parietal | Unknown | Fludarabine | NED at 22 months | Not available | (15) |
| Villeneuve et al, 2018 | 19 | 60 | F | Petrous temporal bone | Vertigo and unilateral right mixed hearing loss | Chemotherapy (rituximab and bendamustine) | NED at 2years | CD20+, CD23+, CD5−, CD10−, BCL-1− and BCL-2+ | (22) |
| Park et al, 2008 | 20 | 18 | M | Left basal ganglia | Right-sided central facial nerve palsy, right-sided weakness, dizziness and dysarthria | Radiation | NED at 22 months | CD20+, CD79a+, CD3−, CD5−, CD10−, BCL-6−, CD23−, MUM1-, ALK-1−, Cyclin D1−, and negative for kappa and lambda LCR | (12) |
| Kelley et al, 2005 | 21 | 53 | M | Right lateral ventricle | Headache and seizure | Excision and chemotherapy (liposomal cytarabine) | NED at 14 months | CD19+, CD20+, CD45+, CD5−, CD10− | (23) |
| Jazy et al, 1980 | 22 | 59 | M | Right temporal | Seizures, visual and hearing impairment | Radiation | NED at 16 months | Not available | (24) |
| Miranda et al, 1996 | 24 | 51 | F | Right frontal | Major motor seizure | Excision and radiation | NED at 14 months | CD19+, CD20+ and CD22+ | (25) |
| Naberhaus et al, 1996 | 25 | 48 | F | Right temporo-parietal | Headaches | Radiation | NED at 36 months | CD20+ | (26) |
| King et al, 1998 | 26 | 60 | F | Cerebellar vermis and right fronto-parietooccipital | Seizures and memory loss | Biopsy and chemotherapy | Died 3 months later due to pneumonia | CD45RB+, CD20+, Cyclin D1 | (27) |
| Hodgson et al, 1999 | 27 | 57 | F | Right sphenoid wing | Headache and mild | Excision photophobia | NED at 6 months | CD20+, kappa LCR, BCL-2+ | (28) |
| Freudenstein et al, 2000 | 28 | 50 | F | Parafalcine and bilateral convexity dura | Headache and seizures | Systemic and intrathecal chemotherapy (MTX) | NED at 36 months | CD20+, LCA+, Vimentin+, CD3−, IgG light chain− and IgG heavy chain− | (29) |
| Neidert et al, 2015 | 29 | 44 | M | Right fronto-parietal dural | Involuntary muscle movements on the left-side of his body | Excision and 36 Gy radiation | NED at 2 years | CD20+, CD45+, BCL-2+, CD79a+, EMA−, CD34−, TDT−, CD99−, Ki-67+ (30%), CD3+, CD5+, CD10+, CD23+ (small population) | (30) |
| Pavlou et al, 2006 | 30 | 73 | F | Left fronto-parietal | Right arm weakness, partial seizures and dysphasia | Excision and chemotherapy (methylpred-nisolone, cytosine and methotrexate, chlorambucil) | Unknown | CD20+, CD79+, BCL-2+, CD10−, BCL-1−, CD5−, MIB-1+ (10%) | (31) |
| Present study | 31 | 59 | F | Left temporal lobe | Dizziness and bilateral leg weakness | Excision | AWD after 6 years | CD20+; CD79a+, CD21+, CD23 follicle+; Ki-67+ 5%; BCL-2+; CD10+ 10%; CD3+, CD5+ CD43 T-cell+, Cyclin D1−, MPO− and CD68- |
AWD, alive with disease; F, female; LCR, light chain restriction; M, male; MPO, myeloperoxidase; NED, no evidence of disease; CP, cerebellopontine; MTX, methotrexate; LCA, leukocyte common antigen; EMA, epithelial membrane antigen; TDT, terminal deoxynucleotide transferase.
Clinically, the differential diagnosis of lymphoma is important; however, due to the dural tail sign, it can be difficult to distinguish it from meningioma based solely on imaging and clinical manifestations. Small lymphocytic lymphoma, chronic lymphocytic leukemia (CLL), lymphoplasmacytic lymphoma (LPL) are other potential diagnoses that require further histological and immunophenotypic analysis for confirmation. Notably, MALT lymphomas express CD20, CD79a and CD38, which can also be seen in LPL (Table II). In addition, CLL expresses CD5 and CD23. In the present case, a 59-year-old woman presented with dizziness and bilateral leg weakness. Immunophenotypically, the patients' lymphoid cells were positive for CD20, CD79a, CD21, CD23, BCL-2, CD3, CD5 and CD43, but were negative for Cylin D1, MPO and CD68. Although the immunohistochemical findings were similar to follicular lymphomas, the cells of follicular lymphoma grew nodular and formed obvious follicular structures at low magnification, which were not observed in the pathological findings of this tumor. Although the specific type of lymphoma cannot be confirmed, MALT lymphoma is more likely based on clinical symptoms, imaging and immunohistochemistry. However, it is unfortunate that genetic analysis of the lesion was not performed to verify and validate the diagnosis and treatments. Additional detection of MYD88, IgM and BRAF would aid in differentiating between LPL/Waldenstrom's macroglobulinemia, hairy-cell leukemia and MALT, increasing the accuracy of diagnosis.
Table II.
Immunohistochemistry of different types of lymphomaa.
| Types of lymphoma | Immunohistochemistry |
|---|---|
| MALT lymphoma (extranodal | CD20+, CD79a+ and |
| marginal zone lymphoma) | CD38+ |
| Lymphoplasmacytic lymphoma/ | CD20+, CD79a+ and |
| Waldenstrom macroglobulinemia | CD38+/IgM+ |
| Follicular lymphoma | CD10+ and BCL-2+ |
| Chronic lymphocytic leukemia | CD5+ and CD23+ |
| Mantle cell lymphoma | CD5+, Cyclin D1−, |
| CD10− and CD23− | |
| Lymphoblastic lymphoma | TDT+ |
| Lymphomatoid granulomatosis | EBER in situ+ |
Adapted from Ueba et al (16). MALT, mucosa-associated lymphoid tissue; TDT, terminal deoxynucleotide transferase; EBER, EB virus-encoded RNA.
In conclusion, MALT lymphomas are often confused with meningioma owing to similarities in imaging and clinical manifestations; thus, clinicians should not jump to conclusions when presented with images that resemble meningiomas, especially containing the dural tail sign. MALT lymphomas are generally indolent, localized lesions that can be cured through surgical resection and radiotherapy. Current evidence suggests that radiotherapy is the most commonly used treatment, and the extent of the dural lesions and leptomeningeal involvement determine the radiation field. As molecular genetic changes are tightly associated with classification, prognosis and treatment of tumors, additional detection of mutated genes is recommended, so as to more effectively treat diseases.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- CLL
chronic lymphocytic leukemia
- CT
computerized tomography
- LPL
lymphoplasmacytic lymphoma
- MALT
mucosa-associated lymphoid tissue
- MPO
myeloperoxidase
- MRI
magnetic resonance imaging
- MZBL
marginal zone B-cell lymphoma
- PCNSL
primary central nervous system lymphoma
- PET-CT
positron emission tomography computerized tomography
Funding Statement
The study was supported by the Key Science and Technology Project of Jiangsu Commission of Health (grant. no. ZD2021051).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JBR, LYC, SXL and JHR confirm the authenticity of all the raw data. Study conception and design was performed by LSD and JBR. Material preparation and data collection were taken by LYC. Analysis and interpretation of data was performed by SXL. Follow-up of the patients was performed by JHR. All authors contributed to manuscript writing. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The report has obtained approval from the Ethics Committee and Institutional Review Board of Huai'an First People's Hospital (Huai'an, China; approval number: KY-2023-035-01).
Patient consent for publication
Written informed consent was obtained from the patient for the publication of the case details and any associated images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nayak L, Pentsova E, Batchelor TT. Primary CNS lymphoma and neurologic complications of hematologic malignancies. Continuum (Minneap Minn) 2015;21:355–372. doi: 10.1212/01.CON.0000464175.96311.0a. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the united states in 2008–2012. Neuro Oncol. 2015;17((Suppl 4)):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holdhoff M, Mrugala MM, Grommes C, Kaley TJ, Swinnen LJ, Perez-Heydrich C, Nayak L. Challenges in the treatment of newly diagnosed and recurrent primary central nervous system lymphoma. J Natl Compr Canc Netw. 2020;18:1571–1578. doi: 10.6004/jnccn.2020.7667. [DOI] [PubMed] [Google Scholar]
- 4.Jesionek-Kupnicka D, Smolewski P, Kupnicki P, Pluciennik E, Zawlik I, Papierz W, Kordek R. Primary extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type in the central nervous system (MZL CNS) presented as traumatic subdural hematoma and subarachnoid bleeding-case report. Clin Neuropathol. 2013;32:384–392. doi: 10.5414/NP300579. [DOI] [PubMed] [Google Scholar]
- 5.Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: Analysis of the surveillance, epidemiology, and end results database. Cancer. 2013;119:629–638. doi: 10.1002/cncr.27773. [DOI] [PubMed] [Google Scholar]
- 6.Weis S, Llenos IC. Primary leptomeningeal B-cell lymphoma of MALT-type in statu nascendi: A case report and review of the literature. Clin Neurol Neurosurg. 2008;110:732–738. doi: 10.1016/j.clineuro.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Goldbrunner R, Stavrinou P, Jenkinson MD, Sahm F, Mawrin C, Weber DC, Preusser M, Minniti G, Lund-Johansen M, Lefranc F, et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021;23:1821–1834. doi: 10.1093/neuonc/noab150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamoshima Y, Sawamura Y, Sugiyama T, Yamaguchi S, Houkin K, Kubota K. Primary central nervous system mucosa-associated lymphoid tissue lymphoma-case report. Neurol Med Chir (Tokyo) 2011;51:527–530. doi: 10.2176/nmc.51.527. [DOI] [PubMed] [Google Scholar]
- 9.Rottnek M, Strauchen J, Moore F, Morgello S. Primary dural mucosa-associated lymphoid tissue-type lymphoma: Case report and review of the literature. J Neurooncol. 2004;68:19–23. doi: 10.1023/B:NEON.0000024704.70250.42. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Kumar D, Kaldjian EP, Bauserman S, Raffeld M, Jaffe ES. Primary low-grade B-cell lymphoma of the dura: A mucosa associated lymphoid tissue-type lymphoma. Am J Surg Pathol. 1997;21:81–87. doi: 10.1097/00000478-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Saggioro FP, Colli BO, Paixão-Becker AN, de Rezende GG, Santos AC, Neder L. Primary low-grade MALT lymphoma of the dura. Histopathology. 2006;49:323–326. doi: 10.1111/j.1365-2559.2006.02433.x. [DOI] [PubMed] [Google Scholar]
- 12.Park I, Huh J, Kim JH, Lee SW, Ryu MH, Kang YK. Primary central nervous system marginal zone B-cell lymphoma of the Basal Ganglia mimicking low-grade glioma: A case report and review of the literature. Clin Lymphoma Myeloma. 2008;8:305–308. doi: 10.3816/CLM.2008.n.043. [DOI] [PubMed] [Google Scholar]
- 13.Goetz P, Lafuente J, Revesz T, Galloway M, Dogan A, Kitchen N. Primary low-grade B-cell lymphoma of mucosa-associated lymphoid tissue of the dura mimicking the presentation of an acute subdural hematoma. Case report and review of the literature. J Neurosurg. 2002;96:611–614. doi: 10.3171/jns.2002.96.3.0611. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson SD, Musleh W, Gurbuxani S, Shafizadeh SF, Lesniak MS. Intracranial mucosa-associated lymphoid tissue (MALT) lymphoma. J Clin Neurosci. 2010;17:666–669. doi: 10.1016/j.jocn.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Venkataraman G, Rizzo KA, Chavez JJ, Streubel B, Raffeld M, Jaffe ES, Pittaluga S. Marginal zone lymphomas involving meningeal dura: Possible link to IgG4-related diseases. Mod Pathol. 2011;24:355–366. doi: 10.1038/modpathol.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueba T, Okawa M, Abe H, Inoue T, Takano K, Hayashi H, Nabeshima K, Oshima K. Central nervous system marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type involving the brain and spinal cord parenchyma. Neuropathology. 2013;33:306–311. doi: 10.1111/j.1440-1789.2012.01350.x. [DOI] [PubMed] [Google Scholar]
- 17.Kambham N, Chang Y, Matsushima AY. Primary low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) arising in dura. Clin Neuropathol. 1998;17:311–317. [PubMed] [Google Scholar]
- 18.Itoh T, Shimizu M, Kitami K, Mitsumori K, Fujita M, Ohnishi A, Nagashima K. Primary extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type in the CNS. Neuropathology. 2001;21:174–180. doi: 10.1046/j.1440-1789.2001.00392.x. [DOI] [PubMed] [Google Scholar]
- 19.Shaia J, Kerr PB, Saini A, Roberti F, Kapil J, Jones R, Aragon-Ching JB. Mucosa-associated lymphoma tissue of the dura presenting as meningioma. South Med J. 2010;103:950–952. doi: 10.1097/SMJ.0b013e3181eb3477. [DOI] [PubMed] [Google Scholar]
- 20.Tu PH, Giannini C, Judkins AR, Schwalb JM, Burack R, O'Neill BP, Yachnis AT, Burger PC, Scheithauer PW, Perry A. Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: A primary low-grade CNS lymphoma that mimics meningioma. J Clin Oncol. 2005;23:5718–5727. doi: 10.1200/JCO.2005.17.624. [DOI] [PubMed] [Google Scholar]
- 21.Lehman NL, Horoupian DS, Warnke RA, Sundram UN, Peterson K, Harsh GR IV. Dural marginal zone lymphoma with massive amyloid deposition: Rare low-grade primary central nervous system B-cell lymphoma. Case report. J Neurosurg. 2002;96:368–372. doi: 10.3171/jns.2002.96.2.0368. [DOI] [PubMed] [Google Scholar]
- 22.Villeneuve A, Rubin F, Bonfils P. Meningeal marginal zone B-cell lymphoma: The meningioma trap. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135:131–132. doi: 10.1016/j.anorl.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Kelley TW, Prayson RA, Barnett GH, Stevens GH, Cook JR, His ED. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue arising in the lateral ventricle. Leuk Lymphoma. 2005;46:1423–1427. doi: 10.1080/10428190500205895. [DOI] [PubMed] [Google Scholar]
- 24.Jazy FK, Shehata WM, Tew JM, Meyer RL, Boss HH. Primary intracranial lymphoma of the dura. Arch Neurol. 1980;37:528–529. doi: 10.1001/archneur.1980.00500570076016. [DOI] [PubMed] [Google Scholar]
- 25.Miranda RN, Glantz LK, Myint MA, Levy N, Jackson CL, Rhodes CH, Glantz MJ, Medeiros LJ. Stage IE non-Hodgkin's lymphoma involving the dura: A clinicopathologic study of five cases. Arch Pathol Lab Med. 1996;120:254–260. [PubMed] [Google Scholar]
- 26.Narberhaus B, Buxó J, Pérez de Olaguer J, Forcadas P, Garcia-Bach M, Aparicio A, Ugarte A. Primary dural lymphoma. Neurologia (Barcelona, Spain) 1996;11:117–119. (In Spanish) [PubMed] [Google Scholar]
- 27.King A, Wilson H, Penney C, Michael W. An unusual case of primary leptomeningeal marginal zone B-cell lymphoma. Clin Neuropathol. 1998;17:326–329. [PubMed] [Google Scholar]
- 28.Hodgson D, David KM, Powell M, Holton JL, Pezzella F. Intracranial extracerebral follicular lymphoma mimicking a sphenoid wing meningioma. J Neurol Neurosurg Psychiatry. 1999;67:251–252. doi: 10.1136/jnnp.67.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freudenstein D, Bornemann A, Ernemann U, Boldt R, Duffner F. Intracranial malignant B-cell lymphoma of the dura. Clin Neuropathol. 2000;19:34–37. [PubMed] [Google Scholar]
- 30.Neidert MC, Leske H, Burkhardt JK, Rushing EJ, Bozinov O. A 44-year old male with right-sided facial numbness. Dura-associated extranodal marginal zone B cell lymphoma (MALT lymphoma) Brain Pathol. 2015;25:113–114. doi: 10.1111/bpa.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavlou G, Pal D, Bucur S, Chakrabarty A, van Hille PT. Intracranial non-Hodgkin's MALT lymphoma mimicking a large convexity meningioma. Acta Neurochir (Wien) 2006;148:791–793. doi: 10.1007/s00701-006-0761-1. discussion 793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.