Abstract
Asthma is a chronic inflammatory airway disease and the airway epithelium is involved in airway inflammation and innate immunity. However, whether circular RNA (circRNA) is involved in the pathogenesis of asthma remains unclear. The present study aimed to determine the functions and molecular mechanisms of circRNA targeting dehydrogenase E1 (circDHTKD1) in the inflammation response of human bronchial epithelial cells. BEAS-2B cells were stimulated with lipopolysaccharide (LPS) to establish a model of in vitro airway inflammation. Cell viability was assessed using Cell Counting Kit-8 assay. CircDHTKD1 was characterised by nucleocytoplasmic isolation and Sanger sequencing. The RNA expression levels of circDHTKD1, microRNA (miR)-338-3p and potential ERK pathway downstream genes were evaluated by reverse transcription-quantitative polymerase chain reaction. Western blot analysis was performed to measure associated protein levels. The levels of inflammatory cytokines were detected by ELISA. The interaction between circDHTKD1 and miR-338-3p was confirmed by dual-luciferase reporter assay. circDHTKD1 expression was significantly upregulated by LPS treatment, whereas miR-338-3p expression was decreased. Furthermore, circDHTKD1 directly targeted miR-338-3p, which negatively regulated expression of E26 transformation specific-1 (ETS1). Inflammatory cytokine and ETS1 expression levels decreased following transfection with small interfering RNA targeting circDHTKD1 or miR-338-3p mimics. In addition, co-transfection with miR-338-3p inhibitor reversed the effects caused by circDHTKD1 knockdown. The knockdown of ETS1 in LPS-induced BEAS-2B cells resulted in decreased cytokine production and inhibition of the ERK signalling pathway. Overall, these results suggested that the knockdown of circDHTKD1 alleviated the LPS-induced production of inflammatory cytokines and activation of the ERK pathway in BEAS-2B cells through the miR-338-3p/ETS1 axis. In summary, circDHTKD1 exacerbated LPS-triggered inflammation responses in BEAS-2B cells by regulating ETS1 expression by interacting with miR-338-3p, suggesting that circDHTKD1 may serve as a potential therapeutic target against asthma.
Keywords: bronchial epithelial cells, circular RNA, microRNA, airway inflammation, ERK signalling pathway
Introduction
Asthma is a chronic airway inflammatory disease that threatens human health. At present, >300 million individuals are diagnosed with asthma worldwide and the total number of patients with asthma is expected to exceed 400 million by 2025(1). The pathological features of asthma include airway remodelling and bronchial hyperresponsiveness (2). As the physical barrier of the airway, the bronchial epithelium is the first line of defence against pathogens, environmental pollutants and allergens. Moreover, the bronchial epithelium is not only involved in airway mucus hypersecretion and airway remodelling but is also related to the airway inflammatory response in asthma (3-5). When bronchial epithelial cells are activated by inflammatory stimuli, they initiate inflammatory cascades and release a variety of pro-inflammatory factors (6). The excessive release of pro-inflammatory factors leads to pulmonary dysfunction, high mucin production and protease-antiprotease imbalance (7,8).
Circular RNAs (circRNAs) are a specific class of non-coding RNAs (ncRNAs), lacking the 5' cap and 3' poly(A) tail of strand RNAs, with covalently closed-loop structures and are resistant to exonuclease degradation (9-10). Several studies suggested that dysregulated circRNAs interfere with protein-coding transcripts at the post-transcriptional level by competing with microRNAs (miRNAs or miRs), thereby affecting development of asthma (11,12). Huang et al (13) reported that in CD4+ T cells, highly expressed hsa_circ_0005519 affects the secretion of IL-13/IL-6 by competitively binding to let-7a-5p, thereby regulating the inflammatory process mediated by T cells. circRNA ERBB2 promotes the proliferation and migration of airway smooth muscle cells (ASMCs) via the miR-98-5p/insulin-like growth factor 1 receptor axis (14). In addition, circVPS33A is highly expressed in plasma of patients with asthma and an in vitro model of airway epithelial inflammation where it regulates high-mobility group box 1 expression via miR-192-5p, resulting in epithelial cell damage (15).
miRNAs are short ncRNAs ~22 nucleotides in length and are closely related to the pathogenesis of asthma (16,17). miRNAs can bind to mRNA in the 3'-untranslated region, which degrades mRNA and inhibits its translation, thereby altering the function of other cells (18). By acting on bronchial epithelial cells, ASMCs and immune cells, miRNAs cause type 1 T helper (Th1)/Th2 polarisation, airway remodelling and chronic epithelial inflammation (19). Recent studies have reported increased miR-338-3p expression in the sera of patients with asthma treated with anti-IL-5 agents (20,21). However, the specific role of miR-338-3p in the inflammatory response in asthma remains unclear.
E26 transformation specific-1 (ETS1) is a proto-oncogene in various cancers (22). The differential expression of ETS1 has also recently been detected in various types of inflammatory disease (23). Th2-polarised CD4+ T cells show increased ETS1 expression and the expression of inflammatory factors is upregulated after the cells are transfected to overexpress ETS1(24). A previous study showed that miR-338-3p targets ETS1 mRNA and inhibits ETS1 expression in gallbladder cancer (25). However, the roles of miR-338-3p and ETS1 in asthma remain unclear.
The present study aimed to investigate the role of circRNA targeting dehydrogenase E1 (circDHTKD1)/miR-338-3p/ETS1 axis in a model of airway epithelial inflammation. The present results may provide understanding of the role of circDHTKD1 in the pathogenesis of asthma and provide novel insights into the molecular mechanisms and therapeutic targets for asthma research.
Materials and methods
Cell culture and treatments
Human immortalized bronchial epithelial cell line (BEAS-2B cell line) was purchased from the Type Culture Collection of the Chinese Academy of Science (Shanghai, China) and cultured using RPMI-1640 supplemented with 10% foetal bovine serum (both Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin (Beijing Solarbio Science & Technology Co., Ltd.) at 37˚C with 5% CO2. To establish an in vitro model of asthma epithelial inflammation, cells were treated with 0, 1, 5 and 10 µg/ml lipopolysaccharide (LPS, Sigma-Aldrich; Merck KGaA) for 24 h at 37˚C when cells had reached a confluence of 70-80%. BEAS-2B cells were used as controls following treatment with an equal volume of PBS for 24 h at 37˚C.
Cell Counting Kit-8 (CCK-8) assay
Cell activity was measured using CCK-8 assay (Dojindo Laboratories, Inc.) according to the manufacturer's instructions. BEAS-2B cells were resuspended in PBS and the density was adjusted to 2x104 cells/ml before plating onto 96-well plates at a density of 5,000 cells/well. After 48 h at 37˚C, 10 µl CCK-8 reagent was added to each well. After 2 h, absorbance values at 450 nm were measured with a microplate reader (BioTek Instruments, Inc.).
Cell transfection
BEAS-2B cells were plated into 6-well plates (5x105 cells/well) and cultured overnight to 60-70% confluency before transfection. All RNA sequences used for cell transfection were obtained from Guangzhou RiboBio Co., Ltd. Transfection complexes containing 50 nM RNAs were transfected into BEAS-2B cells using Lipofectamine 3000® (Invitrogen; Thermo Fisher Scientific, Inc.) and serum-free Opti-MEM (Thermo Fisher Scientific, Inc.) and incubated for 6 h at 37˚C according to the manufacturer's protocols. Reverse transcription-quantitative (RT-q)PCR or western blot analysis was performed 48 h after transfection to assess transfection efficiency. The sequences of small interfering (si)RNAs and miRNA mimics and inhibitors are listed in Table I.
Table I.
siRNA and miR sequences.
| RNA | Sense sequence, 5'→3' | Antisense sequence, 5'→3' |
|---|---|---|
| si-NC | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
| si-circ#1 | GCUGGAAUCUCAGUUGAUCAUTT | AUGAUCAACUGAGAUUCCAGCTT |
| si-circ#2 | UGCUGGAAUCUCAGUUGAUCATT | UGAUCAACUGAGAUUCCAGCATT |
| miR-338-3p mimics | UCCAGCAUCAGUGAUUUUGUUG | CAACAAAAUCACUGAUGCUGGA |
| miR-338-3p mimics NC | UCACAACCUCCUAGAAAGAGUAGA | UCUACUCUUUCUAGGAGGUUGUGA |
| miR-338-3p inhibitor | CAACAAAAUCACUGAUGCUGGA | - |
| miR-338 inhibitor NC | UUCUCCGAACGUGUCACGUTT | - |
| si-ETS1 #1 | GAGCUACGAUAGUUGUGAUTT | AUCACAACUAUCGUAGCUCTT |
| si-ETS1 #2 | GGAAUUACUCACUGAUAAATT | UUUAUCAGUGAGUAAUUCCTT |
| si-ETS1 #3 | AGGGCACCUUCAAGGACUATT | UAGUCCUUGAAGGUGCCCUTT |
si, small interfering; NC, negative control; circ, circular; miR, microRNA; ETS1, E26 transformation specific-1.
RNA-sequencing (RNA-seq)
BEAS-2B cells were treated with LPS at a concentration of 5 µg/ml whereas PBS was used in the control group for 24 h at 37˚C. Total RNA was isolated with RNAiso PLUS (Takara) reagent and mRNA was enriched by Ribo-off rRNA Depletion kit (cat. no. N406; Vazyme Biotech Co., Ltd.). 1% agarose gel electrophoresis and NanoDrop ND-1000 spectrophotometry (Thermo Fisher Scientific, Inc.) were employed to verify quality and integrity of the extracted RNAs. RNA-seq libraries were prepared using the VAHTS® Stranded mRNA-seq V2 Library Prep kit for Illumina® (cat. no. NR612; Vazyme Biotech Co., Ltd.) following the manufacturer's recommendations. Briefly, RNA samples were fragmented and RT of first- and second-strand cDNAs was performed using with random hexamer primers; cDNAs were purified and RNA Adapters were ligated at the 5' and 3' ends of DNA fragments. The quality of the established libraries were examined using 8% agarose gel electrophoresis, then quantified by Qubit 2.0 Fluorometer (Invitrogen). The final strand-specific cDNA library (420 µl of a 10 pM library) was subjected to Illumina® Hiseq X Ten (Illumina, Inc.) platform for 150 bp pair-end sequencing using HiSeq X Ten Reagent Kit (cat. no. FC-501-2501). Sequencing reads from RNA-seq were mapped to the human reference genome (hg19) from UCSC genome database (genome.ucsc.edu/). circRNA identification was performed using CIRI2 software (version 2.0.6; sourceforge.net/projects/ciri/) based on the RNA-seq data.
RNA extraction and RT-qPCR
Total RNA was extracted from BEAS-2B cells placed in a 6-well plate using RNAiso PLUS reagent (Takara, Dalian, China). The cRNA first strand was synthesized by RT using PrimeScript™ RT kit (cat. no. RR037A; Takara Biotechnology Co., Ltd.) under the following reaction conditions: 37˚C for 15 min and 85˚C for 5 sec. qPCR was performed on an MX3000P qPCR system (Agilent Technologies, Inc.) using the ChamQ Universal SYBR qPCR® Master Mix kit (cat. no. Q711; Vazyme Biotech Co., Ltd.), followed by 40 cycles at 95˚C for 15 sec and 64˚C for 30 sec. U6 and GAPDH were used as internal reference genes to normalize the data. Relative RNA expression levels were calculated using the 2-ΔΔCq method (26). The primers were synthesized by Shanghai Bioengineering Co., Ltd. The forward and reverse sequences of the primers used for qPCR are shown in Table II.
Table II.
PCR primer pair sequences.
| Gene | Forward sequence, 5'→3' | Reverse sequence, 5'→3' |
|---|---|---|
| ETS1 | AGGGACAGAGCGGAACTCAAC | AATTGGTCCGCTTCCTGTGTAG |
| GAPDH | CATGAGAAGTATGACAACAGCCT | AGTCCTTCCACGATACCAAAGT |
| miR-338-3p | CGCGTCCAGCATCAGTGATT | AGTGCAGGGTCCGAGGTATT |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| IL-6 | CACTGGTCTTTTGGAGTTTGAG | GGACTTTTGTACTCATCTGCAC |
| IL-8 | AACTGAGAGTGATTGAGAGTGG | ATGAATTCTCAGCCCTCTTCAA |
| TNF-α | AAGGACACCATGAGCACTGAAAGC | AGGAAGGAGAAGAGGCTGAGGAAC |
| VEGF | AGGGCAGAATCATCACGAAGT | AGGGTCTCGATTGGATGGCA |
| circDHTKD1 | CTCCCAACTTCAGAGCCAGG | GCAAGGCCATGATCAACTGAG |
| circRAPGEF5 | CGTCTAACATCTGCGACTCT | TCTGGGAAGGCGTATGTTCA |
| circETNK1 | GGCTTTGGGACTGAAGTTACT | GTTTCCCACGTAACAGCCAA |
| circNID2 | GAAGCTACAGGTGTGAGTGC | TCAGATGCCAAAACTGCCTG |
| circTNS3 | GCCAGTCAGCACAAAGGAG | CTTCCTGAGTCTCCCGCC |
| circPDIA3 | TTTAGAGATGGTGAAGAAGC | ACTCAGGTGCAAGTCTCTTG |
| circGNB2L1 | CCCTGGGTGTGTGCAAATAC | GTTGGTCTCATCCCTGGTCA |
| circTBCD | AGTGACAAGGCCCGAGATG | CAACAAGTTCATCATCCATTCTG |
ETS1, E26 transformation specific-1; circ, circular; miR, microRNA; DHTKD1, dehydrogenase E1 And Transketolase Domain Containing 1; RAPGEF5, Rap Guanine Nucleotide Exchange Factor 5; ETNK1, Ethanolamine Kinase 1; NID2, Nidogen 2; TNS3, Tensin-3; PDIA3, Protein Disulfide Isomerase Family A Member 3; GNB2L1, Guanine Nucleotide-Binding Protein Subunit Beta-2-Like 1; TBCD, Tubulin Folding Cofactor D.
RNA isolation of nuclear and cytoplasmic fractions
PARIS kit (Ambion Life Technologies) was used to isolate nuclear and cytoplasmic RNA from BEAS-2B cells following the manufacturer's instructions. The subcellular localization of circDHTKD1 was analysed using RT-qPCR as aforementioned. GAPDH and NEAT1 were used as cytoplasmic and cytosolic controls, respectively.
Nucleic acid electrophoresis and Sanger sequencing
PCR products were separated by electrophoresis on a 2% agarose gel and gels were cut and sent to Shanghai Sangon Bioengineering Co., Ltd. for sequencing following observation in a UV imaging system.
Dual-luciferase reporter assay
circRNA fragments containing the full length of circDHTKD1 with either the wild-type (WT) or mutated (MUT) binding site for miR-338-3p were synthesized and inserted into the psiCHECK2 vector (Promega Corporation) to generate psiCHECK2-circ-WT and psiCHECK2-circ-MUT, respectively. BEAS-2B were co-transfected with the aforementioned luciferase reporter plasmid and miR-338-3p mimics/miR-338-3p mimics negative control using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instruction. Following 48 h transfection, the cells were lysed immediately and the relative luciferase activity of firefly and Renilla luciferase was determined using a Dual-Luciferase Reporter assay kit (cat. no. FR201; TransGen Biotech Co., Ltd.) following the manufacturer's protocol.
Western blot analysis
Total protein was isolated from BEAS-2B cells using RIPA Lysis Buffer (cat. no. 20-188; Merck KGaA), then quantified with BCA Protein Assay (cat. no. P0012; Beyotime Institute of Biotechnology) and the protein was denatured in a 95˚C water bath for 5 min. Proteins (30 µg/lane) were separated by 10% SDS-PAGE, transferred onto PVDF membranes (Millipore; Merck KGaA), blocked with 5% non-fat milk for 2 h at room temperature and incubated overnight at 4˚C with the following primary antibodies: Anti-ETS1 (cat. no. ab220361; Abcam; 1/1,000); anti-phosphorylated (p)-ERK1/2 (cat. no ab201015; Abcam; 1/1,000); anti-ERK1/2 (cat. no. ab184699; Abcam; 1/8,000) and anti-GAPDH (cat. no. ab181602; Abcam; 1/10,000). This was followed by incubation with rabbit anti-mouse horseradish peroxidase-conjugated secondary antibodies (cat. no. ab6721; Abcam; 1/5,000) for 60 min at room temperature. Protein bands were scanned and imaged using Immobilon® Western HRP Substrate ECL chemiluminescence reagent (cat. no. WBKLS0500; Merck KGaA). Densitometry analysis was performed using ImageJ software (Version 1.53e; National Institutes of Health) with GAPDH as the loading control.
ELISA
BEAS-2B cells were seeded into 12-well plates (1x10^5 cells/well) and treated with or without PBS for 48 h at 37˚C. Subsequently, the cell supernatant was collected and centrifuged at 1,000 x g for 5 min at 37˚C. According to the manufacturer's instruction, the concentrations of inflammatory cytokines IL-6 (cat. no. PI330), IL-8 (cat. no. PI640), TNF-α (cat. no. PT518) and VEGF (cat. no. PV963) were detected using ELISA kits (Beyotime Institute of Biotechnology).
Bioinformatics analysis
Cancer-Specific CircRNAs Database (CSCD) (gb.whu.edu.cn/CSCD/) was applied to annotate and identify exonic circRNAs. The circbank (circbank.cn/) and circRNA interactome (circinteractome.nia.nih.gov/) databases were used to predict downstream miRNAs. In addition, the binding site of miR-338-3p ETS1 and ERK2 was predicted using the TargetScan database (targetscan.org/). The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of miR-338-3p target genes was conducted using the Metascape online tool (version 3.5; metascape.org/) (27).
Statistical analysis
The experimental data from three independent replicates are expressed as the mean ± standard deviation. Data were analysed using SPSS 22.0 (IBM Corp.) and GraphPad Prism 8.0 software (GraphPad Software, Inc.; Dotmatics). Paired Student's t-test was used to assess the difference between two groups, while one-way ANOVA was used to analyse differences between ≥3 groups followed by Tukey's post hoc test for pairwise comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of LPS on human airway epithelial cell viability and inflammatory cytokine secretion
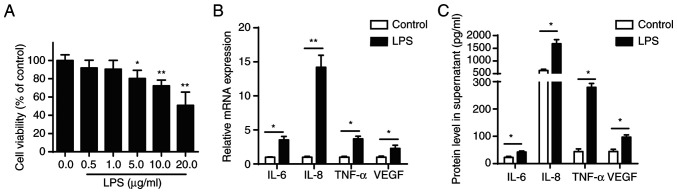
CCK-8 assay was performed to evaluate the effect of different concentrations of LPS (0.1, 1.0, 10.0 and 100.0 µg/ml) on the viability of BEAS-2B cells, which were shown to be concentration-dependent. LPS concentration ≥5 µg/ml significantly inhibited viability of BEAS-2B cells after 24 h treatment (Fig. 1A). Furthermore, RT-qPCR and ELISA confirmed that 5 µg/ml LPS increased secretion of pro-inflammatory cytokines (IL-6, IL-8, TNF-α and VEGF) in BEAS-2B cells (Fig. 1B and C).
Figure 1.
LPS stimulation induces inflammation response in BEAS-2B cells. (A) Cell Counting Kit-8 assay was performed to measure viability of BEAS-2B cells treated with LPS. (B) mRNA levels of IL-6, IL-8, TNF-α and VEGF in BEAS-2B following LPS treatment (5 µg/ml) were analysed by RT-qPCR. (C) Protein levels of IL-6, IL-8, TNF-α and VEGF were measured in supernatant by ELISA. *P<0.05 and **P<0.01. LPS, lipopolysaccharide.
circDHTKD1 is significantly upregulated in LPS-stimulated BEAS-2B cells
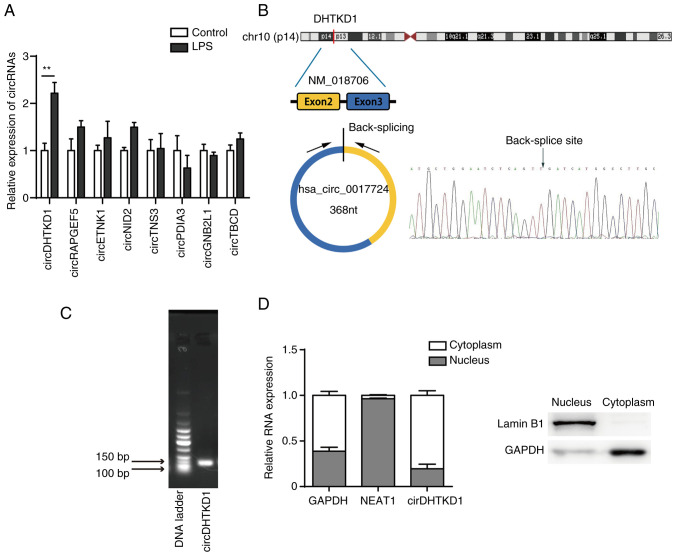
The transcriptome of LPS-treated BEAS-2B cells was sequenced using CIRI2 software for circRNA expression profiling, followed by selection of exonic circRNAs using CSCD (gb.whu.edu.cn/CSCD/). Finally, the top eight upregulated exonic circRNAs identified in BEAS-2B cells between LPS-induced and normal-control were annotated in the circBase database (circbase.org/; Table III). Changes in expression profile were analysed by RT-qPCR and showed a significant upregulation of circDHTKD1 (hsa_circ_0017724) in LPS-induced BEAS-2B cells compared with that in the control group. The fold change was increased compared with that of other circRNAs (Fig. 2A). Based on University of California, Santa Cruz, genome browser (genome.ucsc.edu/) and circBase databases, circDHTKD1 was shown to be circularised from exons 2 and 3 of the DHTKD1 gene on chromosome 10p14 with a splicing length of 368 bp (Fig. 2B). The back-splice junction of circDHTKD1 was confirmed by Sanger sequencing. The RT-PCR product of circDHTKD1 had head-to-tail splicing of the expected size (137 bp fragment) (Fig. 2C), indicating that the product was a circRNA. In addition, the subcellular localisation of circDHTKD1 in BEAS-2B cells was identified by nucleocytoplasmic separation and circDHTKD1 was found to be significantly enriched in the cytoplasm of BEAS-2B cells (Fig. 2D). These data demonstrated that circDHTKD1 was upregulated in LPS-induced BEAS-2B cells and was present in cytoplasm in a circular form.
Table III.
Top eight upregulated exonic circRNAs.
| circRNA | Gene | Position, hg 19 | Spliced sequence length |
|---|---|---|---|
| hsa_circ_0005281 | Tubulin folding cofactor D | chr17:80721840-80730383 | 403 |
| strand: + | |||
| hsa_circ_0001681 | Rap guanine nucleotide exchange factor 5 | chr7:22330793-22357656 | 516 |
| strand: - | |||
| hsa_circ_0002151 | Protein disulphide isomerase family A member 3 | chr15:44046021-44048964 | 197 |
| strand: + | |||
| hsa_circ_0017724 | Dehydrogenase E1 | chr10:12123470-12126750 | 368 |
| strand: + | |||
| hsa_circ_0008664 | Ethanolamine kinase 1 | chr12:22796696-22826594 | 789 |
| strand: + | |||
| hsa_circ_0075402 | Guanine nucleotide-binding protein subunit β-2-like 1 | chr5:180668491-180669345 | 320 |
| strand: - | |||
| hsa_circ_0031930 | Nidogen 2 | chr14:52495439-52527074 | 1996 |
| strand: - | |||
| hsa_circ_0006229 | Tensin-3 | chr7:47384352-47385954 | 369 |
| strand: - |
circRNA, circular RNA.
Figure 2.
circDHTKD1 is upregulated in LPS-treated BEAS-2B cells. (A) circRNA expression in BEAS-2B cells treated with LPS was determined by RT-qPCR. (B) Schematic of biogenesis of circDHTKD1 and Sanger sequencing of the back-splice junction site of circDHTKD1. (C) Agarose gel electrophoresis of circDHTKD1. (D) circDHTKD1 was primarily located in the cytoplasm of BEAS-2B cells, according to the nuclear-cytoplasmic fractionation RNA analysis performed by reverse transcription-quantitative PCR. **P<0.01. LPS, lipopolysaccharide; DHTKD1, dehydrogenase E1; NEAT1, nuclear enriched abundant transcript 1; circ, circular; RAPGEF5, Rap Guanine Nucleotide Exchange Factor 5; ETNK1, Ethanolamine Kinase 1; NID2, Nidogen 2; TNS3, Tensin-3; PDIA3, Protein Disulfide Isomerase Family A Member 3; GNB2L1, Guanine Nucleotide-Binding Protein Subunit Beta-2-Like 1; TBCD, Tubulin Folding Cofactor D.
Knockdown of circDHTKD1 inhibits LPS-induced inflammatory response
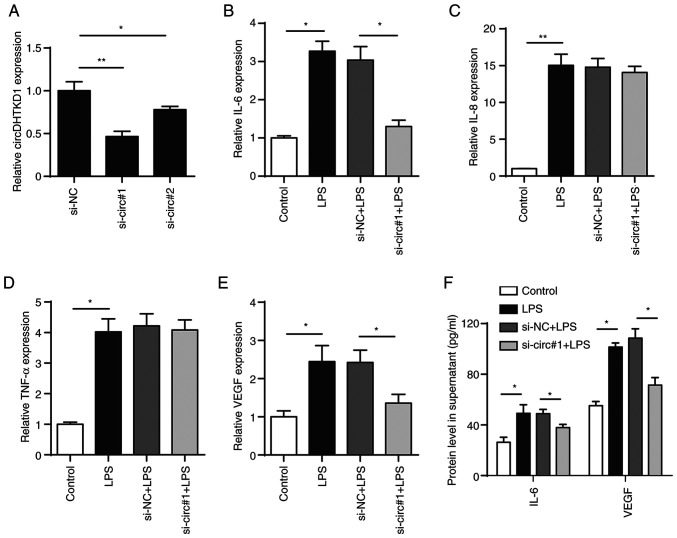
To determine the role of circDHTKD1 in the LPS-induced inflammatory response of BEAS-2B cells, two pairs of siRNAs targeting circDHTKD1 (si-circ#1 and si-circ#2) were designed, both of which effectively interfered with the expression of circDHTKD1. The knockdown of circDHTKD1 caused by si-circ#1 was notably more efficient compared with that caused by si-circ#2 (Fig. 3A). The qPCR results showed that the circDHTKD1 knockdown partially decreased mRNA levels of IL-6 and VEGF but had no significant effect on the mRNA levels of IL-8 and TNF-α (Fig. 3B-E). ELISA confirmed the results of the circDHTKD1 knockdown (Fig. 3F). These results indicated that circDHTKD1 was involved in the regulation of LPS-induced inflammatory responses in BEAS-2B cells.
Figure 3.
Effect of circDHTKD1 knockdown on cell inflammation response. (A) RT-qPCR analysis of circDHTKD1 following transfection of BEAS-2B cells with si-NC, si-circ#1 or si-circ#2 for 48 h. BEAS-2B cells were transfected with si-NC or si-circ#1 followed by treatment with 5 µg/ml lipopolysaccharide. mRNA expression of (B) IL-6, (C) IL-8, (D) TNF-α and (E) VEGF was measured by RT-qPCR. (F) ELISA was used to measure concentrations of IL-6 and VEGF. *P<0.05, **P<0.01. si-NC, small interfering RNA negative control; RT-qPCR, reverse transcription-quantitative PCR; LPS, lipopolysaccharide; DHTKD1, dehydrogenase E1; circ, circular.
circDHTKD1 serves as a sponge for miR-338-3p
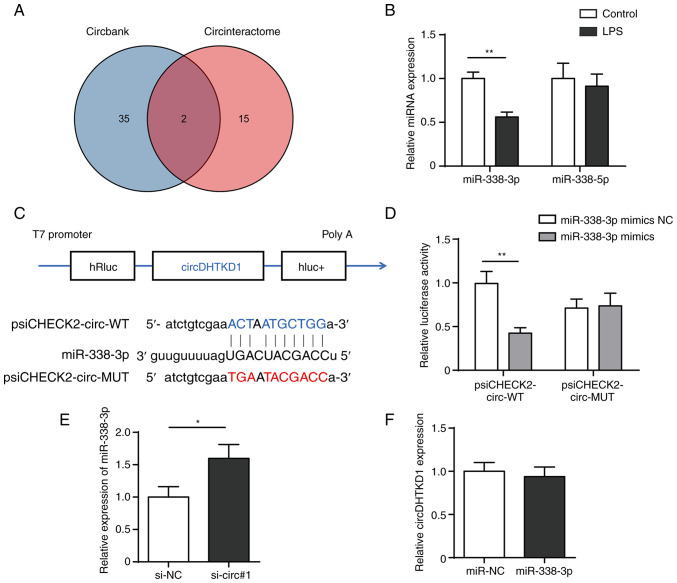
As circRNAs act as miRNA sponges and circDHTKD1 is primarily localised to the cytoplasm, it was hypothesised that circDHTKD1 may be involved in regulation of LPS-induced BEAS-2B cell inflammatory injury response via a competing endogenous RNA (ceRNA) mechanism. The target miRNAs that bind to circDHTKD1 were predicted through two databases, namely circBank (circbank.cn/) and circRNA interactome (circinteractome.nia.nih.gov/). miR-338-5p and miR-338-3p were shared miRNAs in both databases (Fig. 4A). RT-qPCR results showed that the expression of miR-338-3p significantly decreased following 24 h LPS induction, whereas expression of miR-338-5p did not change significantly (Fig. 4B). The dual-luciferase reporter assay showed that overexpression of miR-338-3p significantly reduced the luciferase activity of plasmid psiCHECK2-circ-WT but failed to decrease luciferase activity of psiCHECK2-circ-MUT (Fig. 4C and D). In addition, expression of miR-338-3p was increased after circDHTKD knockdown, whereas no significant changes in circDHTKD1 expression were detected after miR-338-3p overexpression (Fig. 4E and F). The results suggested that circDHTKD1 interfered with the function of miR-338-3p by acting as a sponge for miR-338-3p.
Figure 4.
circDHTKD1 serves as a sponge for miR-338-3p. (A) CircBank and CircInteractome databases were used to screen miRNAs that potentially interact with circDHTKD1. (B) RT-qPCR analysis of expression of miR-338-3p and miR-338-5p in LPS-treated BEAS-2B cells. (C) Schematic of predicted binding sites of circDHTKD1 and miR-338-3p. (D) Dual-luciferase reporter assay was used to validate the interaction between circDHTKD1 and miR-338-3p. (E) miR-338-3p and (F) circDHTKD1 levels were detected using RT-qPCR. *P<0.05, **P<0.01. RT-qPCR, reverse transcription-quantitative PCR; hRluc, Renilla luciferase reporter gene; WT, wild-type; MUT, mutant; NC, negative control; si, small interfering; DHTKD1, dehydrogenase E1; circ, circular; miR, microRNA; LPS, lipopolysaccharide.
ETS1 is a downstream target gene of miR-338-3p
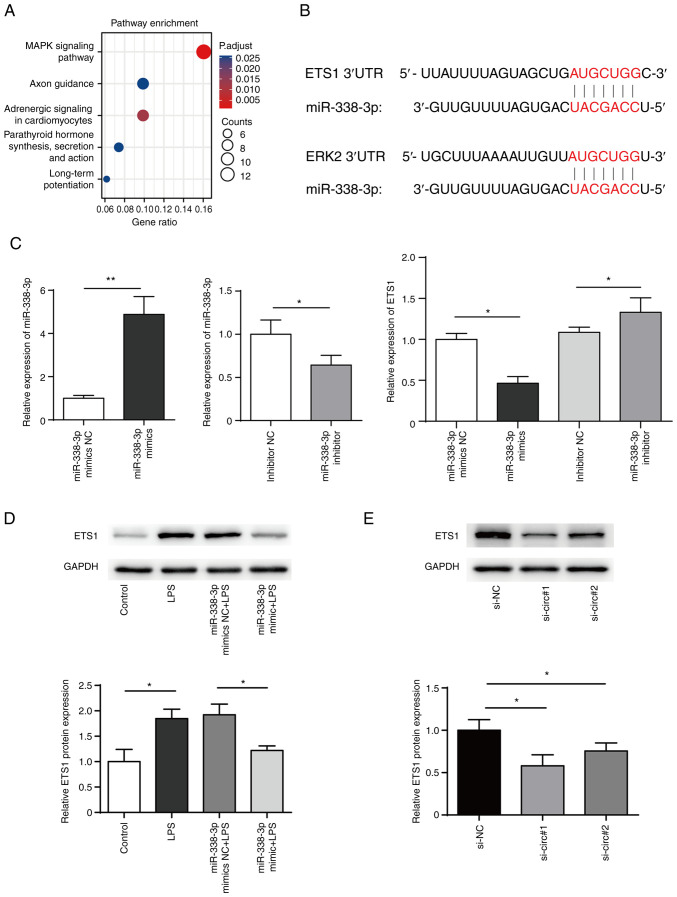
TargetScan (targetscan.org/) was used to predict the target genes of miR-338-3p while Metascape (metascape.org/) was used for cluster analysis of target genes of miR-338. The results suggested that MAPK signaling pathway was significantly enriched (Fig. 5A). Further searches using Kyoto Encyclopedia of Genes and Genomes database (kegg.jp/) revealed that ERK pathway genes (ERK2 and ETS1) may be regulated by miR-338-3p (Fig. 5B). The present results showed that transfection of miR-338-3p mimics in BEAS-2B cells decreased ETS1 expression levels, while inhibition of miR-338-3p increase ETS1 expression levels (Fig. 5C and D). In addition, western blotting showed that ETS1 protein levels significantly decreased following circRNA knockdown in BEAS-2B cells (Fig. 5E).
Figure 5.
ETS1 is a target of miR-338-3p. (A) Pathway enrichment of miR-338-3p. (B) Bioinformatics prediction of the binding site of miR-338-3p to mRNA 3' untranslated region of ETS1 and ERK2. (C) miR-338-3p mimics, miR-338-3p mimics NC, miR-338-3p inhibitor or miR-338-3p inhibitor NC were transfected into BEAS-2B cells and mRNA levels of ETS1 were measured. (D) Expression of ETS1 in BEAS-2B cells transfected with miR-338-3p mimics was measured by western blotting. (E) Expression of ETS1 in BEAS-2B cells transfected with siRNA targeting circDHTKD1 or its NC. *P<0.05, **P<0.01. ETS1, E26 transformation specific-1; NC, negative control; si, small interfering; LPS, lipopolysaccharide; 3'UTR, 3' untranslated region; DHTKD1, dehydrogenase E1; circ, circular.
Knockdown of ETS1 attenuates LPS-induced inflammatory responses in BEAS-2B cells
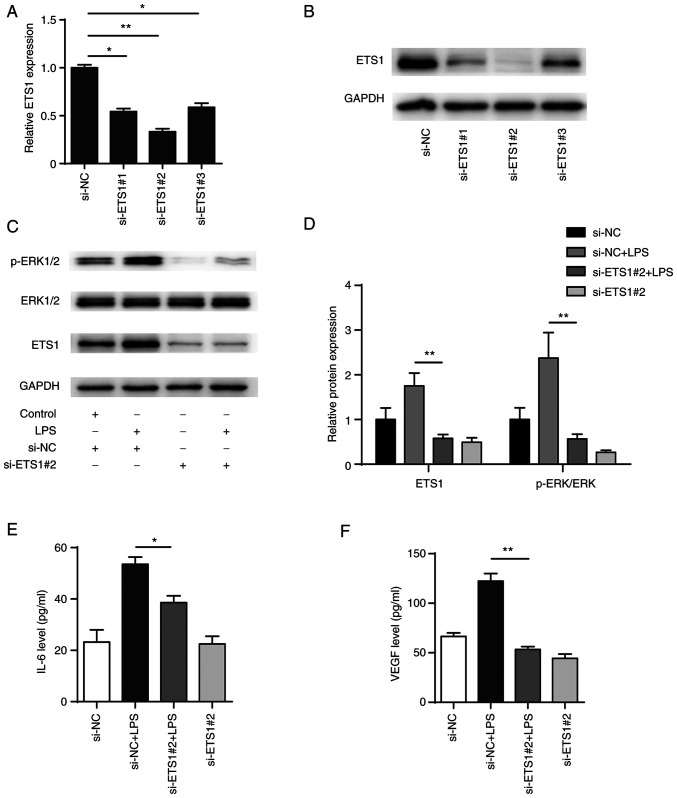
To explore the role of ETS1 in LPS-induced BEAS-2B cells, three siRNA pairs targeting ETS1 were designed (si-ETS1#1, si-ETS1#2 and si-ETS1#3) and transiently transfected into BEAS-2B cells. Following 48 h transfection, the mRNA and protein levels of ETS1 were significantly decreased in all transfection groups, with the si-ETS1#2 transfection showing the highest efficiency in knocking down ETS1 (Fig. 6A and B). In addition, western blotting showed that phosphorylation of ERK1/2 in BEAS-2B cells increased after LPS stimulation and knockdown of ETS1 significantly inhibited both the phosphorylation of ERK1/2 induced by LPS and activation of the ERK signalling pathway (Fig. 6C and D). Also, knockdown of ETS1 decreased the expression levels of IL-6 and VEGF (Fig. 6E and F). The present results indicated that ETS1 was involved in the regulation of inflammatory factors IL-6 and VEGF, as well as activation of the ERK pathway.
Figure 6.
Knockdown of ETS1 attenuates LPS-induced inflammatory responses in BEAS-2B cells. ETS1 levels measured by (A) RT-PCR and (B) western blot assay in BEAS-2B cells following transfection with siRNAs (NC, si-ETS1 #1, #2, #3). (C) Expression levels of key genes of ERK signalling pathway were detected by western blot and (D) semi-quantified. (E) IL-6 and (F) VEGF levels were measured through ELISA. *P<0.05, **P<0.01. ETS1, E26 transformation specific-1; NC, negative control; si, small interfering; LPS, lipopolysaccharide; p, phosphorylated.
circDHTKD1 enhances LPS-induced inflammatory responses in BEAS-2B cells via the miR-338-3p/ETS1 axis
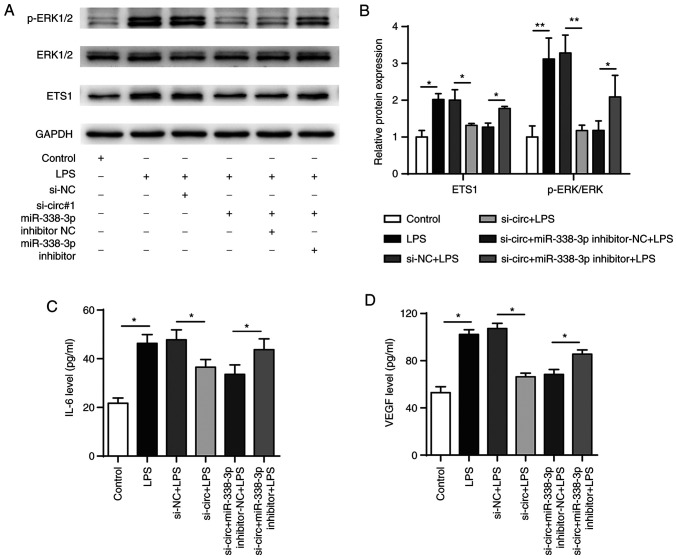
The functional recovery assay showed that LPS treatment resulted in increased expression of p-ERK compared with that in the negative control and that p-ERK levels were significantly decreased when circDHTKD1 was knocked down. This effect was reversed by transfection with miR-338-3p inhibitor (Fig. 7A and B). In addition, transfection with miR-338-3p inhibitor reversed the decrease in IL-6 and VEGF protein levels that was induced by the circDHTKD1 knockdown (Fig. 7C and D). These results collectively demonstrated that circDHTKD1 enhanced LPS-induced inflammatory responses in BEAS-2B cells via the miR-338-3p/ETS1 axis.
Figure 7.
circDHTKD1 promotes LPS-induced BEAS-2B cell inflammation response by regulating the miR-338-3p/ETS1 axis. (A) Combined with LPS treatment, BEAS-2B cells were transfected with si-NC, si-circ#1, si-circ#1 + inhibitor-NC or si-circ#1 + miR-338-3p inhibitor and ETS1, ERK and p-ERK protein expression levels detected by western blot and (B) semi-quantitative analysis (B). Protein expression levels of (C) IL-6 and (D) VEGF in the culture supernatant of BEAS-2B cells were detected by ELISA. *P<0.05, **P<0.01. ETS1, E26 transformation specific-1; NC, negative control; si, small interfering; LPS, lipopolysaccharide; p, phosphorylated; circ, circular; DHTKD1, dehydrogenase E1.
Discussion
Airway epithelial cells are involved in the pathogenesis of asthma (28). Repeated damage to epithelial cells triggers and activates airway remodelling via signalling cascades (29). Several studies have demonstrated that significantly higher levels of IL-6 and VEGF in bronchoalveolar lavage fluid and induced sputum in asthmatic patients are associated with lung function compared with those in the normal population (30-33). Serum TNF-α and IL-8 levels are significantly higher in patients with asthma in acute exacerbation compared with those in stable patients (34). The present study treated BEAS-2B cells with LPS and demonstrated that LPS significantly decreased cell viability while inflammatory factors were significantly upregulated at the mRNA and protein levels, demonstrating that LPS induced bronchial epithelial cells to produce an inflammatory phenotype.
circRNAs are ncRNAs with a closed-loop structure formed by back splicing that perform gene regulation at the transcriptional or post-transcriptional level (35). Bao et al (11) characterised the circRNA expression profile of a mouse asthma model and observed that differentially expressed circRNAs are involved in lipid metabolism, cell adhesion and endocytosis and the pathogenesis of allergic asthma. Several studies have revealed the role of circRNAs in asthmatic airway epithelial inflammation: Jia et al (36) reported that circRNA_406961 regulates particulate matter 2.5 (PM2.5)-induced inflammation in human bronchial epithelial cells by interacting with IL enhancer-binding factor 2 and activating the c-Jun N-terminal kinase/signal transducer and activator of transcription 3 pathway. In addition, circRNA104250 and lncRNAuc001.dgp.1 promote PM2.5-induced inflammatory responses in BEAS-2B cells by co-targeting miR-3607-5p (37). The present study showed that circDHTKD1 was upregulated in BEAS-2B cells treated with LPS. Knockdown of circDHTKD1 resulted in decreased expression levels of pro-inflammatory cytokines. Therefore, circDHTKD1 may serve an inflammatory role in asthma progression.
Aberrantly expressed miR-338-3p is associated with the pathogenesis of various diseases. miR-338-3p is downregulated in the lung tissue of patients with pulmonary fibrosis (38). In addition, miR-338-3p is negatively correlated with VEGF expression levels in hepatoma and vascular endothelial cells (39,40). Recent studies showed that the miR-338-3p expression is increased in the serum of patients with severe asthma treated with anti-IL-5 agents, potentially in association with the MAPK and transforming growth factor β signalling pathways (20,21). circRNAs regulate gene expression by competitively binding to miRNAs and serving as ceRNAs for miRNAs (41,42). The present study showed that the expression of circDHTKD1 was upregulated in LPS-induced BEAS-2B cells, whereas miR-338-3p was downregulated. It was hypothesized that circDHTKD1 regulates the synthesis of IL-6 and VEGF by targeting miR-338-3p. Although online bioinformatics analysis showed that miR-338-3p cannot target and bind IL-6 and VEGF-encoding mRNA, it may affect IL-6 and VEGF protein synthesis and secretion by regulating activation of the ERK pathway.
ETS1 is considered a proto-oncogene and several studies have shown that ETS1 may also be involved in the pathogenesis of inflammatory disease (23,43). In addition, Yang et al (44) reported that ETS1 was targeted by miR-338-3p in 768-O cells. In acute ischemic stroke, the miR-338-3p/ETS1 axis exerts protective effects on cell viability, apoptosis and inflammation (45). Colás-Algora et al (46) reported that the expression of the transcription factor ETS1 is upregulated in endothelial cells stimulated with LPS and ETS1 knockdown decreases expression of E-cadherin and exacerbates disruption of the cell membrane. Studies have demonstrated the involvement of ETS1 in asthma: Zook et al (47) demonstrated that ETS1 is required for IL-33 to induce IL-5 and IL-13 production. Wang et al (24) demonstrated that ETS1 expression is increased in Th2-polarised CD4+ T cells and overexpression of ETS1 results in increased expression of Th2-type cytokines (IL-5 and IL-13). Therefore, ETS1 may regulate the secretion of inflammatory factors in LPS-induced airway epithelial cells. The present findings suggested that ETS1 was involved in the regulation of the production of inflammatory factors IL-6 and VEGF. ETS1 is a key downstream gene of the ERK pathway and is positively regulated by the ERK pathway (48); however, studies have reported that ETS1 is also a key gene in the activation of the ERK pathway (49,50). The present study showed that the circDHTKD1/miR-338-3p/ETS1 axis promoted activation of the LPS-induced ERK pathway and inflammatory factor secretion.
Altogether, the present study demonstrated that circDHTKD1 was upregulated in LPS-induced BEAS-2B cells and circDHTKD1 knockdown alleviated LPS-induced cellular inflammatory responses via the miR-338-3p/ETS1 axis. Although circDHTKD1 may be a potential target for the treatment of asthma, the role of the circ_0001679/miR-338-3p/ETS1 axis in asthma needs validation in clinical samples and animal models.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Centre (Nucleic Acids Res 2022), China National Centre for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences repository, www.ngdc.cncb.ac.cn/gsa-human/browse/HRA003481.
Authors' contributions
FQ, SH and JW designed the study and wrote and edited the manuscript. FQ and JW revised the manuscript. SH and XY performed the experiments and analyzed the data. XC and SZ were involved in the conception of the study. All authors have read and approved the final manuscript. SH and JW confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Busse WW, Rosenwasser LJ. Mechanisms of asthma. J Allergy Clin Immunol. 2003;111:S799–S804. doi: 10.1067/mai.2003.158. [DOI] [PubMed] [Google Scholar]
- 2.Barcik W, Boutin R, Sokolowska M, Finlay BB. The role of lung and gut microbiota in the pathology of Asthma. Immunity. 2020;52:241–255. doi: 10.1016/j.immuni.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol. 2020;145:1499–1509. doi: 10.1016/j.jaci.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gohy S, Hupin C, Ladjemi MZ, Hox V, Pilette C. Key role of the epithelium in chronic upper airways diseases. Clin Exp Allergy. 2020;50:135–146. doi: 10.1111/cea.13539. [DOI] [PubMed] [Google Scholar]
- 5.Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184:1469–1485. doi: 10.1016/j.cell.2021.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Gras D, Chanez P, Vachier I, Petit A, Bourdin A. Bronchial epithelium as a target for innovative treatments in asthma. Pharmacol Ther. 2013;140:290–305. doi: 10.1016/j.pharmthera.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Ursini CL, Cavallo D, Fresegna AM, Ciervo A, Maiello R, Tassone P, Buresti G, Casciardi S, Iavicoli S. Evaluation of cytotoxic, genotoxic and inflammatory response in human alveolar and bronchial epithelial cells exposed to titanium dioxide nanoparticles. J Appl Toxicol. 2014;34:1209–1219. doi: 10.1002/jat.3038. [DOI] [PubMed] [Google Scholar]
- 8.Speth JM, Bourdonnay E, Penke LR, Mancuso P, Moore BB, Weinberg JB, Peters-Golden M. Alveolar epithelial cell-derived prostaglandin E2 serves as a request signal for macrophage secretion of suppressor of cytokine signaling 3 during innate inflammation. J Immunol. 2016;196:5112–5120. doi: 10.4049/jimmunol.1502153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 10.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9(e1003777) doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao H, Zhou Q, Li Q, Niu M, Chen S, Yang P, Liu Z, Xia L. Differentially expressed circular RNAs in a murine asthma model. Mol Med Rep. 2020;22:5412–5422. doi: 10.3892/mmr.2020.11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Chen H, Liu J, Gai L, Yan X, Guo Z, Liu F. Emerging advances of non-coding RNAs and competitive endogenous RNA regulatory networks in Asthma. Bioengineered. 2021;12:7820–7836. doi: 10.1080/21655979.2021.1981796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Z, Cao Y, Zhou M, Qi X, Fu B, Mou Y, Wu G, Xie J, Zhao J, Xiong W. Hsa_circ_0005519 increases IL-13/IL-6 by regulating hsa-let-7a-5p in CD4+ T cells to affect asthma. Clin Exp Allergy. 2019;49:1116–1127. doi: 10.1111/cea.13445. [DOI] [PubMed] [Google Scholar]
- 14.Huang JQ, Wang F, Wang LT, Li YM, Lu JL, Chen JY. Circular RNA ERBB2 contributes to proliferation and migration of airway smooth muscle cells via miR-98-5p/IGF1R signaling in asthma. J Asthma Allergy. 2021;14:1197–1207. doi: 10.2147/JAA.S326058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su Y, Geng L, Ma Y, Yu X, Kang Z, Kang Z. Identification of circular RNA circVPS33A as a modulator in house dust mite-induced injury in human bronchial epithelial cells. Exp Lung Res. 2021;47:368–381. doi: 10.1080/01902148.2021.1974125. [DOI] [PubMed] [Google Scholar]
- 16.Williams AE, Larner-Svensson H, Perry MM, Campbell GA, Herrick SE, Adcock IM, Erjefalt JS, Chung KF, Lindsay MA. MicroRNA expression profiling in mild asthmatic human airways and effect of corticosteroid therapy. PLoS One. 2009;4(e5889) doi: 10.1371/journal.pone.0005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng MJ, Shi F, Qiu C, Peng WK. MicroRNA-181a, -146a and -146b in spleen CD4+ T lymphocytes play proinflammatory roles in a murine model of asthma. Int Immunopharmacol. 2012;13:347–353. doi: 10.1016/j.intimp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Specjalski K, Niedoszytko M. MicroRNAs: Future biomarkers and targets of therapy in asthma. Curr Opin Pulm Med. 2020;26:285–292. doi: 10.1097/MCP.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 19.Rebane A, Akdis CA. MicroRNAs in allergy and asthma. Curr Allergy Asthma Rep. 2014;14(424) doi: 10.1007/s11882-014-0424-x. [DOI] [PubMed] [Google Scholar]
- 20.Cañas JA, Valverde-Monge M, Rodrigo-Muñoz JM, Sastre B, Gil-Martínez M, García-Latorre R, Rial MJ, Gómez-Cardeñosa A, Fernández-Nieto M, Pinillos-Robles EJ, et al. Serum microRNAs as tool to predict early response to Benralizumab in severe Eosinophilic Asthma. J Pers Med. 2021;11(76) doi: 10.3390/jpm11020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rial MJ, Cañas JA, Rodrigo-Muñoz JM, Valverde-Monge M, Sastre B, Sastre J, Del Pozo V. Changes in serum MicroRNAs after Anti-IL-5 biological treatment of severe Asthma. Int J Mol Sci. 2021;22(3558) doi: 10.3390/ijms22073558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Zhou R, Zhou H, Li Q, Hong J, Meng R, Zhu F, Zhang S, Dai X, Peng G, et al. ETS-1 induces endothelial-like differentiation and promotes metastasis in non-small cell lung cancer. Cell Physiol Biochem. 2018;45:1827–1839. doi: 10.1159/000487874. [DOI] [PubMed] [Google Scholar]
- 23.Jiang L, Liang J, Wang T, Meng F, Duan W. ETS proto-oncogene 1 modulates PTP1B expression to participate in high glucose-mediated endothelial inflammation. Acta Biochim Biophys Sin (Shanghai) 2022;54:565–573. doi: 10.3724/abbs.2022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T, Zhou Q, Shang Y. Downregulation of miRNA-451a promotes the differentiation of CD4+ T cells towards Th2 cells by upregulating ETS1 in childhood Asthma. J Innate Immun. 2021;13:38–48. doi: 10.1159/000509714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Yan R, Zhang SN, Zhang HZ, Ruan XJ, Cao Z, Gu XZ. MicroRNA-338-3p inhibits the progression of bladder cancer through regulating ETS1 expression. Eur Rev Med Pharmacol Sci. 2019;23:1986–1995. doi: 10.26355/eurrev_201903_17237. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi A, Tanaseichuk O, Benner C, Chanda SK. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1523) doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 29.Chung KF. p38 mitogen-activated protein kinase pathways in asthma and COPD. Chest. 2011;139:1470–1479. doi: 10.1378/chest.10-1914. [DOI] [PubMed] [Google Scholar]
- 30.Neveu WA, Allard JL, Raymond DM, Bourassa LM, Burns SM, Bunn JY, Irvin CG, Kaminsky DA, Rincon M. Elevation of IL-6 in the allergic asthmatic airway is independent of inflammation but associates with loss of central airway function. Respir Res. 2010;11(28) doi: 10.1186/1465-9921-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang JG, Chen XJ, Liu T, Jiang SJ. FOXP3+ associated with the pro-inflammatory regulatory T and T helper 17 effector cells in asthma patients. Exp Ther Med. 2016;12:2753–2758. doi: 10.3892/etm.2016.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papadaki G, Bakakos P, Kostikas K, Hillas G, Tsilogianni Z, Koulouris NG, Papiris S, Loukides S. Vascular endothelial growth factor and cysteinyl leukotrienes in sputum supernatant of patients with asthma. Respir Med. 2013;107:1339–1345. doi: 10.1016/j.rmed.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol. 2001;107:1034–1038. doi: 10.1067/mai.2001.115626. [DOI] [PubMed] [Google Scholar]
- 34.Jiang XG, Yang XD, Lv Z, Zhuang PH. Elevated serum levels of TNF-α, IL-8, and ECP can be involved in the development and progression of bronchial asthma. J Asthma. 2018;55:111–118. doi: 10.1080/02770903.2017.1318141. [DOI] [PubMed] [Google Scholar]
- 35.Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21:475–490. doi: 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
- 36.Jia Y, Li X, Nan A, Zhang N, Chen L, Zhou H, Zhang H, Qiu M, Zhu J, Ling Y, Jiang Y. Circular RNA 406961 interacts with ILF2 to regulate PM2.5-induced inflammatory responses in human bronchial epithelial cells via activation of STAT3/JNK pathways. Environ Int. 2020;141(105755) doi: 10.1016/j.envint.2020.105755. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Jia Y, Nan A, Zhang N, Zhou H, Chen L, Pan X, Qiu M, Zhu J, Zhang H, et al. CircRNA104250 and lncRNAuc001.dgp.1 promote the PM2.5-induced inflammatory response by co-targeting miR-3607-5p in BEAS-2B cells. Environ Pollut. 2020;258(113749) doi: 10.1016/j.envpol.2019.113749. [DOI] [PubMed] [Google Scholar]
- 38.Rackow AR, Judge JL, Woeller CF, Sime PJ, Kottmann RM. miR-338-3p blocks TGFβ-induced myofibroblast differentiation through the induction of PTEN. Am J Physiol Lung Cell Mol Physiol. 2022;322:L385–L400. doi: 10.1152/ajplung.00251.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang T, Liu W, Zeng XC, Jiang N, Fu BS, Guo Y, Yi HM, Li H, Zhang Q, Chen WJ, Chen GH. Down-regulation of microRNA-338-3p promoted angiogenesis in hepatocellular carcinoma. Biomed Pharmacother. 2016;84:583–591. doi: 10.1016/j.biopha.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 40.Wei H, Cao C, Wei X, Meng M, Wu B, Meng L, Wei X, Gu S, Li H. Circular RNA circVEGFC accelerates high glucose-induced vascular endothelial cells apoptosis through miR-338-3p/HIF-1α/VEGFA axis. Aging (Albany NY) 2020;12:14365–14375. doi: 10.18632/aging.103478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao X, Cai Y, Xu J. Circular RNAs: Biogenesis, mechanism, and function in human cancers. Int J Mol Sci. 2019;20(3926) doi: 10.3390/ijms20163926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kristensen LS, Andersen MS, Stagsted L, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 43.Yan M, Komatsu N, Muro R, Huynh NCN, Tomofuji Y, Okada Y, Suzuki HI, Takaba H, Kitazawa R, Kitazawa S, et al. ETS1 governs pathological tissue-remodeling programs in disease-associated fibroblasts. Nat Immunol. 2022;23:1330–1341. doi: 10.1038/s41590-022-01285-0. [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Zhang Y, Fan H. Downregulation of SBF2-AS1 functions as a tumor suppressor in clear cell renal cell carcinoma by inhibiting miR-338-3p-targeted ETS1. Cancer Gene Ther. 2021;28:813–827. doi: 10.1038/s41417-020-0197-4. [DOI] [PubMed] [Google Scholar]
- 45.Chen C, Wang L, Wang L, Liu Q, Wang C. LncRNA CASC15 promotes cerebral ischemia/reperfusion injury via miR-338-3p/ETS1 axis in acute ischemic stroke. Int J Gen Med. 2021;14:6305–6313. doi: 10.2147/IJGM.S323237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colás-Algora N, García-Weber D, Cacho-Navas C, Barroso S, Caballero A, Ribas C, Correas I, Millán J. Compensatory increase of VE-cadherin expression through ETS1 regulates endothelial barrier function in response to TNFα. Cell Mol Life Sci. 2020;77:2125–2140. doi: 10.1007/s00018-019-03260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zook EC, Ramirez K, Guo X, van der Voort G, Sigvardsson M, Svensson EC, Fu YX, Kee BL. The ETS1 transcription factor is required for the development and cytokine-induced expansion of ILC2. J Exp Med. 2016;213:687–696. doi: 10.1084/jem.20150851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dittmer J. The biology of the Ets1 proto-oncogene. Mol Cancer. 2003;2(29) doi: 10.1186/1476-4598-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plotnik JP, Budka JA, Ferris MW, Hollenhorst PC. ETS1 is a genome-wide effector of RAS/ERK signalling in epithelial cells. Nucleic Acids Res. 2014;42:11928–11940. doi: 10.1093/nar/gku929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ning M, Qin S, Tian J, Wang Y, Liu Q. LncRNA AFAP-AS1 promotes anaplastic thyroid cancer progression by sponging miR-155-5p through ETS1/ERK pathway. Bioengineered. 2021;12:1543–1554. doi: 10.1080/21655979.2021.1918537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Centre (Nucleic Acids Res 2022), China National Centre for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences repository, www.ngdc.cncb.ac.cn/gsa-human/browse/HRA003481.