Abstract
Allantoin is an abundant component of yams and has been known as a skin protectant due to its pharmacological activities. In previous methods for allantoin determination using high-performance liquid chromatography (HPLC), the separation was unsatisfactory. We herein developed a 1H quantitative nuclear magnetic resonance (qNMR) method for quantification of allantoin in the flesh and peel of yams. The method was carried out based on the relative ratio of signals integration of allantoin to a certain amount of the internal standard dimethyl sulfone (DMSO2) and validated in terms of specificity, linearity (range 62.5-2000 μg/ml), sensitivity (limit of detection (LOD) and quantification (LOQ) 4.63 and 14.03 μg/ml, respectively), precision (RSD% 0.02-0.26), and recovery (86.35-92.11%). The method was then applied for the evaluation of allantoin in flesh and peel extracts of four different yams cultivated in Korea.
Keywords: Allantoin, yam, Dioscorea, NMR, qNMR, quantitation
Introduction
Nuclear magnetic resonance (NMR) spectroscopy is a key analytical technique for structures elucidation of small and macromolecules, as well as for the identification of single or multiple compounds in complex matrices [1]. In recent years, quantitative NMR (qNMR) has been well-applicable for the quantification of low molecular weight metabolites in biological fluids or food products with excellently analytical performance [2, 3]. The quantitative inaccuracy of qNMR is less than 2.0%, which is an acceptable limit for precise and accurate quantitation [4]. The 1H qNMR spectroscopy technique is fast and provides higher reliability on the structural prediction of the molecules [5]. Its temperature operation is low; thus preventing the degradation of thermolabile analytes [6]. In addition, the sample preparation for qNMR is of limited complexity and generally compatible with chromatography [7]. In comparison to the traditional chromatographic methods, 1H qNMR spectroscopy technique has not only the above certain advantages but also the possibility to simultaneously determine component structures, no need for prior isolation of the analyte present in a mixture, the possibility of simultaneous quantitative analysis of multiple target analytes in a mixture, no need for individual experimental setup, and reference of the same compound and calibrations, as well as non-invasive and non-destructive character of the method [4, 5]. Essentially, the applications of qNMR in simultaneous purity evaluation of organic molecules have great potential to advance the search for the truth behind their biological activity and to find explanations for problems that require consideration of unexpected chemical diversity due to residual complexity [5, 7].
Allantoin, a diureide of glyoxylic acid, is one of the abundant bioactive components in yams [8]. Allantoin has long been known to enhance the efficacy and desirability of various cosmetic products such as skin creams, lotions, soaps, shampoos, and lipsticks due to its antioxidative, anti-inflammatory, and moisturizing activities [9-11]. Allantoin-treated asthma groups remarkably alleviated airway inflammatory-cell infiltration as well as cytokine mRNA expression in lung tissues [12]. Additionally, allantoin has been proved to be effective on antidiabetic [13, 14], antihypertensive [15], anticancer [14], as well as on cognitive function and hippocampal neurogenesis [10].
To evaluate the contents of allantoin in yam extracts for high-quality raw materials in the development of pharmaceutical or functional food products, an assurance quantitative analytical method is needed. Several methods, including high-performance liquid chromatography (HPLC) methods, for allantoin determination, have been studied [16]. Nevertheless, no information is known in the literature on qNMR methods dealing with the analysis of allantoin in yams up to now [16-19]. Our previous study has revealed a quantitative analysis method using HPLC for allantoin identification in the peel of Dioscorea japonica; however, the method is limited in evaluating allantoin in the different matrices of yam [19]. The aim of this study was to develop a rapid, sensitive, and reliable 1H qNMR spectroscopy-based method for the allantoin quantitation in yams. The method was validated in terms of specificity, linearity, sensitivity (limit of detection (LOD) and quantification (LOQ)), precision, and recovery. The method was then used for the evaluation of allantoin in flesh and peel extracts of four Dioscorea species cultivated in Korea, including Dioscorea bulbifra L., Dioscorea quinqueloba Thunb, Dioscorea batatas Decne, and Dioscorea esculenta (Lour.) Burkil. These findings led to the proposal of a useful method for the analysis of allantoin obtained from various Dioscorea species in particular, plant extracts in general, in further discoveries of allantoin potentials as a functional biomaterial.
Materials and Methods
Chemical and Reference Compounds
Acetonitrile (reagent grade), water (reagent grade), and methanol (reagent grade) were purchased from J.T.Baker (USA). Trifluoroacetic acid (TFA) was purchased from Sigma-Aldrich (USA). Ethanol (extra pure grade) was purchased from Duksan Pure Chemicals Co. (Korea). Waters Alliance 2695 high-performance liquid chromatography (HPLC) (Waters, USA) which was performed on a Hector-M-carbohydrate column (250 × 4.6 mm, 5 μm, RS tech Corporation, Korea) was used for the analysis and the samples was detected by the photodiode array detector (PDA) Waters 2996. Allantoin (analytical standard grade) was purchased from Sigma-Aldrich. Dimethyl sulfoxide (DMSO-d6) was obtained from Sigma-Aldrich. Dimethyl sulfone used as internal standard (IS) was purchased from Sigma-Aldrich. IS solution was prepared in DMSO-d6 at a concentration of 1.0 mg/ml and kept at 4°C. Prior to use the IS solution was left to come to room temperature.
Plant Material and Sample Preparation
Yams were purchased from Taesan Farm (Korea). Their tubers were washed with water and separated into flesh and peel, then dried with a freeze-dryer (Ilshinbiobase, Korea). The freeze-dried peels of four yam species were powdered, and 1 g of dried powder from each sample was sonicated with 250 ml of 50% ethanol for 30 min. The solutions were then kept at 25°C for 12 h. Afterward, the samples were filtered with filter paper (85 g/m2, 0.20 mm, 160 s/100 ml, Ø 110 mm, Hyundai Micro CO., Korea) and evaporated in vacuo. The obtained extracts were weighed and dissolved in methanol to inject into the HPLC system for analysis. The remaining solutions were evaporated under vacuum to dryness and used for 1H NMR analysis. 10.0 ± 0.2 mg of each extract was dissolved in 700 μl IS solution. The solutions containing the extract and calibrant were transferred into 5-mm NMR tubes.
NMR Experimental Parameters
1H NMR spectra were recorded at 700 MHz (Bruker AVANCENeo700) with the standard qNMR conditions: temperature, 298 K; relaxation delay (D1), 60s; flip angle, 90°; acquisition time, 2.34 s; number of scans (nc), 32; and spectral width, 0‒16 ppm. Prior to Fourier transformation (FT) an exponential weighing factor corresponding to a line broadening of 0.3 Hz was applied. The spectra were phased, corrected and integrated automatically using MestReNova software. Where necessary, accurate integration was performed manually for the peaks of interest. The content of allantoin was calculated using the following equation [5]
Where IC is the internal calibrant, A is allantoin, s is the sample, n is the number of protons, Int is integral, MW is the molecular weight, m is the mass, and P is the purity (in %).
Calibration Curves and Validation
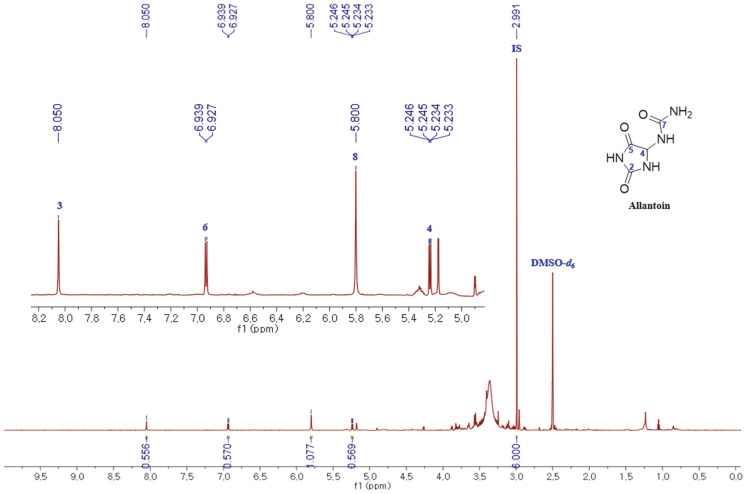
For the preparation of the calibration curve, the exact amount of allantoin (12.9 mg) was weighed and dissolved in 1.29 ml of IS solution. The serial dilution method was used to prepare the desired concentrations (2000, 1000, 500, 250, 125, and 62.5 μg/ml). Each amount of reference mixture was analyzed in triplicate. The quantitation was based on the integration ratio between allantoin protons at δH 8.05, 6.93, 5.80, and 5.24 ppm and dimethyl sulfone protons at δH 2.99 ppm. The method was validated following the ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) guidelines “Validation of Analytical Procedures: Text and Methodology Q2(R1)” [20] for linearity, sensitivity (LOD and LOQ), accuracy, precision, and repeatability. LOD and LOQ were calculated using prepared calibration curves. The intraday precision was determined by analyzing five replicates of spiked samples at three concentration levels of allantoin (500, 250, and 125 μg/ml). The interday precision was assessed by analyzing spiked samples at three concentration levels, 500, 250, and 125 μg/ml, on five consecutive days. The precision was calculated as the relative percent standard deviation (RSD %). Accuracy was determined by recovery experiments spiking a dry extract with or without allantoin, evaluated as the relative percentage error (Er%), and measured by comparing the nominal concentration and the assayed concentration.
Results and Discussion
Metabolic fingerprinting has demonstrated a significant addition to the battery of classical tools for understanding the biochemical metabolites of biological systems at a certain time [21, 22]. NMR and mass spectrometry (MS) are two analytical technologies that have arisen in metabolomics which is focused on the profiling and quantification of natural small compounds. Metabolomic studies make use mostly of hyphenated techniques which rely on chromatography separation (LC) of metabolites coupled to MS to analyze complex mixtures of extracted metabolites [23]. Although LC-MS provides a high resolution and sensitivity, LC methods generally require a long run time, metabolite separation is dependent on the chromatographic column used, detection is limited by the analytes’ ionization ability, and reference compound for peak identity and often suffers from low solubility [6]. On the contrary, 1H NMR is a fast analysis, highly reproducible, and robust quantitative technique [5, 22]. We first quantitated allantoin from eight yam extracts using the HPLC-photodiode array (PDA) method which was reported recently [19]. The chromatograms were obtained at 235 nm. As shown in Fig. 1, the separation was unsatisfactory. Also, the solubility of allantoin in HPLC solvents was not high. Hence, we decided to use the 1H qNMR method for the quantitation of allantoin in the flesh and peel of yams.
Fig. 1. HPLC chromatograms of allantoin and yams flesh and peel extracts monitored at 235 nm.
Method Development
To develop the 1H NMR-based quantification technique for allantoin in yam extracts, several deuterium-labeled solvents including methanol-d4, acetone-d6, acetonitrile-d3, dimethyl sulfoxide (DMSO)-d6, deuterium oxide, as well as mixtures thereof were tested in a series of preliminary 1H NMR experiments as potential solubility and stability of yam extracts and allantoin. DMSO-d6 was selected for sample preparation due to the high solubility of allantoin and extracts, the good separation of NMR key resonances, and the clean baseline in the relevant spectral regions from the obtained spectra [24]. Dimethyl sulfone (DMSO2) was used as an internal standard (IS) for qNMR because of its solubility and stability in DMSO-d6, its non-volatility, and resulting in non-overlapping 1H NMR signals [5] Moreover, the ESI-MS of allantoin in the mixture with IS showed an [M-H]+ ion at m/z 157.3.
It is essential to consider the selection of NMR experimental parameters to perform the qNMR analysis due to their effects on quantitative accuracy and precision. Pulse angle and relaxation delay (D1) are the most important parameters for quantitative experiments and are closely connected [1]. In general, a 90° pulse angle was required for quantitation because of maximum intensity. In such a situation, D1 must be at least five times the longest relaxation time (T1) to guarantee full relaxation and recovery of the signal intensity [5]. Thus, D1 as 60 s and 90°pulse angle were used for the quantitative experiments.
Validation of the 1H qNMR Method
The developed 1H qNMR method was validated in terms of specificity, linearity, sensitivity, precision, and recovery [20]. On the basis of the calibration models, the linearity of the method was confirmed for the concentrations range of 62.5‒2,000 μg/ml of allantoin added to a fixed quantity (0.7 mg) of IS (Fig. 2). The plot of the integral ratio (allantoin/IS) showed a linear trend. The linear regression equation was expressed as y = 0.1303x ‒ 0.0044; where ‘y’ and ‘x’ represent the ratio of integral of allantoin to IS and the concentrations of allantoin, respectively. The correlation coefficient of the calibration function was calculated to be 0.9998. The sensitivity parameters (LOD and LOQ) values were determined as 4.63 and 14.03 μg/ml, respectively (Table 1). Therefore, the sensitivity of the developed method was well adequate considering the working concentrations range of allantoin.
Fig. 2. Linearity test for allantoin signals in the range 62.5‒2000 μg/ml added with a fixed quantity (0.7 mg) of IS.
Table 1.
Linearity of allantoin determination in yams.
| Ratio | Regession eq | Correlation coeff, r2 | Linearity range (μg/ml) | LOD (μg/ml) | LOQ (μg/ml) |
|---|---|---|---|---|---|
| Allantoin/IS | y = 0.1303x-0.0044 | 0.9998 | 62.5‒2000 | 4.63 | 14.03 |
The intraday precision, expressed as RSD values ranged from 0.02 to 0.50%, as shown in Table 2. The interday precision ranged between 0.18 and 0.26% (Table 2). Accuracy was determined in recovery experiments with three different concentrations of allantoin spiked to a yam extract. The recoveries were found to be within the range of 86.35‒92.11%, with RSD% less than 0.17%. Besides that, the results for accuracy were expressed as the relative percentage error (Er%) (Table 3). The estimated accuracy values with the proposed method are within acceptable levels for allantoin. The obtained results indicate that the method could be considered accurate.
Table 2.
Precision data of allantoin in yams (intraday (n = 5 on each day) and interday (n = 5)).
| Ratio | Theoretical concentration (μg/ml) | RSD (%) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Days 1‒5 | ||
| Allantoin/IS | 125 | 0.5 | 0.17 | 0.07 | 0.15 | 0.14 | 0.26 |
| 250 | 0.12 | 0.08 | 0.15 | 0.09 | 0.09 | 0.16 | |
| 500 | 0.14 | 0.05 | 0.06 | 0.02 | 0.05 | 0.18 | |
Table 3.
Accuracy data of allantoin determination in yams (n = 5).
| Ratio | Relative percentage error (Er%) | Recovery (% ± RSD) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 62.5 (μg/ml) | 125 (μg/ml) | 500 (μg/ml) | 62.5 (μg/ml) | 125 (μg/ml) | 500 (μg/ml) | |
| Allantoin/IS | ‒9.76 | ‒7.89 | ‒13.65 | 90.24 ± 0.17 | 92.11 ± 0.15 | 86.35 ± 0.11 |
Method Application
Yams (Dioscorea spp.) are economically valued herbs and are widely used for promoting health and folk medication as well [25]. Reported biological studies revealed that D. quinqueloba extract possesses effects on cardiovascular and inflammatory skin diseases [26, 27]. Sato et al. reported that D. esculenta-induced increase in muscle sex steroid hormone levels helps reduce insulin resistance in type 2 diabetes [28]. The powder and liquid products of Dioscorea alata were found to be promising in the development of blood pressure regulation foods by their antihypertensive effects on spontaneously hypertensive rats [29]. The extract and isolated metabolites of D. batatas were demonstrated to have wide pharmaceutical effects including antioxidant [30], anti-neuroinflammation [31], ethanol-induced gastric ulcer [32], and antidiabetic [13].
In this investigation, the developed 1H qNMR method was further applied for evaluating the content of allantoin in the flesh and peel extracts of four yams varieties, including D. bulbifra, D. quinqueloba, D. batatas, and D. esculenta. Among them, D. bulbifra, D. quinqueloba, and D. batatas were reported to have the promising antioxidant effects [33]. Our results showed that allantoin was the most abundant in the peel of D. bulbifra and least abundant in the D. quinqueloba flesh extract with contents of 62.49 and 3.30 g/kg, respectively, on the basis of weight. As shown in Table 4, allantoin was presented in both flesh and peel of all investigated Dioscorea species. The allantoin content of D. bulbifra, D. batatas, and D. quinqueloba peels is greater than that of their flesh, whereas the content of allantoin in D. esculenta flesh is greater than that of its peel.
Table 4.
qNMR results for allantoin in yams (n = 3).
| Content (g/ kg ± RSD) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Flesh | Peels | |||||||
|
| ||||||||
| D. bulbifra | D. batatas | D. esculenta | D. quinqueloba | D. bulbifra | D. batatas | D. esculenta | D. quinqueloba | |
| Allantoin | 19.14 ± 1.71 | 22.01 ± 2.38 | 16.12 ± 0.84 | 3.30 ± 2.05 | 62.49 ± 0.49 | 25.35 ± 0.36 | 13.55 ± 0.51 | 5.19 ± 1.33 |
As a final remark, we would like to propose a valid quantitative analysis method of allantoin in Dioscorea spp. using 1H qNMR spectroscopy-based technique. Allantoin, widely used in pharmaceutical and cosmetic applications, presents the most abundant in the peel of D. bulbifra. The method was validated in terms of specificity, linearity, sensitivity, precision, and recovery. The results demonstrated that the developed method was suitable for the determination of allantoin in the Dioscorea genus.
Fig. 3. 1H NMR spectra (700 MHz, DMSO-d6) of allantoin from D. bulbifra peel extract (δ 0.0‒10.0 ppm).
The signals for IS, DMSO-d6, and allantoin chemical markers have been assigned.
Acknowlegments
This work was technically supported by Korea Basic Science Institute (National research Facilities and Equipment center) grant funded by the Ministry of Education (2021R1A6C101A416).
Footnotes
Funding
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (IPET) through the Useful Agricultural Life Resources Industry Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (grant number 121049-2).
Conflicts of Interest
The authors have no financial conflicts of interest to declare.
References
- 1.Bharti SK, Roy R. Quantitative 1H NMR spectroscopy. Trends Anal. Chem. 2012;35:5–26. doi: 10.1016/j.trac.2012.02.007. [DOI] [Google Scholar]
- 2.Truzzi E, Marchetti L, Benvenuti S, Righi V, Rossi MC, Gallo V, et al. A novel qNMR application for the quantification of vegetable oils used as adulterants in essential oils. Molecules. 2021;26:5439. doi: 10.3390/molecules26185439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao R, Liu X, Liu Y, Zhai X, Cao T, Wang A, et al. Applications of nuclear magnetic resonance spectroscopy to the evaluation of complex food constituents. Food Chem. 2021;342:128258. doi: 10.1016/j.foodchem.2020.128258. [DOI] [PubMed] [Google Scholar]
- 4.Malz F, Jancke H. Validation of quantitative NMR. J. Pharm. Biomed. Anal. 2005;38:813–823. doi: 10.1016/j.jpba.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 5.Pauli GF, Chen SN, Simmler C, Lankin DC, Gödecke T, Jaki BU, et al. Importance of purity evaluation and the potential of quantitative 1H NMR as a purity assay. J. Med. Chem. 2014;57:9220–9231. doi: 10.1021/jm500734a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemprai P, Mahanta BP, Bora PK, Das DJ, Boruah JLH, Saikia SP, et al. A 1H NMR spectroscopic method for the quantification of propenylbenzenes in the essential oils: Evaluation of key odorants, antioxidants and post-harvest drying techniques for Piper betle L. Food Chem. 2020;331:127278. doi: 10.1016/j.foodchem.2020.127278. [DOI] [PubMed] [Google Scholar]
- 7.Cheilari A, Sturm S, Intelmann D, Seger C, Stuppner H. Head-to-head comparison of ultra-high-performance liquid chromatography with diode array detection versus quantitative nuclear magnetic resonance for the quantitative analysis of the silymarin complex in Silybum marianum fruit extracts. J. Agric. Food Chem. 2016;64:1618–1626. doi: 10.1021/acs.jafc.5b05494. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Liu Y, Chen G. Simultaneous determination of allantoin, choline and L-arginine in Rhizoma Dioscoreae by capillary electrophoresis. J. Chromatogr. A. 2004;1043:317–321. doi: 10.1016/j.chroma.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Chen XB, Matuszewski W, Kowalczyk J. Determination of allantoin in biological, cosmetic, and pharmaceutical samples. J. AOAC Int. 1996;79:628–635. doi: 10.1093/jaoac/79.3.628. [DOI] [PubMed] [Google Scholar]
- 10.Ahn YJ, Park SJ, Woo H, Lee HE, Kim HJ, Kwon G, et al. Effects of allantoin on cognitive function and hippocampal neurogenesis. Food Chem. Toxicol. 2014;64:210–216. doi: 10.1016/j.fct.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 11.Matricardi P, Cencetti C, Peris JE, Melis V, Carbone C, et al. Manca ml, author. Combination of argan oil and phospholipids for the development of an effective liposome-like formulation able to improve skin hydration and allantoin dermal delivery. Int. J. Pharm. 2016;505:204–211. doi: 10.1016/j.ijpharm.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Lee MY, Lee NH, Jung D, Lee JA, Seo CS, Lee H, et al. Protective effects of allantoin against ovalbumin (OVA)-induced lung inflammation in a murine model of asthma. Int. Immunopharmacol. 2010;10:474–480. doi: 10.1016/j.intimp.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Ma J, Meng X, Liu Y, Yin C, Zhang T, Wang P, et al. Effects of a rhizome aqueous extract of Dioscorea batatas and its bioactive compound, allantoin in high fat diet and streptozotocin-induced diabetic mice and the regulation of liver, pancreas and skeletal muscle dysfunction. J. Ethnopharmacol. 2020;259:112926. doi: 10.1016/j.jep.2020.112926. [DOI] [PubMed] [Google Scholar]
- 14.Go HK, Rahman MM, Kim GB, Na CS, Song CH, Kim JS, et al. Antidiabetic effects of yam (Dioscorea batatas) and its active constituent, allantoin, in a rat model of streptozotocin-induced diabetes. Nutrients. 2015;7:8532–8544. doi: 10.3390/nu7105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen MF, Tsai JT, Chen LJ, Wu TP, Yang JJ, Yin LT, et al. Antihypertensive action of allantoin in animals. Biomed Res. Int. 2014;2014:690135. doi: 10.1155/2014/690135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu YC, Ferng LHA, Huang PY. Quantitative analysis of allantoin and allantoic acid in yam tuber, mucilage, skin and bulbil of the Dioscorea species. Food Chem. 2006;94:541–549. doi: 10.1016/j.foodchem.2004.12.006. [DOI] [Google Scholar]
- 17.Yamamoto R, Seki M, Ouchi K, Koyano SI, Nakazawa S, Nagatani Y, et al. A rapid determination of allantoin by highperformance liquid chromatography using tris(hydroxymethyl)aminomethane-HCl buffer as a mobile phase. J. Pharm. Soc. Jpn. 1998;118:310–316. doi: 10.1248/yakushi1947.118.8_310. [DOI] [PubMed] [Google Scholar]
- 18.Ninomiya A, Murata Y, Tada M, Shimoishi Y. Quantitative analysis of allantoin in fresh tubers of Dioscorea opposita 'Tsukuneimo'. J. Japan Soc. Hort. Sci. 2003;72:321–323. doi: 10.2503/jjshs.72.321. [DOI] [Google Scholar]
- 19.Lee M, Kim JM, Kim H, Hahn D. Quantitative analysis of allantoin in Dioscorea japonica peel using an amino bonded-phase HPLC column. J. Food Hyg. Saf. 2021;36:347–352. doi: 10.13103/JFHS.2021.36.4.347. [DOI] [Google Scholar]
- 20.ICH, author. ICH Harmonised Tripartite Guideline Validation of Analytical Procedures: Text and Methodology Q2(R1) Geneva, Switzerland: 2006. https://www.ich.org/page/quality-guidelines. [Google Scholar]
- 21.Lanza IR, Zhang S, Ward LE, Karakelides H, Raftery D, Nair KS. Quantitative metabolomics by 1H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS One. 2010;5:e10538. doi: 10.1371/journal.pone.0010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raletsena MV, Mdlalose S, Bodede OS, Assress HA, Woldesemayat AA, Modise DM. 1H-NMR and LC-MS based metabolomics analysis of potato (Solanum tuberosum L.) cultivars irrigated with fly ash treated acid mine drainage. Molecules. 2022;27:1187. doi: 10.3390/molecules27041187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltran A, Suarez M, Rodríguez MA, Vinaixa M, Samino S, Arola L, et al. Assessment of compatibility between extraction methods for NMR and LC/MS-based metabolomics. Anal. Chem. 2012;84:5838–5844. doi: 10.1021/ac3005567. [DOI] [PubMed] [Google Scholar]
- 24.Nishinami S, Hirano A, Arakawa T, Shiraki K. Effects of allantoin and dimethyl sulfoxide on the thermal aggregation of lysozyme. Int. J. Biol. Macromol. 2018;119:180–185. doi: 10.1016/j.ijbiomac.2018.07.043. [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Cao TQ, Yeo CE, Shin SH, Kim H, Hong DH, et al. Development and validation of quantitative analysis method for phenanthrenes in peels of the Dioscorea genus. J. Microbiol. Biotechnol. 2022;32:976–981. doi: 10.4014/jmb.2206.06037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jegal J, Park NJ, Bong SK, Jegal H, Kim SN, Yang MH. Dioscorea quinqueloba ameliorates oxazolone- and 2,4-dinitrocholorobenzene-induced atopic dermatitis symptoms in murine models. Nutrients. 2017;9:1324–1336. doi: 10.3390/nu9121324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JH, Lee SM, Lee DC. Immunopotentiating effect of protein extract from Dioscorea quinqueloba in stressed mice. Korean J. Food Nutr. 2018;31:252–257. [Google Scholar]
- 28.Sato K, Fujita S, Iemitsu M. Dioscorea esculenta-induced increase in muscle sex steroid hormones is associated with enhanced insulin sensitivity in a type 2 diabetes rat model. FASEB J. 2017;31:793–801. doi: 10.1096/fj.201600874R. [DOI] [PubMed] [Google Scholar]
- 29.Liu YH, Lin YS, Liu DZ, Han CH, Chen CT, Fan M, et al. Effects of different types of yams (Dioscorea alata) products on the blood pressure of spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2009;73:1371–1376. doi: 10.1271/bbb.90022. [DOI] [PubMed] [Google Scholar]
- 30.Lim JS, Hahn D, Gu MJ, Oh J, Lee JS, Kim JS. Anti-inflammatory and antioxidant effects of 2,7-dihydroxy-4,6-dimethoxy phenanthrene isolated from Dioscorea batatas Decne. Appl. Biol. Chem. 2019;62:29. doi: 10.1186/s13765-019-0436-2. [DOI] [Google Scholar]
- 31.Lim JS, Oh J, Yun HS, Lee JS, Hahn D, Kim JS. Anti-neuroinflammatory activity of 6,7-dihydroxy-2,4-dimethoxy phenanthrene isolated from Dioscorea batatas Decne partly through suppressing the p38 MAPK/NF-κB pathway in BV2 microglial cells. J. Ethnopharmacol. 2022;282:114633. doi: 10.1016/j.jep.2021.114633. [DOI] [PubMed] [Google Scholar]
- 32.Byeon S, Oh J, Lim JS, Lee JS, Kim JS. Protective effects of Dioscorea batatas flesh and peel extracts against ethanol-induced gastric ulcer in mice. Nutrients. 2018;10:1680. doi: 10.3390/nu10111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim KM, Kang MK, Kim JS, Kim GC, Choi SY. Physicochemical composition and antioxidant activities of Korean Dioscorea species. J. East Asian Soc. Dietary Life. 2015;25:880–886. doi: 10.17495/easdl.2015.10.25.5.880. [DOI] [Google Scholar]