Abstract
Background
Acne, a commonly treated skin disease, requires patient-centered management due to its varying presentations, chronicity, and impact on health-related quality of life. Despite this, evidence-based clinical guidelines focus primarily on clinical severity of facial acne, omitting important patient- and disease-related factors, including ongoing management.
Objectives
To generate recommendations to support patient-centered acne management, which incorporate priority and prognostic factors beyond conventional clinical severity, traditionally defined by grading the appearance and extent of visible lesions.
Methods
The Personalizing Acne: Consensus of Experts consisted of 17 dermatologists who used a modified Delphi approach to reach consensus on statements regarding patient- and treatment-related factors pertaining to patient-centered acne management. Consensus was defined as ≥75% voting “agree” or “strongly agree.”
Results
Recommendations based on factors such as acne sequelae, location of acne, high burden of disease, and individual patient features were generated and incorporated into the Personalized Acne Treatment Tool.
Limitations
Recommendations are based on expert opinion, which may differ from patients’ perspectives. Regional variations in healthcare systems may not be represented.
Conclusions
The Personalizing Acne: Consensus of Experts panel provided practical recommendations to facilitate individualized management of acne, based on patient features, which can be implemented to improve treatment outcomes, adherence, and patient satisfaction.
Key words: acne care tool, acne guidelines, acne sequelae, consensus, Delphi process, high burden of disease, individual patient features, personalized acne treatment, shared decision-making, truncal acne
Capsule Summary.
-
•
Evidence-based clinical guidelines focus primarily on clinical assessment of visible lesions as a means of defining facial acne severity, omitting important patient- and disease-related factors.
-
•
Recommendations based on these factors contributing to acne severity and chronicity have been incorporated into the Personalised Acne Treatment Tool to support patient-centered acne care.
Introduction
Acne is one of the most common skin diseases. This condition can have significant psychosocial impact due to its ready visibility, slow response to treatment and chronic course, as well as burdensome physical consequences.1,2 The recently published “acne burden surveys” have highlighted the impact of truncal acne and acne scarring on emotional well-being.3,4 These surveys also outlined the need for improvements in the management of truncal acne and acne sequelae.
The Personalizing Acne: Consensus of Experts (PACE) was established to provide recommendations to address gaps in current clinical guidelines and improve patient care. The initial phase of this project published recommendations on truncal acne (chest, shoulders, and back) and acne sequelae, in addition to the Personalized Acne Care Pathway – an acne care roadmap, which was developed to assist healthcare professionals (HCPs) in providing comprehensive long-term acne management.5, 6, 7
Patient-centered acne management is important as varying presentations and impact of acne require different treatment approaches.8, 9, 10 Patients have noted feeling unheard, and trivialized by HCPs, as though their acne fits into 1 sphere of treatment, already predetermined by their practitioner.11,12 Additionally, differing views of acne severity are often held by HCPs and patients,11,13 leading to conflicting interests and lack of patient fulfillment. Implementing a strategy that achieves synergy between HCP and patient is paramount to addressing concerns and dispelling misconceptions.14,15 Interestingly, despite the psychological issues associated with acne, adherence to treatment is generally low. A previous study demonstrated that 27% of patients did not fill all their prescriptions.16 Adherence may be improved by shared decision-making regarding best therapy.17 Thus, placing the patient central to management decisions, listening to their views and expectations, can enhance the HCP–patient relationship and result in improved adherence and treatment outcomes.17
At present, evidence-based clinical guidelines primarily focus on facial acne and base treatment recommendations on clinical severity, which is established by grading of visible, active lesions.8, 9, 10 This severity-based approach omits pertinent aspects of acne such as the patient perspective and acne sequelae. The second phase of the PACE project thus aimed to transcend the conventional assessments in current guidelines by facilitating a patient-centric approach based on individual patient- and treatment-related factors. To address this, PACE developed the Personalized Acne Treatment Tool, a novel tool that can be used by HCPs to help guide individualized treatment decision-making.
Materials and methods
Expert panel
The expert panel comprised 17 expert dermatologists from the US (n = 6), Europe (n = 4), Asia (n = 3), Canada (n = 1), South America (n = 1), the Middle East (n = 1), and Australia (n = 1). Two chairpersons from the panel oversaw the process.
The modified Delphi approach
A modified Delphi process used by the PACE panel has been described previously.5, 6, 7 Between November 2021 and August 2022, 4 e-surveys were conducted to gather information and capture voting responses. To inform and direct the e-survey content, patient- and treatment-related factors pertinent to individualizing acne management were collected using insights from previous recommendations made by the PACE panel,7 clinical experience, and the literature. A premeeting guideline audit was then conducted to assess recommendations relating to these factors and identify any gaps in guidance. The tool was refined over a hybrid (virtual/in-person) meeting with the PACE panel and completion of a workmat activity, with opportunities to provide detailed feedback in the interim (Fig 1).
Fig 1.
The modified Delphi process used by Personalizing Acne: Consensus of Experts to inform and develop the Personalizing Acne Treatment Tool (PATT).
E-survey development and administration
Agreement was measured on each statement using a 5-point scale: “Strongly disagree,” “disagree,” “agree,” “strongly agree,” or “unable to answer.” Consensus was defined as ≥75% voting “agree” or “strongly agree.” Some questions were distributed as multiple choice and several responses could be selected. E-surveys were programmed, administered and responses collated by Ogilvy Health UK to maintain blinding. Topics covered in the e-surveys included statements related to individualizing an acne management approach, specific clinical presentations/scenarios, individual patient features, and general skincare.
Results
Definition of consensus recommendations
Consensus statement voting information is provided in parentheses (eg, 16/17 voted “agree” or “strongly agree”). Some panel members occasionally voted “unable to answer” and were removed from the voting total. Full statements are available in the Supplementary Information, via Mendeley at https://doi.org/10.17632/znmzfszg6k.1 and https://doi.org/10.17632/r664vx8759.1.
Priority factors to consider beyond clinical severity when individualizing a management approach
The PACE panel acknowledged the gaps in current clinical guidelines and agreed that there are additional priority and prognostic factors to consider when individualizing a management approach for patients with acne, such as the presence/risk of future acne-induced scarring (17/17), the presence/risk of acne-induced macular hyperpigmentation (17/17), the presence/future risk of acne-induced macular erythema (15/17), the location of acne (eg, on the face and/or trunk) (16/17), the impact of an individual’s acne on their health-related quality of life (17/17), and the psychosocial impact of an individual’s acne, which is part of their health-related quality of life (17/17).
Assessing Priority Factors
Location of acne
Recommendations to consider when managing truncal acne alongside facial acne are provided in Table I.
Table I.
Recommendations for truncal acne management, based on consensus
| Recommendations |
|---|
|
Discussion points
The panel acknowledged the need for a distinct approach to truncal acne but also highlighted similarities in response to treatment regimens for patients who present with truncal acne alongside facial acne. Selection of combination or monotherapy regimens is dependent on severity, which encompasses the morphology and location of lesions, as well as body surface area affected. Panelists mentioned application of nonsticky, fast-absorbing lotion, foam, or gel formulations for ease of spread over large surface areas. In all cases, shared decision-making is central to adherence as location of lesions, the patient’s lifestyle, and previous treatment history are important factors to discuss with patients to determine what is achievable.
Acne sequelae (and risk thereof)
Recommendations to consider when individualizing a treatment approach for patients with acne sequelae are provided in Table II.
Table II.
Recommendations for individualizing a management approach for patients with acne sequelae, based on consensus
| Recommendations |
|---|
|
Discussion points
Panelists typically escalate treatment for active acne lesions with concomitant acne-induced scarring quicker than other forms of sequelae due to its potential permanence and profound effect on patients.2 Severity of acne-induced scarring is often assessed by the depth, size, number, and morphology of scars, with severe scarring treated more aggressively. Panelists noted that treatment efficacy, potential side effects, and tolerability in the short- and long-term need to be discussed at all stages of treatment.
Burden of disease
Recommendations to consider when individualizing a management approach for patients who present with a high burden of disease are provided in Table III.
Table III.
Recommendations to consider for the management of patients with a high burden of disease, based on consensus
| Considerations |
|---|
|
Discussion points
Establishing a strong relationship with patients is essential to maintain effective communication about disease progression. This involves listening to the patient’s perspective and discussing each aspect of treatment, to ensure continuity of care with relevant support. Panelists agreed that combination therapy may be beneficial in patients with high psychological burden but noted that combination therapies may increase patient burden due to impact on lifestyle, convenience, and cost. Additionally, females may seek early resolution via intensive cosmeceuticals which are often irritating and ineffective, leading to further burden.18
Establishing Patient Perspective
The next step outlined by PATT is to establish the patient perspective. To inform a treatment approach, it is helpful to identify aspects of acne that have been bothering the patient the most in the last 1 to 3 months and subsequently discuss long-term treatment expectations and goals. This, in turn, should affect prioritization and selection of treatment options. From the panelists’ clinical experience, acne-induced macular hyperpigmentation, acne-induced scarring, active lesions, and general appearance were frequent patient concerns.
Considering an Appropriate Treatment Option
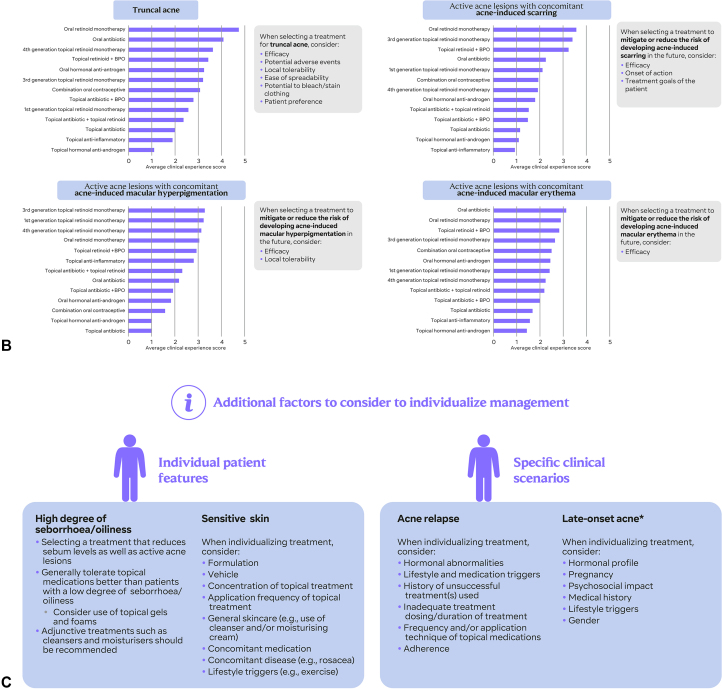
Treatment option schematics are listed for active acne lesions with concomitant acne-induced scarring, acne-induced macular hyperpigmentation, acne-induced macular erythema, and truncal acne based on scoring from PACE panelists (Fig 2, B). For each presentation of acne, the scores reflect the strength of the expert panel’s belief in each treatment class as a potential treatment option, based on clinical experience. These scores are not intended to act as a substitute for evidence-based recommendations in clinical guidelines or indicate which treatments are suitable for first-, second-, or third-line treatment or indicate the use of oral retinoids as first-line therapy. In concert with regulatory advice, scores may be used as a guide for treatment escalation based on the panel’s clinical experience.
Fig 2.
Overview of the Personalized Acne Treatment Tool (PATT) (A) consensus recommendations were incorporated into PATT to form 3 sequential steps: Assessing priority factors, establishing patient perspective, and considering appropriate treatment options. B, For each presentation of acne, the scores reflect the strength of the expert panel’s belief in each treatment class as a potential treatment option, based on clinical experience. These scores are not intended to act as a substitute for evidence-based recommendations in clinical guidelines or indicate ranking of treatments (eg, first-, second-, or third-line). C, Additional factors to consider when individualizing management include patient features and specific clinical scenarios. ∗Defined as first presentation >25 years of age. HRQoL, Health-related quality of life; PACE, Personalizing Acne: Consensus of Experts; PACP, Personalized Acne Care Pathway; PATT, Personalized Acne Treatment Tool.
Recommendations to consider when using antibiotics for patients with acne
Topical and oral antibiotics are commonly prescribed/coprescribed with topical retinoids for the treatment of moderate-to-severe acne.19,20 However, their use is associated with antibiotic resistance and disruption to the microbiome.19,20 Resistance of Cutibacterium acnes to antibiotics can result in a reduced or no response to treatment or recurrence of acne.19 To limit resistance, benzoyl peroxide is added to regimens when long-term antibiotic use is required.19 To minimize the risk of developing antimicrobial resistance, panelists agree that combination regimens that target multiple pathways in acne pathophysiology should be adopted when possible (16/17). When using a systemic antibiotic, its duration of use should be limited to mitigate antimicrobial resistance in patients with acne (17/17).
General skincare
Recommendations to consider when selecting sunscreen, cleansers, and moisturizers are provided in Table IV.
Table IV.
Recommendations to consider when selecting general skincare products
| Skincare product | Recommendations |
|---|---|
| Sunscreen |
|
| |
| Cleansers |
|
| |
| Moisturizers |
|
SPF, Sun protection factor.
Discussion points
Panelists typically consult with patients regarding the use of appropriate adjuncts such as well-tolerated cleansers and noncomedogenic moisturizers. Environmental factors such as temperature and sun exposure (17/17) are considered important and thus, sunscreen is deemed pivotal to help mitigate treatment-induced photosensitivity, to maintain general skin appearance and health, and to prevent the exacerbation of acne-induced macular hyperpigmentation. Overall, the skincare regimen should be evaluated regularly when individualizing a management approach (17/17).
Additional Clinical Presentations
Additional factors to consider when individualizing management for patient features and specific clinical scenarios are provided in Table V and Table VI.
Table V.
Recommendations to consider for individual patient features – sensitive skin and seborrhea/oiliness
| Individual patient feature | Considerations |
|---|---|
| Sensitive skin |
|
| |
| |
| High degree of seborrhea/oiliness |
|
|
Table VI.
Recommendations to consider for specific clinical scenarios – Acne relapse and late onset acne∗
| Clinical scenario | Considerations |
|---|---|
| Acne relapse |
|
| Late-onset acne |
|
Defined as first presentation >25 years of age.
Discussion points
There is notable overlap in skincare recommendations for patients with seborrhea/oiliness and sensitive skin. Panelists consider non-comedogenic, non-aggressive cleansers and moisturizers, coupled with a light texture, broad spectrum sun protection factor ≥30 for both features. However, maintenance treatment for seborrhea/oiliness includes regular cleansing, use of topical retinoids, and oil-free formulations. Constant evaluation and readjustment of products is recommended to ensure tolerability and minimal irritation.
Discussion points
Panelists mentioned educating patients on the chronic nature of acne and discussing the importance of adherence to maintenance treatment even when skin is clear of acne. External factors such as nutrition, sleep quality, and physical activity may be important factors for mitigating late-onset acne.
General discussion
The PATT is designed to place the patient at the center of acne care. Guidelines currently outline a prescriptive approach to acne management, basing treatment selection on clinically determined active acne severity.8, 9, 10 Acne management and care should be extended to a more collaborative and holistic approach between patient and HCP.
PATT incorporates patient- and disease-related factors beyond the clinical grading of acne that contribute to acne chronicity and severity. These include the most relevant aspects of acne impacting the patient, including patient history, specific clinical features, burden of disease, and patient preferences to inform treatment selection. In concert with current clinical guidelines, PATT represents an additional effort to increase the quality of acne management.
Achieving a balance between what the patient is experiencing, by giving equal consideration to patient preferences and aligning those issues with HCP expertise is critical to providing the best treatment outcomes possible.21,22 Essentially, the patient regains autonomy in their healthcare journey,22 but is guided and supported by comprehensible and straightforward recommendations.21 Where possible, treatment decisions should be reached in a collaborative manner, through informed shared decision-making and expert guidance from dermatologists informed by patient value and preferences.23 However, there is currently limited evidence to support the notion that consultations proceed in this way.24 In addition, there is a paucity of patient-oriented treatment goals or patient satisfaction considerations in national and regional clinical guidelines, with the focus being primarily acne severity.25, 26, 27, 28, 29, 30, 31, 32, 33 Furthermore, treatment selection by HCPs may be influenced by relative cost-effectiveness stratified by severity level, inadvertently overlooking patient preference.34
Potential uses for PATT as suggested by HCPs/dermatologists during the symposium “A novel patient-centered approach to acne management” at the European Academy of Dermatology and Venereology 2022, in Milan, include patient education and a medium to guide personalised treatment decision-making with patients. Additionally, 30% believed it would take 4 to 6 minutes to complete the 3-step PATT in practice. Short consultation times mean HCPs are often unable to consult lengthy treatment algorithms in clinical guidelines.35,36 The PATT has been designed as a simple, yet powerful visual to aid decision-making.
The main limitation of the Delphi process was that the patient perspective was not captured, but the expert recommendations were validated by patient feedback. In addition, regional differences in healthcare systems may not have been captured although the expert panel is globally represented.
In essence, the PATT encourages a shift toward a shared decision-making approach and considers the dynamic nature of acne management. Potential iterations include the addition of a dynamic element to foster personalization by HCPs, a version for patient use, primary care practitioners, or nurses with complementary information and recommendations. The PATT can be used in conjunction with the Personalized Acne Care Pathway to create a hybrid tool that aids longitudinal management of acne based on patient- and disease-specific factors. To validate recommendations in the PATT, patient feedback was gathered: 93% of patients reported finding it useful to talk through the treatment decision-making process with their doctor. A future version of the PATT would benefit from incorporating patient-reported outcomes measures to each domain; however, there is an unmet need for robustly developed patient-reported outcomes measures in acne, that are standardized and validated according to The COnsensus-based Standards for the selection of health Measurement INstruments.37
Conclusions
The PACE panel developed the PATT to deliver practical recommendations to facilitate individualized management of acne, based on important and specific patient factors. Recommendations can be implemented to improve treatment adherence and satisfaction, encouraging a more collaborative, integrated management approach to optimize patient outcomes.
Conflicts of interest
All panel members received honoraria from Galderma for participating in this project. Alison M Layton has acted as an advisor or consultant, been chief investigator for research (funded to institution) and/or received honoraria for unrestricted educational events from Almirall, Beiersdorf, Cipher Pharmaceuticals, Galderma, GlaxoSmithKline, La Roche-Posay, LEO Pharma, L’Oreal, Mylan, Origimm, and Proctor and Gamble. Andrew Alexis has received grant/research support (funds to institution) from Abbvie, Almirall, Amgen, Arcutis, Bausch Health, Bristol-Myers Squibb, Cara, Castle, Dermavant, Galderma, LEO Pharma, Novartis, Vyne, acted as a consultant/advisory board attendee for Abbvie, Allergan, Almirall, Amgen, Arcutis, Bausch Health, Beiersdorf, BMS, Cara, Cutera, Dermavant, Eli Lilly, EPI, Galderma, Janssen, LEO Pharma, L’Oreal, Ortho, Pfizer, Sanofi-Regeneron, Sol-Gel, Swiss American, UCB, VisualDx, Vyne. Hilary Baldwin has acted as an investigator, consultant, and/or speaker for Almirall, Bausch Health, Cassiopea, EPI Health, Galderma, La Roche-Posay, L’Oreal, Mayne Pharma, Sol-Gel, Sun Pharma, and Vyne. Vincenzo Bettoli has acted as a consultant, advisory board member, research investigator and received honoraria from AbbVie, Beiersdorf, Bioderma, Biogena, Difa-Cooper, Galderma, Ganassini, GlaxoSmithKline, ICF, LEO Pharma, L’Oreal, Meda, Menarini-Relife, Mylan, Novartis, Pharcos-Biodue, UCB Pharma, and received research support (funds to institution) from AbbVie. James Del Rosso has acted as a research investigator, consultant and/or speaker for Almirall, Bausch Health (Ortho Dermatology), BiopharmX, EPI Health, Galderma, LEO Pharma, Mayne Pharma, Sol-Gel, Sonoma, Sun Pharma, and Vyne Therapeutics (Foamix). Thomas Dirschka has received grants/research support from Almirall, Biofrontera, Galderma, Meda, Schulze & BöhmGmbH, has lectured for Almirall, Biofrontera, Galderma, GlaxoSmithKline, Infectopharm, Janssen-Cilag, LEO Pharma, Meda, Neracare, Novartis, Riemser and is an advisory board member for Almirall, Biofrontera, Dr Pfleger, Galderma, GlaxoSmithKline, Janssen-Cilag, LEO Pharma, Meda, Neracare, Novartis, and Scibase. Brigitte Dréno has acted as investigator, consultant and/or speaker for Almirall, Avène, Bioderma, Fabre, Galderma, La RochePosay, L’Oreal, Novartis, and Sun Pharma. Linda Stein Gold has acted as an investigator/advisor and/or speaker for Almirall, Foamix, Galderma, Novartis, Ortho Derm, Sol-Gel, and Sun Pharma. Julie Harper has acted as a consultant for Almirall, BioPharmX, Cassiopea, Cutera, EPI, Foamix, Galderma, Ortho Derm, Sol-Gel, and Sun Pharma. Joo Yeon Ko has acted as a primary investigator for research (funded to institution) and/or received honoraria for unrestricted educational events from Amorepacific, Eli Lilly, and Galderma. Khaled Al Nuaimi has no relevant disclosures. Hazel H Oon has acted as a speaker, advisory board member and researcher for Galderma, a clinical investigator for Janssen, Novartis, and Pfizer and an advisory board member and speaker for AbbVie, Eli Lilly, and LEO Pharma. Murlidhar Rajagopalan has acted as a speaker and advisor for Galderma India, a consultant for Galderma and a speaker for Janssen, MSD, Novartis, Pfizer, Roche, and Sanofi. Marco Rocha has acted as an advisor, consultant, investigator and/or speaker and received grants/honoraria from Eucerin, FQMMelora, Galderma, Hypera, Johnson & Johnson, La Roche Posay, LEO Pharma, and Pierre-Fabre. Jo-Ann See has acted as an advisor, consultant, advisory board member and/or speaker for Allergan, Galderma, La Roche-Posay, LEO Pharma, L’Oreal, Mayne Pharma, and Viatris. Jonathan Weiss has acted as an investigator/advisor and/or speaker for Almirall, Dr Reddy’s, EPI Health, Foamix, Galderma, Novartis, and Ortho Derm. Jerry Tan has acted as an advisor, consultant, investigator and/or speaker and received grants/honoraria from Almirall, Bausch Health, Boots/Walgreens, Botanix, CeraVe, Cipher Pharmaceuticals, Cutera, Galderma, La Roche-Posay, Novan, Novartis, Pfizer, Promius, Sun Pharma, and Vichy.
Footnotes
Funding sources: Panel members were invited by Galderma, who funded the planning and delivery of this project. Medical writing services, provided by Ogilvy Health UK, were funded by Galderma.
IRB approval status: Not applicable.
References
- 1.Hassan J., Grogan S., Clark-Carter D., et al. The individual health burden of acne: appearance-related distress in male and female adolescents and adults with back, chest and facial acne. J Health Psychol. 2009;14(8):1105–1118. doi: 10.1177/1359105309342470. [DOI] [PubMed] [Google Scholar]
- 2.Sood S., Jafferany M., Sushruth V. Depression, psychiatric comorbidities and psychosocial implications associated with acne vulgaris. J Cosmet Dermatol. 2020;19(12):3177–3182. doi: 10.1111/jocd.13753. [DOI] [PubMed] [Google Scholar]
- 3.Tan J., Beissert S., Cook-Bolden F., et al. Impact of facial and truncal acne on quality of life: a multi-country population-based survey. JAAD Int. 2021;3:102–110. doi: 10.1016/j.jdin.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan J., Beissert S., Cook-Bolden F., et al. Impact of facial atrophic acne scars on quality of life: a multi-country population-based survey. Am J Clin Dermatol. 2022;23(1):115–123. doi: 10.1007/s40257-021-00628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan J., Alexis A., Baldwin H., et al. Gaps and recommendations for clinical management of truncal acne from the Personalising Acne: Consensus of Experts panel. JAAD Int. 2021;5:33–40. doi: 10.1016/j.jdin.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Layton A.M., Alexis A., Baldwin H., et al. Identifying gaps and providing recommendations to address shortcomings in the investigation of acne sequelae by the Personalising Acne: Consensus of Experts panel. JAAD Int. 2021;5:41–48. doi: 10.1016/j.jdin.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan J., Alexis A., Baldwin H., et al. The personalised acne care pathway – recommendations to guide longitudinal management from the personalising acne: consensus of experts. JAAD Int. 2021;5:101–111. doi: 10.1016/j.jdin.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaenglein A.L., Pathy A.L., Schlosser B.J., et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 9.Asai Y., Baibergenova A., Dutil M., et al. Management of acne: Canadian clinical practice guideline. Can Med Assoc J. 2016;188(2):118–126. doi: 10.1503/cmaj.140665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nast A., Dréno B., Bettoli V., et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30(8):1261–1268. doi: 10.1111/jdv.13776. [DOI] [PubMed] [Google Scholar]
- 11.Ip A., Muller I., Geraghty A.W.A., et al. Views and experiences of people with acne vulgaris and healthcare professionals about treatments: systematic review and thematic synthesis of qualitative research. BMJ Open. 2021;11(2) doi: 10.1136/bmjopen-2020-041794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabbrocini G., Cacciapuoti S., Monfrecola G. A qualitative investigation of the impact of acne on health-related quality of life (HRQL): development of a conceptual model. Dermatol Ther. 2018;8:85–99. doi: 10.1007/s13555-018-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas C.L., Kim B., Lam J., et al. Objective severity does not capture the impact of rosacea, acne scarring and photoaging in patients seeking laser therapy. J Eur Acad Dermatol Venereol. 2017;31(2):361–366. doi: 10.1111/jdv.13945. [DOI] [PubMed] [Google Scholar]
- 14.Alsubeeh N.A., Alsharafi A.A., Ahamed S.S., et al. Treatment adherence among patients with five dermatological diseases and four treatment types - a cross-sectional study. Patient Prefer Adherence. 2019;13:2029–2038. doi: 10.2147/PPA.S230921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zureigat M., Fildes K., Hammond A., et al. General practitioners' attitudes towards acne management. Aust J Gen Pract. 2019;48:48–52. doi: 10.31128/AJGP-06-18-4609. [DOI] [PubMed] [Google Scholar]
- 16.Anderson K.L., Dothard E.H., Huang K.E., et al. Frequency of primary nonadherence to acne treatment. JAMA Dermatol. 2015;151(6):623–626. doi: 10.1001/jamadermatol.2014.5254. [DOI] [PubMed] [Google Scholar]
- 17.Eicher L., Knop M., Aszodi N., et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease - strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33(12):2253–2263. doi: 10.1111/jdv.15913. [DOI] [PubMed] [Google Scholar]
- 18.Bagatin E., Proença de Freitas T.H., Machado M.C.R., et al. Adult female acne: a guide to clinical practice. Anas Bras Dermatol. 2019;94(1):62–75. doi: 10.1590/abd1806-4841.20198203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh T.R., Efthimiou J., Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16(3):e23–e33. doi: 10.1016/S1473-3099(15)00527-7. [DOI] [PubMed] [Google Scholar]
- 20.Barbieri J.S., Spaccarelli N., Margolis D.J., et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80(2):538–549. doi: 10.1016/j.jaad.2018.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mühlbacher A.C., Juhnke C. Patient preferences versus physicians' judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy. 2013;11(3):163–180. doi: 10.1007/s40258-013-0023-3. [DOI] [PubMed] [Google Scholar]
- 22.Billings J.A., Krakauer E.L. On patient autonomy and physician responsibility in end-of-life care. Arch Intern Med. 2011;171(9):849–853. doi: 10.1001/archinternmed.2011.180. [DOI] [PubMed] [Google Scholar]
- 23.Charles C., Gafni A., Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc Sci Med. 1997;44(5):681–692. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson F.A., Barry C.A., Britten N., et al. Doctor-patient communication about drugs: the evidence for shared decision making. Soc Sci Med. 2000;50(6):829–840. doi: 10.1016/s0277-9536(99)00376-7. [DOI] [PubMed] [Google Scholar]
- 25.Eichenfield L.F., Krakowski A.C., Piggott C., et al. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics. 2013;131(suppl 3):S163–S186. doi: 10.1542/peds.2013-0490B. [DOI] [PubMed] [Google Scholar]
- 26.Bagatin E., Florez-White M., Arias-Gomez M.I., et al. A. Algorithm for acne treatment: Ibero-Latin American consensus. An Bras Dermatol. 2017;92(5):689–693. doi: 10.1590/abd1806-4841.20177003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Cleach L., Lebrun-Vignes B., Bachelot A., et al. Guidelines for the management of acne: recommendations from a French multidisciplinary group. Br J Dermatol. 2017;177(4):908–913. doi: 10.1111/bjd.15843. [DOI] [PubMed] [Google Scholar]
- 28.Szepietowski J., Kapińska-Mrowiecka M., Kaszuba A., et al. Acne vulgaris: pathogenesis and treatment. Consensus of the Polish Dermatological Society. Przegl Dermatol. 2012;99:649–673. [Google Scholar]
- 29.López-Estebaranz J.L., Herranz-Pinto P., Dréno B. Consensus-based acne classification system and treatment algorithm for Spain. Actas Dermosifiliogr. 2017;108(2):120–131. doi: 10.1016/j.ad.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Oon H.H., Wong S.N., Aw D.C.W., et al. Acne management guidelines by the dermatological society of Singapore. J Clin Aesthet Dermatol. 2019;12(7):34–50. [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi N., Akamatsu H., Iwatsuki K., et al. Japanese Dermatological Association guidelines: guidelines for the treatment of acne vulgaris 2017. J Dermatol. 2018;45(8):898–935. doi: 10.1111/1346-8138.14355. [DOI] [PubMed] [Google Scholar]
- 32.Goh C.L., Abad-Casintahan F., Aw D.C.W., et al. South-East Asia study alliance guidelines on the management of acne vulgaris in South-East Asian patients. J Dermatol. 2015;42(10):945–953. doi: 10.1111/1346-8138.12993. [DOI] [PubMed] [Google Scholar]
- 33.Nast A., Bayerl C., Borelli C., et al. S2k-leitlinie zur therapie der akne [S2k-guideline for therapy of acne] J Dtsch Dermatol Ges. 2010;8(suppl 2):S1–S59. doi: 10.1111/j.1610-0387.2010.07466.x. [DOI] [PubMed] [Google Scholar]
- 34.Mavranezouli I., Welton N.J., Daly C.H., et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47(12):2176–2187. doi: 10.1111/ced.15356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong J.L.C., Vincent R.C., Al-Sharqi A. Dermatology consultations: how long do they take? Future Hosp J. 2017;4(1):23–26. doi: 10.7861/futurehosp.4-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linzer M., Bitton A., Tu S.P., et al. The end of the 15–20 minute primary care visit. J Gen Intern Med. 2015;30(11):1584–1586. doi: 10.1007/s11606-015-3341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Zuuren E.J., Arents B.W.M., Miklas M., et al. Identifying and appraising patient-reported outcome measures on treatment satisfaction in acne: a systematic review. Br J Dermatol. 2021;185(1):36–51. doi: 10.1111/bjd.19675. [DOI] [PMC free article] [PubMed] [Google Scholar]