Abstract
Dorsal striatum, principally comprising of caudate and putamen, is well-known to support motor function but also various higher-order cognitive functions. This is enabled by developing short- and long-range connections to distributed cortical regions throughout the life span, but few studies have examined developmental changes from young children to adults in the same cohort. Here we investigated the development of dorsal-striatal network in a large (n = 476), single-site sample of healthy subjects 3–42 years of age in three groups (children, adolescence, adults). The results showed that the connectivity within the striatum and to sensorimotor regions was established at an early stage of life and remained strong in adolescence, supporting that sensory-seeking behaviours and habit formation are important learning mechanisms during the developmental periods. This connectivity diminished with age, as many behaviours become more efficient and automated. Adolescence demonstrated a remarkable transition phase where the connectivity to dorsolateral prefrontal cortex emerged but connectivity to the dorsomedial prefrontal and posterior brain, which belong to the ventral attentional and default mode networks, was only seen in adults. This prolonged maturation in between-network integration may explain the behavioural characteristics of adolescents in that they exhibit elaborated cognitive performance but also demonstrate high risk-taking behaviours.
Keywords: Resting-state functional connectivity, Dorsal striatum, Putamen, Caudate, Development, Adolescence
Highlights
-
•
Connectivity within-striatum and to sensorimotor region remained through adolescence.
-
•
Connectivity to dorsolateral prefrontal cortex emerged in adolescence.
-
•
Connectivity to attentional and default mode networks was not found until adulthood.
1. Introduction
The striatum is a set of subcortical brain regions that are structurally connected to distributed brain areas across the human cortex as part of cortico-striatothalamo-cortical circuitry. Through parallel and overlapping loops, the striatum integrates information from functionally distinct higher-order cortical brain regions, driving behavioural outputs (Averbeck et al., 2014, Haber, 2003). As such, it has been associated with extensive higher-order cognitive and affective processing beyond its initial attribution to motor-related functions (Gerardin et al., 2004). The dorsal striatum, composed of the bilateral caudate and putamen, is primarily responsible for the motor and cognitive functions, while the ventral striatum, including the nucleus accumbens, is involved in motivation, affective and reward processing (Chen et al., 2020, Pauli et al., 2016).
With its importance in diverse cognitive and motor functions, the striatum is frequently implicated in neurodevelopmental, neuropsychiatric and motor disorders that emerge throughout the lifespan, such as autism spectrum disorder, obsessive-compulsive disorder, major depressive disorder and Parkinson’s disease (Shepherd, 2013). Given the preferential involvement of the dorsal striatum in these functions, establishing its typical developmental trajectory is of critical importance for identifying and understanding deviations in psychopathology. In typical development, mature functional connectivity in the dorsal striatum starts to emerge in adolescence (Peters and Crone, 2017) but long-range connectivity to anterior (e.g., prefrontal cortex) and posterior (e.g., posterior cingulate gyrus) parts of the brain is still not observed until adulthood (Porter et al., 2015). The adolescence period is known for greater risk-taking behaviours and the associated negative outcomes, but it is also a critical phase of life to develop elaborate cognitive learning performance and goal-directed decision-making strategies, which may be linked to maturity of functional connectivity in the dorsal striatum (Peters and Crone, 2017, Shan et al., 2022).
Resting-state functional magnetic resonance imaging (rs-fMRI) examines functional networks in the brain and provides considerable insight into human brain function. rs-fMRI analyses blood oxygen level-dependent (BOLD) signals which are temporally correlated in resting state, with the underlying assumption that brain regions that show BOLD fluctuations in synchrony communicate (Biswal et al., 1995b, Fox and Raichle, 2007). Only a few studies have used rs-fMRI to establish typically developing trajectories of cortico-dorsal striatal functional connectivity, identifying primarily decreasing functional connectivity between the dorsal striatum and cortical regions with age. Through childhood and adulthood, functional connectivity between dorsal striatum and the somatomotor network was reported to decrease (Barber et al., 2019, Greene et al., 2014, Porter et al., 2015), and these decreases were also found between early to mid-adulthood (Manza et al., 2015a) and extended to other subcortical, visual and limbic regions with age (Barber et al., 2019). Higher connectivity with the sensorimotor systems in childhood would underlie the period of acquisition of a wide range of motor skills (e.g., Graybiel and Grafton, 2015), with a decrease from adolescence to adulthood as skills become stable or habitual behaviours. Increased connectivity of the dorsal striatum with age was reported with regions engaged in higher-order cognitive performance such as the default-mode and fronto-parietal networks (Barber et al., 2019, Manza et al., 2015a, Porter et al., 2015). These bidirectional changes align with the developmental model of decreasing stimulus-driven (sensorimotor) to increasing goal-directed (higher-order cognitive) processing with age in the brain (Casey et al., 2005).
Few studies have examined the full age range encompassing children, adolescents and adults and they have had either a relatively small sample size (n = 106, with only 27 participants under 19 years of age) (Porter et al., 2015) or included data from public databases collected across multiple sites (n = 926), with the study’s focus on the relation between striatal development and psychopathology (Barber et al., 2019). Thus, there exists a significant gap in the literature to establish the precise changes that occur between childhood and adolescence, and adolescence and adulthood, and to capture functional connectivity features that may be related to behavioural characteristics in each developmental stage. Furthermore, given the growing awareness of the importance of large sample sizes to establish reliable and replicable neuroimaging findings, there is a need for a larger single-site samples to examine the typical development of cortico-dorsal striatal connectivity (Cui and Gong, 2018, Xia et al., 2019).
Thus, we examined the development in cortico-striatal network in a large (n = 476), single-site sample of healthy subjects aged 3–42 years of age with a focus on the dorsal striatum. We hypothesized that cortico-striatal functional connectivity, particularly to motor areas, would change most rapidly early in the childhood period when many motor skills are being acquired. In addition, long-range connections to anterior and the posterior regions would continue to develop through adolescence and adulthood. In particular, the adolescents would demonstrate a developmental transition in establishing long-range striatal connectivity, supporting their behavioural characteristics.
2. Materials and methods
2.1. Participants and ethics statement
We collected rs-fMRI scans from 476 healthy children, adolescents and adults who participated as controls for a variety of studies at the Hospital for Sick Children in Toronto. The exclusion criteria included intellectual, learning, language, neurological or developmental disabilities and participants 43 years and older, leaving 464 participants. The participants were grouped into children (3–10 years old), adolescents (11–19 years old) and adults (20–42 years old). Written informed consent was obtained from adults and adolescents and the parents of younger children; younger children gave informed verbal assent. All studies were approved by the Research Ethics Board of the Hospital for Sick Children.
2.2. Image data acquisition
Three hundred fifty six of the 476 subjects were scanned using a 3.0-T MRI scanner (MAGNETON Trio with a 12-channel head and neck coil, Siemens Medical Systems, Germany) and remaining 120 subjects were scanned using a post 3.0-T MRI scanner upgrade (MAGNETOM PrismaFIT with a 20-channel head coil, Siemens Medical Systems, Germany). The high resolution structural T1 images (whole brain) were acquired using a three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE) on the Trio in a maximum of 192 axial slices (TR = 2300 ms, TE = 2.96 ms, resolution = 1.0 mm isotropic, flip angle = 9°, FOV = 240 × 256 mm, scan time =5:03 min), and on the PrismaFIT in a maximum of 192 axial slices (TR = 1870 ms, TE = 3.14 ms, resolution = 0.8 mm isotropic, flip angle = 9°, FOV = 240 × 256 mm, scan time = 5:01 min). Resting state images were collected with eyes open and looking at a fixation cross within a circle, using an echo-planar imaging pulse sequence on the Trio (TR = 2340 ms, TE = 30 ms, resolution = 3.5 mm, flip angle = 70°, FOV = 224 × 224 mm, number of slices = 40, volumes = 120, scan time = 5 min), and on the PrismaFIT (TR = 1500 ms, TE = 30 ms, resolution = 3.0 mm, flip angle = 70°, FOV = 222 × 222 mm, number of slices = 50, volumes = 206, scan time = 5:10 min).
2.3. Image preprocessing
All rs-fMRI data preprocessing was performed using AFNI (Analysis of Functional Neuro-Images) (Cox, 1996, Cox and Hyde, 1997, Gold et al., 1998), FMRIB's Software Library (FSL) (Jenkinson et al., 2012, Smith et al., 2004, Woolrich et al., 2008) and locally developed software. The first four volumes of each scan series were discarded for BOLD signal stabilization, and then motion and slice timing correction were implemented. For motion correction, the 3 displacement and 3 rotation realignment parameters were estimated. The fMRI data were smoothed with a Gaussian filter (FWHM 7 mm) and a bandpass temporal filter (0.01 < f < 0.2 Hz) to remove very low frequency drifts and to minimize high frequency physiological noise. The signal contributions from the whole-brain, white matter, CSF and six rigid-body parameters derived from motion correction were bandpass filtered and regressed from the data (Hallquist et al., 2013).
Head motion was evaluated using framewise displacement (FD; Power et al., 2012) for each participant. Participants who had greater than 50 % of volumes with FD > 0.3 mm were excluded from further analyses. In addition, mean FD was used as a covariate in subsequent analyses. 75 participants did not pass quality control process, resulting in 389 participants in the final analysis. In addition, mean FD was used as a covariate in subsequent analyses.
The pre-processed rs-fMRI data were submitted to independent component analyses (ICA) using single-session ICA with Multivariate Exploratory Linear Decomposition into Independent Components (MELODIC, part of the FMRIB Software Library: FSL), which were then further denoised using FMRIB’s ICA-based X-noiseifier. The ICA-FIX is an automatic approach (once hand-trained) for component classifying and denoising motion or physiological artefact. Linear registration to the MNI-152 (T1 = 2 mm) standard template was performed by using each participant’s skull stripped T1-weighted anatomical image.
2.4. Data analyses
We used a standard seed-based functional connectivity approach (Biswal et al., 1995a) to compare the functional connectivity of the subcortical network between the three age groups. The subcortical ROIs included the putamen (MNI coordinates: 28, 5, 2) and the caudate (15, 12, 9). To interpret inter-subject variance, the exact seed coordinates were dilated within a 10 mm spherical ROI until the internal correlation dropped below 0.7 or the number of voxels exceeded 300 (see Supplementary Fig. 1 for example). The first level analysis was implemented using FMRIB’s expert analysis tool (FEAT: https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FEAT) by correlating the mean time series of the ROIs with the time series of all voxels in the brain for single-participant analysis. To identify differences in functional connectivity in the brain between age groups, the second level analysis was then implemented to contrast the first level subcortical network connectivity maps between the three age groups. We used 6 contrasts (children > adolescents, children < adolescents, adolescents > adults, adolescents < adults, children > adults, children < adults) to capture group-specific features while controlling for sex, scanner, and mean FD. All statistical analyses were thresholded using clusters determined by Z > 3.5 and a corrected cluster significance threshold of FWE p < .05. The statistical significance across all findings were corrected over six contrasts for multiple comparisons. In the subsequent analysis, the sex differences across the age groups were also explored. The statistical significance across all findings was corrected over six terms (three age groups [children, adolescents, adults] × two contrasts [male > female, female > male]). The significance was held at pcorrected < 0.05. Both uncorrected and corrected p-values for all findings are now presented in the Tables.
2.5. Supplementary analysis
Additional analyses were conducted for age-related continuous increase or decrease in the caudate and putamen networks. These findings were largely similar to the group findings and are presented in the Supplementary Table 1 and Supplementary Fig. 2.
3. Results
3.1. Participant characteristics
After excluding subjects whose resting state data failed the motion criteria (>50% volumes labeled as motion corrupted via FD), 389 subjects remained (110 aged 3–10 years; 133 aged 11–19 years; 146 aged 20–42 years) in the final analysis. The descriptive statistics for the three age groups are summarized in Table 1. There were no significant differences in sex (see Supplementary Fig. 2 for age and sex distribution in the final sample) or scanner ratios, but the youngest group demonstrated greater head motion and larger ROI volumes.
Table 1.
Participant characteristics.
| Children (C) | Adolescents (AS) | Adults (AD) | Statistics | |
|---|---|---|---|---|
| N | 110 | 133 | 146 | - |
| Age range (years) | 3–10 | 11–19 | 20–42 | - |
| Mean age (years ± sd) | 7.53 ± 2.15 | 14.42 ± 2.29 | 28.98 ± 6.67 | - |
| Sex (M:F) | 62:48 | 71:62 | 90:56 | χ2 = 2.00, p = .37 |
| Mean FD (mm, ± sd) | 0.14 ± 0.03 | 0.12 ± 0.03 | 0.11 ± 0.03 | F = 14.49, p < .001 (C > AS, C > AD) |
| Scanner (pre:post) | 73:37 | 101:32 | 113:33 | χ2 = 4.44, p = .11 |
| Left Caudate ROI (mm3) | 949.09 ± 481.82 | 756.21 ± 341.35 | 668.71 ± 363.59 | F = 16.24, p < .001 (C > AS, C > AD) |
| Right Caudate ROI (mm3) | 967.42 ± 510.37 | 740.87 ± 347.94 | 678.36 ± 431.77 | F = 15.07, p < .001 (C > AS, C > AD) |
| Left Putamen ROI (mm3) | 761.96 ± 418.08 | 558.44 ± 290.49 | 526.19 ± 303.08 | F = 17.32, p < .001 (C > AS, C > AD) |
| Right Putamen ROI (mm3) | 777.02 ± 405.01 | 576.42 ± 259.84 | 528.60 ± 292.19 | F = 20.57, p < .001 (C > AS, C > AD) |
3.2. Caudate network
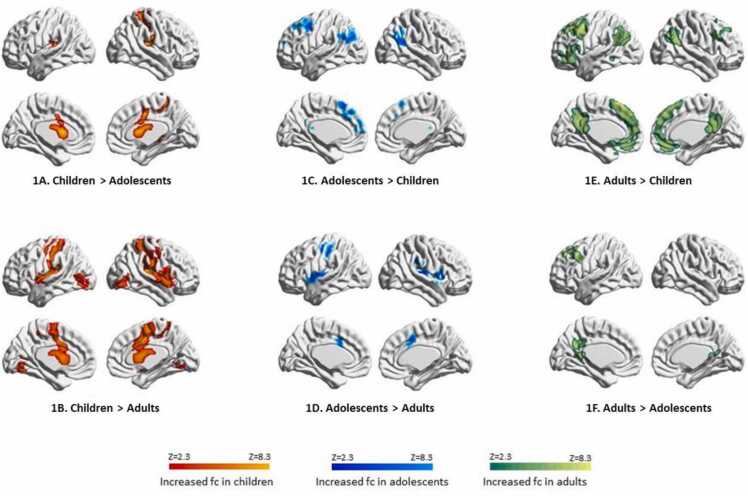
Comparisons between the different age groups revealed distinct developmental patterns in caudate connectivity across the life span. In the caudate network, children demonstrated strong within-caudate connectivity and increased connectivity with somatosensory and motor regions, compared to both adolescents (Supplementary Table 2 and Fig. 1A) and adults (Supplementary Table 3 and Fig. 1B).
Fig. 1.
Age differences in caudate network.
Adolescents showed the development of connections between the caudate and the dorsal attentional network as well as dorsolateral prefrontal cortex, compared to children (Supplementary Table 2 and Fig. 1C), but the connectivity with somatosensory and motor regions still remained stronger compared to adults (Supplementary Table 4 and Fig. 1D).
In the adult caudate network, the caudate had strong connectivity with the default mode network (DMN), particularly the posterior part of DMN, such as posterior cingulate cortex (PCC) and precuneus, compared to both children (Supplementary Table 3, Fig. 1E) and adolescents (Supplementary Table 4 and Fig. 1F).
3.3. Putamen network
Comparisons between the different age groups also revealed some shared and distinct connectivity patterns in the putamen network.
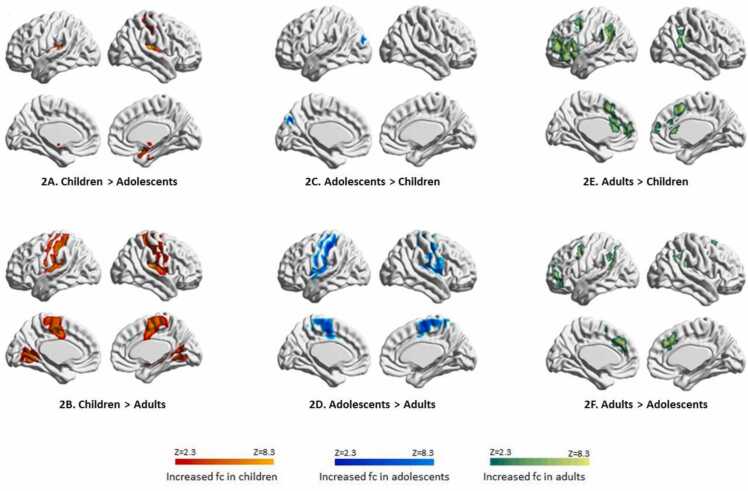
In the putamen network, both children (Supplementary Table 6 and Fig. 2B) and adolescent (Supplementary Table 7 and Fig. 2D) demonstrated strong within-putamen connectivity and the connectivity between the putamen and the sensorimotor regions, compared to adults. Although these patterns of connectivity were stronger in children, the overall patterns were similar and showed minimal changes in adolescence (Supplementary Table 5 and Figs. 2A and 2C).
Fig. 2.
Age differences in putamen network.
In the adult network, the putamen showed strong connectivity with the ventral attentional network as well as dorsolateral and dorsomedial prefrontal cortex, compared to both children (Supplementary Table 6 and Fig. 2E) and adolescents (Supplementary Table 7 and Fig. 2F).
3.4. Sex differences
In the caudate network, significant sex differences were seen only in children, where males demonstrated greater functional connectivity in parietal visuo-motor coordination regions and the supramarginal gyrus, while females showed greater connectivity with the inferior frontal gyrus and dorsolateral prefrontal cortex. No sex differences passed correction in the adolescents nor in the adults (see Table 2).
Table 2.
Sex difference in age groups.
| Network | Age group | Cluster peak |
Regions | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Voxels | puncorr | pcorr | Z | x | y | z | ||||
| Caudate | Children | Male > Female | 2926 | 0.000172 | 0.002** | 4.05 | -30 | -48 | 42 | Visuo-motor coordination area (BA7) Supramarginal gyrus (BA40) |
| Female > Male | 2162 | 0.00139 | 0.005** | 4.19 | -28 | 30 | 16 | Triangular part of inferior frontal gyrus (BA45) Dorsolateral prefrontal cortex (BA46) |
||
| Adolescent | Male > Female | 1132 | 0.0274 | N.S. | 3.79 | -44 | 38 | 28 | Anterior prefrontal cortex (BA10) Dorsolateral prefrontal cortex (BA9) |
|
| Female > Male | - | - | - | - | - | - | - | - | ||
| Adults | Male > Female | - | - | - | - | - | - | - | - | |
| Female > Male | - | - | - | - | - | - | - | - | ||
| Putamen | Children | Male > Female | - | - | - | - | - | - | - | - |
| Female > Male | 2020 | 0.00157 | 0.005** | 3.84 | -12 | 10 | 30 | Premotor and supplementary motor cortex (BA6) Dorsal anterior cingulate cortex (BA32) Primary motor cortex (BA4) |
||
| Adolescents | Male > Female | - | - | - | - | - | - | - | - | |
| Female > Male | 1501 | 0.00646 | 0.016* | 3.96 | -4 | -34 | 14 | Isthmus cingulate gyrus (BA30) Posterior cingulate gyrus (BA23) |
||
| Adults | Male > Female | - | - | - | - | - | - | - | - | |
| Female > Male | 1997 | 0.000361 | 0.002** | 3.7 | -16 | 10 | 60 | Premotor and supplementary motor cortices (BA6) Primary somatosensory cortex (BA1) Supramarginal gyrus (BA40) |
||
Only significant results from uncorrected p < .05 presented in the Table; N.S., Not Significant; **p < .01, *p < .05.
Sex differences were observed over the three age groups in the putamen network, with females demonstrating greater functional connectivity to sensorimotor regions and the cingulate cortex. This pattern was similar in children and adults, although findings were localized more laterally in the adults, while in adolescence, females showed greater functional connectivity to the posterior and isthmus cingulate cortices (Table 2).
4. Discussion
We examined developmental changes of corticostriatal connectivity in caudate and putamen networks in a large single-site sample of participants from 3 to 42 years of age using rs-fMRI and comparing three age groups (children, adolescents and adults). We found that earlier in life both the caudate and putamen shared features of strong within-striatum connectivity and short-range connectivity with sensorimotor regions, consistent with our first hypothesis. In line with our second hypothesis, long-range connectivity to distributed cortical regions increased with age in both networks, which would support higher-order cognitive functions (e.g., Pauli et al., 2016). Interestingly, the final regions to which the caudate and putamen developed connections, and the overall maturational trajectories over time to those connections, differed between the networks. We also found prolonged maturation with age, with distal brain regions becoming more strongly integrated throughout adolescence, gradually developing more connections with the prefrontal areas in adulthood. Adolescence demonstrated a unique transition period as there remained strong connections to sensorimotor regions but also increasing connections to prefrontal cortex.
Sensory learning is critical for early stages of development and our finding with respect to increased connectivity to the sensorimotor areas in young children compared with adults is in line with previous literature. In rs-fMRI studies in infancy and early childhood, cortical networks were largely confined to primary sensory and motor brain regions (Fransson et al., 2011, Lee et al., 2013). Our results showed that connections to the sensorimotor regions in the dorsal-striatal network tended to be stable from childhood to adolescence, and then reduced with age, which was consistent with the previous findings in both functional connectivity (Manza et al., 2015b, Taniwaki et al., 2007) and structural connectivity (Ystad et al., 2010). Dorsal striatum, particularly the dorsolateral part of the striatum, receives massive inputs from somatosensory and motor cortices and has a critical role in forming habitual behaviours and the performance of action sequences (Graybiel and Grafton, 2015, Lipton et al., 2019, Malvaez and Wassum, 2018). Habit formation is a key mechanism for learning at an early stage of life as children learn about their environment through imitating and repeating behaviours of the people around them and processes underlying highly repeated actions in their daily lives require less and less mental effort and eventually become habitual (Yin and Knowlton, 2006). Decreased connectivity to sensorimotor regions with age in the dorsal striatal network may reflect an increase of automated behavioural repertoire and a switch from sensory-based learning to more cognitive, goal-directed learning strategies.
In early adolescence, we found connections emerged between the caudate and dorsal attentional network. The frontal cortex myelination continues throughout adolescence, accelerating after puberty, but myelination is not completed until early adulthood, in contrast to the sensorimotor regions which become fully myelinated in the first years of life (Blakemore and Frith, 2005). The integration of striatum with the dorsal attentional network would facilitate selective attention, such that individuals can prioritize and filter sensory information based on its relevance (Lanssens et al., 2020, Vossel et al., 2014). The ability to incorporate top-down attentional processes, which are regulated by the dorsal attentional network, shows substantial improvement through childhood and adolescence (Rohr et al., 2017), allowing adolescents to engage in sustained attention for longer periods of time and decrease distractions (Dye and Bavelier, 2010, Zhan et al., 2011).
Connections to the DMN in the caudate network, however, developed more slowly and adolescents demonstrated weaker connectivity to the posterior part of the DMN, such as the posterior cingulate cortex (PCC) and precuneus compared to adults. In contrast, the mature putamen network did not show connections with DMN in our study, but instead developed connections with ventral attentional network as well as the dorsolateral and dorsomedial prefrontal cortices. The relations with the DMN in corticostriatal connectivity was reported in the putamen network in a previous study (Manza et al., 2015b); they observed that anti-correlations between DMN and a test-positive network, such as the putamen network, diminished with age from young to middle-aged adulthood. Of note, other investigations have reported that the anti-correlation between cognitive control networks and the DMN were strengthened over adolescence (Barber et al., 2013, Bo et al., 2014, Chai et al., 2014, Uddin et al., 2011). Taken together, the connectivity between the striatum and the DMN is not established in earlier stages of life and demonstrates segregated functions. The increased connectivity observed in early and later adulthood is indicative of greater integration between the networks.
The strong connections to prefrontal cortex in the putamen network were observed only in adulthood, which is consistent with previous findings that the human brain, in particular frontal regions, is not fully mature until individuals reach 20- to 30-years of age (Arain et al., 2013, Johnson et al., 2009, Sowell et al., 2003). The prefrontal cortex has an important role in cognitive control, and specifically the dorsolateral prefrontal cortex (dlPFC) is a crucial region involved in reinforcement learning in corticostriatal connectivity (Jarbo and Verstynen, 2015). Reinforcement learning requires the integration of reward, attention and executive processes as key components of habit formation (Lee and Neuroscience, 2013). The developmental studies have shown that the dlPFC connectivity strengthened with age at an early stage but also declined with age later in life, although several studies have demonstrated that cognitive training can enhance connectivity and better performance (Faraza et al., 2021). Additionally, the dorsomedial prefrontal cortex is critical to social cognition, such as understanding theory of mind, and in demonstrating cognitive empathy and altruistic behaviours (Mitchell, 2009, Preckel et al., 2018, Waytz et al., 2012). Activity patterns in the social brain network showed particularly marked changes during adolescence (Blakemore, 2012).
The results of our study may help to explain child and adolescent learning and how habits are formed. Sensorimotor learning is a critical component of development for young children as they learn a wide range of skills from riding a bicycle, writing, sports to playing a musical instrument, and the increased connectivity in networks between dorsal-striatal and sensorimotor regions seen in young children in this study would support this period of intense acquisition of motor skills.
Adolescence is a unique period and there remained strong connectivity to sensorimotor regions but also development of long-range functional connectivity particularly to frontal areas. Our results extend our understanding of how the brain continues to develop in adolescence; the adolescent brain had more connections to attentional networks and prefrontal regions than younger children, yet was not fully developed compared to the adult population (Dumontheil et al., 2010). These features would support adolescents in developing different sensory preferences and activities but also in experiencing the full range of changes due to transformations in physical, hormonal, cognitive and social maturation (Foulkes and Blakemore, 2018). This combination of major growth in brain structure and function may underlie and support higher-order cognitive learning and performance but the changes also present a risk, experienced as perception of reduced stability, sensation-seeking and risk-taking behaviours (Foulkes and Blakemore, 2016, Fuhrmann et al., 2015, Tamnes et al., 2017).
Sex differences in connectivity with the caudate were seen in the youngest group and across age groups with the putamen, suggesting sex-related influences on brain function. Although structural sex differences have been widely reported (Lenroot and Giedd, 2010, Lenroot and Giedd, 2006, Ruigrok et al., 2014), there have been fewer investigations regarding brain function, with respect to development. A study with a large normative sample (N = 674, age range = 9–22 years) found that males had more between-module functional connectivity with motor and spatial tasks, while females demonstrated more within-module functional connectivity with emotional identification and nonverbal reasoning tasks (Satterthwaite et al., 2015). These differences may support our caudate network findings in children, where we observed greater functional connectivity to parietal visuo-motor regions in males and the inferior frontal and dorsolateral frontal region in females.
In contrast, the sex differences in the putamen network persisted across age groups, with females having greater functional connectivity to sensorimotor regions compared to males. Previous literature shows that the putamen network is not only involved in motor control but also closely engaged in attention, memory and affect, and pain processing, contributing to individuals’ positive and negative sensory-related experiences (Cottam et al., 2016, Starr et al., 2011), and studies have implicated sex disparities in this network function (Hofer et al., 2007, Murray et al., 2021, Whittle et al., 2011). Of note, female adolescents in our study demonstrated greater functional connectivity in the posterior part of cingulate cortex (isthmus and posterior cingulate cortices), which is known for its involvement in memory and pain processing as well as mood symptoms, such as depression (Nielsen et al., 2005, Wei et al., 2021, Whitford et al., 2014) Our findings extend these prior results, showing that sex differences in putamen-to-sensorimotor-connectivity from childhood through to adulthood; future studies could explore this finding in relation to sex disparities in psychiatric conditions, which often begin in adolescence (Aleman et al., 2003, Slewa-Younan et al., 2004).
Several reports have highlighted the importance of this fronto-striatal connectivity during this vulnerable period of development. Studies have shown that social experiences in adolescence had unique effects on brain development (Andrews et al., 2021, Orben et al., 2020). In particular, adversity, such as social isolation, had a direct impact on the dorsomedial striatal pathway, and predicted a shift in the balance of decision-making strategies between goal-directed action and habitual responses in adulthood (Shan et al., 2022). Atypical connection between dorsal striatum and middle frontal gyrus was implicated in internet gaming disorder (Dong et al., 2021). Finally, several recent reports revealed how the COVID-19 pandemic impacted negatively on adolescents’ mental health (Becker et al., 2020, Blackburn et al., 2022, Cost et al., 2021, Thorell et al., 2021, Viner et al., 2022); it would be important future work to determine how these difficulties are related to brain functional organization and behaviour and what interventions would be needed (Dunning et al., 2022).
5. Limitations
The current data have the huge benefit of being collected at one geographic location; however, a scanner upgrade occurred during the study period. We controlled for the scanner effect in the model, but the interpretation of the results should be made carefully in this regard.
Although we examined developmental changes over age in dorsal striatal functional connectivity, care should be taken to interpret the results as they represent differences between three age groups, not developmental changes within each group. Nevertheless, children, adolescents and adults are considered qualitatively different developmental stages and the results should be interpreted in this light.
In addition, developmental processes are complex entities representing both linear and non-linear changes in any developmental period. Although non-linear models have provided new information about developmental changes with multiple inflection points (Grimm et al., 2011, Harring et al., 2021), each model describes a specific pattern of changes over time that requires assumptions based on prior observations and needs extensively large samples (Rutherford et al., 2022). Future studies a much larger number of participants. could address both linear and non-linear changes, not only with age increases but also in connectivity features across the brain regions.
6. Conclusions
Our study provides evidence of age-related changes in caudate and putamen networks across three developmental age ranges (children, adolescents and adults). With age, the caudate network developed connections with the attentional network and stronger connections with the DMN. The putamen network developed connections with the attentional network more slowly and ultimately showed stronger connections with prefrontal cortices. These patterns of development reflect the complexity over these developmental periods. The results of our study have important implications for explaining child and adolescent learning and behaviours such as sensorimotor learning, attention, and habit formation. Future studies should explore sub-divisions of the striatum in association with the evolution of specific cognitive and social skills.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank all participants and their families who took part in the study. We also thank all those involved in participant recruitment and data collection, Vanessa Vogan, Rachel Leung, Julia Young, Sarah Mossad, Julie Sato and Veronica Yuk, as well as Tammy Rayner and Ruth Weiss for their valuable support and contributions. Funding was provided by Canadian Institutes of Health Research (CIHR) to MJT (MOP-106582, MOP-119541, MOP-137115 and MOP-142379). The authors have no conflicts of interest to disclose.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2023.101258.
Appendix A. Supplementary material
Supplementary material
Data Availability
The authors do not have permission to share data.
References
- Aleman A., Kahn R.S., Selten J.P. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch. Gen. Psychiatry. 2003;60:565–571. doi: 10.1001/ARCHPSYC.60.6.565. [DOI] [PubMed] [Google Scholar]
- Andrews J.L., Ahmed S.P., Blakemore S.J. Navigating the social environment in adolescence: the role of social brain development. Biol. Psychiatry. 2021;89:109–118. doi: 10.1016/J.BIOPSYCH.2020.09.012. [DOI] [PubMed] [Google Scholar]
- Arain M., Haque M., Johal L., Mathur P., Nel W., Rais A., Sandhu R., Sharma S. Maturation of the adolescent brain. Neuropsychiatr. Dis. Treat. 2013;9:449. doi: 10.2147/NDT.S39776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck B.B., Lehman J., Jacobson M., Haber S.N. Estimates of projection overlap and zones of convergence within frontal-striatal circuits. J. Neurosci. 2014;34:9497–9505. doi: 10.1523/JNEUROSCI.5806-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A.D., Caffo B.S., Pekar J.J., Mostofsky S.H. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51:156–167. doi: 10.1016/J.NEUROPSYCHOLOGIA.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A.D., Sarpal D.K., John M., Fales C.L., Mostofsky S.H., Malhotra A.K., Karlsgodt K.H., Lencz T. Age-normative pathways of striatal connectivity related to clinical symptoms in the general population. Biol. Psychiatry. 2019;85:966–976. doi: 10.1016/J.BIOPSYCH.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S.P., Breaux R., Cusick C.N., Dvorsky M.R., Marsh N.P., Sciberras E., Langberg J.M. Remote learning during COVID-19: examining school practices, service continuation, and difficulties for adolescents with and without attention-deficit/hyperactivity disorder. J. Adolesc. Health. 2020;67:769–777. doi: 10.1016/j.jadohealth.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med.: Off. J. Soc. Magn. Reson. Med./Soc. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal B., Zerrin Yetkin F., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/MRM.1910340409. [DOI] [PubMed] [Google Scholar]
- Blackburn R.M., Phillips Owen J., Downs J., Gilbert R. COVID-19-related school closures and patterns of hospital admissions with stress-related presentations in secondary school-aged adolescents: weekly time series. Br. J. Psychiatry. 2022:1–3. doi: 10.1192/BJP.2022.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J. Imaging brain development: the adolescent brain. Neuroimage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Frith U. The learning brain: lessons for education: a précis. Dev. Sci. 2005;8:459–465. doi: 10.1111/J.1467-7687.2005.00434.X. [DOI] [PubMed] [Google Scholar]
- Bo J., Lee C.M., Kwak Y., Peltier S.J., Bernard J.A., Buschkuehl M., Jaeggi S.M., Wiggins J.L., Jonides J., Monk C.S., Seidler R.D. Lifespan differences in cortico-striatal resting state connectivity. Brain Connect. 2014;4:166–180. doi: 10.1089/BRAIN.2013.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn. Sci. 2005;9:104–110. doi: 10.1016/J.TICS.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Chai X.J., Ofen N., Gabrieli J.D.E., Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J. Cogn. Neurosci. 2014;26:501–513. doi: 10.1162/JOCN_A_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.Y., Lu K.M., Ko H.A., Huang T.H., Hao J.H.J., Yan Y.T., Chang S.L.Y., Evans S.M., Liu F.C. Parcellation of the striatal complex into dorsal and ventral districts. Proc. Natl. Acad. Sci. USA. 2020;117:7418–7429. doi: 10.1073/PNAS.1921007117/SUPPL_FILE/PNAS.1921007117.SAPP.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cost K.T., Crosbie J., Anagnostou E., Birken C.S., Charach A., Monga S., Kelley E., Nicolson R., Maguire J.L., Burton C.L., Schachar R.J., Arnold P.D., Korczak D.J. Mostly worse, occasionally better: impact of COVID-19 pandemic on the mental health of Canadian children and adolescents. Eur. Child Adolesc. Psychiatry. 2021;1:3. doi: 10.1007/s00787-021-01744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottam W.J., Condon L., Alshuft H., Reckziegel D., Auer D.P. Associations of limbic-affective brain activity and severity of ongoing chronic arthritis pain are explained by trait anxiety. Neuroimage Clin. 2016;12:269–276. doi: 10.1016/J.NICL.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Hyde J.S. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10:171–178. doi: 10.1002/(SICI)1099-1492(199706/08)10:4/5<171::AID-NBM453>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Cui Z., Gong G. The effect of machine learning regression algorithms and sample size on individualized behavioral prediction with functional connectivity features. Neuroimage. 2018;178:622–637. doi: 10.1016/J.NEUROIMAGE.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Dong G.H., Dong H., Wang M., Zhang J., Zhou W., Du X., Potenza M.N. Dorsal and ventral striatal functional connectivity shifts play a potential role in internet gaming disorder. Commun. Biol. 2021:4. doi: 10.1038/S42003-021-02395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I., Houlton R., Christoff K., Blakemore S.J. Development of relational reasoning during adolescence. Dev. Sci. 2010:13. doi: 10.1111/J.1467-7687.2010.01014.X. [DOI] [PubMed] [Google Scholar]
- Dunning D., Ahmed S., Foulkes L., Griffin C., Griffiths K., Leung J.T., Parker J., Piera Pi-Sunyer B., Sakhardande A., Bennett M., Haag C., Montero-Marin J., Packman D., Vainre M., Watson P., Kuyken W., Williams J.M.G., Ukoumunne O.C., Blakemore S.J., Dalgleish T. The impact of mindfulness training in early adolescence on affective executive control, and on later mental health during the COVID-19 pandemic: a randomised controlled trial. Evid. Based Ment. Health. 2022;25:110–116. doi: 10.1136/EBMENTAL-2022-300460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye M.W.G., Bavelier D. Differential development of visual attention skills in school-age children. Vis. Res. 2010;50:452–459. doi: 10.1016/J.VISRES.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraza S., Waldenmaier J., Dyrba M., Wolf D., Fischer F.U., Knaepen K., Kollmann B., Tüscher O., Binder H., Mierau A., Riedel D., Fellgiebel A., Teipel S. Dorsolateral prefrontal functional connectivity predicts working memory training gains. Front. Aging Neurosci. 2021:13. doi: 10.3389/FNAGI.2021.592261/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes L., Blakemore S.J. Is there heightened sensitivity to social reward in adolescence? Curr. Opin. Neurobiol. 2016;40:81–85. doi: 10.1016/J.CONB.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Foulkes L., Blakemore S.J. Studying individual differences in human adolescent brain development. Nat. Neurosci. 2018;21:315–323. doi: 10.1038/S41593-018-0078-4. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/NRN2201. [DOI] [PubMed] [Google Scholar]
- Fransson P., Åden U., Blennow M., Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb. Cortex. 2011;21:145–154. doi: 10.1093/CERCOR/BHQ071. [DOI] [PubMed] [Google Scholar]
- Fuhrmann D., Knoll L.J., Blakemore S.J. Adolescence as a sensitive period of brain development. Trends Cogn. Sci. 2015;19:558–566. doi: 10.1016/J.TICS.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Gerardin E., Pochon J.B., Poline J.B., Tremblay L., Van De Moortele P.F., Levy R., Dubois B., Le Bihan D., Lehéricy S. Distinct striatal regions support movement selection, preparation and execution. Neuroreport. 2004;15:2327–2331. doi: 10.1097/00001756-200410250-00005. [DOI] [PubMed] [Google Scholar]
- Gold S., Christian B., Arndt S., Zeien G., Cizadlo T., Johnson D.L., Flaum M., Andreasen N.C. Functional MRI statistical software packages: a comparative analysis. Hum. Brain Mapp. 1998;6:73–84. doi: 10.1002/(SICI)1097-0193(1998)6:2<73::AID-HBM1>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A.M., Grafton S.T. The striatum: where skills and habits meet. Cold Spring Harb. Perspect. Biol. 2015;7:21691–21692. doi: 10.1101/CSHPERSPECT.A021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D.J., Laumann T.O., Dubis J.W., Ihnen S.K., Neta M., Power J.D., Pruett J.R., Black K.J., Schlaggar B.L. Developmental changes in the organization of functional connections between the Basal Ganglia and cerebral cortex. J. Neurosci. 2014;34:5842–5854. doi: 10.1523/JNEUROSCI.3069-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm K.J., Ram N., Hamagami F. Nonlinear growth curves in developmental research. Child Dev. 2011;82(5):1357. doi: 10.1111/J.1467-8624.2011.01630.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 2003;26:317–330. doi: 10.1016/J.JCHEMNEU.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hallquist M.N., Hwang K., Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 2013;82:208–225. doi: 10.1016/J.NEUROIMAGE.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harring J.R., Strazzeri M.M., Blozis S.A. Piecewise latent growth models: beyond modeling linear-linear processes. Behav. Res. Methods. 2021;53(2):593–608. doi: 10.3758/S13428-020-01420-5. [DOI] [PubMed] [Google Scholar]
- Hofer A., Siedentopf C.M., Ischebeck A., Rettenbacher M.A., Verius M., Felber S., Fleischhacker W.W. Sex differences in brain activation patterns during processing of positively and negatively valenced emotional words. Psychol. Med. 2007;37:109–119. doi: 10.1017/S0033291706008919. [DOI] [PubMed] [Google Scholar]
- Jarbo K., Verstynen T.D. Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. J. Neurosci. 2015;35:3865–3878. doi: 10.1523/JNEUROSCI.2636-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Johnson S.B., Blum R.W., Giedd J.N. Adolescent maturity and the brain: the promise and pitfalls of neuroscience research in adolescent health policy. J. Adolesc. Health. 2009;45:216. doi: 10.1016/J.JADOHEALTH.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanssens A., Pizzamiglio G., Mantini D., Gillebert C.R. Role of the dorsal attention network in distracter suppression based on features. Cogn. Neurosci. 2020;11:37–46. doi: 10.1080/17588928.2019.1683525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Neuroscience S.S.-J. of. The differential effects of reward on space-and object-based attentional allocation. Soc. Neurosci. 2013;33:10625–10633. doi: 10.1523/JNEUROSCI.5575-12.2013. (undefined, 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Morgan B.R., Shroff M.M., Sled J.G., Taylor M.J. The development of regional functional connectivity in preterm infants into early childhood. Neuroradiology. 2013:55. doi: 10.1007/S00234-013-1232-Z. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30:718–729. doi: 10.1016/J.NEUBIOREV.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Sex differences in the adolescent brain. Brain Cogn. 2010;72:46. doi: 10.1016/J.BANDC.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton D.M., Gonzales B.J., Citri A. Dorsal striatal circuits for habits, compulsions and addictions. Front Syst. Neurosci. 2019:13. doi: 10.3389/FNSYS.2019.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M., Wassum K.M. Regulation of habit formation in the dorsal striatum. Curr. Opin. Behav. Sci. 2018;20:67–74. doi: 10.1016/J.COBEHA.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manza P., Zhang S., Hu S., Chao H.H., Leung H.C., Li C., Shan R. The effects of age on resting state functional connectivity of the basal ganglia from young to middle adulthood. Neuroimage. 2015;107:311–322. doi: 10.1016/J.NEUROIMAGE.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manza P., Zhang S., Hu S., Chao H.H., Leung H.C., Li C., Shan R. The effects of age on resting state functional connectivity of the basal ganglia from young to middle adulthood. Neuroimage. 2015;107:311–322. doi: 10.1016/j.neuroimage.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.P. Inferences about mental states. Philos. Trans. R. Soc. B: Biol. Sci. 2009;364:1309–1316. doi: 10.1098/RSTB.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L., Maurer J.M., Peechatka A.L., Frederick B.B., Kaiser R.H., Janes A.C. Sex differences in functional network dynamics observed using coactivation pattern analysis. Cogn. Neurosci. 2021;12:120–130. doi: 10.1080/17588928.2021.1880383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen F.Å., Balslev D., Hansen L.K. Mining the posterior cingulate: segregation between memory and pain components. Neuroimage. 2005;27:520–532. doi: 10.1016/J.NEUROIMAGE.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Orben A., Tomova L., Blakemore S.J. The effects of social deprivation on adolescent development and mental health. Lancet Child Adolesc. Health. 2020;4:634–640. doi: 10.1016/S2352-4642(20)30186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli W.M., O’Reilly R.C., Yarkoni T., Wager T.D. Regional specialization within the human striatum for diverse psychological functions. Proc. Natl. Acad. Sci. USA. 2016;113:1907–1912. doi: 10.1073/PNAS.1507610113/SUPPL_FILE/PNAS.1507610113.SAPP.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., Crone E.A. Increased striatal activity in adolescence benefits learning. Nat. Commun. 2017;1(8):1–9. doi: 10.1038/s41467-017-02174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J.N., Roy A.K., Benson B., Carlisi C., Collins P.F., Leibenluft E., Pine D.S., Luciana M., Ernst M. Age-related changes in the intrinsic functional connectivity of the human ventral vs. dorsal striatum from childhood to middle age. Dev. Cogn. Neurosci. 2015;11:83–95. doi: 10.1016/J.DCN.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preckel K., Kanske P., Singer T. On the interaction of social affect and cognition: empathy, compassion and theory of mind. Curr. Opin. Behav. Sci. 2018 doi: 10.1016/j.cobeha.2017.07.010. [DOI] [Google Scholar]
- Rohr C.S., Vinette S.A., Parsons K.A.L., Cho I.Y.K., Dimond D., Benischek A., Lebel C., Dewey D., Bray S. Functional connectivity of the dorsal attention network predicts selective attention in 4–7 year-old girls. Cereb. Cortex. 2017;27:4350–4360. doi: 10.1093/CERCOR/BHW236. [DOI] [PubMed] [Google Scholar]
- Ruigrok A.N.V., Salimi-Khorshidi G., Lai M.C., Baron-Cohen S., Lombardo M.V., Tait R.J., Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014;39:34. doi: 10.1016/J.NEUBIOREV.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S., Fraza C., Dinga R., Kia S.M., Wolfers T., Zabihi M., Berthet P., Worker A., Verdi S., Andrews D., Han L.K.M., Bayer J.M.M., Dazzan P., McGuire P., Mocking R.T., Schene A., Sripada C., Tso I.F., Duval E.R., Marquand A.F., et al. Charting brain growth and aging at high spatial precision. ELife. 2022:11. doi: 10.7554/ELIFE.72904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Roalf D.R., Ruparel K., Erus G., Vandekar S., Gennatas E.D., Elliott M.A., Smith A., Hakonarson H., Verma R., Davatzikos C., Gur R.E., Gur R.C. Linked sex differences in cognition and functional connectivity in youth. Cereb. Cortex. 2015;25:2383–2394. doi: 10.1093/CERCOR/BHU036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Q., Yu X., Tian Y. Adolescent social isolation shifts the balance of decision-making strategy from goal-directed action to habitual response in adulthood via suppressing the excitatory neurotransmission onto the direct pathway of the dorsomedial striatum. Cereb. Cortex. 2022 doi: 10.1093/CERCOR/BHAC158. [DOI] [PubMed] [Google Scholar]
- Shepherd G.M.G. Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci. 2013 doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slewa-Younan S., Gordon E., Harris A.W., Haig A.R., Brown K.J., Flor-Henry P., Williams L.M. Sex differences in functional connectivity in first-episode and chronic schizophrenia patients. Am. J. Psychiatry. 2004;161:1595–1602. doi: 10.1176/APPI.AJP.161.9.1595/ASSET/IMAGES/LARGE/N212F2.JPEG. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Bannister P.R., de Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., de Stefano N., Brady J.M., Matthews P.M. NeuroImage. Academic Press; 2004. Advances in functional and structural MR image analysis and implementation as FSL; pp. S208–S219. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Thompson P.M., Welcome S.E., Henkenius A.L., Toga A.W. Mapping cortical change across the human life span. Nat. Neurosci. 2003;6:309–315. doi: 10.1038/NN1008. [DOI] [PubMed] [Google Scholar]
- Starr C.J., Sawaki L., Wittenberg G.F., Burdette J.H., Oshiro Y., Quevedo A.S., McHaffie J.G., Coghill R.C. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011;134:1987. doi: 10.1093/BRAIN/AWR117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Herting M.M., Goddings A.L., Meuwese R., Blakemore S.J., Dahl R.E., Güroğlu B., Raznahan A., Sowell E.R., Crone E.A., Mills K.L. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J. Neurosci. 2017;37:3402–3412. doi: 10.1523/JNEUROSCI.3302-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniwaki T., Okayama A., Yoshiura T., Togao O., Nakamura Y., Yamasaki T., Ogata K., Shigeto H., Ohyagi Y., Kira J. ichi, Tobimatsu S. Age-related alterations of the functional interactions within the basal ganglia and cerebellar motor loops in vivo. Neuroimage. 2007;36:1263–1276. doi: 10.1016/J.NEUROIMAGE.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Thorell L.B., Skoglund C., de la Peña A.G., Baeyens D., Fuermaier A.B.M., Groom M.J., Mammarella I.C., van der Oord S., van den Hoofdakker B.J., Luman M., de Miranda D.M., Siu A.F.Y., Steinmayr R., Idrees I., Soares L.S., Sörlin M., Luque J.L., Moscardino U.M., Roch M., Crisci G., Christiansen H. Parental experiences of homeschooling during the COVID-19 pandemic: differences between seven European countries and between children with and without mental health conditions. Eur. Child Adolesc. Psychiatry. 2021:1–13. doi: 10.1007/s00787-020-01706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K.S., Ryali S., Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. Soc. Neurosci. 2011 doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner R., Russell S., Saulle R., Croker H., Stansfield C., Packer J., Nicholls D., Goddings A.L., Bonell C., Hudson L., Hope S., Ward J., Schwalbe N., Morgan A., Minozzi S. School closures during social lockdown and mental health, health behaviors, and well-being among children and adolescents during the first COVID-19 wave: a systematic review. JAMA Pedia. 2022 doi: 10.1001/JAMAPEDIATRICS.2021.5840. [DOI] [PubMed] [Google Scholar]
- Vossel S., Geng J.J., Fink G.R. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waytz A., Zaki J., Neuroscience J.M.-J. of. Response of dorsomedial prefrontal cortex predicts altruistic behavior. Soc. Neurosci. 2012 doi: 10.1523/JNEUROSCI.6193-11.2012. (undefined, 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G.X., Ge L., Chen L.Z., Cao B., Zhang X. Structural abnormalities of cingulate cortex in patients with first‐episode drug‐naïve schizophrenia comorbid with depressive symptoms. Hum. Brain Mapp. 2021;42:1617. doi: 10.1002/HBM.25315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford T.J., Lee S.W., Oh J.S., De Luis-Garcia R., Savadjiev P., Alvarado J.L., Westin C.F., Niznikiewicz M., Nestor P.G., McCarley R.W., Kubicki M., Shenton M.E. Localized abnormalities in the cingulum bundle in patients with schizophrenia: a diffusion tensor tractography study. Neuroimage Clin. 2014;5:93–99. doi: 10.1016/J.NICL.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Yücel M., Yap M.B.H., Allen N.B. Sex differences in the neural correlates of emotion: evidence from neuroimaging. Biol. Psychol. 2011;87:319–333. doi: 10.1016/J.BIOPSYCHO.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T., Beckmann C., Jenkinson M., Smith S.M. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2008;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Xia M., Si T., Sun X., Ma Q., Liu B., Wang L., Meng J., Chang M., Huang X., Chen Z., Tang Y., Xu K., Gong Q., Wang F., Qiu J., Xie P., Li L., He Y. Reproducibility of functional brain alterations in major depressive disorder: evidence from a multisite resting-state functional MRI study with 1434 individuals. Neuroimage. 2019;189:700–714. doi: 10.1016/J.NEUROIMAGE.2019.01.074. [DOI] [PubMed] [Google Scholar]
- Yin H.H., Knowlton B.J. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Ystad M., Hodneland E., Adolfsdottir S., Haász J., Lundervold A.J., Eichele T., Lundervold A. Cortico-striatal connectivity and cognition in normal aging: a combined DTI and resting state fMRI study. Neuroimage. 2010;55:24–31. doi: 10.1016/J.NEUROIMAGE.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Zhan J.Y., Wilding J., Cornish K., Shao J., Xie C.H., Wang Y.X., Lee K., Karmiloff-Smith A., Zhao Z.Y. Charting the developmental trajectories of attention and executive function in Chinese school-aged children. Child Neuropsychol. 2011;17:82–95. doi: 10.1080/09297049.2010.525500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The authors do not have permission to share data.