Abstract
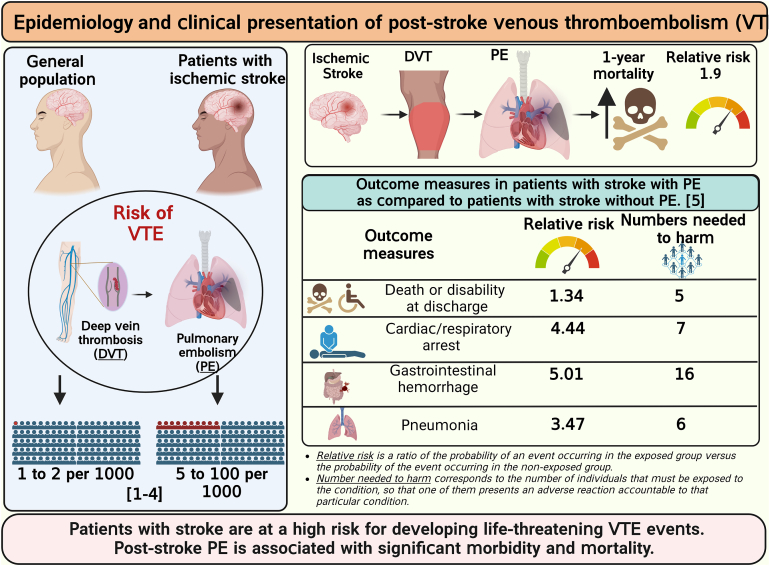
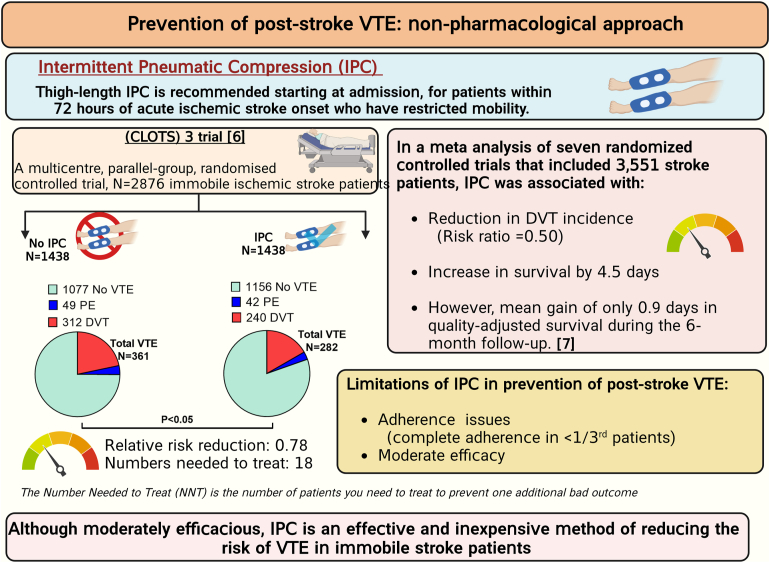
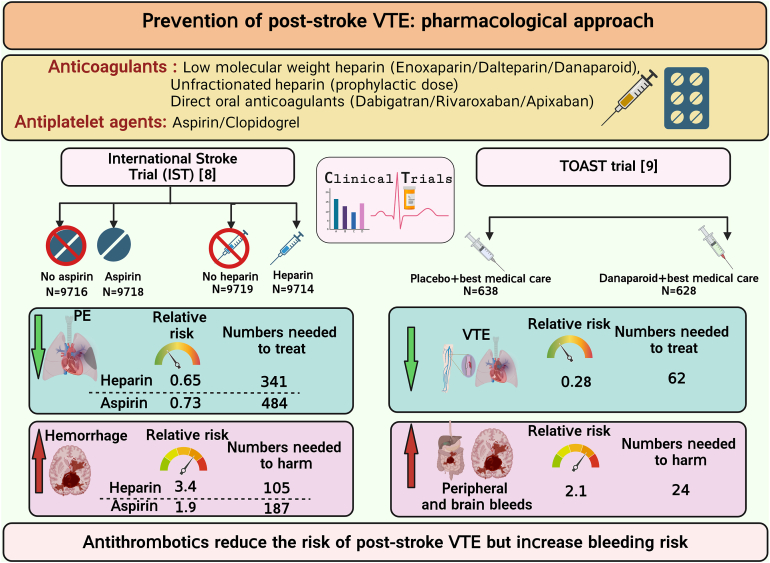
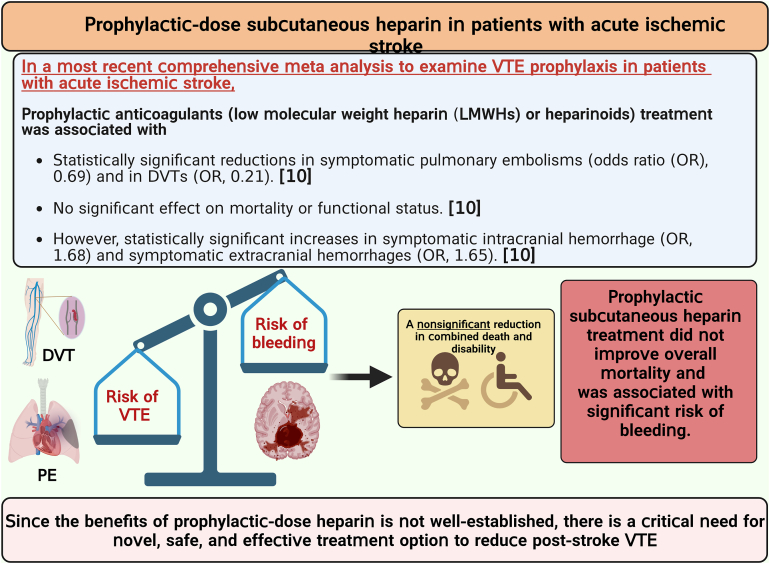
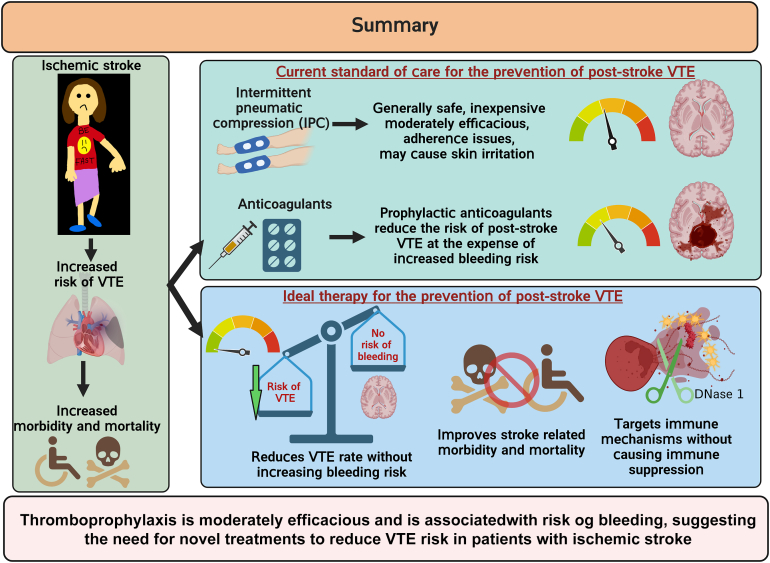
Patients with acute ischemic stroke are at a high risk of venous thromboembolism (VTE), such as deep vein thrombosis (DVT), estimated to affect approximately 80,000 patients with stroke each year in the United States. The prevalence of symptomatic DVT after acute stroke is approximately 10%. VTE is associated with increased rates of in-hospital death and disability, with higher prevalence of in-hospital complications and increased 1-year mortality in patients with stroke. Current guidelines recommend the use of pharmacologic VTE prophylaxis in patients with acute ischemic stroke. However, thromboprophylaxis prevents only half of expected VTE events and is associated with high risk of bleeding, suggesting the need for targeted alternative treatments to reduce VTE risk in these patients. Neutrophils are among the first cells in blood to respond after ischemic stroke. Importantly, coordinated interactions among neutrophils, platelets, and endothelial cells contribute to the development of DVT. In case of stroke and other related immune disorders, such as antiphospholipid syndrome, neutrophils potentiate thrombus propagation through the formation of neutrophil-platelet aggregates, secreting inflammatory mediators, complement activation, releasing tissue factor, and producing neutrophil extracellular traps. In this illustrated review article, we present epidemiology and management of poststroke VTE, preclinical and clinical evidence of neutrophil hyperactivation in stroke, and mechanisms for neutrophil-mediated VTE in the context of stroke. Given the hyperactivation of circulating neutrophils in patients with stroke, we propose that a better understanding of molecular mechanisms leading to neutrophil activation may result in the development of novel therapeutics to reduce the risk of VTE in this patient population.
Keywords: deep vein thrombosis, ischemic stroke, neutrophils, thromboprophylaxis, venous thromboembolism
Essentials
-
•
Patients with acute ischemic stroke are at a high risk of venous thromboembolism (VTE).
-
•
VTE is associated with increased risk of death and disability in patients with stroke.
-
•
Interactions between neutrophils and other cells and factors contribute to risk of VTE.
-
•
Current literature supports a key role of neutrophil-dependent mechanisms in promoting VTE.
Acknowledgment
The authors are thankful to Aadhya Dhanesha (11-year-old daughter of N.D.) for creating an illustration of a stroke patient in slide 11.
Funding
The efforts of N.D. for this publication were supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL158546-01) and by the Career Development Award (20CDA3560123) from American Heart Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The efforts of K.Y.S. for this publication were supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL134959. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
N.D. developed the concepts, wrote the manuscript, and produced the illustrations. N.P., H.K., and C.V. edited the manuscript. J.A. and K.Y.S. cowrote the manuscript and edited the illustrations.
Relationship disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Dr Michelle Sholzberg
References
- 1.Kelly J., Rudd A., Lewis R., Hunt B.J. Venous thromboembolism after acute stroke. Stroke. 2001;32:262–267. doi: 10.1161/01.str.32.1.262. [DOI] [PubMed] [Google Scholar]
- 2.Kelly J., Rudd A., Lewis R.R., Coshall C., Moody A., Hunt B.J. Venous thromboembolism after acute ischemic stroke: a prospective study using magnetic resonance direct thrombus imaging. Stroke. 2004;35:2320–2325. doi: 10.1161/01.STR.0000140741.13279.4f. [DOI] [PubMed] [Google Scholar]
- 3.Rinde L.B., Småbrekke B., Mathiesen E.B., Løchen M.L., Njølstad I., Hald E.M., et al. Ischemic stroke and risk of venous thromboembolism in the general population: the Tromsø study. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg A., Dhanesha N., Shaban A., Samaniego E.A., Chauhan A.K., Leira E.C. Risk of venous thromboembolism in hospitalized patients with acute ischemic stroke versus other neurological conditions. J Stroke Cerebrovasc Dis. 2021;30 doi: 10.1016/j.jstrokecerebrovasdis.2021.106077. [DOI] [PubMed] [Google Scholar]
- 5.Pongmoragot J., Rabinstein A.A., Nilanont Y., Swartz R.H., Zhou L., Saposnik G., et al. Pulmonary embolism in ischemic stroke: clinical presentation, risk factors, and outcome. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration. Dennis M., Sandercock P., Reid J., Graham C., Forbes J., et al. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013;382:516–524. doi: 10.1016/S0140-6736(13)61050-8. [DOI] [PubMed] [Google Scholar]
- 7.Zhang D., Li F., Li X., Du G. Effect of intermittent pneumatic compression on preventing deep vein thrombosis among stroke patients: a systematic review and meta-analysis. Worldviews Evid Based Nurs. 2018;15:189–196. doi: 10.1111/wvn.12288. [DOI] [PubMed] [Google Scholar]
- 8.The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569–1581. [PubMed] [Google Scholar]
- 9.Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. JAMA. 1998;279:1265–1272. [PubMed] [Google Scholar]
- 10.Dennis M., Caso V., Kappelle L.J., Pavlovic A., Sandercock P. European Stroke Organisation. European Stroke Organization (ESO) guidelines for prophylaxis for venous thromboembolism in immobile patients with acute ischaemic stroke. Eur Stroke J. 2016;1:6–19. doi: 10.1177/2396987316628384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan F., Tritschler T., Kahn S.R., Rodger M.A. Venous thromboembolism. Lancet. 2021;398:64–77. doi: 10.1016/S0140-6736(20)32658-1. [DOI] [PubMed] [Google Scholar]
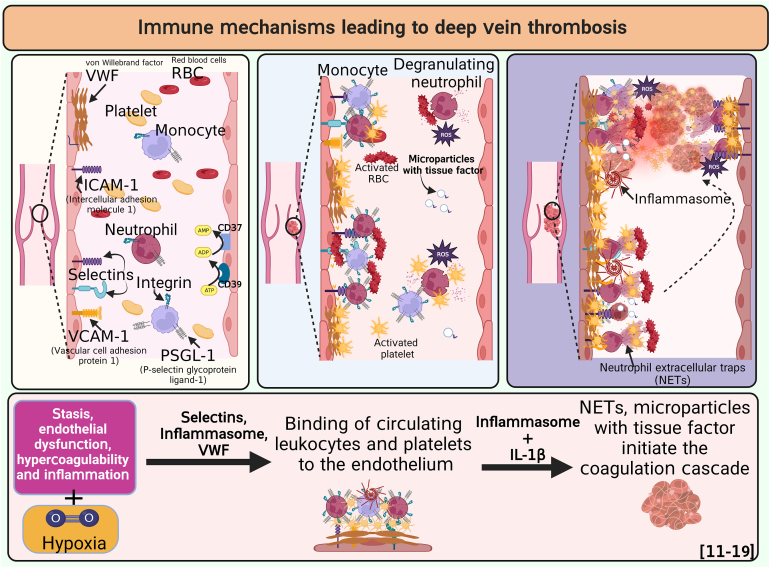
- 12.Budnik I., Brill A. Immune factors in deep vein thrombosis initiation. Trends Immunol. 2018;39:610–623. doi: 10.1016/j.it.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapoor S., Opneja A., Nayak L. The role of neutrophils in thrombosis. Thromb Res. 2018;170:87–96. doi: 10.1016/j.thromres.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhanesha N., Jain M., Doddapattar P., Undas A., Chauhan A.K. Cellular fibronectin promotes deep vein thrombosis in diet-induced obese mice. J Thromb Haemost. 2021;19:814–821. doi: 10.1111/jth.15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs T.A., Brill A., Wagner D.D. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1777–1783. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lester P.A., Diaz J.A., Shuster K.A., Henke P.K., Wakefield T.W., Myers D.D. Inflammation and thrombosis: new insights. Front Biosci (Schol Ed) 2012;4:620–638. doi: 10.2741/s289. [DOI] [PubMed] [Google Scholar]
- 17.Kimball A.S., Obi A.T., Diaz J.A., Henke P.K. The emerging role of NETs in venous thrombosis and immunothrombosis. Front Immunol. 2016;7:236. doi: 10.3389/fimmu.2016.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolberg A.S., Rosendaal F.R., Weitz J.I., Jaffer I.H., Agnelli G., Baglin T., et al. Venous thrombosis. Nat Rev Dis Primers. 2015;1 doi: 10.1038/nrdp.2015.6. [DOI] [PubMed] [Google Scholar]
- 19.Byrnes J.R., Wolberg A.S. Red blood cells in thrombosis. Blood. 2017;130:1795–1799. doi: 10.1182/blood-2017-03-745349. [DOI] [PMC free article] [PubMed] [Google Scholar]
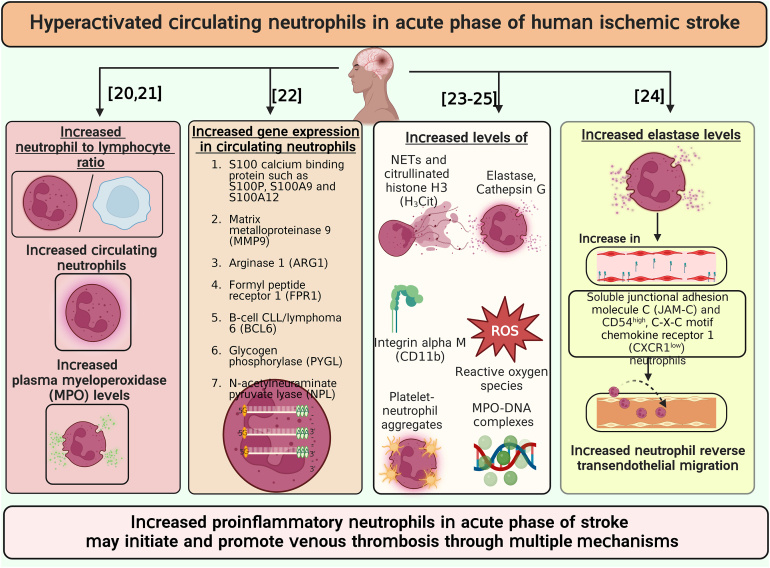
- 20.Maestrini I., Strbian D., Gautier S., Haapaniemi E., Moulin S., Sairanen T., et al. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology. 2015;85:1408–1416. doi: 10.1212/WNL.0000000000002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue J., Huang W., Chen X., Li Q., Cai Z., Yu T., et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26:650–657. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Maestrini I., Tagzirt M., Gautier S., Dupont A., Mendyk A.M., Susen S., et al. Analysis of the association of MPO and MMP-9 with stroke severity and outcome: cohort study. Neurology. 2020;95:e97–e108. doi: 10.1212/WNL.0000000000009179. [DOI] [PubMed] [Google Scholar]
- 23.Tang Y., Xu H., Du X., Lit L., Walker W., Lu A., et al. Gene expression in blood changes rapidly in neutrophils and monocytes after ischemic stroke in humans: a microarray study. J Cereb Blood Flow Metab. 2006;26:1089–1102. doi: 10.1038/sj.jcbfm.9600264. [DOI] [PubMed] [Google Scholar]
- 24.Weisenburger-Lile D., Dong Y., Yger M., Weisenburger G., Polara G.F., Chaigneau T., et al. Harmful neutrophil subsets in patients with ischemic stroke: association with disease severity. Neurol Neuroimmunol Neuroinflamm. 2019;6:e571. doi: 10.1212/NXI.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denorme F., Portier I., Rustad J.L., Cody M.J., de Araujo C.V., Hoki C., et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest. 2022;132 doi: 10.1172/JCI154225. [DOI] [PMC free article] [PubMed] [Google Scholar]
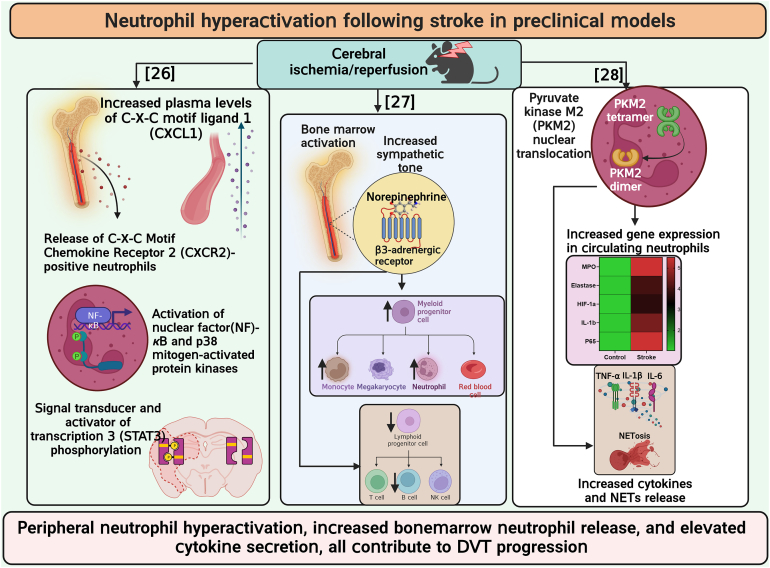
- 26.Denes A., McColl B.W., Leow-Dyke S.F., Chapman K.Z., Humphreys N.E., Grencis R.K., et al. Experimental stroke-induced changes in the bone marrow reveal complex regulation of leukocyte responses. J Cereb Blood Flow Metab. 2011;31:1036–1050. doi: 10.1038/jcbfm.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Courties G., Herisson F., Sager H.B., Heidt T., Ye Y., Wei Y., et al. Ischemic stroke activates hematopoietic bone marrow stem cells. Circ Res. 2015;116:407–417. doi: 10.1161/CIRCRESAHA.116.305207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhanesha N., Patel R.B., Doddapattar P., Ghatge M., Flora G.D., Jain M., et al. PKM2 promotes neutrophil activation and cerebral thromboinflammation: therapeutic implications for ischemic stroke. Blood. 2022;139:1234–1245. doi: 10.1182/blood.2021012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
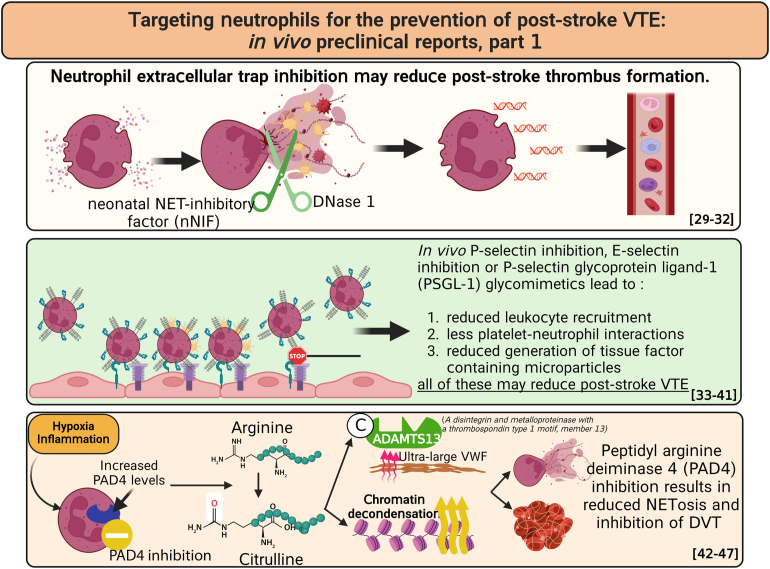
- 29.Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D., Jr., et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Brühl M.L., Stark K., Steinhart A., Chandraratne S., Konrad I., Lorenz M., et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar R., Sonkar V.K., Swamy J., Ahmed A., Sharathkumar A.A., Pierce G.L., et al. DNase 1 protects from increased thrombin generation and venous thrombosis during aging: cross-sectional study in mice and humans. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.021188. [DOI] [PMC free article] [PubMed] [Google Scholar]
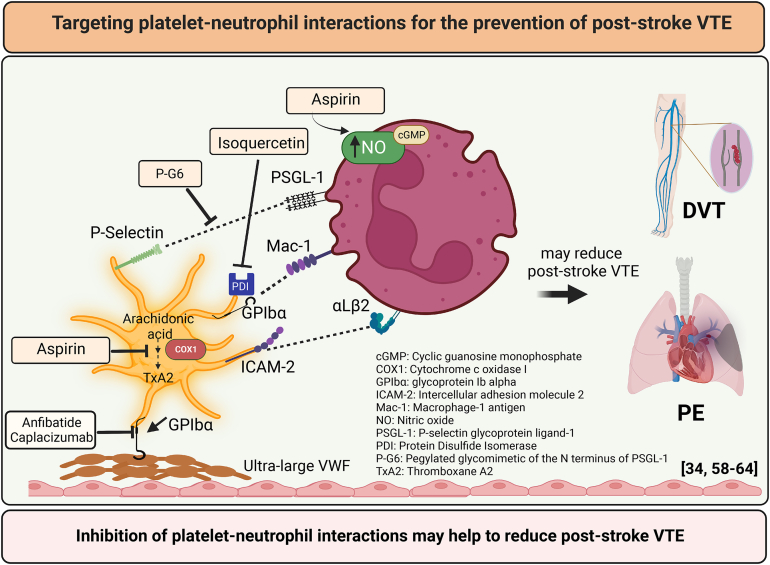
- 32.Brill A., Fuchs T.A., Savchenko A.S., Thomas G.M., Martinod K., De Meyer S.F., et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diaz J.A., Wrobleski S.K., Alvarado C.M., Hawley A.E., Doornbos N.K., Lester P.A., et al. P-selectin inhibition therapeutically promotes thrombus resolution and prevents vein wall fibrosis better than enoxaparin and an inhibitor to von Willebrand factor. Arterioscler Thromb Vasc Biol. 2015;35:829–837. doi: 10.1161/ATVBAHA.114.304457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong D.J., Park D.D., Park S.S., Haller C.A., Chen J., Dai E., et al. A PSGL-1 glycomimetic reduces thrombus burden without affecting hemostasis. Blood. 2021;138:1182–1193. doi: 10.1182/blood.2020009428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers D.D., Wrobleski S.K., Longo C., Bedard P.W., Kaila N., Shaw G.D., et al. Resolution of venous thrombosis using a novel oral small-molecule inhibitor of P-selectin (PSI-697) without anticoagulation. Thromb Haemost. 2007;97:400–407. [PubMed] [Google Scholar]
- 36.Meier T.R., Myers D.D., Jr., Wrobleski S.K., Zajkowski P.J., Hawley A.E., Bedard P.W., et al. Prophylactic P-selectin inhibition with PSI-421 promotes resolution of venous thrombosis without anticoagulation. Thromb Haemost. 2008;99:343–351. doi: 10.1160/TH07-10-0608. [DOI] [PubMed] [Google Scholar]
- 37.Wakefield T.W., Strieter R.M., Downing L.J., Kadell A.M., Wilke C.A., Burdick M.D., et al. P-selectin and TNF inhibition reduce venous thrombosis inflammation. J Surg Res. 1996;64:26–31. doi: 10.1006/jsre.1996.0301. [DOI] [PubMed] [Google Scholar]
- 38.Downing L.J., Wakefield T.W., Strieter R.M., Prince M.R., Londy F.J., Fowlkes J.B., et al. Anti-P-selectin antibody decreases inflammation and thrombus formation in venous thrombosis. J Vasc Surg. 1997;25:816–827. doi: 10.1016/s0741-5214(97)70211-8. [DOI] [PubMed] [Google Scholar]
- 39.Myers D.D., Rectenwald J.E., Bedard P.W., Kaila N., Shaw G.D., Schaub R.G., et al. Decreased venous thrombosis with an oral inhibitor of P selectin. J Vasc Surg. 2005;42:329–336. doi: 10.1016/j.jvs.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 40.Myers D.D., Ning J., Lester P., Adili R., Hawley A., Durham L., et al. E-selectin inhibitor is superior to low-molecular-weight heparin for the treatment of experimental venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10:211–220. doi: 10.1016/j.jvsv.2020.12.086. [DOI] [PubMed] [Google Scholar]
- 41.Culmer D.L., Dunbar M.L., Hawley A.E., Sood S., Sigler R.E., Henke P.K., et al. E-selectin inhibition with GMI-1271 decreases venous thrombosis without profoundly affecting tail vein bleeding in a mouse model. Thromb Haemost. 2017;117:1171–1181. doi: 10.1160/TH16-04-0323. [DOI] [PubMed] [Google Scholar]
- 42.Sundd P., Pospieszalska M.K., Ley K. Neutrophil rolling at high shear: flattening, catch bond behavior, tethers and slings. Mol Immunol. 2013;55:59–69. doi: 10.1016/j.molimm.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ley K., Hoffman H.M., Kubes P., Cassatella M.A., Zychlinsky A., Hedrick C.C., et al. Neutrophils: new insights and open questions. Sci Immunol. 2018;3:4579. doi: 10.1126/sciimmunol.aat4579. [DOI] [PubMed] [Google Scholar]
- 44.Sorvillo N., Mizurini D.M., Coxon C., Martinod K., Tilvawala R., Cherpokova D., et al. Plasma peptidylarginine deiminase IV promotes VWF-platelet string formation and accelerates thrombosis after vessel injury. Circ Res. 2019;125:507–519. doi: 10.1161/CIRCRESAHA.118.314571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis H.D., Liddle J., Coote J.E., Atkinson S.J., Barker M.D., Bax B.D., et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 2015;11:189–191. doi: 10.1038/nchembio.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinod K., Demers M., Fuchs T.A., Wong S.L., Brill A., Gallant M., et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110:8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ansari J., Vital S.A., Yadav S., Gavins F.N.E. Regulating neutrophil PAD4/NOX-dependent cerebrovasular thromboinflammation. Int J Biol Sci. 2023;19:852–864. doi: 10.7150/ijbs.77434. [DOI] [PMC free article] [PubMed] [Google Scholar]
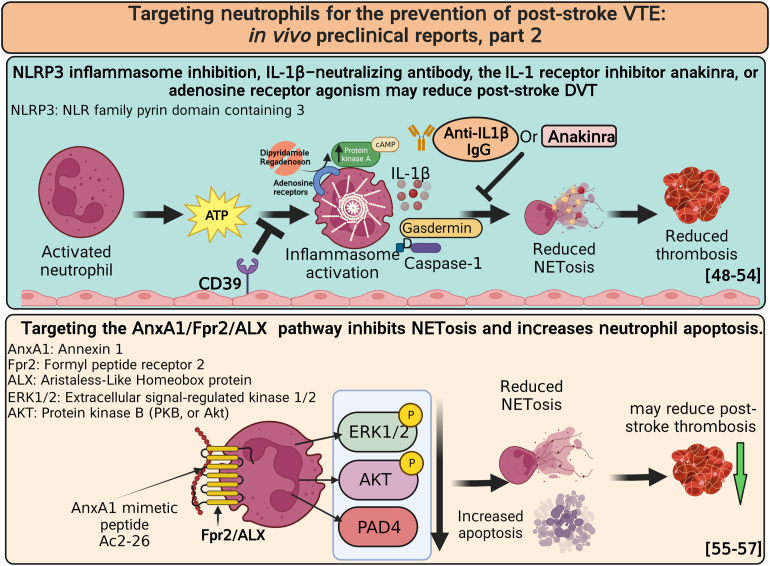
- 48.Colling M.E., Tourdot B.E., Kanthi Y. Inflammation, infection and venous thromboembolism. Circ Res. 2021;128:2017–2036. doi: 10.1161/CIRCRESAHA.121.318225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanthi Y.M., Sutton N.R., Pinsky D.J. CD39: interface between vascular thrombosis and inflammation. Curr Atheroscler Rep. 2014;16:425. doi: 10.1007/s11883-014-0425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav V., Chi L., Zhao R., Tourdot B.E., Yalavarthi S., Jacobs B.N., et al. Ectonucleotidase tri(di)phosphohydrolase-1 (ENTPD-1) disrupts inflammasome/interleukin 1β-driven venous thrombosis. J Clin Invest. 2019;129:2872–2877. doi: 10.1172/JCI124804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anyanwu A.C., Kanthi Y., Fukase K., Liao H., Mimura T., Desch K.C., et al. Tuning the thromboinflammatory response to venous flow interruption by the ectonucleotidase CD39. Arterioscler Thromb Vasc Biol. 2019;39:e118–e129. doi: 10.1161/ATVBAHA.119.312407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., Cui J., Zhang G., Wu C., Abdel-Latif A., Smyth S.S., et al. Inflammasome activation promotes venous thrombosis through pyroptosis. Blood Adv. 2021;5:2619–2623. doi: 10.1182/bloodadvances.2020003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta N., Sahu A., Prabhakar A., Chatterjee T., Tyagi T., Kumari B., et al. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci U S A. 2017;114:4763–4768. doi: 10.1073/pnas.1620458114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali R.A., Gandhi A.A., Meng H., Yalavarthi S., Vreede A.P., Estes S.K., et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun. 2019;10:1916. doi: 10.1038/s41467-019-09801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ansari J., Senchenkova E.Y., Vital S.A., Al-Yafeai Z., Kaur G., Sparkenbaugh E.M., et al. Targeting the AnxA1/Fpr2/ALX pathway regulates neutrophil function, promoting thromboinflammation resolution in sickle cell disease. Blood. 2021;137:1538–1549. doi: 10.1182/blood.2020009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanches J.M., Correia-Silva R.D., Duarte G.H.B., Fernandes A.M.A.P., Sánchez-Vinces S., Carvalho P.O., et al. Role of annexin A1 in NLRP3 inflammasome activation in murine neutrophils. Cells. 2021;10:121. doi: 10.3390/cells10010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vago J.P., Nogueira C.R., Tavares L.P., Soriani F.M., Lopes F., Russo R.C., et al. Annexin A1 modulates natural and glucocorticoid-induced resolution of inflammation by enhancing neutrophil apoptosis. J Leukoc Biol. 2012;92:249–258. doi: 10.1189/jlb.0112008. [DOI] [PubMed] [Google Scholar]
- 58.López-Farré A., Caramelo C., Esteban A., Alberola M.L., Millás I., Montón M., et al. Effects of aspirin on platelet-neutrophil interactions. Role of nitric oxide and endothelin-1. Circulation. 1995;91:2080–2088. doi: 10.1161/01.cir.91.7.2080. [DOI] [PubMed] [Google Scholar]
- 59.Zwicker J.I., Schlechter B.L., Stopa J.D., Liebman H.A., Aggarwal A., Puligandla M., et al. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight. 2019;4 doi: 10.1172/jci.insight.125851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J., Kim K., Jeong S.Y., Chiu J., Xiong B., Petukhov P.A., et al. Platelet protein disulfide isomerase promotes glycoprotein Ibα-mediated platelet-neutrophil interactions under thromboinflammatory conditions. Circulation. 2019;139:1300–1319. doi: 10.1161/CIRCULATIONAHA.118.036323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li B.X., Dai X., Xu X.R., Adili R., Neves M.A.D., Lei X., et al. In vitro assessment and phase I randomized clinical trial of anfibatide a snake venom derived anti-thrombotic agent targeting human platelet GPIbα. Sci Rep. 2021;11 doi: 10.1038/s41598-021-91165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu X.R., Carrim N., Neves M.A., McKeown T., Stratton T.W., Coelho R.M., et al. Platelets and platelet adhesion molecules: novel mechanisms of thrombosis and anti-thrombotic therapies. Thromb J. 2016;14(Suppl 1):29. doi: 10.1186/s12959-016-0100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scully M., Cataland S.R., Peyvandi F., Coppo P., Knöbl P., Kremer Hovinga J.A., et al. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380:335–346. doi: 10.1056/NEJMoa1806311. [DOI] [PubMed] [Google Scholar]
- 64.Li J., Kim K., Barazia A., Tseng A., Cho J. Platelet-neutrophil interactions under thromboinflammatory conditions. Cell Mol Life Sci. 2015;72:2627–2643. doi: 10.1007/s00018-015-1845-y. [DOI] [PMC free article] [PubMed] [Google Scholar]