Key Points
Question
What role did children play in household viral transmission during the COVID-19 pandemic, when enveloped virus rates were low and relative proportions of COVID-19 were at a high?
Findings
In a cohort study of 166 170 households with adults and children using smart thermometers, among 38 787 inferred household transmissions over 3 years, 70.4% had a pediatric index case. Rates dropped during school breaks.
Meaning
These results suggest that children were important viral vectors in households during the pandemic, particularly when school was in session.
This cohort study uses data from commercially available smart thermometers to estimate the role of children in household transmission of SARS-CoV-2 from the beginning of the pandemic until October 2022.
Abstract
Importance
Children’s role in spreading virus during the COVID-19 pandemic is yet to be elucidated, and measuring household transmission traditionally requires contact tracing.
Objective
To discern children’s role in household viral transmission during the pandemic when enveloped viruses were at historic lows and the predominance of viral illnesses were attributed to COVID-19.
Design, Setting, and Participants
This cohort study of a voluntary US cohort tracked data from participatory surveillance using commercially available thermometers with a companion smartphone app from October 2019 to October 2022. Eligible participants were individuals with temperature measurements in households with multiple members between October 2019 and October 2022 who opted into data sharing.
Main Outcomes and Measures
Proportion of household transmissions with a pediatric index case and changes in transmissions during school breaks were assessed using app and thermometer data.
Results
A total of 862 577 individuals from 320 073 households with multiple participants (462 000 female [53.6%] and 463 368 adults [53.7%]) were included. The number of febrile episodes forecast new COVID-19 cases. Within-household transmission was inferred in 54 506 (15.4%) febrile episodes and increased from the fourth pandemic period, March to July 2021 (3263 of 32 294 [10.1%]) to the Omicron BA.1/BA.2 wave (16 516 of 94 316 [17.5%]; P < .001). Among 38 787 transmissions in 166 170 households with adults and children, a median (IQR) 70.4% (61.4%-77.6%) had a pediatric index case; proportions fluctuated weekly from 36.9% to 84.6%. A pediatric index case was 0.6 to 0.8 times less frequent during typical school breaks. The winter break decrease was from 68.4% (95% CI, 57.1%-77.8%) to 41.7% (95% CI, 34.3%-49.5%) at the end of 2020 (P < .001). At the beginning of 2022, it dropped from 80.3% (95% CI, 75.1%-84.6%) to 54.5% (95% CI, 51.3%-57.7%) (P < .001). During summer breaks, rates dropped from 81.4% (95% CI, 74.0%-87.1%) to 62.5% (95% CI, 56.3%-68.3%) by August 2021 (P = .02) and from 83.8% (95% CI, 79.2%-87.5) to 62.8% (95% CI, 57.1%-68.1%) by July 2022 (P < .001). These patterns persisted over 2 school years.
Conclusions and Relevance
In this cohort study using participatory surveillance to measure within-household transmission at a national scale, we discerned an important role for children in the spread of viral infection within households during the COVID-19 pandemic, heightened when schools were in session, supporting a role for school attendance in COVID-19 spread.
Introduction
The role of children in viral spread during the SARS-CoV-2 pandemic has yet to be fully elucidated, but transmission within households plays an important role.1,2,3,4,5,6,7 Measurement traditionally requires labor-intensive and time-consuming contact tracing.1,8,9 We sought to discern within-household transmission using wide-area smart thermometer–based participatory surveillance. Participatory surveillance enables the public to report health-related information using the internet, smartphones, social media, and connected devices.10,11 It is a form of syndromic surveillance that relies on detection of clinical case features discernable before confirmed diagnoses are made.12 Participatory surveillance has been used to assess population health phenomena, providing a timely signal that complements traditional sources.10,13 We used a participatory surveillance cohort to study the role of children in within-household spread of virus during the COVID-19 pandemic, exploiting unique circumstances during a period when rates of enveloped viruses, such as influenza14 and respiratory syncytial virus (RSV),15 were at historical lows and the relative prevalence of viral illnesses attributed to COVID-19 was at a high.
Methods
This is a retrospective cohort study of a nationwide cohort voluntarily using commercially available smartphone-connected thermometers (Kinsa Inc), who opted into data sharing using a companion app between October 1, 2019, and October 29, 2022. This time span was divided into pandemic periods16,17,18: before COVID-19 in the US (October 1, 2019, to February 29, 2020); first outbreak (March 1 to May 15, 2020); second period (May 16 to September 12, 2020); winter wave (September 13, 2020, to March 6, 2021); fourth period (March 7 to July 14, 2021); Delta wave (July 15 to December 18, 2021); Omicron BA.1/BA.2 wave (December 19, 2021, to June 19, 2022); and Omicron BA.4/BA.5 wave (June 20, 2022, to October 29, 2022). The Boston Children’s Hospital institutional review board found the study to be exempt, and informed consent requirements were waived because the study was secondary research using deidentified data. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Participants
Participants aged 18 years or older were considered adults. Children were grouped as younger (ages 0 to 8 years) or older (ages 9 to 17 years).19 The smartphone app let people share a thermometer but recorded data under unique user profiles. Thermometer data included temperature, body location where the temperature was taken, and a timestamp. Age and gender were self-reported. A household was defined as 1 or more individuals using the same thermometer or smartphone. Participants identified in more than 1 household were excluded. For analyses focused on household transmission, readings from a household with just 1 participant were excluded.
Fever Definitions
Fever was defined as a temperature of at least 38.0 °C for rectal and aural readings, 37.8 °C for oral readings and readings from unknown body sites, and 37.2 °C for axillary readings.20 Temperature readings outside the range of 34 °C to 43 °C were excluded as outliers. Each participant’s readings were grouped into episodes that began after a period with no temperature measurements in the past 6 days. Fever onset in a febrile episode was defined as the first body temperature at or exceeding the limits described above.
Transmission Inference
The index case was defined as the participant with the first fever onset in an inferred household transmission sequence. Secondary cases were defined as individuals with fever onset 1 to 7 days after the index case. Other fever transmissions were inferred community transmissions. If more than 1 participant fit the index case definition, the inferred transmission type was defined as unknown.
Inferred Household Transmission Pattern Analysis
We analyzed inferred household transmission patterns in households with both adult and pediatric participants and compared patterns across pandemic periods, considering whether schools were in session or not. These patterns, starting with the index case, were classified as child to child, child to adult, adult to child, and adult to adult. We also analyzed differences when younger and older children were the index case.
Statistical Analysis
Continuous variables were summarized as median values. Discrete variables were summarized as frequencies and percentages. All analyses were performed using R version 4.1.0 (R Foundation for Statistical Computing). All statistical tests were 2-sided, and statistical significance was defined as P < .05. The Granger causality test was used to measure the role of fever as a syndromic signature of viral infection (lmtest package). The Block bootstrap Mann–Kendall trend test was applied for trend detection (modifiedmk package).21 Significance for comparison of rates was tested with a Cochran-Armitage trend test (rstatix package). Spearman rank coefficient was calculated to assess the association between new COVID-19 cases and the proportion of inferred household transmissions with a pediatric index case.
Results
Population Characteristics
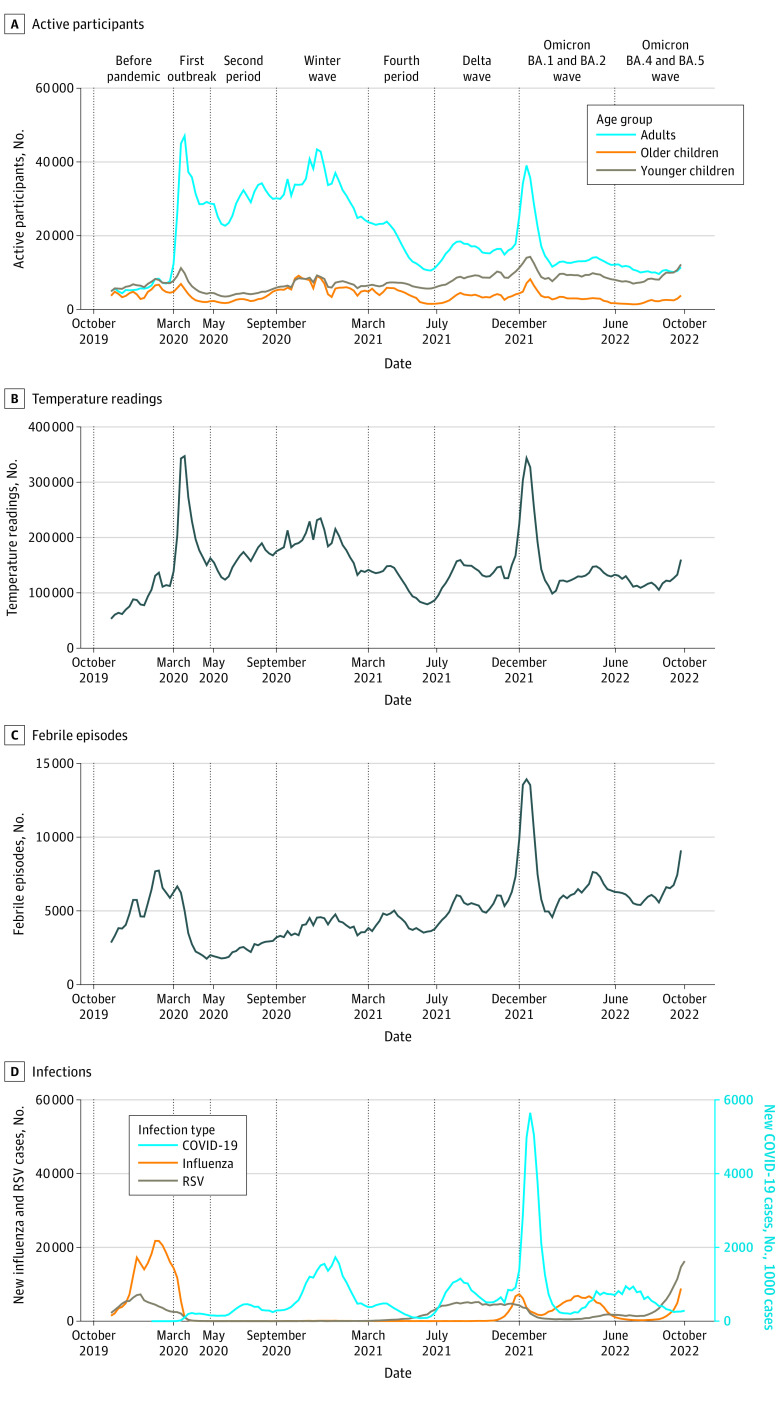
The 1 391 095 participants from 848 591 households took 23 153 925 temperature readings. More than half were from adults (803 116 [57.7%]), and more were from females than males for both adults (470 984 [58.6%] vs 291 871 [36.3%]) and children (284 582 [48.4%] vs 276 918 [47.1%]). There were 3 668 642 (15.8%) readings that met the criteria for fever, comprising 779 092 febrile episodes. The number of participants by age group, temperature readings, and febrile episodes per week are illustrated in Figure 1, along with new US COVID-19 case counts from the Johns Hopkins University Center for Systems Science and Engineering (JHU CSSE)22 and positive case counts for influenza14 and RSV.15 We found that number of febrile episodes forecast new COVID-19 cases (F = 20.0; P < .001), lending validity for using fever syndrome as a proxy for COVID-19 infection. Also, trends for temperature readings and active participants both peaked at the beginning of the pandemic and the Omicron wave.
Figure 1. Time Series From Participatory Surveillance Data and Viral Case Rates From Publicly Available Data Sources.
Vertical lines define the beginning of each pandemic period. US COVID-19 case counts in panel D are sourced from the Johns Hopkins University Center for Systems Science and Engineering,22 the number of positive influenza cases from clinical laboratories using FluView Interactive,14 and the number of positive respiratory syncytial virus (RSV) cases from clinical laboratories in the National Respiratory and Enteric Virus Surveillance System (NREVSS).15
In most households (528 518 [62.3%]), only 1 person shared temperature readings with the app. The remaining 320 073 households (37.7%) included multiple participants (862 577 individuals, 62.0% of all participants) taking 11 945 340 temperature readings (51.6% of all readings). The majority of children were younger (231 462 of 399 209 [58.0%]) and there were more female participants in each age group (Table 1).
Table 1. Characteristics of the Population in Households With 2 or More Participants.
| Characteristics | Children, No. (%) | Adults, No. (%) | |
|---|---|---|---|
| Age 0-8 y (younger) | Age 9-17 y (older) | ||
| Participants | 231 462 (26.8) | 167 747 (19.4) | 463 368 (53.7) |
| Age, median (IQR), y | 4.0 (2.0-7.0) | 12.0 (10.0-14.0) | 38.00 (30.0-50.0) |
| Gender | |||
| Female | 111 124 (48.0) | 83 341 (49.7) | 267 535 (57.7) |
| Male | 110 441 (47.7) | 77 744 (46.3) | 174 290 (37.6) |
| Other | 906 (0.4) | 672 (0.4) | 2490 (0.5) |
| Unknown | 8991 (3.9) | 5990 (3.6) | 19 053 (4.1) |
| No. of readings per participant, median (IQR) | 5.0 (2.0-15.0) | 3.0 (1.0-10.0) | 3.0 (1.0-11.0) |
| No. of episodes per participant, median (IQR) | 2.0 (1.0-3.0) | 1.0 (1.0-2.0) | 1.0 (1.0-3.0) |
| Household composition | |||
| Adults and children | 147 305 (63.6) | 117 888 (70.3) | 250 891 (54.1) |
| Adults only | NA | NA | 212 477 (45.9) |
| Children onlya | 84 157 (36.4) | 49 859 (29.7) | NA |
Households with children only may have had adults in the household who were not using the thermometer.
Household Transmission
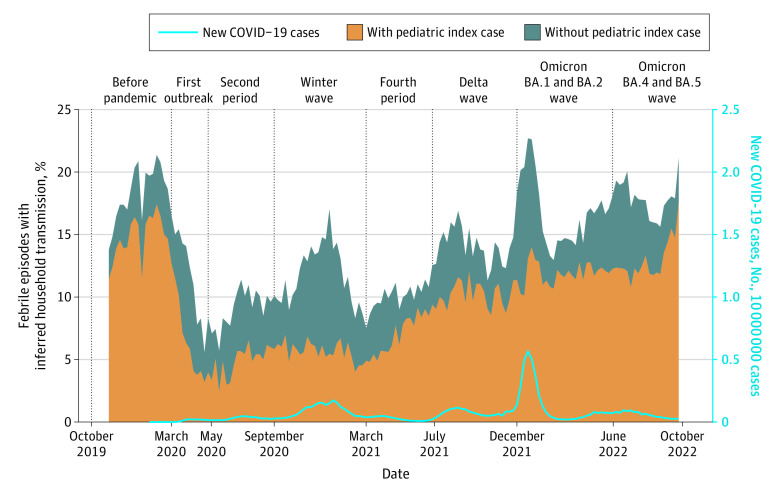
There were 354 602 febrile episodes in households with multiple participants. Of these, 54 506 (15.4%) were considered inferred household transmissions. The percentage of inferred household transmissions increased from the fourth period (3263 of 32 294 [10.1%]) to the Omicron BA.1/BA.2 wave (16 516 of 94 316 [17.5%]) (P < .001) (Figure 2).
Figure 2. Inferred Household Transmissions Among All Febrile Episodes in Households With Multiple Participants With or Without a Pediatric Index Case.
Vertical lines define the beginning of each pandemic period.
Inferred Household Transmission Patterns
There were 166 170 households with both adult and child participants (51.9% of households with multiple participants). These households included 516 159 participants, 265 268 (51.4%) children under 18 years old, who took 6 227 726 temperature readings. The 38 787 inferred household transmissions occurred with the following patterns: 15 819 (40.8%) were child to child, 11 481 (29.6%) child to adult, 7865 (20.3%) adult to child, and 3622 (9.3%) adult to adult. The median (IQR) serial interval between the index and secondary cases was 2 days (IQRs of 1-3 for adult to adult and child to child in the second period, child to child in the winter wave, and adult to adult and adult to child in the Omicron wave; other intervals were 1-4) across all the pandemic periods and transmission patterns, except for the first outbreak where child to child and child to adult was 3 days (1-4 days).
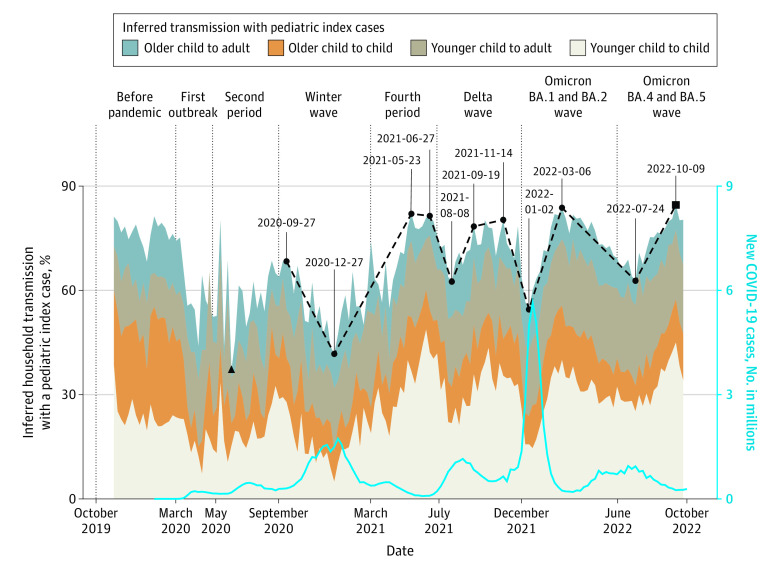
Among all inferred household transmissions, 27 300 (70.4%) started with a pediatric index case but this proportion fluctuated weekly between a low of 36.9% and a high of 87.5% (median [IQR], 70.4% [61.4%-77.6%]) (Figure 3). During all but the second pandemic period, as well as across the whole study period, the percentage of pediatric transmissions was negatively correlated with new COVID-19 cases (Table 2).
Figure 3. Patterns of Inferred Household Transmissions With a Pediatric Index Case.
Lowest and highest percentages during the pandemic are highlighted with black triangle and square points, respectively. Vertical lines define the beginning of each pandemic period. The blue line is the number of new US COVID-19 cases. The points with dates are relatively high and low levels of pediatric transmissions and the dashed line is the trend with these points.
Table 2. Correlation Between Number of New COVID-19 Cases per Week and the Proportion of Inferred Household Transmissions With a Pediatric Index Case.
| Period | No. of wks | Spearman ρ | P value |
|---|---|---|---|
| First outbreak | 11 | −0.627 | .04 |
| Second period | 17 | −0.145 | .58 |
| Winter wave | 25 | −0.667 | <.001 |
| Fourth period | 19 | −0.877 | <.001 |
| Delta wave | 22 | −0.474 | .03 |
| Omicron BA.1/BA.2 wave | 27 | −0.875 | <.001 |
| Omicron BA.3/BA.4 wave | 18 | −0.944 | <.001 |
| Whole study period | 145 | −0.340 | <.001 |
We tracked changes in transmission rates by dates corresponding to the beginning and end of the school years and timing of the winter breaks (Figure 3). Pediatric transmissions started at a high of 68.4% (95% CI, 57.1%-77.8%) during the week of September 27, 2020, and declined to a low of 41.7% (95% CI, 34.3%-49.5%) during the week of December 27, 2020 (0.61 times less frequent; P < .001). The next high point was 82.0% (95% CI, 74.3%-87.8%) during the week of May 23, 2021, which remained stable until June 27, 2021 (81.4%; 95% CI, 74.0%-87.1%). A decrease to 62.5% (95% CI, 56.3%-68.3%) followed by August 8, 2021 (0.77 times less frequent; P = .007). By September 19, 2021, the percentage had increased again to 78.4% (95% CI, 73.4%-82.6%; P = .02) and remained stable until November 14, 2021 (80.3%; 95% CI, 75.1%-84.6%). The next low was 54.5% (95% CI, 51.3%-57.7%) the week of January 2, 2022 (0.68 times less frequent; P = .009). The next increase was to 83.8% (95% CI, 79.2%-87.5%) by March 6, 2022, followed by a decrease to 62.8% (95% CI, 57.1%-68.1%) in the week ending July 24, 2022 (0.75 times less frequent; both P < .001). The final high point of 84.6% (95% CI, 80.6%-87.8%) was during the week of October 9, 2022 (P < .001).
Pediatric Index Cases
Pediatric index cases were examined in 2 age groups, younger (age 0 to 8 years) and older (age 9 to 17 years) (Figure 3). Younger children (17 572, 7.6% of all younger children) were more likely to be the index cases for an inferred household transmission than older children (9,728, 5.8% of all older children) (P < .001).
Discussion
At a time when the relative rate of viral illnesses attributable to COVID-19 was at a high, we were able to take advantage of participatory surveillance methods to gain insight into transmission dynamics among 1.4 million individuals in over 800 000 households using commercially available shared thermometers and a smartphone app. Our findings suggest that children play an important role in within-household viral transmissions. Consistent with demonstrated patterns among other viral illnesses, pediatric-driven transmission was higher when school was in session. During the COVID-19 pandemic, inferred household transmissions increased from the fourth pandemic period (March 7 to July 14, 2021) to the Omicron BA.1/BA.2 wave. More than 70% of household transmissions in households with adults and children were from a pediatric index case, but this percentage fluctuated weekly. Once US schools reopened in fall 2020,23,24,25 children contributed more to inferred within-household transmission when they were in school, and less during summer and winter breaks, a pattern consistent for 2 consecutive school years.
In over 166 000 households with both adults and children, where over 6 million temperature readings were recorded, we found that children represented the majority of index cases after schools reopened in both the 2020-2021 and 2021-2022 school years. However, these transmissions decreased during summer and winter school breaks, which is consistent with prior studies showing school attendance associated with increased respiratory viral spread, and school holidays with decreased spread.26,27,28 While it is known that children play an important role in the spread of respiratory viruses,5,29,30,31 their contribution to the transmission of SARS-CoV-2 has been unclear.1,2,3,4,5 The heterogeneity of prior findings on this topic could be due to several factors including relatively small numbers of households studied, limitations of traditional observational studies,32 and features of the different pandemic periods such as variant type, school policy, and availability of vaccines. In the early pandemic months, school closure was common across the globe,33,34 limiting school transmissions and making children far less important as drivers of SARS-CoV-2 transmission than adults.2,35 However, once schools reopened in fall 2020,23,24,25 children could interact more with others in the community. The number of pediatric COVID-19 cases increased, with evidence that this increase affected overall spread.2,5,6,9,36,37 During the winter wave, children in England were more likely to introduce the virus to households than adults.32 Transmissions from pediatric index cases to household contacts were frequent in California38,39,40 and Colorado.40 During the Delta wave, children were more likely to transmit infection in households in Singapore.41 Consistent with our findings, these studies suggest that the role of children in household transmission became important after school reopened, and changed over time. Our finding that there was more within-household transmission during the Omicron wave is consistent with previous studies.37,42,43
After the winter wave, the percentage of inferred household transmissions with a pediatric index case was negatively correlated with the number of new COVID-19 cases. This is consistent with a previous study showing that during a period of low community transmission, children were the predominant index cases, while during a high community transmission period, adults were.39 Other investigators have shown that the risk of SARS-CoV-2 infection in educational settings correlates with community infection rates,44,45 and that spread among children in school was lower than among adults in the community.46 When the incidence of COVID-19 increases, adults in the community are at higher risk of infection; this may increase the likelihood that adults become the index case in a household transmission and explain the negative correlation we observed. Also, when the COVID-19 incidence is low, overall use of nonpharmaceutical interventions might decrease, leading to increased incidence of non–SARS-CoV-2 pathogens which may be more common in children.
The measurement technique used in this investigation is a form of syndromic surveillance, an approach developed over 2 decades ago.12 The frequency of fevers reported via smartphone-connected thermometers was predictive of population level COVID-19 case counts. This relationship serves to further validate fever measured at home as a syndromic surveillance data source for infectious disease monitoring. In previous studies, actual temperature readings from smart thermometers have been used to detect COVID-19 epicenters,47 and forecast influenza48 and influenza-like illnesses.20 During the COVID-19 pandemic, various citizen-facing participatory surveillance systems were rapidly deployed and used by millions of people.49,50,51,52,53,54 Online surveys underpinned a large remote global health monitoring system and were used for tracing COVID-19 impact trajectories52 and deriving household COVID-19 risk.55 Participatory surveillance systems provide complementary information to the traditional surveillance system and can be an important source of real-time data.13,49,56,57,58 Furthermore, they can capture information about conditions that do not result in a health care visit and its subsequent medical record or insurance claim.
Limitations
This study had several limitations. The study design did not permit laboratory or home testing to confirm viral etiologies. Fever as a syndrome has many etiologies beyond COVID-19. Although confirmatory tests are needed to definitively identify the origin of fever, our study exploited a unique period when the incidence of generally prevalent, non–COVID-19 respiratory viruses plunged, including influenza14 and RSV.15 Although non–SARS-CoV-2 viruses were circulating, we assumed their prevalence was comparatively limited during the study period, with the number of COVID-19 cases22 during the study period being 10 to over 100 times higher than that of influenza14 and RSV15 until August 2022. Parainfluenza59 and human metapneumovirus59 were also rare. Among patients with symptoms hospitalized or presenting to the emergency department (ED), the incidence of rhinoviruses and enteroviruses dropped at the beginning of the pandemic until October 2020, but rose between then and February 2021.59 However, in a community-based study perhaps more reflective of the epidemiology of our home-based cohort, SARS-CoV-2 was more common than rhinovirus from December 2021 to July 2022.60 COVID-19 has been reported to be of very high incidence,22 although generally mild in children,61,62,63 who may be asymptomatic and not develop a fever.64 Given the wide availability of rapid testing at home by December 2020,65 many children with COVID-19 may not have had medical system encounters. We suspect that using rates of children presenting to the ED or hospital with a positive polymerase chain reaction test may underestimate the incidence of COVID-19. Nonetheless, COVID-19 was one of the highest reasons for pediatric ED visits until January 2022, and compared with 2019, visits for non–COVID-19 respiratory illness declined in 2020 and 2021.66 Reassuringly, the number of fevers measured in our cohort was a useful indicator of population-level COVID-19 case counts, supporting our contention that the measured household transmission patterns were reflective of COVID-19 dynamics. Notably, fever patterns also accurately forecasted influenza,20,48 even when other viruses were circulating. A fever-based monitoring approach may, in fact, underestimate COVID-19 rates. Prior work has shown that symptoms varied depending on vaccine status and SARS-CoV-2 variant, with fever less common during the Omicron wave.67,68,69 Changes in age-related presentations varied across studies. One US study69 concluded that percentages of fever among children under age 12 years, adolescents (ages 12 to 18 years), and adults (older than 18 years) were not different among symptomatic individuals testing positive during the Omicron BA.1 period. However, a United Kingdom study68 found that fever was more common in children experiencing symptoms and with positive polymerase chain reaction tests who were younger than 5 years during the Omicron BA.1 and BA.2 periods. During the Omicron waves, children and adolescents had a higher proportion of asymptomatic infection.70
Recruitment and retention of participants that reflect the population is a common challenge for participatory surveillance.71 Structural bias related to access to digital technologies can skew results by socioeconomic and racial factors. Note that an alternative method, contact tracing, may also produce a selection bias, and it cannot achieve the scale of this study. In addition, some groups could have more temperature readings than others. Perhaps parents are more likely to take their children's temperatures than their own, thus overestimating proportions with a pediatric index case. However, in this study, there were more temperature readings per person for adults than children. Antipyretic use could also have affected results, although the thermometers used should have been able to capture the initial fever signal before antipyretic use began. Furthermore, we defined a household as people using the same thermometer or smartphone to report temperature readings. However, there could be other people in the same household who used a different thermometer, did not measure their body temperature, or did not opt into data sharing.
Identifying the transmission chain has challenges, even with detailed conventional epidemiological investigations. Secondary cases could be from outside exposure rather than an infected household member. However, by restricting occurrence to within 1 week after the index case, we sought to mitigate this issue. The median serial interval between the index and secondary case was 2 days.
The quality of self-measured data, even with an FDA-cleared device, may be lower than that collected by a health professional. However, the real-time availability of these data has proven useful for identifying areas that need COVID-19 tests,57 documenting symptoms,13,49,56 forecasting epidemics,72 tracking the spread of infections,56 and identifying disease activity change weeks ahead of traditional disease surveillance.73
Vaccinated individuals may experience mild symptoms,69 so it is possible that the contribution of adults was underestimated due to higher vaccination coverage. Yet, a decreasing trend in the proportion of household transmissions with a pediatric index case was observed during the winter break at the end of 2020, before the initial vaccination protocol was widely completed.74 The companion app used in our study allowed participants to report other symptoms, which could have served as additional indicators if fever became less common and other symptoms became more common. An additional survey could be distributed through the app to collect vaccine status and other information.
A potential confounder is that nonpharmaceutical interventions changed over time and varied across states. Not all schools were open for in-person instruction in fall 2020. About 19% of K-12 schools remained fully online and 50% were using a hybrid model.23,24 In a manual review of 250 school districts, 29% never opened in-person during fall 2020.25 Therefore, the degree to which school breaks in 2020 factored into our results may be underestimated. However, as time went on, more schools chose to reopen for in-person classes.23,24 In the spring and fall 2021, very few school districts, only 2% and 0.5%, respectively, remained fully online.24 The percentage of schools requiring masks decreased from 68% to 7% between fall 2020 and spring 2022.24 In fall 2020, when schools had just reopened, 68.4% of inferred household transmissions started with a pediatric index case. In fall 2021 and 2022, 78.4% and 84.6% did. These latter 2 school years coincided with more in-person classes and fewer requirements for masks and other nonpharmaceutical interventions.
Conclusion
In this cohort study using participatory surveillance to measure within-household viral transmission, children played an important role in viral spread during the SARS-CoV-2 pandemic, at a time when the relative incidence and prevalence of COVID-19 was maximal compared with other circulating viruses. Pediatric transmission was heightened when school was in session and when community levels of COVID-19 were lower, suggesting a substantial role of school attendance in COVID-19 spread. Surveillance with smartphone connected devices enabled infectious disease surveillance and measurement of infection dynamics at a scale unobtainable with traditional methods. The approach brings research into the household without needing study personnel or contact tracers. Future work could validate the inferred transmissions from a participatory network with onsite visits or other contract-tracing outreach for additional data collection and laboratory confirmation. Any system that leverages digital technologies must make every effort to ensure equitable access.
Data Sharing Statement
References
- 1.Lewis NM, Chu VT, Ye D, et al. Household transmission of severe acute respiratory syndrome coronavirus-2 in the United States. Clin Infect Dis. 2021;73(7):1805-1813. doi: 10.1093/cid/ciaa1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y, Bloxham CJ, Hulme KD, et al. A meta-analysis on the role of children in severe acute respiratory syndrome coronavirus 2 in household transmission clusters. Clin Infect Dis. 2021;72(12):e1146-e1153. doi: 10.1093/cid/ciaa1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175(2):143-156. doi: 10.1001/jamapediatrics.2020.4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park YJ, Choe YJ, Park O, et al. ; COVID-19 National Emergency Response Center, Epidemiology and Case Management Team . Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465-2468. doi: 10.3201/eid2610.201315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt M, Plint AC, Tang K, et al. Household transmission of SARS-CoV-2 from unvaccinated asymptomatic and symptomatic household members with confirmed SARS-CoV-2 infection: an antibody-surveillance study. CMAJ Open. 2022;10(2):E357-E366. doi: 10.9778/cmajo.20220026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Salido A. SARS-CoV-2 children transmission: the evidence is that today we do not have enough evidence. Acta Paediatr. 2020;109(9):1912. doi: 10.1111/apa.15396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLean HQ, Grijalva CG, Hanson KE, et al. Household transmission and clinical features of SARS-CoV-2 infections. Pediatrics. 2022;149(3):e2021054178. doi: 10.1542/peds.2021-054178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng OT, Marimuthu K, Koh V, et al. SARS-CoV-2 seroprevalence and transmission risk factors among high-risk close contacts: a retrospective cohort study. Lancet Infect Dis. 2021;21(3):333-343. doi: 10.1016/S1473-3099(20)30833-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawood FS, Porucznik CA, Veguilla V, et al. Incidence rates, household infection risk, and clinical characteristics of SARS-CoV-2 infection among children and adults in Utah and New York City, New York. JAMA Pediatr. 2022;176(1):59-67. doi: 10.1001/jamapediatrics.2021.4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandl KD, McNabb M, Marks N, et al. Participatory surveillance of diabetes device safety: a social media-based complement to traditional FDA reporting. J Am Med Inform Assoc. 2014;21(4):687-691. doi: 10.1136/amiajnl-2013-002127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wójcik OP, Brownstein JS, Chunara R, Johansson MA. Public health for the people: participatory infectious disease surveillance in the digital age. Emerg Themes Epidemiol. 2014;11:7. doi: 10.1186/1742-7622-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandl KD, Overhage JM, Wagner MM, et al. Implementing syndromic surveillance: a practical guide informed by the early experience. J Am Med Inform Assoc. 2004;11(2):141-150. doi: 10.1197/jamia.M1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan AT, Brownstein JS. Putting the public back in public health—surveying symptoms of Covid-19. N Engl J Med. 2020;383(7):e45. doi: 10.1056/NEJMp2016259 [DOI] [PubMed] [Google Scholar]
- 14.US Centers for Disease Control and Prevention . National, regional, and state level outpatient illness and viral surveillance. Last updated April 15, 2023. Accessed December 5, 2022. https://gis.cdc.gov/grasp/fluview/fluportaldashboard.html
- 15.US Centers for Disease Control and Prevention . RSV national trends. Published November 30, 2022. Accessed December 5, 2022. https://www.cdc.gov/surveillance/nrevss/rsv/natl-trend.html
- 16.Surie D, Bonnell L, Adams K, et al. ; IVY Network . Effectiveness of monovalent mRNA vaccines against COVID-19-associated hospitalization among immunocompetent adults during BA.1/BA.2 and BA.4/BA.5 predominant periods of SARS-CoV-2 Omicron variant in the United States—IVY Network, 18 states, December 26, 2021-August 31, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(42):1327-1334. doi: 10.15585/mmwr.mm7142a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leatherby L. What Previous Covid-19 Waves Tell Us About the Virus Now. New York Times. Published October 23, 2021. Accessed August 15, 2022. https://www.nytimes.com/interactive/2021/10/23/us/covid-surges.html
- 18.Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods—United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146-152. doi: 10.15585/mmwr.mm7104e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul LA, Daneman N, Schwartz KL, et al. Association of age and pediatric household transmission of SARS-CoV-2 infection. JAMA Pediatr. 2021;175(11):1151-1158. doi: 10.1001/jamapediatrics.2021.2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackley SF, Pilewski S, Petrovic VS, Worden L, Murray E, Porco TC. Assessing the utility of a smart thermometer and mobile application as a surveillance tool for influenza and influenza-like illness. Health Informatics J. 2020;26(3):2148-2158. doi: 10.1177/1460458219897152 [DOI] [PubMed] [Google Scholar]
- 21.Önöz B, Bayazit M. Block bootstrap for Mann-Kendall trend test of serially dependent data. Hydrol Processes. 2012;26(23):3552-3560. doi: 10.1002/hyp.8438 [DOI] [Google Scholar]
- 22.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533-534. doi: 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honein MA, Barrios LC, Brooks JT. Data and policy to guide opening schools safely to limit the spread of SARS-CoV-2 infection. JAMA. 2021;325(9):823-824. doi: 10.1001/jama.2021.0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MCH Data . COVID-19 IMPACT: School Districts Status Updates for 2022-2023 school year. Last updated April 26, 2023. Accessed December 6, 2022. https://www.mchdata.com/covid19/schoolclosings
- 25.Marianno BD, Hemphill AA, Loures-Elias APS, Garcia L, Cooper D, Coombes E. Power in a pandemic: teachers’ unions and their responses to school reopening. AERA Open. Published online January 26, 2022. doi: 10.1177/23328584221074337 [DOI] [Google Scholar]
- 26.Cauchemez S, Valleron AJ, Boëlle PY, Flahault A, Ferguson NM. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature. 2008;452(7188):750-754. doi: 10.1038/nature06732 [DOI] [PubMed] [Google Scholar]
- 27.Eames KTD. The influence of school holiday timing on epidemic impact. Epidemiol Infect. 2014;142(9):1963-1971. doi: 10.1017/S0950268813002884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson NM, Cummings DAT, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442(7101):448-452. doi: 10.1038/nature04795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viboud C, Boëlle PY, Cauchemez S, et al. Risk factors of influenza transmission in households. Br J Gen Pract. 2004;54(506):684-689. doi: 10.1016/j.ics.2004.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brownstein JS, Kleinman KP, Mandl KD. Identifying pediatric age groups for influenza vaccination using a real-time regional surveillance system. Am J Epidemiol. 2005;162(7):686-693. doi: 10.1093/aje/kwi257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boncristiani HF, Criado MF, Arruda E. Respiratory viruses. In: Schaechter M. Encyclopedia of Microbiology. Plant Sciences Institute; 2009:500-518. [Google Scholar]
- 32.Hyde Z. Difference in severe acute respiratory syndrome coronavirus 2 attack rate between children and adults may reflect bias. Clin Infect Dis. 2022;74(1):152-155. doi: 10.1093/cid/ciab183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viner RM, Russell SJ, Croker H, et al. School closure and management practices during coronavirus outbreaks including COVID-19: a rapid systematic review. Lancet Child Adolesc Health. 2020;4(5):397-404. doi: 10.1016/S2352-4642(20)30095-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donohue JM, Miller E. COVID-19 and school closures. JAMA. 2020;324(9):845-847. doi: 10.1001/jama.2020.13092 [DOI] [PubMed] [Google Scholar]
- 35.Lee B, Raszka WV Jr. COVID-19 transmission and children: the child is not to blame. Pediatrics. 2020;146(2):e2020004879. doi: 10.1542/peds.2020-004879 [DOI] [PubMed] [Google Scholar]
- 36.Goldstein E, Lipsitch M, Cevik M. On the effect of age on the transmission of SARS-CoV-2 in households, schools, and the community. J Infect Dis. 2021;223(3):362-369. doi: 10.1093/infdis/jiaa691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen F, Tian Y, Zhang L, Shi Y. The role of children in household transmission of COVID-19: a systematic review and meta-analysis. Int J Infect Dis. 2022;122:266-275. doi: 10.1016/j.ijid.2022.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu PY, Gragnani CM, Timmerman J, et al. Pediatric household transmission of severe acute respiratory coronavirus-2 infection—Los Angeles County, December 2020 to February 2021. Pediatr Infect Dis J. 2021;40(10):e379-e381. doi: 10.1097/INF.0000000000003251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka ML, Marentes Ruiz CJ, Malhotra S, et al. SARS-CoV-2 transmission dynamics in households with children, Los Angeles, California. Front Pediatr. 2022;9:752993. doi: 10.3389/fped.2021.752993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waltenburg MA, Whaley MJ, Chancey RJ, et al. ; COVID-19 Laboratory & Testing Task Force . Household transmission and symptomology of severe acute respiratory syndrome coronavirus 2 Alpha variant among children—California and Colorado, 2021. J Pediatr. 2022;247:29-37.e7. doi: 10.1016/j.jpeds.2022.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng OT, Koh V, Chiew CJ, et al. Impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination and pediatric age on delta variant household transmission. Clin Infect Dis. 2022;75(1):e35-e43. doi: 10.1093/cid/ciac219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.House T, Riley H, Pellis L, et al. Inferring risks of coronavirus transmission from community household data. Stat Methods Med Res. 2022;31(9):1738-1756. doi: 10.1177/09622802211055853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jalali N, Brustad HK, Frigessi A, et al. Increased household transmission and immune escape of the SARS-CoV-2 Omicron compared to Delta variants. Nat Commun. 2022;13(1):5706. doi: 10.1038/s41467-022-33233-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mensah AA, Sinnathamby M, Zaidi A, et al. SARS-CoV-2 infections in children following the full re-opening of schools and the impact of national lockdown: prospective, national observational cohort surveillance, July-December 2020, England. J Infect. 2021;82(4):67-74. doi: 10.1016/j.jinf.2021.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ladhani SN; sKIDs Investigation Team . Children and COVID-19 in schools. Science. 2021;374(6568):680-682. doi: 10.1126/science.abj2042 [DOI] [PubMed] [Google Scholar]
- 46.Gras-Le Guen C, Cohen R, Rozenberg J, Launay E, Levy-Bruhl D, Delacourt C. Reopening schools in the context of increasing COVID-19 community transmission: the French experience. Arch Pediatr. 2021;28(3):178-185. doi: 10.1016/j.arcped.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chamberlain SD, Singh I, Ariza C, Daitch A, Philips P, Dalziel BD. Real-time detection of COVID-19 epicenters within the United States using a network of smart thermometers. bioRxiv. Preprint posted online April 10, 2020. doi: 10.1101/2020.04.06.20039909 [DOI]
- 48.Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis. 2018;67(3):388-397. doi: 10.1093/cid/ciy073 [DOI] [PubMed] [Google Scholar]
- 49.Drew DA, Nguyen LH, Steves CJ, et al. ; COPE Consortium . Rapid implementation of mobile technology for real-time epidemiology of COVID-19. Science. 2020;368(6497):1362-1367. doi: 10.1126/science.abc0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koehlmoos TP, Janvrin ML, Korona-Bailey J, Madsen C, Sturdivant R. COVID-19 self-reported symptom tracking programs in the United States: framework synthesis. J Med Internet Res. 2020;22(10):e23297. doi: 10.2196/23297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Güemes A, Ray S, Aboumerhi K, et al. A syndromic surveillance tool to detect anomalous clusters of COVID-19 symptoms in the United States. Sci Rep. 2021;11(1):4660. doi: 10.1038/s41598-021-84145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Astley CM, Tuli G, Mc Cord KA, et al. Global monitoring of the impact of the COVID-19 pandemic through online surveys sampled from the Facebook user base. Proc Natl Acad Sci U S A. 2021;118(51):e2111455118. doi: 10.1073/pnas.2111455118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desjardins MR. Syndromic surveillance of COVID-19 using crowdsourced data. Lancet Reg Health West Pac. 2020;4:100024. doi: 10.1016/j.lanwpc.2020.100024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26(7):1037-1040. doi: 10.1038/s41591-020-0916-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lessler J, Grabowski MK, Grantz KH, et al. Household COVID-19 risk and in-person schooling. Science. 2021;372(6546):1092-1097. doi: 10.1126/science.abh2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nomura S, Yoneoka D, Shi S, et al. An assessment of self-reported COVID-19 related symptoms of 227,898 users of a social networking service in Japan: has the regional risk changed after the declaration of the state of emergency? Lancet Reg Health West Pac. 2020;1:100011. doi: 10.1016/j.lanwpc.2020.100011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leal-Neto OB, Santos FAS, Lee JY, Albuquerque JO, Souza WV. Prioritizing COVID-19 tests based on participatory surveillance and spatial scanning. Int J Med Inform. 2020;143:104263. doi: 10.1016/j.ijmedinf.2020.104263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wirth FN, Johns M, Meurers T, Prasser F. Citizen-centered mobile health apps collecting individual-level spatial data for infectious disease management: scoping review. JMIR Mhealth Uhealth. 2020;8(11):e22594. doi: 10.2196/22594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rankin DA, Spieker AJ, Perez A, et al. ; NVSN Network Investigators . Circulation of rhinoviruses and/or enteroviruses in pediatric patients with acute respiratory illness before and during the COVID-19 pandemic in the US. JAMA Netw Open. 2023;6(2):e2254909. doi: 10.1001/jamanetworkopen.2022.54909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hansen C, Perofsky AC, Burstein R, et al. Trends in risk factors and symptoms associated with SARS-CoV-2 and rhinovirus test positivity in King County, Washington, June 2020 to July 2022. JAMA Netw Open. 2022;5(12):e2245861. doi: 10.1001/jamanetworkopen.2022.45861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toba N, Gupta S, Ali AY, et al. COVID-19 under 19: a meta-analysis. Pediatr Pulmonol. 2021;56(6):1332-1341. doi: 10.1002/ppul.25312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iijima H, Kubota M, Ogimi C. Clinical characteristics of pediatric patients with COVID-19 between Omicron era vs. pre-Omicron era. J Infect Chemother. 2022;28(11):1501-1505. doi: 10.1016/j.jiac.2022.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088-1095. doi: 10.1111/apa.15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Z, Wu P, Wang J, et al. Assessing the asymptomatic proportion of SARS-CoV-2 infection with age in China before mass vaccination. J R Soc Interface. 2022;19(195):20220498. doi: 10.1098/rsif.2022.0498 [DOI] [Google Scholar]
- 65.US Food and Drug Administration . Coronavirus (COVID-19) Update: FDA Authorizes Antigen Test as First Over-the-Counter Fully At-Home Diagnostic Test for COVID-19. U.S. Food and Drug Administration. December 15, 2020. Accessed April 13, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-antigen-test-first-over-counter-fully-home-diagnostic
- 66.Radhakrishnan L, Carey K, Hartnett KP, et al. Pediatric emergency department visits before and during the COVID-19 pandemic—United States, January 2019-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(8):313-318. doi: 10.15585/mmwr.mm7108e1 [DOI] [PubMed] [Google Scholar]
- 67.Butt AA, Dargham SR, Loka S, et al. Coronavirus disease 2019 disease severity in children infected with the Omicron variant. Clin Infect Dis. 2022;75(1):e361-e367. doi: 10.1093/cid/ciac275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vihta KD, Pouwels KB, Peto TE, et al. ; COVID-19 Infection Survey team . Omicron-associated changes in SARS-CoV-2 symptoms in the United Kingdom. Clin Infect Dis. 2022;76(3):e133-e141. doi: 10.1101/2022.01.18.22269082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marquez C, Kerkhoff AD, Schrom J, et al. COVID-19 symptoms and duration of rapid antigen test positivity at a community testing and surveillance site during pre-Delta, Delta, and Omicron BA.1 periods. JAMA Netw Open. 2022;5(10):e2235844. doi: 10.1001/jamanetworkopen.2022.35844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu W, Guo Y, Zhang S, Kong Y, Shen Z, Zhang J. Proportion of asymptomatic infection and nonsevere disease caused by SARS-CoV-2 Omicron variant: a systematic review and analysis. J Med Virol. 2022;94(12):5790-5801. doi: 10.1002/jmv.28066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smolinski MS, Crawley AW, Olsen JM, Jayaraman T, Libel M. Participatory disease surveillance: engaging communities directly in reporting, monitoring, and responding to health threats. JMIR Public Health Surveill. 2017;3(4):e62. doi: 10.2196/publichealth.7540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mavragani A, Gkillas K. COVID-19 predictability in the United States using Google Trends time series. Sci Rep. 2020;10(1):20693. doi: 10.1038/s41598-020-77275-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kogan NE, Clemente L, Liautaud P, et al. An early warning approach to monitor COVID-19 activity with multiple digital traces in near real time. Sci Adv. 2021;7(10):eabd6989. doi: 10.1126/sciadv.abd6989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.US Centers for Disease Control and Prevention . Archive: COVID-19 vaccination and case trends by age group, United States. Published online July 1, 2021. Accessed April 6, 2023. https://data.cdc.gov/Vaccinations/Archive-COVID-19-Vaccination-and-Case-Trends-by-Ag/gxj9-t96f
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement