Abstract
Background:
Microbiota restoration is highly effective to treat recurrent Clostridioides difficile infection (CDI) in observational studies (cure rates >90%) but efficacy in controlled clinical trials appears to be lower.
Objectives:
To perform an updated meta-analysis to assess the efficacy of microbiota restoration for recurrent CDI in open-label registered prospective clinical trials compared to randomized controlled trials (RCTs).
Design:
A systematic review and meta-analysis was conducted.
Data Sources and Methods:
A systematic search of various databases was performed up to July 2022 to identify studies of interest. Clinical trials of microbiota restoration for recurrent CDI with clinical resolution with one dose were included. We calculated weighted pooled rates (WPRs) with 95% confidence intervals (CIs).
Results:
In all, 19 clinical trials with 1176 recurrent CDI patients were included. Of the patients treated with microbiota restoration, 897 experienced a clinical cure with a single microbiota restoration therapy (WPR, 78%; 95% CI, 71–85%). There was significant heterogeneity among studies with an I2 of 88%. Analysis of trials with a control arm (non-microbiota restoration) revealed CDI resolution in 373 of 523 patients (WPR, 72%; 95% CI, 60–82%) with microbiota restoration. Among the nine open-label clinical trials, CDI resolution was seen in 524 of 653 patients after initial microbiota restoration (WPR, 84%; 95% CI, 74–92%). Comparison of resolution rates between RCTs and open-label trials revealed a lower cure rate in RCTs compared to open-label trials (WPR, 73 versus 84%, p < 0.0001).
Conclusions:
Microbiota restoration in a randomized controlled setting leads to lower resolution rates compared to open label and observational settings, likely due to stricter definitions and inclusion criteria. Resolution rates in open-label studies were similar to observational studies.
Keywords: C difficile, Microbiota restoration, clinical cure, controlled trials, fecal microbiota transplantation, meta-analysis
Introduction
Clostridioides difficile infection (CDI) is the most common healthcare-associated infection in the United States with over 50% of patients developing recurrences after two or more episodes. Microbiota replacement therapy (MRT) is used to treat recurrent CDI by restoring a healthy gut microbiome. Guidelines from the Infectious Diseases Society of America and Society of Healthcare Epidemiology of America recommend MRT after appropriate antibiotic treatment after two or more CDI recurrences in patients who have failed appropriate antibiotic treatments. 1
The efficacy of MRT for recurrent CDI in observational studies is more than 85% but efficacy in controlled clinical trials appears to be lower. 2 Our 2017 meta-analysis showed an overall cure rate of 76% in clinical trial settings with efficacy being lower (67%) in trials with a comparator group compared to open-label trials. 3 Most trials included in that meta-analysis had different methodologies including recurrent CDI diagnostic and inclusion criteria, MRT preparations, and comparator group leading to a significant heterogeneity. These inconsistencies have resulted in limiting the generalizability of these results and pose a caution in positioning MRT as a therapy for CDI.
Since the earlier systematic review and meta-analysis, more evidence from trials regarding use of MRT has emerged. These have included phase III trials of fecal microbiota transplantation (FMT) and standardized live biotherapeutics for recurrent CDI. We performed an updated meta-analysis with the latest evidence to reassess the efficacy of microbiota restoration in clinical trials.
Methods
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines to conduct this meta-analysis. 4
Selection criteria and data search
A systematic search of electronic databases including Ovid MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, and Scopus, along with abstracts and press releases from major gastroenterology and infectious diseases conferences, was performed up to July 2022. The search strategy was designed and conducted independently by Mayo Clinic library staff and two study investigators (S.K. and R.T.). A controlled vocabulary supplemented with keywords was used to search for studies that used FMT for CDI. Main keywords used in the search were the following: Clostridium difficile, C diff, C difficile, Clostridium difficile infection, CDI, Clostridium difficile–associated diarrhea or CDAD, AND faecal or faeces or fecal or feces or stool or microbiota, with infusion or transplant or transfer or instill or reconstitute or donor or bacteriotherapy. The search was limited to English-language publications.
Studies considered in this meta-analysis were prospective clinical trials that included a study population of patients with recurrent CDI who were treated with microbiota restoration via any delivery modality.
Data abstraction
Two investigators independently abstracted data to a predetermined collection form (S.K. and R.T.). Data collected for each study included study setting and design, year of publication, number of patients, patient characteristics, indication for FMT, FMT route, type of donor used for FMT, duration of follow-up, and outcomes. Discrepancies in data collection were resolved by consensus, referring to the original article.
Outcomes assessed
In our primary analysis, we calculated the clinical resolution rate with single microbiota restoration treatment with stool transplant or a live biotherapy in a controlled setting. We did not include patients treated with multiple MRTs after clinical failure with initial MRT in our primary analysis.
Statistical analysis
Our primary outcome of the pooled analysis was clinical cure rates. The random-effects model described by DerSimonian and Laird was used to calculate the weighted pooled rate (WPR). 5 We calculated WPRs with corresponding 95% confidence interval (CI) for the overall analysis as well as subgroup analyses. Data were weighted on sample size in each trial to calculate WPR. We assessed heterogeneity within groups with the I2 statistic, which estimates the proportion of total variation across studies that is due to heterogeneity in study patients, design, or interventions rather than chance; I2 values > 50% suggest substantial heterogeneity. All p values reported are two-tailed. For all tests (except for heterogeneity), a p value of <0.05 was considered statistically significant. Calculations were performed and graphs constructed with MetaXL meta-analysis software (version 5.3; EpiGear International Pty Ltd, Sunrise Beach, Queensland, Australia).
Risk of bias assessment
We use the Cochrane collaboration risk of bias tool to assess the methodologic quality of the included trials. 6 The Cochrane risk of bias tool consists of fixed domains of bias that focus on aspects of trial design, reporting, and conduct. The items assessed using this tool included methods used to generate the randomization schedule and conceal allocation, blinding, completeness of outcome data, and evidence of selective outcome reporting.
Results
Search results
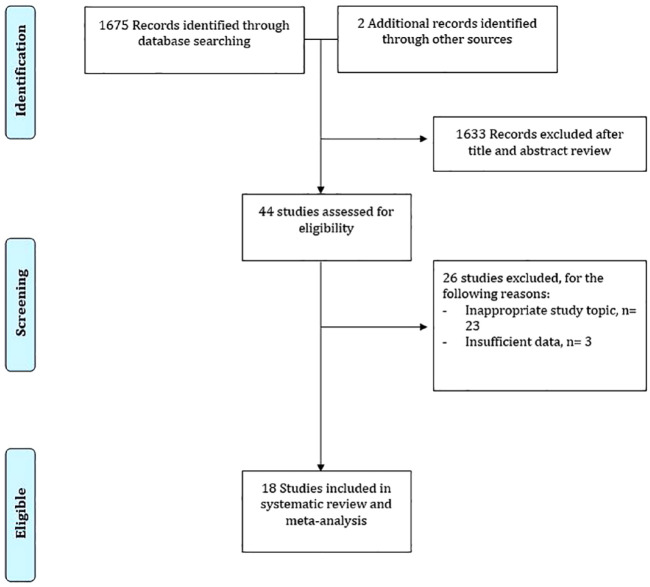
We found a total of 1677 unique studies using the described search strategies. The titles and abstracts were screened for all the studies and a total of 44 relevant articles were selected. Of the 44 relevant articles, we excluded 26 for various reasons and included a total of 18 studies in the final meta-analysis (Figure 1).
Figure 1.
Detailed search strategy for inclusion of studies.
Characteristics of the included studies
In all, 19 clinical trials reported in 18 studies with 1176 recurrent CDI patients were included.2,7–23 Of the included trials, 10 had a control arm and for the remaining 9, all patients received a microbiota restoration therapy as open label. For trials with a control arm, six trials used antibiotics followed by placebo and three used standard antibiotics (vancomycin or fidaxomicin) only. Data from two RBX2660 trials have been presented as a combined report and was included as a single study. Follow-up ranged from 8 to 24 weeks. The characteristics of the included studies are described in Table 1.
Table 1.
Characteristics of the included studies.
| Study | Study design | Sample size | Location | Indication of FMT | Time period | Microbiota delivery modality | Type of MRT | Type of control group | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Van Nood et al. 7 | Open-label randomized trial with control arm | 42 (FMT group: 16) | The Netherlands | Recurrent CDI | January 2008 –April 2010 | Nasoduodenal tube | Infusion of donor feces | Standard vancomycin regimen with or without bowel lavage | 10 weeks |
| Cammarota et al. 8 | Open-label randomized trial with control arm | 39 (FMT group:20) | Italy | Recurrent CDI | July 2013–June 2014 | Colonoscopy | Fresh stool | Vancomycin taper | 10 weeks |
| Kelly et al. 9 | Double-blind randomized trial with control arm | 46 (FMT group:22) | USA | Recurrent CDI | November 2012–March 2015 | Colonoscopy | Donor stool | FMT with patient’s own stool | 8 weeks |
| Hota et al. 10 | Open-label randomized control trial with control arm | 30 (FMT group:16) | Canada | Recurrent CDI | NA | Enema | Fresh stool | Vancomycin taper | 120 days |
| McGovern et al. 2 | Double-blind randomized trial with control arm (phase II) | 89 (59: SER-109; 30: placebo) | USA | Recurrent CDI | May 2015–October 2016 | Oral | SER-109 | Placebo | 8 weeks |
| Hvas et al. 12 | Open-label randomized trial with control arm | 64 (FMT group: 24) | Denmark | Recurrent CDI | April 2016–June 10, 2018 | Colonoscopy or nasojejunal tube | Frozen stool | Fidaxomicin or vancomycin | 8 weeks |
| Feuerstadt et al. 11 | Double-blind randomized placebo-controlled trial (phase III) | 182 (89 in SER-109 group) | USA and Canada | Recurrent CDI | July 2017–September 2020 | Oral | SER-109 | Placebo | 16 weeks |
| RBX2660 | Double-blind randomized trial with control group (phase II and phase III) | 352 (221 received RBX2660) | USA | Recurrent CDI | NA | Enema | RBX2660 | Placebo | 8 weeks |
| Louie et al. 14 | Randomized double-blind trial with control arm | 78 | UK | Recurrent CDI | NA | Oral | V303 | Placebo | 24 |
| Youngster et al. 15 | Open-label trial | 20 | USA | Recurrent + refractory CDI | NA | 6/10 nasogastric tube8/10 colonoscopy | Frozen stool | 8 weeks | |
| Youngster et al. 16 | Open-label trial | 20 | USA | Recurrent + refractory CDI | August 2013–June 2014 | Oral | Frozen stool | 8 weeks | |
| Kao et al. 17 | Open-label trial | 116 | Canada | Recurrent CDI | October 2014–September 2016 | Oral/colonoscopy | Frozen stool | 12 weeks | |
| Orenstein et al. 18 | Open-label trial | 31 | USA | Recurrent CDI | August 2013–December 2013 | Enema | RBX2660 | 8 weeks | |
| Lee et al. 19 | Open-label trial | 178 | Canada | Recurrent + refractory CDI | July 2012–September 2014 | Enema | Frozen stoolFresh stool | 13 weeks | |
| Khanna et al. 20 | Open-label trial | 30 | USA | Recurrent CDI | NA | Oral | SER-109 | 8 weeks | |
| Jiang et al. 22 | Open-label trial | 72 | USA | Recurrent CDI | September 2013–April 2016 | Colonoscopy | Fresh stoolFrozen stoolLyophilized stool | 8 weeks | |
| Jiang et al. 21 | Open-label trial | 65 | USA | Recurrent CDI | NA | Oral and enema | Lyophilized stool | 8 weeks | |
| Allegretti et al. 23 | Open-label trial | 132 | USA and Canada | Recurrent CDI | NA | Oral | CP101 | 24 weeks |
CDI, Clostridioides difficile infection; NA, not applicable.
Risk of bias
The risk of bias assessment for all included studies is described in Table 2. All trials had appropriate reporting and incomplete outcome data assessment. All randomized controlled trials (RCTs) used appropriate methods for random sequence generation. All open-label trials were considered as moderate bias due to lack of blinding and random sequence generation (Table 2).
Table 2.
Risk of bias assessment in the included studies.
| Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data addressed | Selective reporting |
|---|---|---|---|---|---|---|
| Van Nood et al. 7 | + | + | – | – | + | + |
| Cammarota et al. 8 | + | + | – | – | + | + |
| Kelly et al. 9 | + | + | + | – | + | + |
| Hota et al. 10 | + | + | + | – | + | + |
| McGovern et al. 2 | + | + | – | – | + | + |
| Hvas et al. 12 | + | + | + | – | + | + |
| Feuerstadt et al. 11 | NA | NA | – | – | + | + |
| RBX 2660 | NA | NA | – | – | + | + |
| Louie et al. 14 | NA | NA | – | – | + | + |
| Youngster 15 | NA | NA | – | – | + | + |
| Youngster 16 | NA | NA | – | – | + | + |
| Kao et al.17 | NA | NA | – | – | + | + |
| Orenstein et al. 18 | NA | NA | – | – | + | + |
| Kao et al. | NA | NA | – | – | + | + |
| Orenstein et al. 18 | NA | NA | – | – | + | + |
| Lee et al. 19 | NA | NA | – | – | + | + |
| Khanna et al. 20 | NA | NA | – | – | + | + |
| Jiang et al. 22 | NA | NA | – | – | + | + |
| Jiang et al. 21 | NA | NA | – | – | + | + |
| Allegretti et al. 23 | NA | NA | – | – | + | + |
NA, not applicable.
Clinical cure with single MRT
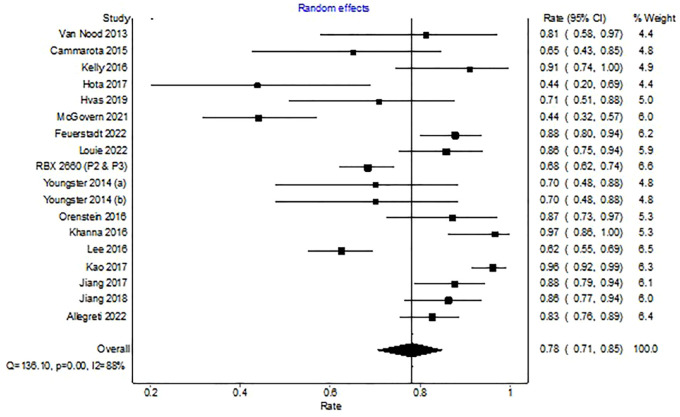
In the 19 trials reporting on 1176 patients treated with a single microbiota restoration therapy, 897 experienced a clinical cure overall (WPR, 78%; 95% CI, 71–85%). There was significant heterogeneity among studies with an I2 of 88% (Figure 2).
Figure 2.
Forest plot depicting clinical resolution with microbiota replacement among clinical trials.
Clinical cure with MRT in trials with a control arm
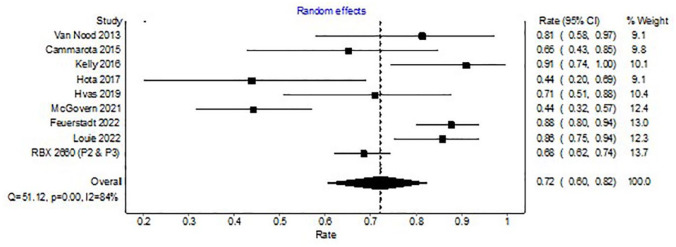
Analysis of 10 trials with a control arm (non-microbiota restoration) revealed CDI resolution in 373 of 523 patients (WPR, 72%; 95% CI, 60–82%) with microbiota restoration. There was significant heterogeneity among the included studies with an I2 of 84% (Figure 3).
Figure 3.
Forest plot depicting clinical resolution with microbiota replacement among clinical trials with control arm.
Clinical cure in control arm
Analysis of the 10 trials with non-microbiota restoration revealed CDI resolution in 201 of 397 patients with antibiotics (WPR, 52%, 95% CI, 43–60%). There was significant heterogeneity among the included studies with an I2 of 61%.
Comparison of cure rates with microbiota restoration versus antibiotics showed higher cure rate with microbiota restoration [WPR 72%, (95% CI, 60–82%) versus 52% (95% CI, 43–60%); p < 0.0001].
Clinical cure with MRT in open-label trials
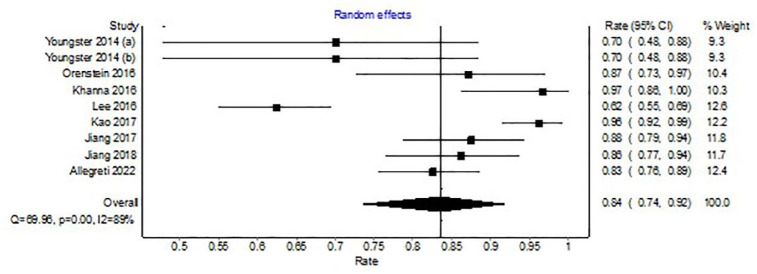
Among the nine open-label clinical trials, CDI resolution was seen in 524 of 653 patients after initial microbiota restoration (WPR, 84%, 95% CI, 74–92%). There was significant heterogeneity among the included studies with an I2 of 89% (Figure 4).
Figure 4.
Forest plot depicting clinical resolution with microbiota replacement among open-label trials.
Comparison of cure rates between clinical trials with a control arm and those without revealed a lower cure rate in trials with a control group [WPR; 72% (95% CI, 60–82%) versus 84% (95% CI, 74–92%); p < 0.0001].
Discussion
In this study, we demonstrate that the efficacy of microbiota restoration for recurrent CDI was lower in trials with a comparator group compared to open-label trials of MRT. Among the included trials, there was a noteworthy variation in methodology, control group, route of administration, and type of microbiota restoration therapy used. The cure rate in the control group receiving antibiotics only was significantly lower compared to microbiota restoration.
The low-efficacy rate noted in clinical trials with a comparator group likely stems from strict inclusion and exclusion criteria and strict definition of cure in controlled trials. A recent meta-analysis, including both observational studies and clinical trials (n = 45), found the efficacy of MRT to be 84% following a single dose and reported high-cure rates both in observational and controlled settings. However, it may be noted that the efficacy in subgroup analysis of clinical trials (open label and RCT) was noted to be 72% likely from lower cure rates in RCT. 24 Our pooled analysis with updated literature shows similar results.
We also found significant heterogeneity among the included trials. One study evaluated the heterogeneity among randomized clinical trials for MRT and found significant differences in study methodology, control groups, prior antibiotic treatment, number of FMT administrations, and time to clinical outcomes assessed. 25 All these heterogeneous aspects lead to differences in estimated efficacy rates as well as limiting the generalizability of the results.
Trials included in our study are foundational studies to access the efficacy of MRT in recurrent CDI. Future studies may consider accessing the use of MRT for severe and fulminant CDI. There have been studies regarding the use of FMT in severe and fulminant disease and a recent meta-analysis including 10 studies (8 case series, 1 case–control, and 1 randomized study) suggested that FMT was safe and effective for severe fulminant CDI. 26 In addition, there have been prior studies looking at the predictors of FMT failure from real-world data and found several predictors including old age, poor quality of bowel preparation, concurrent inflammatory bowel disease (IBD), and peri FMT use of non-CDI antibiotics. Given that most of the trials have excluded patients with concurrent IBD and patients taking antibiotics, it would be interesting to access the efficacy of MRT among these high-risk patients. 27
The strength of our study includes comprehensive literature review with large population from clinical trials. Our study has several limitations. There was lack of microbiome data in most of the trials; hence, we were not able to explore the effect of donor and recipient microbiome. Other factors that may have affected FMT include antibiotic exposure and prior hospitalizations. Those were not reported uniformly and calls for more uniform reporting of FMT trials.
Conclusion
In conclusion, data from open-label trials and observational studies suggest that while MRT is an effective option for recurrent CDI, results vary based on the study design. Newer data from clinical trials are extremely promising for the use of MRT for recurrent CDI. There are still opportunities for optimization of future trials which include boarder patient population, more consistent approach for the inclusion of patients with standardization of products, and universal follow-up durations.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848231174293 for Resolution rates in clinical trials for microbiota restoration for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis by Raseen Tariq, Darrell S. Pardi and Sahil Khanna in Therapeutic Advances in Gastroenterology
Acknowledgments
None.
Footnotes
ORCID iD: Sahil Khanna  https://orcid.org/0000-0002-7619-8338
https://orcid.org/0000-0002-7619-8338
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Raseen Tariq, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA.
Darrell S. Pardi, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA
Sahil Khanna, Division of Gastroenterology and Hepatology, Mayo Clinic, 200 1st Street SW, Rochester, MN 55905, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Raseen Tariq: Conceptualization; Data curation; Writing – original draft.
Darrell S. Pardi: Writing—review & editing.
Sahil Khanna: Conceptualization; Data curation; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: S.K. receives research support from Rebioitx/Ferring, Vedanta, Finch, Seres, and Pfizer. He serves as a consultant for Probiotech, Takeda, Niche, and Immuron. D.S.P. has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda. He has consulted for Vedanta, Seres, AbbVie, Immunic, and Otsuka.
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
References
- 1.Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the infectious diseases society of America (IDSA) and society for healthcare epidemiology of America (SHEA): 2021 focused update guidelines on management of clostridioides difficile infection in adults. Clin Infect Dis 2021; 73: e1029–e1044. [DOI] [PubMed] [Google Scholar]
- 2.McGovern BH, Ford CB, Henn MR, et al. SER-109, an investigational microbiome drug to reduce recurrence after clostridioides difficile infection: lessons learned from a phase 2 trial. Clin Infect Dis 2021; 72: 2132–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tariq R, Pardi DS, Bartlett MG, et al. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent clostridium difficile infection: a systematic review and meta-analysis. Clin Infect Dis 2019; 68: 1351–1358. [DOI] [PubMed] [Google Scholar]
- 4.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DerSimonian R, Laird N.Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368: 407–415. [DOI] [PubMed] [Google Scholar]
- 8.Cammarota G, Ianiro G, Gasbarrini A.Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol 2014; 48: 693–702. [DOI] [PubMed] [Google Scholar]
- 9.Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent clostridium difficile infection: a randomized trial. Ann Intern Med 2016; 165: 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hota SS, Sales V, Tomlinson G, et al. Oral vancomycin followed by fecal transplantation versus tapering oral vancomycin treatment for recurrent clostridium difficile infection: an open-label, randomized controlled trial. Clin Infect Dis 2017; 64: 265–271. [DOI] [PubMed] [Google Scholar]
- 11.Feuerstadt P, Louie TJ, Lashner B, et al. SER-109, an oral microbiome therapy for recurrent clostridioides difficile infection. N Engl J Med 2022; 386: 220–229. [DOI] [PubMed] [Google Scholar]
- 12.Hvas CL, Dahl Jorgensen SM, Jorgensen SP, et al. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent clostridium difficile infection. Gastroenterology 2019; 156: 1324–1332. [DOI] [PubMed] [Google Scholar]
- 13.Khanna S, Assi M, Lee C, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent clostridioides difficile infection. Drugs 2022; 82: 1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louie TJ, Golan Y, Khanna S, et al. 109: An 8-strain, rationally defined bacterial consortium, Ve303, reduces the risk of clostridioides difficile infection (Cdi) recurrence compared with placebo in adults at high risk for recurrence: results of the phase 2 consortium study. Gastroenterology 2022; 162: S-21. [Google Scholar]
- 15.Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis 2014; 58: 1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youngster I, Russell GH, Pindar C, et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014; 312: 1772–1778. [DOI] [PubMed] [Google Scholar]
- 17.Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent clostridium difficile infection: a randomized clinical trial. JAMA 2017; 318: 1985–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orenstein R, Dubberke E, Hardi R, et al. Safety and durability of RBX2660 (Microbiota Suspension) for recurrent clostridium difficile infection: results of the PUNCH CD study. Clin Infect Dis 2016; 62: 596–602. [DOI] [PubMed] [Google Scholar]
- 19.Lee CH, Steiner T, Petrof EO, et al. Frozen vs Fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent clostridium difficile infection: a randomized clinical trial. JAMA 2016; 315: 142–149. [DOI] [PubMed] [Google Scholar]
- 20.Khanna S, Pardi DS, Kelly CR, et al. A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent clostridium difficile infection. J Infect Dis 2016; 214: 173–181. [DOI] [PubMed] [Google Scholar]
- 21.Jiang ZD, Jenq RR, Ajami NJ, et al. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: a randomized clinical trial. PLoS One 2018; 13: e0205064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang ZD, Ajami NJ, Petrosino JF, et al. Randomised clinical trial: faecal microbiota transplantation for recurrent Clostridium difficile infection - fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment Pharmacol Ther 2017; 45: 899–908. [DOI] [PubMed] [Google Scholar]
- 23.Allegretti JR, Kelly CR, Fischer M, et al. Tu1519: CP101, An Investigational Microbiome Therapeutic for the Prevention of Recurrent C. Difficile infection: a combined analysis of the Prism3 (Randomized Placebo-Controlled) and prism-ext (open-label) trials. Gastroenterology 2022; 162: S-995–S-996. [Google Scholar]
- 24.Baunwall SMD, Lee MM, Eriksen MK, et al. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis. EClinicalMedicine 2020; 29–30: 100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feuerstadt P, Aroniadis OC, Svedlund FL, et al. Heterogeneity of randomized controlled trials of fecal microbiota transplantation in recurrent clostridioides difficile infection. Dig Dis Sci 2022; 67: 2763–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song YN, Yang DY, Veldhuyzen van Zanten S, et al. Fecal microbiota transplantation for severe or fulminant clostridioides difficile infection: systematic review and meta-analysis. J Can Assoc Gastroenterol 2022; 5: e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beran A, Sharma S, Ghazaleh S, et al. Predictors of fecal microbiota transplant failure in clostridioides difficile infection: an updated meta-analysis. J Clin Gastroenterol 2023; 57: 389–399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848231174293 for Resolution rates in clinical trials for microbiota restoration for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis by Raseen Tariq, Darrell S. Pardi and Sahil Khanna in Therapeutic Advances in Gastroenterology