Abstract
Background
Compared with adults with normal glucose metabolism, those with prediabetes tend to be frail. However, it remains poorly understood whether frailty could identify adults who are most at risk of adverse outcomes related to prediabetes.
Objective
We aimed to systematically evaluate the associations between frailty, a simple health indicator, and risks of multiple adverse outcomes including incident type 2 diabetes mellitus (T2DM), diabetes-related microvascular disease, cardiovascular disease (CVD), chronic kidney disease (CKD), eye disease, dementia, depression, and all-cause mortality in late life among middle-aged adults with prediabetes.
Methods
We evaluated 38,950 adults aged 40 years to 64 years with prediabetes using the baseline survey from the UK Biobank. Frailty was assessed using the frailty phenotype (FP; range 0-5), and participants were grouped into nonfrail (FP=0), prefrail (1≤FP≤2), and frail (FP≥3). Multiple adverse outcomes (ie, T2DM, diabetes-related microvascular disease, CVD, CKD, eye disease, dementia, depression, and all-cause mortality) were ascertained during a median follow-up of 12 years. Cox proportional hazards regression models were used to estimate the associations. Several sensitivity analyses were performed to test the robustness of the results.
Results
At baseline, 49.1% (19,122/38,950) and 5.9% (2289/38,950) of adults with prediabetes were identified as prefrail and frail, respectively. Both prefrailty and frailty were associated with higher risks of multiple adverse outcomes in adults with prediabetes (P for trend <.001). For instance, compared with their nonfrail counterparts, frail participants with prediabetes had a significantly higher risk (P<.001) of T2DM (hazard ratio [HR]=1.73, 95% CI 1.55-1.92), diabetes-related microvascular disease (HR=1.89, 95% CI 1.64-2.18), CVD (HR=1.66, 95% CI 1.44-1.91), CKD (HR=1.76, 95% CI 1.45-2.13), eye disease (HR=1.31, 95% CI 1.14-1.51), dementia (HR=2.03, 95% CI 1.33-3.09), depression (HR=3.01, 95% CI 2.47-3.67), and all-cause mortality (HR=1.81, 95% CI 1.51-2.16) in the multivariable-adjusted models. Furthermore, with each 1-point increase in FP score, the risk of these adverse outcomes increased by 10% to 42%. Robust results were generally observed in sensitivity analyses.
Conclusions
In UK Biobank participants with prediabetes, both prefrailty and frailty are significantly associated with higher risks of multiple adverse outcomes, including T2DM, diabetes-related diseases, and all-cause mortality. Our findings suggest that frailty assessment should be incorporated into routine care for middle-aged adults with prediabetes, to improve the allocation of health care resources and reduce diabetes-related burden.
Keywords: frailty, adverse outcomes, diabetes, prediabetes, prospective study
Introduction
In 2021, the International Diabetes Federation estimated that there were more than 500 million adults with prediabetes among those aged 20 years to 79 years worldwide [1]. As an intermediate hyperglycemia state, prediabetes increases the risk of diabetes [2] and diabetes-related complications (eg, cardiovascular disease [CVD], chronic kidney disease [CKD], and dementia) [3]; the latter contributes to a large proportion of diabetes-related burden [4,5]. The latest guidelines from the American Diabetes Association (ADA) recommend annual diabetes screening for adults with prediabetes [6]. However, this is challenged by emerging evidence showing the very low rates of diabetes progression among older adults with prediabetes [7]. Conversely, middle-aged adults (ie, <65 years) with prediabetes should be monitored for adverse outcomes, which is of high value and appropriate [8].
Prediabetes is highly heterogeneous, impeding the application of a one-size-fits-all health management strategy. Recently, a simple health aging indicator—frailty—has been demonstrated to be able to predict the risk of adverse outcomes (eg, CVD and mortality) [9-12] even in the younger population [13]. Frailty is defined as a state of decreased reserve and resistance to stressors, characterized by functional decline in multiple systems [9]. Frailty and disorders of glucose metabolism share common physiological mechanisms, such as insulin resistance [14,15] and chronic inflammation [15,16]. Frailty has been found to be an important risk factor for disability [17], fracture [18], CVD [19,20], hospitalization [20], intensive care unit admission [20], and mortality [20,21] among adults with diabetes. A few studies have shown that frailty incidence is slightly higher in older adults with prediabetes compared with those with normal glucose metabolism [22]. Only 1 prospective study recently reported that frailty was positively associated with the progression of prediabetes to type 2 diabetes mellitus (T2DM), as well as higher risks of CVD and all-cause mortality, in middle-aged and older adults with prediabetes [23]. However, whether these positive associations remain in those aged less than 65 years is not yet clear. In addition, impaired glucose metabolism is also associated with higher risks of CKD [3], eye disease (eg, cataract) [24], dementia [3], and depression [25]. However, relatively little is known about whether frailty could identify middle-aged adults with prediabetes who are most at risk of these adverse outcomes.
Therefore, we performed a prospective cohort study among 38,950 middle-aged adults with prediabetes from the UK Biobank (UKB). Using a widely validated frailty measurement—frailty phenotype (FP) [9]—the objective of this study was to systematically evaluate the associations of frailty with the risk of multiple adverse outcomes, including incident T2DM, diabetes-related microvascular disease, CVD, CKD, eye disease, dementia, depression, and all-cause mortality.
Methods
Study Participants
The UKB is a large-scale health research study with a long-term follow-up that began in 2006 to 2010 with the recruitment of approximately 500,000 adults in the United Kingdom [26]. Adults in the UKB were recruited through 22 assessment centers across England, Scotland, and Wales. Data were collected through a touch screen questionnaire and verbal interviews (eg, demographic, health, lifestyle variables), physical measures (eg, handgrip strength), and biological sample collection (eg, blood). Since recruitment, all adults have given consent for the UKB to follow up to determine the incidence of health outcomes through links to health-related records (eg, hospital inpatient episodes and death registrations), and only about 0.3% of the adults have been lost to follow-up because they left the United Kingdom or withdrew consent for future linkage. The protocol of the UKB is available online [27]. At baseline, there were 405,319 middle-aged adults (age: 40-64 years), of whom 43,133 had prediabetes. Prediabetes was defined by a nonfasting glycated hemoglobin (HbA1c) level of 5.7% to 6.4% (39-47 mmol/mol) following the ADA criteria [6]. After the exclusion of adults with prevalent cancer (n=2386) or with missing data on frailty (n=6) and covariates (eg, ethnicity, educational level; n=1791), 38,950 middle-aged adults with prediabetes were included in the final analytic samples. Additionally, because the number of prevalent cases for each outcome varied, we assembled different analytic samples for each outcome (see details in Figure 1).
Figure 1.
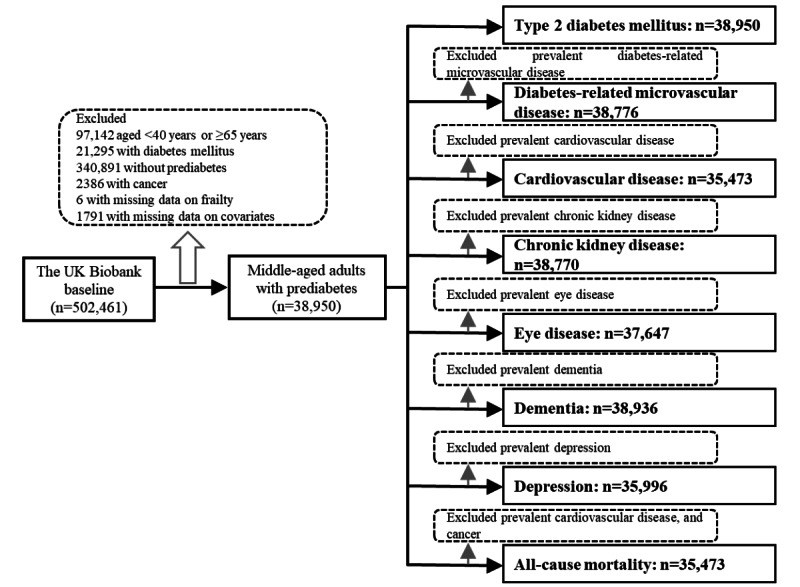
Flow chart of the sample for analyses.
Ethical Considerations
The UKB was approved by the North West Multi-Centre Research Ethics Committee (11/NW/0382). Written informed consent from all participants was obtained. The data used in this study were anonymized and de-identified for privacy and confidentiality protection.
Outcomes
In this study, the outcomes included T2DM, diabetes-related microvascular disease (including retinopathy, neuropathy, and nephropathy), diabetes-related macrovascular disease (ie, CVD including ischemic heart disease and stroke), CKD, eye disease (including cataract and glaucoma), dementia, depression, and all-cause mortality.
We defined prevalent and incident T2DM using a UKB algorithm that combined self-reported medical history and medication information (for the ascertainment of prevalent cases only), as well as linked hospital admissions records (Table S1 in Multimedia Appendix 1). In addition, according to the ADA criteria [6], undiagnosed prevalent T2DM cases were identified using random glucose (≥11.1 mmol/L) or HbA1c (≥6.5% [48 mmol/mol]) levels. We ascertained prevalent and incident cases of diabetes-related microvascular disease through linked hospital admissions records using the International Statistical Classification of Diseases and Related Health Problems, 9th version (ICD-9) and 10th version (ICD-10; Table S1 in Multimedia Appendix 1). We ascertained prevalent and incident cases of CVD, CKD, eye disease, dementia, and depression using self-reported medical history (for the ascertainment of prevalent cases only) and linked hospital admissions records using ICD-9 and ICD-10 (Table S1 in Multimedia Appendix 1). We ascertained death through linkage to national death registries. For analyses of each outcome, the time to event was calculated from the baseline (ie, the years 2006-2010) to the occurrence of the specific disease outcome, death, loss to follow-up, or end of follow-up (the year 2021), whichever came first. For instance, for analysis of incident T2DM, time to event was calculated from the baseline to the occurrence of T2DM, death, loss to follow-up, or end of follow-up, whichever came first.
Frailty Measurement
We used FP, a widely used physical frailty measurement proposed by Fried et al [9]. FP was evaluated using 5 criteria (unintentional weight loss, exhaustion, weakness, slow gait speed, and low physical activity) and was used previously in the UKB [28]. Of the 5 criteria, weakness was assessed using objectively measured handgrip strength; the other 4 criteria were assessed using a self-reported questionnaire (see details in Table 1). The FP score ranged from 0 to 5, with a higher score indicating greater frailty. Participants were categorized into nonfrail (FP score=0), prefrail (FP score≥1 and ≤2), and frail (FP score≥3), as done in previous studies [9,28].
Table 1.
The 5 criteria for the frailty phenotype in the UK Biobank.
| Number | Criteria description | Categories |
| 1 | Unintentional weight loss: Participants were asked “Compared with one year ago, has your weight changed?” | 1: “Yes, loss weight”; 0: Others |
| 2 | Exhaustion: Participants were asked “Over the past 2 weeks, how often have you felt tired or had little energy?” | 1: “More than half the days or nearly every day”; 0: Others |
| 3 | Weakness: Weakness was measured using grip strength with a Jamar J00105 hydraulic hand dynamometer (Lafayette Instrument). Participants were asked to complete a grip assessment for both hands once. The maximal value of the right and left hands was used. | 1: (1) Men: ≤29 kg for BMI ≤24 kg/m2; ≤30 kg for BMI 24.1-26 kg/m2; ≤30 kg for BMI 26.1-28 kg/m2; or ≤32 kg for BMI >28 kg/m2; (2) Women: ≤17 kg for BMI ≤23 kg/m2; ≤17.3 kg for BMI 23.1-26 kg/m2; ≤18 kg for BMI 26.1-29 kg/m2; or ≤21 kg for BMI >29 kg/m2; 0: Others |
| 4 | Slow gait speed: Participants were asked “How would you describe your usual walking pace?” | 1: “Slow pace”; 0: Others |
| 5 | Low physical activity: Participants were asked “In the last 4 weeks, did you spend any time doing light DIYa activity, heavy DIY activity, or strenuous sports?” | 1: “None or light activity with a frequency of once per week or less”; 0: Others |
aDIY: do it yourself.
Covariates
Baseline data on age, sex (female or male), ethnicity (White, mixed race, South Asian, Black, Chinese, or other background), educational level (high, intermediate, or low), occupational status (working, retired, or other), alcohol consumption (never or special occasions only, 1 to 3 times per month, 1 to 4 times per week, or daily or almost daily), smoking status (never, previous smoker, or current smoker), healthy diet (yes or no), and family history of disease (including diabetes, CVD, dementia, and depression) were collected through a questionnaire interview. The Townsend deprivation index (TDI) was calculated based on areas before participants were recruited in the UKB. BMI was calculated as measured weight/height2 (kg/m2).
Statistical Analyses
Baseline characteristics of the complete analyzed sample and by frailty status are presented as median (IQRs) and number (percentage) for continuous variables and categorical variables, respectively. Kruskal-Wallis tests and chi-square tests were used to compare the differences in characteristics by frailty status.
To evaluate the associations between frailty status (nonfrail, prefrail, and frail) and adverse outcomes, Cox proportional hazards regression models were performed. The Schoenfeld residuals test was used to verify the proportional hazard assumption, and no significant violation was found. We calculated hazard ratios (HRs) and corresponding 95% CIs using 2 models. Model 1 was adjusted for age and sex. Model 2 was further adjusted for ethnicity, educational level, occupational status, TDI, alcohol consumption, smoking status, healthy diet, BMI, and family history of disease based on Model 1. Additionally, we calculated HRs (95% CIs) for adverse outcomes per 1-point increase in FP score.
Several sensitivity analyses were conducted to confirm the robustness of the results. First, we compared the characteristics of included and excluded study participants. Second, to minimize the influence of reverse causality, we repeated the main analyses after excluding those without 2 years of follow-up. Third, to reduce the influence of poor health on frailty status, we repeated the main analyses after excluding participants with poor self-rated health status at baseline. Fourth, to account for the influence of missing data on results, we performed multiple imputations by chained equations [29] for missing values and repeated the primary analyses. Finally, we validated the associations between frailty and adverse outcomes among adults with T2DM. For adults with T2DM, HbA1c level (≥7.0% [≥53 mmol/mol] or <7.0% [<53 mmol/mol]), diabetes medication use (oral antidiabetes drug only, insulin, or neither), diabetes duration (in years), and prevalent diabetes-related microvascular disease (except for incident diabetes-related microvascular disease) were also included in Model 2.
We used SAS version 9.4 (SAS Institute) and R version 3.6.3 (2020-02-29) to conduct all statistical analyses. To account for multiple testing, we used Bonferroni correction in all analyses (P<.006).
Results
Baseline Characteristics
Among the 38,950 participants with prediabetes, the median age was 58.6 (IQR 53.1-62.0) years, and the majority were women (21,155/38,950, 54.3%) and White (34,705/38,950, 89.1%; Table 2). The prevalences of prefrailty and frailty were 49.1% (19,122/38,950) and 5.9% (2289/38,950), respectively. Prefrail and frail adults were more likely to be women, have a lower educational level, and have a higher level of TDI and BMI, compared with the nonfrail adults. Table 2 shows the detailed baseline characteristics by frailty status.
Table 2.
Baseline characteristics of study participants with prediabetes by frailty status.
| Variables | Total (n=38,950) | Nonfrail (n=17,539) | Prefrail (n=19,122) | Frail (n=2289) | P valuea | |
| Age (years), median (IQR) | 58.6 (53.1 to 62.0) | 59.0 (53.7 to 62.1) | 58.3 (52.7 to 61.8) | 58.3 (52.9 to 61.7) | <.001 | |
| Gender, n (%) | <.001 | |||||
|
|
Female | 21,155 (54.3) | 8928 (50.9) | 10,771 (56.3) | 1456 (63.6) |
|
|
|
Male | 17,795 (45.7) | 8611 (49.1) | 8351 (43.7) | 833 (36.4) |
|
| Ethnicity, n (%) | <.001 | |||||
|
|
White | 34,705 (89.1) | 16,075 (91.7) | 16,719 (87.4) | 1911 (83.5) |
|
|
|
Mixed | 339 (0.9) | 137 (0.8) | 180 (0.9) | 22 (1.0) |
|
|
|
South Asian | 1558 (4.0) | 449 (2.6) | 943 (4.9) | 166 (7.3) |
|
|
|
Black | 1474 (3.8) | 563 (3.2) | 796 (4.2) | 115 (5.0) |
|
|
|
Chinese | 261 (0.7) | 104 (0.6) | 139 (0.7) | 18 (0.8) |
|
|
|
Other background | 613 (1.6) | 211 (1.2) | 345 (1.8) | 57 (2.5) |
|
| Educational levelb, n (%) | <.001 | |||||
|
|
High | 11,198 (28.7) | 5647 (32.2) | 5156 (27.0) | 395 (17.3) |
|
|
|
Intermediate | 12,464 (32.0) | 5728 (32.7) | 6165 (32.2) | 571 (24.9) |
|
|
|
Low | 15,288 (39.3) | 6164 (35.1) | 7801 (40.8) | 1323 (57.8) |
|
| Occupational status, n (%) | <.001 | |||||
|
|
Working | 23,793 (61.1) | 11,059 (63.1) | 11,892 (62.2) | 842 (36.8) |
|
|
|
Retired | 10,407 (26.7) | 5095 (29.0) | 4710 (24.6) | 602 (26.3) |
|
|
|
Other | 4750 (12.2) | 1385 (7.9) | 2520 (13.2) | 845 (36.9) |
|
| Townsend deprivation index, median (IQR) | –1.7 (–3.5 to 1.2) | –2.2 (–3.7 to 0.3) | –1.4 (–3.2 to 1.6) | 0.5 (–2.3 to 3.6) | <.001 | |
| BMI (kg/m2), median (IQR) | 28.5 (25.4 to 32.1) | 27.5 (24.8 to 30.8) | 29.2 (25.9 to 32.9) | 31.6 (27.8 to 36.4) | <.001 | |
| Smoking status, n (%) | <.001 | |||||
|
|
Never | 19,301 (49.6) | 8963 (51.1) | 9366 (49.0) | 972 (42.5) |
|
|
|
Previous | 12,788 (32.8) | 5929 (33.8) | 6137 (32.1) | 722 (31.5) |
|
|
|
Current | 6861 (17.6) | 2647 (15.1) | 3619 (18.9) | 595 (26.0) |
|
| Alcohol consumption, n (%) | <.001 | |||||
|
|
Never or special occasions only | 9939 (25.5) | 3308 (18.9) | 5551 (29.0) | 1080 (47.2) |
|
|
|
1 to 3 times per month | 4919 (12.6) | 2045 (11.7) | 2587 (13.5) | 287 (12.5) |
|
|
|
1 to 4 times per week | 17,545 (45.0) | 8674 (49.5) | 8176 (42.8) | 695 (30.4) |
|
|
|
Daily or almost daily | 6547 (16.8) | 3512 (20.0) | 2808 (14.7) | 227 (9.9) |
|
| Healthy diet, n (%) | <.001 | |||||
|
|
No | 9146 (23.5) | 3444 (19.6) | 4930 (25.8) | 772 (33.7) |
|
|
|
Yes | 29,804 (76.5) | 14,095 (80.4) | 14,192 (74.2) | 1517 (66.3) |
|
| Glycated hemoglobin (mmol/mol), median (IQR) | 40.4 (39.6 to 42.0) | 40.3 (39.5 to 41.6) | 40.5 (39.6 to 42.1) | 40.9 (39.8 to 42.6) | <.001 | |
| Prevalent diseases, n (%) | ||||||
|
|
Cardiovascular disease | 3477 (8.9) | 1157 (6.6) | 1835 (9.6) | 485 (21.2) | <.001 |
|
|
Chronic kidney disease | 180 (0.5) | 55 (0.3) | 88 (0.5) | 37 (1.6) | <.001 |
|
|
Eye disease | 1303 (3.3) | 520 (3.0) | 646 (3.4) | 137 (6.0) | <.001 |
|
|
Dementia | 14 (0.0) | 4 (0.0) | 7 (0.0) | 3 (0.1) | .37 |
|
|
Depression | 2954 (7.6) | 812 (4.6) | 1636 (8.6) | 506 (22.1) | <.001 |
| Family history, n (%) | ||||||
|
|
Diabetes mellitus | 11,197 (28.7) | 4716 (26.9) | 5720 (29.9) | 761 (33.2) | <.001 |
|
|
Cardiovascular disease | 23,633 (60.7) | 10,448 (59.6) | 11,711 (61.2) | 1474 (64.4) | <.001 |
|
|
Dementia | 4733 (12.2) | 2164 (12.3) | 2283 (11.9) | 286 (12.5) | .44 |
|
|
Depression | 5146 (13.2) | 2087 (11.9) | 2637 (13.8) | 422 (18.4) | <.001 |
aGenerated using chi-square and Kruskal-Wallis tests for categorical and continuous variables, respectively.
bEducational level was classified as high (college or university degree), intermediate (A/AS levels or equivalent, O levels/General Certificate of Secondary Education levels or equivalent), and low (none of the above).
Frailty and Risks of Adverse Outcomes in Middle-aged Adults With Prediabetes
During a median follow-up of 12 years, there were 5289 incident T2DM cases, 2657 incident diabetes-related microvascular disease cases, 3234 incident CVD cases, 1439 incident CKD cases, 3525 incident eye disease cases, 325 incident dementia cases, 1265 incident depression cases, and 2016 deaths. We found that frail participants developed more adverse outcomes than did their prefrail and nonfrail counterparts over the 12-year follow-up (Figure 2).
Figure 2.
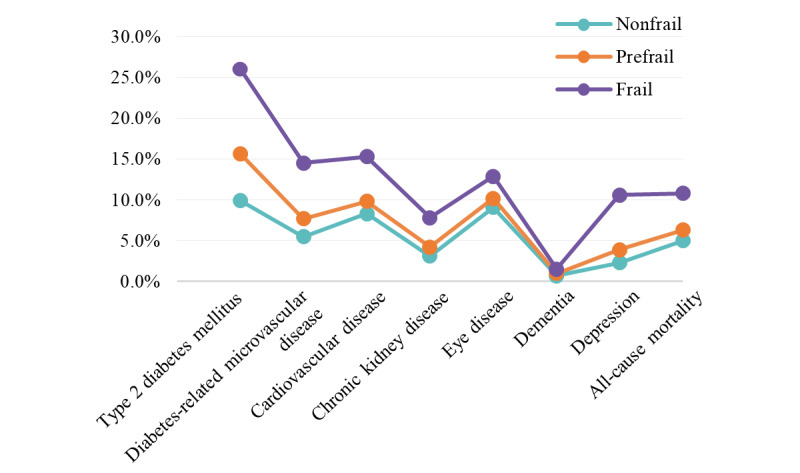
Age-adjusted incidence of adverse outcomes among UKB participants with prediabetes during 12 years of follow-up. UKB: UK Biobank.
Table 3 shows the associations between frailty and the risks of multiple adverse outcomes in middle-aged adults with prediabetes. In the age- and sex-adjusted model, both prefrailty and frailty were associated with higher risks of all adverse outcomes (all P for trend <.001). After further adjusting for additional covariates, these associations remained statistically significant. When comparing prefrail participants with their nonfrail counterparts, the multivariable-adjusted HRs were 1.35 (95% CI 1.27-1.43) for T2DM, 1.29 (95% CI 1.18-1.40) for diabetes-related microvascular disease, 1.17 (95% CI 1.08-1.26) for CVD, 1.22 (95% CI 1.09-1.37) for CKD, 1.12 (95% CI 1.04-1.20) for eye disease, 1.57 (95% CI 1.23-2.01) for dementia, 1.48 (95% CI 1.30-1.68) for depression, and 1.25 (95% CI 1.14-1.38) for all-cause mortality. For frail participants, the multivariable-adjusted HRs were 1.73 (95% CI 1.55-1.92) for T2DM, 1.89 (95% CI 1.64-2.18) for diabetes-related microvascular disease, 1.66 (95% CI 1.44-1.91) for CVD, 1.76 (95% CI 1.45-2.13) for CKD, 1.31 (95% CI 1.14-1.51) for eye disease, 2.03 (95% CI 1.33-3.09) for dementia, 3.01 (95% CI 2.47-3.67) for depression, and 1.81 (95% CI 1.51-2.16) for all-cause mortality, compared with their nonfrail counterparts. Additionally, with each 1-point increase in FP score, the incidence risks of these adverse outcomes significantly increased by 10% to 42% (Model 2).
Table 3.
Associations between frailty and adverse health outcomes among middle-aged adults with prediabetes.
| Outcomes | Frailty status | P value for trenda | Hazard ratio (HR) per 1-point increase | |||
|
|
Nonfrail | Prefrail | Frail | |||
| Type 2 diabetes mellitus (n=38,950) | ||||||
|
|
Number of events/person-years | 1724/207,929 | 2965/218,522 | 600/23,890 | —b | — |
|
|
Model 1c, HR (95% CI) | Reference | 1.70 (1.61-1.81) | 3.37 (3.07-3.71) | <.001 | 1.45 (1.42-1.49) |
|
|
Model 2d, HR (95% CI) | Reference | 1.35 (1.27-1.43) | 1.73 (1.55-1.92) | <.001 | 1.19 (1.16-1.23) |
| Diabetes-related microvascular disease (n=38,776) | ||||||
|
|
Number of events/person-years | 926/212,240 | 1417/226,956 | 314/25,445 | — | — |
|
|
Model 1c, HR (95% CI) | Reference | 1.54 (1.42-1.67) | 3.23 (2.84-3.68) | <.001 | 1.45 (1.40-1.50) |
|
|
Model 2d, HR (95% CI) | Reference | 1.29 (1.18-1.40) | 1.89 (1.64-2.18) | <.001 | 1.24 (1.19-1.29) |
| Cardiovascular disease (n=35,473) | ||||||
|
|
Number of events/person-years | 1314/195,009 | 1651/201,855 | 269/20,099 | — | — |
|
|
Model 1c, HR (95% CI) | Reference | 1.31 (1.22-1.41) | 2.39 (2.09-2.72) | <.001 | 1.29 (1.25-1.34) |
|
|
Model 2d, HR (95% CI) | Reference | 1.17 (1.08-1.26) | 1.66 (1.44-1.91) | <.001 | 1.16 (1.12-1.21) |
| Chronic kidney disease (n=38,770) | ||||||
|
|
Number of events/person-years | 513/213,304 | 758/228,894 | 168/25,929 | — | — |
|
|
Model 1c, HR (95% CI) | Reference | 1.47 (1.31-1.64) | 3.01 (2.53-3.58) | <.001 | 1.43 (1.36-1.50) |
|
|
Model 2d, HR (95% CI) | Reference | 1.22 (1.09-1.37) | 1.76 (1.45-2.13) | <.001 | 1.23 (1.16-1.30) |
| Eye disease (n=37,647) | ||||||
|
|
Number of events/person-years | 1470/202,556 | 1792/216,687 | 263/24,132 | — | — |
|
|
Model 1c, HR (95% CI) | Reference | 1.20 (1.12-1.29) | 1.62 (1.42-1.85) | <.001 | 1.17 (1.13-1.21) |
|
|
Model 2d, HR (95% CI) | Reference | 1.12 (1.04-1.20) | 1.31 (1.14-1.51) | <.001 | 1.10 (1.06-1.14) |
| Dementia (n=38,936) | ||||||
|
|
Number of events/person-years | 111/215,549 | 181/232,270 | 33/26,881 | — | — |
|
|
Model 1c, HR (95% CI) | Reference | 1.69 (1.34-2.15) | 2.87 (1.94-4.23) | <.001 | 1.41 (1.28-1.56) |
|
|
Model 2d, HR (95% CI) | Reference | 1.57 (1.23-2.01) | 2.03 (1.33-3.09) | <.001 | 1.29 (1.16-1.44) |
| Depression (n=35,996) | ||||||
|
|
Number of events/person-years | 387/204,125 | 687/209,605 | 191/19,994 | — | — |
|
|
Model 1c, HR (95% CI) | Reference | 1.71 (1.51-1.94) | 4.97 (4.18-5.92) | <.001 | 1.63 (1.55-1.71) |
|
|
Model 2d, HR (95% CI) | Reference | 1.48 (1.30-1.68) | 3.01 (2.47-3.67) | <.001 | 1.42 (1.34-1.50) |
| All-cause mortality (n= 35,473 ) | ||||||
|
|
Number of events/person-years | 783/195,777 | 1047/204,710 | 186/20,940 | — | — |
|
|
Model 1c, HR (95% CI) | Reference | 1.39 (1.27-1.53) | 2.65 (2.26-3.12) | <.001 | 1.35 (1.29-1.41) |
|
|
Model 2d, HR (95% CI) | Reference | 1.25 (1.14-1.38) | 1.81 (1.51-2.16) | <.001 | 1.21 (1.16-1.27) |
aCalculated to test linear trend using frailty status (3 categories) as a continuous variable.
bNot applicable.
cModel 1 was adjusted for age and sex.
dModel 2 was further adjusted for ethnicity, educational level, occupational status, Townsend deprivation index, alcohol consumption, smoking status, healthy diet, BMI, and family history of disease based on Model 1.
Sensitivity Analyses
The differences in characteristics between included and excluded participants were observed. Those who were excluded were more likely to be older, women, non-White, and frail (Table S2 in Multimedia Appendix 1). Robust results were generally observed when excluding the participants with less than 2 years of follow-up (Table S3 in Multimedia Appendix 1), excluding the participants with poor self-rated health status at baseline (Table S4 in Multimedia Appendix 1), or imputing missing data on frailty and covariates (Table S5 in Multimedia Appendix 1). In addition, we confirmed that frailty was positively associated with the risks of diabetes-related microvascular disease, CVD, CKD, eye diseases, dementia, depression, and all-cause mortality in middle-aged adults with T2DM, and these associations were independent of factors related to diabetes severity at baseline (Table S6 in Multimedia Appendix 1).
Discussion
Principal Findings
In a large sample of UKB participants with prediabetes, we, for the first time, demonstrated that both prefrailty and frailty were associated with higher risks of multiple adverse outcomes, including T2DM, diabetes-related microvascular disease, CVD, CKD, eye disease, dementia, depression, and all-cause mortality. Our findings support the heterogeneity of prediabetes in middle-aged adulthood and suggest that assessing frailty status among middle-aged adults with prediabetes may help to identify those who were most at risk of subsequent adverse outcomes.
We observed a nearly twice higher prevalence of frailty among middle-aged adults with prediabetes (ie, 5.9%) in this study than that in general adults (ie, 3.3%) from the UKB as well [28]. Similarly, the prevalence of frailty among older adults with diabetes [30] is almost twice as high as that in those without diabetes (20.1% vs 12%) [31]. It seems that adults with glucose metabolism disorders are experiencing an accelerated aging process [32]. Multiple age-related metabolic disturbances are present in adults with prediabetes, including chronic inflammation, hyperglycemia, insulin resistance, and β-cell dysfunction [2,16], creating a pathophysiological environment that contributes to frailty. Given the sharp increase in frailty after the age of 65 years [33], our findings suggest that there is a need for early identification of frailty, an aging indicator, in this middle-aged population with prediabetes.
To the best of our knowledge, this study provided new evidence on the associations between frailty and higher risks of a series of adverse outcomes in middle-aged adults with prediabetes. A few studies on the relationship between frailty and adverse outcomes included middle-aged adults with diabetes as part of the study sample [19,20,34]. One prospective study of 998 African Americans aged 49 years to 65 years has shown that frail adults with diabetes had an increased risk of mortality [21]. Except for this study, only 1 study conducted in middle-aged and older adults with prediabetes found that frailty was associated with the progression of prediabetes to diabetes, as well as higher risks of CVD and all-cause mortality [23]. This large prospective study (n=38,950) showed that frailty was positively associated with higher risks of more outcomes including CKD, eye disease, and dementia in middle-aged adults with prediabetes.
This study draws attention to the accelerated aging process in adults with prediabetes, which may lead to rapid diabetes progression and contribute to the development of diabetes-related complications [32]. Nutritional and pharmacological anti-aging interventions have been revealed to help mitigate or reverse the accelerated aging process [35]. A recent review suggested that the most effective and easiest intervention strategy targeting frailty is to combine strength exercise and protein supplements in primary care [36]. Thus, our findings implicate that frailty assessment might help primary care providers identify the subpopulation at higher risk of adverse outcomes even in middle-aged adults with prediabetes in communities. It is worth noting that the application of technological solutions in assessing frailty is constantly expanding [37,38]. The major types of technologies include information and telecommunications technology–based platforms, smartphones, remote monitoring, and wearable sensors and devices [39]. For example, a frailty prediction model based on a points system and integrated into a mobile app for Android phones has been developed in the clinical setting, enabling professionals to identify frailty using clinical information and further improve decision-making [40]. With the aid of these technological tools, frailty screening becomes more convenient and flexible. Next, early preventive and interventive programs targeting frailty in adults with prediabetes are urgently needed. On the one hand, they may directly help reduce the occurrence of T2DM; on the other hand, they may indirectly help reduce diabetes-related burden. Meanwhile, pharmacologic interventions or other aggressive approaches to diabetes prevention are also encouraged [41,42]. Before formal implementation, considerably more research on the effectiveness and cost-effectiveness of interventional programs in this population is required.
Strengths and Limitations
The major strengths of this study were the large sample of middle-aged adults with prediabetes, the long follow-up time, rich phenotype data, and linked hospital admissions records, enabling us to systematically evaluate the prospective associations between frailty and multiple adverse outcomes. There were several potential limitations. First, the UKB was not representative of the sampling population, and the majority of included adults were White. Also, there were differences in baseline characteristics between included and excluded participants. Thus, selection bias existed in this study, and the results may not be generalizable to populations from other countries. Second, transitions in frailty status may occur over time [43], and evidence has suggested that transitions in frailty status were associated with adverse outcomes [44]. However, repeated measurements of frailty were lacking; thus, we were unable to estimate the influence of frailty transitions on the subsequent adverse outcomes in this study. Future longitudinal studies incorporating data on frailty transition are needed. Third, multiple outcomes were considered in this study, and thus, type Ⅰ errors inevitably increased. To reduce the possibility of chance findings, we used Bonferroni correction. Finally, because of the observational study design, we could not draw a causal inference.
Conclusion
In this prospective cohort study of middle-aged UKB participants with prediabetes, both prefrailty and frailty were significantly associated with increased risks of multiple adverse outcomes, including T2DM, diabetes-related microvascular disease, CVD, CKD, eye disease, dementia, depression, and all-cause mortality. The findings underscore the importance of frailty assessment in routine care for middle-aged adults with prediabetes. Detecting frailty at an early stage (ie, accelerated aging) and implementing timely targeted interventions may help to improve the allocation of health care resources and to reduce diabetes-related burden.
Acknowledgments
This research was conducted using the UK Biobank resource under application number 61856. We wish to acknowledge the UK Biobank participants who formed the sample that made the data available. This study was supported by a grant from the National Natural Science Foundation of China (82171584); the Fundamental Research Funds for the Central Universities, Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province (2020E10004); and Zhejiang University Global Partnership Fund (188170-11103). TMG is supported by the Claude D. Pepper Older Americans Independence Center at Yale School of Medicine from the National Institute on Aging (P30AG021342) and the National Center for Advancing Translational Sciences (UL1TR001863). The funders had no role in the study design; data collection, analysis, or interpretation; in the writing of the report; or in the decision to submit the article for publication.
Abbreviations
- ADA
American Diabetes Association
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- FP
frailty phenotype
- HbA1c
glycated hemoglobin
- HR
hazard ratio
- ICD-9
International Statistical Classification of Diseases and Related Health Problems, 9th version
- ICD-10
the International Statistical Classification of Diseases and Related Health Problems, 10th version
- T2DM
type 2 diabetes mellitus
- TDI
Townsend deprivation index
- UKB
UK Biobank
Supplementary tables.
Data Availability
The data sets analyzed during this study are available at [45].
Footnotes
Conflicts of Interest: None declared.
References
- 1.IDF Diabetes Atlas. International Diabetes Federation. [2023-05-04]. https://diabetesatlas.org/
- 2.Tabák Adam G, Herder C, Rathmann W, Brunner EJ, Kivimäki Mika. Prediabetes: a high-risk state for diabetes development. Lancet. 2012 Jun 16;379(9833):2279–2290. doi: 10.1016/S0140-6736(12)60283-9. https://europepmc.org/abstract/MED/22683128 .S0140-6736(12)60283-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlesinger S, Neuenschwander M, Barbaresko J, Lang A, Maalmi H, Rathmann W, Roden M, Herder C. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: umbrella review of meta-analyses of prospective studies. Diabetologia. 2022 Feb;65(2):275–285. doi: 10.1007/s00125-021-05592-3. https://europepmc.org/abstract/MED/34718834 .10.1007/s00125-021-05592-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019 Jan;62(1):3–16. doi: 10.1007/s00125-018-4711-2. http://hdl.handle.net/10044/1/72664 .10.1007/s00125-018-4711-2 [DOI] [PubMed] [Google Scholar]
- 5.Whicher CA, O'Neill S, Holt RIG. Diabetes in the UK: 2019. Diabet Med. 2020 Feb;37(2):242–247. doi: 10.1111/dme.14225. [DOI] [PubMed] [Google Scholar]
- 6.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA, on behalf of the American Diabetes Association 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023 Jan 01;46(Suppl 1):S19–S40. doi: 10.2337/dc23-S002. https://europepmc.org/abstract/MED/36507649 .148056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rooney MR, Rawlings AM, Pankow JS, Echouffo Tcheugui JB, Coresh J, Sharrett AR, Selvin E. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med. 2021 Apr 01;181(4):511–519. doi: 10.1001/jamainternmed.2020.8774. https://europepmc.org/abstract/MED/33555311 .2775594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam K, Lee SJ. Prediabetes-a risk factor twice removed. JAMA Intern Med. 2021 Apr 01;181(4):520–521. doi: 10.1001/jamainternmed.2020.8773. https://europepmc.org/abstract/MED/33555307 .2775591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 10.Sergi G, Veronese N, Fontana L, De Rui M, Bolzetta F, Zambon S, Corti M, Baggio G, Toffanello ED, Crepaldi G, Perissinotto E, Manzato E. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol. 2015 Mar 17;65(10):976–983. doi: 10.1016/j.jacc.2014.12.040. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(15)00088-1 .S0735-1097(15)00088-1 [DOI] [PubMed] [Google Scholar]
- 11.Soysal P, Veronese N, Thompson T, Kahl KG, Fernandes BS, Prina AM, Solmi M, Schofield P, Koyanagi A, Tseng P, Lin P, Chu C, Cosco TD, Cesari M, Carvalho AF, Stubbs B. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res Rev. 2017 Jul;36:78–87. doi: 10.1016/j.arr.2017.03.005.S1568-1637(17)30024-7 [DOI] [PubMed] [Google Scholar]
- 12.Aguayo GA, Vaillant MT, Donneau A, Schritz A, Stranges S, Malisoux L, Chioti A, Guillaume M, Muller M, Witte DR. Comparative analysis of the association between 35 frailty scores and cardiovascular events, cancer, and total mortality in an elderly general population in England: An observational study. PLoS Med. 2018 Mar;15(3):e1002543. doi: 10.1371/journal.pmed.1002543. https://hdl.handle.net/2268/238181 .PMEDICINE-D-17-03490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan J, Yu C, Guo Y, Bian Z, Sun Z, Yang L, Chen Y, Du H, Li Z, Lei Y, Sun D, Clarke R, Chen J, Chen Z, Lv J, Li L, China Kadoorie Biobank Collaborative Group Frailty index and all-cause and cause-specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health. 2020 Dec;5(12):e650–e660. doi: 10.1016/S2468-2667(20)30113-4. https://linkinghub.elsevier.com/retrieve/pii/S2468-2667(20)30113-4 .S2468-2667(20)30113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clegg A, Hassan-Smith Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol. 2018 Sep;6(9):743–752. doi: 10.1016/S2213-8587(18)30110-4. https://eprints.whiterose.ac.uk/129306/ S2213-8587(18)30110-4 [DOI] [PubMed] [Google Scholar]
- 15.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006 May;29(5):1130–1139. doi: 10.2337/diacare.2951130.29/5/1130 [DOI] [PubMed] [Google Scholar]
- 16.Lü Qingguo, Tong N, Liu Y, Li N, Tang X, Zhao J, Cao H, Li D, Gou L, Zhang Y, Wan J, Jiang L. Community-based population data indicates the significant alterations of insulin resistance, chronic inflammation and urine ACR in IFG combined IGT group among prediabetic population. Diabetes Res Clin Pract. 2009 Jun;84(3):319–324. doi: 10.1016/j.diabres.2009.03.002.S0168-8227(09)00115-6 [DOI] [PubMed] [Google Scholar]
- 17.Castro-Rodríguez M, Carnicero JA, Garcia-Garcia FJ, Walter S, Morley JE, Rodríguez-Artalejo F, Sinclair AJ, Rodríguez-Mañas L. Frailty as a major factor in the increased risk of death and disability in older people with diabetes. J Am Med Dir Assoc. 2016 Oct 01;17(10):949–955. doi: 10.1016/j.jamda.2016.07.013.S1525-8610(16)30291-2 [DOI] [PubMed] [Google Scholar]
- 18.Li G, Prior JC, Leslie WD, Thabane L, Papaioannou A, Josse RG, Kaiser SM, Kovacs CS, Anastassiades T, Towheed T, Davison KS, Levine M, Goltzman D, Adachi JD, CaMos Research Group Frailty and risk of fractures in patients with type 2 diabetes. Diabetes Care. 2019 Apr;42(4):507–513. doi: 10.2337/dc18-1965.dc18-1965 [DOI] [PubMed] [Google Scholar]
- 19.Pandey A, Khan MS, Garcia K, Simpson F, Bahnson J, Patel KV, Singh S, Vaduganathan M, Bertoni A, Kitzman D, Johnson K, Lewis CE, Espeland MA. Association of baseline and longitudinal changes in frailty burden and risk of heart failure in type 2 diabetes-findings from the Look AHEAD Trial. J Gerontol A Biol Sci Med Sci. 2022 Dec 29;77(12):2489–2497. doi: 10.1093/gerona/glac094. https://europepmc.org/abstract/MED/35453142 .6572670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao C, Wang J, Chien K, COhort of GEriatric Nephrology in NTUH (COGENT) study group Both pre-frailty and frailty increase healthcare utilization and adverse health outcomes in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2018 Sep 27;17(1):130. doi: 10.1186/s12933-018-0772-2. https://cardiab.biomedcentral.com/articles/10.1186/s12933-018-0772-2 .10.1186/s12933-018-0772-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chode S, Malmstrom TK, Miller DK, Morley JE. Frailty, diabetes, and mortality in middle-aged African Americans. J Nutr Health Aging. 2016;20(8):854–859. doi: 10.1007/s12603-016-0801-3. [DOI] [PubMed] [Google Scholar]
- 22.Chhetri JK, Zheng Z, Xu X, Ma C, Chan P. The prevalence and incidence of frailty in pre-diabetic and diabetic community-dwelling older population: results from Beijing longitudinal study of aging II (BLSA-II) BMC Geriatr. 2017 Feb 08;17(1):47. doi: 10.1186/s12877-017-0439-y. https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-017-0439-y .10.1186/s12877-017-0439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He D, Li J, Li Y, Zhu J, Zhou T, Xu Y, Wu Q, Cheng Z, Chen Q, Liu Z, Zhu Y. Frailty is associated with the progression of prediabetes to diabetes and elevated risks of cardiovascular disease and all-cause mortality in individuals with prediabetes and diabetes: Evidence from two prospective cohorts. Diabetes Res Clin Pract. 2022 Dec;194:110145. doi: 10.1016/j.diabres.2022.110145.S0168-8227(22)00959-7 [DOI] [PubMed] [Google Scholar]
- 24.Feldman-Billard S, Dupas B. Eye disorders other than diabetic retinopathy in patients with diabetes. Diabetes Metab. 2021 Nov;47(6):101279. doi: 10.1016/j.diabet.2021.101279.S1262-3636(21)00062-8 [DOI] [PubMed] [Google Scholar]
- 25.Wang F, Wang S, Zong Q, Zhang Q, Ng CH, Ungvari GS, Xiang Y. Prevalence of comorbid major depressive disorder in type 2 diabetes: a meta-analysis of comparative and epidemiological studies. Diabet Med. 2019 Aug;36(8):961–969. doi: 10.1111/dme.14042. [DOI] [PubMed] [Google Scholar]
- 26.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015 Mar;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. https://dx.plos.org/10.1371/journal.pmed.1001779 .PMEDICINE-D-12-02351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UK Biobank: Protocol for a large-scale prospective epidemiological resource. UK Biobank. 2007. Mar 21, [2023-05-04]. https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf .
- 28.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. The Lancet Public Health. 2018 Jul;3(7):e323–e332. doi: 10.1016/s2468-2667(18)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Buuren SV, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J. Stat. Soft. 2011;45(3):1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 30.Kong L, Lyu Q, Yao H, Yang L, Chen S. The prevalence of frailty among community-dwelling older adults with diabetes: A meta-analysis. Int J Nurs Stud. 2021 Jul;119:103952. doi: 10.1016/j.ijnurstu.2021.103952.S0020-7489(21)00095-X [DOI] [PubMed] [Google Scholar]
- 31.O'Caoimh R, Sezgin D, O'Donovan MR, Molloy DW, Clegg A, Rockwood K, Liew A. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021 Jan 08;50(1):96–104. doi: 10.1093/ageing/afaa219.5928224 [DOI] [PubMed] [Google Scholar]
- 32.Palmer AK, Gustafson B, Kirkland JL, Smith U. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia. 2019 Oct;62(10):1835–1841. doi: 10.1007/s00125-019-4934-x. https://europepmc.org/abstract/MED/31451866 .10.1007/s00125-019-4934-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raymond E, Reynolds CA, Dahl Aslan AK, Finkel D, Ericsson M, Hägg S, Pedersen NL, Jylhävä J. Drivers of Frailty from Adulthood into Old Age: Results from a 27-Year Longitudinal Population-Based Study in Sweden. J Gerontol A Biol Sci Med Sci. 2020 Sep 25;75(10):1943–1950. doi: 10.1093/gerona/glaa106. https://europepmc.org/abstract/MED/32348465 .5826936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liccini A, Malmstrom TK. Frailty and sarcopenia as predictors of adverse health outcomes in persons with diabetes mellitus. J Am Med Dir Assoc. 2016 Sep 01;17(9):846–851. doi: 10.1016/j.jamda.2016.07.007.S1525-8610(16)30254-7 [DOI] [PubMed] [Google Scholar]
- 35.Ros M, Carrascosa JM. Current nutritional and pharmacological anti-aging interventions. Biochim Biophys Acta Mol Basis Dis. 2020 Mar 01;1866(3):165612. doi: 10.1016/j.bbadis.2019.165612. https://linkinghub.elsevier.com/retrieve/pii/S0925-4439(19)30335-7 .S0925-4439(19)30335-7 [DOI] [PubMed] [Google Scholar]
- 36.Travers J, Romero-Ortuno R, Bailey J, Cooney M. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019 Jan;69(678):e61–e69. doi: 10.3399/bjgp18X700241. https://bjgp.org/lookup/pmidlookup?view=long&pmid=30510094 .bjgp18X700241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mugueta-Aguinaga I, Garcia-Zapirain B. Is technology present in frailty? Technology a back-up tool for dealing with frailty in the elderly: a systematic review. Aging Dis. 2017 Apr;8(2):176–195. doi: 10.14336/AD.2016.0901. https://europepmc.org/abstract/MED/28400984 .ad-8-2-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallucci A, Trimarchi PD, Abbate C, Tuena C, Pedroli E, Lattanzio F, Stramba-Badiale M, Cesari M, Giunco F. ICT technologies as new promising tools for the managing of frailty: a systematic review. Aging Clin Exp Res. 2021 Jun;33(6):1453–1464. doi: 10.1007/s40520-020-01626-9.10.1007/s40520-020-01626-9 [DOI] [PubMed] [Google Scholar]
- 39.Cruz AM, Monsalve L, Ladurner A, Jaime LF, Wang D, Quiroga DA. Information and communication technologies for managing frailty: a systematic literature review. Aging Dis. 2021 Jun;12(3):914–933. doi: 10.14336/AD.2020.1114. https://europepmc.org/abstract/MED/34094651 .ad-12-3-914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aznar-Tortonda V, Palazón-Bru A, la Rosa DMF, Espínola-Morel V, Pérez-Pérez BF, León-Ruiz AB, Gil-Guillén VF. Detection of frailty in older patients using a mobile app: cross-sectional observational study in primary care. Br J Gen Pract. 2020 Jan;70(690):e29–e35. doi: 10.3399/bjgp19X706577. https://bjgp.org/lookup/pmidlookup?view=long&pmid=31685541 .bjgp19X706577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diabetes Prevention Program Research Group. Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009 Nov 14;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. https://europepmc.org/abstract/MED/19878986 .S0140-6736(09)61457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M, Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001 May 03;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 43.Anker D, Carmeli C, Zwahlen M, Rodondi N, Santschi V, Henchoz Y, Wolfson C, Chiolero A. How blood pressure predicts frailty transitions in older adults in a population-based cohort study: a multi-state transition model. Int J Epidemiol. 2022 Aug 10;51(4):1167–1177. doi: 10.1093/ije/dyab210. https://boris.unibe.ch/id/eprint/160189 .6398053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Li T, Li C, Liu C, Lin W, Lin C, Yang C, Yang S, Lin C. Frailty, transition in frailty status and all-cause mortality in older adults of a Taichung community-based population. BMC Geriatr. 2019 Jan 28;19(1):26. doi: 10.1186/s12877-019-1039-9. https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-019-1039-9 .10.1186/s12877-019-1039-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.UK Biobank. [2023-05-04]. https://www.ukbiobank.ac.uk/enable-your-research/register .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.
Data Availability Statement
The data sets analyzed during this study are available at [45].


