Abstract
Viral infections have led to many public health crises and pandemics in the last few centuries. Neurotropic virus infection-induced viral encephalitis (VE), especially the symptomatic inflammation of the meninges and brain parenchyma, has attracted growing attention due to its high mortality and disability rates. Understanding the infectious routes of neurotropic viruses and the mechanism underlying the host immune response is critical to reduce viral spread and improve antiviral therapy outcomes. In this review, we summarize the common categories of neurotropic viruses, viral transmission routes in the body, host immune responses, and experimental animal models used for VE study to gain a deeper understanding of recent progress in the pathogenic and immunological mechanisms under neurotropic viral infection. This review should provide valuable resources and perspectives on how to cope with pandemic infections.
Keywords: Neurotropic viruses, Viral encephalitis, Meningeal immunity, Experimental animal models
INTRODUCTION
Viral encephalitis (VE) is a major global disease, with an incidence rate of 1.4 cases per 100 000 inhabitants (Silva, 2013). Following infection with a variety of neurotropic viruses, VE can cause acute intracranial inflammatory injury of the meninges and brain parenchyma (Ludlow et al., 2016). Clinical VE pathogens are primarily neurotropic RNA viruses, such as Japanese encephalitis virus (JEV), Zika virus (ZIKV), West Nile virus (WNV), Dengue virus (DV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and DNA viruses, such as herpes simplex virus 1 and 2 (HSV-1 and HSV-2), varicella zoster virus (VZV), and cytomegalovirus (CMV) (Kennedy, 2005; Silva, 2013; Spudich & Nath, 2022). The typical incubation period for primary infection is approximately six days, during which the prodromal phase may present with symptoms such as mild fever, sore throat, cough, nausea, vomiting, myalgia, and fatigue (Bale, 2015). Diagnostic strategies include clinical and laboratory testing as well as neuroimaging. Acute viral invasion of the central nervous system (CNS) can increase mortality and disability if treatment is delayed. A window exists during which time epidemiological analysis and diagnosis can confirm the infection route, genome, antigen, and specific immunoglobulin M (IgM) and immunoglobulin G (IgG) of the invading virus (Venkatesan et al., 2013). However, specific therapeutic approaches for effectively curing VE after viral infection remain limited, emphasizing the need for more intensive basic investigations on viral invasion routes, VE pathogenesis, and host immunity post-infection to accelerate the development of novel diagnostic and therapeutic strategies.
The brain is a relatively well-protected organ and contains several cellular barriers, including the blood-brain barrier (BBB), which acts as a defense mechanism to prevent entry of dangerous factors and drugs from the peripheral circulation into the CNS (de Lima et al., 2020). Natural human infection from fresh or postmortem samples of VE is extremely rare, posing a challenge in the study of VE pathogenesis and immune defense in humans. However, the transmission and life cycle of neurotropic viruses have been extensively investigated in animal models, such as artiodactyls, domestic birds, and mosquitoes. With the advance of VE animal models, especially non-human primate (NHP) and rodent models, our understanding of virus infection routes, pathogenesis, and immunity has greatly improved. In addition to classic invasion via blood circulation and peripheral nerves, we recently showed that meningeal lymphatic vessel (MLV) endothelial cells can be infected by JEV and vesicular stomatitis virus (VSV) and transport viral particles to cervical lymph nodes (CLNs) (Li et al., 2022). The unique origin and definite role of meningeal immune cells in health and disease have become interesting fields of study (Brioschi et al., 2021; Cugurra et al., 2021; Niu et al., 2022), remaining elusive until recently (de Lima et al., 2020). In the present review, we provide a comprehensive summary of prevalent neurotropic viruses, potential routes of neurotropic viral invasion, and host immune defense. Furthermore, we discuss reported animal models with neurotropic viral loading. This review should provide useful information for further investigation into the diagnosis and treatment of VE in the future.
CATEGORIES OF NEUROTROPIC VIRUSES
Viruses that invade the nervous system are collectively known as neurotropic viruses. Neurotropic viruses include RNA and DNA viruses from various families, including Bunyaviridae, Flaviviridae, Bornaviridae, Herpesviridae, Orthomyxoviridae, Paramyxoviridae, Picornaviridae, Retroviridae, Polyomaviridae, Rhabdoviridae, and Togaviridae (see Table 1 for details). Following CNS infection, inflammation can arise in distinct anatomical regions, such as the meninges (meningitis), brain (encephalitis), and spinal cord (myelitis), or simultaneously in multiple regions (meningoencephalitis, encephalomyelitis). Neurotropic viral infection of the CNS can cause acute inflammatory lesions, as well as chronic inflammatory or non-inflammatory lesions. Various neurological disorders, such as Guillain-Barre syndrome, multiple sclerosis, narcolepsy, and lethargic encephalitis, are considered to be delayed onset virus-induced diseases (Ludlow et al., 2016). Perineural virus infection-induced nerve inflammation, nerve damage, and neurological complications pose considerable threats to human and animal health and can cause substantial economic losses. A deeper understanding of the mechanism underlying the invasion of neurotropic viruses in the nervous system will provide a valuable theoretical foundation for proper treatment.
Table 1. Categories of neurotropic viruses.
| Viral family | Virus | Genome | Reference |
| dsDNA: Double strand DNA. ssRNA: Single strand RNA. +: Positive-sense. –: Negative-sense. | |||
| Herpesviridae | Herpes simplex virus-1, HSV-1 | dsDNA | Bradshaw & Venkatesan, 2016; Whitley, 2015 |
| Varicella zoster virus, VZV | dsDNA | Nagel et al., 2020 | |
| Cytomegalovirus, CMV | dsDNA | Cheeran et al., 2009 | |
| Human herpes virus 6, HHV-6 | dsDNA | Agut et al., 2015; Kimberlin & Whitley, 1998 | |
| Epstein-Barr virus, EBV | dsDNA | Houen et al., 2020; Tselis, 2014 | |
| Pseudorabies virus, PRV | dsDNA | Liu et al., 2021 | |
| Flaviviridae | Japanese encephalitis virus, JEV | + ssRNA | Redant et al., 2020 |
| West Nile virus, WNV | + ssRNA | Klein, 2021; Sips et al., 2012 | |
| Zika virus, ZIKV | + ssRNA | Christian et al., 2019; Klein, 2021; White et al., 2016 | |
| Tickborne encephalitis virus, TBEV | + ssRNA | Cvjetković et al., 2016; Kubinski et al., 2020 | |
| Dengue virus, DV | + ssRNA | Trivedi & Chakravarty, 2022; Verma et al., 2011 | |
| St Louis encephalitis virus, SLEV | + ssRNA | Marques et al., 2017 | |
| Picornaviridae | Poliovirus, PV | + ssRNA | Brownell et al., 2015; Verboon-Maciolek et al., 2008 |
| Enterovirus 71, EV71 | + ssRNA | Solomon et al., 2010 | |
| Human parechovirus, HPeV | + ssRNA | Verboon-Maciolek et al., 2008 | |
| Coxsackievirus A16, CV-A16 | + ssRNA | Hooi et al., 2020 | |
| Rhabdoviridae | Rabies virus, RABV | – ssRNA | Hemachudha et al., 2013 |
| Vesicular stomatitis virus, VSV | – ssRNA | Beier et al., 2011; Sabin & Olitsky, 1937 | |
| Retroviridae | Human immunodeficiency virus, HIV | + ssRNA | González-Scarano & Martín-García, 2005 |
| Human T cell lymphotropic virus, HTLV | + ssRNA | Cabre et al., 2000 | |
| Polyomaviridae | John Cunningham virus, JCV | dsDNA | Ferenczy et al., 2012 |
| Orthomyxoviridae | Influenza A virus | Segmented - RNA | Takahashi et al., 1995; van Riel et al., 2015 |
| Paramyxoviridae | Measles virus | – ssRNA | Fisher et al., 2015 |
| Mumps virus, MuV | – ssRNA | Rubin et al., 1998 | |
| Togaviridae | Chikungunya virus, CHIKV | + ssRNA | Das et al., 2015; Klein, 2021 |
| Equine encephalitis virus, EEV | + ssRNA | Ludlow2016 | |
| Venezuelan equine encephalitis virus, VEEV | + ssRNA | Ludlow et al., 2016 | |
| Western equine encephalitis virus, WEEV | + ssRNA | Ludlow2016 | |
| Eastern equine encephalitis virus, EEEV | + ssRNA | Ludlow et al., 2016 | |
| Bornaviridae | Bornavirus | – ssRNA | Jordan & Ian Lipkin, 2001 |
| Bunyaviridae | La Crosse virus, LCV | Segmented – RNA |
McJunkin et al., 2001 |
| Rift Valley fever virus, RVFV | Segmented – RNA | Albe et al., 2019 | |
| Toscana virus, TOSV | Segmented – RNA | Gori Savellini et al., 2019 | |
| Coronaviridae | Severe acute respiratory syndrome coronavirus 2, SARS-CoV-2 | + ssRNA | Erickson et al., 2021; Meinhardt et al., 2021 |
Flaviviruses (FVs) are enveloped, positive-sense, single-stranded RNA viruses (ssRNA) carried by mosquitoes and ticks and are regarded as neurotropic viruses due to their significant neuroinvasive characteristics. A small percentage of infected individuals may exhibit neurological symptoms, such as acute encephalitis, meningitis, and acute flaccid paralysis, while long-term effects include Parkinsonism, dystonia, and cognitive changes. Classical neurotropic FVs include the JEV, ZIKV, WNV, DV, tick-borne encephalitis virus (TBEV), and St Louis encephalitis virus (SLEV).
Horses, pigs, birds (corvid species), and dogs are natural reservoirs of FVs. For example, the JEV replicates and remains in porcine tonsils for up to 25 days, enabling persistence in seasons when mosquitoes are inactive (García-Nicolás et al., 2018). WNV is maintained in nature in a mosquito-bird-mosquito transmission cycle primarily involving Culex mosquitoes (Turell et al., 2001). Aedes mosquitoes, namely Aedes aegypti, Aedes albopictus, Aedes scutellaris, and Aedes polynesiensis, are recognized vectors for the transmission of DV infection. TBEV is transmitted from the saliva of infected ticks within minutes of a tick bite (Lindquist & Vapalahti, 2008). Humans are regarded as “dead-end hosts” because they are infected accidentally by FV-carrying mosquitoes.
The primary sites of FV infection include subcortical nuclei (substantia nigra and thalamus), anterior horn neurons, and neocortex, with different neurological signs occurring in some individuals. About 80% of human WNV infections are asymptomatic (Mostashari et al., 2001), while those with symptoms are characterized by the acute onset of fever, headache, fatigue, malaise, muscle pain, weakness, difficulty concentrating, and neck pain or stiffness (Watson et al., 2004). In neuroinvasive WNV disease, infection of spinal motor neurons (anterior horn cells) causes acute asymmetric flaccid paralysis, similar to that seen with poliomyelitis (Li et al., 2003). Many patients with WNV-induced encephalitis exhibit movement disorders, including severe tremors and parkinsonism (Sejvar et al., 2003). While most JEV infections present with either mild symptoms (fever and headache) or remain asymptomatic, those that develop encephalitis can suffer significant morbidity and mortality. Patients with meningoencephalitis may progress to a permanent neurological deficit or ultimately death (Salimi et al., 2016). JEV neuroinvasion in patients can cause reduced levels of consciousness associated with seizures, movement disorders, and flaccid paralysis, as well as perivascular and CNS inflammation (Johnson et al., 1985). ZIKV infection during pregnancy leads to an increased risk of fetal growth restriction and fetal CNS malformations, resulting in long-term structural and neurological defects (da Silva & Gao, 2016). Similarly, tick-borne encephalitis can cause acute meningoencephalitis with or without myelitis.
HSV-1, HSV-2, and VZV are members of the herpes family of DNA viruses and are characterized by double-stranded DNA genomes located within a capsid consisting of 162 capsomers. Among the herpes family viruses that infect the nervous system, HSV is one of the most common pathogens of infectious human encephalitis.
Herpesviruses have developed very specific mechanisms to evade host defenses and establish latency by shutting down lytic replication. Following primary HSV-1 infection, which is typically asymptomatic, the virus becomes latent in trigeminal and other cranial nerve ganglia, after which it spreads via axons of the trigeminal nerve into the frontal and temporal lobes. In immunocompetent adults, more than 90% of herpes simplex virus encephalitis (HSE) cases are due to HSV-1. HSE symptoms include headache, fever, and neck stiffness, with associated convulsions and dysfunction of the frontotemporal lobes (Bradshaw & Venkatesan, 2016). Approximately 80% of neonatal encephalitis cases are caused by HSV-2. Neonates present with systemic findings (alterations in body temperature, lethargy, respiratory distress, anorexia, vomiting, cyanosis) and neurological signs (irritability, bulging fontanels, seizures, and coma) (Overall, 1994). VZV causes varicella (chickenpox) and herpes zoster. Varicella usually results in mild to moderate illness in immunocompetent patients but may cause serious complications in infants and elderly individuals, such as CNS involvement, pneumonia, secondary bacterial infections, and death (Heininger & Seward, 2006). Typical herpes zoster presents with vesicular eruptions distributed unilaterally within a dermatome, sometimes preceded by paresthesia, itching, and pain, a condition termed preherpetic neuralgia (Gilden et al., 1991).
Rabies (RABV) and rabies-related viruses belong to the Lyssavirus genus of the Rhabdoviridae family. The small, negative-stranded RNA genome (12 kb) of RABV encodes five proteins. RABV is a prototypical neurotropic virus transmitted in the saliva of infected animals (predominantly dogsbut also other species such as bats, foxes, raccoon dogs, raccoons, mongooses, and skunks) via bites and scratches, which infects host neurons almost exclusively. After successful completion of the virus cycle, host death occurs due to the exhaustion of infected neurons, accompanied by structural damage and severe neuronal dysfunction (Hemachudha et al., 2013).
Recent studies have reported that SARS-CoV-2 infection is associated with encephalopathy, encephalitis, especially meningoencephalitis, and other complications (Pilotto et al., 2021). More than one-third of patients show mild or moderate disturbance in consciousness (Pilotto et al., 2021) and autopsy reports have revealed the presence of SARS-CoV-2 in the brain tissue of COVID-19 patients (Maury et al., 2021). At present, however, the mechanism underlying SARS-CoV-2 neurotropism remains unclear.
Prions, which are unusual proteinaceous infectious agents, can cause neuropathies (Aguzzi et al., 2007), with some strains targeting the CNS as the primary target organ. Among these prions, the scrapie prion protein (PrPSc), a misfolded host-derived membrane glycolipoprotein cellular prion protein (PrPC), can cause various fatal neurodegenerative diseases, including transmissible spongiform encephalopathies (TSEs) such as scrapie in sheep, chronic wasting disease (CWD) in deer, bovine spongiform encephalopathy (BSE) in cattle (known as “mad cow disease”), and Creutzfeldt-Jakob disease (CJD) in humans. Aberrant prion protein conformations accumulate in the CNS, causing spongiform changes in the brain and eventually death. Generally, prion transmission between distinct species (e.g., transmission of human prions into hamsters) is restricted by the species barrier. The infectious conformer of this protein (PrPSc) is predicted to recruit and convert the normal conformer (PrPC) into the PrPSc form by interacting with specific regions of the protein, thus completing the ‘replication’ process during infection (Tuite & Serio, 2010).
TRANSMISSION ROUTE OF VIRUS IN THE BODY
Most viral infections started at barrier sites, such as epithelial or endothelial cells on the peripheral surface, causing a tissue-specific antiviral response. If the virus is not effectively eliminated at the site of primary infection, it will spread to other tissues and organs. Once the virus reaches its target tissue, it rapidly replicates within the cells, leading to an overactivation of the innate immune response, causing a local or systemic “inflammatory storm” (Koyuncu et al., 2013).
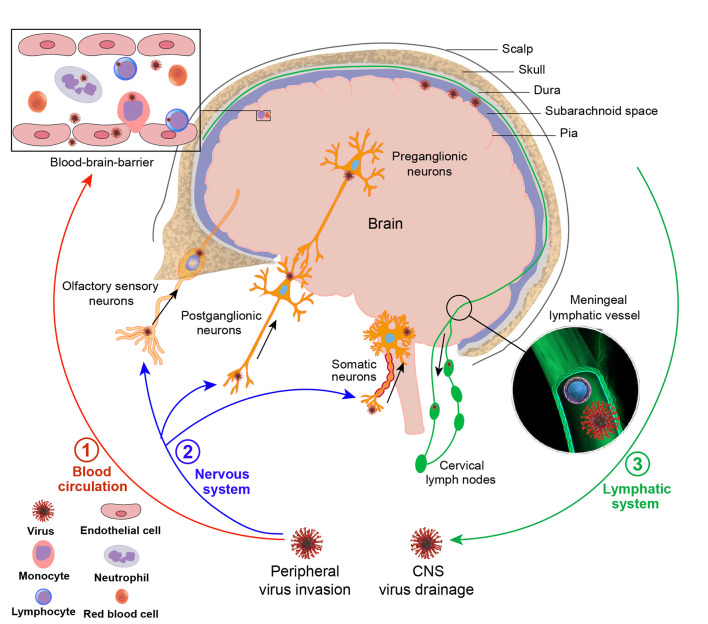
The CNS possesses unique anatomical features, including the BBB and an absence of conventional lymphatic vessels within the parenchyma. The BBB is a special structure of blood vessels composed of endothelial cells, astrocytes, and pericytes (Ballabh et al., 2004). Tightly connected vascular endothelial cells in the BBB precisely regulate the exchange of ions, macromolecules, and cells between blood vessels and the brain to ensure the normal operation of nerve function and prevent toxic substances and pathogens from entering the brain (Daneman & Prat, 2015). Despite the highly complex defense system that protects the CNS, certain viruses can evade the protective barriers through different strategies. There are three main routes for invading CNS defenses (Figure 1).
Figure 1.
Viral transmission routes in the body
Although the CNS is protected by a highly complex barrier system, certain viruses still manage to enter the CNS and cause disease. One pathway is via blood circulation, whereby viruses infect leukocytes in the blood, which then pass through the BBB into the CNS during normal turnover of perivascular leukocytes or tight junction disruption of vessel endothelial cells. Viruses can also directly enter the CNS via the BBB by infecting vascular endothelial cells. In addition, viruses can migrate through peripheral nerve infection to enter the CNS, e.g., via peripheral motor neurons at axonal terminals, peripheral sensory neurons, and olfactory nerves. In addition to viral invasion routes, viruses can also drain from the CNS to the CLNs via the MLVs.
Virus-infected leukocytes enter the CNS via the BBB
Certain viruses can enter the CNS via a “Trojan horse” mechanism, in which infected leukocytes carry pathogens from the blood across the BBB. Infection of monocytes and/or macrophages is a major mechanism used by lentiviruses, including simian immunodeficiency virus (SIV) and human immunodeficiency virus (HIV), to migrate across vascular barriers of the CNS (Alexaki & Wigdahl, 2008; Clay et al., 2007). Infected monocytes pass through the BBB during normal turnover of perivascular macrophages or due to the production of proinflammatory mediators that compromise the barrier, such as CC-chemokine ligand 2 (CCL2) (Ancuta et al., 2006; Roberts et al., 2010). Adhesion molecules also play a crucial role in cellular migration, with vascular cell-adhesion molecule-1 (VCAM-1) mediating mononuclear cell migration into the brain during HIV and SIV infection (Sasseville et al., 1994). Furthermore, JCV is believed to remain latent in the lymphoid organs, neuronal tissue, and kidney, but may reactivate under severe immunosuppression and infiltrate the brain via the "Trojan horse" mechanism, resulting in progressive multifocal leukoencephalopathy, a demyelinating disease of the CNS with a high mortality rate (Chapagain & Nerurkar, 2010). In addition, following WNV infection, the systemic levels of tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) increase macrophage inhibitory factor (MIF) and adhesion molecule (ICAM) expression at the BBB and promote tight junction disruption, thereby facilitating the adhesion and entry of WNV-infected neutrophils into the CNS (Arjona et al., 2007; Bai et al., 2010; Dai et al., 2008; Roe et al., 2012).
Virus enters the CNS by infecting microvascular endothelial cells
Other viruses can directly enter the CNS via the BBB by infecting vascular endothelial cells. Epithelial barrier integrity relies on tight junction complexes composed of transmembrane proteins located on the plasma membranes of adjacent endothelial and epithelial cells. Disruption of these tight junction complexes is a well-documented route for the invasion of certain viruses, such as the influenza virus, Ebola virus (EBOV), hepatitis C virus (HCV), and HIV (Meertens et al., 2008). The HIV-Tat protein increases the permeability of brain endothelial cells by down-regulating occludin mRNA levels in microvascular brain cells to increase HIV neuroinvasion (Xu et al., 2012a). Arboviruses can enter the skin through insect bites, then transmigrate to secondary lymphoid tissues and eventually enter the bloodstream, causing systemic infections and inflammation-induced disruption of the BBB, thus allowing the virus to invade the CNS (Ransohoff et al., 2003; Wu et al., 2000). WNV capsid degradation of the claudin protein disrupts epithelial barrier tight junctions and function (Medigeshi et al., 2009; Xu et al., 2012b). Replication of ZIKV, JEV, and HCV in brain microvascular endothelial cells (BMVECs) does not cause cytopathy but can increase vascular endothelial monolayer permeability (Al-Obaidi et al., 2017; Mustafá et al., 2019). Epstein-Barr virus (EBV) can infect human BBB cells, leading to increased production of proinflammatory mediators that result in immune cell adherence, which is implicated in the onset of MS (Casiraghi et al., 2011). Some viruses (e.g., WNV, HCV, HTLV-1, JCV, EBV, and human cytomegalovirus (HCMV)) can enter the CNS through the transcytosis of cerebrovascular endothelial cells and infection of nerve cells (Liou & Hsu, 1998; Papa et al., 2017). Poliovirus (PV) enters human brain microvascular endothelial cells (HBMECs) through dynamin-dependent caveolar endocytosis, facilitated by the association between the PV receptor (PVR) and SH2 domain-containing protein tyrosine phosphatase 2 (SHP-2) following virus attachment to the PVR, causing paralytic poliomyelitis by replicating within motor neurons of the brain and spinal cord (Ohka et al., 2012). Some regions of the CNS, such as the choroid plexus and periventricular organs, are not fully protected by the BBB and can be targeted as viral entry points (van den Pol et al., 1999).
Virus enters the CNS through peripheral nerve infection
Viruses can also enter the CNS via migration through peripheral nerve infection. RABV can infect myocytes through saliva and subsequently enter peripheral motor neurons at axonal terminals, where it eventually infects the CNS through strictly unidirectional (retrograde) transneuronal transfer (Ugolini, 2011). Similarly, PV can infect mucosal epithelial cells after ingestion and invade the CNS via peripheral motor nerves (Racaniello, 2006). Retrograde axonal transport in neuronal cells may represent a major transmission route of enterovirus 71 (EV71) in mice, spreading from skeletal muscle to motoneuron junctions, peripheral motor nerves, then motor nuclei in the CNS (Tan et al., 2014). In addition, HSV-1 can infect keratinocytes and migrate to peripheral sensory neurons and may invade the CNS via the trigeminal nerve or olfactory sensory neurons after primary oropharyngeal infection (Mori et al., 2005). FVs can spread to the CNS via axonal transport from the periphery during viremia, as found in other neurotropic viruses such as RABV, PV, and HSV. Furthermore, WNV exhibits bidirectional spread in neurons, with axonal transport promoting viral entry into the CNS, followed by acute limb paralysis (Samuel et al., 2007). Moreover, VSV, Nipah virus (Munster et al., 2012), influenza virus (van Riel et al., 2015), RABV (Constantine, 1962), bovine herpesvirus 5 (Lee et al., 1999), and equine herpesvirus 9 (Narita et al., 2001) are proposed to enter the CNS via the olfactory nerve. SARS-CoV-2 may also invade the CNS through the olfactory bulb, spreading into functional areas such as the hippocampus, thalamus, and medulla oblong to induce brain inflammation (Meinhardt et al., 2021).
Virus is drained from the CNS to CLNs via meningeal lymphatic vessels (MLVs)
While previous studies on viral dissemination have primarily focused on the BBB and peripheral nerves, recent research has revealed the potential involvement of MLVs in viral spread during neurotropic virus infection. Specifically, Li et al. (2022) demonstrated that JEV migrates from the CNS to CLNs, with inoculation of suckling mice with deep CLNs (dCLNs) and superfical CLNs (sCLNs) tissue homogenates from intracerebrally JEV-infected mice resulting in a morbidity rate exceeding 40%. These results indicate that viruses draining from the CNS to the CLNs maintain their infectivity and may trigger an immune response in the CLNs. Li et al. (2022) also intracerebrally injected a recombinant VSV expressing green fluorescent protein (VSV-GFP) into the mouse brains and observed colocalization of VSV-GFP and lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), podoplanin (PDPN), and prospero homeobox 1 (PROX1) in the meninges, suggesting that the virus can drain from the CNS to the CLNs via the MLVs (Figure 1).
IMMUNE RESPONSES TO VE
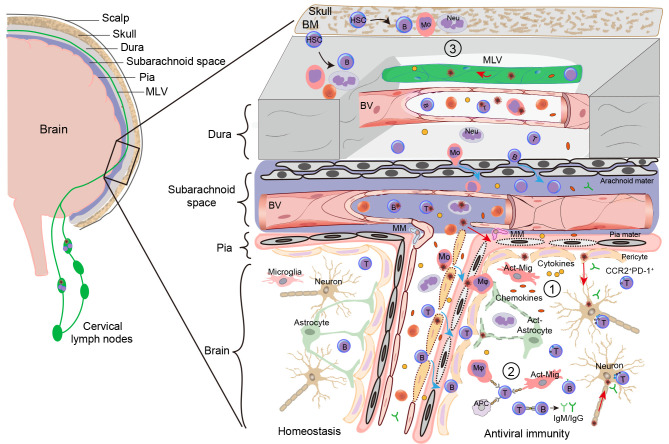
Clinical observations and experimental models have provided strong evidence that both innate and adaptive immune responses play important roles in the pathophysiology of VE (Figure 2) (Chen et al., 2019; Suthar et al., 2013; Yshii et al., 2015). Following neurotropic viral entry into the CNS, antiviral immune responses are immediately induced by innate immune cells, such as microglia, astrocytes, dendritic cells (DCs), and infiltrated macrophages, as well as other immune cells. Given the toxic effects of proinflammatory cytokines/chemokines, and a breached BBB, peripherally circulating leukocytes, such as monocytes, neutrophils, and lymphocytes, can infiltrate the CNS. Viral antigens, presented by antigen-presenting cells (APCs), activate CNS-infiltrating CD8+ T cells, which differentiate into effector cytotoxic T lymphocytes (CTLs). CTLs directly eliminate infected cells by producing cytotoxic molecules, such as perforin and granzyme B (Wong & Pamer, 2003). CTLs also clear infected cells by releasing apoptotic ligands, including Fas ligand (FasL) and TNF-α-related apoptosis-inducing ligand (TRAIL) (Shrestha & Diamond, 2007; Shrestha et al., 2012). Ferroptosis is also involved in neurotropic viral clearance and brain injury (Yan et al., 2023; Zhang et al., 2022a). Furthermore, CD8+ T cell-derived interferon γ (IFN-γ) plays an essential role in restraining intracranial viral infection and clearing viruses from infected neurons (Garber et al., 2019; Griffin & Metcalf, 2011). Additionally, a small group of CD8+ T cells can transform into brain-resident memory T (bTRM) cells (Steinbach et al., 2016). After viral reinfection, bTRM cells rapidly produce cytotoxic molecules to prevent virus infection (Mockus et al., 2019). Finally, successful antiviral immunity is acquired with virus-specific IgM and IgG/IgA antibodies secreted from B cell-transformed plasma cells to decrease viral spread and neutralize circulating viral particles (Lam et al., 2020).
Figure 2.
Meningeal and parenchymal immunity during VE
VE refers to acute intracranial inflammatory lesions and involves the meninges and brain parenchyma. Various viruses invade the CNS through blood/lymphoid circulation and peripheral nerve migration. Damaged neurons release signals (such as ATP and cytokines) to recruit microglia, which initiate innate immunity ①. Cytokines and chemokines are released from activated microglia (Act-Mig) and astrocytes (see text for details). Thereafter, peripheral neutrophils (Neu), monocytes (Mo), and APCs (including DCs) infiltrate the brain parenchyma via increased BBB permeability. IFN is produced to enlarge the antiviral effects and APCs present antigens to T cells and later to B cells, constituting adaptive immunity ②. CD8+ T cells produce granzyme and perforin to clear infected cells, including neurons, vascular endothelial cells, and pericytes. Plasmacytes differentiated from mature B cells secrete specific IgM and IgG antibodies to neutralize viral particles and restrict their spread. Meningeal immunity ③ has also recently been reported to play an important role in VE. Both myeloid and B cells differentiate from HSCs, which originate from skull bone marrow (BM) or meninges, and meningeal macrophages (MMs) extravasate from the pia mater or cross the arachnoid mater into the brain parenchyma. Additionally, viruses can infect and transmit from MLVs into CLNs to enhance peripheral immunity. Red arrow: Viral invasion/transmission; Blue arrow: Immune cell infiltration.
The role of meningeal immunity has gained increasing attention in recent years, particularly regarding its contribution to VE (de Lima et al., 2020). MLVs can transport viruses into the CLNs to regulate peripheral immunity (Li et al., 2022), while meningeal macrophages directly protect against lymphocytic CMV (LCMV) neuroinfection (Figure 2) (Rebejac et al., 2022). Furthermore, evidence suggests that innate and adaptive immune responses exhibit both beneficial and detrimental roles in antiviral effects (Reagin & Funk, 2022). Regulating immunological balance between viral clearance and neuronal damage is important to increase survival and decrease the sequela in infected patients.
Innate immunity in VE
The innate immune system establishes the first line of defense against neurotropic viruses in the CNS. Microglia, astrocytes, monocyte-derived macrophages, neutrophils, natural killer (NK) cells, and DCs play critical roles in the innate response against viral invasion. Type I IFN is important for host survival, with mice lacking IFN-α/β receptors showing significantly increased susceptibility to neurotropic viruses (Byrnes et al., 2000; Fiette et al., 1995; Müller et al., 1994). IFN-β has an important neuroprotective effect in the CNS and can induce the production of neurotrophic factors (Boutros et al., 1997). Rapid IFN-β response after infection reduces viral transmission and inhibits viral replication before the initiation of specific adaptive immune responses (Boutros et al., 1997). Virally infected neurons can also produce a relatively low level of type I IFN for CNS defense (Delhaye et al., 2006).
Innate immune responses are mediated by pattern recognition receptors (PRRs), including retinoic acid-inducible gene-I-like receptors (RLRs), nucleotide oligomerization domain-like receptors (NLRs), C-type lectin receptors (CLRs), Toll-like receptors (TLRs), absent in melanoma-2 (AIM2)-like receptors (ALRs), and cytoplasmic DNA sensor cyclic GMP-AMP synthase (cGAS) (Miller et al., 2021). Following recognition of pathogen-associated molecular pattern molecules (PAMPs), such as viral RNA or DNA, mRNA metabolism, and viral protein expression, PRRs can modify their conformational structures to initiate downstream production of type I IFN and proinflammatory cytokines by infected cells (Wilkins & Gale, 2010). Virus-infected cells can also produce virus-derived small RNAs (vsiRNAs), including small interfering RNAs (siRNAs), microRNAs (miRNAs), and Piwi-interacting RNAs (piRNAs) (Ding, 2010; Parameswaran et al., 2010; Pfeffer et al., 2004). Multiple herpesviruses use viral miRNAs to regulate innate receptor recognition and the signaling pathways of IFN production and function (Chen et al., 2022). Moreover, designed peptides targeting viral suppressors of RNAi (VSR) can effectively silence cognate EV-71 RNA in vivo and in vitro. This evidence implicates the involvement of vsiRNAs in the modulation of antiviral immunity and potential therapeutic strategies (Fang et al., 2021). Thus, both PPRs and vsiRNAs play important roles in host immune defense against viral infection (Ding, 2010).
Damaged neurons can also produce chemokine CX3CL1, which binds to its receptor CX3CR1 expressed in microglia and macrophages. Activation of these cells plays an important role in immune protection of the body during the early stage of infection (Jung et al., 2000; Maciejewski-Lenoir et al., 1999). In addition to sensing adenosine triphosphate (ATP) signals through the purinergic receptor P2Y12, residential microglia are also recruited and activated around infected neurons to enhance IFN production, proinflammatory cytokine release, and phagocytic activity (Fekete et al., 2018). Microglia and astrocytes respond quickly, producing antiviral and proinflammatory mediators. During JEV infection, proinflammatory molecules, such as RANTES, TNF-α, IL-1-α, IL-6, IL-12, IL-18, IL-1β, CCL2, C-X-C motif chemokine ligand 9 (CXCL9), CXCL10, and CXCL11, and proinflammatory enzymes, such as cyclooxygenase-2 and inducible nitric oxide synthase (iNOS), are up-regulated (Cheeran et al., 2005). In vivo, astrocytes can produce CXCL10, CXCL11, and CCL5 to transfer virus-specific CD4+ and CD8+ T cells (Glass et al., 2005; Klein et al., 2005; Lane et al., 2006). DCs also play important roles in T cell activation and adaptive immune response initiation during JEV infection (Li et al., 2011; Sooryanarain et al., 2012). DCs can be rapidly activated following neurotropic virus infection to release proinflammatory cytokines and chemokines, such as type I IFN, TNF, IL-1β, CCL2, CCL3, and CCL5 (Martina et al., 2008; Shrestha et al., 2008; Silva et al., 2007). Following BBB breach and chemokine release, infiltrated monocytes are differentiated into macrophages, which are then recruited and activated in the CNS. Removal of polymorphonuclear leukocytes by mouse monoclonal antibodies Gr-1 treatment has highlighted their crucial functions in viral clearance (Bai et al., 2010). Using single-cell RNA-sequencing (scRNA-seq), a recent study identified a novel subset of cells, named microglia-like cells, during herpes simplex encephalitis, which show high expression of Retnlg, Cxcr2, and Il1f9 and contribute to increased CNS inflammation (Uyar et al., 2022). Another study based on WNV-inclusive scRNA-seq reported that only a few L929 cells respond and exhibit robust transcription of IFN-β (O’Neal et al., 2019). The use of scRNA-seq to investigate heterogeneity of innate immunity will greatly expand our understanding of innate immunity in VE. Collectively, innate immunity is involved in the recognition of neurotropic viruses, presentation of viral antigens, and initiation of antiviral responses.
Adaptive immunity in VE
Under physiological conditions, the BBB prevents immune cells in blood circulation from entering the brain parenchyma, and APCs, such as DCs, are absent in the brain parenchyma (McMenamin, 1999). It is widely accepted that during CNS infection, pathogenic antigens are transported by cerebrospinal fluid (CFS) to draining lymph nodes (dLNs), where antigen presentation immediately occurs (Cserr & Knopf, 1992; Harling-Berg et al., 1999).
Neutrophils and DCs are primarily recruited to the CNS after viral infection (Templeton et al., 2008; Zuo et al., 2006). Neutrophils interact with endothelial cells through adhesion molecules that promote the disintegration of tight junction complexes, leading to the breakdown of the BBB. Neutrophils can also secrete matrix metalloproteinase-9 (MMP-9) to degrade the extracellular matrix and basement membrane of the BBB, further promoting BBB permeability (Kjeldsen et al., 1994). After BBB impairment, DCs appear within a few days of CNS virus infection and migrate from the CNS to CLNs via chemokine CCL3, thereby sensitizing virus-specific T cells (Trifilo & Lane, 2004). Mouse CD11chi DCs can induce the differentiation of CD4+ T cells into inflammatory T-helper 17 (Th17) cells, increase the number of anti-inflammatory regulatory T (Treg) cells in lymphoid tissue and CNS, and play a protective role in the CNS during fatal neuritis (Chiou et al., 2005; Kim et al., 2015). CD4+ and CD8+ T cells are recruited into the infected CNS, to some extent, by chemokines CXCL9 and CXCL10 (Stiles et al., 2006; Walsh et al., 2007). In addition, CCR5 contributes to T cell recruitment in the CNS (Chen et al., 2001; Glass & Lane, 2003). CD4+ T cells secrete IFN-γ to support the function of CD8+ T cells (Weinger et al., 2013), which are the main antiviral effector cells during CNS infection. IFN-γis also critical for the elimination of viruses in glial cells (Bergmann et al., 1999, 2003, 2006). CD8+ T cells produce IFN-γ, granulosa B, and perforin (Ramakrishna et al., 2004), which participate in the elimination of virus-infected astrocytes (Lin et al., 1997). IFN-γ helps oligodendrocytes control viral replication (González et al., 2006; Parra et al., 1999). Recent mass cytometry of infiltrating immune cells revealed a new subset of PD-1+CCR2+CD8+ T cells that may play important roles in viral defense (Zhang et al., 2019a). In MHV and Sindbis virus (SINV) encephalitis models, T cells promote B cell proliferation and differentiation via secretion of cytokines IL-10 and IL-21 (Linterman et al., 2010; Phares et al., 2011; Puntambekar et al., 2011). B cells clear virions from the CNS through powerful non-complement-dependent, non-cytolytic mechanisms. During RABV infection, antibodies against RABV glycoproteins inhibit viral RNA transcription and prevent viral spread between cells (Dietzschold et al., 1992). Antibodies can also sensitize NK cells and macrophages, inducing antibody-dependent cell-mediated cytolysis of virus-infected cells (Dietzschold et al., 1992). In acute infection, virus-specific antibody-secreting cells (ASCs) play an important role in achieving non-cytolytic viral clearance. In addition, because viral RNA is difficult to completely eradicate from target tissues, the long-term presence of ASCs in the CNS can prevent viral reactivation (Metcalf & Griffin, 2011). Human memory T cells contribute to defense against JEV infection (Turtle et al., 2016). Previous studies have indicated that IFN-γ responses of asymptomatic individuals infected with JEV are primarily mediated by CD8+ T cells, whereas IFN-γ responses of JEV-recovered individuals are primarily mediated by CD4+ T cells, suggesting that distinct clinical outcomes in JEV infection may be associated with CD4+ and CD8+ T cell responses. (Aleyas et al., 2009, 2012; Falasco et al., 1990).
Understanding the relationship between different immune cell infiltration and disease prognosis can help guide the prediction and treatment of clinical VE.
Meningeal immunity in VE
The CNS also replies on meningeal immune defense, which consists of the meningeal lymphatic system, glymphatic system, immune cells, and cytokines (de Lima et al., 2020; Louveau, 2018; Rua & McGavern, 2018). The crucial roles of meningeal immunity have been confirmed in many studies of different CNS diseases, such as stroke, Alzheimer’s disease (AD), VE, and cancer (Chen et al., 2020; Da Mesquita et al., 2018; Hu et al., 2020; Li et al., 2022; Song et al., 2020).
Recent research revealed that hematopoietic stem cells (HSCs) reside in the meninges under steady-state conditions. These meningeal HSCs are an important origin of leukocytes that supplement immune cells in the CNS (Niu et al., 2022). Reports also indicate that meningeal B cells derived locally from the calvaria at the CNS border are educated and negatively selected by CNS-specific antigens and may play an essential role in maintaining immune privilege within the CNS (Brioschi et al., 2021; Wang et al., 2021). In addition, recent study found that a pool of meningeal monocytes and neutrophils is supplied from the adjacent skull and vertebral bone marrow, but not from circulated blood. Under spinal injury and neuroinflammation, the meningeal myeloid cells can infiltrate the CNS and may serve a critical function in affecting the infection of these diseases (Cugurra et al., 2021). Furthermore, in our previous work, we found that neurotropic viruses, including JEV and HSV-1, can infect and replicate in lymphatic endothelial cells (LECs). In vivo, JEV can spread into dCLNs through the MLVs to activate the peripheral immune response for CNS viral clearance. Moreover, pretreatment of vascular endothelial growth factor C (VEGF-C), a well-known cytokine for MLV expansion, can improve the effects of antiviral infection (Li et al., 2022). MHC-II+ meningeal macrophages are also reported to play a critical role in protecting against LCMV neuroinfection via regulation of the IFN-I signaling pathway (Rebejac et al., 2022). These studies indicate that MLVs and meninge-resident immune cells may exhibit unique functions in immune defense of the CNS, including protection against neurotropic virus infections. The recent confirmation of functional lymphatic vessels in the brain meninges raises the possibility of an alternative drainage route of macromolecules and immune cells in the cerebrospinal fluid (CSF) into the CLNs. Following the initial discovery of MLVs, whole-mount immunolabeling and imaging revealed that most sinus T cells and MHCII+ cells, as well as some CD11c+ and B220+ cells, are found within the MLVs (Louveau et al., 2015). Several studies have reported that naive CD4+ T cells and Tomato-labeled CD19+ splenocytes primarily accumulate in the dCLNs and sCLNs after intracisternal magna injection into the CSF of naive mice (Brioschi et al., 2021; Louveau et al., 2018). Thus, MLVs may serve as a migratory route for B and T cells exiting the CNS compartment. MLVs are also involved in the regulation of immune cells under pathological conditions. Enhanced drainage of MLVs promotes the transport of tumor-related antigens and DCs from intracranial tumor tissue to dCLNs, thereby promoting the enhancement of CD8+ T cell initiation in dCLNs and the rapid clearance of tumors (Da Mesquita et al., 2018). Ablation of dorsal MLVs can reduce CNS-derived autoantigen drainage, thus alleviating the inflammatory response of brain-reactive T cells, delaying experimental autoimmune encephalomyelitis (EAE) onset, and diminishing pathology (Hsu et al., 2019; Louveau et al., 2018). However, the molecular mechanism underlying the cross-talk among the MLVs, skull, vertebral bone marrow-derived or meningeal immune cells, and cytokines is still unclear. Thus, further investigations focusing on the skull, meningeal ecosystem, and local immunity among these regions are required.
EXPERIMENTAL ANIMAL MODELS OF NEUROTROPIC VIRAL INFECTION
Different experimental animal models are required to investigate the viral life cycle, viral invasion routes in hosts, antiviral immunity, neuropathogenesis, clinical outcomes, and therapeutic strategies. Here, we focus on experimental animal species, including NHPs, artiodactyls, domestic birds, and mosquitoes, and individual routes in experimental animal models of neurotropic viral infection.
NHPs
NHP-based research has played a crucial role in understanding the neuropathogenesis of neurotropic viral infection, especially fetal infection from ZIKV (Haese et al., 2021). ZIKV infection in adult macaques is generally limited to pathologies of rash, fever, and conjunctivitis (Hirsch et al., 2017). Following subcutaneous infection, viremia can be observed as early as one day post-infection (dpi) and is usually cleared by 10 dpi (Dudley et al., 2016). During this period, ZIKV RNA can also be detected in saliva, lacrimal fluid, CSF, urine, semen, and vaginal swabs (Li et al., 2016c). These evidences suggest that the virus will develop rapid and widespread infection in the body. Fetal death in pregnancy and microcephaly in newborn babies are the most serious outcomes of ZIKV infection in humans. In NHP models, a four-fold higher rate of fetal loss occurs in ZIKV-infected rhesus macaques compared to ZIKV-unexposed animals (Dudley et al., 2018). The neurological pathologies and histopathologies found in the brains of macaque newborns are similar to those found in human neonates, including loss of neuroprogenitor cells and reduced brain size (Adams Waldorf et al., 2018; Seferovic et al., 2018). In several NHP models, certain CNS abnormalities have not yet been manifested clinically (Mavigner et al., 2018). NHP models have also been used to study the pathologies of TBEV, WNV, and DV infection. However, the low quantity of offspring and high experimental costs restrict large-scale basic and translational research. Surprisingly, the Chinese tree shrew shows benefits of safety, efficacy, and predictability for studying the neural mechanisms underlying brain diseases, including VE (Yao, 2017). With the successful application of gene-editing technology in tree shrew models (Li et al., 2017) and the release of the tree shrew genome database (Fan et al., 2014), a more powerful animal model for investigating VE should be developed in the coming years.
Rodents
Rodents, such as mice and rats, are the most common animals used for studies of neurotropic virus pathogenesis. Wild-type (WT) mice and rats are sensitive to certain neurotropic viruses, such as JEV and HSV-1, but exhibit less consistent development of encephalitis under ZIKV, DV, and WNV infection (Kennedy, 2005; Miura et al., 1988). Thus, several gene-editing and humanized mouse models have been developed to study VE. AG129 mice, which lack both type I and II interferon (IFN) responses, generate reproducible viremia and neurological symptoms, including tremors, following ZIKV infection, with peak viremia (107 plaque-forming units (PFU)/mL) at 2 dpi, high viral titers in the spleen (1 dpi) and brain (3 dpi), and robust viral replication in the testes. (Rossi et al., 2016). Recently, several groups have reported on human angiotensin-converting enzyme 2 (hACE2) transgenic mouse models for SARS-CoV-2, confirming that hACE2 is the target of SARS-CoV-2 and that the virus can rapidly spread into tissues (Bao et al., 2020; Jiang et al., 2020; Sun et al., 2020). Rodent models are also valuable tools for studying pathology and immune responses and for testing potential therapeutics and vaccines (see Table 2). Although rodents are genetically and evolutionarily distant from NHPs and humans, their dependable reproductive ability and gene-editing capability make them useful tools for studying neurotropic encephalitis.
Table 2. Animal models of neurotropic viruses.
| Species/Strain | Viral strain | Route of infection | References |
| i.c.: Intracutaneous; i.d.: Intradermal; i.m.: Intramuscular; i.n.: Intranasal; i.p.: Intraperitoneal; i.v.: Intravenous; s.c.: Subcutaneous. | |||
| NHPs | |||
| Macaca sylvanus | TBEV | s.c. | Kenyon et al., 1992; Süss et al., 2007 |
| ZIKV | s.c. | Adams Waldorf et al., 2016, 2018 | |
| Rhesus macaque | WNV | i.d. | Verstrepen et al., 2014 |
| DV | s.c./i.d. | Li et al., 2013 | |
| HCMV | i.p. | Tarantal et al., 1998 | |
| ZIKV | s.c. | Dudley et al., 2016; Martinot et al., 2018 | |
| SARS-CoV-2 | Intratracheal/i.n./ocular | Gao et al., 2020; Munster et al., 2020; Shan et al., 2020 | |
| Callithrix jacchus | WNV | i.d. | Verstrepen et al., 2014 |
| Marmoset | ZIKV | s.c. | Dudley et al., 2018 |
| Squirrel monkey | ZIKV | i.d. | de Alcantara et al., 2021 |
| Olive baboon | ZIKV | s.c. | Gurung et al., 2019 |
| Tree shrew | HSV-1/2 | i.v./i.p./s.c./ocular | Darai et al., 1978; Li et al., 2016b |
| Influenza | i.n. | Yang et al., 2013 | |
| Coxsackie virus A16 | Nasal spraying | Li et al., 2014 | |
| ZIKV | s.c. | Zhang et al., 2019b | |
| SARS-CoV-2 | Oral/i.n./ocular | Xu et al., 2020 | |
| Rodents | |||
| C57BL/6 | JEV | i.v./i.p./footpad | Grossberg & Scherer, 1966; Miura et al., 1988 |
| DV | i.p. | Byrne et al., 2021 | |
| HSV-1 | i.n./i.v./corneal | Xiao et al., 2001 | |
| EV71 | Intracranial | Luo et al., 2019 | |
| BABl/c | JEV | i.p. | Saxena et al., 2008 |
| DV | i.p./i.c. | Byrne et al., 2021; Li et al., 2013 | |
| μMT | WNV | Footpad | Diamond et al., 2003a |
| sIgM-/- | WNV | Footpad | Diamond et al., 2003b |
| RAG1-/- | WNV | Footpad | Throsby et al., 2006 |
| Ifnar1-/- | ZIKV | i.v./footpad | Lazear et al., 2016 |
| IRF3-/- IRF5-/- IRF7-/- |
ZIKV | Retro-orbital | • Lazear et al., 2016; Li et al., 2016a |
| AG129 | ZIKV | i.v./i.p./footpad/s.c. | Aliota et al., 2016; Rossi et al., 2016; Sumathy et al., 2017; Xie et al., 2011 |
| DV | i.p./i.v. | Brewoo et al., 2012; Fuchs et al., 2014 | |
| NSG transplanted with CD34+ HSPC | DV | Footpad/era via mosquito biting | Cox et al., 2012 |
| HepG2-grafted SCID | DV | i.p. | An et al., 1999 |
| hSCARB2-transgenic | CV-A16 | i.n. | Chen et al., 2021 |
| HFH4-hACE2 | SARS-CoV-2 | i.n. | Jiang et al., 2020 |
| hACE2 transgene | SARS-CoV -2 | i.n./i.g. | Bao et al., 2020; Sun et al., 2020 |
| K18-hACE2 | SARS-CoV-2 | i.n. | Winkler et al., 2020 |
| C3H/He | RABV | i.m. | Mifune et al., 1980 |
| ICR | RABV | Footpad | Smith, 1981 |
| Kunming | RABV | i.m. | Zhang et al., 2016 |
| ZIKV | s.c. | Yu et al., 2017 | |
| Rat | HSV-1/2 | Oral mucosa/i.c. | Bergström et al., 1991; Hirsch et al., 1984 |
| RABV | i.m. | Ren et al., 2021 | |
| Golden hamster | RABV | i.m. | Zhang et al., 2016 |
| SARS-CoV-2 | i.n. | Chan et al., 2020 | |
| Artiodactyls | |||
| Pig/swine | JEV | Oronasal/s.c. | Ricklin et al., 2016 |
| Beagle | RABV | i.m. | Fekadu et al., 1982 |
| Horse | WNV | s.c. | Meyer et al., 1931 |
| Cattle | JEV | s.c. | Kimura et al., 2010 |
| Domestic birds | |||
| Chicken | JEV | s.c. | Fan et al., 2019 |
| Duckling | JEV | s.c. | Xiao et al., 2018 |
| Great egret | JEV | s.c. | Nemeth et al., 2012 |
| Mosquitoes | |||
| Culex tritaeniorhynchus | JEV | Intrathoracic | Buescher et al., 1959 |
| Culex pipiens | JEV | Intrathoracic | Hameed et al., 2019 |
| Aedes aegypti | DV | Midgut | Choy et al., 2020 |
| Aedes albopictus | DV | Salivary glands | Pompon et al., 2017 |
| Aedes aegypti | ZIKV | Intrathoracic | Boorman & Porterfield, 1956 |
| Aedes aegypti/ unilineatus/ vittatus/ luteocephalus | ZIKV | Oral | Diagne et al., 2015 |
| Aedes albopictus | ZIKV | Oral | Wong et al., 2013 |
| Culex p. quinquefasciatus | ZIKV | Oral | Guo et al., 2016 |
| Culex annulirostris | ZIKV | Oral | Duchemin et al., 2017 |
Other animal models
Artiodactyls, domestic birds, and mosquitoes have also been used for studying the transmission cycles of neurotropic viruses. Some viruses can replicate in mosquitoes and their zoonotic life cycle can be maintained in vertebrate hosts. While pigs and domestic birds can potentially act as amplifying or reservoir hosts, humans are considered dead-end hosts (Hameed et al., 2021). Understanding these transmission cycles will help to develop preventive measures, such as vector control and vaccination in animals.
Diagnosis of neurotropic virus diseases
In 2013, the International Encephalitis Consortium released guidelines related to case definitions, diagnostic algorithms, and priorities for diagnosing encephalitis (Venkatesan et al., 2013). The diagnostic strategies present clinical, neuroimaging, and laboratory tests, including major and minor criteria, with presumed viral infectious encephalitis given priority examination (as per Table 3) (Fillatre et al., 2017; Venkatesan et al., 2013). The Consortium also proposed an etiological examination algorithm, including CSF examination, skin and serum antibody detection, and other peripheral examinations, including skin changes, tracheoscopy biopsy, throat swab, and stool and urine culture.
Table 3. Diagnostic tests preferred for suspected etiology.
| Causative agents | Diagnostic tests |
| CSF: Cerebrospinal fluid; EBNA: Epstein-Barr nuclear antigen; RT-PCR: Reverse transcription polymerase chain reaction; VCA: Viral capsid antigen. | |
| HSV-1/2 | HSV-1/2 PCR: if negative and highly suspected, repeat within 3–7 days with CSF sent for HSV PCR; if test available, consider HSV CSF IgG and IgM in addition |
| VZV | CSF: VZV IgG |
| Enterovirus | CSF: EV PCR; Sensitivity may be low, if test available, consider throat swab and stool sent for EV PCR |
| EBV | EBV serology: VCA IgG and IgM and EBNA IgG |
| HHV-6 | CSF: HHV-6 PCR/Photoconductive relay |
| Influenza | Culture/Antigen detection/Respiratory secretion PCR |
| JEV | CSF/serum: JEV PCR/CSF: IgM/Serology: IgM |
| ZIKV/DV/ CHIKV | CSF/serum: RT-PCR/CSF: IgM/Serology: IgG and IgM |
| Measles virus | Plasma/CSF serology/CSF: PCR |
| CMV | CSF: PCR/IgM |
| JCV | CSF: RT-PCR/IgM/Serology: IgG and IgM/Plasma antigen |
| Rabies/ABLV | Rabies/ABLV testing: serological analysis of serum and CSF; viral isolation or RT-PCR from saliva; tests for viral antigen or histopathology on either a brain biopsy or full-thickness biopsy of nape of neck |
Perspectives for animal model use in VE research
For decades, scientists and physicians have pursued innovative therapeutic strategies to combat viral infections, including IFN, immunoglobulin, and ribavirin treatment, due to increasing clinical demands. Standard therapeutic compounds that target receptors or enzymes involved in essential viral functions have focused on host cell factors, with drug resistance, cytotoxicity, and cellular side effects remaining significant disadvantages. As such, computational screening of small molecular drugs, nucleic acid-based antivirals, and monoclonal antibodies that target virus-conserved proteins provides an alternative strategy to target the development of viral replication (Joe et al., 2022; Laulund et al., 2020; Lundin et al., 2006). Classic drug screening is a cost-effective and time-efficient technique to identify potential drug candidates, allowing hundreds of candidates to be tested at the cellular level in vitro, using viral titers as readouts. For example, remdesivir and chloroquine, which are effective at inhibiting SARS-CoV-2 replication, can be rapidly screened in vitro (Wang et al., 2020). However, further study is required to examine pharmacokinetics and drug metabolism in vivo, particularly given the presence of the BBB.
Animal models are indispensable for investigating human diseases and therapeutic interventions. Although rodents are widely used, certain pathological phenotypes and immune responses cannot be fully recapitulated in small animals and in vitro culture systems. NHP models are ideal experimental tools for studying pathology, immunity, and therapeutic efficacy. However, limitations in terms of animal feeding, inbreeding, and long experimental periods have restricted the use of NHP models in VE research. Thus, the development of viable animal models, such as the Chinese tree shrew, may provide early diagnostic tools and contribute to the development of effective therapies.
CONCLUSIONS AND FUTURE PERSPECTIVES
Identifying and classifying neurotropic virus species can help epidemiologists and clinicians to respond quickly and accelerate basic research. In the current review, we discussed the categories of common neurotropic viruses (Table 1), which remain the primary pathogens of VE. Newly identified SARS-CoV-2 can enter the CNS and generate neuroinflammation (Meinhardt et al., 2021), thus we included SARS-CoV-2 as a new neurotropic viral candidate. Compared to DNA viruses, neurotropic RNA viruses are endowed with the ability to mutate frequently, leading to larger infectious populations. The invasion and transmission routes of neurotropic viruses in humans are becoming increasingly diverse. Beyond classical invasion routes of the BBB, peripheral nerve migration, and microvascular endothelial cells, the MLV system also exhibits the ability to infect and transport neurotropic viruses from the CNS to the periphery (Li et al., 2022). This beneficial behavior can enhance peripheral immunity against intracranial viral infection. However, the efflux function of MLVs can be damaged (Li et al., 2022). With aged individuals exhibiting recession MLV function, dysfunction of MLVs can promote amyloid-β deposition in the meninges and aggravate parenchymal amyloid-β accumulation in transgenic mouse models of AD (Da Mesquita et al., 2018). These findings suggest that neurotropic virus-infected aged individuals may experience an increased risk of developing neurodegenerative diseases, such as AD or Parkinson’s disease. Further investigation is necessary to understand the impact of neurotropic virus-infected MLVs in triggering or accelerating the development of neurodegenerative diseases. Studies have shown that IL-6 induced by genotoxic stress may promote lymphangiogenesis in the bones, including the cranium, which may contribute to bone and hematopoietic regeneration (Biswas et al., 2023). However, it is still unclear whether and how the intracranial inflammatory cytokines induced by viral infection in the CNS, contributes to the expansion and functional impairment of MLVs. Apart from VEGF-C, the specific factors, such as inflammatory cytokines and chemokines, that modulate the proliferation, migration, and differentiation of LECs and influence the structure and function of MLVs in the meninges during viral infection are yet to be determined.
The generation of type I IFN in response to intracranial neurotropic viruses has been demonstrated in parenchymal neurons and immune cells, including residential microglia and peripheral-infiltrating leukocytes. Upon mobilization, B lymphocytes will encounter viral antigens that stimulate their maturation and differentiation into plasmacytes, which then secrete virus-specific antibodies to neutralize viral particles (Figure 2). Interestingly, meningeal immunity was recently discovered to play an important role in antiviral immune defense in the CNS (Li et al., 2022; Rebejac et al., 2022). Immune cells, including many myeloid cells, are harbored in the subarachnoid lymphatic-like membrane (SLYM) and may participate in CNS immunity (Møllgård et al., 2023). Mucosal-associated invariant (MAIT) cells in the meninges preserve meningeal barrier integrity and restrict neuroinflammation in the brain (Zhang et al., 2022b). These findings imply that resident immune cells in the meningeal ecosystem possess many unexplored features in immune defense against diseases, including VE. The importance of peripheral-infiltrating immune cells in the clearance of intracranial pathogens is indisputable. However, it remains unclear which cells take priority in the mobilization of resident immune cells in the meninges or periphery. In addition, the routes through which meningeal immune cells shuttle between the peripheral lymph nodes and the brain parenchyma are yet to be explored. Of course, several barriers still exist in the field. First, using LEC-specific Cre recombinase or photoinitiators to ablate MLVs can result in fetal death or low-deleting area and efficiency. Second, monitoring the transmission paths of viruses and dynamic migration of immune cells in real-time in the meninges and deep brain parenchyma is challenging. Third, observing lymphangiogenesis of the MLVs with LEC-specific lineage tracing in diseased animal models remains difficult.
As summarized in this review, common animal model-based experiments can provide first-hand evidence of the pathologies of VE, including routes of infection (Table 2). While NHP models are invaluable for pathological and therapeutic development studies of VE, the difficulty in consecutively obtaining brain samples from individual animals has restricted cellular mechanism studies of pathogenesis. In vitro organoid co-culture systems are beneficial supplements, although they cannot fully simulate pathological conditions of VE. However, such systems have the advantage of cell tropism in combination with the expression of receptors necessary for viral entry and are useful models for drug screening and therapeutic testing (Antonucci & Gehrke, 2019; Depla et al., 2022). Finally, improving the delivery efficiency and accuracy of antiviral drugs remains an unsolved clinical issue. Engineering precision nanoparticle drug delivery systems (NDDS) (Mitchell et al., 2021) and virus-based nanoparticles (VNPs) (Li et al., 2019) may provide new insights to ameliorate those problems. For example, FDA-approved Plegridy is an injectable nanomedicine for relapsing forms of multiple sclerosis that offers low-dosing frequency (Mitchell et al., 2021). VNPs work well with different types of cargo, including inorganic nanoparticles and proteins (Li et al., 2019). Modulating the function of MLVs in animal models of disease, including VE, may improve disease outcomes (Da Mesquita et al., 2018; Hu et al., 2020; Li et al., 2022). Furthermore, altering drug delivery and administration sites, such as the meninges, may provide an improved therapeutic strategy.
However, how to integrate animal models and advanced live tracing systems to consecutively visualize the spread of neurotropic viruses, lymphangiogenesis, and mobilization of immune cells in the brain is still a challenge. How to improve outcomes for VE patients with neoteric therapeutics and modified drug delivery also remain urgent tasks.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
D.Y., X.J.L., and D.Z.T. wrote the original draft and constructed the figures and tables. X.L.L. and B.W. conceptualized, wrote, and edited the manuscript. All authors read and approved the final version of the manuscript.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81825011, 81930038, 81961160738), Program of Shanghai Academic/Technology Research Leader (22XD1400800), and Strategic Priority Research Program of the Chinese Academy of Sciences (XDB19030200)
Contributor Information
Xiu-Li Li, Email: ldyy_lixl@lzu.edu.cn.
Bin Wei, Email: weibinwhy@shu.edu.cn.
References
- Adams Waldorf KM, Nelson BR, Stencel-Baerenwald JE, et al Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nature Medicine. 2018;24(3):368–374. doi: 10.1038/nm.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, et al Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nature Medicine. 2016;22(11):1256–1259. doi: 10.1038/nm.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agut H, Bonnafous P, Gautheret-Dejean A Laboratory and clinical aspects of human herpesvirus 6 infections. Clinical Microbiology Reviews. 2015;28(2):313–335. doi: 10.1128/CMR.00122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A, Heikenwalder M, Polymenidou M Insights into prion strains and neurotoxicity. Nature Reviews Molecular Cell Biology. 2007;8(7):552–561. doi: 10.1038/nrm2204. [DOI] [PubMed] [Google Scholar]
- Albe JR, Boyles DA, Walters AW, et al Neutrophil and macrophage influx into the central nervous system are inflammatory components of lethal Rift Valley fever encephalitis in rats. PLoS Pathogens. 2019;15(6):e1007833. doi: 10.1371/journal.ppat.1007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexaki A, Wigdahl B HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. PLoS Pathogens. 2008;4(12):e1000215. doi: 10.1371/journal.ppat.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleyas AG, George JA, Han YW, et al Functional modulation of dendritic cells and macrophages by Japanese encephalitis virus through MyD88 adaptor molecule-dependent and -independent pathways. The Journal of Immunology. 2009;183(4):2462–2474. doi: 10.4049/jimmunol.0801952. [DOI] [PubMed] [Google Scholar]
- Aleyas AG, Han YW, Patil AM, et al Impaired cross-presentation of CD8α+CD11c+ dendritic cells by Japanese encephalitis virus in a TLR2/MyD88 signal pathway-dependent manner. European Journal of Immunology. 2012;42(10):2655–2666. doi: 10.1002/eji.201142052. [DOI] [PubMed] [Google Scholar]
- Aliota MT, Caine EA, Walker EC, et al Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Neglected Tropical Diseases. 2016;10(4):e0004682. doi: 10.1371/journal.pntd.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Obaidi MMJ, Bahadoran A, Har LS, et al Japanese encephalitis virus disrupts blood-brain barrier and modulates apoptosis proteins in THBMEC cells. Virus Research. 2017;233:17–28. doi: 10.1016/j.virusres.2017.02.012. [DOI] [PubMed] [Google Scholar]
- An J, Kimura-Kuroda J, Hirabayashi Y, et al Development of a novel mouse model for dengue virus infection. Virology. 1999;263(1):70–77. doi: 10.1006/viro.1999.9887. [DOI] [PubMed] [Google Scholar]
- Ancuta P, Wang JB, Gabuzda D CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. Journal of Leukocyte Biology. 2006;80(5):1156–1164. doi: 10.1189/jlb.0206125. [DOI] [PubMed] [Google Scholar]
- Antonucci J, Gehrke L Cerebral organoid models for neurotropic viruses. ACS Infectious Diseases. 2019;5(12):1976–1979. doi: 10.1021/acsinfecdis.9b00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjona A, Foellmer HG, Town T, et al Abrogation of macrophage migration inhibitory factor decreases West Nile virus lethality by limiting viral neuroinvasion. Journal of Clinical Investigation. 2007;117(10):3059–3066. doi: 10.1172/JCI32218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai FW, Kong KF, Dai JF, et al A paradoxical role for neutrophils in the pathogenesis of West Nile virus. The Journal of Infectious Diseases. 2010;202(12):1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale JF Jr Virus and immune-mediated encephalitides: epidemiology, diagnosis, treatment, and prevention. Pediatric Neurology. 2015;53(1):3–12. doi: 10.1016/j.pediatrneurol.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiology of Disease. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Bao LL, Deng W, Huang BY, et al The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583(7818):830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- Beier KT, Saunders A, Oldenburg IA, et al Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(37):15414–15419. doi: 10.1073/pnas.1110854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CC, Altman JD, Hinton D, et al Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. The Journal of Immunology. 1999;163(6):3379–3387. doi: 10.4049/jimmunol.163.6.3379. [DOI] [PubMed] [Google Scholar]
- Bergmann CC, Lane TE, Stohlman SA Coronavirus infection of the central nervous system: host-virus stand-off. Nature Reviews Microbiology. 2006;4(2):121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CC, Parra B, Hinton DR, et al Perforin-mediated effector function within the central nervous system requires IFN-γ-mediated MHC up-regulation. The Journal of Immunology. 2003;170(6):3204–3213. doi: 10.4049/jimmunol.170.6.3204. [DOI] [PubMed] [Google Scholar]
- Bergström T, Svennerholm B, Conradi N, et al Discrimination of herpes simplex virus types 1 and 2 cerebral infections in a rat model. Acta Neuropathologica. 1991;82(5):395–401. doi: 10.1007/BF00296551. [DOI] [PubMed] [Google Scholar]
- Biswas L, Chen JY, De Angelis J, et al Lymphatic vessels in bone support regeneration after injury. Cell. 2023;186(2):382–397.e24. doi: 10.1016/j.cell.2022.12.031. [DOI] [PubMed] [Google Scholar]
- Boorman JPT, Porterfield JS A simple technique for infection of mosquitoes with viruses transmission of Zika virus. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1956;50(3):238–242. doi: 10.1016/0035-9203(56)90029-3. [DOI] [PubMed] [Google Scholar]
- Boutros T, Croze E, Yong VW Interferon-beta is a potent promoter of nerve growth factor production by astrocytes. Journal of Neurochemistry. 1997;69(3):939–946. doi: 10.1046/j.1471-4159.1997.69030939.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw MJ, Venkatesan A Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics. 2016;13(3):493–508. doi: 10.1007/s13311-016-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewoo JN, Kinney RM, Powell TD, et al Immunogenicity and efficacy of chimeric dengue vaccine (DENVax) formulations in interferon-deficient AG129 mice. Vaccine. 2012;30(8):1513–1520. doi: 10.1016/j.vaccine.2011.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioschi S, Wang WL, Peng V, et al Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science. 2021;373(6553):eabf9277. doi: 10.1126/science.abf9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell AD, Reynolds TQ, Livingston B, et al Human parechovirus-3 encephalitis in two neonates: acute and follow-up magnetic resonance imaging and evaluation of central nervous system markers of inflammation. Pediatric Neurology. 2015;52(2):245–249. doi: 10.1016/j.pediatrneurol.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Buescher EL, Scherer WF, Rosenberg MZ, et al Ecologic studies of Japanese encephalitis virus in Japan. II. Mosquito infection. The American Journal of Tropical Medicine and Hygiene. 1959;8(6):651–664. doi: 10.4269/ajtmh.1959.8.651. [DOI] [PubMed] [Google Scholar]
- Byrne AB, García AG, Brahamian JM, et al A murine model of dengue virus infection in suckling C57BL/6 and BALB/c mice. Animal Models and Experimental Medicine. 2021;4(1):16–26. doi: 10.1002/ame2.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes AP, Durbin JE, Griffin DE Control of Sindbis virus infection by antibody in interferon-deficient mice. Journal of Virology. 2000;74(8):3905–3908. doi: 10.1128/JVI.74.8.3905-3908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabre P, Smadja D, Cabié A, et al HTLV-1 and HIV infections of the central nervous system in tropical areas. Journal of Neurology, Neurosurgery & Psychiatry. 2000;68(5):550–557. doi: 10.1136/jnnp.68.5.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casiraghi C, Dorovini-Zis K, Horwitz MS. 2011. Epstein-Barr virus infection of human brain microvessel endothelial cells: a novel role in multiple sclerosis. Journal of Neuroimmunology, 230(1–2): 173–177.
- Chan JFW, Zhang AJ, Yuan SF, et al Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in a golden syrian hamster model: implications for disease pathogenesis and transmissibility. Clinical Infectious Diseases. 2020;71(9):2428–2446. doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapagain ML, Nerurkar VR Human polyomavirus JC (JCV) infection of human B lymphocytes: a possible mechanism for JCV transmigration across the blood-brain barrier. The Journal of Infectious Diseases. 2010;202(2):184–191. doi: 10.1086/653823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran MCJ, Hu SX, Sheng WS, et al Differential responses of human brain cells to West Nile virus infection. Journal of Neurovirology. 2005;11(6):512–524. doi: 10.1080/13550280500384982. [DOI] [PubMed] [Google Scholar]
- Cheeran MCJ, Lokensgard JR, Schleiss MR Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clinical Microbiology Reviews. 2009;22(1):99–126. doi: 10.1128/CMR.00023-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BP, Kuziel WA, Lane TE Lack of CCR2 results in increased mortality and impaired leukocyte activation and trafficking following infection of the central nervous system with a neurotropic coronavirus. The Journal of Immunology. 2001;167(8):4585–4592. doi: 10.4049/jimmunol.167.8.4585. [DOI] [PubMed] [Google Scholar]
- Chen JM, Wang LM, Xu H, et al Meningeal lymphatics clear erythrocytes that arise from subarachnoid hemorrhage. Nature Communications. 2020;11(1):3159. doi: 10.1038/s41467-020-16851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Deng Y, Pan DL MicroRNA regulation of human herpesvirus latency. Viruses. 2022;14(6):1215. doi: 10.3390/v14061215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Li H, Yang JX, et al A hSCARB2-transgenic mouse model for Coxsackievirus A16 pathogenesis. Virology Journal. 2021;18(1):84. doi: 10.1186/s12985-021-01557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZZ, Zhong D, Li GZ The role of microglia in viral encephalitis: a review. Journal of Neuroinflammation. 2019;16(1):76. doi: 10.1186/s12974-019-1443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SS, Liu H, Chuang CK, et al Fitness of Japanese encephalitis virus to Neuro-2a cells is determined by interactions of the viral envelope protein with highly sulfated glycosaminoglycans on the cell surface. Journal of Medical Virology. 2005;76(4):583–592. doi: 10.1002/jmv.20406. [DOI] [PubMed] [Google Scholar]
- Choy MM, Ng DHL, Siriphanitchakorn T, et al A non-structural 1 protein G53D substitution attenuates a clinically tested live dengue vaccine. Cell Reports. 2020;31(6):107617. doi: 10.1016/j.celrep.2020.107617. [DOI] [PubMed] [Google Scholar]
- Christian KM, Song HJ, Ming GL Pathophysiology and mechanisms of Zika virus infection in the nervous system. Annual Review of Neuroscience. 2019;42:249–269. doi: 10.1146/annurev-neuro-080317-062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay CC, Rodrigues DS, Ho YS, et al Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. Journal of Virology. 2007;81(21):12040–12048. doi: 10.1128/JVI.00133-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine DG Rabies transmission by nonbite route. Public Health Reports. 1962;77(4):287–289. doi: 10.2307/4591470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Mota J, Sukupolvi-Petty S, et al Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. Journal of Virology. 2012;86(14):7637–7649. doi: 10.1128/JVI.00534-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr HF, Knopf PM Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunology Today. 1992;13(12):507–512. doi: 10.1016/0167-5699(92)90027-5. [DOI] [PubMed] [Google Scholar]
- Cugurra A, Mamuladze T, Rustenhoven J, et al Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science. 2021;373(6553):eabf7844. doi: 10.1126/science.abf7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvjetković IH, Cvjetković D, Patić A, et al. 2016. Tick-borne encephalitis virus infection in humans. Medicinski Pregled, 69(3–4): 93–98.
- Da Mesquita S, Louveau A, Vaccari A, et al Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. 2018;560(7717):185–191. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva SR, Gao SJ Zika virus: an update on epidemiology, pathology, molecular biology, and animal model. Journal of Medical Virology. 2016;88(8):1291–1296. doi: 10.1002/jmv.24563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai JF, Wang PH, Bai FW, et al ICAM-1 participates in the entry of West Nile virus into the central nervous system. Journal of Virology. 2008;82(8):4164–4168. doi: 10.1128/JVI.02621-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Prat A The blood-brain barrier. Cold Spring Harbor Perspectives in Biology. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darai G, Schwaier A, Komitowski D, et al Experimental infection of Tupaia belangeri (tree shrews) with herpes simplex virus types 1 and 2. The Journal of Infectious Diseases. 1978;137(3):221–226. doi: 10.1093/infdis/137.3.221. [DOI] [PubMed] [Google Scholar]
- Das T, Hoarau JJ, Bandjee MCJ, et al. 2015. Multifaceted innate immune responses engaged by astrocytes, microglia and resident dendritic cells against Chikungunya neuroinfection. Journal of General Virology, 96(Pt 2): 294–310.
- de Alcantara BN, Imbeloni AA, de Brito Simith Durans D, et al Histopathological lesions of congenital Zika syndrome in newborn squirrel monkeys. Scientific Reports. 2021;11(1):6099. doi: 10.1038/s41598-021-85571-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima KA, Rustenhoven J, Kipnis J Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annual Review of Immunology. 2020;38:597–620. doi: 10.1146/annurev-immunol-102319-103410. [DOI] [PubMed] [Google Scholar]
- Delhaye S, Paul S, Blakqori G, et al Neurons produce type I interferon during viral encephalitis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(20):7835–7840. doi: 10.1073/pnas.0602460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depla JA, Mulder LA, de Sá RV, et al Human brain organoids as models for central nervous system viral infection. Viruses. 2022;14(3):634. doi: 10.3390/v14030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagne CT, Diallo D, Faye O, et al Potential of selected senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infectious Diseases. 2015;15:492. doi: 10.1186/s12879-015-1231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Shrestha B, Marri A, et al B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. Journal of Virology. 2003a;77(4):2578–2586. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Sitati EM, Friend LD, et al A critical role for induced IgM in the protection against West Nile virus infection. Journal of Experimental Medicine. 2003b;198(12):1853–1862. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B, Kao M, Zheng YM, et al Delineation of putative mechanisms involved in antibody-mediated clearance of rabies virus from the central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(15):7252–7256. doi: 10.1073/pnas.89.15.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding SW RNA-based antiviral immunity. Nature Reviews Immunology. 2010;10(9):632–644. doi: 10.1038/nri2824. [DOI] [PubMed] [Google Scholar]
- Duchemin JB, Mee PT, Lynch SE, et al Zika vector transmission risk in temperate Australia: a vector competence study. Virology Journal. 2017;14(1):108. doi: 10.1186/s12985-017-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DM, Aliota MT, Mohr EL, et al A rhesus macaque model of Asian-lineage Zika virus infection. Nature Communications. 2016;7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DM, Van Rompay KK, Coffey LL, et al Miscarriage and stillbirth following maternal Zika virus infection in nonhuman primates. Nature Medicine. 2018;24(8):1104–1107. doi: 10.1038/s41591-018-0088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Rhea EM, Knopp RC, et al Interactions of SARS-CoV-2 with the Blood-Brain barrier. International Journal of Molecular Sciences. 2021;22(5):2681. doi: 10.3390/ijms22052681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falasco RF, Robinson E, Faja BW Problems encountered by recent graduates in establishing dental practices. The Journal of the Michigan Dental Association. 1990;72(1):15–19. [PubMed] [Google Scholar]
- Fan Y, Yu DD, Yao YG Tree shrew database (TreeshrewDB): a genomic knowledge base for the Chinese tree shrew. Scientific Reports. 2014;4:7145. doi: 10.1038/srep07145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YC, Liang JJ, Chen JM, et al NS2B/NS3 mutations enhance the infectivity of genotype I Japanese encephalitis virus in amplifying hosts. PLoS Pathogens. 2019;15(8):e1007992. doi: 10.1371/journal.ppat.1007992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Liu ZZ, Qiu Y, et al. 2021. Inhibition of viral suppressor of RNAi proteins by designer peptides protects from enteroviral infection in vivo. Immunity, 54(10): 2231–2244. e6.
- Fekadu M, Shaddock JH, Baer GM Excretion of rabies virus in the saliva of dogs. The Journal of Infectious Diseases. 1982;145(5):715–719. doi: 10.1093/infdis/145.2.715. [DOI] [PubMed] [Google Scholar]
- Fekete R, Cserép C, Lénárt N, et al Microglia control the spread of neurotropic virus infection via P2Y12 signalling and recruit monocytes through P2Y12-independent mechanisms. Acta Neuropathologica. 2018;136(3):461–482. doi: 10.1007/s00401-018-1885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczy MW, Marshall LJ, Nelson CDS, et al Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clinical Microbiology Reviews. 2012;25(3):471–506. doi: 10.1128/CMR.05031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiette L, Aubert C, Müller U, et al Theiler's virus infection of 129Sv mice that lack the interferon α/β or interferon γ receptors. Journal of Experimental Medicine. 1995;181(6):2069–2076. doi: 10.1084/jem.181.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillatre P, Crabol Y, Morand P, et al Infectious encephalitis: management without etiological diagnosis 48 hours after onset. Médecine et Maladies Infectieuses. 2017;47(3):236–251. doi: 10.1016/j.medmal.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DL, Defres S, Solomon T Measles-induced encephalitis. QJM:An International Journal of Medicine. 2015;108(3):177–182. doi: 10.1093/qjmed/hcu113. [DOI] [PubMed] [Google Scholar]
- Fuchs J, Chu HY, O'Day P, et al Investigating the efficacy of monovalent and tetravalent dengue vaccine formulations against DENV-4 challenge in AG129 mice. Vaccine. 2014;32(48):6537–6543. doi: 10.1016/j.vaccine.2014.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Bao LL, Mao HY, et al Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber C, Soung A, Vollmer LL, et al T cells promote microglia-mediated synaptic elimination and cognitive dysfunction during recovery from neuropathogenic flaviviruses. Nature Neuroscience. 2019;22(8):1276–1288. doi: 10.1038/s41593-019-0427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Nicolás O, Braun RO, Milona P, et al Targeting of the nasal mucosa by Japanese encephalitis virus for non-vector-borne transmission. Journal of Virology. 2018;92(24):e01091–18. doi: 10.1128/JVI.01091-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden DH, Dueland AN, Cohrs R, et al Preherpetic neuralgia. Neurology. 1991;41(8):1215–1218. doi: 10.1212/WNL.41.8.1215. [DOI] [PubMed] [Google Scholar]
- Glass WG, Lane TE Functional expression of chemokine receptor CCR5 on CD4+ T cells during virus-induced central nervous system disease. Journal of Virology. 2003;77(1):191–198. doi: 10.1128/JVI.77.1.191-198.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass WG, Lim JK, Cholera R, et al Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. Journal of Experimental Medicine. 2005;202(8):1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JM, Bergmann CC, Ramakrishna C, et al Inhibition of interferon-γ signaling in oligodendroglia delays coronavirus clearance without altering demyelination. The American Journal of Pathology. 2006;168(3):796–804. doi: 10.2353/ajpath.2006.050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Scarano F, Martín-García J The neuropathogenesis of AIDS. Nature Reviews Immunology. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gori Savellini G, Anichini G, Gandolfo C, et al Toscana virus non-structural protein NSs acts as E3 ubiquitin ligase promoting RIG-I degradation. PLoS Pathogens. 2019;15(12):e1008186. doi: 10.1371/journal.ppat.1008186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE, Metcalf T Clearance of virus infection from the CNS. Current Opinion in Virology. 2011;1(3):216–221. doi: 10.1016/j.coviro.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg SE, Scherer WF The effect of host age, virus dose and route of inoculation on inapparent infection in mice with Japanese encephalitis virus. Experimental Biology and Medicine. 1966;123(1):118–124. doi: 10.3181/00379727-123-31418. [DOI] [PubMed] [Google Scholar]
- Guo XX, Li CX, Deng YQ, et al Culex pipiens quinquefasciatus: a potential vector to transmit Zika virus. Emerging Microbes & Infections. 2016;5(9):e102. doi: 10.1038/emi.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung S, Reuter N, Preno A, et al Zika virus infection at mid-gestation results in fetal cerebral cortical injury and fetal death in the olive baboon. PLoS Pathogens. 2019;15(1):e1007507. doi: 10.1371/journal.ppat.1007507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haese NN, Roberts VHJ, Chen A, et al Nonhuman primate models of Zika virus infection and disease during pregnancy. Viruses. 2021;13(10):2088. doi: 10.3390/v13102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed M, Liu K, Anwar MN, et al The emerged genotype I of Japanese encephalitis virus shows an infectivity similar to genotype III in Culex pipiens mosquitoes from China. PLoS Neglected Tropical Diseases. 2019;13(9):e0007716. doi: 10.1371/journal.pntd.0007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed M, Wahaab A, Nawaz M, et al Potential role of birds in Japanese encephalitis virus zoonotic transmission and genotype shift. Viruses. 2021;13(3):357. doi: 10.3390/v13030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harling-Berg CJ, Park TJ, Knopf PM Role of the cervical lymphatics in the Th2-type hierarchy of CNS immune regulation. Journal of Neuroimmunology. 1999;101(2):111–127. doi: 10.1016/S0165-5728(99)00130-7. [DOI] [PubMed] [Google Scholar]
- Heininger U, Seward JF Varicella. The Lancet. 2006;368(9544):1365–1376. doi: 10.1016/S0140-6736(06)69561-5. [DOI] [PubMed] [Google Scholar]
- Hemachudha T, Ugolini G, Wacharapluesadee S, et al Human rabies: neuropathogenesis, diagnosis, and management. The Lancet Neurology. 2013;12(5):498–513. doi: 10.1016/S1474-4422(13)70038-3. [DOI] [PubMed] [Google Scholar]
- Hirsch AJ, Smith JL, Haese NN, et al Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathogens. 2017;13(3):e1006219. doi: 10.1371/journal.ppat.1006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JM, Johansson SL, Vahlne A Effect of snuff and herpes simplex virus-1 on rat oral mucosa: possible associations with the development of squamous cell carcinoma. Journal of Oral Pathology & Medicine. 1984;13(1):52–62. doi: 10.1111/j.1600-0714.1984.tb01400.x. [DOI] [PubMed] [Google Scholar]
- Hooi YT, Ong KC, Tan SH, et al Coxsackievirus A16 in a 1-day-old mouse model of central nervous system infection shows lower neurovirulence than enterovirus A71. Journal of Comparative Pathology. 2020;176:19–32. doi: 10.1016/j.jcpa.2020.02.001. [DOI] [PubMed] [Google Scholar]
- Houen G, Trier NH, Frederiksen JL Epstein-barr virus and multiple sclerosis. Frontiers in Immunology. 2020;11:587078. doi: 10.3389/fimmu.2020.587078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Rayasam A, Kijak JA, et al Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nature Communications. 2019;10(1):229. doi: 10.1038/s41467-018-08163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XT, Deng QP, Ma L, et al Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Research. 2020;30(3):229–243. doi: 10.1038/s41422-020-0287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang RD, Liu MQ, Chen Y, et al Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell. 2020;182(1):50–58.e8. doi: 10.1016/j.cell.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe S, Salam AAA, Neogi U, et al Antiviral drug research for Japanese encephalitis: an updated review. Pharmacological Reports. 2022;74(2):273–296. doi: 10.1007/s43440-022-00355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RT, Burke DS, Elwell M, et al Japanese encephalitis: immunocytochemical studies of viral antigen and inflammatory cells in fatal cases. Annals of Neurology. 1985;18(5):567–573. doi: 10.1002/ana.410180510. [DOI] [PubMed] [Google Scholar]
- Jordan I, Ian Lipkin W Borna disease virus. Reviews in Medical Virology. 2001;11(1):37–57. doi: 10.1002/rmv.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, et al Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Molecular and Cellular Biology. 2000;20(11):4106–4114. doi: 10.1128/MCB.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PGE Viral encephalitis. Journal of Neurology. 2005;252(3):268–272. doi: 10.1007/s00415-005-0770-7. [DOI] [PubMed] [Google Scholar]
- Kenyon RH, Rippy MK, McKee KT Jr, et al Infection of Macaca radiata with viruses of the tick-borne encephalitis group. Microbial Pathogenesis. 1992;13(5):399–409. doi: 10.1016/0882-4010(92)90083-Z. [DOI] [PubMed] [Google Scholar]
- Kim JH, Choi JY, Kim SB, et al CD11chi dendritic cells regulate Ly-6Chi monocyte differentiation to preserve immune-privileged CNS in lethal neuroinflammation. Scientific Reports. 2015;5:17548. doi: 10.1038/srep17548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin DW, Whitley RJ Human herpesvirus-6: neurologic implications of a newly-described viral pathogen. Journal of Neurovirology. 1998;4(5):474–485. doi: 10.3109/13550289809113492. [DOI] [PubMed] [Google Scholar]
- Kimura T, Sasaki M, Okumura M, et al Flavivirus encephalitis: pathological aspects of mouse and other animal models. Veterinary Pathology. 2010;47(5):806–818. doi: 10.1177/0300985810372507. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L, Sengelov H, Lollike K, et al Isolation and characterization of gelatinase granules from human neutrophils. Blood. 1994;83(6):1640–1649. doi: 10.1182/blood.V83.6.1640.1640. [DOI] [PubMed] [Google Scholar]
- Klein RS Encephalitic arboviruses of africa: emergence, clinical presentation and neuropathogenesis. Frontiers in Immunology. 2021;12:769942. doi: 10.3389/fimmu.2021.769942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Lin E, Zhang B, et al Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. Journal of Virology. 2005;79(17):11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncu OO, Hogue IB, Enquist LW Virus infections in the nervous system. Cell Host & Microbe. 2013;13(4):379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubinski M, Beicht J, Gerlach T, et al. 2020. Tick-borne encephalitis virus: a quest for better vaccines against a virus on the rise. Vaccines (Basel), 8(3): 451.
- Lam JH, Smith FL, Baumgarth N B cell activation and response regulation during viral infections. Viral Immunology. 2020;33(4):294–306. doi: 10.1089/vim.2019.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TE, Hardison JL, Walsh KB. 2006. Functional diversity of chemokines and chemokine receptors in response to viral infection of the central nervous system. In: Lane TE. Chemokines and Viral Infection. Berlin, Heidelberg: Springer, 1–27.
- Laulund ASB, Trøstrup H, Lerche CJ, et al Synergistic effect of immunomodulatory S100A8/A9 and ciprofloxacin against Pseudomonas aeruginosa biofilm in a murine chronic wound model. Pathogens and Disease. 2020;78(5):ftz027. doi: 10.1093/femspd/ftz027. [DOI] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, et al A mouse model of Zika virus pathogenesis. Cell Host & Microbe. 2016;19(5):720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BJ, Weiss ML, Mosier D, et al Spread of bovine herpesvirus type 5 (BHV-5) in the rabbit brain after intranasal inoculation. Journal of Neurovirology. 1999;5(5):474–484. doi: 10.3109/13550289909045376. [DOI] [PubMed] [Google Scholar]
- Li CH, Yan LZ, Ban WZ, et al Long-term propagation of tree shrew spermatogonial stem cells in culture and successful generation of transgenic offspring. Cell Research. 2017;27(2):241–252. doi: 10.1038/cr.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HD, Saucedo-Cuevas L, Regla-Nava JA, et al Zika virus infects neural progenitors in the adult mouse brain and alters proliferation. Cell Stem Cell. 2016a;19(5):593–598. doi: 10.1016/j.stem.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Loeb JA, Shy ME, et al Asymmetric flaccid paralysis: a neuromuscular presentation of West Nile virus infection. Annals of Neurology. 2003;53(6):703–710. doi: 10.1002/ana.10575. [DOI] [PubMed] [Google Scholar]
- Li JP, Liao Y, Zhang Y, et al Experimental infection of tree shrews (Tupaia belangeri) with Coxsackie virus A16. Zoological Research. 2014;35(6):485–491. doi: 10.13918/j.issn.2095-8137.2014.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LH, Li ZR, Wang EL, et al Herpes simplex virus 1 infection of tree shrews differs from that of mice in the severity of acute infection and viral transcription in the peripheral nervous system. Journal of Virology. 2016b;90(2):790–804. doi: 10.1128/JVI.02258-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LL, Xu CC, Zhang WJ, et al Cargo-compatible encapsulation in virus-based nanoparticles. Nano Letters. 2019;19(4):2700–2706. doi: 10.1021/acs.nanolett.9b00679. [DOI] [PubMed] [Google Scholar]
- Li XF, Deng YQ, Yang HQ, et al A chimeric dengue virus vaccine using Japanese encephalitis virus vaccine strain SA14–14-2 as backbone is immunogenic and protective against either parental virus in mice and nonhuman primates. Journal of Virology. 2013;87(24):13694–13705. doi: 10.1128/JVI.00931-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Dong HL, Huang XY, et al Characterization of a 2016 clinical isolate of Zika virus in non-human primates. eBioMedicine. 2016c;12:170–177. doi: 10.1016/j.ebiom.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Qi LL, Yang D, et al Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system. Nature Neuroscience. 2022;25(5):577–587. doi: 10.1038/s41593-022-01063-z. [DOI] [PubMed] [Google Scholar]
- Li YM, Ye J, Yang XH, et al Infection of mouse bone marrow-derived dendritic cells by live attenuated Japanese encephalitis virus induces cells maturation and triggers T cells activation. Vaccine. 2011;29(4):855–862. doi: 10.1016/j.vaccine.2010.09.108. [DOI] [PubMed] [Google Scholar]
- Lin MT, Stohlman SA, Hinton DR Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. Journal of Virology. 1997;71(1):383–391. doi: 10.1128/jvi.71.1.383-391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist L, Vapalahti O Tick-borne encephalitis. The Lancet. 2008;371(9627):1861–1871. doi: 10.1016/S0140-6736(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Beaton L, Yu D, et al IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. Journal of Experimental Medicine. 2010;207(2):353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou ML, Hsu CY Japanese encephalitis virus is transported across the cerebral blood vessels by endocytosis in mouse brain. Cell and Tissue Research. 1998;293(3):389–394. doi: 10.1007/s004410051130. [DOI] [PubMed] [Google Scholar]
- Liu QY, Wang XJ, Xie CH, et al A novel human acute encephalitis caused by pseudorabies virus variant strain. Clinical Infectious Diseases. 2021;73(11):e3690–e3700. doi: 10.1093/cid/ciaa987. [DOI] [PubMed] [Google Scholar]
- Louveau A. 2018. Meningeal immunity, drainage, and tertiary lymphoid structure formation. In: Dieu-Nosjean MC. Tertiary Lymphoid Structures. New York: Humana Press, 31–45.
- Louveau A, Herz J, Alme MN, et al CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nature Neuroscience. 2018;21(10):1380–1391. doi: 10.1038/s41593-018-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, et al Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow M, Kortekaas J, Herden C, et al Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathologica. 2016;131(2):159–184. doi: 10.1007/s00401-015-1511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin KE, Good L, Strömberg R, et al Biological activity and biotechnological aspects of peptide nucleic acid. Advanceds in Genetics. 2006;56:1–51. doi: 10.1016/S0065-2660(06)56001-8. [DOI] [PubMed] [Google Scholar]
- Luo Z, Su R, Wang WB, et al EV71 infection induces neurodegeneration via activating TLR7 signaling and IL-6 production. PLoS Pathogens. 2019;15(11):e1008142. doi: 10.1371/journal.ppat.1008142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski-Lenoir D, Chen SZ, Feng LL, et al Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia. The Journal of Immunology. 1999;163(3):1628–1635. doi: 10.4049/jimmunol.163.3.1628. [DOI] [PubMed] [Google Scholar]
- Marques RE, Del Sarto JL, Rocha RPF, et al Development of a model of Saint Louis encephalitis infection and disease in mice. Journal of Neuroinflammation. 2017;14(1):61. doi: 10.1186/s12974-017-0837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina BEE, Koraka P, van den Doel P, et al DC-SIGN enhances infection of cells with glycosylated West Nile virus in vitro and virus replication in human dendritic cells induces production of IFN-α and TNF-α. Virus Research. 2008;135(1):64–71. doi: 10.1016/j.virusres.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Martinot AJ, Abbink P, Afacan O, et al Fetal neuropathology in Zika virus-infected pregnant female rhesus monkeys. Cell. 2018;173(5):1111–1122.e10. doi: 10.1016/j.cell.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury A, Lyoubi A, Peiffer-Smadja N, et al. 2021. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: a narrative review for clinicians. Revue Neurologique, 177(1–2): 51–64.
- Mavigner M, Raper J, Kovacs-Balint Z, et al Postnatal Zika virus infection is associated with persistent abnormalities in brain structure, function, and behavior in infant macaques. Science Translational Medicine. 2018;10(435):eaao6975. doi: 10.1126/scitranslmed.aao6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McJunkin JE, de los Reyes EC, Irazuzta JE, et al La Crosse encephalitis in children. New England Journal of Medicine. 2001;344(11):801–807. doi: 10.1056/NEJM200103153441103. [DOI] [PubMed] [Google Scholar]
- McMenamin PG Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. Journal of Comparative Neurology. 1999;405(4):553–562. doi: 10.1002/(SICI)1096-9861(19990322)405:4<553::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Medigeshi GR, Hirsch AJ, Brien JD, et al West nile virus capsid degradation of claudin proteins disrupts epithelial barrier function. Journal of Virology. 2009;83(12):6125–6134. doi: 10.1128/JVI.02617-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meertens L, Bertaux C, Cukierman L, et al The tight junction proteins claudin-1, -6, and -9 are entry cofactors for hepatitis C virus. Journal of Virology. 2008;82(7):3555–3560. doi: 10.1128/JVI.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J, Radke J, Dittmayer C, et al Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nature Neuroscience. 2021;24(2):168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- Metcalf TU, Griffin DE Alphavirus-induced encephalomyelitis: antibody-secreting cells and viral clearance from the nervous system. Journal of Virology. 2011;85(21):11490–11501. doi: 10.1128/JVI.05379-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KF, Haring CM, Howitt B The etiology of epizootic encephalomyelitis of horses in the san joaquin valley, 1930. Science. 1931;74(1913):227–228. doi: 10.1126/science.74.1913.227. [DOI] [PubMed] [Google Scholar]
- Mifune K, Shichijo A, Makino Y, et al A mouse model for the pathogenesis and postexposure prophylaxis of rabies. Microbiology and Immunology. 1980;24(9):835–845. doi: 10.1111/j.1348-0421.1980.tb02888.x. [DOI] [PubMed] [Google Scholar]
- Miller KN, Victorelli SG, Salmonowicz H, et al Cytoplasmic DNA: sources, sensing, and role in aging and disease. Cell. 2021;184(22):5506–5526. doi: 10.1016/j.cell.2021.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MJ, Billingsley MM, Haley RM, et al Engineering precision nanoparticles for drug delivery. Nature Reviews Drug Discovery. 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Goto N, Suzuki H, et al Strain difference of mouse in susceptibility to Japanese encephalitis virus infection. Experimental Animals. 1988;37(4):365–373. doi: 10.1538/expanim1978.37.4_365. [DOI] [PubMed] [Google Scholar]
- Mockus TE, Ren HM, Shwetank, et al To go or stay: the development, benefit, and detriment of tissue-resident memory CD8 T cells during central nervous system viral infections. Viruses. 2019;11(9):842. doi: 10.3390/v11090842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møllgård K, Beinlich FRM, Kusk P, et al A mesothelium divides the subarachnoid space into functional compartments. Science. 2023;379(6627):84–88. doi: 10.1126/science.adc8810. [DOI] [PubMed] [Google Scholar]
- Mori I, Nishiyama Y, Yokochi T, et al Olfactory transmission of neurotropic viruses. Journal of Neurovirology. 2005;11(2):129–137. doi: 10.1080/13550280590922793. [DOI] [PubMed] [Google Scholar]
- Mostashari F, Bunning ML, Kitsutani PT, et al Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. The Lancet. 2001;358(9278):261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- Müller U, Steinhoff U, Reis LF, et al Functional role of type I and type II interferons in antiviral defense. Science. 1994;264(5167):1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Munster VJ, Feldmann F, Williamson BN, et al Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020;585(7824):268–272. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Prescott JB, Bushmaker T, et al Rapid Nipah virus entry into the central nervous system of hamsters via the olfactory route. Scientific Reports. 2012;2:736. doi: 10.1038/srep00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafá YM, Meuren LM, Coelho SVA, et al Pathways exploited by flaviviruses to counteract the blood-brain barrier and invade the central nervous system. Frontiers in Microbiology. 2019;10:525. doi: 10.3389/fmicb.2019.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel MA, Niemeyer CS, Bubak AN Central nervous system infections produced by varicella zoster virus. Current Opinion in Infectious Diseases. 2020;33(3):273–278. doi: 10.1097/QCO.0000000000000647. [DOI] [PubMed] [Google Scholar]
- Narita M, Uchimura A, Kawanabe M, et al Invasion and spread of equine herpesvirus 9 in the olfactory pathway of pigs after intranasal inoculation. Journal of Comparative Pathology. 2001;124(4):265–272. doi: 10.1053/jcpa.2000.0461. [DOI] [PubMed] [Google Scholar]
- Nemeth N, Bosco-Lauth A, Oesterle P, et al North American birds as potential amplifying hosts of Japanese encephalitis virus. The American Journal of Tropical Medicine and Hygiene. 2012;87(4):760–767. doi: 10.4269/ajtmh.2012.12-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu CX, Yu JJ, Zou T, et al Identification of hematopoietic stem cells residing in the meninges of adult mice at steady state. Cell Reports. 2022;41(6):111592. doi: 10.1016/j.celrep.2022.111592. [DOI] [PubMed] [Google Scholar]
- Ohka S, Nihei CI, Yamazaki M, et al Poliovirus trafficking toward central nervous system via human poliovirus receptor-dependent and -independent pathway. Frontiers in Microbiology. 2012;3:147. doi: 10.3389/fmicb.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal JT, Upadhyay AA, Wolabaugh A, et al West nile virus-inclusive single-cell RNA sequencing reveals heterogeneity in the type i interferon response within single cells. Journal of Virology. 2019;93(6):e01778–18. doi: 10.1128/JVI.01778-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JC Jr Herpes simplex virus infection of the fetus and newborn. Pediatric Annals. 1994;23(3):131–136. doi: 10.3928/0090-4481-19940301-06. [DOI] [PubMed] [Google Scholar]
- Papa MP, Meuren LM, Coelho SVA, et al Zika virus infects, activates, and crosses brain microvascular endothelial cells, without barrier disruption. Frontiers in Microbiology. 2017;8:2557. doi: 10.3389/fmicb.2017.02557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran P, Sklan E, Wilkins C, et al Six RNA viruses and forty-one hosts: viral small RNAs and modulation of small RNA repertoires in vertebrate and invertebrate systems. PLoS Pathogens. 2010;6(2):e1000764. doi: 10.1371/journal.ppat.1000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra B, Hinton DR, Marten NW, et al IFN-γ is required for viral clearance from central nervous system oligodendroglia. The Journal of Immunology. 1999;162(3):1641–1647. doi: 10.4049/jimmunol.162.3.1641. [DOI] [PubMed] [Google Scholar]
- Pfeffer S, Zavolan M, Grasser FA, et al Identification of virus-encoded microRNAs. Science. 2004;304(5671):734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- Phares TW, Marques CP, Stohlman SA, et al Factors supporting intrathecal humoral responses following viral encephalomyelitis. Journal of Virology. 2011;85(6):2589–2598. doi: 10.1128/JVI.02260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A, Masciocchi S, Volonghi I, et al Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) encephalitis is a cytokine release syndrome: evidences from cerebrospinal fluid analyses. Clinical Infectious Diseases. 2021;73(9):e3019–e3026. doi: 10.1093/cid/ciaa1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompon J, Manuel M, Ng GK, et al Dengue subgenomic flaviviral RNA disrupts immunity in mosquito salivary glands to increase virus transmission. PLoS Pathogens. 2017;13(7):e1006535. doi: 10.1371/journal.ppat.1006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntambekar SS, Bergmann CC, Savarin C, et al Shifting hierarchies of interleukin-10-producing T cell populations in the central nervous system during acute and persistent viral encephalomyelitis. Journal of Virology. 2011;85(13):6702–6713. doi: 10.1128/JVI.00200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello VR One hundred years of poliovirus pathogenesis. Virology. 2006;344(1):9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Ramakrishna C, Stohlman SA, Atkinson RA, et al Differential regulation of primary and secondary CD8+ T cells in the central nervous system. The Journal of Immunology. 2004;173(10):6265–6273. doi: 10.4049/jimmunol.173.10.6265. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Kivisäkk P, Kidd G Three or more routes for leukocyte migration into the central nervous system. Nature Reviews Immunology. 2003;3(7):569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Reagin KL, Funk KE The role of antiviral CD8+ T cells in cognitive impairment. Current Opinion in Neurobiology. 2022;76:102603. doi: 10.1016/j.conb.2022.102603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebejac J, Eme-Scolan E, Arnaud Paroutaud L, et al Meningeal macrophages protect against viral neuroinfection. Immunity. 2022;55(11):2103–2117.e10. doi: 10.1016/j.immuni.2022.10.005. [DOI] [PubMed] [Google Scholar]
- Redant V, Favoreel HW, Dallmeier K, et al Efficient control of Japanese encephalitis virus in the central nervous system of infected pigs occurs in the absence of a pronounced inflammatory immune response. Journal of Neuroinflammation. 2020;17(1):315. doi: 10.1186/s12974-020-01974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren MS, Mei H, Zhou JJ, et al Early diagnosis of rabies virus infection by RPA-CRISPR techniques in a rat model. Archives of Virology. 2021;166(4):1083–1092. doi: 10.1007/s00705-021-04970-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin ME, García-Nicolás O, Brechbühl D, et al Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nature Communications. 2016;7:10832. doi: 10.1038/ncomms10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TK, Buckner CM, Berman JW Leukocyte transmigration across the blood-brain barrier: perspectives on neuroAIDS. Frontiers in Bioscience. 2010;15(2):478–536. doi: 10.2741/3631. [DOI] [PubMed] [Google Scholar]
- Roe K, Kumar M, Lum S, et al West Nile virus-induced disruption of the blood-brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. Journal of General Virology. 2012;93(6):1193–1203. doi: 10.1099/vir.0.040899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi SL, Tesh RB, Azar SR, et al Characterization of a Novel Murine Model to Study Zika Virus. The American Journal of Tropical Medicine and Hygiene. 2016;94(6):1362–1369. doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rua R, McGavern DB Advances in meningeal immunity. Trends in Molecular Medicine. 2018;24(6):542–559. doi: 10.1016/j.molmed.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin SA, Pletnikov M, Carbone KM Comparison of the neurovirulence of a vaccine and a wild-type mumps virus strain in the developing rat brain. Journal of Virology. 1998;72(10):8037–8042. doi: 10.1128/JVI.72.10.8037-8042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin AB, Olitsky PK Influence of host factors on neuroinvasiveness of vesicular stomatitis virus: II. Effect of age on the invasion of the peripheral and central nervous systems by virus injected into the leg muscles or the eye. Journal of Experimental Medicine. 1937;66(1):35–57. doi: 10.1084/jem.66.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi H, Cain MD, Klein RS Encephalitic arboviruses: emergence, clinical presentation, and neuropathogenesis. Neurotherapeutics. 2016;13(3):514–534. doi: 10.1007/s13311-016-0443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Wang H, Siddharthan V, et al Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17140–17145. doi: 10.1073/pnas.0705837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasseville VG, Newman W, Brodie SJ, et al Monocyte adhesion to endothelium in simian immunodeficiency virus-induced AIDS encephalitis is mediated by vascular cell adhesion molecule-1/α4β1 integrin interactions. American Journal of Pathology. 1994;144(1):27–40. [PMC free article] [PubMed] [Google Scholar]
- Saxena V, Mathur A, Krishnani N, et al. 2008. Kinetics of cytokine profile during intraperitoneal inoculation of Japanese encephalitis virus in BALB/c mice model. Microbes and Infection, 10(10–11): 1210–1217.
- Seferovic M, Martín CSS, Tardif SD, et al Experimental zika virus infection in the pregnant common marmoset induces spontaneous fetal loss and neurodevelopmental abnormalities. Scientific Reports. 2018;8(1):6851. doi: 10.1038/s41598-018-25205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar JJ, Haddad MB, Tierney BC, et al Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290(4):511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- Shan C, Yao YF, Yang XL, et al. 2020. Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in Rhesus macaques. Cell Research, 30(8): 670–677.
- Shrestha B, Diamond MS Fas ligand interactions contribute to CD8+ T-cell-mediated control of West Nile virus infection in the central nervous system. Journal of Virology. 2007;81(21):11749–11757. doi: 10.1128/JVI.01136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Pinto AK, Green S, et al CD8+ T cells use TRAIL to restrict West Nile virus pathogenesis by controlling infection in neurons. Journal of Virology. 2012;86(17):8937–8948. doi: 10.1128/JVI.00673-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Zhang B, Purtha WE, et al Tumor necrosis factor alpha protects against lethal West Nile virus infection by promoting trafficking of mononuclear leukocytes into the central nervous system. Journal of Virology. 2008;82(18):8956–8964. doi: 10.1128/JVI.01118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MC, Guerrero-Plata A, Gilfoy FD, et al Differential activation of human monocyte-derived and plasmacytoid dendritic cells by West Nile virus generated in different host cells. Journal of Virology. 2007;81(24):13640–13648. doi: 10.1128/JVI.00857-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MTT Viral encephalitis. Arquivos de Neuro-Psiquiatria. 2013;71(9B):703–709. doi: 10.1590/0004-282X20130155. [DOI] [PubMed] [Google Scholar]
- Sips GJ, Wilschut J, Smit JM Neuroinvasive flavivirus infections. Reviews in Medical Virology. 2012;22(2):69–87. doi: 10.1002/rmv.712. [DOI] [PubMed] [Google Scholar]
- Smith JS Mouse model for abortive rabies infection of the central nervous system. Infection and Immunity. 1981;31(1):297–308. doi: 10.1128/iai.31.1.297-308.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T, Lewthwaite P, Perera D, et al Virology, epidemiology, pathogenesis, and control of enterovirus 71. The Lancet Infectious Diseases. 2010;10(11):778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- Song E, Mao TY, Dong HP, et al VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577(7792):689–694. doi: 10.1038/s41586-019-1912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooryanarain H, Ayachit V, Gore M. 2012. Activated CD56+ lymphocytes (NK+NKT) mediate immunomodulatory and anti-viral effects during Japanese encephalitis virus infection of dendritic cells in-vitro. Virology, 432(2): 250–260.
- Spudich S, Nath A Nervous system consequences of COVID-19. Science. 2022;375(6578):267–269. doi: 10.1126/science.abm2052. [DOI] [PubMed] [Google Scholar]
- Steinbach K, Vincenti I, Kreutzfeldt M, et al Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. Journal of Experimental Medicine. 2016;213(8):1571–1587. doi: 10.1084/jem.20151916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles LN, Hosking MP, Edwards RA, et al Differential roles for CXCR3 in CD4+ and CD8+ T cell trafficking following viral infection of the CNS. European Journal of Immunology. 2006;36(3):613–622. doi: 10.1002/eji.200535509. [DOI] [PubMed] [Google Scholar]
- Sumathy K, Kulkarni B, Gondu RK, et al Protective efficacy of Zika vaccine in AG129 mouse model. Scientific Reports. 2017;7:46375. doi: 10.1038/srep46375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SH, Chen Q, Gu HJ, et al A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host & Microbe. 2020;28(1):124–133.e4. doi: 10.1016/j.chom.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süss J, Gelpi E, Klaus C, et al Tickborne encephalitis in naturally exposed monkey (Macaca sylvanus) Emerging Infectious Diseases. 2007;13(6):905–907. doi: 10.3201/eid1306.061173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Diamond MS, Gale M Jr West Nile virus infection and immunity. Nature Reviews Microbiology. 2013;11(2):115–128. doi: 10.1038/nrmicro2950. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Yamada T, Nakajima S, et al The substantia nigra is a major target for neurovirulent influenza A virus. Journal of Experimental Medicine. 1995;181(6):2161–2169. doi: 10.1084/jem.181.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SH, Ong KC, Wong KT Enterovirus 71 can directly infect the brainstem via cranial nerves and infection can be ameliorated by passive immunization. Journal of Neuropathology & Experimental Neurology. 2014;73(11):999–1008. doi: 10.1097/NEN.0000000000000122. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Salamat MS, Britt WJ, et al Neuropathogenesis induced by rhesus cytomegalovirus in fetal rhesus monkeys (Macaca mulatta) The Journal of Infectious Diseases. 1998;177(2):446–450. doi: 10.1086/514206. [DOI] [PubMed] [Google Scholar]
- Templeton SP, Kim TS, O'Malley K, et al Maturation and localization of macrophages and microglia during infection with a neurotropic murine coronavirus. Brain Pathology. 2008;18(1):40–51. doi: 10.1111/j.1750-3639.2007.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throsby M, Geuijen C, Goudsmit J, et al Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile Virus. Journal of Virology. 2006;80(14):6982–6992. doi: 10.1128/JVI.00551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilo MJ, Lane TE The CC chemokine ligand 3 regulates CD11c+CD11b+CD8α- dendritic cell maturation and activation following viral infection of the central nervous system: implications for a role in T cell activation. Virology. 2004;327(1):8–15. doi: 10.1016/j.virol.2004.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi S, Chakravarty A Neurological complications of dengue fever. Current Neurology and Neuroscience Reports. 2022;22(8):515–529. doi: 10.1007/s11910-022-01213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tselis AC Epstein-Barr virus infections of the nervous system. Handbook of Clinical Neurology. 2014;123:285–305. doi: 10.1016/B978-0-444-53488-0.00013-4. [DOI] [PubMed] [Google Scholar]
- Tuite MF, Serio TR The prion hypothesis: from biological anomaly to basic regulatory mechanism. Nature Reviews Molecular Cell Biology. 2010;11(12):823–833. doi: 10.1038/nrm3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell MJ, Sardelis MR, Dohm DJ, et al Potential North American vectors of West Nile virus. Annals of the New York Academy of Sciences. 2001;951(1):317–324. doi: 10.1111/j.1749-6632.2001.tb02707.x. [DOI] [PubMed] [Google Scholar]
- Turtle L, Bali T, Buxton G, et al Human T cell responses to Japanese encephalitis virus in health and disease. Journal of Experimental Medicine. 2016;213(7):1331–1352. doi: 10.1084/jem.20151517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini G Rabies virus as a transneuronal tracer of neuronal connections. Advances in Virus Research. 2011;79:165–202. doi: 10.1016/B978-0-12-387040-7.00010-X. [DOI] [PubMed] [Google Scholar]
- Uyar O, Dominguez JM, Bordeleau M, et al Single-cell transcriptomics of the ventral posterolateral nucleus-enriched thalamic regions from HSV-1-infected mice reveal a novel microglia/microglia-like transcriptional response. Journal of Neuroinflammation. 2022;19(1):81. doi: 10.1186/s12974-022-02437-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Mocarski E, Saederup N, et al Cytomegalovirus cell tropism, replication, and gene transfer in brain. Journal of Neuroscience. 1999;19(24):10948–10965. doi: 10.1523/JNEUROSCI.19-24-10948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riel D, Verdijk R, Kuiken T The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. The Journal of Pathology. 2015;235(2):277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- Venkatesan A, Tunkel AR, Bloch KC, et al Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clinical Infectious Diseases. 2013;57(8):1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verboon-Maciolek MA, Groenendaal F, Hahn CD, et al Human parechovirus causes encephalitis with white matter injury in neonates. Annals of Neurology. 2008;64(3):266–273. doi: 10.1002/ana.21445. [DOI] [PubMed] [Google Scholar]
- Verma R, Sharma P, Garg RK, et al Neurological complications of dengue fever: experience from a tertiary center of north India. Annals of Indian Academy of Neurology. 2011;14(4):272–278. doi: 10.4103/0972-2327.91946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstrepen BE, Fagrouch Z, van Heteren M, et al Experimental infection of rhesus macaques and common marmosets with a European strain of West Nile virus. PLoS Neglected Tropical Diseases. 2014;8(4):e2797. doi: 10.1371/journal.pntd.0002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KB, Edwards RA, Romero KM, et al Expression of CXC chemokine ligand 10 from the mouse hepatitis virus genome results in protection from viral-induced neurological and liver disease. The Journal of Immunology. 2007;179(2):1155–1165. doi: 10.4049/jimmunol.179.2.1155. [DOI] [PubMed] [Google Scholar]
- Wang ML, Cao RY, Zhang LK, et al Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen DY, Xu D, et al Early developing B cells undergo negative selection by central nervous system-specific antigens in the meninges. Immunity. 2021;54(12):2784–2794.e6. doi: 10.1016/j.immuni.2021.09.016. [DOI] [PubMed] [Google Scholar]
- Watson JT, Pertel PE, Jones RC, et al Clinical characteristics and functional outcomes of West Nile Fever. Annals of Internal Medicine. 2004;141(5):360–365. doi: 10.7326/0003-4819-141-5-200409070-00010. [DOI] [PubMed] [Google Scholar]
- Weinger JG, Marro BS, Hosking MP, et al The chemokine receptor CXCR2 and coronavirus-induced neurologic disease. Virology. 2013;435(1):110–117. doi: 10.1016/j.virol.2012.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MK, Wollebo HS, David Beckham J, et al Zika virus: an emergent neuropathological agent. Annals of Neurology. 2016;80(4):479–489. doi: 10.1002/ana.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley RJ. 2015. Herpes simplex virus infections of the central nervous system. Continuum (Minneap Minn), 21(6): 1704–1713.
- Wilkins C, Gale M Jr Recognition of viruses by cytoplasmic sensors. Current Opinion in Immunology. 2010;22(1):41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler ES, Bailey AL, Kafai NM, et al SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nature Immunology. 2020;21(11):1327–1335. doi: 10.1038/s41590-020-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Pamer EG CD8 T cell responses to infectious pathogens. Annual Review of Immunology. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- Wong PSJ, Li MZI, Chong CS, et al Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Neglected Tropical Diseases. 2013;7(8):e2348. doi: 10.1371/journal.pntd.0002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SJL, Grouard-Vogel G, Sun W, et al Human skin Langerhans cells are targets of dengue virus infection. Nature Medicine. 2000;6(7):816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- Xiao CG, Wang X, Cui GH, et al Possible pathogenicity of Japanese encephalitis virus in newly hatched domestic ducklings. Veterinary Microbiology. 2018;227:8–11. doi: 10.1016/j.vetmic.2018.10.016. [DOI] [PubMed] [Google Scholar]
- Xiao SY, Guzman H, Zhang H, et al West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerging Infectious Diseases. 2001;7(4):714–721. doi: 10.3201/eid0704.017420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XP, Wang QY, Xu HY, et al Inhibition of dengue virus by targeting viral NS4B protein. Journal of Virology. 2011;85(21):11183–11195. doi: 10.1128/JVI.05468-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Yu DD, Ma YH, et al COVID-19-like symptoms observed in Chinese tree shrews infected with SARS-CoV-2. Zoological Research. 2020;41(5):517–526. doi: 10.24272/j.issn.2095-8137.2020.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RF, Feng XY, Xie X, et al HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9. Brain Research. 2012a;1436:13–19. doi: 10.1016/j.brainres.2011.11.052. [DOI] [PubMed] [Google Scholar]
- Xu ZK, Waeckerlin R, Urbanowski MD, et al West nile virus infection causes endocytosis of a specific subset of tight junction membrane proteins. PLoS One. 2012b;7(5):e37886. doi: 10.1371/journal.pone.0037886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Zheng WJ, Jiang Y, et al Transcriptomic reveals the ferroptosis features of host response in a mouse model of Zika virus infection. Journal of Medical Virology. 2023;95(1):e28386. doi: 10.1002/jmv.28386. [DOI] [PubMed] [Google Scholar]
- Yang ZF, Zhao J, Zhu YT, et al The tree shrew provides a useful alternative model for the study of influenza H1N1 virus. Virology Journal. 2013;10:111. doi: 10.1186/1743-422X-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YG Creating animal models, why not use the Chinese tree shrew (Tupaia belangeri chinensis)? Zoological Research. 2017;38(3):118–126. doi: 10.24272/j.issn.2095-8137.2017.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yshii L, Gebauer C, Bernard-Valnet R, et al Neurons and T cells: understanding this interaction for inflammatory neurological diseases. European Journal of Immunology. 2015;45(10):2712–2720. doi: 10.1002/eji.201545759. [DOI] [PubMed] [Google Scholar]
- Yu JH, Liu XL, Ke CW, et al Effective suckling C57BL/6, kunming, and BALB/c mouse models with remarkable neurological manifestation for Zika virus infection. Viruses. 2017;9(7):165. doi: 10.3390/v9070165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Qi LL, Li T, et al PD1+CCR2+CD8+ T cells infiltrate the central nervous system during acute japanese encephalitis virus infection. Virologica Sinica. 2019a;34(5):538–548. doi: 10.1007/s12250-019-00134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang NN, Zhang L, Deng YQ, et al Zika virus infection in tupaia belangeri causes dermatological manifestations and confers protection against secondary infection. Journal of Virology. 2019b;93(8):e01982–18. doi: 10.1128/JVI.01982-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RY, Sun C, Chen XM, et al COVID-19-related brain injury: the potential role of ferroptosis. Journal of Inflammation Research. 2022a;15:2181–2198. doi: 10.2147/JIR.S353467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang SF, Li LT, et al Ineffectiveness of rabies vaccination alone for post-exposure protection against rabies infection in animal models. Antiviral Research. 2016;135:56–61. doi: 10.1016/j.antiviral.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Bailey JT, Xu E, et al Mucosal-associated invariant T cells restrict reactive oxidative damage and preserve meningeal barrier integrity and cognitive function. Nature Immunology. 2022b;23(12):1714–1725. doi: 10.1038/s41590-022-01349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Stohlman SA, Hoskin JB, et al Mouse hepatitis virus pathogenesis in the central nervous system is independent of IL-15 and natural killer cells. Virology. 2006;350(1):206–215. doi: 10.1016/j.virol.2006.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]