This phase 3 randomized clinical trial conducted in France investigates whether systemic weekly radiotherapy replanning compared with standard intensity-modulated radiotherapy decreases xerostomia in adult patients with squamous cell oropharyngeal cancer.
Key Points
Question
Does a maximalist strategy of systematic weekly replanning of radiotherapy for locally advanced oropharyngeal cancer improve salivary gland function and decrease xerostomia?
Findings
In this phase 3 randomized clinical trial of 132 patients randomized to receive adaptive radiotherapy or standard intensity-modulated radiotherapy (IMRT), adaptive radiotherapy compared with standard IMRT did not significantly improve salivary flow assessed by paraffin stimulation, patient-reported outcome scores, or rates of toxic effects.
Meaning
Adaptive radiotherapy vs IMRT did not show a benefit of decreasing xerostomia in patients with oropharyngeal cancer.
Abstract
Importance
Xerostomia is a major toxic effect associated with intensity-modulated radiotherapy (IMRT) for oropharyngeal cancers.
Objective
To assess whether adaptive radiotherapy (ART) improves salivary function compared with IMRT in patients with head and neck cancer.
Design, Setting, and Participants
This phase 3 randomized clinical trial was conducted in 11 French centers. Patients aged 18 to 75 years with stage III-IVB squamous cell oropharyngeal cancer treated with chemoradiotherapy were enrolled between July 5, 2013, and October 1, 2018. Data were analyzed from November 2021 to May 2022.
Interventions
The patients were randomly assigned (1:1) to receive standard IMRT (without replanning) or ART (systematic weekly replanning).
Main Outcomes and Measures
The primary end point was the frequency of xerostomia, measured by stimulating salivary flow with paraffin. Secondary end points included salivary gland excretory function measured using technetium-99m pertechnetate scintigraphy, patient-reported outcomes (Eisbruch xerostomia-specific questionnaire and the MD Anderson Symptom Inventory for Head and Neck Cancer questionnaire), early and late toxic effects, disease control, and overall and cancer-specific survival.
Results
A total of 132 patients were randomized, and after 1 exclusion in the ART arm, 131 were analyzed: 66 in the ART arm (mean [SD] age at inclusion, 60 [8] years; 57 [86.4%] male) and 65 in the standard IMRT arm (mean [SD] age at inclusion, 60 [8] years; 57 [87.7%] male). The median follow-up was 26.4 months (IQR, 1.2-31.3 months). The mean (SD) salivary flow (paraffin) at 12 months was 630 (450) mg/min in the ART arm and 584 (464) mg/min in the standard arm (P = .64). The mean (SD) excretory function of the parotid gland at 12 months, measured by scintigraphy, improved in the ART arm (48% [17%]) compared with the standard arm (41% [17%]) (P = .02). The 2-year-overall survival was 76.9% (95% CI, 64.7%-85.4%) in both arms.
Conclusions and Relevance
This randomized clinical trial did not demonstrate a benefit of ART in decreasing xerostomia compared with standard IMRT. No significant differences were found in secondary end points except for parotid gland excretory function, as assessed by scintigraphy, or in survival rates.
Trial Registration
ClinicalTrials.gov Identifier: NCT01874587
Introduction
Radiotherapy with chemotherapy or cetuximab is the standard of care for patients with locally advanced squamous cell carcinoma of the head and neck.1,2,3 The recommended radiotherapy technique is intensity-modulated radiotherapy (IMRT),4 which has been proven to reduce the dose to the parotid gland (PG) and subsequently decrease xerostomia.5,6,7 However, xerostomia remains a major issue causing difficulties in swallowing, speaking, loss of taste, and dental caries, with direct effects on the patient’s quality of life. Xerostomia is mainly caused by radiation-induced damage to the PG and, to a lesser extent, to the submandibular glands.8 Intensity-modulated radiotherapy is classically based on a single initial planning computed tomography (CT) scan, whereas large anatomical variations can be observed during the treatment course.9,10,11,12 These variations may result in PG overdose and, therefore, an increased risk of xerostomia.9,10,11,12
By performing 1 or several replanning sessions, adaptive radiotherapy (ART) aims to correct PG overdose during treatment.11,13,14,15,16 A dosimetric benefit of ART has been reported in the literature, with a decrease in the mean PG dose up to 10 Gy compared with the dose delivered without ART.9,11,13,16,17,18,19 Few studies, mostly retrospective, have suggested a potential clinical benefit of ART, with an increase in quality of life and/or in local control of the disease.15,16,20,21,22,23 However, to our knowledge, no phase 3 trial has distinctly demonstrated the benefit of ART in head and neck cancer or in any tumor localization.
We hypothesized that ART might decrease the rate of xerostomia in locally advanced oropharyngeal carcinoma compared with standard CT scan–based single-planning IMRT. This study reports the results of the ARTIX phase 3 randomized clinical trial (RCT) comparing ART with standard IMRT in patients with locally advanced oropharyngeal cancer.
Methods
Study Design and Participants
The ARTIX trial was a parallel-group, multicenter RCT comparing ART with standard IMRT for patients with locally advanced oropharyngeal cancer (trial protocol in Supplement 1). This study was conducted in 11 French centers. Patients were enrolled between July 5, 2013, and October 1, 2018. Eligible patients had histologically documented stage III-IVB squamous cell oropharyngeal cancer according to the Union for International Cancer Control and the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (Seventh Edition) and had to be treated with chemoradiotherapy. Patients had an Eastern Cooperative Oncology Group Performance Status Scale score of 2 or lower and were aged 18 to 75 years. Immunohistochemical staining for p16 was performed. The main exclusion criteria were previous head or neck radiotherapy, surgical resection of the primary tumor and/or lymph node, previous malignant tumor except nonmelanoma skin cancer, preexisting salivary gland disease, tumor involvement of both PGs, or previous or concurrent illness that would compromise the completion of treatment or follow-up. Written informed consent was obtained from all the patients. This study was conducted in accordance with the principles of the Declaration of Helsinki.24 The study was approved by the French Institutional Review Board and was registered at ClinicalTrials.gov (NCT01874587). The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Randomization and Masking
Patients were randomly assigned using a central electronic automated system (1:1 ratio) to receive standard IMRT or ART. Computer-generated randomization with minimization was used to stratify patients based on tumor stage (AJCC, Seventh Edition), human papillomavirus status (based on p16 expression), concomitant chemotherapy (platinum, cetuximab, or carboplatin plus fluorouracil), and IMRT technique (tomotherapy, volumetric modulated arc therapy/arc therapy, or step-and-shoot technique).
Treatment Procedures
All patients received a total dose of 70 Gy in 35 fractions (2 Gy/fraction/d), with a simultaneous integrated boost technique25 and concomitant chemotherapy. The following chemotherapy regimens were given: cisplatin, 100 mg/m2 every 3 weeks26; cetuximab at an initial dose of 400 mg/m2 7 days before the start of RT, followed by 250 mg/m2 weekly3; or carboplatin (70 mg/m2/d each day for 4 days every 3 weeks) and fluorouracil (600 mg/m2/d each day for 4 days every 3 weeks).27
The radiation protocol is detailed in the eMethods in Supplement 2. Contours and dose-volume constraints were set according to the Groupe d’Oncologie Radiothérapie Tête Et Cou recommendations.28 In particular, for the PG, the dose constraints were a mean dose less than 30 Gy and a median dose less than 26 Gy.29 Treatment parameters were reviewed retrospectively by the quality assurance review committee (eTables 1 and 2 in Supplement 2).
For patients in the ART arm, a weekly CT scan was performed using the same protocol as the initial planning CT scan. The dose distribution was computed using the same constraints as those used in the initial planning. A maximum of 5 days was allowed between each weekly CT scan and the start of the treatment using a new dose distribution. One replanning, based on the radiation oncologist’s decision, was allowed in the standard IMRT arm. In both arms, during the treatment course, daily in-room imaging (2-dimensional kilovoltage imaging, cone-beam CT [CBCT], or megavoltage CT) corrected setup errors greater than 5 mm.
Outcomes
The primary end point was the frequency of xerostomia, defined by salivary quantification, 12 months after the end of radiotherapy. The salivary flow was measured for primary analysis using stimulation by paraffin wax chewing before radiotherapy (baseline) and at 6, 12, 18, and 24 months after radiotherapy. The patient chewed paraffin wax for 2 minutes, and while continuing chewing, saliva was collected for 5 minutes. A salivary flow of 500 mg/min or less was used as the threshold for xerostomia.30
The secondary end points were salivary gland excretory function measured by scintigraphy; patient-reported outcomes (PROs); early and late toxic effects; overall, cancer-specific, and progression-free survival; and occurrence of second cancer. Salivary gland excretory function was measured by dynamic image acquisition after injection of technetium-99m pertechnetate and oral administration of 10 mL of lemon juice to stimulate salivary secretion before treatment and 12 months after the end of radiotherapy.31 All salivary scintigraphy images were centrally reviewed and analyzed. Patient-reported outcomes were measured using both the Eisbruch xerostomia-specific questionnaire32 and the MD Anderson Symptom Inventory for Head and Neck Cancer questionnaire for health-related quality of life33 collected at 3, 6, 12, 18, and 24 months after the end of radiotherapy. Toxic effects were recorded weekly during radiotherapy, monthly for the first 3 months after radiotherapy, and quarterly until the end of follow-up using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Toxic effects were defined as acute if they occurred during the first 3 months following the end of treatment and late if they occurred 3 months or more after the end of radiotherapy. Only toxic effects occurring at a frequency greater than 1% were reported.
Tumor evolution was assessed by clinical evaluation every 3 months and CT and/or positron emission tomography and CT scans at 3, 15, and 24 months. Locoregional control was defined as the absence of progressive disease according to the Response Evaluation Criteria in Solid Tumors, version 1.1 in the primary tumor and lymph node area. Overall survival (OS) was defined as the time from randomization to the date of death from any cause or the last follow-up. Cancer-specific survival was defined as the time from randomization to the date of death related to head and neck cancer or last follow-up (deaths related to other causes were censored). Progression-free survival (PFS) was defined as the time from randomization to the date of cancer recurrence, death from any cause, or last follow-up. Time to progression was defined as the time from randomization to the date of cancer recurrence (locoregional and/or metastatic) or the last follow-up (deaths were censored).
Statistical Analysis
The study was designed to detect a 25% decrease in xerostomia (assessed by salivary flow after paraffin stimulation) in the ART arm, with an expected xerostomia rate in the standard IMRT arm equal to 60%, a 5% 1-sided type I error rate, and 90% power. An interim analysis by an independent data monitoring committee was planned at 50% inclusion. The independent data monitoring committee had to decide in cases of excessive 1-year local disease recurrence to stop the study early. The stopping guideline was defined using a 1% 1-sided type I error rate.
Quantitative data were described by means and SDs or medians and IQRs and qualitative data by absolute and relative frequencies. Survival curves were plotted using Kaplan-Meier estimators and described by median time and 6-, 12-, 18-, and 24-month survival rates. For all the survival and late-toxic-effects analyses, hazard ratios (HRs) and their 95% CIs were estimated using Cox proportional hazards regression models. Appropriate nonparametric tests were used to compare the 2 arms of the study (ie, Wilcoxon rank sum test, Fisher exact test, and log-rank test). Wilcoxon signed rank tests were used to compare paired measures, such as salivation flow and questionnaire scores, with baseline.
Sensitivity analyses concerning the primary end point were conducted using logistic regression models considering stratification covariates and possible confounding factors after stepwise multivariable selection. Results were presented as odds ratios (ORs) of xerostomia in the standard arm vs the replanning arm with a 2-sided 95% CI. The ORs and CIs were also presented by subgroups using forest plots. One-sided P < .05 was considered as significant. Since a single primary outcome with 2 treatment groups was defined, no adjustment for multiplicity was required. Thus, findings from secondary outcomes should be considered as exploratory only.
The main analyses were conducted for the modified intent-to-treat (ITT) population, defined by the overall patients receiving at least 1 dose of chemotherapy and 1 radiotherapy session as assigned by the randomization procedure. In case of missing data related to the primary end point, data imputation was also performed with adjacent measurements (eg, xerostomia diagnosed after 18 months was considered present at 12 months; in the absence of xerostomia, the 12-month value was kept missing). Analyses were also conducted for the per-protocol (PP) population, defined as the ITT population without any of the following major protocol violations: nonadherence to written informed consent, failure to meet eligibility criteria, nonadherence to the randomization assignment, and more than 30 days between diagnosis CT scan and study treatment start. Data were analyzed from November 2021 to May 2022, using the R software package, version 4.1.0 (R Foundation for Statistical Computing).
Results
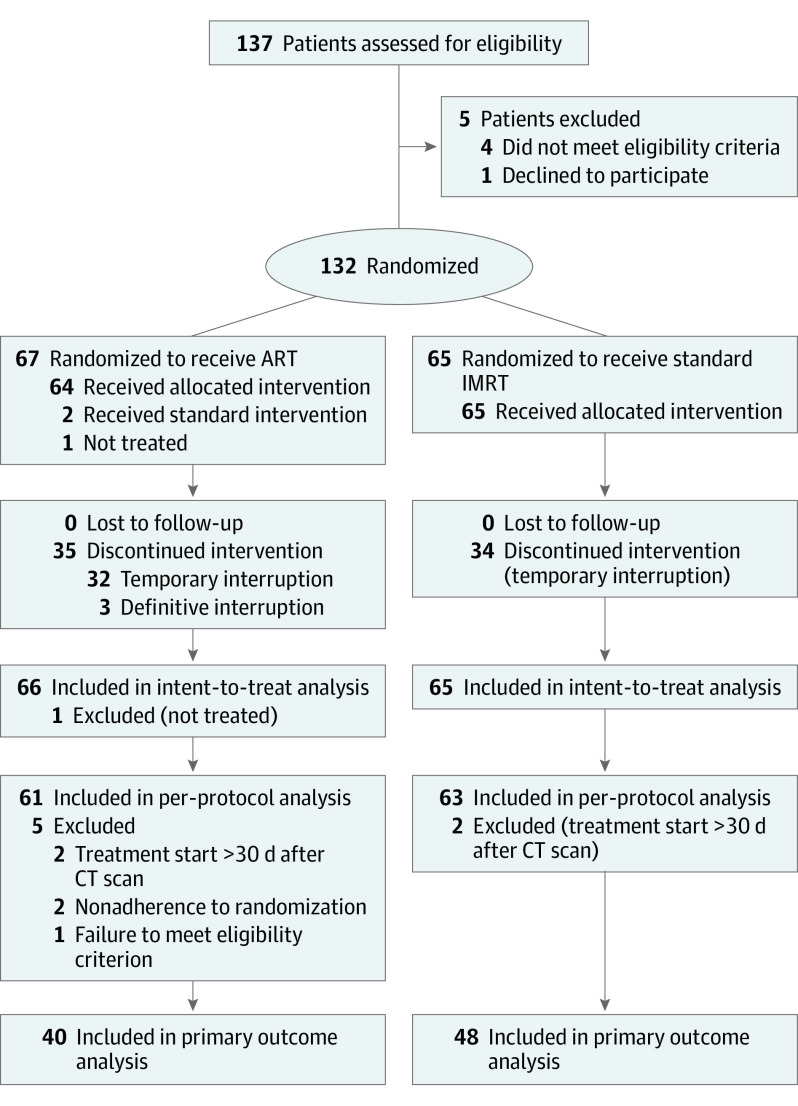
From July 5, 2013, to October 1, 2018, 132 patients were recruited and randomized. One patient in the ART arm did not receive any treatment and was therefore excluded from the analysis. Thus, there were 66 patients in the ART (mean [SD] age at inclusion, 60 [8] years; 9 [13.6%] female; 57 [86.4%] male) and 65 patients in the standard IMRT arm (mean [SD] age at inclusion, 60 [8] years; 8 [12.3%] female; 57 [87.7%] male). Figure 1 shows the CONSORT diagram. The median time between randomization and the first day of radiotherapy was 9 days (IQR, 4-14 days). Table 1 shows the patient and tumor characteristics at baseline. No significant differences were found between the 2 arms. Treatment characteristics are shown in Table 2. Replanning was performed weekly as expected in 62 patients (93.9%) in the ART arm. In the standard arm, 7 patients (10.8%) had 1 replanning session, based on the physician’s decision, at a mean (SD) time of 23 (11.3) days from the start of radiotherapy. Interim analysis was performed for 87 patients (66.4%) in October 2018. Regarding disease control, no significant difference was found, allowing the study to continue.
Figure 1. CONSORT Diagram.
ART indicates adaptive radiotherapy; CT, computed tomography; and IMRT, intensity-modulated radiotherapy.
Table 1. Demographic and Clinical Data of the ITT Study Population.
| Characteristic | Patientsa | |
|---|---|---|
| Replanning arm (n = 66) | Standard arm (n = 65) | |
| Patients | ||
| Sex | ||
| Female | 9 (13.6) | 8 (12.3) |
| Male | 57 (86.4) | 57 (87.7) |
| Age at inclusion, mean (SD), y | 60 (8) | 60 (8) |
| WHO performance status | ||
| 0 | 27 (40.9) | 26 (40.0) |
| 1 | 34 (51.5) | 37 (56.9) |
| 2 | 5 (7.6) | 2 (3.1) |
| Tobacco smoking | ||
| Active smoker | 23 (34.8) | 19 (29.2) |
| Former smoker | 34 (51.5) | 38 (58.5) |
| Nonsmoker | 9 (13.6) | 8 (12.3) |
| Pack-years, mean (SD), No. | 40 (23) | 38 (21) |
| Alcoholism | ||
| Yes | 21 (31.8) | 20 (30.8) |
| Weaned | 20 (30.3) | 25 (38.5) |
| No or occasional | 25 (37.9) | 20 (30.8) |
| Diabetes | ||
| Insulin dependent | 2 (3.0) | 3 (4.6) |
| Non–insulin dependent | 7 (10.6) | 5 (7.7) |
| None | 57 (86.4) | 57 (87.7) |
| Clear, fair-skin phototype | 21 (31.8) | 18 (27.7) |
| Tumors | ||
| Histologic characteristics | ||
| Squamous cell carcinoma poorly differentiated | 17/63 (27.0) | 15/61 (24.6) |
| Squamous cell carcinoma well differentiated | 46/63 (73.0) | 46/61 (75.4) |
| p16 Expression positive | 28/65 (43.1) | 27/65 (41.5) |
| Primary tumor localization | ||
| Base of the tongue, anterior wall | 15 (22.7) | 17 (26.2) |
| Pharynx, posterior wall | 1 (1.5) | 1 (1.5) |
| Several regions | 38 (57.6) | 32 (49.2) |
| Tonsillar region, lateral wall | 12 (18.2) | 15 (23.1) |
| Tumor laterality | ||
| Bilateral | 5 (7.6) | 3 (4.6) |
| Left | 27 (40.9) | 29 (44.6) |
| Medial | 7 (10.6) | 5 (7.7) |
| Right | 27 (40.9) | 28 (43.1) |
| Largest diameter of the primary tumor, mean (SD), mm | 41 (13) | 41 (15) |
| Lymph nodes | ||
| Involvement | ||
| Homolateral | 34 (51.5) | 36 (55.4) |
| Contralateral | 0 | 4 (6.2) |
| Bilateral | 23 (34.8) | 13 (20.0) |
| None | 9 (13.6) | 12 (18.5) |
| Conglomerate of lymph nodes | 17 (30.9) | 14 (26.9) |
| Lymph nodes involved if no conglomerate, mean (SD), No. | 2.97 (1.78) | 2.39 (1.05) |
| N stage | ||
| N0 | 9 (13.6) | 12 (18.5) |
| N1 | 5 (7.6) | 5 (7.7) |
| N2a | 1 (1.5) | 0 |
| N2b | 28 (42.4) | 29 (44.6) |
| N2c | 20 (30.3) | 17 (26.2) |
| N3 | 3 (4.5) | 2 (3.1) |
| AJCC tumor staging | ||
| III | 17 (25.8) | 19 (29.2) |
| IVa | 43 (65.2) | 43 (66.2) |
| IVb | 6 (9.1) | 3 (4.6) |
Abbreviations: AJCC, American Joint Committee on Cancer; ITT, intent to treat; WHO, World Health Organization.
Data are presented as the number (percentage) of patients unless otherwise indicated.
Table 2. Treatment Characteristics of the ITT Patient Groups.
| Characteristic | Patientsa | P valueb | |
|---|---|---|---|
| Replanning arm (n = 66) | Standard arm (n = 65) | ||
| Type of chemotherapy | |||
| Fluorouracil and carboplatin | 9 (13.6) | 9 (13.8) | .43 |
| Cisplatin | 42 (63.6) | 47 (72.3) | |
| Cetuximab | 15 (22.7) | 9 (13.8) | |
| IMRT modality, by tomotherapy vs arc therapy | 17 (25.8) | 16 (24.6) | >.99 |
| CT scans including initial planning, No. | |||
| 1 | 2 (3.0) | 58 (89.2) | <.001 |
| 2 | 2 (3.0) | 7 (10.8) | |
| 6 | 51 (77.3) | 0 | |
| 7 | 11 (16.7) | 0 | |
| ≥35 Cycles of radiotherapy | 63 (95.5) | 65 (100) | .24 |
| Interruption of treatment | |||
| No | 33 (50.0) | 32 (49.2) | .30 |
| Definitive | 3 (4.5) | 0 | |
| Temporary | 30 (45.5) | 33 (50.8) | |
| Overall duration of radiotherapy, mean (SD), d | 50.4 (8.6) | 51.6 (4.3) | .81 |
| Reason for interruptionsc | |||
| Not related to toxic effects | 22 (33.3) | 27 (41.5) | .36 |
| Toxic effects | 11 (16.7) | 6 (9.2) | |
| No interruption | 33 (50.0) | 32 (49.2) | |
Abbreviations: CT, computed tomography; IMRT, intensity-modulated radiotherapy; ITT, intent to treat.
Data are presented as the number (percentage) of patients unless otherwise indicated.
Fisher exact test or Wilcoxon rank sum test.
Interruption was defined as at least 1 day.
No significant differences were found in the initial dosimetry parameters (eTables 1 and 2 in Supplement 2). eTables 3 and 4 in Supplement 2 show the dosimetry parameters for each replan in the ART arm. Dose constraints were respected for each replan.
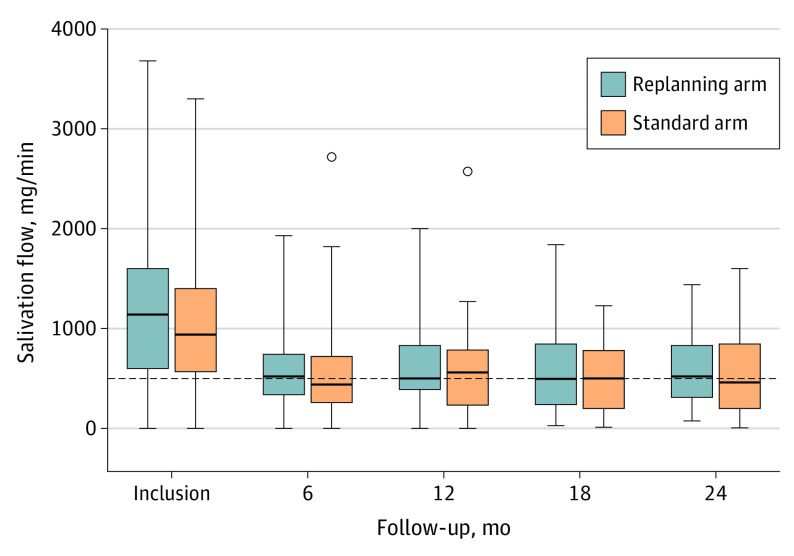
The median follow-up time was 26.4 months (IQR, 1.2-31.3 months). At baseline, the mean (SD) salivary flow after stimulation with paraffin was 1028 (729) mg/min in the standard arm and 1231 (855) mg/min in the experimental group (P = .16). At 12 months, the mean (SD) stimulated salivary flow was 584 (464) mg/min in the standard arm and 630 (450) mg/min in the ART arm (P = .64) (Figure 2). Nineteen of 40 patients (47.5%) in the ART arm were considered to experience xerostomia (<500 mg/min) compared with 23 of 48 patients (47.9%) in the standard arm (P > .99); data were missing for 26 (39.4%) and 17 (26.2%) patients in the ART and standard arms, respectively. After data imputation based on adjacent measures, no significant difference was found for 20 of 45 patients (44.4%) in the ART arm with xerostomia compared with 24 of 52 (46.2%) in the standard arm (P > .99).
Figure 2. Changes in Salivary Flow After Stimulation by Paraffin in the Intent-to-Treat Population.
Salivary flow was calculated as the weight of the saliva sample in milligrams divided by the sample collection time in minutes. Xerostomia was defined as a salivary flow of less than 500 mg/min (horizontal dashed line).30 The horizontal line within the boxes indicates the median; the lower and upper ends of the boxes, the first and third quartiles, respectively; whiskers, ranges; and dots, outliers.
At baseline, the mean (SD) excretory function of the PG estimated by salivary scintigraphy was not different between the 2 groups (56% [16%] and 55% [15%] for the ART and standard arms, respectively; P = .81). The mean (SD) PG excretory function at 12 months showed a significant improvement in the ART arm (48% [17%]) compared with the standard arm (41% [17%]) (P = .02). No difference in excretory function was observed in the submaxillary glands (eTable 5 in Supplement 2).
In both groups, PRO scores were significantly worse at 3 and 6 months than at baseline. No differences were found between the 2 arms at any study time point (eFigures 1 and 2 in Supplement 2).
No differences in acute toxic effects were found between the 2 arms (eTable 6 in Supplement 2). The rate of acute xerostomia of grade 2 or greater was 28.8% (19 of 66) in the ART arm compared with 23.1% (15 of 65) in the standard arm (P = .46). Regarding late toxic effects, the rate of xerostomia of grade 2 or greater at 12 months was 51.8% (95% CI, 37.5%-62.9%) in the ART arm compared with 53.5% (95% CI, 39.4%-64.3%) in the standard arm (P = .98). No significant differences were found in the rates of other late toxic effects between the 2 treatment arms (eTable 7 and eFigure 3 in Supplement 2).
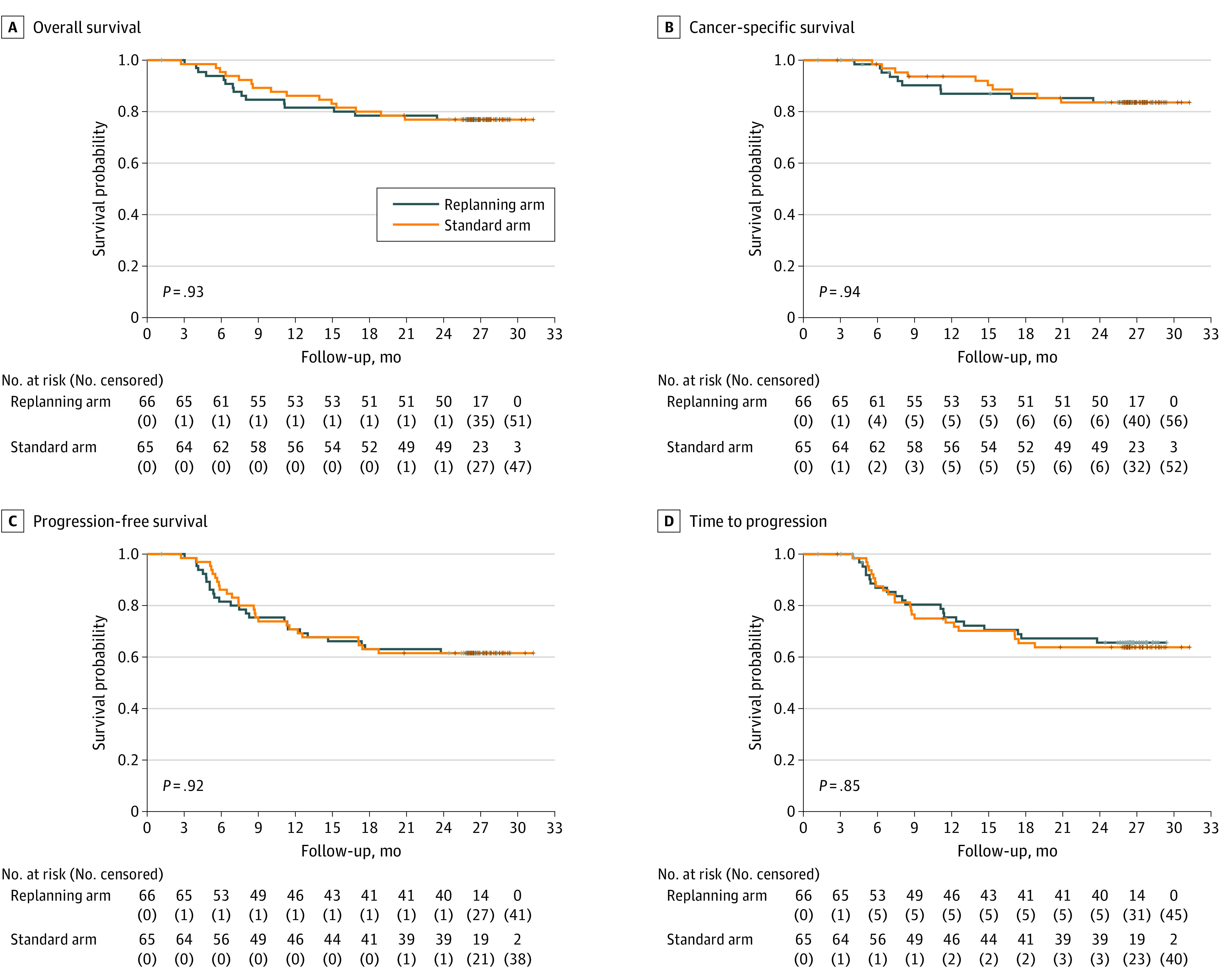
Regarding the efficacy of the treatment at 12 months, the total number of locoregional recurrences was 18 of 57 (31.6%) in the ART arm and 19 of 63 (30.2%) in the standard arm (P > .99). A total of 30 deaths were reported, with 15 in each group. Twenty deaths (66.7%) were due to oropharyngeal cancer (n = 10 in both groups). The OS rates at 12 and 24 months were 81.5% (95% CI, 69.8%-89.1%) and 76.9% (95% CI, 64.7%-85.4%), respectively, in the ART arm, compared with 86.2% (95% CI, 75.1%-92.5%) and 76.9% (95% CI, 64.6%-85.4%), respectively, in the standard arm. The OS curves did not differ between the groups at 2 years (P = .93) (Figure 3). The 2-year PFS was 61.5% (95% CI, 48.6%-72.1%) in the ART arm compared with 61.5% (95% CI, 48.6%-72.1%) in the standard arm (P = .92). The 2-year locoregional recurrence rate was 23.7% (95% CI, 12.0%-43.7%) in the ART arm compared with 22.5% (95% CI, 11.4%-32.8%) in the standard arm (P = .87). The 2-year distant metastasis rate was 13.9% (95% CI, 4.4%-22.4%) in the ART arm compared with 18.4% (95% CI, 7.9%-27.7%) in the standard arm (P = .51).
Figure 3. Survival Curves for the Intent-to-Treat Population.
All analyses were also conducted in the PP population (eTables 8-10 and eFigures 4-9 in Supplement 2). The results of the PP analysis were consistent with those of the ITT analysis.
Discussion
To our knowledge, this is the first phase 3 RCT of ART in head and neck cancer or any other tumor localization. We tested a systematic maximalist weekly replanning ART34 approach for locally advanced oropharyngeal cancer treated with chemoradiotherapy. Adaptive radiotherapy appeared to be technically feasible and safe and did not decrease local disease control and survival. However, this expensive and intensive process did not translate into a clinical benefit. Our study did not demonstrate a clinical benefit of ART. Regarding clinical outcomes of both arms, the results of this study agree with data previously published,4,35,36 whether for toxic effects, with an expected rate of xerostomia of 40% to 50%, or efficacy, with a 2-year overall survival of around 80%.
Although the clinical benefit of ART compared with standard IMRT was reported in a few nonrandomized studies with respect to improvement in quality of life16,22,37 and/or in local disease control,22,38 the present study did not find such a benefit. This could be due to several reasons. First, the study may have overestimated the benefit of ART, assuming a 25% decrease in xerostomia at 1 year between the 2 arms.39 Second, even if a significant improvement in PG function measured by scintigraphy was found in the ART arm related to PG sparing, no difference in salivary excretion was found for the submandibular gland. This result highlights the importance of an approach sparing the whole salivary glands and not only the PG to decrease xerostomia. The use of a 3-mm planning target volume margin owing to daily CBCT may allow reduction of the dose to the salivary glands. Of note, multiple factors are associated with xerostomia (eg, doses to the oral cavity and all salivary glands, quality of planning), and adjusting all these factors may be required to decrease xerostomia. Third, the study results may be related to the complexity of the xerostomia assessment. Even if IMRT had demonstrated better PG sparing, this benefit in salivary function did not translate into an improvement in PRO scores.5,6 Finally, dysphagia is another frequent adverse effect closely related to xerostomia and has greater consequences for quality of life than xerostomia, with potential effects on patients’ responses to the questionnaires.40
It is likely that ART is not necessary for all patients but only for a subset of patients.23,41,42 Based on only pretreatment parameters, the present study could not identify a subgroup of patients with a potential clinical benefit of ART in sensitivity analysis (eFigure 4 in Supplement 2).
Limitations
The study had limitations. The salivary flow after stimulation by paraffin was used as the primary end point, and whole saliva samples were collected, thus evaluating the whole salivary gland function and not only the PG.31 However, this method is well correlated with PROs in the literature39 and has the advantage of simplicity, even if chewing and spitting may be uncomfortable and unreliable. Another quantitative method involves using scintigraphy to assess the salivary function of each gland. However, scintigraphy is operator dependent. To address this limitation, we chose to perform all scintigraphy analyses by an experienced nuclear medicine physician (A.D.). Finally, PRO questionnaires may be complex for certain patients.
Adaptive radiotherapy could be used to spare another organ at risk, such as pharyngeal constrictors,43 which could have improved the quality of life of the patients. Another strategy could have been to propose ART for dose escalation in the tumor to increase local control, as in the ongoing European ARTFORCE study.44 Moreover, recent software advancements allow real-time adaptive radiotherapy to be performed to adapt the treatment to the daily shape of the patients as viewed by CBCT or onboard magnetic resonance imaging.45
Conclusions
This study is, to our knowledge, the first phase 3 RCT testing the benefits of ART for reducing xerostomia in patients with oropharyngeal cancer. Despite a maximal and labor-intensive approach, the primary end point was not reached, with no benefit of ART in decreasing xerostomia, and only 1 of the secondary end points was reached. A potential benefit of ART was found only in PG function assessed using salivary scintigraphy. Adaptive radiotherapy appears to be feasible and safe, with no unfavorable effects on local disease control and OS. These findings highlight the need to identify the subset of patients who may benefit from a personalized ART strategy aided by recent advances in the treatment planning system integrating artificial intelligence and onboard imaging, such as magnetic resonance imaging.
Trial Protocol
eMethods. Radiotherapy Treatment Procedures
eTable 1. Initial Planning CT Dosimetric Parameters for the Planned Target Volume (ITT)
eTable 2. Initial Planning CT Dosimetric Parameters for the Organs at Risk (ITT)
eTable 3. Weekly Dosimetric Parameters for the Target Volume in the ART Arm (ITT)
eTable 4. Weekly Dosimetric Parameters for the Organs at Risk in the ART Arm (ITT)
eTable 5. Excretory Function of Salivary Glands Measured by Scintigraphy (ITT)
eTable 6. Acute Grade ≥2 and Grade ≥3 Toxic Effects Occurrence by Treatment Arm (ITT)
eTable 7. Two-Year Grade ≥2 and Grade ≥3 Toxic Effects Rates by Treatment Arm (ITT)
eTable 8. Demographic and Clinical Data of the Study Population (PP)
eTable 9. Treatment Characteristics of the Patient Groups (PP)
eTable 10. Excretory Function of Salivary Glands Measured by Scintigraphy (PP)
eFigure 1. Trend of Eisbruch Scoring in the Treatment Arm (ITT)
eFigure 2. Trend of the MDASI-HN Questionnaire in the Treatment Arm (ITT)
eFigure 3. Cumulative Incidence Rate of Xerostomia (CTCAE 4.0 grade ≥2 and grade ≥3) by Treatment Arm (ITT)
eFigure 4. Sensitivity Analysis: Impact of the Treatment Arm on Xerostomia (Assessed by Simulation Flow After Paraffin Stimulation) in Subgroup Analyses (ITT)
eFigure 5. Flowchart of the Patient Population (Intent-to-Treat and per Protocol Analyses)
eFigure 6. Evolution of the Salivary Flow After Stimulation by Paraffin (PP)
eFigure 7. Trend of Eisbruch Scoring in the Treatment Arm (PP)
eFigure 8. Evolution of MDASI HN Questionnaire by Treatment Arm (PP)
eFigure 9. Survival Curves of the Study (PP)
eReferences
Data Sharing Statement
References
- 1.Pignon JP, Bourhis J, Domenge C, Designé L; MACH-NC Collaborative Group . Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. Lancet. 2000;355(9208):949-955. doi: 10.1016/S0140-6736(00)90011-4 [DOI] [PubMed] [Google Scholar]
- 2.Pignon JP, le Maître A, Maillard E, Bourhis J; MACH-NC Collaborative Group . Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4-14. doi: 10.1016/j.radonc.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 3.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567-578. doi: 10.1056/NEJMoa053422 [DOI] [PubMed] [Google Scholar]
- 4.Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393(10166):40-50. doi: 10.1016/S0140-6736(18)32779-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25(31):4873-4879. doi: 10.1200/JCO.2007.11.5501 [DOI] [PubMed] [Google Scholar]
- 6.Nutting CM, Morden JP, Harrington KJ, et al. ; PARSPORT Trial Management Group . Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127-136. doi: 10.1016/S1470-2045(10)70290-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981-991. doi: 10.1016/j.ijrobp.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 8.Chambers MS, Rosenthal DI, Weber RS. Radiation-induced xerostomia. Head Neck. 2007;29(1):58-63. doi: 10.1002/hed.20456 [DOI] [PubMed] [Google Scholar]
- 9.Castelli J, Simon A, Louvel G, et al. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat Oncol. 2015;10:6. doi: 10.1186/s13014-014-0318-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn PH, Chen CC, Ahn AI, et al. Adaptive planning in intensity-modulated radiation therapy for head and neck cancers: single-institution experience and clinical implications. Int J Radiat Oncol Biol Phys. 2011;80(3):677-685. doi: 10.1016/j.ijrobp.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 11.Jensen AD, Nill S, Huber PE, Bendl R, Debus J, Münter MW. A clinical concept for interfractional adaptive radiation therapy in the treatment of head and neck cancer. Int J Radiat Oncol Biol Phys. 2012;82(2):590-596. doi: 10.1016/j.ijrobp.2010.10.072 [DOI] [PubMed] [Google Scholar]
- 12.Castelli J, Simon A, Lafond C, et al. Adaptive radiotherapy for head and neck cancer. Acta Oncol. 2018;57(10):1284-1292. doi: 10.1080/0284186X.2018.1505053 [DOI] [PubMed] [Google Scholar]
- 13.Capelle L, Mackenzie M, Field C, Parliament M, Ghosh S, Scrimger R. Adaptive radiotherapy using helical tomotherapy for head and neck cancer in definitive and postoperative settings: initial results. Clin Oncol (R Coll Radiol). 2012;24(3):208-215. doi: 10.1016/j.clon.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 14.Duma MN, Kampfer S, Schuster T, Winkler C, Geinitz H. Adaptive radiotherapy for soft tissue changes during helical tomotherapy for head and neck cancer. Strahlenther Onkol. 2012;188(3):243-247. doi: 10.1007/s00066-011-0041-8 [DOI] [PubMed] [Google Scholar]
- 15.Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83(3):986-993. doi: 10.1016/j.ijrobp.2011.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao L, Wan Q, Zhou Y, Deng X, Xie C, Wu S. The role of replanning in fractionated intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiother Oncol. 2011;98(1):23-27. doi: 10.1016/j.radonc.2010.10.009 [DOI] [PubMed] [Google Scholar]
- 17.Dewan A, Sharma S, Dewan AK, et al. Impact of adaptive radiotherapy on locally advanced head and neck cancer—a dosimetric and volumetric study. Asian Pac J Cancer Prev. 2016;17(3):985-992. doi: 10.7314/APJCP.2016.17.3.985 [DOI] [PubMed] [Google Scholar]
- 18.Olteanu LA, Berwouts D, Madani I, et al. Comparative dosimetry of three-phase adaptive and non-adaptive dose-painting IMRT for head-and-neck cancer. Radiother Oncol. 2014;111(3):348-353. doi: 10.1016/j.radonc.2014.02.017 [DOI] [PubMed] [Google Scholar]
- 19.Schwartz DL, Garden AS, Shah SJ, et al. Adaptive radiotherapy for head and neck cancer–dosimetric results from a prospective clinical trial. Radiother Oncol. 2013;106(1):80-84. doi: 10.1016/j.radonc.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 20.Kataria T, Gupta D, Goyal S, et al. Clinical outcomes of adaptive radiotherapy in head and neck cancers. Br J Radiol. 2016;89(1062):20160085. doi: 10.1259/bjr.20160085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai YL, Yang SN, Liang JA, et al. Impact of body-mass factors on setup displacement in patients with head and neck cancer treated with radiotherapy using daily on-line image guidance. Radiat Oncol. 2014;9:19. doi: 10.1186/1748-717X-9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H, Hu W, Wang W, Chen P, Ding W, Luo W. Replanning during intensity modulated radiation therapy improved quality of life in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2013;85(1):e47-e54. doi: 10.1016/j.ijrobp.2012.09.033 [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee S, Maulik S, Prasath S, et al. Xerostomia quality of life and resource requirements following parotid sparing adaptive radiotherapy in head and neck cancers: results of a prospective cohort study (study ID CTRI/2017/11/010683). Radiother Oncol. 2022;168:250-255. doi: 10.1016/j.radonc.2022.01.020 [DOI] [PubMed] [Google Scholar]
- 24.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 25.Studer G, Huguenin PU, Davis JB, Kunz G, Lütolf UM, Glanzmann C. IMRT using simultaneously integrated boost (SIB) in head and neck cancer patients. Radiat Oncol. 2006;1:7. doi: 10.1186/1748-717X-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21(1):92-98. doi: 10.1200/JCO.2003.01.008 [DOI] [PubMed] [Google Scholar]
- 27.Bourhis J, Sire C, Graff P, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): an open-label phase 3 randomised trial. Lancet Oncol. 2012;13(2):145-153. doi: 10.1016/S1470-2045(11)70346-1 [DOI] [PubMed] [Google Scholar]
- 28.Tao Y, Auperin A, Blanchard P, et al. Concurrent cisplatin and dose escalation with intensity-modulated radiotherapy (IMRT) versus conventional radiotherapy for locally advanced head and neck squamous cell carcinomas (HNSCC): GORTEC 2004-01 randomized phase III trial. Radiother Oncol. 2020;150:18-25. doi: 10.1016/j.radonc.2020.05.021 [DOI] [PubMed] [Google Scholar]
- 29.Dirix P, Nuyts S. Evidence-based organ-sparing radiotherapy in head and neck cancer. Lancet Oncol. 2010;11(1):85-91. doi: 10.1016/S1470-2045(09)70231-1 [DOI] [PubMed] [Google Scholar]
- 30.Pedersen AM, Bardow A, Jensen SB, Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002;8(3):117-129. doi: 10.1034/j.1601-0825.2002.02851.x [DOI] [PubMed] [Google Scholar]
- 31.Wu VWC, Leung KY. A review on the assessment of radiation induced salivary gland damage after radiotherapy. Front Oncol. 2019;9:1090. doi: 10.3389/fonc.2019.01090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50(3):695-704. doi: 10.1016/S0360-3016(01)01512-7 [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal DI, Mendoza TR, Chambers MS, et al. Measuring head and neck cancer symptom burden: the development and validation of the M. D. Anderson symptom inventory, head and neck module. Head Neck. 2007;29(10):923-931. doi: 10.1002/hed.20602 [DOI] [PubMed] [Google Scholar]
- 34.Zhang P, Simon A, Rigaud B, et al. Optimal adaptive IMRT strategy to spare the parotid glands in oropharyngeal cancer. Radiother Oncol. 2016;120(1):41-47. doi: 10.1016/j.radonc.2016.05.028 [DOI] [PubMed] [Google Scholar]
- 35.Lee NY, Ferris RL, Psyrri A, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22(4):450-462. doi: 10.1016/S1470-2045(20)30737-3 [DOI] [PubMed] [Google Scholar]
- 36.Gebre-Medhin M, Brun E, Engström P, et al. ARTSCAN III: a randomized phase III study comparing chemoradiotherapy with cisplatin versus cetuximab in patients with locoregionally advanced head and neck squamous cell cancer. J Clin Oncol. 2021;39(1):38-47. doi: 10.1200/JCO.20.02072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen AM, Yoshizaki T, Hsu S, Mikaeilian A, Cao M. Image-guided adaptive radiotherapy improves acute toxicity during intensity-modulated radiation therapy for head and neck cancer. J Radiat Oncol. 2018;7(2):139-145. doi: 10.1007/s13566-017-0336-1 [DOI] [Google Scholar]
- 38.Chen AM, Daly ME, Cui J, Mathai M, Benedict S, Purdy JA. Clinical outcomes among patients with head and neck cancer treated by intensity-modulated radiotherapy with and without adaptive replanning. Head Neck. 2014;36(11):1541-1546. doi: 10.1002/hed.23477 [DOI] [PubMed] [Google Scholar]
- 39.Lin A, Helgeson ES, Treister NS, et al. The impact of head and neck radiotherapy on salivary flow and quality of life: results of the ORARAD study. Oral Oncol. 2022;127:105783. doi: 10.1016/j.oraloncology.2022.105783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramaekers BL, Joore MA, Grutters JP, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi: 10.1016/j.oraloncology.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 41.Brouwer CL, Steenbakkers RJ, Langendijk JA, Sijtsema NM. Identifying patients who may benefit from adaptive radiotherapy: does the literature on anatomic and dosimetric changes in head and neck organs at risk during radiotherapy provide information to help? Radiother Oncol. 2015;115(3):285-294. doi: 10.1016/j.radonc.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 42.Castelli J, Simon A, Rigaud B, et al. A nomogram to predict parotid gland overdose in head and neck IMRT. Radiat Oncol. 2016;11:79. doi: 10.1186/s13014-016-0650-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weppler S, Quon H, Schinkel C, et al. Patient-reported outcomes-guided adaptive radiation therapy for head and neck cancer. Front Oncol. 2021;11:759724. doi: 10.3389/fonc.2021.759724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heukelom J, Hamming O, Bartelink H, et al. Adaptive and innovative Radiation Treatment FOR improving Cancer treatment outcomE (ARTFORCE); a randomized controlled phase II trial for individualized treatment of head and neck cancer. BMC Cancer. 2013;13:84. doi: 10.1186/1471-2407-13-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boeke S, Mönnich D, van Timmeren JE, Balermpas P. MR-guided radiotherapy for head and neck cancer: current developments, perspectives, and challenges. Front Oncol. 2021;11:616156. doi: 10.3389/fonc.2021.616156 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Radiotherapy Treatment Procedures
eTable 1. Initial Planning CT Dosimetric Parameters for the Planned Target Volume (ITT)
eTable 2. Initial Planning CT Dosimetric Parameters for the Organs at Risk (ITT)
eTable 3. Weekly Dosimetric Parameters for the Target Volume in the ART Arm (ITT)
eTable 4. Weekly Dosimetric Parameters for the Organs at Risk in the ART Arm (ITT)
eTable 5. Excretory Function of Salivary Glands Measured by Scintigraphy (ITT)
eTable 6. Acute Grade ≥2 and Grade ≥3 Toxic Effects Occurrence by Treatment Arm (ITT)
eTable 7. Two-Year Grade ≥2 and Grade ≥3 Toxic Effects Rates by Treatment Arm (ITT)
eTable 8. Demographic and Clinical Data of the Study Population (PP)
eTable 9. Treatment Characteristics of the Patient Groups (PP)
eTable 10. Excretory Function of Salivary Glands Measured by Scintigraphy (PP)
eFigure 1. Trend of Eisbruch Scoring in the Treatment Arm (ITT)
eFigure 2. Trend of the MDASI-HN Questionnaire in the Treatment Arm (ITT)
eFigure 3. Cumulative Incidence Rate of Xerostomia (CTCAE 4.0 grade ≥2 and grade ≥3) by Treatment Arm (ITT)
eFigure 4. Sensitivity Analysis: Impact of the Treatment Arm on Xerostomia (Assessed by Simulation Flow After Paraffin Stimulation) in Subgroup Analyses (ITT)
eFigure 5. Flowchart of the Patient Population (Intent-to-Treat and per Protocol Analyses)
eFigure 6. Evolution of the Salivary Flow After Stimulation by Paraffin (PP)
eFigure 7. Trend of Eisbruch Scoring in the Treatment Arm (PP)
eFigure 8. Evolution of MDASI HN Questionnaire by Treatment Arm (PP)
eFigure 9. Survival Curves of the Study (PP)
eReferences
Data Sharing Statement