Abstract
Neurovascular networks play significant roles in the metabolism and regeneration of many tissues and organs in the human body. Blood vessels can transport sufficient oxygen, nutrients, and biological factors, while nerve fibers transmit excitation signals to targeted cells. However, traditional scaffolds cannot satisfy the requirement of stimulating angiogenesis and innervation in a timely manner due to the complexity of host neurovascular networks. Three-dimensional (3D) printing, as a versatile and favorable technique, provides an effective approach to fabricating biological scaffolds with biomimetic architectures and multimaterial compositions, which are capable of regulating multiple cell behaviors. This review paper presents a summary of the current progress in 3D-printed biomaterials for vascularized and innervated tissue regeneration by presenting skin, bone, and skeletal muscle tissues as an example. In addition, we highlight the crucial roles of blood vessels and nerve fibers in the process of tissue regeneration and discuss the future perspectives for engineering novel biomaterials. It is expected that 3D-printed biomaterials with angiogenesis and innervation properties can not only recapitulate the physiological microenvironment of damaged tissues but also rapidly integrate with host neurovascular networks, resulting in accelerated functional tissue regeneration.
Keywords: 3D printing, Biomaterials, Vascularization, Innervation, Tissue regeneration
1. Introduction
216Despite the remarkable progresses in the field of biomaterials and tissue engineering in the past few decades, the treatment of large tissue defects caused by diseases, traumas, and surgery still remains a huge challenge[1-3]. The main reason for the failure of tissue engineering scaffolds is attributed to the impaired or delayed integration with host system[4-6]. Unsatisfactory integration is always associated with the formation of fibrous tissue and long-term inflammatory response, resulting in failed tissue repair. Hence, the transplantation of autografts is still considered the gold standard in clinical practice, but it is limited by the shortage of donor sites and secondary damages[1,6]. From these perspectives, innovative tissue-engineering scaffolds with rapid host integration capacity are urgently needed for tissue regeneration.
217Tissues and organs in the human body are composed of multiple cell types and surrounding extracellular matrix (ECM) through three-dimensional (3D) self-assembly and further regulated by vascular and nervous system[7]. Blood vessels and nerve fibers are densely distributed within many tissues/organs, which are essential in tissue regeneration and functional recovery[1,6]. Generally, blood vessels and capillary networks continuously supply oxygen, nutrients, and growth factors to accelerate the process of regeneration[8]. Nerve fibers can transport excitation signals to targeted tissues to maintain its physiological excitation functions[9]. Moreover, a growing line of evidence has proven that nerve fibers also actively take part in tissue regeneration through secreting various neuropeptides and neurotrophic factors[10]. Previous studies have confirmed that insufficient vascularization and innervation can lead to delayed tissue regeneration. In addition, blood vessels and nerve fibers within tissues are closely coupled and interact with each other[1]. For instance, blood vessels provide nutrients for the formation and development of neural networks, while nerve fibers could stimulate angiogenesis via secreting neuropeptides[11-13]. Therefore, given the crucial roles of blood vessels and nerve fibers in tissue regeneration and functions recovery, ideal tissue regenerative scaffolds should possess the capacity of inducing vascularization and innervation via integrating with host neurovascular networks.
Unfortunately, traditional tissue-engineering scaffolds are mainly focusing on single type of tissue regeneration, and unable to regulate multiple cell types, resulting in insufficient vascularization and innervation. In addition, since different types of cells (tissue-related cells, endothelial cells, and neural cells) require different microenvironments for their proliferation and differentiation activities, it is required to fabricate scaffolds with complex composition and heterogeneous structures to satisfy the requirement of vascularization, innervation, and tissue regeneration[4]. 3D printing, as a rapidly developing technique, has become a versatile platform to precisely regulate the hierarchical structure and spatial distribution of multiple materials[14]. 3D printing makes it possible to fabricate biological scaffolds with multifunctional properties that can facilitate tissue regeneration and integration with vascular and nervous system. Moreover, in recent years, 3D bioprinting, an emerging subset of 3D printing technique, has attracted much attention for fabricating biomimetic multicellular constructs for complex tissue regeneration[15]. Capitalizing upon the advantages of the controllable distribution of multiple cells, 3D bioprinting offers a convenient and effective approach to stimulating vascularization and innervation by precisely depositing endothelial cells and neural cells into the constructs.
In this review, we first introduce the significant roles of blood vessels and nerve fibers in the regeneration of skin, bone, and skeletal muscle tissues, then highlight that both angiogenesis and innervation are indispensable for tissue regeneration and functions recovery. Subsequently, current strategies of 3D-printed biomaterials for vascularized and innervated tissue regeneration are summarized, respectively. Finally, the conclusions and future perspectives of 3D-printed biomaterials for vascularized and innervated tissue regeneration are provided.
2. Role of neurovascular networks in tissue regeneration
Many tissues (skin, bone, skeletal muscle tissues, etc.) are densely vascularized with blood vessels and innervated with nerve fibers, which play key roles in tissue metabolism, homeostasis, and repair (Figure 1)[1,2]. Blood vessels are able to continuously supply oxygen, nutrients, and growth factors to tissue-resident cells to maintain their metabolic activity[8]. Besides, several angiogenesis-related growth factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), also show positive effect on tissue regeneration[16]. For example, VEGF could bind to the receptors expressed on tissueresident cells to enhance their bioactivity[17]. Furthermore, it should be emphasized that the distance between cells and capillary vessels should be less than 200 μm, otherwise the diffusion and transportation of oxygen would be impaired, eventually reducing viability of internal cells[18]. However, severe tissue injury is usually accompanied by blood vessel damage, which impairs the transportation of oxygen and nutrients to wound sites, thus resulting in nonhealing wounds[19]. Hence, rapid induction of vascularization has become a key design criterion for developing novel tissue regenerative biomaterials[20].
Figure 1.

(A) The hierarchical structure of skin tissues[21]. Reprinted from Ashrafi M, Baguneid Mand, Bayat A, Acta Dermato-Venereologica, 2016, 96: 587–594, From ref. [21] licensed under Creative Commons Attribution 4.0 International License. (B) Schematic representation of the structure of bone tissues. Adapted from Marieb and Mallet, Human Anatomy, 1997. (C) Anatomy of the hierarchical organization of skeletal muscle tissues[41]. Reprinted from Samandari M, Quint J, Rodriguez-delaRosa A, et al., Advanced Materials, 2022, 34: 2105883. Copyright © 2022 John Wiley and Sons.
Similar to blood vessels, nerve fibers play essential roles in modulating the development and homeostasis of targeted tissues[1]. Moreover, nerves are also responsible for receiving information from the external environments, which are subsequently processed in central nervous system to initiate physiological responses[9]. For example, as the largest organ of human body, the skin has the ability of sensing external stimuli, such as pain, temperature, and mechanical force, which are mediated by the complex neural networks within skin[21]. Cutaneous receptors can receive the external signals and then send them to central nervous system via nerve fibers[22]. Furthermore, cutaneous nerve fibers also play significant roles in wound healing[23,24]. For example, cutaneous nerve fibers can increase blood supply around the wound beds through inducing neurogenic inflammation in the early phase of wound healing[19]. Besides, numerous218 neuropeptides (calcitonin gene-related peptide [CGRP], substance P [SP], etc.) and neurotrophic factors (nerve growth factors [NGF], brain-derived neurotrophic factor [BDNF], etc.) secreted by nerve fibers have been demonstrated to be beneficial to collagen deposition and wound contraction[3,21,23,25]. Hence, repairing the damaged neural networks is crucial for accelerating wound healing efficiency and restoring its biological functions (such as sensing pain, temperature, and touch perception), which is also beneficial to improve the life quality of patients[19].
In addition, nerves are distributed throughout the periosteum and bone marrow, and are responsible for transmitting different excitation electrical signals to targeted bone tissues[26,27]. It is well known that bone metabolism and homeostasis are tightly regulated by peripheral nervous system[28]. At present, researchers found that nerves could regulate bone metabolism through the following ways. First, sensory and sympathetic nerve fibers release different types of neuropeptides such as CGRP, SP, semaphorin 3 A (Sema 3A), and norepinephrine (NE), which could regulate the biological behaviors of bone-related cells (Figure 2A)[29]. For example, CGRP are able to upregulate the expression of osteocalcin and transcription factor-4 (ATF4) of osteoblast, and inhibit OPG/ RANKL-mediated osteoclastogenesis[30]. CGRP also bind to specific TRP1 receptor expressed on bone mesenchymal stem cells (BMSCs) and then activate Erk1/2 signaling pathway, thus promoting the osteogenic differentiation of BMSCs[31]. Moreover, NE, an important neurotransmitter of sympathetic nerve fibers, plays a crucial role in bone remodeling via binding with β-adrenergic receptors[32,33]. NE could reduce bone formation through inhibiting the differentiation of osteoblast and promoting bone resorption through the activation of osteoclast[34]. Second, recent studies found that neurotrophic factors could not only support the bioactivity of neurons but also actively participate in the process of bone regeneration and remodeling (Figure 2B)[10]. For example, nerve growth factors (NGF) can promote the proliferation and differentiation of osteoblast via binding with tropomyosin receptor kinase A (TrkA) receptors[35]. It is demonstrated that NGF-TrkA signaling pathway plays essential roles in bone healing with ingrowth of blood vessels and nerve fibers[36]. Similarly, brain-derived neurotrophic factor (BDNF) has the ability of stimulating the differentiation of osteoblast and then promoting bone formation via binding219 with tropomyosin receptor kinase B (TrkB) receptors[37]. BDNF also participates in the process of bone remodeling by inducing osteoclast formation[38]. Third, recent studies found that peripheral glial cell also contribute to bone metabolism and development[39]. For example, paracrine factors (platelet-derived growth factor-AA [PDGF-AA] and oncostatin M [OSM]) secreted by Schwann cells can regulate the cell behaviors of BSMCs and then participate in the process of bone regeneration[40]. Moreover, Cai et al. found that Schwann cells co-cultured with osteoblast could promote the proliferation, differentiation, and calcium nodules deposition activity of osteoblast[39]. Taken together, neural system actively participates in bone development and remodeling through various pathways.
Figure 2.

(A) The mechanism of peripheral nerves regulates the migration of mesenchymal stem cells and osteogenesis[29]. Reprinted from Wang XD, Li SY, Zhang SJ, et al., Theranostics, 2020, 10(11): 4839–4850. From ref. [29] licensed under Creative Commons Attribution 4.0 International License. (B) The potential roles of neurotrophic factors in bone regeneration and remodeling[10]. Reprinted from Su YW, Zhou XF, Foster BK, et al., Journal of Cellular Physiology, 2018, 233(3): 2133–2145. Copyright © 2017 John Wiley and Sons.
Skeletal muscle tissue is the heaviest tissue in the human body, accounting for about 45% of the body’s mass[41]. Muscle tissues are densely innervated with the peripheral nerves by forming neuromuscular junctions (NMJs), which are vital for the metabolism, maturation, and contraction of skeletal muscle[42]. It is well known that the dynamic movement of body is realized through contractile force generated by skeletal muscle tissues[2]. Specifically, somatic motor neurons trigger the release of acetylcholine (Ach) from the axon terminal through generating action potentials. Subsequently, the released Ach can bind to acetylcholine receptor (AchR) on the surface of the myofiber at NMJs, and eventually initiate the contraction of skeletal muscle[43]. However, volumetric muscle loss (VML) is always accompanied by significant motoneuron axotomy damage, resulting in permanent functional impairment[2]. Hence, reconstructing NJMs is of great significance to restore its physiological functions after VML.
In human body, blood vessel networks are distributed throughout tissues/organs for supplying nutrients and removing metabolic wastes. Nervous system extending highly branched neural fiber networks into targeted tissues/organs to establish communication by transmitting electrical signals.[44]. Developmental cues direct the formation of neural and vascular networks in an ordered manner with overlapping patterns to match the architectural and functional demands of tissues. Moreover, due to the anatomical similarity, blood vessels and nerves have a close interaction with each other[45]. For instance, blood vessels need to provide sufficient nutrients for supporting the development of nerves, while nerves also regulate vasodilation and vasoconstriction via transmitting signals[46]. Moreover, previous studies also found that blood vessels and nerve fibers are able to share same signals and receptors[47]. For example, NGF, one of the most important neurotrophic factors for neuron maturation, has a positive effect on the proliferation and migration activity of endothelial cells through binding to its surface TrkA receptors[48,49]. Similarly, VEGF could also promote the survival of neuron and axonal outgrowth[50]. Overall, considering the significant roles of blood vessels and nerve fibers in tissue regeneration, developing novel neurovascularized biomaterials based on 3D printing technology is highly demanded.
3. Skin tissues
3.1. 3D printing for skin tissue engineering
At present, auto-transplantation still remains the best strategy in clinical setting for treating severe and critical-size skin defects, in which skin tissue is harvested from one part of the body and subsequently grafted to injury site in the same220 patient[1]. It should be emphasized that skin tissues used for transplantation usually harvested from the hidden part of human body, such as hip, inner thigh, and head. Therefore, the amount of available skin grafts was limited for the patients. Besides, the risk of donor site infection and blood loss should also be seriously considered during the surgical process of auto-transplantation[51]. Therefore, developing novel skin grafts is urgently needed. However, insufficient and ineffective integration with host neurovascular system are the major issues. 3D printing technique is considered an innovative treatment option to solve these problems. The specific control of structures, as well as incorporation of multiple materials and biological factors, enables the ability of 3D-printed wound dressings to rapidly integrate with host neurovascular systems[14]. Moreover, 3D bioprinting of biomimetic multicellular skin scaffolds also contributes to the early induction of angiogenesis and innervation, which can accelerate the healing process of skin defects[52].
3.2. 3D-printed biomaterials for vascularized skin regeneration
Increasing studies showed that many bioactive molecules exhibited positive effect on angiogenesis, such as growth factors, exosomes, peptides, platelet-rich plasma (PRP), and gas molecules[53-62]. At present, numerous biological factors (VEGF, bFGF, etc.) have been integrated into 3D-printed scaffolds for vascularized skin regeneration[63]. For example, Alizadehgiashi et al. designed VEGF- incorporated multifunctional hydrogel wound dressing via 3D printing technology (Figure 3A)[64]. By regulating the architectures and shapes of scaffolds, the release profile of growth factors could be easily controlled in an ordered manner to match the physiological process of wound healing. As a result, 3D-printed hydrogel scaffolds possessed satisfactory effect on granulation tissue formation and vascularization. However, from the perspective of clinical applications, there are still some problems that need to be solved. First, the complex preparation processes (3D scan, CAD model, biomaterials deposition, postprocess, etc.) of 3D-printed scaffolds would inevitably impair the bioactivity of VEGF and increase the cost[65]. Second, due to the short half-life period of growth factors, the rigorous conditions for preservation and transportation of VEGF- laden scaffolds would further increase the financial stress of patients[66].
Figure 3.
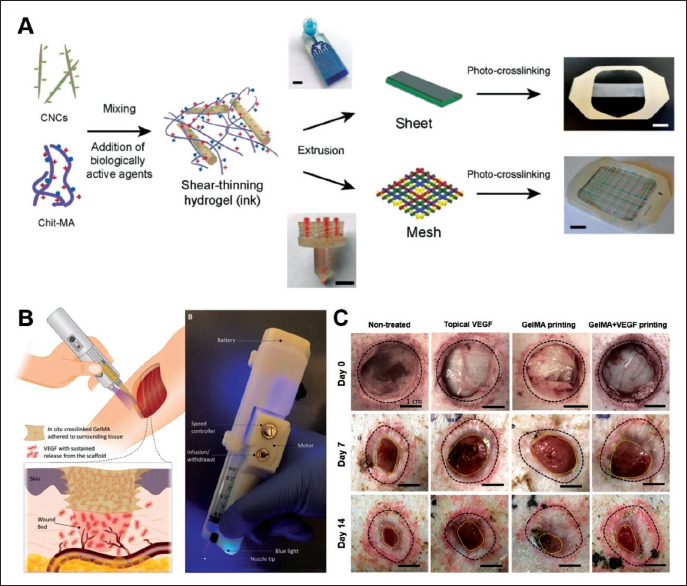
(A) Schematic representation of the preparation of 3D-printed skin wound dressings[64]. Reprinted from Alizadehgiashi M, Nemr C R, Chekini M, et al., ACS Nano, 2021, 15(7): 12375–12387, Copyright © 2021 American Chemical Society. (B) Schematic representation of in-situ 3D printing strategy and the images of handheld 3D printer. (C) Representative photographs of the wounds after 7 days and 14 days treatments[69]. Reprinted from Nuutila K, Samandari M, Endo Y, et al., Bioactive Materials, 2022, 8: 296–308, From ref. [69] licensed under Creative Commons Attribution 4.0 license.
221In situ printing technique provides a potential approach for overcoming these problems. Briefly, in situ printing strategy allows the biomaterials to be directly deposited onto the wound site during surgery, which significantly simplifies the preparation process and avoids preservation and transportation of growth factors-laden scaffolds before surgery[67,68]. Therefore, this strategy obviously saved the cost of preservation and transportation of scaffolds. For example, Nuutila et al. developed in situ 3D-printed VEGF- gelatin methacryloyl (GelMA) hydrogel scaffold for wound healing via a custom-made handheld printer (Figure 3B)[69]. Briefly, VEGF-GelMA precursor was directly deposited onto wound beds and then integrated with host tissues after in situ crosslinked by blue light. The strategy of in situ printing can greatly shorten the preparation time of scaffolds, which is beneficial to maintaining the biological activity of VEGF. Moreover, in situ crosslinking strategy could enhance the adhesion strength of scaffolds to wound beds, which further simplified the procedures of surgery. As a result, the developed VEGF-GelMA scaffolds had a great performance in stimulating angiogenesis and enhancing the quality of skin regeneration in skin defects model (Figure 3C).
Exosomes, one of the most important secretory products of cells, are collectively a type of emerging bioactive agent for regulating cell–cell communications[70]. Many studies have shown that exosomes can promote tissue repair, due to its immune regulation and angiogenesis ability[71]. For example, Hu et al. fabricated a cryogenic 3D-printed hydrogel wound dressing with the incorporation of BMSCs-derived exosomes for diabetic wound healing[72]. Exosomes could be sustainably released from the 3D-printed hydrogel scaffolds and capable to promote the differentiation activities of human umbilical vein endothelial cells (HUVECs). Furthermore, the developed scaffolds possessed the ability of stimulating the process of angiogenesis and increasing the blood flow of wound beds, resulting in accelerated wound healing.
Peptides, formed by amino acid sequences with the connection of peptide bond, have been confirmed to be beneficial to angiogenesis and wound healing[73]. For instance, Chu et al., prepared proangiogenic peptide nanofiber-integrated GelMA hydrogel scaffolds via 3D printing technology for vascularized skin regeneration[74]. The pro-angiogenesis ability of peptide nanofiber was similar to growth factors. Besides, the interconnected macroporous structure of scaffolds could provide physical cues for the proliferation and migration of endothelial cells, and induce the ingrowth of host blood vessels, resulting in early vascularization.
Apart from biological factors, inorganic materials have attracted great attention for the regeneration of skin defects, owing to the positive effect of bioactive elements (Mg, Si, Sr, Fe, Mn, P, etc.) on angiogenesis, immunomodulation, collagen deposition and wound healing[5,75-81]. Until now, various inorganic materials have been prepared and incorporated into wound dressings to improve its biological activity. For example, Ma et al. developed a 3D-printed inorganic/organic composite wound dressing for the treatment of deep skin burns[82]. Diatomite (DE, SiO2·nH2O), the natural siliceous skeleton, was incorporated into GelMA matrix to serve as a bioactive agent to sustainably release Si ions, which is beneficial to cell proliferation and vascularization. As a result, 3D-printed composite wound dressings could promote the angiogenesis-related genes expression of HUVECs in vitro and the formation of new blood vessels in vivo. Hence, the incorporation of inorganic biomaterials has been regarded as an effective and inexpensive strategy to endow the 3D-printed scaffolds with enhanced angiogenic bioactivities.
In addition to acellular wound dressings, 3D bioprinting of cellular living system with biomimetic dermal-epidermal physiological structures for use in skin regeneration has gained huge attention[83,84]. However, the low cell viability and differentiation capacities have limited its further application. To overcome these shortcomings, our group developed a strontium silicate (SS)-containing multicellular system with vascularization-induced properties (Figure 4)[85]. SS microparticles were integrated into bioinks to act as stable biological agent to promote the differentiation of encapsulated endothelial cells through continuous release of Sr and Si ions. As a result, the SS- containing biomimetic skin constructs can rapidly integrate with host tissues and induce vascularization, resulting in accelerated skin regeneration in vivo. In another work of our group, inspired by the immune modulation effects on angiogenesis, Wu et al. developed manganese silicate (MS)- containing bioinks with immunomodulatory properties[86]. The Mn and Si ions could provide a beneficial immune microenvironment for stimulating angiogenesis through modulating macrophages into anti-inflammatory M2 phenotype.
Figure 4.

3D-bioprinted biomimetic multicellular scaffolds promoting vascularized skin regeneration. (A) The specific bilayered distribution of human dermal fibroblasts (HDFs) and HUVECs in the 3D-bioprinted scaffolds. (B) Live/dead staining of the cells within the scaffolds after co-cultured for 1 day and 10 days. (C) Images of acute wounds at different times. (D) Images of chronic wounds at different times. (E) Immunohistochemical staining images of CD31 antibody[85]. Reprinted from Ma J, Qin C, Wu J, et al., Advanced Healthcare Materials, 2021, 2100523. Copyright © 2021 John Wiley and Sons.
3.3. 3D-printed biomaterials for innervated skin regeneration
Skin is an electroactive tissue with conductivity values ranging from 0.1 to 2.6 mS cm-1[61,87]. It is reported that skin defects can trigger the formation of endogenous electric fields, and these electric fields have been confirmed to promote cell migration and wound contraction[88]. Besides, neural cells are known to be sensitive to electrical signals. For example, Sebastian et al. had confirmed that electrical signals stimulation can accelerate wound healing by promoting re-innervation[89,90]. Therefore, the incorporation 222of electroactive materials is a feasible approach to endowing 3D-printed scaffolds with bioelectrical properties, which enables to stimulate innervation and accelerate wound healing[91]. For example, Peng et al. prepared a self- adaptive delivery chip by 3D coaxial printing technique, which allows stepwise release of multiple biochemical and bioelectrical components to promote rapid skin nerves restoration and excitation (Figure 5)[92]. Electroactive materials GO-polyethyleneimine (CGP) and GO- polypyrrole-alginate (GPA) were distributed in the core and shell of the 3D-printed scaffolds, respectively, which could provide a conductive microenvironment to promote cutaneous nerve regeneration. The bioelectrical signals and released plasmid DNAs (pDNAs) synergistically directed the neural differentiation of mesenchymal stem cells (MSCs), which could further differentiate into neural cells with electrophysiological activities. The in vivo results further confirmed that this 3D-printed self-adaptive scaffold can stimulate the cutaneous nerves regeneration with excitation function recovery within 23 days.
Figure 5.

Schematic representation of design and fabrication of 3D-printed chips. First: biomaterials synthesis and chip engineering. Second: smart delivery of biological factors and electrical signal cues to wound beds can stimulate the neural differentiation of MSCs and excitation function recovery[92]. Reprinted from Peng L H, Xu X H, Huang Y F, et al., Advanced Functional Materials, 2020, 30: 2001751. Copyright © 2020 John Wiley and Sons.
Apart from electroactive materials, biological factors, mRNA, platelet, and exosomes have been demonstrated to promote neurogenesis and wound healing[93-96]. For example, neurotrophic factors (NGF, BDNF, etc.) play significant roles in the survival, differentiation, and functions of sensory nerves through binding with its specific receptors[23]. Recently, Chinese traditional medicine ginseng-derived exosomes have been proven to stimulate the neurogenic differentiation and maturation of MSCs, possessing huge potential in cutaneous nerves regeneration[94]. Moreover, increasing evidence indicated that bioactive ions play important roles in nerve tissue regeneration by participating in many biological processes (such as DNA/RNA synthesis and enzyme activation)[95]. In a recent study of our group, we found that zinc silicate nanoparticles exhibited outstanding neurogenic activity by releasing bioactive Zn and Si ions in a sustainable manner[97]. Besides, the positive effects of zinc silicate nanoparticles on cutaneous innervation were also confirmed in a deep second-degree skin burn model. Therefore, integrating these bioactive agents into 3D-printed scaffolds would be ideal for the regeneration of skin nerves.
4. Bone tissues
4.1. 3D printing for bone tissue engineering
Due to the limited self-healing ability of bone tissues, numerous bone regenerative biomaterials were developed and applied to repair large-scale bone defects, such as bioceramics, metals, and polymers[98,99]. 3D printing possesses the capacity to repair bone defects via simulating223 the complex hierarchical structure, organic–inorganic components, and physiological properties of bone tissues[100]. For example, 3D-printed bone regenerative scaffolds with multi-channel structures could promote host cells infiltration and serve as cell delivery platforms for new tissue regeneration[101]. Besides, integrating bioactive factors into 3D-printed scaffolds could mimic the microenvironments in bone formation, then accelerate the process of bone repair[102,103]. Moreover, 3D bioprinting has brought promise to prepare biomimetic bone constructs with precise distribution of multiple cells[104-106]. Biomimetic 3D cell-laden construct could highly mimic the hierarchical structure and cellular components of native bone tissues, enabling rapid integration with host systems and accelerated healing rate.
4.2. 3D-printed biomaterials for vascularized bone regeneration
Bone scaffolds with macroporous or channel structures are beneficial to the penetration of cells and ingrowth of host blood vessels. It is well known that 3D printing technology could easily control the structure of scaffolds from macroscale to microscale[107]. For example, our group developed lotus-like biomimetic scaffolds via a modified 3D printing technique (Figure 6A)[108]. Compared to traditional 3D-printed scaffolds stacked by solid structs, the multichannel structs were capable to enhance oxygen/ nutrients transports and promote the early angiogenesis inside the implanted scaffolds. The in vivo results further confirmed the satisfactory effects of channel structures on vascularization. Similarly, Hann et al. prepared perfusable vascular networks-based biomimetic bone scaffolds by combining stereolithography (SLA) and fused deposition modeling (FDM) 3D printing technology[109]. The perfusable channels can provide appropriate microenvironments for vasculogenesis and angiogenesis. In order to enhance the tissue regeneration capacity inside the hollow-channel structs of the 3D-printed scaffolds, Wang et al. developed a smart scaffold with hollow-pipe channel structures and stimuli-responsive features by using microfluidic 3D printing technique (Figure 6B)[110]. The channel dimensions showed reversible swelling and shrinkage properties under near-infrared light irradiation, which is beneficial to the infiltration of external cells into hollow channels. As a result, these near-infrared-responsive channels could obviously promote the deep infiltration of host vessel into scaffolds and effectively accelerate bone regeneration in vivo.
Figure 6.

(A) The preparation and application of 3D-printed lotus root-like scaffolds for promoting bone regeneration and blood vessels infiltration[108]. Reprinted from Feng C, Zhang W, Deng C, et al., Advanced Science, 2017, 4: 1700401. Copyright © 2017 John Wiley and Sons. (B) Schematic representation of microfluidic assisted-3D printed stimuli-responsive scaffolds with biomimetic hollow channels for promoting bone regeneration[110]. Reprinted from Wang X, Yu Y, Yang C, et al., Advanced Functional Materials, 2021, 2105190. Copyright © 2021 John Wiley and Sons. (C) 3D printing of Haversian bone-mimicking scaffolds for delivering BMSCs and HUVECs and promoting vascularized bone regeneration[111]. Reprinted from Zhang M, Lin R, Wang X, et al., Science Advances, 2020, 6: eaaz6725. From ref. [111] licensed under Creative Commons Attribution 4.0 license.
224In addition, designing a complex hierarchical structure scaffold similar to native bone tissues is a promising approach to accelerating bone regeneration[100]. For instance, Zhang et al. fabricated a Haversian canals-biomimetic bioceramic scaffold by using digital laser processing-based 3D printing technique (Figure 6C)[111]. The porosity and mechanical strength of the biomimetic scaffolds could be accurately regulated by the custom design software. Moreover, the hierarchical structure of biomimetic scaffolds can provide a platform to modulate multicellular distributions and cell– cell interactions. Besides, the Haversian bone-mimicking scaffolds could also serve as a multicellular delivery system for simultaneously inducing angiogenesis and osteogenesis in vitro, and promoting vascularized bone regeneration in a rabbit femoral defects model.
Plenty of bioactive agents, such as growth factors, drugs, liposomes, enzymes and small molecules, have been shown to possess excellent pro-angiogenesis properties[103,112-117]. Hence, the incorporation of proangiogenesis agents into 3D-printed bone regenerative scaffolds has been regarded as a promising strategy for vascularized bone regeneration. For example, Han et al. designed a lotus seedpod mimetic drug-loaded 3D-printed bioceramic scaffold for accelerating the healing process of bone defects[116]. Deferoxamine@lipsome (DFO@lip)- laden GelMA microsphere was fabricated via microfluidics technology and then integrated into 3D-printed β-TCP scaffolds. The controlled release of DFO could not only stimulate angiogenesis by upregulating hypoxia-inducible factor 1 alpha (HIF-1α) expression but also promote225 the mineralization of extracellular matrix. In another study, Ha et al. developed dual-drug delivery bone scaffolds via sacrificial templates-assisted 3D printing technology[118]. First, pro-angiogenesis small-molecule drugs, dimethyloxalylglycine (DMOG) and bone forming peptide-1 (BFP), were incorporated into mesoporous silica nanoparticles (MSNs). Subsequently, DMOG/MSN were loaded on the surface of the scaffolds, and BFP/MSN were embedded into the scaffolds to achieve the sequential delivery of dual drugs. 3D-printed composite scaffolds possess satisfactory angiogenesis and osteogenesis ability both in vitro and in vivo.
In addition, 3D-printed scaffolds with injury microenvironment response characteristics can obviously improve the viability and function of endogenous cells, thus promoting tissue regeneration. For example, Yang et al. designed enzyme-functionalized bone tissue regenerative scaffolds with the integration of glucose oxidase (GOx) and catalase (CAT) enzymes[119]. The cascade catalytic reaction of GOx and CAT enzymes could alleviate the hyperglycemic microenvironments and continuously consume oxygen, leading to the formation of hypoxic microenvironment, which further stimulates the neovascularization process. As a result, the scaffolds could obviously upregulate the expression of angiogenesis-related gene markers (such as VEGF and HIF-1α) of HUVECs, and enhance its osteogenic activity, further confirming its potential for vascularized bone regeneration.
4.3. 3D-printed biomaterials for innervated bone regeneration
As previously mentioned, nerve fibers actively take part in the process of bone regeneration and remodeling[28]. Hence, simultaneous regeneration of neural elements in the newly formed bone tissues is essential for functional bone regeneration. The incorporation of neurotrophic agents or neural cells into 3D-printed scaffolds is a promising approach to achieving innervated bone regeneration. For example, Li et al. developed ossification center microenvironment-mimicking 3D-bioprinted bone constructs for innervated bone regeneration (Figure 7)[120]. With the integration of NGF@Laponite (NGF@ Lap) nanomaterials, the constructs continuously released NGF for a long time, which is similar to the microenvironment of ossification center with high concentration of NGF. 226Besides, NGF and Lap could effectively stimulate the gene expression and secretion of CGRP in dorsal root ganglion (DRG) sensory neurons, and eventually stimulate osteogenic differentiation of BMSCs. In another study, Fitzpatrick et al. developed a multifunctional 3D-printed scaffold for vascularized and innervated bone regeneration via incorporating osteo-inductive factor bone morphogenetic protein-2 (BMP2), pro-angiogenic factors (VEGF) and neurotrophic growth factors (NGF) into silk-hydroxyapatite bone cements[121]. As a result, the functionalized scaffolds had tri-effects on stimulating the osteogenic differentiation of MSCs, proliferation and migration activities of endothelial cells, and neurogenic differentiation of neural stem cells (NSCs).
Figure 7.

(A) The fabrication process of the 3D-bioprinted ossification center microenvironments biomimetic bone constructs. The bioinks were composed of GelMA, AlgMA and NGF@Laponite. The constructs were crosslinked by UV light and Ca2+ ions. (B) Mechanism of the 3D bioprinted constructs promoting the regeneration of bone defects[120]. Reprinted from Li W, Miao W, Liu Y, et al., Advanced Functional Materials, 2022, 202109871. Copyright © 2021 John Wiley and Sons.
Apart from neurotrophic factors, previous studies found that Mg promotes bone fracture healing by inducing the secretion of CGRP from sensory nerves, confirming the essential role of sensory nerves in bone healing[13,122]. Mg ions released from the implants could enter DRG neurons through the TRPM (transient receptor potential melastatin) channel and magnesium transporter1 (MAGT1), thus promoting the synthesis and secretion of CGRP as well as osteogenesis. Moreover, it is reported that Si ions are able to induce the synthesis and secretion of Sema 3A in the DRGs via activating the PI3K-Akt-mTOR signaling pathway[123]. Silicon-stimulated DRGs condition medium can stimulate the osteogenesis-related gene expression of BMSCs and angiogenesis of endothelial cells by Sema 3A. Therefore, the integration of neural-active elements into 3D-printed scaffolds is a good option for innervated bone regeneration.
In addition, neural cells-based therapy holds great potential in stimulating innervation in engineered bone tissue. It is reported that the interaction of neural cells and bone cells is beneficial to innervation and osteogenesis[39]. For example, Zhang et al. fabricated tree-like bioceramic (TLB) bone regenerative scaffolds for delivering bone- related and nerve-related cells, which could simultaneously promote innervated bone regeneration[124]. BMSCs and Schwann cells were distributed on different leaf blades of the TLB scaffold. Moreover, the gradient micro-structure of the surface of leaf blades could simultaneously promote the osteogenesis-related gene expression of BMSCs and neurogenesis-related gene expression of Schwann cells in the co-culture scaffolds. As a result, bone regeneration with innervation was observed after the implantation of TLB scaffold. Moreover, in order to accelerate the process of bone repair, mimicking the cellular distribution of natural bone tissues is beneficial to the fabrication of highly integrated bone scaffolds. In a recent study, Zhang et al. developed a biomimetic 3D multicellular neural- bone construct for bone regeneration with innervation by combining multicellular 3D bioprinting technology with nanocomposite bioinks (Figure 8)[125]. Based on the native distribution of bone tissue and skeletal nerves, neural cells (Schwann cells) and bone-related cells (BMSCs) were specifically deposited in the top layer and bottom layer of the constructs, respectively. Moreover, bioactive calcium silicate nanowires were added into GelMA bioinks to serve as biological agents to enhance cell viability and regulate cell behaviors. Both Schwann cells and BMSCs spread well to form cell-networks during the culture periods. Moreover, calcium silicate nanowires incorporated nanocomposite bioinks could not only stimulate the expression of osteogenesis-related genes and proteins of BMSCs but also promote the expression of neurogenesis-related genes and proteins of Schwann cells. Most interestingly, these biomimetic neural-bone constructs obviously promoted new bone formation and induce ingrowth of host nerves after the constructs were implanted into the defects, thereby promoting innervated bone regeneration. Hence, the incorporation of neural cell is a promising strategy for bone regeneration with enhanced innervation capacity.
Figure 8.

(A) Schematic representation of the preparation and application of 3D-bioprinted biomimetic multicellular neural-bone constructs for promoting bone formation and innervation. (B) The specific location and morphology of BMSCs and Schwann cells within the constructs. (C) Immunohistochemical staining results of bone markers (OCN and OPN) and neural markers (NF and CGRP) after treatment for 4 and 8 weeks[125]. Reprinted from Zhang H, Qin C, Wu J, et al., Nano Today, 2022, 46: 101584. Copyright (2022), with permission from Elsevier.
5. Skeletal muscle tissues
5.1. 3D printing for skeletal muscle tissue engineering
Owing to the complex and hierarchical structure of skeletal muscle tissues, it remains a huge challenge for traditional strategies to fabricate artificial muscle constructs with biological characteristics. Besides, sufficient vascularization and innervation are quite necessary for skeletal muscle regeneration with functional recovery[6]. 3D printing is able to recapitulate the structure of skeletal muscle tissues by precisely regulating the specific distribution and arrangement of multiple materials and growth factors[41]. The aligned filaments of 3D-printed scaffolds could provide topological cues for inducing alignment and differentiation of muscle cells[126-128]. Moreover, 3D bioprinting of engineered muscle constructs with multimaterial structures and multicellular components could meet the requirement of vascularization and innervation, enabling in promoting functional skeletal muscle regeneration[41].
5.2. 3D-printed biomaterials for vascularized skeletal muscle regeneration
Two main strategies for stimulating vascularization in skeletal muscle tissue engineering include in situ vascularization and prevascularization. The first approach is inducing the infiltration of host blood vessels through regulating the physicochemical or biological properties of 3D-printed scaffolds. For instance, scaffolds with high porosity or multichannel structures can recruit more host cells and biological factors to participate in the process of tissue regeneration and induce the ingrowth of host227 blood vessels to deliver nutrients[129]. Besides, scaffolds functionalized with bioactive molecules (growth factors, cytokines, etc.) have also gained much attention for promoting host vessel infiltration[130]. For example, Quint et al. developed growth factors-releasing 3D scaffolds for the repairment of skeletal muscle defects[131]. The bioinks composed of GelMA hydrogels and VEGF-Laponite nanoparticles could be in situ deposited in the injury site by using a partially automated handheld printer. The long-term sustained release of VEGF from the scaffolds could obviously regulate the injury environment to increase CD31+ capillaries, reduce fibrous, and improve anabolic response, thereby promoting the functional muscle recovery. In another study, Said et al. developed a fibroblast growth factor-9 (FGF9)-loaded electrospun poly (ester amide) fiber mat for improving angiogenesis in ischemic muscle[132]. The locally released FGF9 had a great performance in stimulating formation of microvascular networks and reducing interstitial fibrosis, resulting in improved locomotion.
In prevascularization strategy, researchers attempted to incorporate endothelial cells into engineered skeletal muscle constructs to form vascular network in vitro. After implanted into the defects, the preformed micro- vascular networks could successfully integrate with host vascular system and were infiltrated with red blood cells, resulting in enhanced vascularization[133]. For example, Choi et al. prepared a prevascularized 3D muscle scaffolds that are equipped with highly biomimetic hierarchical architecture of natural muscles through coaxial extrusion 3D bioprinting of cell-laden bioinks[134]. Muscle cells- laden decellularized skeletal muscle extracellular matrix (mdECM) bioinks and endothelial cells-laden vascular228 tissue-derived decellularized extracellular matrix (vdECM) bioinks were distributed in the core and shell of the filaments through extrusion from a coaxial nozzle in order to simulate the complex structure of muscle fibers coupled by blood vessels. Immunofluorescence staining results indicated that coaxial 3D-bioprinted muscle construct possessed the capacity of promoting endothelial network formation and muscle maturation. Encouraged by the satisfactory in vitro outcomes, the in vivo performance of prevascularized 3D-bioprinted muscle constructs was evaluated in a volumetric muscle loss model. As a result, 3D-bioprinted muscles constructs had the maximum recovery of muscle tissue weight and minimum fibrosis as compared to other groups. Besides, functional blood vessels with lumen structures, formation of NMJs and integration with host neural system could be observed in the implanted area. Taken together, this study demonstrated that 3D bioprinting of biomimetic prevascularized muscle construct is an effective strategy for treating volumetric muscle loss.
5.3. 3D-printed biomaterials for innervated skeletal muscle regeneration
Similar to the aforementioned vascularization strategies, promoting host neural infiltration and fabricating preinnervated constructs contribute to enhanced innervation[6]. In the first strategy, tissue-engineered muscle constructs functionalized with biochemical signals and micro-topographical cues are capable to promote host neural infiltration and formation of NMJs after implanted into muscle defects[135]. For example, Lee et al. fabricated self-aligned 3D skeletal muscle constructs through in situ creating aligned surface topological microstructures of 3D-printed muscle constructs[136]. The fibrillation and leaching process of poly (vinyl alcohol) induced the formation of aligned topographical structures, which further promoted the directional arrangement of muscle progenitor cells. The self-aligned constructs obviously accelerated the integration with host neural networks, leading to rapid functional muscle recovery.
Taking the advantages of 3D bioprinting technology, it is practical to fabricate a preinnervated tissue engineering muscle construct with the integration of neural progenitor cells or differentiated neurons[137,138]. The coculture of neural cells and myoblast in 3D constructs enable the formation of NMJs in vitro, which is beneficial to the survival, differentiation, and maturation of myoblast. In a recent study, Kim et al. developed neural stem cells- containing 3D-bioprinted muscle construct to promote muscle regeneration and functional recovery (Figure 9)[139]. 2D co-culture assay was firstly performed to explore the cross-talking of human neural stem cells (hNSCs) on human muscle progenitor cells (hMPCs). It was found that the ratio of hNSCs and hMPCs at 1:300 was optimal for the formation of myotube, the neural differentiation of hNSCs and the NMJs formation. Then, the hNSCs were integrated into the bioengineered skeletal muscle constructs via a multichannel 3D bioprinting technique. According to the results, hNSCs-integrated constructs showed significant improvement of myofiber formation, neural differentiation, and NMJs formation in vitro. Moreover, the in vivo results further demonstrated the rapid integration with host neural networks and the vascularization of implanted hNSCs-integrated constructs, leading to enhanced function restoration of muscle tissues. Taken together, tissue engineering-based strategies possess great potential in promoting functional muscle regeneration.
Figure 9.

(A) Schematic representation of the preparation of 3D-bioprinted neural cell-integrated skeletal muscle constructs. (B) Assessment of 3D- bioprinted muscle constructs: (i) the design of printing path; (ii) gross photographs of the bioprinted constructs; (iii) live/dead staining images; and (iv) quantitative cell viability (%) at day 1. (C) Immunofluorescence staining images of the formation of NMJs, neural differentiation of hNSCs and vascularization in vivo[139]. Reprinted from Kim J H, Kim I, Seol Y J, et al., Nature Communications, 2020, 11(1025). From ref. [139] licensed under Creative Commons Attribution 4.0 license.
6. Conclusions and perspectives
In this review paper, we highlighted the essential role of vascular system and nervous system in functional tissue regeneration and summarized recent advances of 3D-printed biomaterials for vascularized and innervated tissue regeneration. In general, vascularization can accelerate the process of tissue regeneration through providing sufficient oxygen and nutrients. Meanwhile, innervation actively participates in the process of tissue regeneration and is indispensable for the functional recovery of damaged tissues. Furthermore, blood vessels and nerve fibers closely distributed with each other and have synergistic effect on tissue regeneration. However, there are very few reports about biomaterials that can simultaneously induce vascularization and innervation, which are mainly attributed to the difficulty of regulating multiple cells.
As previously described, several strategies have been proven to be beneficial to vascularization, such as fabricating macroporous/channel structures and integrating pro- angiogenic factors and cells. However, innervations have always been overlooked in the past few decades when it comes to designing tissue regenerative scaffolds. The development of biomaterials for promoting innervated tissue regeneration is still in its infancy stage. Hence, more biomaterials that are capable of inducing innervation should be developed and the underlying mechanism should be explored. The design criterion of pro-innervation biomaterials can be considered from the following aspects:
-
(i)
Given the physiological properties of nerve fibers, biomaterials that have been extensively applied in neural tissue engineering field such as electrical stimulation and electroactive materials may be potentially useful for innervation[130]. As previously mentioned, several studies have confirmed that the application of electrical stimulation or electroactive materials have positive effect on promoting 229innervation[128,140]. Hence, novel electroactive materials could be developed and integrated into 3D-printed scaffolds to enhance their biological activity.
-
(ii)
Due to the anatomically coupled distribution of blood vessels and nerve fibers, it is worth exploring whether pro-angiogenetic biomaterials have potential impact on innervation. Moreover, the intrinsic mechanisms of blood vessels and nerve fibers also need to be further investigated.
-
(iii)
Stem cells-based therapy might be a feasible approach for innervation. Plenty of studies have confirmed that stimulating the neural differentiation of stem cells could accelerate innervation and host nerve fibers infiltration.
In addition to material design, scaffolds also need to match the host physiological microenvironment. For ideal tissue-engineering scaffolds, their mechanical strength, porosity and degradation rate are required to be similar to native tissues[20]. However, it is virtually impossible for traditional strategies to fabricate scaffolds to meet all these requirements. 3D printing opens new avenues for fabricating ideal tissue-engineering scaffolds with appropriate physical properties. Therefore, with the help of 3D printing technology, vascularized and innervated tissue regeneration are expected to be achieved through the integration of multiple functional materials in a controlled manner. Moreover, taking the advantages of 3D bioprinting technology, multiple types of cells could be integrated into 3D-bioprinted constructs[52]. The prevascularized and preinnervated constructs can quickly integrate with host vascular and nervous systems after implanted into the defect sites, leading to obviously vascularized and innervated tissue regeneration. In addition, more characterization needs to be performed to elucidate the mechanism of cells–materials and cells– cells interactions during the process of vascularized and innervated tissue regeneration. Taken together, we expect 230that strategies based on 3D printing of biomaterials could offer a new direction for complex tissue regeneration with functional recovery.
Acknowledgments
None.
Funding
This work was supported by Natural Science Foundation of China (32130062, 32225028), Technology Commission of Shanghai Municipality (21DZ1205600), CAS Project for Young Scientists in Basic Research Grant (No. YSRB073) and Shanghai Pilot Program for Basic Research-Chinese Academy of Science, Shanghai Branch (JCYJ-SHFY-2022-003).
Conflict of interest
The authors declare no conflicts of interest.
Author contributions
Conceptualization: Hongjian Zhang
Supervision: Chengtie Wu
Writing – original draft: Hongjian Zhang
Writing – review & editing: Chengtie Wu
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data
Not applicable.
References
- 1.Marrella A, Lee TY, Lee DH, et al. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater Today. 2018;21(4):362–376. doi: 10.1016/j.mattod.2017.10.005. https://doi.org/10.1016/j.mattod.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eugenis I, Wu D, Rando TA. Cells, scaffolds, and bioactive factors: Engineering strategies for improving regeneration following volumetric muscle loss. Biomaterials. 2021;278:121173. doi: 10.1016/j.biomaterials.2021.121173. https://doi.org/10.1016/j.biomaterials.2021.121173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weng T, Wu P, Zhang W, et al. Regeneration of skin appendages and nerves: Current status and further challenges. J Trans Med. 2020;18(1):1–17. doi: 10.1186/s12967-020-02248-5. https://doi.org/10.1186/s12967-020-02248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan Q-Q, Qin W-P, Shen M-J, et al. Simultaneous regeneration of bone and nerves through materials and architectural design: Are we there yet? Adv Funct Mater. 2020;30(48):2003542. https://doi.org/10.1002/adfm.202003542. [Google Scholar]
- 5.Ma J, Wu C. Bioactive inorganic particles-based biomaterials for skin tissue engineering. Exploration. 2022;2(5):20210083. doi: 10.1002/EXP.20210083. https://doi.org/10.1002/EXP.20210083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert-Honick J, Grayson W. Vascularized and innervated skeletal muscle tissue engineering. Adv Healthc Mater. 2020;9(1):1900626. doi: 10.1002/adhm.201900626. https://doi.org/10.1002/adhm.201900626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beachley VZ, Wolf MT, Sadtler K, et al. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat Methods. 2015;12(12):1197-+. doi: 10.1038/nmeth.3619. https://doi.org/10.1038/nmeth.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marenzana M, Arnett TR. 2013. The key role of the blood supply to bone. Bone Res 1 203–215. https://doi.org/10.4248/br201303001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das S, Gordian-Velez WJ, Ledebur HC, et al. 2020. Innervation: The missing link for biofabricated tissues and organs. NPJ Regen Med 5 1 1 17. https://doi.org/10.1038/s41536-020-0096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su Y-W, Zhou X-F, Foster BK, et al. Roles of neurotrophins in skeletal tissue formation and healing. J Cell Physiol. 2018;233(3):2133–2145. doi: 10.1002/jcp.25936. https://doi.org/10.1002/jcp.25936. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Mao C, Zhuo L, et al. 2015. Beta-nerve growth factor promotes neurogenesis and angiogenesis during the repair of bone defects. Neural Regen Res 10 7 1159 1165. https://doi.org/10.4103/1673-5374.160114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mi J, Xu J, Yao H, et al. Calcitonin gene-related peptide enhances distraction osteogenesis by increasing angiogenesis. Tissue Eng Part A. 2021;27(1-2):87–102. doi: 10.1089/ten.TEA.2020.0009. https://doi.org/10.1089/ten.tea.2020.0009. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, Chen H, Jiang Y, et al. CGRP regulates the dysfunction of peri-implant angiogenesis and osseointegration in streptozotocin-induced diabetic rats. Bone. 2020;139:115464. doi: 10.1016/j.bone.2020.115464. https://doi.org/10.1016/j.bone.2020.115464. [DOI] [PubMed] [Google Scholar]
- 14.Bittner SM, Guo JL, Melchiorri A, et al. 2018. Threedimensional printing of multilayered tissue engineering scaffolds. Mater Today 21 8 861 874. https://doi.org/10.1016/j.mattod.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinrich MA, Liu W, Jimenez A, et al. 3D bioprinting: From benches to translational applications. Small. 2019;15(23):1805510. doi: 10.1002/smll.201805510. https://doi.org/10.1002/smll.201805510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, Wang Z. Influence of mesenchymal stem cells with endothelial progenitor cells in co-culture on osteogenesis and angiogenesis: An in vitro study. Arch Med Res. 2013;44(7):504–513. doi: 10.1016/j.arcmed.2013.09.009. https://doi.org/10.1016/j.arcmed.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99(15):9656–9661. doi: 10.1073/pnas.152324099. https://doi.org/10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laschke MW, Harder Y, Amon M, et al. Angiogenesis in tissue engineering: Breathing life into constructed tissue substitutes. Tissue Eng. 2006;12(8):2093–2104. doi: 10.1089/ten.2006.12.2093. https://doi.org/10.1089/ten.2006.12.2093. [DOI] [PubMed] [Google Scholar]
- 19.Blais M, Parenteau-Bareil R, Cadau S, et al. Concise review: Tissue-engineered skin and nerve regeneration in burn treatment. Stem Cells Transl Med. 2013;2(7):545–551. doi: 10.5966/sctm.2012-0181. http://doi.org/10.5966/sctm.2012-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koons GL, Diba M, Mikos AG. Materials design for bone-tissue engineering. Nat Rev Mater. 2020;5(8):584–603. https://doi.org/10.1038/s41578-020-0204-2. [Google Scholar]
- 21.Ashrafi M, Baguneid M, Bayat A. 2016. The role of neuromediators and innervation in cutaneous wound healing. Acta Dermato-Venereol 96 5 587-+ https://doi.org/10.2340/00015555-2321 [DOI] [PubMed] [Google Scholar]
- 22.Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445(7130):858–865. doi: 10.1038/nature05662. https://doi.org/10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 23.Cheret J, Lebonvallet N, Buhe V, et al. Influence of sensory neuropeptides on human cutaneous wound healing process. J Dermatol Sci. 2014;74(3):193–203. doi: 10.1016/j.jdermsci.2014.02.001. http://doi.org/10.1016/j.jdermsci.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Theocharidis G, Veves A. Autonomic nerve dysfunction and impaired diabetic wound healing: The role of neuropeptides. Autonomic Neurosci Basic Clin. 2020;223:102610. doi: 10.1016/j.autneu.2019.102610. https://doi.org/10.1016/j.autneu.2019.102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blais M, Mottier L, Germain M-A, et al. Sensory neurons accelerate skin reepithelialization via substance P in an innervated tissue-engineered wound healing model. Tissue Eng Part A. 2014;20(15-16):2180–2188. doi: 10.1089/ten.tea.2013.0535. https://doi.org/10.1089/ten.tea.2013.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sayilekshmy M, Hansen RB, Delaisse J-M, et al. Innervation is higher above bone remodeling surfaces and in cortical pores in human bone: Lessons from patients with primary hyperparathyroidism. Sci Rep. 2019;9:5361. doi: 10.1038/s41598-019-41779-w. https://doi.org/10.1038/s41598-019-41779-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hohmann EL, Elde RP, Rysavy JA, et al. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232(4752):868–871. doi: 10.1126/science.3518059. https://doi.org/10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- 28.Wan Q-Q, Qin W-P, Ma Y-X, et al. Crosstalk between bone and nerves within bone. Adv Sci. 2021;8(7):2003390. doi: 10.1002/advs.202003390. https://doi.org/10.1002/advs.202003390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X-D, Li S-Y, Zhang S-J, et al. The neural system regulates bone homeostasis via mesenchymal stem cells: a translational approach. Theranostics. 2020;10(11):4839–4850. doi: 10.7150/thno.43771. https://doi.org/10.7150/thno.43771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He H, Chai J, Zhang S, et al. CGRP may regulate bone metabolism through stimulating osteoblast differentiation and inhibiting osteoclast formation. Mol Med Rep. 2016;13(5):3977–3984. doi: 10.3892/mmr.2016.5023. https://doi.org/10.3892/mmr.2016.5023. [DOI] [PubMed] [Google Scholar]
- 31.Graessel S. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res Ther. 2014;16(6):485. doi: 10.1186/s13075-014-0485-1. https://doi.org/10.1186/s13075-014-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore RE, Smith CK, Bailey CS, et al. Characterization of beta-adrenergic receptors on rat and human osteoblastlike cells and demonstration that beta-receptor agonists can stimulate bone resorption in organ culture. Bone Miner. 1993;23(3):301–315. doi: 10.1016/s0169-6009(08)80105-5. https://doi.org/10.1016/S0169-6009(08)80105-5. [DOI] [PubMed] [Google Scholar]
- 33.Katayama Y, Battista M, Kao W-M, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. https://doi.org/10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 34.Elefteriou F. Impact of the autonomic nervous system on the skeleton. Physiol Rev. 2018;98(3):1083–1112. doi: 10.1152/physrev.00014.2017. https://doi.org/10.1152/physrev.00014.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun S, Diggins NH, Gunderson ZJ, et al. No pain no gain? The effects of pain-promoting neuropeptides and neurotrophins on fracture healing. Bone. 2020;131:115109. doi: 10.1016/j.bone.2019.115109. https://doi.org/10.1016/j.bone.2019.115109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyers CA, Lee S, Sono T, et al. A neurotrophic mechanism directs sensory nerve transit in cranial bone. Cell Rep. 2020;31(8):107696. doi: 10.1016/j.celrep.2020.107696. https://doi.org/10.1016/j.celrep.2020.107696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Q, Lei L, Yu T, et al. Effect of brain-derived neurotrophic factor on the neurogenesis and osteogenesis in bone engineering. Tissue Eng Part A. 2018;24(15-16):1283–1292. doi: 10.1089/ten.TEA.2017.0462. https://doi.org/10.1089/ten.tea.2017.0462. [DOI] [PubMed] [Google Scholar]
- 38.Ai L-S, Sun C-Y, Zhang L, et al. Inhibition of BDNF in multiple myeloma blocks osteoclastogenesis via down-regulated stroma-derived RANKL expression both in vitro and in vivo. PLoS One. 2012;7(10):e46287. doi: 10.1371/journal.pone.0046287. https://doi.org/10.1371/journal.pone.0046287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai X-x, Luo E, Yuan Q. 2010. Interaction between schwann cells and osteoblasts in vitro. Int J Oral Sci 2 2 74 81. https://doi.org/10.4248/ijos10039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones RE, Salhotra A, Robertson KS, et al. 2019. Skeletal stem cell-schwann cell circuitry in mandibular repair. Cell Rep 28 11 2757 2766.e5. https://doi.org/10.1016/j.celrep.2019.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samandari M, Quint J, Rodriguez-delaRosa A, et al. Bioinks and bioprinting strategies for skeletal muscle tissue engineering. Adv Mater. 2022;34(12):21105883. doi: 10.1002/adma.202105883. https://doi.org/10.1002/adma.202105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilbert-Honick J, Iyer SR, Somers SM, et al. Engineering 3D skeletal muscle primed for neuromuscular regeneration following volumetric muscle loss. Biomaterials. 2020;255:120154. doi: 10.1016/j.biomaterials.2020.120154. https://doi.org/10.1016/j.biomaterials.2020.120154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raffa P, Easler M, Urciuolo A. 2022. Three-dimensional in vitro models of neuromuscular tissue. Neural Regen Res 17 4 759 766. https://doi.org/10.4103/1673-5374.322447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carmeliet P. 2003. Blood vessels and nerves: Common signals, pathways and diseases. Nat Rev Genet 4 9 710 720. https://doi.org/10.1038/nrg1158 [DOI] [PubMed] [Google Scholar]
- 45.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436(7048):193–200. doi: 10.1038/nature03875. https://doi.org/10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 46.Morotti M, Vincent K, Brawn J, et al. 2014. Peripheral changes in endometriosis-associated pain. Hum Reprod Update 20 5 717 736. https://doi.org/10.1093/humupd/dmu021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raab S, Plate KH. 2007. Different networks, common growth factors: Shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol 113 6 607 626. https://doi.org/10.1007/s00401-007-0228-3 [DOI] [PubMed] [Google Scholar]
- 48.Troullinaki M, Alexaki V-I, Mitroulis I, et al. 2019. Nerve growth factor regulates endothelial cell survival and pathological retinal angiogenesis. J Cell Mol Med 23 4 2362 2371. https://doi.org/10.1111/jcmm.14002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emanueli C, Salis MB, Pinna A, et al. 2002. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 106 17 2257 2262. https://doi.org/10.1161/01.CIR.0000033971.56802.C5 [DOI] [PubMed] [Google Scholar]
- 50.Hecking I, Stegemann LN, Theis V, et al. Neuroprotective effects of VEGF in the enteric nervous system. Int J Mol Sci. 2022;23(12):6756. doi: 10.3390/ijms23126756. https://doi.org/10.3390/ijms23126756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang Y, Zhang L, Ji Y, et al. 2023. A non-invasive smart scaffold for bone repair and monitoring. Bioact Mater 19 499 510. https://doi.org/10.1016/j.bioactmat.2022.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy SV, De Coppi P, Atala A. 2020. Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng 4 4 370 380. https://doi.org/10.1038/s41551-019-0471-7 [DOI] [PubMed] [Google Scholar]
- 53.Cheng L, Cai Z, Ye T, et al. Injectable polypeptideprotein hydrogels for promoting infected wound healing. Adv Funct Mater. 2020;30(25):2001196. https://doi.org/10.1002/adfm.202001196. [Google Scholar]
- 54.Yang H, Lai C, Xuan C, et al. Integrin-binding prosurvival peptide engineered silk fibroin nanosheets for diabetic wound healing and skin regeneration. Chem Eng J. 2020;398:125617. https://doi.org/10.1016/j.cej.2020.125617. [Google Scholar]
- 55.Yao S, Wang Y, Chi J, et al. Porous MOF microneedle array patch with photothermal responsive nitric oxide delivery for wound healing. Adv Sci. 2022;9(3):2103449. doi: 10.1002/advs.202103449. https://doi.org/10.1002/advs.202103449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen H, Guo Y, Zhang Z, et al. 2022. Symbiotic algae- bacteria dressing for producing hydrogen to accelerate diabetic wound healing. Nano Lett 22 1 229 237. https://doi.org/10.1021/acs.nanolett.1c03693 [DOI] [PubMed] [Google Scholar]
- 57.Yao S, Chi J, Wang Y, et al. Zn-MOF encapsulated antibacterial and degradable microneedles array for promoting wound healing. Adv Healthc Mater. 2021;10(12):2100056. doi: 10.1002/adhm.202100056. https://doi.org/10.1002/adhm.202100056. [DOI] [PubMed] [Google Scholar]
- 58.Yin M, Wu J, Deng M, et al. 2021. Multifunctional magnesium organic framework-based microneedle patch for accelerating diabetic wound healing. Acs Nano 15 11 17842 17853. https://doi.org/10.1021/acsnano.1c06036 [DOI] [PubMed] [Google Scholar]
- 59.Deng Z, Li M, Hu Y, et al. Injectable biomimetic hydrogels encapsulating Gold/metal-organic frameworks nanocomposites for enhanced antibacterial and wound healing activity under visible light actuation. Chem Eng J. 2021;420:129668. https://doi.org/10.1016/j.cej.2021.129668. [Google Scholar]
- 60.Xiao J, Zhu Y, Huddleston S, et al. 2018. Copper metal-organic framework nanoparticles stabilized with folic acid improve wound healing in diabetes. ACS Nano 12 2 1023 1032. https://doi.org/10.1021/acsnano.7b01850 [DOI] [PubMed] [Google Scholar]
- 61.Zhao X, Wu H, Guo B, et al. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials. 2017;122:34–47. doi: 10.1016/j.biomaterials.2017.01.011. https://doi.org/10.1016/j.biomaterials.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 62.Ibanez RIR, do Amaral RJFC, Simpson CR, et al. 3D printed scaffolds incorporated with platelet-rich plasma show enhanced angiogenic potential while not inducing fibrosis. Adv Funct Mater. 2022;32(10):2109915. https://doi.org/10.1002/adfm.202109915. [Google Scholar]
- 63.Wang X, Yu Y, Yang C, et al. Dynamically responsive scaffolds from microfluidic 3D printing for skin flap regeneration. Adv Sci. 2022;9(22):2201155. doi: 10.1002/advs.202201155. https://doi.org/10.1002/advs.202201155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alizadehgiashi M, Nemr CR, Chekini M, et al. Multifunctional 3D-printed wound dressings. ACS Nano. 2021;15(7):12375–12387. doi: 10.1021/acsnano.1c04499. https://doi.org/10.1021/acsnano.1c04499. [DOI] [PubMed] [Google Scholar]
- 65.Singh S, Choudhury D, Yu F, et al. 2020. In situ bioprinting— Bioprinting from benchside to bedside? Acta Biomater 101 14-25 https://doi.org/10.1016/j.actbio.2019.08.045 [DOI] [PubMed] [Google Scholar]
- 66.Kong L, Wu Z, Zhao H, et al. Bioactive injectable hydrogels containing desferrioxamine and bioglass for diabetic wound healing. ACS Appl Mater Interface. 2018;10(36):30103–30114. doi: 10.1021/acsami.8b09191. https://doi.org/10.1021/acsami.8b09191. [DOI] [PubMed] [Google Scholar]
- 67.Albanna M, Binder KW, Murphy SV, et al. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci Rep. 2019;9:1856. doi: 10.1038/s41598-018-38366-w. https://doi.org/10.1038/s41598-018-38366-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hakimi N, Cheng R, Leng L, et al. Handheld skin printer: In situ formation of planar biomaterials and tissues. Lab Chip. 2018;18(10):1440–1451. doi: 10.1039/c7lc01236e. https://doi.org/10.1039/c7lc01236e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nuutila K, Samandari M, Endo Y, et al. In vivo printing of growth factor-eluting adhesive scaffolds improves wound healing. Bioact Mater. 2022;8:296–308. doi: 10.1016/j.bioactmat.2021.06.030. https://doi.org/10.1016/j.bioactmat.2021.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Phan J, Kumar P, Hao D, et al. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell- free therapy. J Extracell Vesicles. 2018;7(1):1522236. doi: 10.1080/20013078.2018.1522236. https://doi.org/10.1080/20013078.2018.1522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu Y, Tao R, Chen L, et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnol. 2021;19(1):150. doi: 10.1186/s12951-021-00894-5. https://doi.org/10.1186/s12951-021-00894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu Y, Wu B, Xiong Y, et al. Cryogenic 3D printed hydrogel scaffolds loading exosomes accelerate diabetic wound healing. Chem Eng J. 2021;426:130634. https://doi.org/10.1016/j cej.2021.130634. [Google Scholar]
- 73.Thapa RK, Diep DB, Tonnesen HH. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020;103:52–67. doi: 10.1016/j.actbio.2019.12.025. https://doi.org/10.1016/j.actbio.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 74.Chu B, He J-m, Wang Z, et al. Proangiogenic peptide nanofiber hydrogel/3D printed scaffold for dermal regeneration. Chem Eng J. 2021;424:128146. https://doi.org/10.1016/j.cej.2020.128146. [Google Scholar]
- 75.Yu Q, Han Y, Tian T, et al. Chinese sesame stick- inspired nano-fibrous scaffolds for tumor therapy and skin tissue reconstruction. Biomaterials. 2019;194:25–35. doi: 10.1016/j.biomaterials.2018.12.012. https://doi.org/10.1016/j.biomaterials.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 76.Yu Q, Han Y, Wang X, et al. Copper silicate hollow microspheres-incorporated scaffolds for chemophotothermal therapy of melanoma and tissue healing. ACS Nano. 2018;12(3):2695–2707. doi: 10.1021/acsnano.7b08928. https://doi.org/10.1021/acsnano.7b08928. [DOI] [PubMed] [Google Scholar]
- 77.Wang X, Xue J, Ma B, et al. Black bioceramics: Combining regeneration with therapy. Adv Mater. 2020;32(48):2005140. doi: 10.1002/adma.202005140. https://doi.org/10.1002/adma.202005140. [DOI] [PubMed] [Google Scholar]
- 78.Xu C, Xu Y, Yang M, et al. Black-phosphorus- incorporated hydrogel as a conductive and biodegradable platform for enhancement of the neural differentiation of mesenchymal stem cells. Adv Funct Mater. 2020;30(39):2000177. https://doi.org/10.1002/adfm.202000177. [Google Scholar]
- 79.Saghiri MA, Asatourian A, Orangi J, et al. Functional role of inorganic trace elements in angiogenesis-Part I: N Fe Se, P Au and Ca. Crit Rev Oncol Hematol. 2015;96(1):129–142. doi: 10.1016/j.critrevonc.2015.05.010. https://doi.org/10.1016/j.critrevonc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 80.Ma W, Ma H, Qiu P, et al. Sprayable beta-FeSi2 composite hydrogel for portable skin tumor treatment and wound healing. Biomaterials. 2021;279:121225. doi: 10.1016/j.biomaterials.2021.121225. https://doi.org/10.1016/j.biomaterials.2021.121225. [DOI] [PubMed] [Google Scholar]
- 81.Sheng L, Zhang Z, Zhang Y, et al. A novel “hot spring”- mimetic hydrogel with excellent angiogenic properties for chronic wound healing. Biomaterials. 2021;264:120414. doi: 10.1016/j.biomaterials.2020.120414. https://doi.org/10.1016/j.biomaterials.2020.120414. [DOI] [PubMed] [Google Scholar]
- 82.Ma J, Wu J, Zhang H, et al. 3D printing of diatomite incorporated composite scaffolds for skin repair of deep burn wounds. Int J Bioprint. 2022;8(3):163–175. doi: 10.18063/ijb.v8i3.580. https://doi.org/10.18063/ijb.v8i3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy SV, Atala A. 2014. 3D bioprinting of tissues and organs. Nat Biotech 32 8 773 785. https://doi.org/10.1038/nbt.2958 [DOI] [PubMed] [Google Scholar]
- 84.Zhou F, Hong Y, Liang R, et al. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials. 2020;258:120287. doi: 10.1016/j.biomaterials.2020.120287. 10.1016/j.biomaterials.2020.120287. [DOI] [PubMed] [Google Scholar]
- 85.Ma J, Qin C, Wu J, et al. 3D printing of strontium silicate microcylinder-containing multicellular biomaterial inks for vascularized skin regeneration. Adv Healthc Mater. 2021;10(16):2100523. doi: 10.1002/adhm.202100523. https://doi.org/10.1002/adhm.202100523. [DOI] [PubMed] [Google Scholar]
- 86.Wu J, Qin C, Ma J, et al. An immunomodulatory bioink with hollow manganese silicate nanospheres for angiogenesis. Appl Mater Today. 2021;23:101015. https://doi.org/10.1016/j.apmt.2021.101015. [Google Scholar]
- 87.Fan L, Xiao C, Guan P, et al. Extracellular matrixbased conductive interpenetrating network hydrogels with enhanced neurovascular regeneration properties for diabetic wounds repair. Adv Healthc Mater. 2022;11(1):2101556. doi: 10.1002/adhm.202101556. https://doi.org/10.1002/adhm.202101556. [DOI] [PubMed] [Google Scholar]
- 88.Wang J, Lin J, Chen L, et al. Endogenous electricfield-coupled electrospun short fiber via collecting wound exudation. Adv Mater. 2022;34(9):2108325. doi: 10.1002/adma.202108325. https://doi.org/10.1002/adma.202108325. [DOI] [PubMed] [Google Scholar]
- 89.Sebastian A, Volk SW, Halai P, et al. 2017. Enhanced neurogenic biomarker expression and reinnervation in human acute skin wounds treated by electrical stimulation. J Investig Dermatol 137 3 737 747. https://doi.org/10.1016/j.jid.2016.09.038 [DOI] [PubMed] [Google Scholar]
- 90.Emmerson E. 2017. Efficient healing takes some nerve: Electrical stimulation enhances innervation in cutaneous human wounds. J Investig Dermatol 137 3 543 545. https://doi.org/10.1016/j.jid.2016.10.018 [DOI] [PubMed] [Google Scholar]
- 91.Tan M-h, Xu X-h, Yuan T-j, et al. Self-powered smart patch promotes skin nerve regeneration and sensation restoration by delivering biological-electrical signals in program. Biomaterials. 2022;283:121413. doi: 10.1016/j.biomaterials.2022.121413. https://doi.org/10.1016/j.biomaterials.2022.121413. [DOI] [PubMed] [Google Scholar]
- 92.Peng L-H, Xu X-H, Huang Y-F, et al. Self-adaptive all-in-one delivery chip for rapid skin nerves regeneration by endogenous mesenchymal stem cells. Adv Funct Mater. 2020;30(40):2001751. https://doi.org/10.1002/adfm.202001751. [Google Scholar]
- 93.Qian Z, Wang H, Bai Y, et al. 2020. Improving chronic diabetic wound healing through an injectable and selfhealing hydrogel with platelet-rich plasma release. ACS Appl Mater Interface 12 50 : 55659 55674 https://doi.org/10.1021/acsami.0c17142 [DOI] [PubMed] [Google Scholar]
- 94.Xu X-H, Yuan T-J, Dad HA, et al. 2021. Plant exosomes as novel nanoplatforms for microRNA transfer stimulate neural differentiation of stem cells in vitro and in vivo. Nano Lett 21 19 8151 8159. https://doi.org/10.1021/acs.nanolett.1c02530 [DOI] [PubMed] [Google Scholar]
- 95.Brokesh AM, Gaharwar AK. 2020. Inorganic biomaterials for regenerative medicine. ACS Appl Mater Interface 12 5 5319 5344. https://doi.org/10.1021/acsami.9b17801 [DOI] [PubMed] [Google Scholar]
- 96.Sun L, Wang M, Chen S, et al. 2019. Molecularly engineered metal-based bioactive soft materials—Neuroactive magnesium ion/polymer hybrids. Acta Biomater 85: 310–319. https://doi.org/10.1016/j.actbio.2018.12.040 [DOI] [PubMed] [Google Scholar]
- 97.Zhang H, Ma W, Ma H, et al. Spindle-like zinc silicate nanoparticles accelerating innervated and vascularized skin burn wound healing. Adv Healthc Mater, 2022;11(10):2102359. doi: 10.1002/adhm.202102359. https://doi.org/10.1002/adhm.202102359. [DOI] [PubMed] [Google Scholar]
- 98.Li T, Zhai D, Ma B, et al. 3D printing of hot dog-like biomaterials with hierarchical architecture and distinct bioactivity. Adv Sci. 2019;6(19):1901146. doi: 10.1002/advs.201901146. https://doi.org/10.1002/advs.201901146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y, Xu J, Mi J, et al. Biodegradable magnesium combined with distraction osteogenesis synergistically stimulates bone tissue regeneration via CGRP-FAK-VEGF signaling axis. Biomaterials. 2021;275:120984. doi: 10.1016/j.biomaterials.2021.120984. https://doi.org/10.1016/j.biomaterials.2021.120984. [DOI] [PubMed] [Google Scholar]
- 100.Li T, Chang J, Zhu Y, et al. 3D printing of bioinspired biomaterials for tissue regeneration. Adv Healthc Mater. 2020;9(23):2000208. doi: 10.1002/adhm.202000208. https://doi.org/10.1002/adhm.202000208. [DOI] [PubMed] [Google Scholar]
- 101.Zhang W, Feng C, Yang G, et al. 2017. 3D-printed scaffolds with synergistic effect of hollow-pipe structure and bioactive ions for vascularized bone regeneration. Biomaterials 135 85 95. https://doi.org/10.1016/j.biomaterials.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 102.Chung JJ, Yoo J, Sum BST, et al. 3D printed porous methacrylate/silica hybrid scaffold for bone substitution. Adv Healthc Mater. 2021;10(12):2100117. doi: 10.1002/adhm.202100117. https://doi.org/10.1002/adhm.202100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang C, Lai J, Li K, et al. 2021. Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization. Bioact Mater 6 1 137 145. https://doi.org/10.1016/j.bioactmat.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Byambaa B, Annabi N, Yue K, et al. Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv Healthc Mater. 2017;6(16):1700015. doi: 10.1002/adhm.201700015. https://doi.org/10.1002/adhm.201700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Piard C, Baker H, Kamalitdinov T, et al. Bioprinted osteon-like scaffolds enhance in vivo neovascularization. Biofabrication. 2019;11(2):025013. doi: 10.1088/1758-5090/ab078a. https://doi.org/10.1088/1758-5090/ab078a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun X, Ma Z, Zhao X, et al. 2021. Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus. Bioact Mater 6 3 757 769. https://doi.org/10.1016/j.bioactmat.2020.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wan Z, Zhang P, Liu Y, et al. 2020. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater 101 26 42. https://doi.org/10.1016/j.actbio.2019.10.038 [DOI] [PubMed] [Google Scholar]
- 108.Feng C, Zhang W, Deng C, et al. 3D printing of lotus root-like biomimetic materials for cell delivery and tissue regeneration. Adv Sci. 2017;4(12):1700401. doi: 10.1002/advs.201700401. https://doi.org/10.1002/advs.201700401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hann SY, Cui H, Esworthy T, et al. 2021. Dual 3D printing for vascularized bone tissue regeneration. Acta Biomater 123 263 274. https://doi.org/10.1016/j.actbio.2021.01.012 [DOI] [PubMed] [Google Scholar]
- 110.Wang X, Yu Y, Yang C, et al. Microfluidic 3D printing responsive scaffolds with biomimetic enrichment channels for bone regeneration. Adv Funct Mater. 2021;31(40):2105190. https://doi.org/10.1002/adfm.202105190. [Google Scholar]
- 111.Zhang M, Lin R, Wang X, et al. 3D printing of Haversian bone-mimicking scaffolds for multicellular delivery in bone regeneration. Sci Adv. 2020;6(12):eaaz6725. doi: 10.1126/sciadv.aaz6725. https://doi.org/10.1126/sciadv.aaz6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang L, Hu P, Jiang H, et al. Mild hyperthermia- mediated osteogenesis and angiogenesis play a critical role in magnetothermal composite-induced bone regeneration. Nano Today. 2022;43:101401. https://doi.org/10.1016/j.nantod.2022.101401. [Google Scholar]
- 113.Yu X, Wang X, Li D, et al. Mechanically reinforced injectable bioactive nanocomposite hydrogels for in-situ bone regeneration. Chem Eng J. 2022;433:132799. https://doi.org/10.1016/j.cej.2021.132799. [Google Scholar]
- 114.Zhu D, Lu B, Yang Q, et al. Lanthanum-doped mesoporous bioglasses/chitosan composite scaffolds enhance synchronous osteogenesis and angiogenesis for augmented osseous regeneration. Chem Eng J. 2021;405:127077. https://doi.org/10.1016/j.cej.2020.127077. [Google Scholar]
- 115.Yin J, Pan S, Guo X, et al. Nb2C MXene-functionalized scaffolds enables osteosarcoma phototherapy and angiogenesis/osteogenesis of bone defects. Nano-Micro Lett. 2021;13(1):30. doi: 10.1007/s40820-020-00547-6. https://doi.org/10.1007/s40820-020-00547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Han X, Sun M, Chen B, et al. 2021. Lotus seedpod-inspired internal vascularized 3D printed scaffold for bone tissue repair. Bioact Mater 6 6 1639 1652. https://doi.org/10.1016/j.bioactmat.2020.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gu J, Zhang Q, Geng M, et al. 2021. Construction of nanofibrous scaffolds with interconnected perfusable microchannel networks for engineering of vascularized bone tissue. Bioact Mater 6 10 3254 3268. https://doi.org/10.1016/j.bioactmat.2021.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ha Y, Ma X, Li S, et al. Bone microenvironmentmimetic scaffolds with hierarchical microstructure for enhanced vascularization and bone regeneration. Adv Funct Mater. 2022;32(20):2200011. https://doi.org/10.1002/adfm.202200011. [Google Scholar]
- 119.Yang C, Zheng Z, Younis MR, et al. 3D printed enzyme-functionalized scaffold facilitates diabetic bone regeneration. Adv Funct Mater. 2021;31(20):2101372. https://doi.org/10.1002/adfm.202101372. [Google Scholar]
- 120.Li W, Miao W, Liu Y, et al. Bioprinted constructs that mimic the ossification center microenvironment for targeted innervation in bone regeneration. Adv Funct Mater. 2022;32(9):2109871. https://doi.org/10.1002/adfm.202109871. [Google Scholar]
- 121.Fitzpatrick V, Martin-Moldes Z, Deck A, et al. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials. 2021;276:120995. doi: 10.1016/j.biomaterials.2021.120995. https://doi.org/10.1016/j.biomaterials.2021.120995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y, Xu J, Ruan YC, et al. 2016. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med 22 10 1160 1169. https://doi.org/10.1038/nm.4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma Y-X, Jiao K, Wan Q-Q, et al. Silicified collagen scaffold induces semaphorin 3A secretion by sensory nerves to improve in-situ bone regeneration. Bioact Mater. 2022;9:475–490. doi: 10.1016/j.bioactmat.2021.07.016. https://doi.org/10.1016/j.bioactmat.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang M, Qin C, Wang Y, et al. 3D printing of tree-like scaffolds for innervated bone regeneration. Addit Manuf. 2022;54:102721. https://doi.org/10.1016/j.addma.2022.102721. [Google Scholar]
- 125.Zhang H, Qin C, Zhang M, et al. Calcium silicate nanowires-containing multicellular bioinks for 3D bioprinting of neural-bone constructs. Nano Today. 2022;46:101584. https://doi.org/10.1016/j.nantod.2022.101584. [Google Scholar]
- 126.Li T, Hou J, Wang L, et al. 2022. Bioprinted anisotropic scaffolds with fast stress relaxation bioink for engineering 3D skeletal muscle and repairing volumetric muscle loss. Acta Biomater 156 21 36’. https://doi.org/10.1016/j.actbio.2022.08.037 [DOI] [PubMed] [Google Scholar]
- 127.Hwangbo H, Lee H, Jin E-J, et al. 2022. Bio-printing of aligned GelMa-based cell-laden structure for muscle tissue regeneration. Bioact Mater 8 57 70. https://doi.org/10.1016/j.bioactmat.2021.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim W, Lee H, Lee C K, et al. A bioprinting process supplemented with in situ electrical stimulation directly induces significant myotube formation and myogenesis. Adv Funct Mater. 2021;31(51):2105170. https://doi.org/10.1002/adfm.202105170. [Google Scholar]
- 129.Panayi AC, Smit L, Hays N, et al. 2020. A porous collagen-GAG scaffold promotes muscle regeneration following volumetric muscle loss injury. Wound Repair Regen 28 1 61 74. https://doi.org/10.1111/wrr.12768 [DOI] [PubMed] [Google Scholar]
- 130.Zhang Z, Klausen LH, Chen M, et al. Electroactive scaffolds for neurogenesis and myogenesis: graphene-based nanomaterials. Small. 2018;14(48):1801983. doi: 10.1002/smll.201801983. https://doi.org/10.1002/smll.201801983. [DOI] [PubMed] [Google Scholar]
- 131.Quint JP, Mostafavi A, Endo Y, et al. In vivo printing of nanoenabled scaffolds for the treatment of skeletal muscle injuries. Adv Healthc Mater. 2021;10(10):2002152. doi: 10.1002/adhm.202002152. https://doi.org/10.1002/adhm.202002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Said SS, Yin H, Elfarnawany M, et al. Fortifying angiogenesis in ischemic muscle with FGF9-loaded electrospun poly(ester amide) fibers. Adv Healthc Mater. 2019;8(8):1801294. doi: 10.1002/adhm.201801294. https://doi.org/10.1002/adhm.201801294. [DOI] [PubMed] [Google Scholar]
- 133.Gholobova D, Terrie L, Gerard M, et al. Vascularization of tissue-engineered skeletal muscle constructs. Biomaterials. 2020;235:119708. doi: 10.1016/j.biomaterials.2019.119708. https://doi.org/10.1016/j.biomaterials.2019.119708. [DOI] [PubMed] [Google Scholar]
- 134.Choi Y-J, Jun Y-J, Kim D Y, et al. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials. 2019;206:160–169. doi: 10.1016/j.biomaterials.2019.03.036. https://doi.org/10.1016/j.biomaterials.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 135.Qazi TH, Mooney DJ, Pumberger M, et al. 2015. Biomaterials based strategies for skeletal muscle tissue engineering: Existing technologies and future trends. Biomaterials 53 502 521. https://doi.org/10.1016/j.biomaterials.2015.02.110 [DOI] [PubMed] [Google Scholar]
- 136.Lee H, Kim W, Lee J, et al. Self-aligned myofibers in 3D bioprinted extracellular matrix-based construct accelerate skeletal muscle function restoration. Appl Phys Rev. 2021;8(2):021405. doi: 10.1063/5.0039639. https://doi.org/10.1063/5.0039639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ostrovidov S, Salehi S, Costantini M, et al. 3D bioprinting in skeletal muscle tissue engineering. Small. 2019;15(24):1805530. doi: 10.1002/smll.201805530. https://doi.org/10.1002/smll.201805530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gao G, Cui X. 2016. Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotech Lett 38 2 203 211. https://doi.org/10.1007/s10529-015-1975-1 [DOI] [PubMed] [Google Scholar]
- 139.Kim JH, Kim I, Seol Y-J, et al. Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function. Nat Commun. 2020;11(1):1025. doi: 10.1038/s41467-020-14930-9. https://doi.org/10.1038/s41467-020-14930-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mi J, Xu J-K, Yao Z, et al. Implantable electrical stimulation at dorsal root ganglions accelerates osteoporotic fracture healing via calcitonin gene-related peptide. Adv Sci. 2022;9(1):2103005. doi: 10.1002/advs.202103005. https://doi.org/10.1002/advs.202103005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


