Abstract
The absolute shortage of compatible liver donors and the growing number of potential recipients have led scientists to explore alternative approaches to providing tissue/ organ substitutes from bioengineered sources. Bioartificial regeneration of a fully functional tissue/organ replacement is highly dependent on the right combination of engineering tools, biological principles, and materiobiology horizons. Over the past two decades, remarkable achievements have been made in hepatic tissue engineering by converging various advanced interdisciplinary research approaches. Three-dimensional (3D) bioprinting has arisen as a promising state-of-the-art tool with strong potential to fabricate volumetric liver tissue/organ equivalents using viscosity- and degradation-controlled printable bioinks composed of hydrous microenvironments, and formulations containing living cells and associated supplements. Source of origin, biophysiochemical, or thermomechanical properties and crosslinking reaction kinetics are prerequisites for ideal bioink formulation and realizing the bioprinting process. In this review, we delve into the forecast of the potential future utility of bioprinting technology and the promise of tissue/organ- specific decellularized biomaterials as bioink substrates. Afterward, we outline various methods of decellularization, and the most relevant studies applying decellularized bioinks toward the bioengineering of in vitro liver models. Finally, the challenges and future prospects of decellularized material-based bioprinting in the direction of clinical regenerative medicine are presented to motivate further developments.
Keywords: Biofabrication, Bioprinting, Decellularization, Bioink, Liver tissue engineering, Translational regenerative medicine
1. Introduction
340341Human body is the most complex and marvelously evolved structure on earth, composed of many different cell types, tissues, and organs, performing numerous specialized biological functions. It has a limited capability to properly repair or self-regenerate most, if not all, of its complex tissues and organs when natural biological, structural, or mechanical integrity is severely compromised. Medical treatment of cell/tissue/organ failure because of cellular damage, impairment of critical tissue function, or devastating deficits is a paramount public health concern. In the case of vital organs (e.g., liver), lack of adequate treatment or replacement of damaged organs without proper treatment for progressive chronic diseases means certain death for the patient[1,2]. Tissue/organ repair and transplantation are viable options for treating pathologies in patients with organ dysfunction, depending on the intensity and severity of the disease or associated complications. Technological advances in cell, tissue, and organ transplantation procedures have proven to help improve the overall health of patients and increase survival rates while reducing the risk of side effects[3]. However, scarcity of optimal donors, difficulty in human leukocyte antigens (HLA) matching, risk of graft rejection, induction of postoperative immune intolerance, and the toxicity of the lifelong use of pharmacological immunosuppression are some of the daunting issues confronting the field of innovative transplant procedures. Given the significant increase in critical organ dysfunction due to a variety of aberrant factors, the list of patients waiting for lifesaving organ transplants is growing significantly at an overwhelming rate worldwide. With the alarming increase in the incidence of end-stage liver failure and the ongoing disparity between organ supply/demand ratio, transplant clinicians, and researchers are working frantically to develop advanced alternative therapeutic approaches to engineer bioartificial tissue grafts or bioequivalents of organs[4-8].
Scientific advances and technological breakthroughs to recapitulate the biological cascade of native tissues have a broad spectrum of potential biomedical applications. Thus, the research domains of tissue engineering and regenerative medicine have boosted as an immediate response to the urgency of alternative means of developing biologically active three-dimensional (3D) tissue and organ surrogates to help save lives as well as to address many of the inadequacies associated with currently intractable diseases. The original concept of the tissue engineering research field was formally proposed in a historic milestone research paper reported in Science by Langer and Vacanti in 1993, which detailed for the first time the practical application and properties of biodegradable scaffolds as a 3D-culture substrate[9]. In classical tissue engineering strategies, living cells, biocompatible scaffolds, and bioassistants (growth factors and hormones) are generally considered interdependent essential “building blocks” for the successful manufacturing of tissue-engineered products. Recent advances in tissue engineering to repair and regenerate damaged tissue have attracted significant interest from transplant clinicians and interdisciplinary researchers. Owing to the outstanding advantages, tissue engineering and regenerative medicine means are arguably the only therapeutic alternatives that apply biological and engineering principles to generate tissue or organ substitutes with native-like structural and functional features[9,10]. In addition to clinical translations of bioengineered constructs, other uses include personalized drug screening, drug repositioning, deconvolution of biophysiological and pathological signals, high-content analysis, disease modeling, and morphogenesis studies[11-13].
Contemporary tissue engineering strategies mainly rely on biocompatible porous scaffolds or hydrogels incorporated with living cells and associated supplements[9]. However, it is difficult to generate finely tuned therapeutically relevant biological structures using traditional methods. Ultimately, generating multiscale functional tissue and organ replacements with sufficient maturity remains a major obstacle in translational regenerative medicine[10,17-20]. Therefore, there continues to be an emphasis on the development and refinement of bioengineering strategies that either use living cells exclusively or incorporate biocompatible biomaterials for the large-scale automated biomanufacturing of heterogeneous and fully functional replacements for critical organs such as the liver, kidney, heart, and lung. Consequently, 3D bioprinting has appeared as a rapidly growing tool that utilizes computer-aided manufacturing techniques to produce clinically valuable bioinic structures with desired biological, structural, and biomechanical complexities for repairing/replacing diseased/damaged tissues/organs[21-27].
In this review, an overview of bioprinting technologies, bioink requirements, and fundamentals of decellularization methods is explained. Thereafter, we outline the recent representative studies for the adoption of liver-specific decellularized materials in the formulation of bioinks for liver tissue bioprinting applications. Finally, the current challenges in bioprinting research as well as the future 342perspectives of the organ-specific bioink research domain are presented.
2. Overview of three-dimensional bioprinting research
3D printing relies on preprogrammed digital blueprints or predetermined model devised by computer-aided design data to generate scalable and reproducible 3D-physical constructs by sequentially depositing materials of interest in a classical layer-by-layer format. The possibility of using additive manufacturing in biomedical field kickstarted a race for the convergence of printing engineering, material chemistry, and cell biology/tissue engineering. Currently, bioprinting techniques based on extrusion, inkjet, laser, and stereolithography are being extensively explored for the precise construction of bioartificial soft- to-hard tissue-like structures for drug screening, disease modeling and eventual clinical applications[22,28-33]. The working principles of these techniques are different, and each approach has its own uniqueness, but they are not free from the shortcomings that affect the manufacturing process and bioprinted constructs. The production of viable biostructures emulating natural tissues/organs features highly relies on the appropriate choice of bioprinting method and adopted bioink materials. For examples, inkjet technology is considered suitable for bioprinting of small constructs to repair tissue defects. However, the application of inkjet methods remains limited due to shear and thermal stresses on cells. Furthermore, the need for low-viscosity bioink significantly affects the mechanical stiffness, rigidity, and stability of inkjet-printed structures. Extrusion bioprinting allows the printing of bioink materials of different viscosities, which opens up a wider choice of biomaterials for printing the equivalent of larger tissues and organ-like structures. However, this affects the process for finer resolution and the phenotype and behavior of encapsulated cells. Unlike inkjet and extrusion printing, laser-assisted bioprinting technology uses energy from a laser source to produce cellular and tissue patterns at relatively high resolution. The main advantage of laser-assisted printing is the better survival rate of cells after printing because no nozzles are required and the bioink material does not come in direct contact with dispensing or ejection components. However, there are several drawbacks, including cost, thermal damage to cells, and cytotoxicity from laser and photoinitiators. Stereolithography is another promising bioprinting method that can produce patterned structures with higher resolution and precision. Still, its application is limited because it requires only light-curable biomaterials with high physical and chemical properties. Stereolithography does not use a nozzle, but like laser-based methods, it exhibits some drawbacks related to exposure to harmful ultraviolet (UV) light.
Although the bioprinting field is still in its infancy, combination of materiobiology, computer-aided design, and printing techniques has enabled the successful bioprinting of various biomimicking constructs[34,45]. Compared to traditional tissue engineering methodologies, bioprinting techniques offer several advantageous properties that are not achievable with conventional approaches[28,37,39,44,46-50]. Functionality and maturity of bioprinted constructs are prerequisites. Indeed, these two issues are the main constraints in the transition of bioprinted tissue products from the laboratory to the clinical setting[51-62]. To understand the basic concepts and operating principles of printing technologies suitable for bioprinting applications, the readers can refer to more specialized reviews[63-69].
2.1. Overview of bioink and key requirements
Bioink refers to a printable cocktail of hydrogel embedded with cells and bioactive molecules that provide a 3D microenvironment to support cell growth, proliferation, migration, differentiation, and postprinting maturation[70-73]. Bioink not only constrains embedded cells and bioactive components to build complex structures but also provides a hydrous 3D-microenvironment conducive to the permeation of oxygen, nutrients, and other soluble metabolites. An ideal cell-laden bioink exhibits excellent liquid absorption, wetting and swelling properties for regulating cell infiltration, motility, adhesion, and remodeling[74]. Hence, finding a cytocompatible ECM surrogate with appropriate physiochemical and biological properties is the basic requirement for the preparation of an ideal bioink and cell encapsulation[75-78]. The reinforcement of novel materials in the bioink formulation process is not only crucial to modulate the rigidity or stiffness of the structures, and to protect the biological performance of cells during the printing process but also to ensure the functionality of the cells embedded within 3D-bioprinted constructs. Research is still ongoing to design novel bioinks using natural, synthetic, or semisynthetic materials (Figure 1). Critical milestones in bioinks design and their formulation are determined by the physiomechanical characteristics (viscosity, viscoelasticity, porosity, topology, architectural fidelity, tensile strength, rigidity, and stiffness), biochemical properties (composition, crosslinking, gelation kinetics, biodegradability, degradation rate, insolubility in cell culture medium, and immunological compatibility) of the target tissues/organs (Figure 2)[79]. The implementation of bioprinting technologies for the successful fabrication of viable 3D-printed structures and their clinical translations are directly linked to bioink cytocompatibility, stability, and sustainability. Therefore, important determinants of bioinks, such as biocompatibility, nonimmunogenic 343degradability, tuneability molecular weight, and material concentration must be optimized in the preprinting stages to ensure efficient printability and reproducibility of the bioprinted products.
Figure 1.
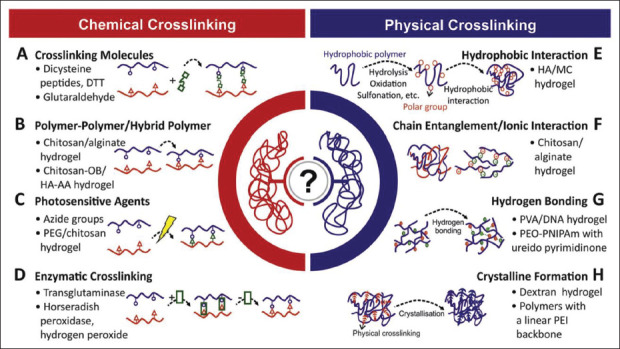
Crosslinking methods used in the synthesis of different kinds of hydrogels. Adapted from ref.[74], with copyright permission under the terms of the CC-BY-NC-ND 3.0 license.
Figure 2.
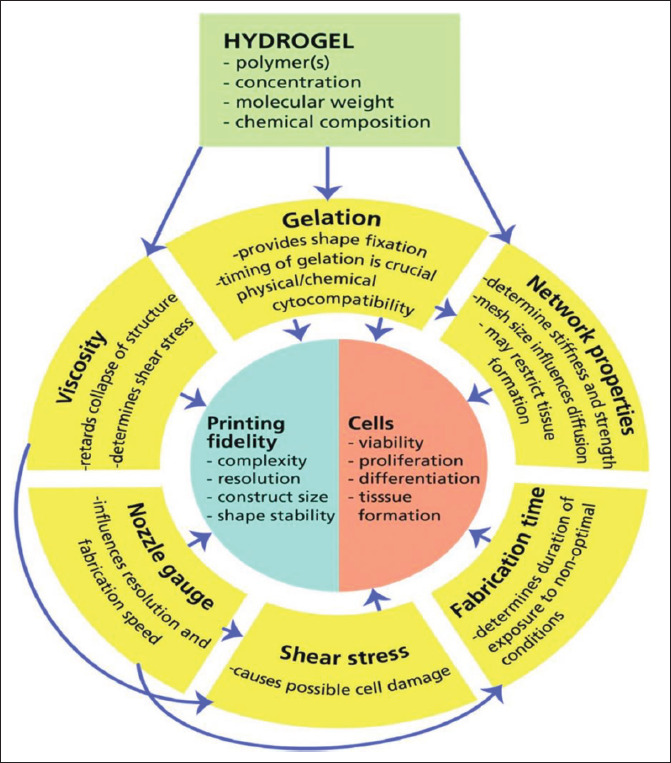
Important variables in hydrogel design and synthesis for biofabrication-bioprinting applications. Adapted from ref.[81], with copyright permission.
Several studies have demonstrated the design and synthesis of a wide variety of cytocompatible bioink materials that can be printed alone as scaffolds or as living cells embedded hydrogel for the 3D bioprinting of spatially defined tissue constructs[73,80-87]. These bioink materials are mostly prepared from natural, synthetic, and semisynthetic polymer systems featuring typical native-like extracellular matrix (ECM)-mimicking features. Although commonly used natural biopolymers (e.g., alginate, collagen, chitosan, gelatin, hyaluronic acid, and agarose) offer biophysical and biochemical resemblances with the native ECM; the printability, mechanical integrity, batch-to-batch variability, and cell-adhesive properties of natural biopolymers are not yet fully compatible with existing bioprinting modalities. Synthetic bioinks, on the other hand, are generally synthesized by chemically modifying polymeric materials. Synthetic biomaterials are often tailored with more supramolecular chemistries and mostly exhibit acceptable rheological, crosslinking, and gelling properties. Synthetic polymers such as polyethylene glycol (PEG), polyethylene oxide (PEO), polyvinylpyrrolidone (PVP), polylactic-glycolic acid (PLGA), polylactic acid (PLA), polycaprolactone (PCL), and gelatin methacryloyl (GelMA) are commonly used for 3D bioprinting applications[88,89].
Arguably, recent advances in the design of a variety of bio-interesting materials using different chemical, physical, and enzymatic crosslinking strategies have enabled the formulation of several bioinks[90-92]. Although bioinks based on natural and synthetic or semisynthetic materials are endowed with proregenerative biophysiochemical attributes that are, to some extent, adaptable to the requirements of biologically active tissue regeneration, they are still far from ideal bioinks for establishing complex tissue- engineered products for clinical applications (Figure 3). Poor cytocompatibility, anchorage-providing scaffolds, cellular recognition, immunomodulation, and tissuespecific degradation of synthetic materials are among the limiting factors hindering the wider application of synthetic polymers in the bioengineering of complex, and functional hierarchical structures. Thus, there remains a significant tradeoff between the printing methods, the printing process efficiency, and the rheological and physicochemical parameters of bioinks[93].
Figure 3.
Consideration of bioink formulation and translational path to the clinical setting. The text in the boxes indicates the desired features of bioinks from design to formulation to translational applications. Adapted from ref.[93], with copyright permission under the terms of the CC-BY-NC-ND 3.0 license.
3. Decellularized extracellular matrix as potential bioink material
344Decellularization refers to the process of complete elution of cells and some antigenic components, primarily from animal and human tissues and organs, while maintaining the inherent 3D microstructural, physicochemical, and biomechanical properties essential for preserving specialized cell functions and regulating tissue integrity in acellular matrix templates[94-96]. Decellularization of specific organs and tissues can be typically accomplished by chemical, physical, and enzymatic approaches (Figure 4). A combination of these methods can also be used to increase the effectiveness of the decellularization procedures, avoid detrimental effects associated with certain decellularization techniques, and balance the removal of cellular components with the preservation of extracellular components[97-106]. It is noteworthy that decellularized-acellular matrices retain predominantly fibrous and nonantigenic extracellular macromolecules, such as glycoproteins (collagen, elastin, fibronectin, and laminin), glycosaminoglycans, proteoglycans, and growth factors. In contrast, reconstitution of the original bioactive composition, natural organization, and hierarchal structure of the tissue-specific microenvironment is synthetically impossible (Figure 5).
Figure 4.
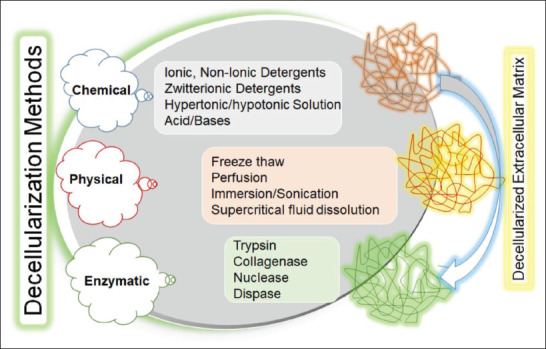
Concept of decellularization approaches based on chemical, physical, and enzymatic procedures.
Figure 5.
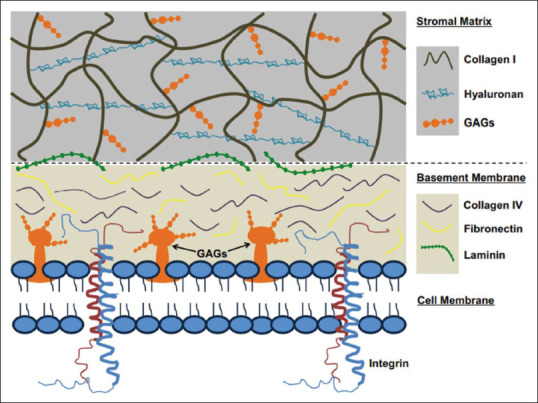
Endogenous extracellular matrix components-based biomaterials suitable for tissue engineering and regenerative medicine applications. Adapted from ref.[97], with copyright permission.
There is an increasing interest in the biofabrication/ bioprinting of tissue-specific bioequivalents in terms of anatomical, functional, and architectural features for studying the fundamental biological and pathophysiology processes, mechanotransductive responses, drug response profiling, cytotoxicity screening, and development of personalized tissue-based therapies. To 345achieve these goals, development and selection of organ/ tissue-specific decellularized matrix-derived bioink is considered one of the promising tools for bioprinting research[107-114]. Researchers are integrating the advantages of organ-specific decellularized extracellular matrices (dECM) with supramolecular surface functionalization and surface chemistry remodeling to function as selective bioink substrates for target tissues and organs. The prerequisites for the broad application of bioinks derived from decellularized materials are as follows: selection of the tissue/organ, decellularization and purification, biochemical, topographical and rheological characterizations, and postdecellularization modifications. Generally, decellularized matrices are conjugated with other biocompatible materials in order to enhance the rheological and viscoelastic properties of the bioinks, or utilization of the same for 3D bioprinting of stable, mature, sustainable, functional, and clinically relevant human-scale tissue and organ equivalents[82,109,110,112]. Applications of dECM-based bioinks for different tissues/organs are shown in Figure 6[90,115,116].
Figure 6.
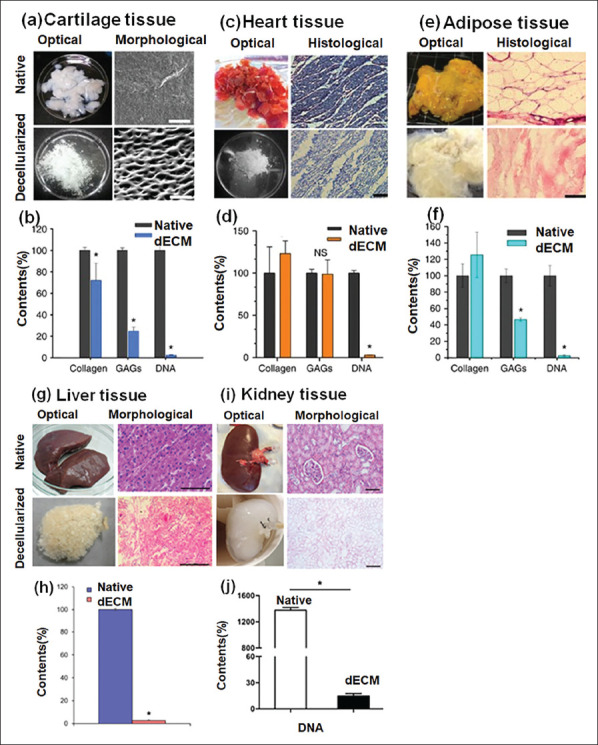
(a–j): Decellularization process and preparation of organ-specific bioink using different tissue sources. Native and decellularized extracellular matrix and its contents: (a-b) cartilage, (c-d) heart, and (e-f) adipose tissue (reproduced from ref.[90] under CC-BY-NC-ND 3.0 license, copyright 2014 Nature Publishing Group), (g-h) liver (reproduced with permission from ref.[115], copyright 2017 American Chemical Society), (i-j) kidney (reproduced with permission from ref.[116], copyright 2018 John Wiley & Sons, Inc.).
3.1. Liver-derived dECM bioinks for liver tissue engineering
As decellularized materials preserve tissue-specific biophysical and biochemical properties that are difficult to emulate with other synthetic and semisynthetic polymers or biopolymers isolated from natural sources, liver dECM is considered one of the most promising bioink materials that can provide an efficient supporting framework to specific hepatic cells and orchestrate reciprocal interactions between cells by providing tissue-specific dynamic microenvironment. Moreover, liver-specific decellularized matrices are composed of specialized biopolymers (e.g., collagen, elastin, fibrin, glycosaminoglycans), and retain many biochemical, biophysical, and biomechanical signaling molecules of tissue/organ of origin[117-123].
Several studies have begun to adopt dECM-based bioinks to replicate organ’s microarchitecture and346 physiological features in 3D bioprinted constructs. Skardal et al. conducted an interesting study to fabricate liver constructs by implementing dECM/HA/gelatin blended bioink utilizing an extrusion-based bioprinting approach[124]. To prepare bioinks, the authors used two- crosslinker and two-stage polymerization chemistry. In the first approach, multi-arm polyethylene glycol acrylate (PEG 4-Arm) was used as crosslinker and (4-(2-hydroxyethoxy) phenyl-(2-propyl) ketone as photoinitator. In the second strategy, multi-arm polyethylene glycol acrylate (PEG 8-Arm) alkyne was used as a crosslinker. The pre-bioink was formed through the UV light irradiation and thiolalkyne polymerization reaction. Liver spheroids comprised of primary human hepatocytes, primary human stellate cells, and primary human Kupffer cells were encapsulated in the blended solution. For functional assessment, bioprinted structures were maintained for 14 days. Using this model, the authors demonstrated that the printed spheroids maintained consistent viability rate, and recapitulated hepatic functions, such as albumin secretion and urea synthesis.
Similarly, Lee et al. developed 3D liver constructs using porcine liver-derived decellularized material and polycaprolactone-based hybrid bioink solution supplemented with human hepatocellular carcinoma cells and human bone marrow-derived mesenchymal stem cells[125]. The authors demonstrated that after incubating the printed constructs in a culture medium for seven days, the human hepatocellular carcinoma cells were able to produce liver-specific functions (albumin and urea secretion). Interestingly, human bone marrow-derived mesenchymal stem cells showed an enhanced differentiation process (Figure 7). Yu et al. prepared photo-crosslinkable bioink solutions using dECM derived from pig liver, gelatin, methacryloyl prepolymer, and lithium phenyl-2,4,6 trimethylbenzoylphosphinate[126]. They used a custom- built digital light processing (DLP)-based scanningless and continuous 3D bioprinting system for the biofabrication of liver structures. Biopatterned constructs based on hepatocytes derived from human induced pluripotent stem cells (hiPSCs) were cultured for seven days, and cell viability and expression levels of cell-specific genes were evaluated. Live/dead staining showed that biopatterned constructs were able to maintain viability throughout the experimental period. In addition, high-magnification bioimaging confirmed the formation of clusters and hexagonal patterns over seven days. Kim et al. prepared a composite bioink solution based on porcine liver-derived dECM, gelatin, and hyaluronic acid[127]. The authors used multidispensing bioprinting system equipped with a Nano master SMP-III for creating the micropatterns of primary mouse hepatocytes. Their findings demonstrated that introducing dECM microparticles into the composite bioink significantly improved 3D printability and mechanical integrity. Overall347 data showed that the micropatterned structures-maintained viability for one week and recapitulated hepatic functions. Lewis et al. aimed to control the creation and formation of the biliary tract using decellularized bioink derived from female Yorkshire pigs (Figure 7)[128]. The authors employed sacrificial poloxamer Pluronic F-127 as a support structure to control the geometric distribution and orientation of the in vitro biliary tree model. Computational image analysis showed that Pluronic F-127 enabled efficient biopatterning of hepatocytes/cholangiocytes and facilitated the alignment of stable tubular structures with controlled 2D geometry. The authors used dual printing parameters to extrude cell-laden dECM pre-gel solution into the F-127 structures for the formation of biliary structures. Lee et al. used porcine liver-derived decellularized bioink material to co-culture hepatocytes and biofabricate biliary system models using a cell printing/liver-on-chip model[129]. The authors incorporated decellularized material with poly (ethylene/vinyl acetate) for structure printing in a layer- by-layer format. To prepare the liver-on-a-chip model, microporous vascular and biliary fluidic channels with media reservoirs were printed to mimic the vascular and biliary systems. The model also demonstrated excellent hepatic function and drug responsiveness. The results demonstrated that the integration of biliary fluidic channel facilitated the generation of biliary system and recapitulated hepatic functions. The proposed model also showed excellent hepatic functions and drug responsiveness. Wang et al. used digital light processing (DLP) bioprinting setup to print photocurable methacrylated gelatin-based bioink containing porcine liver dECM and human-induced hepatocytes (hiHep cells) to fabricate microtissue structures via photo-crosslinking with lithium phenyl-2,4,6-trimet hylbenzoylphosphinate[130]. The proposed bioink showed improvements in printability, cell viability, and hepatic functions post-printing. Jeong et al. used porcine livers- derived decellularized materials to evaluate the influence of various detergent types on liver-decellularized matrixbased bioinks and bioprintability[131]. The proposed bioinks embedded with primary mouse hepatocyte-spheroids displayed excellent performance. The printed constructs formed clusters and maintained good cytocompatibility for 14 days.
Figure 7.
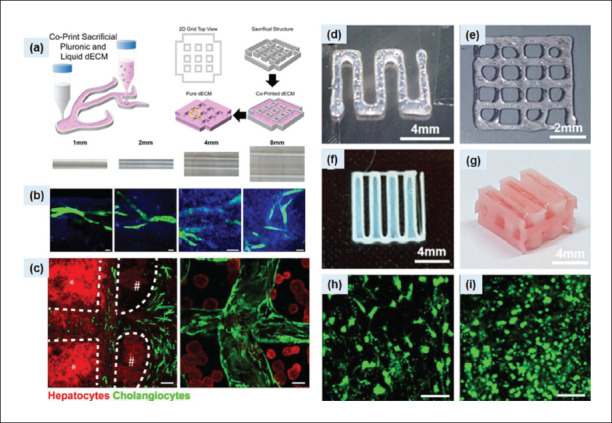
(a) Schematic of the dual printing process using pure dECM and dECM with Pluronic F-127. (b) Influences of strut with different widths on the alignment of ductal structure. Cholangiocyte duct structures (green) and collagen fibrils. (c) Hepatocyte/cholangiocyte co-culture and formation of biliary structure after 7 days. Images were reproduced from ref.[128], with copyright permission. (d and e) Characteristics of bioprinted HepG2 constructs based using decellularized materials based bioink. (f and g) 2D printing patterns and 3D hybrid structures. (h) Cell viability of BMMSCs and (i) HepG2 cells on day 7.
Recently, Khati et al. applied decellularization technology to produce temperature-sensitive multi-material bioink348349comprised of the porcine liver-derived acellular matrix, gelatin, polyethylene glycol, and tyrosine[132]. The authors used gelatin as a rheology enhancer, polyethylene glycol as a crosslinker, and mushroom tyrosinase for enzymatic crosslinking and mechanical integrity improvement of the bioprinted constructs. To ensure maximum cell viability and proliferation, they embedded HepG2 in the bioink solution and maintained the printed structures for 1 week. The results demonstrated that the printed structures were able to maintain HepG2 survival and liver-specific function for 1 week.
4. Future outlook and perspectives
3D bioprinting offers tremendous potential necessary to overcome the obstacles associated with conventional tissue engineering and regenerative medicine approaches. This transformative technology possesses several advantages essential to control the spatiotemporal orientation of cellladen bioprinted constructs for clinical translation. Over the past several years, bioprinting has been widely explored in the biofabrication of human spare parts, and some of the printed structures are already in the distinct clinical phases (e.g., hydroxyapatite bone, cartilage, ear, and nose)[133-135].
In the current scenario, considering bioprinting technology as an option to manufacture transplantable tissue/organs is far too optimistic. Multicellular biological structures (e.g., liver) are highly complex with built-in hierarchical organizations and zonations, and 3D-bioprinted equivalents must replicate key anatomical and morphological features. To date, bioprinting has been used to print a variety of tissue architectures (parts of the liver, heart, nephron, lung, muscle, cartilage, and kidney) or cancer models. However, despite some achievements, several key challenges still need to be addressed, especially in the biofabrication of extremely complex tissue/organs. It is worth noting that many bioprinted constructs are commonly used as in vitro models for basic developmental, morphogenetic, pharmacokinetic, and drug response 350studies. Efficient implementation of bioprinting technology for GMP-compliant biomanufacturing of clinical-grade tissue/organ substitutes suitable for transplantation will require a multitude of technological and bioink material-related research advancements. The fundamental limitations in achieving complex, implantable, clinical- grade bioprinted structures include poor resolution, dimensions, speed, accuracy, and precision. To produce biomimetic constructs with accurate geometric and compositional attributes, bioink must be printed at a reasonable resolution ideally comparable to the average size of human body cells (10–20 μm). Identification of functionally graded biomaterials in the formulation of bioink is another limiting factor in bioprinting research and mandatory regulatory approvals.
Despite many efforts in bioink formulation, the design and development of tissue/organ-specific bioinks (with minimum sol–gel transition and crosslinking duration without nozzle clogging) suitable for specific bioprinting of functionally graded bioconstructs are still limited. To fabricate industrial-scale bioprinted structures for implantation or repair/replacement of damaged/diseased portions, bioink precursors should be stable, reliable, printable, biocompatible, cytocompatible, biodegradable, bioactive, and commercially available. In addition to the essential features of bioprinting, bioink should be organ-specific with regeneration-promoting properties, providing an ideal platform for angiogenesis in culture, and avoiding immune rejection after surgical transplantation. Nonetheless, highly complex ultrastructural and biomechanical features of ECM vary from tissue to tissue or organ to organ, which makes it difficult to reconstitute using other natural, synthetic, and semisynthetic polymers. Thus, the reconstruction of structural delicacy and complexity of multicellular human organs, mimicry of biological mechanism of organ developmental stages, specialized vascular networks, and innervation patterns are some of the most critical challenges. Formulation of bioink recapitulating the complexity of native tissue/ organ-specific matrices is still an open challenge.
Fortunately, the emerging concept of using tissue/ organ decellularization technology to design and formulate acellular matrix-based cell-laden bioinks provides the potential toward the biofabrication of specific tissue/ organ bioequivalents. Briefly, decellularization method has evolved as an attractive technology for removing cellular components from source tissue/organ while preserving important constituents of ECM. Decellularized matrix is considered the most biomimetic, reliable, and instructive biomaterial compared to other natural, synthetic, or synthetic materials for the formulation of translational bioink substrates that can induce or control a vast number of cellular processes essential for cell growth, tissue repair, regeneration, and homeostasis through embedded physical, chemical, and biological cues. Typically, chemical, biological, physical, or combative methods are used for decellularization. Although experimental procedures for decellularizing nearly all tissues in the body have been well studied, there is still no consensus on the optimal protocol to use for each tissue/organ of various species. This is because each tissue has different characteristics in terms of source, donor age, size of tissue/organ, abundance of ECM contents, morphological appearance, anatomical location, cytoarchitecture, and cellular density. Therefore, when performing decellularization treatments, it is essential to recognize that one protocol may not yield effective results for all tissue types[136-149].
The common procedure for preparing bioink using liver decellularized bioink is to solubilize the extracted and purified ECM crystals, enzymatically (pepsin) digest them, and adjust the pH and ion concentration. Although decellularized liver materials have remarkable biophysicochemical properties, their low mechanical strength makes it difficult to maintain stability, stiffness, shape fidelity, and maturity of the biostructures during and after the printing phase. To overcome these problems, solubilized decellularized bioink materials can be further biofunctionalized with enhancers that are important to synchronously improve the mechanical, rheological, and biological properties of the original bioink. Overall, crosslinked dECM-derived bioinks can significantly improve structural stability, cell encapsulation ability, mechanical strength, material bonding, and printability comparable to nondeformable tissues/organs. Recently, the application of conjugated bioinks using decellularized matrices and gelatin derivatives has attracted much attention. For example, by adding methacrylic acid groups to gelatin derivatives, it is possible to synthesize dECM-GelMA composites that form hydrogels by photo-crosslinking via a UV crosslinking mechanism. Crosslinking modification with methacrylic acid has been demonstrated to significantly improve the mechanical integrity of bioinks based on decellularized materials. It is clear that the preparation of decellularized bioink materials and their biological, physical, and mechanical integrity is highly dependent on the method of decellularization, concentration of cell contents, gelation rate, physical, chemical, and enzymatic crosslinking mechanisms.
While 3D bioprinting is undoubtedly the future hope for automated manufacturing of more stable bioartificial tissues and organ substitutes within a predictable timeframe, this technology is still in its infancy. More advanced biomaterials engineering and crosslinking strategies to biofunctionalize decellularized matrices with351 functionally innovative biomaterials and drug delivery media must be explored to bridge the gap between experimental research and practical personalized regenerative medicine applications. Thus, further advances are urgently needed to solve the challenging problems associated with the multiscale manufacturing of clinical- grade bioengineered products for application in tissue repair and regeneration[150]. Furthermore, the current intrinsic shortcomings of bioprinting technologies, which are essential for reconstructing vascularized, hierarchical biological structures with organ-specific biochemical or biomechanical characteristics that resemble their native counterparts, also need to be addressed through interdisciplinary collaborative research efforts.
5. Conclusion
Using bioprinting technology and bioink materials to print and recapitulate tissue/organ function have a broad spectrum of biomedical applications. This automated approach has significantly boosted the tissue engineering and regenerative medicine research. Despite its remarkable advancement and encouraging results, 3D bioprinting technology still needs to be validated to generate fully functional and mechanically robust bioprinted liver substitutes suitable for transplantation. There are still areas for improvements in using the bioprinting approach for clinical applications. Further advancements, including advances in the printing resolution, and the development of functionally graded organ-specific ECM based-bioinks will enable the bioprinting field to satisfy myriads of pragmatic biomedical applications, such as the development of patient-specific tissue/organ equivalents with defined geometric arrangements and directed 3D bioassembly for personalized drug screening, liver reconstruction, and repair damaged tissue or whole organ.
Acknowledgments
None.
Funding
This work was primarily funded by KFSH&RC for Tissue and Organ Bioengineering Research Group (RAC# 2150012). Any opinions, findings, conclusions, or recommendations expressed herein are those of the author(s) and do not necessarily reflect the views of the KFSH&RC-Organ transplant Centre of excellence.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Conceptualization: Tanveer Ahmad Mir
Supervision: Tanveer Ahmad Mir
Visualization: Shadil Ibrahim Wani, Alaa Alzhrani, Kenichi Arai, Bilal Ahmed Mir, Shadab Kazmi
Writing – original draft: Tanveer Ahmad Mir, Makoto Nakamura, Shinji Sakai, Shintaroh Iwanaga
Writing – review & editing: Abdullah M. Assiri, Dieter C.
Broering
All authors have given approval for the final version of the draft for submission.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data
Not applicable.
References
- 1.Abouna GM. The humanitarian aspects of organ transplantation. Transpl Intl, 2001;14:117–123. doi: 10.1007/s001470050859. https://doi.org/10.1007/s001470050859. [DOI] [PubMed] [Google Scholar]
- 2.Godown J, McKane M, Wujcik K, et al. Expanding the donor pool: Regional variation in pediatric organ donation rates. Pediatr Transplant. 2016;20:1093–1097. doi: 10.1111/petr.12779. https://doi.org/10.1111/petr.12779. [DOI] [PubMed] [Google Scholar]
- 3.Cippà PE. New ideas for old problems: How scientific advances can change the future of organ transplantation. Transpl Intl, 2019;32:561–562. doi: 10.1111/tri.13419. https://doi.org/10.1111/tri.13419. [DOI] [PubMed] [Google Scholar]
- 4.Kitajima T, Kuno Y, Ivanics T, et al. Improved survival with higher-risk donor grafts in liver transplant with acute- on-chronic liver failure. Transplant Direct. 2022;8:e1283. doi: 10.1097/TXD.0000000000001283. https://doi.org/10.1097/TXD.0000000000001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amin A, Panayotova G, Guarrera JV. Hypothermic machine perfusion for liver graft preservation. Curr Opin Organ Transplant, 2022;27:98–105. doi: 10.1097/MOT.0000000000000973. https://doi.org/10.1097/MOT.0000000000000973. [DOI] [PubMed] [Google Scholar]
- 6.Kojima H, Yasuchika K, Fukumitsu K, et al. Establishment of practical recellularized liver graft for blood perfusion using primary rat hepatocytes and liver sinusoidal endothelial cells. Am J Transplant. 2018;18:1351–1359. doi: 10.1111/ajt.14666. https://doi.org/10.1111/ajt.14666Citation. [DOI] [PubMed] [Google Scholar]
- 7.352Sampaziotis F, Muraro D, Tysoe OC, et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science, 2021;371:839–846. doi: 10.1126/science.aaz6964. https://doi.org/10.1126/science.aaz6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antarianto RD, Pragiwaksana A, Septiana WL, et al. Hepatocyte differentiation from iPSCs or MSCs in decellularized liver scaffold: Cell-ECM adhesion, spatial distribution, and hepatocyte maturation profile. Organogenesis, 2021;18:2061263. doi: 10.1080/15476278.2022.2061263. https://doi.org/10.1080/15476278.2022.2061263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langer R, Vacanti JP. Tissue engineering. Science. 1999;260:920–926. doi: 10.1126/science.8493529. https://doi.org/10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 10.Ashammakhi N, GhavamiNejad A, Tutar R, et al. Highlights on advancing frontiers in tissue engineering. Tissue Eng Part B Rev, 2022;28:633–664. doi: 10.1089/ten.teb.2021.0012. https://doi.org/10.1089/ten.TEB.2021.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park KM, Shin YM, Kim K, et al. Tissue engineering and regenerative medicine 2017: A year in review. Tissue Eng Part B Rev, 2018;24:327–344. doi: 10.1089/ten.TEB.2018.0027. https://doi.org/10.1089/ten.TEB.2018.0027. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi J, Kikuchi A, Aoyagi T, et al. Cell sheet tissue engineering: Cell sheet preparation, harvesting/ manipulation, and transplantation. J Biomed Mater Res A. 2019;107:955–967. doi: 10.1002/jbm.a.36627. https://doi.org/10.1002/jbm.a.36627. [DOI] [PubMed] [Google Scholar]
- 13.Mehrban N, Teoh GZ, Birchall M. 2015. 3D bioprinting for tissue engineering: Stem cells in hydrogels. Int J Bioprint 6-19. https://doi.org/10.18063/IJB.2016.01.006 [Google Scholar]
- 14.Fayon A, Menu P, Omar RE. Cellularized small-caliber tissue-engineered vascular grafts: Looking for the ultimate gold standard, NPJ Regen Med. 2021;6:46. doi: 10.1038/s41536-021-00155-x. https://doi.org/10.1038/s41536-021-00155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadtler K, Singh A, Wolf MT, et al. Design, clinical translation and immunological response of biomaterials in regenerative medicine. Nat Rev Mater. 2016;1:1–17. https://doi.org/10.1038/natrevmats.2016.40. [Google Scholar]
- 16.Ohashi K, Yokoyama T, Yamato M, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13:880–885. doi: 10.1038/nm1576. https://doi.org/10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 17.Mazza G, Al-Akkad W, Rombouts K, et al. Liver tissue engineering: From implantable tissue to whole organ engineering, Hepatol Commun. 2018;2:131–141. doi: 10.1002/hep4.1136. https://doi.org/10.1002/hep4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollister SJ. Scaffold design and manufacturing: From concept to clinic. Adv Mater. 2009;21:3330–3342. doi: 10.1002/adma.200802977. https://doi.org/10.1002/adma.200802977. [DOI] [PubMed] [Google Scholar]
- 19.Huang D, Gibeley SB, Xu C, et al. Engineering liver microtissues for disease modeling and regenerative medicine. Adv Funct Mater. 2020;30:1909553. doi: 10.1002/adfm.201909553. https://doi.org/10.1002/adfm.201909553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho S, Discher DE, Leong KW, et al. Challenges and opportunities for the next generation of cardiovascular tissue engineering. Nat Methods, 2022;19:1064–1071. doi: 10.1038/s41592-022-01591-3. https://doi.org/10.1038/s41592-022-01591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mir TA, Nakamura M. Three-dimensional bioprinting: Toward the era of manufacturing human organs as spare parts for healthcare and medicine, Tissue Eng Part B Rev. 2017;23:245–256. doi: 10.1089/ten.TEB.2016.0398. https://doi.org/10.1089/ten.TEB.2016.0398. [DOI] [PubMed] [Google Scholar]
- 22.Moroni L, Burdick JU, Highley C, et al. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat Rev Mater, 2018;3:21–37. doi: 10.1038/s41578-018-0006-y. https://doi.org/10.1038/s41578-018-0006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mir TA, Iwanaga S, Kurooka T, et al. Biofabrication offers future hope for tackling various obstacles and challenges in tissue engineering and regenerative medicine: A perspective. Int J Bioprint, 2019;5:153. doi: 10.18063/ijb.v5i1.153. https://doi.org/10.18063/ijb.v5i1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arai K, Yoshida T, Okabe M, et al. Fabrication of 3D-culture platform with sandwich architecture for preserving liver-specific functions of hepatocytes using 3D bioprinter. J Biomed Mater Res A. 2017;105:1583–1592. doi: 10.1002/jbm.a.35905. https://doi.org/10.1002/jbm.a.35905. [DOI] [PubMed] [Google Scholar]
- 25.Bertassoni LE. Bioprinting of complex multicellular organs with advanced functionality-recent progress and challenges ahead. Adv Mater, 2022;34:e2101321. doi: 10.1002/adma.202101321. https://doi.org/10.1002/adma.202101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chae S, Yong U, Park W, et al. 3D cell-printing of gradient multi-tissue interfaces for rotator cuff regeneration. Bioact Mater, 2023;19:611–625. doi: 10.1016/j.bioactmat.2022.05.004. https://doi.org/10.1016/j.bioactmat.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gantumur E, Nakahata M, Kojima M, et al. Extrusionbased bioprinting through glucose-mediated enzymatic hydrogelation. Int J Bioprint, 2020;6:250. doi: 10.18063/ijb.v6i1.250. https://doi.org/10.18063/ijb.v6i1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groll J, Boland T, Blunk T, et al. Biofabrication: reappraising the definition of an evolving field. Biofabrication, 2016;8(1):013001. doi: 10.1088/1758-5090/8/1/013001. https://doi.org/10.1088/1758-5090/8/1/013001. [DOI] [PubMed] [Google Scholar]
- 29.353Mironov V, Boland T, Trusk T, et al. 2003. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol 21 157-161. https://doi.org/10.1016/S0167-7799(03)00033-7 [DOI] [PubMed] [Google Scholar]
- 30.Kang KH, Hockaday LA, Duan B, et al. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mat Res A. 2013;101(5):1255–1264. doi: 10.1002/jbm.a.34420. https://doi.org/10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sears NA, Seshadri DR, Dhavalikar PS, et al. A review of three-dimensional printing in tissue engineering. Tissue Eng Part B Rev. 2015:298–310. doi: 10.1089/ten.TEB.2015.0464. https://doi.org/10.1089/ten.teb.2015.0464. [DOI] [PubMed] [Google Scholar]
- 32.Elomaa L, Yang YP. Additive manufacturing of vascular grafts and vascularized tissue constructs. Tissue Eng Part B Rev. 2017;5:436–450. doi: 10.1089/ten.teb.2016.0348. https://doi.org/10.1089/ten.teb.2016.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matai I, Kaur G, Seyedsalehi A, et al. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi: 10.1016/j.biomaterials.2019.119536. https://doi.org/10.1016/j.biomaterials.2019.119536. [DOI] [PubMed] [Google Scholar]
- 34.Moroni L, Boland T, Burdick JA, et al. Biofabrication: A guide to technology and terminology. Trends Biotechnol. 2018;36:384–402. doi: 10.1016/j.tibtech.2017.10.015. https://doi.org/10.1016/j.tibtech.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Pereira RF, Bártolo PJ. 3D bioprinting of photocrosslinkable hydrogel constructs. J Appl Polym Sci. 2015;132:42458. https://doi.org/10.1002/app.42458. [Google Scholar]
- 36.Mandrycky C, Wang Z, Kim K, et al. 2016. 3D bioprinting for engineering complex tissues. Biotechnol Adv, 34, 422 434 https://doi.org/10.1016/j.biotechadv.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saunders RE, Derby B. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int Mater Rev. 2014;59:430–448. https://doi.org/10.1179/1743280414Y.0000000040. [Google Scholar]
- 38.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. https://doi.org/10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 39.Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials, 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. https://doi.org/10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Guillemot F, Souquet A, Catros S, et al. High- throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater, 2010;6:2494–2500. doi: 10.1016/j.actbio.2009.09.029. https://doi.org/10.1016/j.actbio.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 41.Ozbolat IT, Moncal KK, Gudapati H. Evaluation of bioprinter technologies. Addit Manuf, 2017;13:179–200. https://doi.org/10.1016/j.addma.2016.10.003. [Google Scholar]
- 42.Ouyang L, Highley CB, Sun W, et al. A generalizable strategy for the 3D bioprinting of hydrogels from nonviscous photo-crosslinkable inks. Adv Mater, 2017;29:1604983. doi: 10.1002/adma.201604983. https://doi.org/10.1002/adma.201604983. [DOI] [PubMed] [Google Scholar]
- 43.Duan B, Hockaday LA, Kang KH, et al. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013;101:1255–1264. doi: 10.1002/jbm.a.34420. https://doi.org/10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu W, Ma X, Gou M, et al. 3D printing of functional biomaterials for tissue engineering. Curr Opin Biotechnol. 2016;40:103–112. doi: 10.1016/j.copbio.2016.03.014. https://doi.org/10.1016/j.copbio.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 45.Sarig-Nadir O, Livnat N, Zajdman R, et al. 2009. Laser photoablation of guidance microchannels into hydrogels directs cell growth in three dimensions. Biophys J 96, 4743 4752 https://doi.org/10.1016/j.bpj.2009.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park S, Choi G, Kim JM, et al. 3D printing model of abdominal cavity of liver transplantation recipient to prevent large-forsize syndrome. Int J Bioprint, 2022;8(4):609. doi: 10.18063/ijb.v8i4.609. https://doi.org/10.18063/ijb.v8i4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie F, Sun L, Pang Y, et al. Three-dimensional bioprinting of primary human hepatocellular carcinoma for personalized medicine. Biomaterials, 2021;265:120416. doi: 10.1016/j.biomaterials.2020.120416. https://doi.org/10.1016/j.biomaterials.2020.120416. [DOI] [PubMed] [Google Scholar]
- 48.Bouwmeester MC, Bernal PN, Oosterhoff LA, et al. Bioprinting of human liver-derived epithelial organoids for toxicity studies. Macromol Biosci. 2021;21(12):e2100327. doi: 10.1002/mabi.202100327. https://doi.org/10.1002/mabi.202100327. [DOI] [PubMed] [Google Scholar]
- 49.Taymour R, Kilian D, Ahlfeld T, et al. 3D bioprinting of hepatocytes: Core–shell structured co-cultures with fibroblasts for enhanced functionality. Sci Rep. 2021;11:5130. doi: 10.1038/s41598-021-84384-6. https://doi.org/10.1038/s41598-021-84384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura M, Mir TA, Arai K, et al. Bioprinting with pre-cultured cellular constructs towards tissue engineering of hierarchical tissues. Int J Bioprint. 2015;1(1):39–48. https://dx.doi.org/10.18063/IJB.2015.01.007. [Google Scholar]
- 51.Ma L, Wu Y, Li Y, et al. Current advances on 3D-bioprinted liver tissue models. Adv Healthc Mater. 2020;9(24):e2001517. doi: 10.1002/adhm.202001517. https://doi.org/10.1002/adhm.202001517. [DOI] [PubMed] [Google Scholar]
- 52.Xu K, Han Y, Huang Y, et al. The application of 3D bioprinting in urological diseases. Mat Today Bio. 2022;2(16):100388. doi: 10.1016/j.mtbio.2022.100388. https://doi.org/10.1016/j.mtbio.2022.100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.354Murphy SV, De Coppi P, Atala A. Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng. 2019;4:370–380. doi: 10.1038/s41551-019-0471-7. https://doi.org/10.1038/s41551-019-0471-7. [DOI] [PubMed] [Google Scholar]
- 54.Weekes A, Bartnikowski N, Pinto N, et al. Biofabrication of small diameter tissue-engineered vascular grafts. Acta Biomater. 2022;138:92–111. doi: 10.1016/j.actbio.2021.11.012. https://doi.org/10.1016/j.actbio.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Taormina G, Sciancalepore C, Messori M, et al. 3D printing processes for photocurable polymeric materials: Technologies, materials, and future trends. J Appl Biomater Funct Mater. 2018;16:151–160. doi: 10.1177/2280800018764770. https://doi.org/10.1177/2280800018764770. [DOI] [PubMed] [Google Scholar]
- 56.Krkobabić M, Medarević D, Pešić N, et al. Digital light processing (DLP) 3D printing of atomoxetine hydrochloride tablets using photoreactive suspensions. Pharmaceutics, 2020;12:E833. doi: 10.3390/pharmaceutics12090833. https://doi.org/10.3390/pharmaceutics12090833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ozbolat IT. Bioprinting scale-up tissue and organ constructs for transplantation, Trends Biotechnol. 2015;33:395–400. doi: 10.1016/j.tibtech.2015.04.005. https://doi.org/10.1016/j.tibtech.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Rengier F, Mehndiratta A, Tengg-Kobligk HV, et al. 3D printing based on imaging data: Review of medical applications. Int J Comput Assist Radiol Surg, 2010;5:335–341. doi: 10.1007/s11548-010-0476-x. https://doi.org/10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 59.Stampfl J, Baudis S, Heller C, et al. Photopolymers with tunable mechanical properties processed by laser-based highresolution stereolithography. J Micromech Microeng. 2008;18:125014. https://doi.org/10.1088/0960-1317/18/12/125014. [Google Scholar]
- 60.Tumbleston JR, Shirvanyants D, Ermoshkin N, et al. Additive manufacturing Continuous liquid interface production of 3D objects. Science, 2015;347:1349–1352. doi: 10.1126/science.aaa2397. https://doi.org/10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- 61.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. https://doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 62.Guillotin B, Guillemot F. Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol. 2011;29:183–190. doi: 10.1016/j.tibtech.2010.12.008. https://doi.org/10.1016/j.tibtech.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 63.Arai K, Iwanaga S, Toda H, et al. Three-dimensional inkjet biofabrication based on designed images. Biofabrication. 2011;3:034113. doi: 10.1088/1758-5082/3/3/034113. https://iopscience.iop.org/article/10.1088/1758-5082/3/3/034113. [DOI] [PubMed] [Google Scholar]
- 64.Choudhury D, Anand S, Naing MW. The arrival of commercial bioprinters—Towards 3D bioprinting revolution. Int J Bioprint. 2018;4:139. doi: 10.18063/IJB.v4i2.139. https://doi.org/10.18063/IJB.v4i2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Liu B, Pei B, et al. Inkjet bioprinting of biomaterials. Chem Rev. 2020;120:10793–10833. doi: 10.1021/acs.chemrev.0c00008. https://doi.org/10.1021/acs.chemrev.0c00008. [DOI] [PubMed] [Google Scholar]
- 66.Gu Z, Fu J, Lin H, et al. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J Pharm Sci, 2020;15:529–557. doi: 10.1016/j.ajps.2019.11.003. https://doi.org/10.1016/j.ajps.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levato R, Jungst T, Scheuring RG, et al. 2020. From shape to function: The next step in bioprinting. Adv Mater 1906423 https://doi.org/10.1002/adma.201906423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlos Mota C, Camarero-Espinosa S, Baker MB, et al. Bioprinting: From tissue and organ development to in vitro models. Chem Rev. 2020;120(19):10547–10607. doi: 10.1021/acs.chemrev.9b00789. https://doi.org/10.1021/acs.chemrev.9b00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jorgensen AM, Yoo JJ, Anthony A. Solid organ bioprinting: Strategies to achieve organ function. Chem Rev, 2020;120(19):11093–11127. doi: 10.1021/acs.chemrev.0c00145. https://doi.org/10.1021/acs.chemrev.0c00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Li J, Li Y, et al. Biomimetic bioinks of nanofibrillar polymeric hydrogels for 3D bioprinting. Nano Today, 2021;39:101180. https://doi.org/10.1016/j.nantod.2021.101180. [Google Scholar]
- 71.Lee J, Lee S, Ahmad T, et al. Human adipose-derived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials. 2020;255:120192. doi: 10.1016/j.biomaterials.2020.120192. https://doi.org/10.1016/j.biomaterials.2020.120192. [DOI] [PubMed] [Google Scholar]
- 72.Kim EM, Lee YB, Kim SJ, et al. Fabrication of coreshell spheroids as building blocks for engineering 3D complex vascularized tissue. Acta Biomater, 2019;100:158–172. doi: 10.1016/j.actbio.2019.09.028. https://doi.org/10.1016/j.actbio.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 73.Nakamura M, Iwanaga S, Henmi C, et al. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication, 2010;2(1):014110. doi: 10.1088/1758-5082/2/1/014110. https://iopscience.iop.org/article/10.1088/1758-5082/2/1/014110 [DOI] [PubMed] [Google Scholar]
- 74.George J, Hsu CC, Ba LT, et al. Neural tissue engineering with structured hydrogels in CNS models and therapies. Biotechnol Adv. 2020;42:107370. doi: 10.1016/j.biotechadv.2019.03.009. https://doi.org/10.1016/j biotechadv.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 75.Pereira RF, Bártolo PJ. 3D photo-fabrication for tissue engineering and drug delivery. Engineering. 2015;1:90–112. https://doi.org/10.15302/J-ENG-2015015. [Google Scholar]
- 76.Unagolla JM, Jayasuriya AC. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl Mater Today, 2020;18:100479. doi: 10.1016/j.apmt.2019.100479. https://doi.org/10.1016/j.apmt.2019.100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.355Sharma S, Tiwari S. A review on biomacromolecular hydrogel classification and its applications. Int J Biol Macromol. 2020;162:737–747. doi: 10.1016/j.ijbiomac.2020.06.110. https://doi.org/10.1016/j.ijbiomac.2020.06.110. [DOI] [PubMed] [Google Scholar]
- 78.Ebhodaghe SO. Hydrogel based biopolymers for regenerative medicine applications: A critical review. Intl J Polym Mater Polym Biomater. 2020;71:155–172. https://doi.org/10.1080/00914037.2020.1809409. [Google Scholar]
- 79.Catoira MC, Fusaro L, Francesco DD, et al. Overview of natural hydrogels for regenerative medicine applications. J Mater Sci Mater Med. 2019;30:115. doi: 10.1007/s10856-019-6318-7. https://doi.org/10.1007/s10856-019-6318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zieliński PS, Gudet PKR, Rikmanspoel T, et al. 3D printing of bio-instructive materials: Toward directing the cell. Bioact Mater. 2022;19:292–327. doi: 10.1016/j.bioactmat.2022.04.008. https://doi.org/10.1016/j.bioactmat.2022.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malda J, Visser J, Melchels FP, et al. 25th anniversary article: Engineering hydrogels for biofabrication. Adv Mater, 2013;25:5011–5028. doi: 10.1002/adma.201302042. https://doi.org/10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 82.Kim BS, Das S, Jang J, et al. Decellularized extracellular matrix-based bioinks for engineering tissue- and organspecific microenvironments. Chem Rev. 2020;120:10608–10661. doi: 10.1021/acs.chemrev.9b00808. https://doi.org/10.1021/acs.chemrev.9b00808. [DOI] [PubMed] [Google Scholar]
- 83.Lee SC, Gillispie G, Prim P, et al. Physical and chemical factors influencing the printability of hydrogelbased extrusion bioinks. Chem Rev. 2020;120:10834–10886. doi: 10.1021/acs.chemrev.0c00015. https://doi.org/10.1021/acs.chemrev.0c00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou K, Sun Y, Yang J, et al. Hydrogels for 3D embedded bioprinting: A focused review on bioinks and support baths. J Mater Chem B. 2022;10(12):1897–1907. doi: 10.1039/d1tb02554f. https://doi.org/10.1039/D1TB02554F. [DOI] [PubMed] [Google Scholar]
- 85.Arai K, Tsukamoto Y, Yoshida H, et al. The development of cell-adhesive hydrogel for 3D printing. Int J Bioprint. 2016;2(2):153–162. https://dx.doi.org/10.18063/IJB.2016.02.002. [Google Scholar]
- 86.Soman SS, Govindraj M, Al Hashimi N, et al. Bioprinting of human neural tissues using a sustainable marine tunicate derived bioink for translational medicine applications. Int J Bioprint. 2022;8(4):604. doi: 10.18063/ijb.v8i4.604. https://doi.org/10.18063/ijb.v8i4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang M, Li W, Hao J, et al. Molecularly cleavable bioinks facilitate high-performance digital light processingbased bioprinting of functional volumetric soft tissues. Nat Commun, 2022;13:3317. doi: 10.1038/s41467-022-31002-2. https://doi.org/10.1038/s41467-022-31002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Yuan X, Yao B, et al. Tailoring bioinks of extrusion-based bioprinting for cutaneous wound healing. Bioact Mater. 2022;17:178–194. doi: 10.1016/j.bioactmat.2022.01.024. https://doi.org/10.1016/j.bioactmat.2022.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khoeini R, Nosrati H, Akbarzadeh A, et al. Natural and synthetic bioinks for 3D bioprinting. Adv NanoBiomed Res. 2021;1:2000097. https://doi.org/10.1002/anbr.202000097. [Google Scholar]
- 90.Pati F, Jang J, Ha DH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun, 2014;5:3935. doi: 10.1038/ncomms4935. https://doi.org/10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cui X, Li J, Hartanto Y, et al. Advances in extrusion 3D bioprinting: A focus on multicomponent hydrogel-based bioinks. Adv Healthc Mater. 2020;9:e1901648. doi: 10.1002/adhm.201901648. https://doi.org/10.1002/adhm.201901648. [DOI] [PubMed] [Google Scholar]
- 92.Sakai S, Nakahata M. Horseradish peroxidase catalyzed hydrogelation for biomedical, biopharmaceutical, and biofabrication applications. Chem Asian J. 2017;12:3098–3109. doi: 10.1002/asia.201701364. https://doi.org/10.1002/asia.201701364. [DOI] [PubMed] [Google Scholar]
- 93.Gu Y, Forget A, Shastri VP. Biobridge: An outlook on translational bioinks for 3D bioprinting. Adv Sci (Weinh), 2022;9:e2103469. doi: 10.1002/advs.202103469. https://doi.org/10.1002/advs.202103469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. https://doi.org/10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X, Chen X, Hong H, et al. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact Mater. 2022;10:15–31. doi: 10.1016/j.bioactmat.2021.09.014. https://doi.org/10.1016/j.bioactmat.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nicolas J, Magli S, Rabbachin L, et al. 3D extracellular matrix mimics: Fundamental concepts and role of materials chemistry to influence stem cell fate. Biomacromolecules, 2020;21:1968–1994. doi: 10.1021/acs.biomac.0c00045. https://doi.org/10.1021/acs.biomac.0c00045. [DOI] [PubMed] [Google Scholar]
- 97.Kuraitis D, Giordano C, Ruel M, et al. Exploiting extracellular matrix-stem cell interactions: A review of natural materials for therapeutic muscle regeneration. Biomaterials, 2012;33(2):428–443. doi: 10.1016/j.biomaterials.2011.09.078. https://doi.org/10.1016/j.biomaterials.2011.09.078. [DOI] [PubMed] [Google Scholar]
- 98.Jain P, Rauer SB, Möller M, et al. Mimicking the natural basement membrane for advanced tissue engineering. Biomacromolecules. 2022;23:3081–3103. doi: 10.1021/acs.biomac.2c00402. https://doi.org/10.1021/acs.biomac.2c00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.356Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34. doi: 10.1016/j.ymeth.2015.03.005. https://doi.org/10.1016/j.ymeth.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 100.Willemse J, Tienderen G, Hengel E, et al. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials. 2022;284:121473. doi: 10.1016/j.biomaterials.2022.121473. [DOI] [PubMed] [Google Scholar]
- 101.Liao J, Xu B, Zhang R, et al. Applications of decellularized materials in tissue engineering: Advantages, drawbacks and current improvements, and future perspectives. J Mater Chem B. 2020;8:10023–10049. doi: 10.1039/d0tb01534b. https://doi.org/10.1039/d0tb01534b. [DOI] [PubMed] [Google Scholar]
- 102.Lu P, Weaver VM, Werb Z. The extracellular matrix: A dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. https://doi.org/10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meng F, Almohanna F, Altuhami A, et al. Vasculature reconstruction of decellularized liver scaffolds via gelatinbased re-endothelialization. J Biomed Res Part A. 2018;4:107. doi: 10.1002/jbm.a.36551. https://doi.org/10.1002/jbm.a.36551. [DOI] [PubMed] [Google Scholar]
- 104.Ponticos M, Smith BD. Extracellular matrix synthesis in vascular disease: Hypertension, and atherosclerosis. J Biomed Res. 2014;28:25–39. doi: 10.7555/JBR.27.20130064. https://doi.org/10.7555/JBR.27.20130064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lorenzo P, Bayliss MT, Heinegård D. Altered patterns and synthesis of extracellular matrix macromolecules in early osteoarthritis. Matrix Biol. 2004;23:381–391. doi: 10.1016/j.matbio.2004.07.007. https://doi.org/10.1016/j.matbio.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 106.Meng F, Assiri AM, Dhar D, et al. Whole liver engineering: A promising approach to develop functional liver surrogates. Liver Int. 2017;37:1759–1772. doi: 10.1111/liv.13444. https://doi.org/10.1111/liv.13444. [DOI] [PubMed] [Google Scholar]
- 107.Hussey GS, Dziki JL, Badylak SF. Extracellular matrixbased materials for regenerative medicine. Nat Rev Mater. 2018;3:159–173. https://doi.org/10.1038/s41578-018-0023-x. [Google Scholar]
- 108.Zhang X, Liu Y, Zuo Q, et al. 3D bioprinting of biomimetic bilayered scaffold consisting of decellularized extracellular matrix and silk fibroin for osteochondral repair. Int J Bioprint. 2021;74:401. doi: 10.18063/ijb.v7i4.401. https://doi.org/10.18063/ijb.v7i4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen FM, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci, 2016;53:86–168. doi: 10.1016/j.progpolymsci.2015.02.004. https://doi.org/10.1016/j.progpolymsci.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim MK, Jeong WW, Lee SM, et al. Decellularized extracellular matrix-based bio-ink with enhanced 3D printability and mechanical properties. Biofabrication, 2020;12:025003. doi: 10.1088/1758-5090/ab5d80. https://doi.10.1088/1758-5090/ab5d80. [DOI] [PubMed] [Google Scholar]
- 111.Rueda-Gensini L, Serna JA, Cifuentes J, et al. Graphene oxide-embedded extracellular matrix-derived hydrogel as a multiresponsive platform for 3D bioprinting applications. Int J Bioprint. 2021;7(3):353. doi: 10.18063/ijb.v7i3.353. https://doi.org/10.18063/ijb.v7i3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang R, Xu J, Huang C. Effect of Ionic crosslinking on morphology and thermostability of biomimetic supercritical fluids-decellularized dermalbased composite bioscaffolds for bioprinting applications. Int J Bioprint. 2023;9(1):625. doi: 10.18063/ijb.v9i1.625. https://doi.org/10.18063/ijb.v9i1.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zheng J, Liu Y, Hou C, et al. Ovary-derived decellularized extracellular matrix-based bioink for fabricating 3D primary ovarian cells-laden structures for mouse ovarian failure correction. Int J Bioprint. 2022;8(3):597. doi: 10.18063/ijb.v8i3.597. https://doi.org/10.18063/ijb.v8i3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Choudhury D, Tun HW, Wang T, et al. Organ-derived decellularized extracellular matrix: A game changer for bioink manufacturing? Trends Biotechnol. 2018;36(8):787–805. doi: 10.1016/j.tibtech.2018.03.003. https://doi.org/10.1016/j.tibtech.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 115.Lee H, Han W, et al. Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromolecules, 2017;18:1229–1237. doi: 10.1021/acs.biomac.6b01908. https://doi.org/10.1021/acs.biomac.6b01908. [DOI] [PubMed] [Google Scholar]
- 116.Ali M, Kumar A, Yoo JJ, et al. A photo-crosslinkable kidney ECM-derived bioink accelerates renal tissue formation. Adv Healthc Mater. 2019;8(7):e1800992. doi: 10.1002/adhm.201800992. https://doi.org/10.1002/adhm.201800992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Willemse J, Verstegen MMA, Vermeulen A, et al. Fast, robust and effective decellularization of whole human livers using mild detergents and pressure controlled perfusion. Mater Sci Eng C Mater Biol Appl, 2020;108:110200. doi: 10.1016/j.msec.2019.110200. https://doi.org/10.1016/j.msec.2019.110200. [DOI] [PubMed] [Google Scholar]
- 118.Felgendreff P, Schindler C, Mussbach F, et al. Identification of tissue sections from decellularized liver scaffolds for repopulation experiments. Heliyon, 2021;7:e06129. doi: 10.1016/j.heliyon.2021.e06129. https://doi.org/10.1016/j.heliyon.2021.e06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shimoda H, Yagi H, Higashi H, et al. Decellularized liver scaffolds promote liver regeneration after partial hepatectomy. Sci Rep. 2019;9:12543. doi: 10.1038/s41598-019-48948-x. https://doi.org/10.1038/s41598-019-48948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tajima K, Yagi H, Kitagawa Y. Human-scale liver harvest and decellularization for preclinical research. Methods Mol Biol. 2018;1577:327–335. doi: 10.1007/7651_2018_195. https://doi.org/10.1007/7651_2018_195. [DOI] [PubMed] [Google Scholar]
- 121.Zhang Z, Xu M, Mazza G, et al. Decellularized human liver scaffold-based three-dimensional culture system facilitate hepatitis B virus infection. J Biomed Mater Res A, 2019;107(8):1744–1753. doi: 10.1002/jbm.a.36690. https://doi.org/10.1002/jbm.a.36690. [DOI] [PubMed] [Google Scholar]
- 122.357Mazza G, Rombouts K, et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep, 2015;5:13079. doi: 10.1038/srep13079. https://doi.org/10.1038/srep13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mazza G, Al-Akkad W, Telese A, et al. Rapid production of human liver scaffolds for functional tissue engineering by high shear stress oscillation-decellularization. Sci Rep. 2017;7:5534. doi: 10.1038/s41598-017-05134-1. https://doi.org/10.1038/s41598-017-05134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Skardal A, Devarasetty M, Kang H-W, et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater, 2015;25:24–34. doi: 10.1016/j.actbio.2015.07.030. https://doi.org/10.1016/j.actbio.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 125.Lee H, Han W, Kim H, et al. Development of liver decellularized extracellular matrix bioink for threedimensional cell printing-based liver tissue engineering. Biomacromolecules. 2017;18:1229–1237. doi: 10.1021/acs.biomac.6b01908. https://doi.org/10.1021/acs.biomac.6b01908. [DOI] [PubMed] [Google Scholar]
- 126.Yu C, Ma X, Zhu W, et al. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials. 2019;194:1–13. doi: 10.1016/j.biomaterials.2018.12.009. https://doi.org/10.1016/j.biomaterials.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim MK, Jeong W, Lee SM, et al. Decellularized extracellular matrix-based bio-ink with enhanced 3D printability and mechanical properties. Biofabrication, 2020;12:025003. doi: 10.1088/1758-5090/ab5d80. https://doi.org/10.1088/1758-5090/ab5d80. [DOI] [PubMed] [Google Scholar]
- 128.Lewis PL, Yan M, Su J, et al. Directing the growth and alignment of biliary epithelium within extracellular matrix hydrogels. Acta Biomater. 2019;85:84–93. doi: 10.1016/j.actbio.2018.12.039. https://doi.org/10.1016/j.actbio.2018.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee H, Chae S, Kim JY, et al. Cell-printed 3D liver- on-a-chip possessing a liver microenvironment and biliary system. Biofabrication, 2019;11:025001. doi: 10.1088/1758-5090/aaf9fa. https://doi.org/10.1088/1758-5090/aaf9fa. [DOI] [PubMed] [Google Scholar]
- 130.Mao Q, Wang Y, Li Y, et al. Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater Sci Eng C Mater Biol Appl, 2020;109:110625. doi: 10.1016/j.msec.2020.110625. https://doi.org/10.1016/j.msec.2020.110625. [DOI] [PubMed] [Google Scholar]
- 131.Jeong W, Kim MK, Kang HW. Effect of detergent type on the performance of liver decellularized extracellular matrix-based bio-inks. J Tissue Eng, 2021;12:2041731421997091. doi: 10.1177/2041731421997091. https://doi.org/10.1177/2041731421997091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khati V, Ramachandraiah H, Pati F, et al. 3D bioprinting of multi-material decellularized liver matrix hydrogel at physiological temperatures. Biosensors (Basel) 2022;12:521. doi: 10.3390/bios12070521. https://doi.org/10.3390/bios12070521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou G, Jiang H, Yin Z, et al. In vitro regeneration of patient-specific ear-shaped cartilage and its first clinical application for auricular reconstruction. EBioMedicine. 2018;28:287–302. doi: 10.1016/j.ebiom.2018.01.011. https://doi.org/10.1016/j.ebiom.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Raya-Rivera AM, Esquiliano D, Fierro-Pastrana R, et al. 2014. Tissue-engineered autologous vaginal organs in patients: A pilot cohort study. Lancet, 384, 329 336 https://doi.org/10.1016/S0140-6736(14)60542-0 [DOI] [PubMed] [Google Scholar]
- 135.Zopf DA, Hollister SJ, Nelson ME, et al. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med, 2013;368:2043–2045. doi: 10.1056/NEJMc1206319. https://doi.org/10.1056/NEJMc1206319. [DOI] [PubMed] [Google Scholar]
- 136.Poel WE. Preparation of acellular homogenates from muscle samples. Science. 1948;108:390–391. doi: 10.1126/science.108.2806.390-a. https://doi.org/10.1126/science.108.2806.390-a. [DOI] [PubMed] [Google Scholar]
- 137.Hjelle JT, Carlson EC, Brendel K, et al. Biosynthesis of basement membrane matrix by isolated rat renal glomeruli. Kidney Int. 1979;15:20–32. doi: 10.1038/ki.1979.3. https://doi.org/10.1038/ki.1979.3. [DOI] [PubMed] [Google Scholar]
- 138.Badylak SF, Tullius R, Kokini K, et al. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res, 1995;29:977–985. doi: 10.1002/jbm.820290809. https://doi.org/10.1002/jbm.820290809. [DOI] [PubMed] [Google Scholar]
- 139.Ott HC, Matthiesen TS, Goh SK, et al. Perfusion- decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. https://doi.org/10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 140.Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med, 2010;16:927–933. doi: 10.1038/nm.2193. https://doi.org/10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 141.Petersen TH, Calle EA, Zhao L, et al. 2010. Tissue- engineered lungs for in vivo implantation. Science, 329, 538 541 https://doi.org/10.1126/science.1189345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Uygun BE, Soto-Gutierrez AS, Yagi H, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–820. doi: 10.1038/nm.2170. https://doi.org/10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Song JJ, Guyette JP, Gilpin SE, et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–651. doi: 10.1038/nm.3154. https://doi.org/10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saldin LT, Cramer MC, Velankar SS, et al. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017;49:1–15. doi: 10.1016/j.actbio.2016.11.068. https://doi.org/10.1016/j.actbio.2016.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.358Garreta E, Oria R, Tarantino C, et al. Tissue engineering by decellularization and 3D bioprinting. Mater Today, 2017;20:166–178. https://doi.org/10.1016/j.mattod.2016.12.005. [Google Scholar]
- 146.Chimene D, Kaunas R, Gaharwar AK. Hydrogel bioink reinforcement for additive manufacturing: A focused review of emerging strategies. Adv Mater. 2020;32:e1902026. doi: 10.1002/adma.201902026. https://doi.org/10.1002/adma.201902026. [DOI] [PubMed] [Google Scholar]
- 147.Jang J, Park HJ, Kim SW, et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair, Biomaterials, 2017;112:264–274. doi: 10.1016/j.biomaterials.2016.10.026. https://doi.org/10.1016/j.biomaterials.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 148.Abaci A, Guvendiren M. Designing decellularized extracellular matrix-based bioinks for 3D bioprinting. Adv Healthc Mater. 2020;9(24):e2000734. doi: 10.1002/adhm.202000734. https://doi.org/10.1002/adhm.202000734. [DOI] [PubMed] [Google Scholar]
- 149.Fu RH, Wang YC, Liu SH, et al. Decellularization and recellularization technologies in tissue engineering. Cell Transplant. 2014;23:621–630. doi: 10.3727/096368914X678382. https://doi.org/10.3727/096368914×678382. [DOI] [PubMed] [Google Scholar]
- 150.Hussey GS, Dziki JL, Badylak SF. Extracellular matrixbased materials for regenerative medicine. Nat Rev Mater, 2018;3:159–173. https://doi.org/10.1038/s41578-018-0023-x. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


