Abstract
Many of the estimated 1.4 million adults with congenital heart defects (CHDs) in the United States are lost to follow-up (LTF) despite recommendations for ongoing cardiology care. Using 2016 to 2019 CH STRONG (Congenital Heart Survey To Recognize Outcomes, Needs, and well-beinG) data, we describe cardiac care among community-based adults with CHD, born in 1980 to 1997, identified through state birth defects registries. Our estimates of LTF were standardized to the CH STRONG eligible population and likely more generalizable to adults with CHD than clinic-based data. Half of our sample were LTF and more than 45% had not received cardiology care in over 5 years. Of those who received care, only 1 in 3 saw an adult CHD physician at their last encounter. Not knowing they needed to see a cardiologist, being told they no longer needed cardiology care, and feeling “well” were the top reasons for LTF, and only half of respondents report doctors discussing the need for cardiac follow-up.
Many of the estimated 1.4 million adults with congenital heart defects (CHD) in the United States are lost to follow-up (LTF), despite recommendations for ongoing cardiology care.1 Remaining in cardiac care is associated with CHD complexity, higher education, and receiving care from adult CHD providers,2 but data are limited to patients in adult congenital heart disease (ACHD) care centers or administrative databases that exclude those who are currently out of care or receiving care from noncongenital cardiologists. Therefore, we estimate the community receipt of recommended cardiac care and LTF.
Methods
Using the 2016 to 2019 CH STRONG (Congenital Heart Survey To Recognize Outcomes, Needs, and well-beinG) data, we describe cardiac care among community-based adults with CHD, born in 1980 to 1997, identified through state birth defects registries.3 Severe CHD was classified using diagnostic codes selected by cardiologists.3 LTF was calculated using 2018 guidelines by defect.4 The most severe diagnosis per participant was mapped to recommendations under New York Heart Association classification A. The descriptive statistics included chi-square and analysis of variance analyses. To reduce potential for response bias, the LTF estimate was standardized to site and year of birth, gender assigned at birth, maternal race/ethnicity, and CHD severity across all CH STRONG eligible respondents. The prevalence ratio were estimated with Poisson regression, and standard errors were calculated using conditional standardization and the delta method on 9,312 eligible individuals (including non-respondents). All analyses were conducted in R (https://www.R-project.org/) and SAS (SAS Institute Inc., Cary, North Carolina). A p > 0.05 was considered significant.
Results
Of 1,656 respondents, 3.4% did not have a CHD represented in the guidelines5 and 1.4% did not report their last cardiology visit, leaving 1,576 individuals for analysis. Participant characteristics are described in Table 1. After standardization, 45.6% last saw a cardiologist over 5 years ago (30.4%) or never (15.2%), and 50.4% were LTF at time of survey. No association was seen between the type of cardiologist visited most recently and LTF (Figure 1); however, there was a negative correlation between age and care from a pediatric cardiologist (p <0.01; Figure 1).
Table 1.
Demographic characteristics and factors associated with LTF among young adults with congenital heart defects, CH STRONG 2016–2019
| Losttocardiacfollow–up | ||||||
|---|---|---|---|---|---|---|
| All cases N=1576 | Yes n=758 (%*) | PR (95% CI) | ||||
| Bivariate | Multivariable | Male | Female | |||
| Severe CHD | ||||||
| No | 1032 | 611(60) | ||||
| Yes | 544 | 147 (27) | 0.5 (0.4 – 0.5) | 0.7 (0.6 – 0.9) | 0.82 (0.6 – 1.1) | 0.63 (0.5 – 0.9) |
| Race/ethnicity | ||||||
| Non–Hispanic White | 1080 | 507 (53) | ||||
| Non–Hispanic Black | 224 | 113(54) | 1.1 (0.9 – 1.3) | 1.1 (0.9– 1.3) | 1.1 (0.7 – 1.5) | 1.1 (0.8 – 1.4) |
| Hispanic | 164 | 68 (52) | 0.9 (0.7 – 1.1) | 1.0 (0.7 – 1.2) | 0.9 (0.6 – 1.4) | 1.0 (0.6 – 1.4) |
| Non–Hispanic other | 94 | 62 (73) | 1.4 (1.1 – 1.8) | 1.3 (1.0 – 1.7) | 1.6 (1.1 – 2.3) | 1.1 (0.7 – 1.7) |
| Worked at least part–time past 12 months | ||||||
| No | 400 | 168 (46) | ||||
| Yes | 1162 | 580 (52) | 1.2 (1.0 – 1.4) | 1.1 (0.9 – 1.4) | 1.1 (0.8 – 1.5) | 1.1 (0.8 – 1.4) |
| Denied disability | ||||||
| No | 1249 | 641 (52) | ||||
| Yes | 201 | 63 (34) | 0.6 (0.5 – 0.8) | 0.9 (0.7 – 1.2) | 0.8 (0.5 – 1.2) | 1.0 (0.6– 1.4) |
| Cardiac comorbidity | ||||||
| No | 1172 | 596 (54) | ||||
| Yes | 404 | 162 (43) | 0.79 (0.7 – 0.9) | 0.9 (0.8 – 1.1) | 0.8 (0.6 – 1.1) | 0.9 (0.7 – 1.2) |
| Used emergency room past 12 months | ||||||
| No | 1076 | 551 (53) | ||||
| Yes | 499 | 207 (44) | 0.8 (0.7 – 0.9) | 0.9 (0.8 – 1.1) | 0.9 (0.7 – 1.2) | 0.9 (0.7– 1.2) |
| Admitted to hospital past 12 months | ||||||
| No | 1374 | 693 (53) | ||||
| Yes | 201 | 65 (36) | 0.6 (0.5 – 0.8) | 0.8 (0.6 – 1.0) | 0.6 (0.2 – 0.9)) | 0.9 (0.6– 1.3) |
| Type of insurance | ||||||
| Private | 886 | 413 (48) | ||||
| Public | 442 | 190 (46) | 0.9 (0.8 – 1.1) | 1.0 (0.9 – 1.3) | 1.1 (0.8 – 1.4) | 1.03 (0.8 – 1.3) |
| Other | 73 | 35 (52) | 1.0 (0.7 – 1.4) | 1.1 (0.8 – 1.6) | 1.3 (0.7 – 2.4) | 1.1 (0.6 – 1.7) |
| None | 156 | 105 (66) | 1.4 (1.2 – 1.8) | 1.3 (1.0 – 1.7) | 1.3 (0.9 – 2.0) | 1.3 (0.9 – 1.8) |
| Concern with health in general | ||||||
| Not at all –not very | 745 | 411(56) | ||||
| Somewhat – very | 793 | 332 (46) | 0.8 (0.7 – 0.9) | 1.1 (0.9 – 1.4) | 1.1 (0.8 – 1.5) | 1.18 (0.9– 1.56) |
| Concern with overall heart health | ||||||
| Not at all –not very | 793 (52) | 467 (59) | ||||
| Somewhat – very | 740 (48) | 277 (41) | 0.6 (0.6 – 0.7) | 0.8 (0.7 – 1.0) | 0.9 (0.6 – 1.2) | 0.8 (0.6– 1.1) |
| Concern with ability to have children | ||||||
| Not at all –not very | 1108 (73) | 578 (54) | ||||
| Somewhat – very | 421 (28) | 162 (45) | 0.7 (0.6 – 0.9) | 0.8 (0.7 – 0.9) | 1.0 (0.7 – 1.4) | 0.8 (0.6 – 0.9) |
| Healthcare provider discussed need for follow–up | ||||||
| Yes | 814 (52) | 172 (24) | ||||
| No | 744 (48) | 582 (74) | 3.7 (3.1– 4.4) | 3.1 (2.5 – 3.8) | 2.8 (2.1–3.7) | 3.3 (2.5 – 4.5) |
Values represent percentages after sample standardization to site, birthyear, gender, maternal race/ethnicity, and CHD severity across all respondents and nonrespondents.
Variables tested but not contributory in bivariate analyses: Sex at birth; age; comorbid noncardiac birth defect; high school completion; delayed care because of cost; Unable to pay for care past 12 months; additional noncardiac comorbidity; changed provider because of insurance past 12 months.
CH STRONG = Congenital Heart Survey To Recognize Outcomes, Needs, and well-beinG; CHD = congenital heart defect; CI = confidence interval; LTF = lost to cardiac care follow-up; PR = prevalence ratio.
Figure 1. Data shown represent.
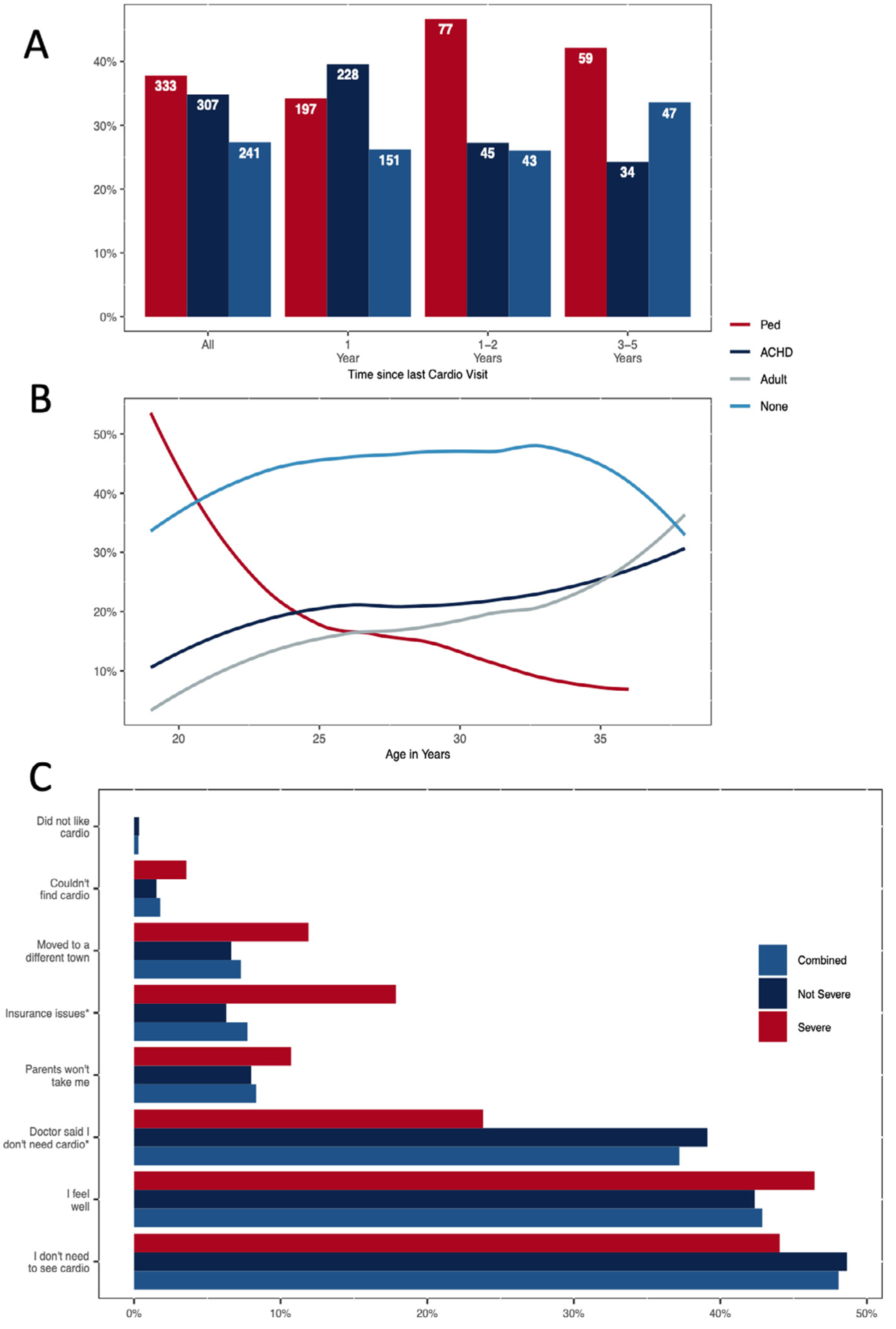
(A) time since last cardiology visit and (B) age at survey by type of cardiologist last seen for the 881 young adults with CHD who saw a cardiologist in past 5 years and (C) reasons why the remaining 659 young adults did not see a cardiologist by CHD severity, CH STRONG, 2016 to 2019. *p <0.05.
Having severe CHD, concerns about the ability to have children and a provider who discussed the need for lifelong cardiac care were associated with lower risk of LTF. Among men, hospital admission in the past 12 months was associated with reduced LTF; among women, severe CHD and concern with ability to have children were associated with reduced LTF (Table 1).
The top reasons for not seeing cardiology care in the past 5 years were not knowing they needed to see a cardiologist (48%) and feeling well (43%; Figure 1). In addition, 24% with severe and 39% with nonsevere CHD reported that a doctor told them they no longer needed to see a cardiologist and 18% and 6%, respectively, reported insurance issues as barriers to seeing a cardiologist (both p <0.05).
Conclusions
The recent estimates of LTF, based on single centers and 1 nonclinic cohort, are 26% internationally (Canada, United States, Belgium, Sweden) and 34% in the United States.5 Our estimates of LTF were standardized to the CH STRONG eligible population and likely more generalizable to adults with CHD than clinic-based data. Half of our sample were LTF, according to recommendations for their specific CHD, and more than 45% had not received cardiology care in over 5 years. Of those who received care, only 1 in 3 saw an ACHD physician at their last encounter.
Individuals with CHD commonly leave cardiac care during adolescence and, for those who return, the median gap in CHD care is 10 years.6 The receipt of cardiac care in the previous 5 years was highest for the youngest (aged 19 years) and oldest (aged 38 years) participants. We observed an increasing trend in ACHD care with age, but an equal or greater percentage of those aged 35–38 years received their care from an adult cardiologist without CHD training, despite the guidelines specifying transition to an ACHD cardiologist. Within the 3 CH STRONG sites, there are between 1 – 3 million patients per ACHD physician, and this shortage of board-certified ACHD physicians will increase as the ACHD population in the United States conitnues to grow.7
CHD severity is a predictor for staying in cardiac care and people with less complex lesions are at increased risk for LTF, mirroring our findings.1,5 Biologic gender was not associated with LTF in our analyses; however, it modified other associations with LTF. Studies found mixed results for socioeconomic associations with LTF, and we did not find associations between race/ethnicity, delay of care because of cost, or employment and LTF.2,8,9 Associations between type of cardiologist last seen and LTF are mixed;2,8,9 we did not find an association. A study identified the lack of or change in insurance contributing to LTF,1 similar to our findings, while another did not.2
The strengths of this manuscript lie in the community-based CH STRONG cohort. Clinic samples are likely skewed toward individuals who have transitioned and may underrepresent those LTF early in adulthood. The limitations include categorical responses for last cardiology visit not always aligning with the guidelines. Our data are based on self- or proxy report and prone to recall issues. Potential for response bias is present so our LTF estimate was standardized to account for this.
Only half of respondents report doctors discussing the need for cardiac follow-up. Not knowing they needed to see a cardiologist, being told they no longer needed cardiology care, and feeling “well” were the top reasons for LTF. Patients with ACHD discussing the need for cardiology care with physicians were 3 times more likely to stay in care.10 Starting in childhood, cardiologists and primary care providers may increase awareness and prevent LTF by discussing need for cardiac follow-up with their patients.
This work is funded by a grant through the Centers for Disease Control and Prevention (Atlanta, Georgia) and the March of Dimes (Arlington, Virginia), 5U38OT000199 and U38O2000199. This analysis has been replicated by Amanda Dorsey.
Abbreviations:
- lifelong care
lifelong care
- Fontan
Fontan
- LTF
Lost to follow-up
- CHD
Congenital heart defects
- ACHD
adult congenital heart disease
- TOF
Tetralogy of Fallot
Footnotes
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
References
- 1.Gurvitz M, Valente AM, Broberg C, Cook S, Stout K, Kay J, Ting J, Kuehl K, Earing M, Webb G, Houser L, Opotowsky A, Harmon A, Graham D, Khairy P, Gianola A, Verstappen A, Landzberg M, Alliance for Adult Research in Congenital Cardiology (AARCC) and Adult Congenital Heart Association. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART-ACHD (The Health, Education, and Access Research Trial). J Am Coll Cardiol 2013;61:2180–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kollengode MS, Daniels CJ, Zaidi AN. Loss of follow-up in transition to adult CHD: a single-centre experience. Cardiol Young 2018;28: 1001–1008. [DOI] [PubMed] [Google Scholar]
- 3.Farr SL, Klewer SE, Nembhard WN, Alter C, Downing KF, Andrews JG, Collins RT, Glidewell J, Benavides A, Goudie A, Riehle-Colarusso T, Overman L, Riser AP, Oster ME. Rationale and design of CH STRONG: congenital Heart Survey To Recognize Outcomes, Needs, and well-being. Am Heart J 2020;221:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139: e698–e800. [DOI] [PubMed] [Google Scholar]
- 5.Moons P, Skogby S, Bratt EL, Z€uhlke L, Marelli A, Goossens E. Discontinuity of cardiac follow-up in young people with congenital heart disease transitioning to adulthood: a systematic review and meta-analysis. J Am Heart Assoc 2021;10:e019552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeung E, Kay J, Roosevelt GE, Brandon M, Yetman AT. Lapse of care as a predictor for morbidity in adults with congenital heart disease. Int J Cardiol 2008;125:62–65. [DOI] [PubMed] [Google Scholar]
- 7.Ezzeddine FM, Moe T, Ephrem G, Kay WA. Do we have the ACHD physician resources we need to care for the burgeoning ACHD population? Congenit Heart Dis 2019;14:511–516. [DOI] [PubMed] [Google Scholar]
- 8.Mackie AS, Rempel GR, Rankin KN, Nicholas D, Magill-Evans J.Risk factors for loss to follow-up among children and young adults with congenital heart disease. Cardiol Young 2012;22:307–315. [DOI] [PubMed] [Google Scholar]
- 9.Norris MD, Webb G, Drotar D, Lisec A, Pratt J, King E, Akanbi F, Marino BS. Prevalence and patterns of retention in cardiac care in young adults with congenital heart disease. J Pediatr 2013;163:902–904. e1. [DOI] [PubMed] [Google Scholar]
- 10.Skogby S, Goossens E, Johansson B, Moons P, Bratt EL. Qualitative study of facilitators and barriers for continued follow-up care as perceived and experienced by young people with congenital heart disease in Sweden. BMJ Open 2021;11:e049556. [DOI] [PMC free article] [PubMed] [Google Scholar]


