Abstract
The appearance of multidrug-resistant bacteria and the formation of bacterial biofilms have necessitated the development of alternative antimicrobial therapeutics. Antibiotics conjugated with or embedded in nano-drug carriers show a great potential and advantage over free drugs, but the mass proportion of carriers generally exceeds 90% of the nano-drug, resulting in low drug loading and limited therapeutic output. Herein, we fabricated a nanocarrier using antibiotics as the building blocks, minimizing the use of carrier materials, significantly increasing the drug loading content and treatment effect. Firstly, we conjugated betaine carboxylate with ciprofloxacin (CIP) through an ester bond to form the amphiphilic conjugate (CIP-CB), which self-assembled into micelles (CIP-CBMs) in aqueous solutions, with a CIP loading content as high as 65.4% and pH-induced surface charge reversal properties. Secondly, a model photosensitizer (5, 10, 15, 20-tetraphenylporphyrin (TPP)) was encapsulated in CIP-CBMs, generating infection-targeted photodynamic/antibiotic combined nanomedicines (denoted as TPP@CIP-CBMs). Upon accumulation at infection sites or in deep bacterial biofilms, the ester bond between the betaine carboxylate and CIP is cleaved to release free TPP and CIP, leading to a synergetic antibacterial and antibiofilm activity in vitro and in vivo.
Keywords: Antibiotics based micelles, Chemo-photodynamic therapy, Antibiofilm, pH responsive, Multidrug-resistant
Graphical abstract
1. Introduction
Bacterial infections threaten human health and safety around the world. Millions of people suffered from bacterial infections each year, which caused high mortality and huge economic losses. This situation has been greatly alleviated by the discovery of antibiotics and their widespread biomedical applications [1,2]. However, the emergence of multidrug-resistant (MDR) bacteria due to antibiotic misuse exacerbated bacterial infections [3]. MDR bacteria are reported to cause nearly one million deaths per year; by 2050, they are expected to cause 10 million deaths and up to $100 trillion in economic losses annually [4]. At present, the development of new antibiotics is far slower than the evolution of drug-resistant bacteria [5,6]. The problem is further amplified when bacteria form biofilms, where the extracellular polymeric substances in the biofilm will prevent the penetration of antibiotics and shield the immune response [7,8]. Therefore, further improvement of the killing efficiency of current antibiotics against MDR bacteria and their deep penetration into matured bacterial biofilm are the key to prolonging clinical applications of conventional antibiotics.
The exploration of drug delivery systems (DDS) with reduced doses and enhanced bioavailability of antibiotics is as important as the development of new antibiotics in combating MDR bacteria and bacterial biofilms [9]. In the past decades, a wide variety of DDS, such as polymeric nanogels, vesicles, liposomes and micelles have been developed and applied in the treatment of bacterial infections [10], [11], [12], [13]. Among them, polymeric micelles have been the most widely used drug delivery platforms due to their outstanding advantages, including passive bacteria targeting, minimal side effects, enhanced drug solubility and improved pharmacokinetics. Nevertheless, polymer carriers, which are the major component of nanomedicines, have no other therapeutic activity other than being delivery carriers, resulting in polymeric nanomedicines with a drug loading capacity of less than 10% [14]. In addition, the complex composition of the polymer carrier is another obstacle to their clinical application.
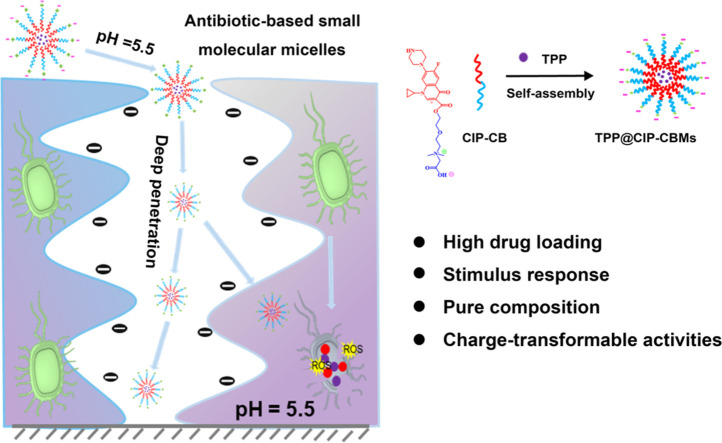
Amphiphilic small-molecules can also assemble into micelles in water with a core-shell structure similar to polymeric micelles [15]. Unlike polymers, small-molecule surfactants have a distinct and uniform chemical composition, and the spatial structure of their self-assembled aggregates can be precisely designed [16]. For example, we recently prepared dandelion flower-like micelles using surface cross-linked small molecular micelles (SCMs) as seed micelles, with spatially well-organized structures and external stimuli-triggered fast release, which showed the seed SCMs travelling a long distance [17]. Some hydrophobic antibiotics can be easily connected with hydrophilic molecules to form amphiphilic conjugates. We thus hypothesize here that the drug loading capacity can be greatly improved by using hydrophobic antibiotics as the hydrophobic part of amphiphilic conjugates. The antibiotic-based amphiphilic conjugates will self-assemble into micelles, which can accumulate at infection sites and biofilms by an enhanced permeability and retention effect [18]. Unlike traditional drug carriers, which are inert and constitute the majority of the nanomedicine, hydrophobic antibiotics play two roles in the antibiotics-based micelles, i.e., drug carriers and therapeutic drugs. The hydrophobic core of the micelles can be used to encapsulate another hydrophobic therapeutic agent, e.g., a photosensitizer (PS) for photodynamic therapy (PDT), resulting in combined or synergetic antibacterial activities [19,20]. PDT is a novel technology to fight MDR bacterial infections, using reactive oxygen species (ROS) produced by the PS [21]. However, the poor water-solubility, bad biocompatibility and non-selectivity of PSs limit the clinical application of PDT. In addition, the lifetime of ROS is very short (3.5 µs), and their distance of diffusion in aqueous solutions is usually less than ca. 100 nm, which greatly reduces the therapeutic effect of PDT on biofilms, resulting in failure to treat bacterial infections [22]. Therefore, encapsulating PSs in carriers with infection-targeting and deep biofilm penetration activities will greatly enhance the therapeutic output of PDT and reduce its systemic toxicity. ROS can oxidatively damage the cell membrane and cell wall of the bacteria, resulting in an increased permeability of the bacterial cells [23]. Thus, a synergetic antibacterial and antibiofilm therapy would be realized by encapsulating a PS in antibiotic-based micelles that mainly target internal structures of the bacterium, such as bacterial nucleic acid and protein synthesis machinery [24,25].
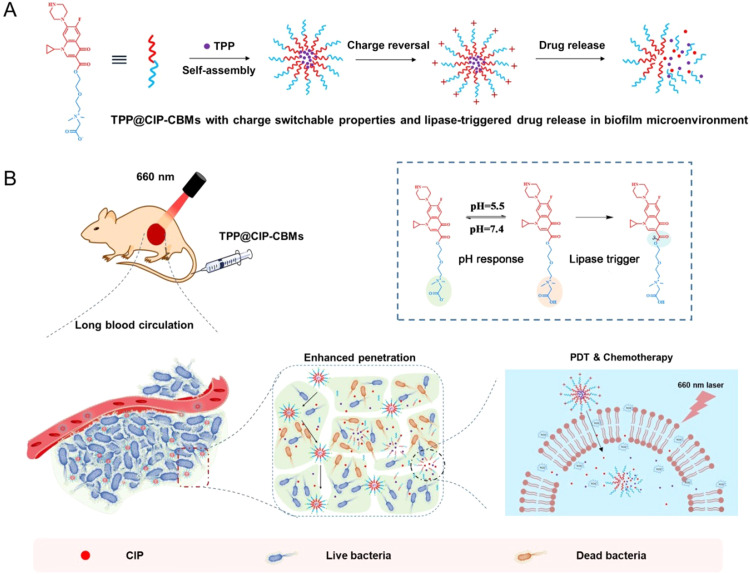
As a proof of concept, we conjugated betaine carboxylate with ciprofloxacin (CIP) through an ester bond to form the amphiphilic conjugate (CIP-CB), which was self-assembled into micelles with pH-induced charge reversal properties (CIP-CBMs) [13]. A model PS (5, 10, 15, 20-tetraphenylporphyrin (TPP)) was encapsulated in the CIP-CBMs, resulting in infection-targeted PDT/antibiotic combined nanomedicines (denoted as TPP@CIP-CBMs). A negative surface charge of the TPP@CIP-CBMs in a normal physiological environment (pH 7.4) could facilitate a long circulation of nano-drugs in the blood (Scheme 1). When reaching sites of bacterial infection, the TPP@CIP-CBMs present a net positive surface charge as a result of the protonation of betaine carboxylate in acidic microenvironments of the infection [26,27]. The positive surface charge facilitates both an enhanced electrostatic interaction between the micelles and the bacteria cells and their deep penetration into matured biofilms [28]. Light irradiation on the infection sites triggers the production of ROS, which damage the cell membrane of bacteria, resulting in bacterial inactivation and enhanced antibiotic penetration into the bacterial cells. Furthermore, overexpressed lipase at the bacterial infection sites induces hydrolysis of the micelles and the release of CIP against drug-resistant bacteria and the eradication of mature biofilms.
Scheme 1.
(A) The construction of photosensitizer-encapsulated micelles (TPP@CIP-CBMs) with pH and lipase response. (B) Schematic illustration of surface charge switchable photodynamic-chemotherapy for bacterial infections using TPP@CIP-CBMs, and the electrostatic interaction between TPP@CIP-CBMs and bacterial cells, their lipase-triggered drug release and photodynamic chemotherapy bactericidal properties.
2. Materials and methods
2.1. Materials
2-[2-(Dimethylamino) ethoxy] ethanol (DMAEE) was purchased from Aladdin. Ciprofloxacin (CIP), di‑tert‑butyl decarbonate (Boc2O), tert‑butyl bromoacetate, 5, 10, 15, 20-tetraphenylporphyrin (TPP), 2-(1H-benzotriazole-1-yl)-1, 1, 3, 3-tetramethyluronium tetrafluoroborate (TBTU) and Nile Red were obtained from Huaxia Reagent. N,N-Diisopropylethylamine (DIPEA) was obtained from Best-Reagent. Dioxane, sodium hydroxide (NaOH), potassium carbonate (K2CO3) and dichloromethane (CH2Cl2) were obtained from Zhiyuan Reagent. Trifluoroacetic acid (TFA), diethyl ether, acetone, 1,3-diphenylisobenzofuran (DPBF), trypticase soy liquid medium (TSB, AOBOX, Hengshui), trypticase soy agar medium (TSA, AOBOX, Hengshui) were obtained from Hengli Reagent. A LIVE/DEAD BacLight bacterial viability kit (L-7012) was obtained from Invitrogen. The bacterial strains of Staphylococcus aureus (S. aureus, ATCC 43,300), Escherichia coli (E. coli, ATCC 35,218), Methicillin-resistant Staphylococcus aureus (MRSA, ATCC 43,300) and Extended spectrum beta-lactamase (ESBL) producing E. coli (EBSLE. coli, ATCC 51,299) were purchased from Guangdong Microbiology Culture Center.
2.2. Preparation and characterizations of CIP-CBMs
20 mg of CIP-CB were added in 20 ml PBS (pH 7.4), and then the above mixture was ultrasonically treated for 120 s to obtain a uniformly distributed CIP-CB micelles (CIP-CBMs, 1 mg/ml). The zeta potential and size distribution of the CIP-CBMs was measured at different pH values by a Nano-ZS 90 Nanosizer. Repeat the experiment at least three times. The morphology of micelles was obtained by transmission electron microscope (TEM) (Tecnai G2F20S-TWIN instrument, 120 kV). The critical micelle concentration (CMC) was obtained by a Fluorescence spectrometer (HORIBA, Japan).
2.3. Drug loading and drug release
TPP was entrapped into CIP-CBMs by the following method: 10 mg TPP were added in 10 ml methylene chloride, and the methylene chloride in the flask is removed by rotary evaporator. A film is formed at the bottom of the flask. Then 20 ml PBS (pH 7.4) with 20 mg CIP-CB was added with magnetic stirring to result TPP loaded CIP-CBMs (TPP@CIP-CBMs). The excess TPP was removed using a filter (0.45 µm). TPP concentration in the solution was measured by a UV spectrophotometer (UnicamUA500, Thermoelectronics Corporation). The drug loading (DL) content were obtained by the following equations: DL (%) = WTPP/WCP × 100%, Where WTPP was the weight of TPP in the nanodrug TPP@CIP-CBMs, Wcp was the weight of nanocomposite TPP@CIP-CBMs.
2.4. In vitro drug release
The drug release of CIP and TPP in the TPP@CIP-CBMs system was obtained using a dialysis method. Briefly, the dialysis bag (MW = 1,000 Da) containing 2 ml freshly prepared dispersions was immersed in 20 mM buffer solution at different pH values with or without 1.0 mg/ml lipase, and then were incubated at 37 °C. 2 ml buffer solution was collected and supplemented at a specific time point. The amount of CIP and TPP was calculated by high performance liquid chromatography (HPLC) (GE Waters, America) and UV–vis spectrophotometer, respectively.
2.5. 1O2 generation assay
To evaluate the ROS generation ability of TPP@CIP-CBMs, 1,3-diphenyl-isobenzofuran (DPBF) was used. DPBF (30 µl, 9 mM) was added into TPP@CIP-CBMs solution (3 ml, 200 µg/ml, CIP doses) in PBS with or without 1.0 mg/ml lipase. The mixture was added to a quartz cuvette and irradiated by a 50 mW/cm2 laser (660 nm) for 35 min. The 1O2 generated from laser irradiated TPP@CIP-CBMs will consume DPBF which has a characteristic adsorption at ∼415 nm. Therefore, the UV–vis spectrum absorbance of DPBF at 415 nm was determined every 5 min.
2.6. In vitro antibacterial test
Gram-positive S. aureus (ATCC 29,213), Gram-negative E. coli (ATCC 35,218), drug-resistant bacteria ESBL E. coli (ATCC 51,299) and MRSA (ATCC 43,300) were used for antibacterial activity test. The antibacterial activities of the TPP@CIP-CBMs were determined by minimal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). Briefly, the bacteria were coated on agar plates and cultured for 24 h at 37 °C, and then incubated in trypticase soy liquid medium (TSB) about 12 h. Finally, a bacterial suspension (3 × 108 CFU/ml) was obtained. Firstly, the TPP@CIP-CBMs was put under a UV light to sterilize for 2 h. Secondly, 150 µl TPP@CIP-CBMs suspensions were added to 50 µl bacterial dispersions (1 × 105 CFU/ml) with a 50 mW/cm2 laser irradiation (660 nm) for 20 min, which was then incubated for 16 h. The MIC values were taken as the lowest concentration (CIP doses) at which bacterial growth remained absent. After being incubated at 37 °C for 16 h, the MBC values were determined by no visible growth of bacteria on LB agar plates, and the lowest concentration (CIP doses) at which colony formation was absent. Repeat the experiment at least three times.
2.7. TEM observation of nanomedicine-bacteria interaction
In order to verify the interaction between TPP@CIP-CBMs and bacteria, the morphological changes of bacteria before and after treatment with TPP@CIP-CBMs were studied by TEM. Firstly, the bacteria were purified. And then TPP@CIP-CBMs (1 ml, 200 µg/ml) and 2.0 ml bacteria solution were added to a centrifuge tube. After irradiated by laser (660 nm, 50 mW/cm2) for 20 min, the solution was cultivated in a thermostatic incubator for 8 h at 37 °C. The solution was centrifuged for 30 min at 4,000 rpm, and then redispersed in 2 ml buffer solution. Buffer solution under the same conditions was treated in the same way as the control group. 2.5% glutaraldehyde was added to the solutions of the two groups to fix the bacterial morphology at 4 °C [29].
2.8. Antibiofilm activities in vitro
The S. aureus and MRSA grow on Fisher cover glass and form biofilm. Firstly, Fisher brand cover slides were incubated in a 1 ml (1 × 106 CFU/ml) suspension of S. aureus and MRSA at 37 °C for 7 d The unbound planktonic bacteria were removed a with sterile PBS buffer. The 7-day-old S. aureus biofilms were exposed to the buffer solution (pH 5.5 or 7.4, 200 µl) of CIP-CBMs and TPP@CIP-CBMs at varied CIP concentrations (6.25–100 µg/ml) for 24 h. Free CIP, CIP-CBMs/TPP mixtures (named as CIP-CBMs+TPP) with the same CIP and/or TPP concentrations were used as comparisons. After incubation for 24 h, the biofilms were irradiated by near-infrared (NIR) laser for 20 min (660 nm, 50 mW/cm2). Subsequently, bacterial suspension was removed, the remaining biofilms were washed with sterilized physiological saline several times and dispersed into 1 ml PBS by ultrasound to quantify by plate counting. Confocal laser scanning microscopy (CLSM, Olympus FV1000, Japan) was used to observe the viability of bacteria in the residual biofilms and the live and dead bacteria were labeled by live/dead backlight bacterial viability kit (L-7012, Invitrogen) as described previously [30]. At last, in another batch of experiments, scanning electron microscopy (SEM) was also used to observe the morphology of residual biofilm [31].
2.9. Crystal violet staining assay
The 7-day-old MRSA biofilms for 7 d were exposed to 200 µl CIP-CBMs and TPP@ CIP-CBMs for 24 h at varied CIP concentrations from 6.25 µg/ml to 100 µg/ml. Free CIP, CIP-CBMs/TPP mixtures (named as CIP-CBMs+TPP) with the same CIP and/or TPP concentrations were used as comparisons. After 24 h, the biofilms were irradiated by NIR laser (660 nm, 50 mW/cm2) for 20 min. Next, bacterial liquid was discarded and the residue biofilms were washed with sterilized PBS (pH 7.4) to remove planktonic bacteria. The residual biofilm was dyed with 0.1% crystal violet for 10 min to evaluate the dispersion activity of the micelles. Finally, the biofilms were rinsed with water until it becomes colorless. Crystal violet was dissolved with ethanol (80%, v/v), and the residual biofilms quantified at 590 nm by enzymolysis assay (Biotek, China).
2.10. Bacterial dispersal after treatment of TPP@CIP-CBMs and laser irradiation
According to published literature [32], the release of live bacteria from biofilms in all groups with different treatments was evaluated by determining the numbers of CFU in the supernatants. In brief, before and after 10 min of treatment with different formulations, the supernatants of biofilm-containing slides were collected and serially diluted in PBS. Then the corresponding bacterial dilutions were plated on LB agar plates, and then cultivated at 37 °C for 24 h to count the numbers of CFU.
2.11. Penetration of CIP-CBMs into biofilms
To study the penetration of nanomaterials into mature bacterial biofilms, Nile red loaded CIP-CBMs were obtained using by the similar thin-film hydration method as that of preparing TPP@CIP-CBMs [33]. SYTO-9 (30 µl) was used to label bacterial cells in biofilms for 15 min at 4 °C. After stirring overnight, the biofilms were cultured with Nile red loaded CIP-CBMs for 2 h. Finally, the biofilm was washed twice with saline, and then observed by CLSM.
2.12. Biocompatibility
The hemolytic test was used to evaluate the blood compatibility. The specific steps were as follows: eyeballs were used to obtain fresh blood stabilized with heparin sodium, 3 ml fresh blood was added to 6 ml buffer solution (pH 7.4), centrifuged for 15 min at 1,500 rpm, and the supernatant containing serum, ruptured red blood cells and other substances affecting the experimental results was removed. The samples in each group were diluted with PBS (pH 7.4), and the concentrations were 12.5, 25, 50, 100 and 200 µg/ml, with PBS (pH7.4) as the negative control group and deionized water as the positive control group. 200 µl (8%) red blood cell suspension was added to each sample and incubated in orbital shaker at 37 °C for 2 h. Subsequently, the supernatant was collected by centrifugation at 1,500 rpm, its absorbance at 570 nm was detected by enzyme marker. The percentage hemolysis of the RBCs was obtained by the following equation: Hemolysis (%) = (Asample − Anegative)/(Apositive − Anegative) × 100%.Repeat the experiment at least three times.
2.13. Cell viability assay
Firstly, 3T3 fibroblasts were used as model cells and cultured in DMEM medium containing 100 µg/ml streptomycin, 100 µg/ml penicillin and 10% (v/v) fetal bovine serum (FBS). The whole culture process was carried out in the Japanese Sanyo McO-18aic cell incubator at 37 °C, containing 5% CO2. Secondly, 3T3 fibroblasts (6,000 cells per well) were inoculated on 96-well plates. The original medium was replaced with 200 µl CIP, CIP-CBMs and TPP@CIP-CBMs with different concentrations after 24 h of culture. And then the cells were incubated for 24 h with 5% CO2 at 37 °C. After treatment, each well was filled with 10 µl MTT solution (5 mg/ml). The medium was removed after 4 h treatment, and each well was filled with 150 µl DMSO to avoid light. After 10 min, the absorbance at 545 nm was measured using a microplate reader (Ultraspec 2000, Pharmacia Biotech).
2.14. In vivo pharmacokinetic study
Animal Ethics Committee in West China Hospital, Sichuan University, Chengdu, China, approved the protocols for mice experiments and the approval number of in vivo experiment is 20220307004. In order to study the serum pharmacokinetics of CIP, TPP, CIP-CBMs and TPP@CIP-CBMs, Kunming mice (male, ∼20 g) were randomly divided into four groups, with free access to water and food. The drug (CIP 20 mg/kg) and saline were injected via the tail vein. Blood samples were collected from the eyes of the mice at predetermined time points, and immediately centrifuged at 4 °C (3,000 g). Then, the solutions were added to acetonitrile to precipitate protein for 1 min. Following further centrifugation (10,000 rpm, 10 min), the concentration of CIP and TPP in the supernatant was detected by a HPLC system.
2.15. Antibacterial experiment in vivo
The mice wound infection model was used for evaluating the antibacterial effect of TPP@CIP-CBMs in vivo. Kunming mice (20–25 g) were obtained from Chengdu Dossy Experimental Animals. MRSA was employed to form the wound-infecting skin due to the common cause of skin infection. The bacterial infection model was established by subcutaneously inoculating 3 × 108 CFU/ml MRSA into the right side of the back of the mice. After 24 h treatment, the mice were infected, and then the drugs were injected via the tail vein. Using PBS as a control, samples with a concentration of 200 µg/ml were injected. PBS+NIR, CIP+NIR, TPP@CIP-CBMs+NIR groups were irradiated with NIR for 10 min after 5 h drug injection (660 nm, 50 mW/cm2) [34]. After 7 d continuous treatment, the drug was stopped and observed until the wound of the mice was completely healed. The wound healing of the mice and the weight of the mice were monitored every day. After the wound was completely healed, the wound tissue was excised, diluted in 15 ml normal saline, and counted on a plate. Hearts, livers, lungs, spleens and kidneys of mice were removed for hematoxylin and eosin (H&E) staining analysis.
2.16. Statistical analysis
All the measurement data are the average value and average standard deviation (SD) of three different test results. Student's t-test was used to assess the statistical significance, and the significance levels were as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
3. Results and discussion
3.1. Preparation and characterization of CIP-CBMs and TPP@CIP-CBMs
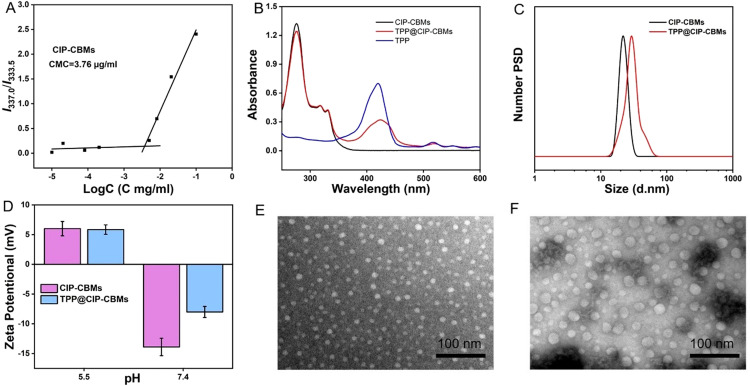
In this study, an amphiphilic conjugate was synthesized using a four-step process, as shown in Scheme S1. The amine group of CIP was protected by di‑tert‑butyl decarbonate, which resulted in Boc-CIP. The carboxyl group of Boc-CIP was reacted with the hydroxyl group of 2-[2-(dimethylamino) ethoxy] ethanol (DMAEE) by esterification, to form Boc-CIP-DMAEE. The tertiary amine of Boc-CIP-DMAEE was quaternized by tert‑butyl bromoacetate (Boc-CIP-CB-tBu). Finally, CIP-CB was obtained by removing the tert‑butyl ester groups of Boc-CIP-CB-tBu. The chemical structure of CIP-CB and all intermediates were characterized by 1H and 13C NMR spectra and high resolution ESI-MS (Figs. S1–S4). CMC of CIP-CB was determined to be 3.76 µg/ml by fluorescence spectroscopy using pyrene as the fluorescent probe (Fig. 1A) [35]. At concentrations higher than the CMC, the amphiphilic conjugate CIP-CB self-assembled into micelles (CIP-CBMs) in aqueous solutions. TPP was encapsulated into the CIP-CBMs by the thin-film hydration method, which resulted in TPP@CIP-CBMs. A characteristic UV–vis adsorption at 425 nm, corresponding to TPP, was observed in the UV–vis spectrum of the TPP@CIP-CBMs (Fig. 1B), suggesting successful loading of TPP. The size and ξ potential of the CIP-CBMs and TPP@CIP-CBMs were measured by dynamic laser scattering (DLS) at various pH values. The hydrodynamic diameter of CIP-CBMs and TPP@CIP-CBMs determined by DLS were about ∼20 nm and ∼30 nm with uniform size distribution, respectively (Fig. 1C). The ξ potentials of the CIP-CBMs and TPP@CIP-CBMs were pH-dependent (Fig. 1D). At physiological conditions (pH 7.4), the CIP-CBMs and TPP@CIP-CBMs showed a net negative charge (∼−15 mV for CIP-CBMs and ∼−8 mV for TPP@CIP-CBMs) as a result of carboxyl deprotonation. The negative surface charge of the CIP-CBMs and TPP@CIP-CBMs will help to prolong blood circulation time [36]. In contrast, in acidic environments a net positive surface charge was found for CIP-CBMs and TPP@CIP-CBMs as a result of carboxyl protonation. An acidic microenvironment is usually found at bacterial biofilms of infection sites, and the two micelles would be expected to target infectious sites and deeply penetrate into bacterial biofilms [37,38]. The morphology and size of the CIP-CBMs and TPP@CIP-CBMs were also observed by TEM micrographs. The spherical micelles with a relatively uniform size distribution of ∼15 nm and ∼20 nm were observed for CIP-CBMs and TPP@CIP-CBMs, respectively, which is smaller than the diameter measured by DLS, as a result of the dehydrated state of the micelles (Fig. 1E and F). The CIP-CBMs and TPP@CIP-CBMs had good stability during long-term storage in biocompatible solution. As shown in Fig. S5, the particle sizes of the both CIP-CBMs and TPP@CIP-CBMs were uniformly maintained in 10% FBS solution over an incubation time of 48 h.
Fig. 1.
Characterization of CIP-CBMs and TPP@CIP-CBMs. (A) CMC of CIP-CB; (B) UV–vis absorption spectra; (C) hydrodynamic diameter distributions; (D) zeta potentials at different pH values (pH 7.4 and 5.5); TEM images of (E) CIP-CBMs and (F) TPP@CIP-CBMs (scale bar: 100 nm).
3.2. Drug release and ROS production capacity of TPP@CIP-CBMs
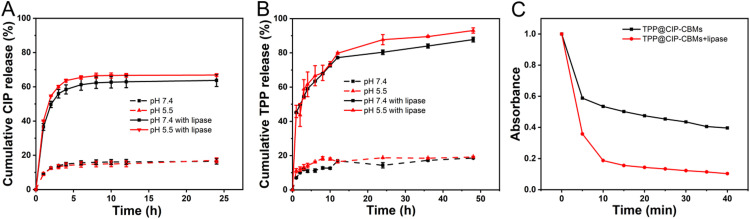
The CIP content of the CIP-CBMs was about 65.4% (w/w), which was much higher than that loaded in inert carriers [39]. TPP was encapsulated in the CIP-CBMs with a loading capacity of 9.0% (w/w) and an encapsulation efficiency of 74.2%. In the presence of lipase, the ester bond between the betaine carboxylate and CIP will be cleaved, causing the disassembly of the micelles and the release of CIP and TPP. The release profiles of CIP and TPP over time were analyzed with or without lipase, under pH 5.5 or pH 7.4. As shown in Fig. 2A and B, the CIP and TPP release were inhibited significantly under physiological or acidic conditions without lipase, and about 15% of CIP and 18% of TPP being released during 25 h of incubation. In contrast, CIP release was accelerated significantly in the presence of lipase, which also caused the collapse of the micelles, resulting in the burst release of encapsulated TPP.
Fig. 2.
(A) CIP release profile from CIP-CBMs and (B) TPP release from TPP@CIP-CBMs under different pH values (pH 5.5 and 7.4), with or without lipase, at 37 °C; (C) the change in absorption at 415 nm of DPBF solutions with TPP@CIP-CBMs nanoparticles upon irradiation at 660 nm, with or without lipase.
1,3-Diphenylisobenzofuran (DPBF), which can consume ROS generated by TPP@CIP-CBMs under 660 nm laser light irradiation (50 mW/cm2, 660 nm), was used as a 1O2 indicator [40]. DBPF and TPP@CIP-CBMs alone displayed no obvious UV–vis adsorption decrease under light irradiation, as a result of their good photostability. However, a mixture of DBPF and TPP@CIP-CBMs showed a rapid decrease in the absorbance of DPBF, suggesting an increased ROS generation by irradiation. Notably, the presence of lipase caused a further decrease in the absorbance of DPBF, indicating an increased ROS generation. This is attributed to lipase responsiveness of the TPP@CIP-CBM, which could increase ROS production by releasing TPP from the micelles (Fig. 2C). Lipase is widely present in microbial flora because it participates in bacterial lipid metabolism [24,41]. Thus, PSs encapsulated into CIP-CBMs could be transported into infection sites due to the acid-induced surface charge reversable properties of the CIP-CBMs. TPP can then be released, triggered by overexpressed lipase, which will increase ROS production and enhance biocidal efficiency under light irradiation.
3.3. The antibacterial activity of TPP@CIP-CBMs
S. aureus (Gram positive bacteria), E. coli (Gram negative bacteria), and their drug resistant strains, i.e., MRSA and ESBL E. coli, were selected as model bacteria to study the antibacterial activities of CIP-CBMs and TPP@CIP-CBMs. The MIC and MBC values are showed in Table 1, and the corresponding test photos are listed in Fig. S6. The CIP-CBMs displayed lower MIC and MBC values than free CIP, especially in acidic solutions (pH 5.5), due to the enhanced static electronic interactions between CIP-CBMs and bacterial cells. Notably, TPP-loaded CIP-CBMs showed further enhanced biocidal activities against all the tested strains compared to CIP-CBMs, both in neutral and acidic conditions. We encapsulated TPP into surface charge reversible CIP-CBMs to deliver TPP to bacterial cell surfaces, which can resolve the main problems of ROS in their disinfection process: the very short lifetime and limited diffusion length in aqueous solution. In order to verify that the encapsulation of TPP into CIP-CBMs was essential for the synergetic antibacterial activities of PDT and chemotherapy, the same amount of TPP was mixed with CIP-CBMs (denoted as TPP+CIP-CBMs) and their MIC and MBC were evaluated as for the TPP@CIP-CBMs. As shown in Table 1, the TPP+CIP-CBMs showed higher MIC and MBC values than those of the TPP@CIP-CBMs, suggesting the decreased antibacterial efficiency of TPP+CIP-CBMs. Furthermore, CIP-CBMs and TPP@CIP-CBMs were mixed with S. aureus, irradiated with a laser, and the morphological changes of the bacterial cell were observed by TEM. The morphologies of bacterium before treatment with CIP-CBMs and TPP@CIP-CBMs solution were structurally complete (Fig. 3). However, after culturing with TPP@CIP-CBMs and irradiating with a laser, the bacterial outer membrane was damaged and bacterial cytoplasm had leaked, compared with the CIP-CBMs. The results suggest that CIP-CBMs can deliver TPP to the bacterial surface, and the light irradiation triggers the production of ROS, which damages the bacterial cell wall and cell membrane, resulting in bacterial inactivation and enhanced antibiotic penetration into the bacterial cells [20,29].
Table 1.
MIC and MBC of different groups against E. coli, S. aureus, ESBLE. coli and MRSA.
| Sample* | pH | MIC (CIP µg/ml) |
MBC (CIP µg/ml) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| E. coli | S. aureus | ESBLE. coli | MRSA | E. coli | S. aureus | ESBLE. coli | MRSA | ||
| CIP | – | 25 | 25 | 100 | 100 | 50 | 50 | 200 | 100 |
| CIP-CBMs | 7.4 | 6.25 | 12.5 | 50 | 50 | 12.5 | 12.5 | 50 | 100 |
| 5.5 | 3.12 | 6.25 | 25 | 50 | 6.25 | 6.25 | 25 | 50 | |
| TPP + CIP-CBMs | 7.4 | 3.12 | 6.25 | 50 | 50 | 6.25 | 6.25 | 50 | 100 |
| 5.5 | 1.56 | 3.12 | 12.5 | 25 | 3.12 | 6.25 | 25 | 50 | |
| TPP@CIP-CBMs | 7.4 | 1.56 | 3.12 | 6.25 | 12.5 | 3.12 | 6.25 | 6.25 | 25 |
| 5.5 | 0.78 | 1.56 | 3.12 | 12.5 | 1.56 | 3.12 | 3.12 | 12.5 | |
All MIC and MBC values refer to CIP concentrations.
Fig. 3.
The TEM images of MRSA after treatment with PBS, CIP-CBMs and TPP@CIP-CBMs (dark or light).
3.4. Antibiofilm activities of TPP@CIP-CBMs
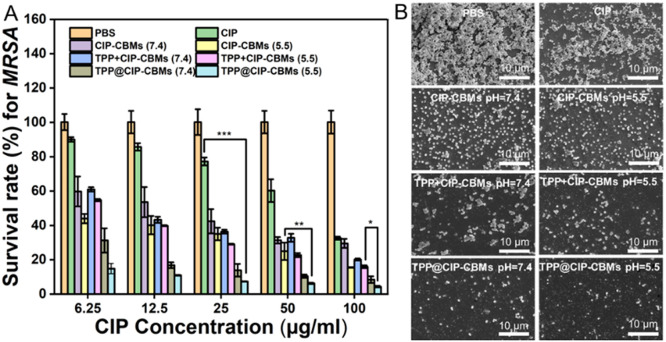
The formation of bacterial biofilm would further enhance bacterial tolerance to antibiotics and even antibacterial nanoparticles [42]. Therefore, we subsequently explored whether the nanoparticles could penetrate and remove the MRSA and S. aureus biofilms (Figs. 4 and S7–S11). First, 7-day-old mature MRSA biofilms were exposed to suspensions of CIP-CBMs and TPP@CIP-CBMs, with varied CIP concentrations, for 24 h. The biofilms were subsequently irradiated with a laser for 20 min. The standard plate counting assay, crystal violet staining assay, SEM observation, and the live/dead staining assay were used to study the antibiofilm activities of CIP-CBMs and TPP@CIP-CBMs. After treatment with different concentrations of CIP-CBMs and TPP@CIP-CBMs, Fig. S10 showed the photograph of the agar plates, and Fig. 4A showed the number of MRSA bacteria, Free CIP and TPP+CIP-CBMs were used as controls. All samples displayed dose dependent antibiofilm activities. Free CIP at 100 µg/ml destroyed about 68% of bacteria embedded in the biofilm, while the CIP-CBMs killed 85% of bacteria at the same CIP concentration in an acidic environment, indicating the enhanced antibiofilm activities of CIP-CBMs. Notably, TPP encapsulated CIP-CBMs (TPP@CIP-CBMs) killed 90% of bacteria embedded in the biofilm with a CIP concentration of 50 µg/ml in a neutral environment. The antibiofilm efficiency of TPP@CIP-CBMs was further enhanced in an acidic solution, with 96% of bacteria in the biofilm being killed at the same CIP dosage. The crystal violet staining was used to evaluate the total amount of biofilm biomass, which was in good agreement with that observed in colony assay methods (Fig. S11). We ascribed the enhanced antibiofilm activities of the TPP@CIP-CBMs to the synergetic antibiofilm activities of PDT and chemotherapy. Considering the short lifetime and limited diffusion length of ROS in aqueous solution [43], we hypothesized that the encapsulation of a PS in surface charge transformable micelles could increase the penetration depth of the PS in mature biofilms, for excellent antibiofilm outcomes of PDT. To verify this hypothesis, firstly, Nile red-loaded CIP-CBMs were used to study the penetration depth of the CIP-CBMs in mature biofilms. At acidic conditions (pH 5.5), an intense red fluorescence was found throughout all the biofilms, while much less red fluorescence was found at neutral conditions (pH 7.4), presumably as a result of pH-triggered charge transforming activities (Fig. S12) [44]. The deep penetration of CIP-CBMs into the biofilms may improve the antibiofilm outcomes of PSs encapsulated in micelles.
Fig. 4.
In vitro elimination properties of nanomaterials against MRSA biofilms. (A) The survival rate of MRSA after treatment with different groups. (B) SEM images of MRSA biofilm after treatment with different groups. The CIP concentration of CIP, CIP-CBMs, TPP + CIP-CBMs, and TPP@CIP-CBMs was 100 µg/mL (scale bar: 10 µm). *P < 0.05, **P < 0.01, ***P < 0.001.
Furthermore, TPP/CIP-CBMs mixtures with the same amount of TPP and CIP as the TPP@CIP-CBMs were used as controls to study their antibiofilm activities. As shown in Fig. 4A and S10, the TPP+CIP-CBMs displayed inferior antibiofilm properties compared with TPP@CIP-CBMs; this suggested that the encapsulation of the PS into the surface charge transformable micelles improved the antibacterial and antibiofilm outcomes of PDT. The morphological changes of the bacterial cells and biofilms were examined by SEM. For the PBS-treated group, large bacterial aggregates were presented in the 7-day-old MRSA biofilm (Fig. 4B). Treatment with CIP-CBMs and TPP+CIP-CBMs led to an enhanced disruption in the biofilms compared to the free drug treatment. Notably, the biofilm mass was mostly eliminated, and non-aggregated wrinkled bacteria cells and severe structural destruction were found in the TPP@CIP-CBMs treated groups, as a result of the synergetic antibiofilm activities of PDT and CIP-CBMs, which was consistent with the results of plate counting (Figs. 4A and S10).
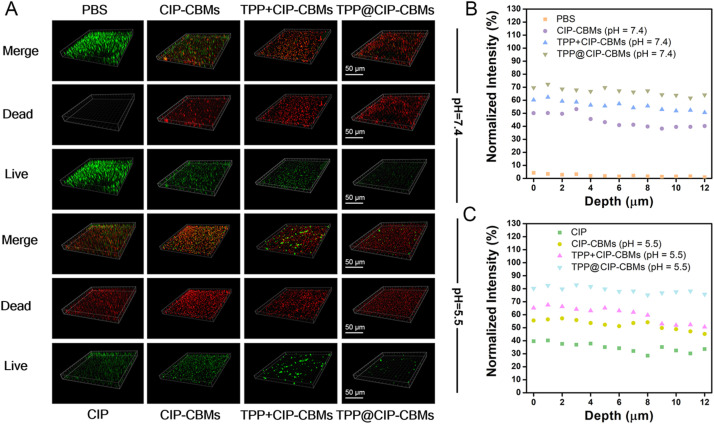
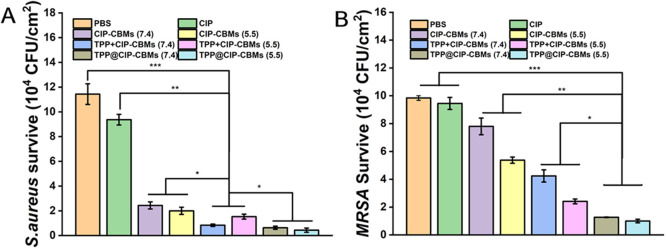
The antibiofilm activities of the TPP@CIP-CBMs were further studied using three-dimensional CLSM observation. As shown in Fig. 5, the whole biofilms with full live (green) bacteria were observed in the control group, while biofilms treated with free CIP resulted in partial cell death (red). In comparison, the CIP-CBMs and TPP+CIP-CBMs treatment groups resulted in a reduction in the number of live bacteria, especially in acidic solutions. Notably, after treatment with TPP@CIP-CBMs, most bacteria within the residual biofilms were effectively removed. Bacteria dispersed from biofilms can cause a possible spread of infection or even septic shock [45]. Therefore, before and after the different treatments, the viability of the bacteria in the supernatants was tested by the colony counting method. As expected, the number of colonies forming units (CFUs) in the supernatant was significantly reduced in the TPP@CIP-CBMs treatment compared to the other treatment and control groups, indicating that the treatment did not lead to a spread of live bacteria (Fig. 6). Notably, the TPP@CIP-CBMs group showed a much greater reduction in dispersed bacteria than the TPP+CIP-CBMs group. This was because the TPP in the TPP@CIP-CBMs group could easily penetrate and diffuse the entire biofilm, efficiently killing bacteria under NIR irradiation [32,46], while the TPP in the TPP+CIP-CBMs+NIR group could not penetrate into deep biofilm. The above results suggest that TPP@CIP-CBMs can effectively remove biofilm-encased bacteria by synergistic photodynamic and antibiotic therapy.
Fig. 5.
(A) CLSM images and (B, C) normalized intensity of red channels of MRSA biofilms co-cultured with PBS, CIP, CIP-CBMs, TPP + CIP-CBMs, and TPP@CIP-CBMs at pH 7.4 or 5.5 for 24 h at 37 °C (scale bar: 50 µm).
Fig. 6.
CFUs in the supernatants of S. aureus (A) and MRSA (B) biofilms after treatment with various formulations (n = 4).
3.5. Cytotoxicity and hemolysis
The preliminary biocompatibility of nanocomposites was investigated for biomedical applications. As shown in Fig. 7A and B, the CIP-CBMs and TPP@CIP-CBMs displayed less than 10% hemolysis at a concentration as high as 200 µg/ml, indicating the good hemocompatibility of the nanocomposites and their potential in antibiofilm therapy in vivo. In addition, after 24 h incubation with 3T3 fibroblasts, the CIP-CBMs and TPP@CIP-CBMs groups display good cytocompatibility; even at a concentration of 50 µg/ml, more than 80% live cells were found for these two micelles (Fig. 7C), presumably as a result of the negative surface charge for their high cytocompatibility [29].
Fig. 7.
Biocompatibility of TPP@CIP-CBMs. (A) Photographs after coculturing with blood cells for 2 h and centrifugation. (B) Percentage of hemolysis measured using a microplate reader. (C) Cell viability of 3T3 cells, measured by the MTT assay after treatment withCIP, CIP-CBMs and TPP@CIP-CBMs.
3.6. In vivo pharmacokinetic study
To investigate blood circulation ability of micelles, the pharmacokinetic of TPP@CIP-CBMs was evaluated in male Kunming mice prior to evaluating in vivo antibacterial activities. Briefly, Kunming mice were intravenously administered TPP, CIP, CIP-CBMs and TPP@CIP-CBMs via the tail vein. Blood samples were collected from the eyes of the mice at predetermined time points. As shown in Table 2, the pharmacokinetic parameter after drug intravenous injection was summarized. The half-life (T1/2) of the free CIP group was 1.65 h. Compared with the CIP group parameters, the CIP-CBMs and TPP@CIP-CBMs increased the T1/2 to 4.49 and 4.95 h respectively. Notably, the T1/2 of the TPP in CIP-CBMs was 4.88, up to 9-fold higher than those of the TPP group, respectively. The results suggest that the CIP-CBMs can effectively deliver TPP to the bacterial infection site, resulting in synergetic antibacterial activities of PDT and chemotherapy.
Table 2.
Pharmacokinetic parameters of mice after systemic administration of CIP, TPP, CIP-CBMs, and TPP@CIP-CBMs (mean ± SD, n = 3).
| Parametersa | TPP | CIP | CIP-CBMs | TPP@CIP-CBMs |
|
|---|---|---|---|---|---|
| TPP | CIP-CBMs | ||||
| AUC (µg/mL h) | 7.64 ± 0.23 | 215.78 ± 9.82 | 895.98 ± 22.35 | 86.53 ± 6.81 | 863.52 ± 10.51 |
| MRT (h) | 0.41 ± 0.03 | 1.42 ± 0.16 | 6.36 ± 0.27 | 6.81 ± 0.22 | 6.95 ± 0.52 |
| T1/2 (h) | 0.52 ± 0.09 | 1.65 ± 0.19 | 4.49 ± 0.34 | 4.88 ± 0.38 | 4.95 ± 0.42 |
AUC: area under the curve; MRT: mean residence time; T1/2: half life time.
3.7. Antibacterial activities in vivo
In order to investigate targeting ability of the infectious site, the in vivo antibacterial activity of TPP@CIP-CBMs was evaluated using a back subcutaneous MRSA-infected mice model (Fig. 8A). A linear incision (∼1 cm) was made using a sterile knife on the right side of each mouse back and 200 µl MRSA suspension (108 CFU/ml) was added. After infection for 24 h, the mice were treated with PBS, free CIP, CIP-CBMs, or TPP@CIP-CBMs (CIP concentration: 200 µg/ml, 200 µl), respectively. Five hours after the injection, the mice were irradiated with NIR with a power density of 50 mW/cm2 for 10 min. The treatment lasted for 7 d, and body weight and the lesion of the mice were observed every day until the end of the treatment.
Fig. 8.
In vivo therapeutic effect of the TPP@CIP-CBMs in a mouse model with MRSA infection. (A) Establishment and treatment of subcutaneous abscess in mice; (B) photographs of abscesses of mice in different experimental groups; (C) corresponding statistical analysis of the bacterial viability. Data are presented as mean ± SD (n = 3); (E) the percentage of the infected area and (D) body weight change of different groups of mice with time.
As shown in Fig. 8B and E, mice in the free CIP, CIP-CBMs, TPP@CIP-CBMs, CIP + NIR, CIP-CBMs+NIR and TPP@CIP-CBMs+NIR groups showed a partial recovery of lesions, with about 20% wound area remaining. As expected, the NIR-irradiated TPP@CIP-CBMs group demonstrated almost full recovery on Day 18, with 97% elimination of the infection sites. The anti-infection activities were further investigated by executing the mice, harvesting and homogenizing their inflammation tissues, and plate spreading and counting bacterial CFU on LB agar plates. Compared with the PBS treated group, the CIP group showed about 40% MRSA elimination, while the CIP-CBMs group demonstrated more than 60% bacteria elimination, suggesting enhanced antibacterial activities against MRSA in vivo compared to free CIP at the same concentration (Fig. S13 and 8C). Notably, no obvious live MRSA remained for the TPP@CIP-CBMs+ NIR group, suggesting the synergetic antibacterial activities of PDT and chemotherapy. The above results indicate the promising antibacterial potential of TPP@CIP-CBMs against MRSA infections. No significant body weight change was observed in any of the experimental mice during the whole observation process (Fig. 8E). Histological analysis of the infectious tissues demonstrated that there were almost no inflammatory cells, cell debris, or tissue damage in the TPP@CIP-CBMs + NIR group (Fig. 9A). Furthermore, Masson's trichrome staining showed intact established collagen fibers and dermal layer in the TPP@CIP-CBMs+NIR group, providing further evidence of wound healing (Fig. 9B). Additionally, the main organs (heart, liver, spleen, lung and kidney) excised from the mice did not show obvious damage, indicating that the TPP@CIP-CBMs have good therapeutic biosafety in vivo (Fig. S14).
Fig. 9.
(A) Histological photomicrographs and (B) Masson's staining of skin tissue sections of infected mice after completion of the in vivo antibacterial activity experiment (scale bars: 100 µm).
4. Conclusion
In conclusion, we created a strategy to construct an antibiotic delivery system by directly using antibiotics as major components of nanocarriers, thereby significantly increasing drug loading. Amphiphilic antibiotic-based prodrugs were prepared by conjugating hydrophobic CIP with hydrophilic betaine carboxylate via ester bonds. The CIP-CB formed micelles with a CIP loading content as high as 65.4% (w/w). Meanwhile, a model photosensitizer, TPP, was loaded into the CIP-CBMs to form PDT/chemotherapy combined antibacterial and antibiofilm nano-systems. The TPP@CIP-CBMs showed pH-induced surface charge transformable activities and the lipase-triggered release of TPP and CIP. Under NIR irradiation, the TPP@CIP-CBMs could effectively kill S. aureus and MRSA, eradicating biofilms due to infection targeting and synergetic activity of PDT and chemotherapy. Furthermore, the in vivo synergetic antibacterial effects of the TPP@CIP-CBMs were verified in a MRSA-infected skin knife injury model. Overall, novel antibiotic-based micelles combined with PDT, with good biocompatibility, will be a powerful new weapon to combat pathogen infections.
Conflicts of interest
The authors declare that there is no conflicts of interest.
Acknowledgements
This work was financially supported by Fundamental Research Funds for the Central Universities (2020NYB10).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2023.100810.
Contributor Information
Yuchen Jiang, Email: jiangyuchen@swun.edu.cn.
Yongchao Yao, Email: john-daljon-1962@foxmail.com.
Jianbin Luo, Email: luojb1971@163.com.
Appendix. Supplementary materials
References
- 1.Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 2.Sun A., He X., Ji X., Hu D., Pan M., Zhang L., et al. Current research progress of photopolymerized hydrogels in tissue engineering. Chin Chem Lett. 2021;32:2117–2126. [Google Scholar]
- 3.Murray C.J.L., Ikuta K.S., Sharara F., Swetschinski L., Robles Aguilar G., Gray A., et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roope L.S.J., Smith R.D., Pouwels K.B., Buchanan J., Abel L., Eibich P., et al. The challenge of antimicrobial resistance: what economics can contribute. Science. 2019;364:eaau4679. doi: 10.1126/science.aau4679. [DOI] [PubMed] [Google Scholar]
- 5.Lyu Y., Yang X., Goswami S., Gorityala B.K., Idowu T., Domalaon R., et al. Amphiphilic tobramycin–lysine conjugates sensitize multidrug resistant gram-negative bacteria to rifampicin and minocycline. J Med Chem. 2017;60:3684–3702. doi: 10.1021/acs.jmedchem.6b01742. [DOI] [PubMed] [Google Scholar]
- 6.Lyu Y., Domalaon R., Yang X., Schweizer F. Amphiphilic lysine conjugated to tobramycin synergizes legacy antibiotics against wild-type and multidrug-resistant Pseudomonas aeruginosa. Pept Sci. 2019;111:e23091. doi: 10.1002/bip.23091. [DOI] [PubMed] [Google Scholar]
- 7.Prince A.S. Biofilms, antimicrobial resistance, and airway infection. N Engl J Med. 2002;347:1110–1111. doi: 10.1056/NEJMcibr021776. [DOI] [PubMed] [Google Scholar]
- 8.Tian S., Su L., Liu Y., Cao J., Yang G., Ren Y., et al. Self-targeting, zwitterionic micellar dispersants enhance antibiotic killing of infectious biofilms-an intravital imaging study in mice. Sci Adv. 2020;6 doi: 10.1126/sciadv.abb1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makabenta J.M.V., Nabawy A., Li C.H., Schmidt-Malan S., Patel R., Rotello V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol. 2021;19:23–36. doi: 10.1038/s41579-020-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding X., Wang A., Tong W., Xu F.J. Biodegradable antibacterial polymeric nanosystems: a new hope to cope with multidrug-resistant bacteria. Small. 2019;15 doi: 10.1002/smll.201900999. [DOI] [PubMed] [Google Scholar]
- 11.Chen J., Wang F., Liu Q., Du J. Antibacterial polymeric nanostructures for biomedical applications. Chem Commun. 2014;50:14482–14493. doi: 10.1039/c4cc03001j. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z., Liu X., Duan Y., Huang Y. Infection microenvironment-related antibacterial nanotherapeutic strategies. Biomaterials. 2022;280 doi: 10.1016/j.biomaterials.2021.121249. [DOI] [PubMed] [Google Scholar]
- 13.Yin M., Yang M., Yan D., Yang L., Wan X., Xiao J., et al. Surface-charge-switchable and size-transformable thermosensitive nanocomposites for chemo-photothermal eradication of bacterial biofilms in vitro and in vivo. ACS App Mater Interfaces. 2022;14:8847–8864. doi: 10.1021/acsami.1c24229. [DOI] [PubMed] [Google Scholar]
- 14.Huang P., Wang D., Su Y., Huang W., Zhou Y., Cui D., et al. Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug–drug conjugate for cancer therapy. J Am Chem Soc. 2014;136:11748–11756. doi: 10.1021/ja505212y. [DOI] [PubMed] [Google Scholar]
- 15.Feng J., Luo Q., Chen Y., Li B., Luo K., Lan J., et al. DOTA functionalized cross-linked small-molecule micelles for theranostics combining magnetic resonance imaging and chemotherapy. Bioconjugate Chem. 2018;29:3402–3410. doi: 10.1021/acs.bioconjchem.8b00565. [DOI] [PubMed] [Google Scholar]
- 16.Yao Y., Yu Y., Wan X., Yan D., Chen Y., Luo J., et al. Azobenzene-based cross-linked small-molecule vesicles for precise oxidative damage treatments featuring controlled and prompt molecular release. Chem Mater. 2021;33:7357–7366. [Google Scholar]
- 17.Yao Y., Xu D., Zhu Y., Dai X., Yu Y., Luo J., et al. Dandelion flower-like micelles. Chem. Sci. 2020;11:757–762. doi: 10.1039/c9sc05741b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia Q., Song Q., Li P., Huang W. Rejuvenated photodynamic therapy for bacterial infections. Adv Healthcare Mater. 2019;8 doi: 10.1002/adhm.201900608. [DOI] [PubMed] [Google Scholar]
- 19.Hu X., Zhang H., Wang Y., Shiu B.C., Lin J.H., Zhang S., et al. Synergistic antibacterial strategy based on photodynamic therapy: progress and perspectives. Chem Eng J. 2022;450 [Google Scholar]
- 20.Gao Q., Huang D., Deng Y., Yu W., Jin Q., Ji J., et al. Chlorin e6 (Ce6)-loaded supramolecular polypeptide micelles with enhanced photodynamic therapy effect against Pseudomonas aeruginosa. Chem Eng J. 2021;417 [Google Scholar]
- 21.Liu Z., Cao T., Xue Y., Li M., Wu M., Engle J.W., et al. Self-amplified photodynamic therapy through the 1O2-mediated internalization of photosensitizers from a Ppa-bearing block copolymer. Angew Chem Int Ed. 2020;59:3711–3717. doi: 10.1002/anie.201914434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng Y., Coradi Tonon C., Ashraf S., Hasan T. Photodynamic and antibiotic therapy in combination against bacterial infections: efficacy, determinants, mechanisms, and future perspectives. Adv Drug Delivery Rev. 2021;177 doi: 10.1016/j.addr.2021.113941. [DOI] [PubMed] [Google Scholar]
- 23.Baquero F., Levin B.R. Proximate and ultimate causes of the bactericidal action of antibiotics. Nat Rev Microbiol. 2021;19:123–132. doi: 10.1038/s41579-020-00443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komnatnyy V.V., Chiang W.C., Tolker-Nielsen T., Givskov M., Nielsen T.E. Bacteria-triggered release of antimicrobial agents. Angew Chem Int Ed. 2014;53:439–441. doi: 10.1002/anie.201307975. [DOI] [PubMed] [Google Scholar]
- 25.Bai Y., Hu Y., Gao Y., Wei X., Li J., Zhang Y., et al. Oxygen self-supplying nanotherapeutic for mitigation of tissue hypoxia and enhanced photodynamic therapy of bacterial keratitis. ACS Appl Mater Interfaces. 2021;13:33790–33801. doi: 10.1021/acsami.1c04996. [DOI] [PubMed] [Google Scholar]
- 26.Xie X., Sun T., Xue J., Miao Z., Yan X., Fang W., et al. Ag nanoparticles cluster with pH-triggered reassembly in targeting antimicrobial applications. Adv Funct Mater. 2020;30 [Google Scholar]
- 27.Yin M., Qiao Z., Yan D., Yang M., Yang L., Wan X., et al. Ciprofloxacin conjugated gold nanorods with pH induced surface charge transformable activities to combat drug resistant bacteria and their biofilms. Mater Sci Eng C. 2021;128 doi: 10.1016/j.msec.2021.112292. [DOI] [PubMed] [Google Scholar]
- 28.Deng Q., Sun P., Zhang L., Liu Z., Wang H., Ren J., et al. Porphyrin MOF dots–based, function-adaptive nanoplatform for enhanced penetration and photodynamic eradication of bacterial biofilms. Adv Funct Mater. 2019;29 [Google Scholar]
- 29.Qiao Z., Yao Y., Song S., Yin M., Yang M., Yan D., et al. Gold nanorods with surface charge-switchable activities for enhanced photothermal killing of bacteria and eradication of biofilm. J Mater Chem B. 2020;8:3138–3149. doi: 10.1039/d0tb00298d. [DOI] [PubMed] [Google Scholar]
- 30.Ma W., Chen X., Fu L., Zhu J., Fan M., Chen J., et al. Ultra-efficient antibacterial system based on photodynamic therapy and CO gas therapy for synergistic antibacterial and ablation biofilms. ACS Appl Mater Interfaces. 2020;12:22479–22491. doi: 10.1021/acsami.0c01967. [DOI] [PubMed] [Google Scholar]
- 31.Chen M., Wei J., Xie S., Tao X., Zhang Z., Ran P., et al. Bacterial biofilm destruction by size/surface charge-adaptive micelles. Nanoscale. 2019;11:1410–1422. doi: 10.1039/c8nr05575k. [DOI] [PubMed] [Google Scholar]
- 32.Cui T., Wu S., Sun Y., Ren J., Qu X. Self-propelled active photothermal nanoswimmer for deep-layered elimination of biofilm in vivo. Nano Lett. 2020;20:7350–7358. doi: 10.1021/acs.nanolett.0c02767. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y., Busscher H.J., Zhao B., Li Y., Zhang Z., van der Mei H.C., et al. Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano. 2016;10:4779–4789. doi: 10.1021/acsnano.6b01370. [DOI] [PubMed] [Google Scholar]
- 34.Wang S., Fang Y., Zhang Z., Jin Q., Ji J. Bacterial infection microenvironment sensitive prodrug micelles with enhanced photodynamic activities for infection control. Colloid Interface Sci Commun. 2021;40 [Google Scholar]
- 35.Mohr A., Talbiersky P., Korth H.G., Sustmann R., Boese R., Bläser D., et al. A new pyrene-based fluorescent probe for the determination of critical micelle concentrations. J Phys Chem B. 2007;111:12985–12992. doi: 10.1021/jp0731497. [DOI] [PubMed] [Google Scholar]
- 36.Lee J.S., Ankone M., Pieters E., Schiffelers R.M., Hennink W.E., Feijen J. Circulation kinetics and biodistribution of dual-labeled polymersomes with modulated surface charge in tumor-bearing mice: comparison with stealth liposomes. J Control Release. 2011;155:282–288. doi: 10.1016/j.jconrel.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y., Jin Y., Chen W., Wang J., Chen H., Sun L., et al. Construction of nanomaterials with targeting phototherapy properties to inhibit resistant bacteria and biofilm infections. Chem Eng J. 2019;358:74–90. [Google Scholar]
- 38.Gao Y., Wang J., Chai M., Li X., Deng Y., Jin Q., et al. Size and charge adaptive clustered nanoparticles targeting the biofilm microenvironment for chronic lung infection management. ACS Nano. 2020;14:5686–5699. doi: 10.1021/acsnano.0c00269. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y., Ren T., Gou J., Zhang L., Tao X., Tian B., et al. Strategies for improving the payload of small molecular drugs in polymeric micelles. J Control Release. 2017;261:352–366. doi: 10.1016/j.jconrel.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 40.Yu F., Chen C., Yang G., Ren Z., Cao H., Zhang L., et al. An acid-triggered porphyrin-based block copolymer for enhanced photodynamic antibacterial efficacy. Sci China: Chem. 2021;64:459–466. [Google Scholar]
- 41.Su Y., Zhao L., Meng F., Wang Q., Yao Y., Luo J. Silver nanoparticles decorated lipase-sensitive polyurethane micelles for on-demand release of silver nanoparticles. Colloids Surf B. 2017;152:238–244. doi: 10.1016/j.colsurfb.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z., Zhao X., Yu B., Zhao N., Zhang C., Xu F.J. Rough carbon–iron oxide nanohybrids for near-infrared-II light-responsive synergistic antibacterial therapy. ACS Nano. 2021;15:7482–7490. doi: 10.1021/acsnano.1c00894. [DOI] [PubMed] [Google Scholar]
- 43.Yu Z., Sun Q., Pan W., Li N., Tang B. A near-infrared triggered nanophotosensitizer inducing domino effect on mitochondrial reactive oxygen species burst for cancer therapy. ACS Nano. 2015;9:11064–11074. doi: 10.1021/acsnano.5b04501. [DOI] [PubMed] [Google Scholar]
- 44.Qiao Z., Yao Y., Song S., Yin M., Luo J. Silver nanoparticles with pH induced surface charge switchable properties for antibacterial and antibiofilm applications. J Mater Chem B. 2019;7:830–840. doi: 10.1039/c8tb02917b. [DOI] [PubMed] [Google Scholar]
- 45.Teirlinck E., Xiong R., Brans T., Forier K., Fraire J., Van Acker H., et al. Laser-induced vapour nanobubbles improve drug diffusion and efficiency in bacterial biofilms. Nat Commun. 2018;9:4518. doi: 10.1038/s41467-018-06884-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao B., Lyu X., Wang C., Lu S., Xing D., Hu X. Rational collaborative ablation of bacterial biofilms ignited by physical cavitation and concurrent deep antibiotic release. Biomaterials. 2020;262 doi: 10.1016/j.biomaterials.2020.120341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.