Abstract
Mutations in a common extracellular domain of fibroblast growth factor receptor (FGFR)-2 isoforms (type IIIb and IIIc) cause craniosynostosis syndrome and chondrodysplasia syndrome. FGF10, a major ligand for FGFR2-IIIb and FGFR1-IIIb, is a key participant in the epithelial-mesenchymal interactions required for morphogenetic events. FGF10 also regulates preadipocyte differentiation and early chondrogenesis in vitro, suggesting that FGF10-FGFR signaling may be involved in craniofacial skeletogenesis in vivo. To test this hypothesis, we used a tet-on doxycycline-inducible transgenic mouse model (FGF10 Tg) to overexpress Fgf10 from embryonic day 12.5. Fgf10 expression was 73.3-fold higher in FGF10 Tg than in wild-type mice. FGF10 Tg mice exhibited craniofacial anomalies, such as a short rostrum and mandible, an underdeveloped (cleft) palate, and no tympanic ring. Opposite effects on chondrogenesis in different anatomical regions were seen, e.g., hyperplasia in the nasal septum and hypoplasia in the mandibular condyle. We found an alternative splicing variant of Fgfr2-IIIb with a predicted translation product lacking the transmembrane domain, and suggesting a soluble form of FGFR2-IIIb (sFGFR2-IIIb), differentially expressed in some of the craniofacial bones and cartilages. Thus, excessive FGF10 may perturb signal transduction of the FGF-FGFR, leading to craniofacial skeletal abnormalities in FGF10 Tg mice.
Keywords: FGF10, FGFR2, Craniofacial skeleton, Chondrogenesis
Highlights
-
•
Mice overexpressing FGF10 showed craniofacial anomalies.
-
•
Mice overexpressing FGF10 showed the opposing effects on chondrogenesis depending on anatomical sites.
-
•
A soluble form of FGFR2-IIIb was expressed in some of the craniofacial bones and cartilages.
1. Introduction
Craniofacial development requires highly organized cell-to-cell communication, often involving secreted factors and their cognate receptors that play roles in spatiotemporally-controlled events (Leitch et al., 2020; Wilkie and Morriss-Kay, 2001). Fibroblast growth factor (FGF)/FGF receptor (FGFR) signaling is one such essential pathway in craniofacial development (Moosa and Wollnik, 2016; Wilkie and Morriss-Kay, 2001). Members of the FGF family can be grouped into seven subfamilies, based on such criteria as sequence similarity and evolutionary relationships (Ornitz and Itoh, 2015). FGFs can act as intracrine, paracrine and endocrine factors via binding to a variety of FGFR family members, which signal through four different pathways: the JAK/STAT, Ras/MAPK, PI3K/Akt and PKC pathways (Ornitz and Itoh, 2015; Ornitz and Marie, 2015; Su et al., 2014).
FGFRs are encoded by four distinct genes (Fgfr1–4), and their transcripts are composed of two or three extracellular immunoglobulin (Ig)-like loops, a single transmembrane domain and two cytoplasmic tyrosine kinase domains (Ornitz and Itoh, 2015). Alternative splicing of exons encoding the third Ig-like loops in Fgfr1–3 generate IIIb and IIIc isoforms (Ornitz and Itoh, 2015). Mutations of FGFRs alter ligand-binding specificity and affinity, and, in some cases, constitutively activate receptor tyrosine kinases in a ligand-independent manner. These mutations are present in the common extracellular domain of FGFRs and are responsible for chondrodysplasia and craniosynostosis in congenital syndromes such as Apert syndrome, Crouzon syndrome and Pfeiffer syndrome (Ornitz and Itoh, 2015; Wilkie and Morriss-Kay, 2001).
Of 22 FGF family members, FGF10 binds to FGFR2-IIIb and other FGFRs including FGFR1-IIIb with high and low affinity, respectively (Zhang et al., 2006). Both Fgf10 and Fgfr2-IIIb knockout mice are viable until birth and show similar deformities of multiple tissues requiring epithelial-mesenchymal interactions for development including the lung, salivary gland, palate and limb bud, with complete truncation of the fore- and hindlimbs (De Moerlooze et al., 2000; Hajihosseini et al., 2009; Rice et al., 2004; Sekine et al., 1999). The functional significance of FGF10 in rodent and human mesenchymal cells in vitro has also been reported. For example, neutralizing antibodies against FGF10 inhibited proliferation and adipogenic differentiation of mouse 3T3-L1 cells in association with a reduction of C/EBPα levels (Sakaue et al., 2002). Conflicting evidence exists on FGF10 activities in skeletogenic cells. For example, FGF10 was reported not to affect proliferation or differentiation of primary mouse calvaria osteoblastic cells, rib cage chondrocyte differentiation or osteoclast formation in mouse bone marrow cells cocultured with osteoblasts (Shimoaka et al., 2002). FGF10 inhibited cell proliferation in human growth plate chondrocyte cultures (Olney et al., 2004), while overexpression of Fgf10 in organ cultures and micromass cell cultures of fetal rat mandibular processes enhanced chondrogenic differentiation (Terao et al., 2011). Taken together, these results raise the possibility that FGF10 may directly target bone and cartilage, but the role of FGF10 in osteo/chondrogenic cells in vivo remains to be clarified. To address this issue, we prepared transgenic mice overexpressing Fgf10 and analyzed in detail the pronounced effects of excess FGF10 on development of the craniofacial skeleton.
2. Materials and methods
2.1. Transgenic mice overexpressing Fgf10 (FGF10 Tg mice)
Transgenic mice constitutively expressing CMV-rtTA (Parsa et al., 2010) and tet(o)Fgf10 (Clark et al., 2001) were provided by the Jackson Laboratory (Bar Harbor, ME) and by Dr. Jeffrey A. Whitsett (Cincinnati Children's Hospital Medical Center, OH), respectively. All mice were conventionally reared and fed with a regular diet. Mice were genotyped as described (http://www.jax.org/index.html). To obtain fetuses ubiquitously overexpressing Fgf10 (CMV-rtTAtg/+;tet(o)Fgf10tg/tg and/or CMV-rtTAtg/+; tet(o)Fgf10 tg/+) and control fetuses (tet(o)Fgf10tg/tg and/or tet(o)Fgf10tg/+) as littermates, female mice (tet(o)Fgf10tg/tg) were bred with male mice (CMV-rtTAtg/+;tet(o)Fgf10tg/+). The day of vaginal plug detection was defined as embryonic day (E) 0.5. Fgf10 expression was induced in pregnant mice by ad libitum water containing 2 mg/mL of doxycycline hyclate (Dox; Sigma-Aldrich, St Louis, MO) in 5 % sucrose from E12.5. Note that Dox in drinking water is delivered to organs within 4 h and reaches a maximum after 24 h (Kistner et al., 1996) and that pregnant mice were not affected by Dox (Fedorov et al., 2001). Five animals were used in each group. Animal use and procedures were approved by the Committee of Animal Experimentation at Hiroshima University (#A15–142).
2.2. Microcomputed tomography (μCT) analysis
Neonates were analyzed using μCT (Skyscan 1176; Bruker, Billerica, MA) under the following conditions: voltage 40 kV, current 600 μA, image pixel size 17.5 μm, and no filter. Images were reconstructed using NRecon (Bruker).
2.3. Whole-mount skeletal staining
Whole-mount skeletal staining was performed as described previously (Kimmel and Trammell, 1981). Briefly, neonates were skinned and eviscerated, and stained with 0.14 % Alcian blue and 0.12 % Alizarin red S in ethanol and glacial acetic acid. Specimens were then cleared in 2 % potassium hydroxide and stored in 50 % (vol/vol) glycerin.
2.4. Paraffin sections and histology
Fetuses at E18.5 were fixed in 4 % paraformaldehyde in PBS at 4 °C overnight and demineralized in 10 % EDTA in PBS at 4 °C for a few days, followed by dehydration and paraffin embedding. Deparaffinized sections (5 μm thickness) were stained with hematoxylin and eosin (HE). For immunohistochemistry, sections were pretreated with 0.1 % hyaluronidase in TBS (20 mM Tris-HCl, 150 mM NaCl, pH 7.6) at 37 °C for 60 min, followed by Protein Block (DAKO, Glostrup, Denmark) for 30 min at room temperature. Sections were then incubated with primary antibodies against type X collagen (1:1000; Cosmo Bio, Tokyo, Japan) at 4 °C overnight. Alexa Fluor 594 goat anti-rabbit IgG (1:500; Life Technologies, Carlsbad, CA) was used as a secondary antibody, and sections were mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, CA). Normal rabbit IgG was used as a negative control.
2.5. RT-PCR and sequence analysis
Mandibular condylar cartilages were isolated from E18.5 fetuses (Control, n = 8–13; FGF10 Tg, n = 7–15) carried by 2–3 pregnant mice in each experiment and pooled for RNA isolation. Tissues were collected form wild-type neonate mice for RNA isolation. Total RNA was isolated using TriPure Isolation Reagent (Roche Diagnostics, Indianapolis, IN) and reverse transcribed using ReverTraAce, according to the manufacturer's instructions (Toyobo, Osaka, Japan). Gene expression was quantified by real-time PCR (StepOnePlus real-time PCR system, Life Technologies) using Fast SYBR Green Master Mix (Life Technologies). PCR was performed with KOD Fx Neo polymerase (Toyobo). Primers used are as follows: Fgf10, 5′-AGC GGG ACC AAG AAT GAA G-3′, 5′-GCT GTT GAT GGC TTT GAC G-3′; Sox9, 5′-CTA TCT TCA AGG CGC TGC AA-3′, 5′-GTC GGT TTT GGG AGT GGT G-3′; H4c, 5′-CGG TGT GCT GAA GGT GTT CC-3′, 5′-ACC GCC GAA TCC GTA GAG AG-3′; Runx2, 5′-TTC TGC CTC TGG CCT TCC TC-3′, 5′-AAG GGC CCA GTT CTG AAG CA-3′; Col2a1, 5′-GTG GAG CAG CAA GAG CAA GG-3′, 5′-CTG GAC GTT AGC GGT GTT GG-3′; Col10a1, 5′-GGC AGA GGA AGC CAG GAA AG-3′, 5′-TTA GCA GCA GAA AGG GTA TTT GAG G-3′; Fgfr2-IIIb, 5′-CTC ACT GTC CTG CCC AAA CA-3′, 5′-CAC CAT GCA GGC GAT TAA GA-3′; Fgfr2-IIIc, 5′-GCT TCA TCT GCC TGG TCT TGG-3′, 5′-TGG GAG ATT TGG TAT TTG GTT GG-3′; Actb, 5′-GGC TGT ATT CCC CTC CAT CG-3′, 5′-GCC TCG TCA CCC ACA TAG GA-3′ (forward and reverse, respectively). Actb was used as an internal control. PCR products were analyzed by electrophoresis in a 2 % agarose gel and stained with ethidium bromide (Wako Pure Chemical Industries, Osaka, Japan). PCR products of Fgfr2-IIIb were cloned into pcDNA3.1 vector and sequenced by Eurofins Genomics (Tokyo, Japan). The obtained sequence was analyzed in the GenBank database using NCBI BLAST search tools (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
2.6. Statistical analysis
Data are expressed as mean ± SD. Non-numerical data are shown as representative results of three independent experiments. Statistical differences were evaluated using F-test followed by Student's or Welch's t-test. A p-value <0.05 was considered statistically significant.
3. Results
3.1. Craniofacial skeletal anomalies in FGF10 Tg mice
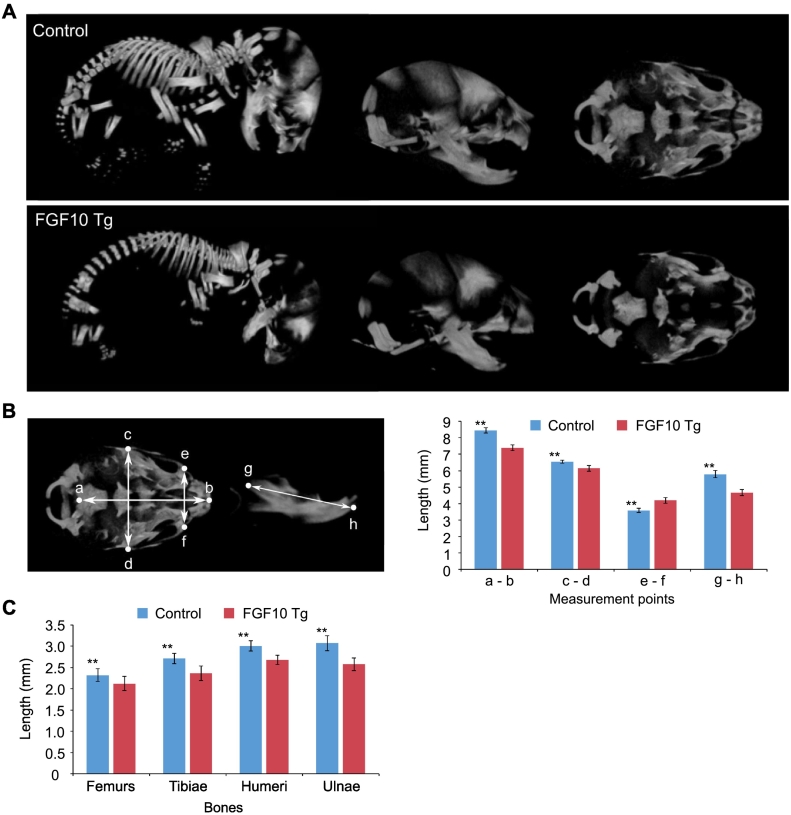
In keeping with the observation that early skeletogenesis in mice is seen in limbs at E11.5–13.5 (Kaufman, 1992) and in the craniofacial complex (chondrocranium) at E11 (McBratney-Owen et al., 2008), we induced Fgf10 expression from E12.5. FGF10 Tg mice were viable until birth but died soon after. Amongst the substantial anomalies detected by whole-mount skeletal staining of FGF10 Tg neonates, significant craniofacial shape abnormalities were seen, such as a shorter rostrum and mandible compared to control (Fig. 1A, B). FGF10 Tg mice also exhibited defects in cranial bones and sutures, especially coronal, sagittal, and lambdoid sutures (Fig. 1B, C). Overexpression of Fgf10 also caused cleft palate and tympanic ring defects (Fig. 1D). Cartilage dysplasia was observed especially in the frontonasal cartilage, mandibular condylar cartilage, mandibular angular cartilage and the rostral process of Meckel's cartilage (Fig. 1C–E). Skeletal anomalies were not restricted to the craniofacial complex; for example, mineralized phalanges were absent (Fig. 1B). On the other hand, whole-mount staining revealed no obvious cartilage-bone defects in the limbs or vertebrae of FGF10 Tg mice, although limb bones appeared to be shorter. Results of μCT analyses largely mirrored the results of whole-mount skeletal staining and demonstrated in more detail the craniofacial bone dysmorphologies including of the parietal bone, interparietal bone, supraoccipital bone, and exoccipital bone (Fig. 2A). Quantification confirmed the significantly smaller cranial size and mandibular length and the larger palatal width in the temporal region (Fig. 2B) as well as the shortened limb bones (Fig. 2C) in FGF10 Tg versus control mice. We focused the rest of our analyses on the craniofacial complex.
Fig. 1.
Skeletal anomalies in FGF10 Tg mice. (A) Gross appearance of neonate mice. (B–E) Skeletons of neonates stained with Alizarin red and Alcian blue. Superior (C) and inferior (D) views of craniofacial skeletons and lateral view of mandibles (E). Mandibles were removed to show inferior view of the cranial base (D). Tympanic rings (arrowheads) were observed in control but not FGF10 Tg mice. Cleft palate (arrow) in FGF10 Tg mice. Rulers are in millimeters (A, B, E). Scale bars, 5 mm (C, D).
Fig. 2.
μCT analysis of craniofacial skeletons. (A) Three-dimensional reconstructions from μCT images of control and FGF10 Tg neonates. Whole skeletons (left), and lateral (middle) and inferior (right) views of craniofacial skeletons are shown. Mandibles were removed to show inferior view of the cranial base. (B) Morphological evaluation of the craniofacial skeletons. The landmarks (a–h) shown in the left column were used for the axial length measurements shown in the right column. (C) Morphological evaluation of long bones. Error bars indicate SEM. n = 10. **, p < 0.01.
3.2. Histological and gene expression analyses of craniofacial skeletal anomalies in FGF10 Tg mice
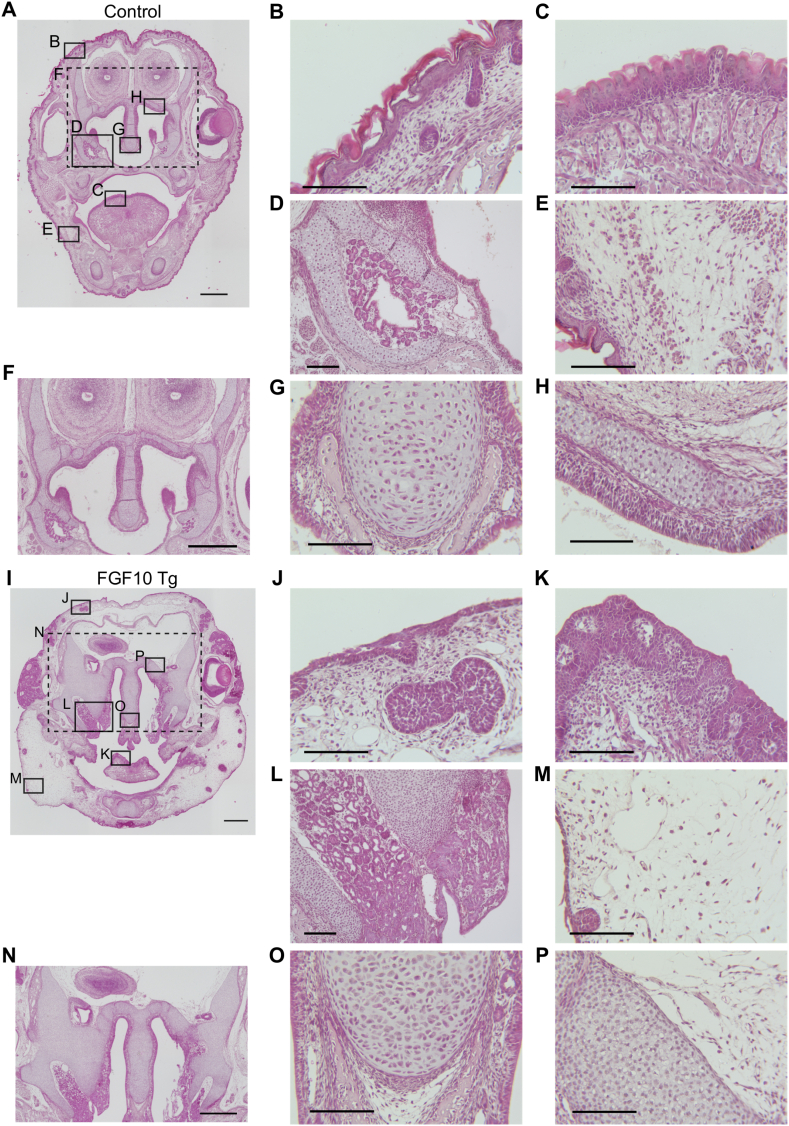
HE staining was performed to assess histological changes in craniofacial skeleton of FGF10 Tg compared to littermate control mice (Fig. 3A, I). Fgf10 overexpression induced marked anomalies of epithelial cells, with lack of keratinization (Fig. 3B, J), invagination of epithelial cells into connective tissue (Fig. 3C, K), and hyperplasia of exocrine glands (Fig. 3D, L). Hyperplasia of adipose tissue was also evident (Fig. 3E, M). In contrast to the hypoplasia seen in the mandibular condylar cartilage (Fig. 1), hyperplasia of cartilage in the nasal septum, nasal concha, and cranial base was observed in FGF10 Tg neonates (Fig. 3F–H, N–P).
Fig. 3.
Histology of craniofacial skeletons. Coronal sections of control (A–H) and FGF10 Tg (I-P) fetuses were stained with HE. Higher magnifications of areas enclosed by solid lines in (A) and (I) are indicated in (B–E, G, H) and (J–M, O, P), respectively. The skin (B, J), tongue (C, K), nasal gland (D, L), and adipose tissue (E, M) are shown. Areas enclosed by dashed lines in (A) and (I) correspond to (F) and (N), respectively. Cartilages around the nasal septum (G, O), nasal concha, and cranial base (H, P) are shown (F, N). Scale bars, 500 μm (A, F, I, N); 100 μm (B–E, G, H, J–M, O, P).
Immunostaining of type X collagen, a marker of hypertrophic chondrocytes, demonstrated a reduction of the hypertrophic zone in mandibular condylar cartilage in FGF10 Tg compared to control mice (Fig. 4A–D). To characterize the molecular basis of the hypoplasia, we compared expression levels of Fgf10 and osteochondrogenesis-related genes between control and FGF10 Tg mandibular condylar cartilage. Fgf10 expression was 73.3-fold higher (average of 3 experiments) in FGF10 Tg compared to control specimens (Fig. 4E), while amongst the osteochondrogenesis-related genes analyzed, Runx2 was increased and Col10a1 was decreased (Fig. 4F). Taken together with the result that no difference was seen in the expression of H4c, a proliferation marker, these results suggest that an excess of Fgf10 may inhibit the terminal differentiation of chondrocytes, resulting in cartilage and bone hypoplasia.
Fig. 4.
Histological and gene expression analyses of the mandibular condylar cartilage. Coronal sections of the condylar cartilage in control (A, C) and FGF10 Tg (B, D) fetuses were stained with HE (A, B) and antibodies against type X collagen (C, D). Higher magnifications of areas enclosed by solid lines in left panels are shown in the right panels (A, B). Areas enclosed by solid lines (phase contrast) correspond to type X collagen (red) immunohistochemistry (C, D). DAPI (blue) was used for nuclear staining. Scale bars, 500 μm (left in A and B); 200 μm (right in A and B); 100 μm (C, D). Levels of Fgf10 (E) and osteochondrogenic genes (F) in the mandibular condylar cartilage of control and FGF10 Tg mice. Relative mRNA levels in the control are set at 1. For Fgf10 gene, three independent experiments are shown. For osteochondrogenic genes, error bars indicate SD. n = 3. *, p < 0.05.
3.3. Expression profiles of Fgf10 and receptors Fgfr2-IIIb and Fgfr2-IIIc in mouse tissues
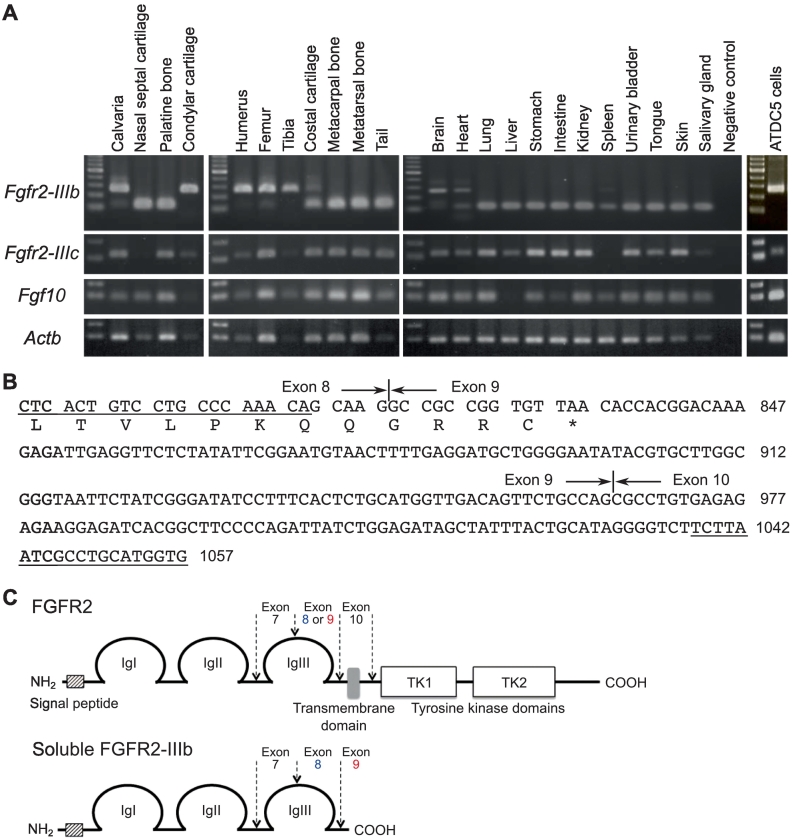
To explore the mechanism underlying the regulation of osteochondrogenesis by FGF10 signaling, we analyzed the expression profiles of the Fgf10, Fgfr2-IIIb, and Fgfr2-IIIc genes by RT-PCR in multiple tissues of wild-type neonatal mice and the prechondrogenic mouse cell line ATDC5 (Fig. 5A). Most of the tissues analyzed as well as the ATDC5 cells expressed all three transcripts, but an unexpected longer RT-PCR product of Fgfr2-IIIb was identified in both ATDC5 cells and in the cartilages and bones exhibiting dysplasia in FGF10 Tg mice (Fig. 1, Fig. 2). Sequencing of the longer RT-PCR product identified sequences corresponding to exon 8 and 9, encoding IIIb and IIIc respectively (Fig. 5B), and sharing high sequence similarity (99.62 %) with an alternative splicing variant of Fgfr2 (GenBank accession number EF143332, from nucleotides 796 to 1057). The inclusion of exon 9 in Fgfr2-IIIb mRNA may result in a frameshift and subsequent premature stop codon soon after the immunoglobulin (Ig)-like domain III (Fig. 5B, C). Consequently, a truncated form of FGFR2-IIIb lacking the transmembrane and tyrosine kinase domains may be produced and secreted as a soluble form (sFGFR2-IIIb) (Fig. 5C).
Fig. 5.
(A) Expression profiles of Fgfr2 and Fgf10 genes in wild-type mouse tissues and the prechondrogenic cell line ATDC5; the ß-actin gene (Actb) was used as internal control. (B) cDNA sequence and deduced amino acid sequence of Fgfr2. Asterisk shows stop codon. PCR primers used are indicated by underlined letters. The numbers on the right indicate the nucleotide positions corresponding to the GenBank accession number EF143332. (C) Schematic structures of FGFR2 and soluble FGFR2-IIIb (sFGFR2-IIIb).
4. Discussion
We report here that high (∼73-fold) overexpression of Fgf10 in mouse fetuses induced developmental anomalies in craniofacial cartilage and bone. The consequences of Fgf10 overexpression on differentiation of various lineages and the associated signaling factor regulatory pathways is known to be complex. For example, modest overexpression of Fgf10 (2- to 4-fold) under the control of the Ipf1 promoter maintained Notch signaling activation throughout the pancreatic epithelium, leading to the impaired differentiation of pancreatic cells (Hart et al., 2003). On the other hand, somewhat higher overexpression of Fgf10 (11-fold) attenuated bleomycin-induced lung fibrosis at least in part via a reduction in TGF-β expression and activity (Gupte et al., 2009). Some or all of the skeletal anomalies we observe with high overexpression of Fgf10 in our Tg mice may result in changes of expression and/or activities of other signaling factors also involved in skeletal development (see below) or may result, at least in part, from the high overexpression overwhelming the FGF signaling pathway itself, issues that require additional studies.
Actions of FGF10 appear to be mediated primarily by communication between FGF10-producing mesenchymal cells and specific FGFR-expressing epithelial cells (Ornitz and Itoh, 2015), resulting in FGF10-induced increases in epithelial cell proliferation (Igarashi et al., 1998; Marchese et al., 2001). This mitogenic effect of FGF10 on epithelial cells may have caused lung dysplasia in our Tg mice, leading to their death soon after birth, as is the case in mouse fetuses infected with FGF10 adenoviruses (Gonzaga et al., 2008). We also infer that the excess Fgf10 in our Tg mice accelerates proliferation and impedes differentiation of epithelial cells, leading to a defective cornified layer and hyperplasia of glands (Fig. 3J–L). The hyperplasia of adipose tissues (Fig. 3M) in the FGF10 Tg mice is also consistent with the observation that FGF10 coordinates the proliferation and differentiation of adipocytes by activating CEBP/α and PPARγ (Ohta and Itoh, 2014; Sakaue et al., 2002).
Our results are consistent with the view that overexpression of Fgf10 reduced chondrocyte hypertrophy in mandibular condylar cartilage (Fig. 4A–D) as a consequence of increased expression of Runx2, a transcription factor required for chondrocyte differentiation (Fig. 4F). Although knockout and transgenic mouse studies have shown that Runx2 expressed in prehypertrophic chondrocytes triggers hypertrophy of chondrocytes by activating Ihh and Col10a1 (Yoshida et al., 2004; Zheng et al., 2003), loss- and gain-of-function mouse models have also shown that the long-lasting expression of Runx2 in perichondrocytes inhibits chondrocyte proliferation and hypertrophy (Hinoi et al., 2006). FGF10 Tg mice also showed thickening of the cartilage in the nasal septum, nasal concha, and cranial base (Fig. 3). These phenotypic anomalies are also observed in the Fgfr2C342Y Crouzon syndrome mouse model (Perrine et al., 2022), while the Fgfr2S252W Apert syndrome mouse model shows no cartilage abnormalities in the cranial base (Kim et al., 2020). Despite activating the same receptor, different mutations in Fgfr2 give rise to different effects even at very close sites such as the nasal septum and cranial base, paralleling the differential effects that we see in FGF10 Tg mice which exhibit hypoplasia in mandibular condylar cartilage but hyperplasia of cartilage in the closely positioned nasal septum and cranial base. These diverse effects at different albeit closely positioned sites may reflect the fact that cartilages of the chondrocranium form individually, appearing at different points of embryonic time and maturing according to their own developmental schedule (see Perrine et al., 2022 for detailed discussion). Such developmental differences suggest that FGF10 could affect the chondrocytes of different sites based on developmental time-dependent changes in other regulatory molecules in the environment and/or in specific cells of different cartilage zones (e.g., proliferative versus hypertrophic), such as Runx2, as mentioned above and/or the potential alternatively spliced variant of Fgfr2-IIIb that we have identified in some bones and cartilages (see below). Further studies will be necessary to dissect the mechanisms underlying the aberrant chondrogenesis in the FGF10 Tg mice.
Mutations in the FGF signaling pathway are the most common cause of craniosynostosis, characterized by the premature fusion of cranial sutures (Ornitz and Itoh, 2015; Wilkie and Morriss-Kay, 2001). However, FGF10 Tg mice exhibited the opposite phenotype, with wider sutures (Fig. 1C, 2A). This suggests that excess FGF10 affects not only endochondral ossification but also intramembranous ossification. Intramembranous ossification is known to be regulated by several signaling pathways, including FGFs (Ornitz and Marie, 2015), as exemplified for example by the severe defects in cranial bones seen in double knockout mice lacking Fgf9 and Fgf18 (Hung et al., 2016). Amongst possible mechanisms when FGF is in excess is the induction of SOX2, which has been shown to result in the inhibition of osteoblast differentiation and parietal bone hypoplasia (Holmes et al., 2011), consistent with the hypoplastic cranial bones with enlarged cranial sutures seen in our Tg mice. We also cannot discount possible indirect effects on osteoblast lineage cells of FGF10 excess via other non-osteoblast lineage cells in the developing chondrocranium and dermal cranium tissue complex. Elucidating potential osteoblast lineage autonomous versus nonautonomous mechanisms of FGF10 on cranial sutures will be addressed in future.
We identified a potential alternative splicing variant of Fgfr2-IIIb in some bones and cartilages of wild-type mice (Fig. 5). This variant, sFGFR2-IIIb, may act as a decoy receptor for FGF10 and/or other FGF subfamilies including FGF3, FGF7, and FGF22 to modulate its (their) signal transduction. Considering the fact that human FGFR2 forms heterodimers with human FGFR1 in vitro (Wang et al., 1997), heterodimerization of sFGFR2-IIIb and FGFR1 could also participate in the FGF signaling pathway. Thus, it is notable that the cartilages and bones where sFgfr2-IIIb was expressed (no expression of membranous type Fgfr2-IIIb) were those exhibiting hypoplasia in the FGF10 Tg mice. An excess of FGF10 may trap sFGFR2-IIIb and allow extraordinary FGF signaling by the remainder and/or other FGFs. For example, a surplus of FGF10 may bind to FGFR1 involved in the terminal maturation of mouse hypertrophic chondrocytes (Jacob et al., 2006). Indeed, constitutive FGFR1 activity causes osteoglophonic dysplasia, characterized by multiple osteochondrogenic anomalies such as rhizomelic dwarfism, craniosynostosis, and low bone mineral density (White et al., 2005). On the other hand, we found that cartilages and bones expressing the membranous Fgfr2-IIIb showed either hyperplasia or no phenotypes (Fig. 1, Fig. 2, Fig. 5A). FGF10 is a key factor for epithelial-mesenchymal interaction and induces the expression of ectodermal FGF8 (Itoh, 2016) that promotes chondrogenesis during chick cranial development (Abzhanov and Tabin, 2004). Thus, sFGFR2-IIIb scavenging by an excess of FGF10 may elicit abundant FGF8 that may accelerate the growth of cartilage in the nasal septum, nasal concha, and cranial base of FGF10 Tg mice (Fig. 3).
In conclusion, we made the unexpected observation of diverse site-specific dysmorphologies in development of the craniofacial skeletal in FGF10 Tg mice and site-specific expression of an alternative splicing variant of Fgfr2-IIIb, sFgfr2-IIIb. Further studies are necessary to dissect the regulatory mechanisms underlying these diverse effects of overexpression of FGF10 in relation to sFGFR2-IIIb.
CRediT authorship contribution statement
Hirotaka Yoshioka: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Visualization, Writing – review & editing. Kazuko Kagawa: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft. Tomoko Minamizaki: Conceptualization, Investigation, Methodology. Masashi Nakano: Investigation. Jane E. Aubin: Supervision, Writing – review & editing. Katsuyuki Kozai: Supervision. Kazuhiro Tsuga: Supervision. Yuji Yoshiko: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgements
We thank Dr. Jeffrey A. Whitsett for providing the tet(o)Fgf10 mice and Dr. Koh-ichi Kuremoto for his generous support. This work was supported by JSPS KAKENHI (#17K11804).
Contributor Information
Hirotaka Yoshioka, Email: yoshioka@iuhw.ac.jp.
Yuji Yoshiko, Email: yskyuji@yahoo.co.jp.
Data availability statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
- Abzhanov A., Tabin C.J. Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev. Biol. 2004;273:134–148. doi: 10.1016/j.ydbio.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Clark J.C., Tichelaar J.W., Wert S.E., Itoh N., Perl A.-K.T., Stahlman M.T., Whitsett J.A. FGF-10 disrupts lung morphogenesis and causes pulmonary adenomas in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L705–L715. doi: 10.1152/ajplung.2001.280.4.L705. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L., Spencer-Dene B., Revest J.M., Hajihosseini M., Rosewell I., Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Fedorov L.M., Tyrsin O.Y., Krenn V., Chernigovskaya E.V., Rapp U.R. Tet-system for the regulation of gene expression during embryonic development. Transgenic Res. 2001;10:247–258. doi: 10.1023/a:1016632110931. [DOI] [PubMed] [Google Scholar]
- Gonzaga S., Henriques-Coelho T., Davey M., Zoltick P.W., Leite-Moreira A.F., Correia-Pinto J., Flake A.W. Cystic adenomatoid malformations are induced by localized FGF10 overexpression in fetal rat lung. Am. J. Respir. Cell Mol. Biol. 2008;39:346–355. doi: 10.1165/rcmb.2007-0290OC. [DOI] [PubMed] [Google Scholar]
- Gupte V.V., Ramasamy S.K., Reddy R., Lee J., Weinreb P.H., Violette S.M., Guenther A., Warburton D., Driscoll B., Minoo P., Bellusci S. Overexpression of fibroblast growth factor-10 during both inflammatory and fibrotic phases attenuates bleomycin-induced pulmonary fibrosis in mice. Am. J. Respir. Crit. Care Med. 2009;180:424–436. doi: 10.1164/rccm.200811-1794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajihosseini M.K., Duarte R., Pegrum J., Donjacour A., Lana-Elola E., Rice D.P., Sharpe J., Dickson C. Evidence that Fgf10 contributes to the skeletal and visceral defects of an Apert syndrome mouse model. Dev. Dyn. 2009;238:376–385. doi: 10.1002/dvdy.21648. [DOI] [PubMed] [Google Scholar]
- Hart A., Papadopoulou S., Edlund H. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev. Dyn. 2003;228:185–193. doi: 10.1002/dvdy.10368. [DOI] [PubMed] [Google Scholar]
- Hinoi E., Bialek P., Chen Y.-T., Rached M.-T., Groner Y., Behringer R.R., Ornitz D.M., Karsenty G. Runx2 inhibits chondrocyte proliferation and hypertrophy through its expression in the perichondrium. Genes Dev. 2006;20:2937–2942. doi: 10.1101/gad.1482906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G., Bromage T.G., Basilico C. The Sox2 high mobility group transcription factor inhibits mature osteoblast function in transgenic mice. Bone. 2011;49:653–661. doi: 10.1016/j.bone.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.H., Schoenwolf G.C., Lewandoski M., Ornitz D.M. A combined series of Fgf9 and Fgf18 mutant alleles identifies unique and redundant roles in skeletal development. Dev. Biol. 2016;411:72–84. doi: 10.1016/j.ydbio.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M., Finch P.W., Aaronson S.A. Characterization of recombinant human fibroblast growth factor (FGF)-10 reveals functional similarities with keratinocyte growth factor (FGF-7) J. Biol. Chem. 1998;273:13230–13235. doi: 10.1074/jbc.273.21.13230. [DOI] [PubMed] [Google Scholar]
- Itoh N. FGF10: a multifunctional mesenchymal–epithelial signaling growth factor in development, health, and disease. Cytokine Growth Factor Rev. 2016;28:63–69. doi: 10.1016/j.cytogfr.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Jacob A.L., Smith C., Partanen J., Ornitz D.M. Fibroblast growth factor receptor 1 signaling in the osteo-chondrogenic cell lineage regulates sequential steps of osteoblast maturation. Dev. Biol. 2006;296:315–328. doi: 10.1016/j.ydbio.2006.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman M. Academic Press; 1992. The Atlas of Mouse Development. [Google Scholar]
- Kim B., Shin H., Kim W., Kim H., Cho Y., Yoon H., Baek J., Woo K., Lee Y., Ryoo H. PIN1 attenuation improves midface hypoplasia in a mouse model of Apert syndrome. J. Dent. Res. 2020;99:223–232. doi: 10.1177/0022034519893656. [DOI] [PubMed] [Google Scholar]
- Kimmel C.A., Trammell C. A rapid procedure for routine double staining of cartilage and bone in fetal and adult animals. Stain. Technol. 1981;56:271–273. doi: 10.3109/10520298109067325. [DOI] [PubMed] [Google Scholar]
- Kistner A., Gossen M., Zimmermann F., Jerecic J., Ullmer C., Lubbert H., Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1996;93:10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch V.D., Bassett J.H.D., Williams G.R. Role of thyroid hormones in craniofacial development. Nat. Rev. Endocrinol. 2020;16:147–164. doi: 10.1038/s41574-019-0304-5. [DOI] [PubMed] [Google Scholar]
- Marchese C., Felici A., Visco V., Lucania G., Igarashi M., Picardo M., Frati L., Torrisi M.R. Fibroblast growth factor 10 induces proliferation and differentiation of human primary cultured keratinocytes. J. Invest. Dermatol. 2001;116:623–628. doi: 10.1046/j.0022-202x.2001.01280.x. [DOI] [PubMed] [Google Scholar]
- McBratney-Owen B., Iseki S., Bamforth S.D., Olsen B.R., Morriss-Kay G.M. Development and tissue origins of the mammalian cranial base. Dev. Biol. 2008;322:121–132. doi: 10.1016/j.ydbio.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosa S., Wollnik B. Altered FGF signalling in congenital craniofacial and skeletal disorders. Semin. Cell Dev. Biol. 2016;53:115–125. doi: 10.1016/j.semcdb.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Ohta H., Itoh N. Roles of FGFs as adipokines in adipose tissue development, remodeling, and metabolism. Front. Endocrinol. 2014;5:18. doi: 10.3389/fendo.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney R.C., Wang J., Sylvester J.E., Mougey E.B. Growth factor regulation of human growth plate chondrocyte proliferation in vitro. Biochem. Biophys. Res. Commun. 2004;317:1171–1182. doi: 10.1016/j.bbrc.2004.03.170. [DOI] [PubMed] [Google Scholar]
- Ornitz D.M., Itoh N. The fibroblast growth factor signaling pathway. Dev. Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D.M., Marie P.J. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 2015;29:1463–1486. doi: 10.1101/gad.266551.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa S., Kuremoto K., Seidel K., Tabatabai R., MacKenzie B., Yamaza T., Akiyama K., Branch J., Koh C.J., Alam D.A., Klein O.D., Bellusci S. Signaling by FGFR2b controls the regenerative capacity of adult mouse incisors. Development. 2010;137:3743–3752. doi: 10.1242/dev.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine S.M.M., Pitirri M.K., Durham E.L., Kawasaki M., Zheng H., Chen D.Z., Kawasaki K., Richtsmeier J.T. A dysmorphic mouse model reveals developmental interactions of chondrocranium and dermatocranium. eLife. 2022;11 doi: 10.7554/eLife.76653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R., Spencer-Dene B., Connor E.C., Gritli-Linde A., McMahon A.P., Dickson C., Thesleff I., Rice D.P.C. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J. Clin. Invest. 2004;113:1692–1700. doi: 10.1172/JCI20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue H., Konishi M., Ogawa W., Asaki T., Mori T., Yamasaki M., Takata M., Ueno H., Kato S., Kasuga M., Itoh N. Requirement of fibroblast growth factor 10 in development of white adipose tissue. Genes Dev. 2002;16:908–912. doi: 10.1101/gad.983202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine K., Ohuchi H., Fujiwara M., Yamasaki M., Yoshizawa T., Sato T., Yagishita N., Matsui D., Koga Y., Itoh N., Kato S. Fgf10 is essential for limb and lung formation. Nat. Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- Shimoaka T., Ogasawara T., Yonamine A., Chikazu D., Kawano H., Nakamura K., Itoh N., Kawaguchi H. Regulation of osteoblast, chondrocyte, and osteoclast functions by fibroblast growth factor (FGF)-18 in comparison with FGF-2 and FGF-10. J. Biol. Chem. 2002;277:7493–7500. doi: 10.1074/jbc.M108653200. [DOI] [PubMed] [Google Scholar]
- Su N., Jin M., Chen L. Role of FGF/FGFR signaling in skeletal development and homeostasis: learning from mouse models. Bone Res. 2014;2:14003. doi: 10.1038/boneres.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao F., Takahashi I., Mitani H., Haruyama N., Sasano Y., Suzuki O., Takano-Yamamoto T. Fibroblast growth factor 10 regulates Meckel’s cartilage formation during early mandibular morphogenesis in rats. Dev. Biol. 2011;350:337–347. doi: 10.1016/j.ydbio.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Wang F., Kan M., McKeehan K., Jang J.-H., Feng S., McKeehan W.L. A homeo-interaction sequence in the ectodomain of the fibroblast growth factor receptor. J. Biol. Chem. 1997;272:23887–23895. doi: 10.1074/jbc.272.38.23887. [DOI] [PubMed] [Google Scholar]
- White K.E., Cabral J.M., Davis S.I., Fishburn T., Evans W.E., Ichikawa S., Fields J., Yu X., Shaw N.J., McLellan N.J., McKeown C., FitzPatrick D., Yu K., Ornitz D.M., Econs M.J. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am. J. Hum. Genet. 2005;76:361–367. doi: 10.1086/427956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie A.O.M., Morriss-Kay G.M. Genetics of craniofacial development and malformation. Nat. Rev. Genet. 2001;2:458–468. doi: 10.1038/35076601. [DOI] [PubMed] [Google Scholar]
- Yoshida C.A., Yamamoto H., Fujita T., Furuichi T., Ito K., Inoue K., Yamana K., Zanma A., Takada K., Ito Y., Komori T. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 2004;18:952–963. doi: 10.1101/gad.1174704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Ibrahimi O.A., Olsen S.K., Umemori H., Mohammadi M., Ornitz D.M. Receptor specificity of the fibroblast growth factor family. J. Biol.Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q., Zhou G., Morello R., Chen Y., Garcia-Rojas X., Lee B. Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte–specific expression in vivo. J. Cell Biol. 2003;162:833–842. doi: 10.1083/jcb.200211089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.