Abstract
The efficient delivery of small interfering RNA (siRNA) to the targeted cells significantly affects the regulation of the overexpressed proteins involved in the progression of several genetic diseases. SiRNA molecules in naked form suffer from low internalization across the cell membrane, high susceptibility to degradation by nuclease enzyme and low stability, which hinder their efficacy. Therefore, there is an urge to develop a delivery system that can protect siRNA from degradation and facilitate their uptake across the cell membrane. In this study, the cationic lipid (GL67) was exploited, in addition to DC-Chol and DOPE lipids, to design an efficient liposomal nanocarrier for siRNA delivery. The physiochemical characterizations demonstrated that the molar ratio of 3:1 has proper particle size measurements from 144 nm to 332 nm and zeta potential of −9 mV to 47 mV that depends on the ratio of the GL67 in the liposomal formulation. Gel retardation assay exhibited that increasing the percentage of GL67 in the formulations has a good impact on the encapsulation efficiency compared to DC-Chol. The optimal formulations of the 3:1 M ratio also showed high metabolic activity against A549 cells following a 24 h cell exposure. Flow cytometry findings showed that the highest GL67 lipid ratio (100 % GL67 and 0 % DC-Chol) had the highest percentage of cellular uptake. The lipoplex nanocarriers based on GL67 lipid could potentially influence treating genetic diseases owing to the high internalization efficiency and safety profile.
Keywords: GL67 lipid, Liposomes, Gene therapy, siRNA delivery, A549 cells
1. Introduction
Gene therapy delivers genetic materials into cells to treat genetic disorders by introducing a normal and functional copy of a defective gene (Batty and Lillicrap, 2019) or an abnormal allele with a new function (Navarro et al., 2016, Levine et al., 2017). Other strategies for gene therapy involve CRISPR-Cas–mediated mutation repair or gene editing and silencing genetic abnormality using small interfering RNA (siRNA) (Maurer et al., 2018, Kristen et al., 2019, Gillmore et al., 2021). The latter shows many significant advantages over the other strategies of gene therapy. The mechanism of RNA interference by double-stranded RNA (dsRNA) was a scientific breakthrough discovered in 1998 by Prof. Andrew Fire and Prof. Craig Mello that granted them the Nobel Prize in 2006. The gene-silencing phenomenon involves the specific degradation of a targeted messenger RNA (mRNA) by generating 21 to 26 nt RNA segments that guide endonucleolytic cleavage of the mRNA (Fire et al., 1998, Grishok et al., 2001, Fire, 2007). Using siRNA reduces the possibility of DNA mutations as it does not directly interact with chromosomal DNA but targets messenger RNA (mRNA) in the cytoplasm through complementing base pairing to inhibit the process of gene transcription (Bennett and Swayze 2010).
Additionally, gene silencing using siRNA efficiently limits precisely targeted mRNA and provides immense advantages in treatment cost and preparation. However, there are several challenges in delivering naked siRNAs due to their physicochemical properties, such as hydrophilicity, negative charge, instability and cytotoxicity. The hydrophilic nature of siRNA complicates its entry into the cell through passive transport, which generally results in inefficient uptake (Wang et al., 2010). Consequently, naked and unmodified siRNAs have a short half-life of < 5 min to (15–60 mins) due to their fast degradation by RNAases and serum endonucleases and rapid excretion (Bartlett and Davis, 2007, Gao et al., 2009, Paul et al., 2022). Moreover, using a high concentration of naked siRNA could lead to an off-target effect (Persengiev et al., 2004).
For this reason, many siRNA delivery systems have been developed to overcome cellular barriers and transport siRNA to the intracellular target site (Wang et al., 2010). Lipid-based siRNA delivery systems, particularly liposomes, are preferable siRNA carriers owing to their FDA approval as a safe delivery system for human use (Ozpolat et al., 2014). Although many studies demonstrated the successful delivery of siRNA by neutral liposomes (Landen et al., 2005), cationic lipids have shown promising results for siRNA delivery than other lipid-based siRNA delivery systems (Xia et al., 2016). Cationic lipids interact electrostatically with the anionic siRNA to form lipoplexes with multilamellar structures that prevent siRNA degradation and enhance siRNA cellular internalization. There are three parts of cationic lipids, namely a cationic functional group, a lipophilic hydrocarbon tail, and a connecting linker, which define the transfection efficiency and toxicity of the carrier. In contrast, the ratio of lipids and siRNA is vital in optimizing a positive zeta potential that can enhance their cellular uptake (Wang et al., 2010). Many novel cationic lipids were designed and developed to improve gene delivery and overcome physiological barriers.
The cationic lipid N4-cholesteryl-spermine, also known as Genzyme Lipid (GL67), is one of the most effective non-viral gene carriers comprising spermine as a headgroup that bond in a T-shape configuration to a cholesterol anchor. GL67 was found to be very effective for gene delivery of a plasmid encoding chloramphenicol acetyltransferase (CAT) with 100-fold more than one of the most commonly used cationic lipids for siRNA delivery, dimethylaminoethane-carbamoyl-cholesterol liposome (DC-Chol) (Caplen et al., 1995, Lee et al., 1996). In another preclinical study, GL67A, consisting of a mixture of GL67–DOPE–DMPE-PEG5000, performed better as an aerosol for gene delivery to the sheep lung than other gene transfer agents such as polyethyleneimine and polyethylene glycol-substituted lysine (McLachlan et al., 2011). Also, GL67 has demonstrated a potential carrier for delivering siRNA to lung cells. An in vivo study conducted by Griesenbach et al. showed a modest reduction in the expression of β-galactosidase in the respiratory tract of a mouse lung by 30 % using a GL67/siRNA complex (Griesenbach et al., 2006). However, few studies have examined the efficiency of liposome nanoparticles based on GL67 lipid for siRNA delivery.
When comparing the structure of GL67 and DC-Chol, both have cholesterol moiety, which does not have a role in the cationic properties of those two cationic lipids. The cationic properties are caused by the functional group that binds to the cholesterol moiety. N',N'-dimethylaminoethane moiety is the functional group responsible for the cationic properties of DC-Chol, which contains secondary and tertiary amines. It is expected that the structure of GL67 can be more cationic than DC-Chol due to the presence of a spermine functional group that contains two primary amines, a secondary amine and a tertiary amine.
To our knowledge, the comparison between DC-Chol and GL67 regarding physiochemical properties, encapsulation and cellular uptake efficiencies has yet to be studied or investigated extensively. Therefore, this study assessed the delivery of siRNA using liposomal nanocarriers composed of different percentages of two cationic liposomes, namely, GL67 and DC-Chol, and a helper lipid, DOPE. The physicochemical properties of the prepared formulations were evaluated in terms of particle size, zeta potential, encapsulation efficiency, cytotoxicity, and cellular uptake. The result of this work may provide insight into the possibility of using GL67 as a carrier for siRNAs.
2. Materials and methods
2.1. Materials
Dulbecco’s Modified Eagle’s Medium (DMEM) and Accutase® solution were purchased from Sigma-Aldrich (Gillingham, UK), while Opti-MEM™ Reduced-Serum Medium was obtained from Thermo Fisher Scientific (Northumberland, UK). SYBR™ Safe and Lipofectamine 2000™ were bought from Invitrogen (Warrington, UK). The cationic lipids 3ß-[N-(N',N'-dimethylaminoethane)-carbamoyl]cholesterol hydrochloride (DC-Cholesterol), N4-cholesteryl-spermine HCl salt, which is also known as Genzyme Lipid 67 (GL67) and zwitterionic lipid 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) were all purchased from Avanti-polar lipids (Birmingham, UK). Human alveolar type II epithelial lung carcinoma cell line (i.e., A549) was obtained from the American type culture collection (ATCC; CCL-185). Silencer negative control no.1 siRNA and Cy3-siRNA were bought from Thermo Fisher Scientific (Northumberland, UK). CellTiter 96® Aqueous one solution cell proliferation assay (MTS assay) was obtained from Promega (Southampton, UK). The LDH assay kit was purchased from Sigma-Aldrich (Gillingham, UK). Distilled water was generated through Milli Q (Millipore Corporation, Bedford, MA, USA) and applied throughout the study.
2.2. Liposomal nanoparticles preparation
Following the modified method, the thin-film hydration method was applied to formulate liposome nanoparticles (Alzahrani et al., 2022). Briefly, the two cationic lipids used in this work, DC-Chol and GL67 and the neutral lipid (i.e., DOPE), were initially dispersed in the organic solvent according to the manufacturer’s instructions (methanol–chloroform mixture of 5:1 ratio). The three lipids were mixed in a round-bottom flask with different molar ratios. Then, the organic solvent was evaporated by nitrogen gas until a thin film formed at the bottom of the flask. Next, the developed thin film was hydrated with distilled water containing siRNA molecules; then, it was vortexed for a few seconds until the lipid was dispersed. Once the dispersed lipids interact with water, they form a lamellar (either bilayer or multilamellar) structure. Subsequently, the mixture was transferred into an Avanti mini-extruder quipped with a 200 nm polycarbonate filter and passed 21 times to reduce the overall particles size and convert the multilamellar vesicles (MLV) to unilamellar vesicles (ULV) with the proper size. The molar ratio between the cationic lipid moiety and DOPE lipid in the formulation was adjusted to be 1:1 M ratio. The liposomal nanoparticles were prepared in three different molar ratios of cationic lipid unit to siRNA phosphate backbone unit, which was 1:1, 2:1 and 3:1, with an increased molar ratio of GL67 lipid in the formulation as shown in Table 1.
Table 1.
Formulations were prepared at different molar ratios between cationic lipid unit to siRNA phosphate backbone unit and different content of cationic lipids.
| Formulations | Molar ratio |
Molar cationic lipids percentage |
||
|---|---|---|---|---|
| GL67 | DC-Chol | |||
| A1 | 1:1 | 0 % | 100 % | |
| A2 | 20 % | 80 % | ||
| A3 | 40 % | 60 % | ||
| A4 | 60 % | 40 % | ||
| A5 | 80 % | 20 % | ||
| A6 | 100 % | 0 % | ||
| B1 | 2:1 | 0 % | 100 % | |
| B2 | 20 % | 80 % | ||
| B3 | 40 % | 60 % | ||
| B4 | 60 % | 40 % | ||
| B5 | 80 % | 20 % | ||
| B6 | 100 % | 0 % | ||
| C1 | 3:1 | 0 % | 100 % | |
| C2 | 20 % | 80 % | ||
| C3 | 40 % | 60 % | ||
| C4 | 60 % | 40 % | ||
| C5 | 80 % | 20 % | ||
| C6 | 100 % | 0 % | ||
2.3. Particle size and zeta potential measurement
Zetasizer Nano Series (Malvern Instruments, Malvern, UK) was used to measure the particle size and zeta potential of all prepared lipoplexes. The measurements were conducted in distilled water at pH 7.4 and ambient temperature, and the results were presented as three independent measurements' mean ± standard deviation (SD).
2.4. Determination of encapsulation efficiency (EE%) of lipoplexes
Gel retardation assay was used to evaluate the loading efficiency of siRNA into liposomal nanoparticles and to determine the optimum complexed formulation. Following the preparation of liposomes, 20 µL of each formulation was loaded into 1 % agarose gel and was mixed with SYBR™ Safe; then, the samples were run for 45–60 min at 60 V. Subsequently, the gel was imaged under a UV imaging system (BioRad Gel Doc™ EZ System gel documentation system, California, USA) to detect the siRNA loading efficiency.
2.5. In vitro cytotoxicity assessment of lipoplexes
In vitro cytotoxicity of the lipoplex formulations was evaluated using two colorimetric assays, MTS and LDH, following a 24 h cell exposure of lipoplexes to the A549 cells. The in vitro metabolic activity assessment of the liposomal formulations was performed using the MTS assay, according to (Alzahrani et al., 2022). At the same time, the membrane integrity of A549 cells was evaluated using an LDH assay similar to (Alkahtani et al., 2021a, Alkahtani et al., 2021b). DMEM supplemented with streptomycin 100 µg/mL, penicillin 100 U/mL, and 10 % (v/v) fetal bovine serum (FBS) was used to feed the living cells. Trypsin solution was then incubated with the cells for a few minutes to harvest the cells from the flask, then seeding 1.5 × 104 cells per well into 96-well plates. The cells were incubated overnight in a cell culture incubator at 37 °C and 5 % CO2. 100 µL of six different lipoplexes encapsulated with siRNA with a molar ratio of 3:1 was then exposed to the human cells for 24 h. Cells incubated with DMEM only were used as a negative control, whereas cells incubated with 0.1 % triton x-100 were used as a positive control.
For the MTS assay, the consumed medium was aspirated from each well, and 100 µL of fresh DMEM was added, followed by 20 µL of the MTS reagent. The cells were incubated for 2–3 h at 37 °C and 5 % CO2. Cytation 3 absorbance microplate reader (BIOTEK Instruments Inc., Winooski, VT, USA) was used at 492 nm to measure the ability of the cells to produce formazan color upon the living, and the following equation calculated the relative cell viability (%) (Alyami et al., 2022):
| (1) |
where S is the absorbance of the cells treated with the applied lipoplexes, T is the absorbance of the cells treated with triton x-100, and H is the absorbance of the cells treated with DMEM. The results were presented as the mean ± SD of at least three independent measurements.
For the LDH assay, 50 μL of the treated samples containing DMEM and the cytoplasmic LDH enzyme released from the disrupted cellular membrane was transferred to a new 96-well plate. Next, 100 μL of the LDH reaction mixture was added to each well and incubated at room temperature for 30 min. Next, the absorbance at 492 nm was measured using the Cytation 3 microplate reader. The percentage of LDH release and relative cell viability was calculated using the following equations (Alkahtani et al., 2021a, Alkahtani et al., 2021b):
| (2) |
| (3) |
where S is the absorbance of the cells treated with the lipoplexes, H is the absorbance of the negative cytotoxicity control, and T is the absorbance of the positive cytotoxicity control.
2.6. Cellular uptake assessment of Cy3-lipoplexes
A549 cells were seeded onto 24-well plates at a seeding density of 5 × 104 cells/well and cultured overnight in 500 µL of DMEM. Cells were then washed with phosphate buffer saline (PBS; pH 7.4), and the samples of the cy3-siRNA loading liposomes were added to the cells in OptiMEM® serum-reduced medium and incubated for 24 h. The transfection reagent Lipofectamine® was used as a positive control, following the manufacturer's protocol. Next, the cells were harvested from the wells using Accutase® solution and transferred into Eppendorf tubes. The cellular uptake was assessed using flow cytometry (Beckman Coulter Cytomics FC500 Flow cytometer, USA), with a minimum of 10,000 events set at FL2 and a medium flow rate of 30 µL/min. The targeted cell population was gated to investigate the change in fluorescent intensity in cell groups treated with cy3-siRNA. The results are histograms of cy3-siRNA fluorescence intensity against the cell count.
2.7. Statistical analysis
Microsoft Excel 2019 software (Microsoft Corporation, Redmond, MA, USA) was used to calculate the mean and the SD of this study’s results. Student's t-tests were performed to compare two group means, and a p-value of < 0.05 was considered statistically significant. * displays p < 0.05, and 'ns' indicates non-significant (p > 0.05).
3. Results
3.1. Particle size and zeta potential measurement
The particle size and zeta potential were measured to find the suitable molar ratio between the cationic lipid of the liposomes to the phosphate backbone of the siRNA and assess the impact of GL67 and DC-Chol contents on both parameters. In Table 2, the particle size and zeta potential were determined after the preparation of liposomal formulations at a molar ratio of 1:1. The particle size showed that increasing the content of GL67 lipid could raise the size of the particles, as the measured sizes were increased from 292 nm to 557 nm, in 0 % and 100 % content of GL67 lipid, respectively. Additionally, it can be observed that the negative charge of the zeta potential was decreased from −26 mV in the A1 formulation, which contained 100 % DC-Chol, to −11 mV in A6, which had 100 % GL67.
Table 2.
Particle size and zeta potential of the formulations prepared at a 1:1 M ratio.
| Formulations | Particle size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|
| A1 | 292 ± 61 | 0.379 ± 0.08 | −26 ± 3 |
| A2 | 90 ± 7 | 0.684 ± 0.04 | −21 ± 7 |
| A3 | 137 ± 17 | 0.602 ± 0.05 | –32 ± 3 |
| A4 | 228 ± 9 | 0.376 ± 0.05 | –22 ± 0.3 |
| A5 | 152 ± 5 | 0.267 ± 0.01 | −21 ± 4 |
| A6 | 557 ± 223 | 0.599 ± 0.03 | −11 ± 3 |
Since the zeta potential is still negatively charged, the molar ratio was increased to 2:1, as shown in Table 1. A slight increase in the particle sizes was obtained upon increasing the contents of GL67 lipid, with the B1 formulation (0 % of GL67 lipid) and B6 formulation (100 % of GL67 lipid) measuring 307 nm and 367 nm, respectively. Zeta potential results exhibited that the negative charge of liposomes was decreased by increasing the content of the cationic GL67 lipid in the formulation. The zeta potential of the highest content of GL67 lipid (i.e., B6) was shown to be −2 mV, closer to the neutral charge.
However, since the cellular uptake of the lipoplex nanoparticles is expected to occur when the net charge is positively charged, the ratio of the cationic lipid was increased to reach the molar ratio of 3:1 (Table 4). The particle sizes of all the formulations at this ratio were lower than 340 nm, and the size was increased by increasing the contents of GL67 lipid from 144 nm (C1) to 311 nm (C6). At the 3:1 ratio, only one formulation has a negative zeta potential value (C1) with only cationic DC-Chol. The positive Zeta potential values tend to increase by increasing the GL67 ratio in the formulation, which reached 47 mV in C6 (100 % of GL67 lipid).
Table 4.
Particle size and zeta potential of the formulations prepared at a 3:1 M ratio.
| Formulation | Particle size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|
| C1 | 144 ± 18 | 0.482 ± 0.06 | −9 ± 2 |
| C2 | 302 ± 27 | 0.412 ± 0.05 | 6 ± 1 |
| C3 | 332 ± 57 | 0.602 ± 0.13 | 28 ± 1 |
| C4 | 315 ± 34 | 0.563 ± 0.11 | 25 ± 1 |
| C5 | 162 ± 16 | 0.261 ± 0.01 | 29 ± 1 |
| C6 | 311 ± 2 | 0.364 ± 0.02 | 47 ± 2 |
3.2. Determination of encapsulation efficiency (EE%) of lipoplexes
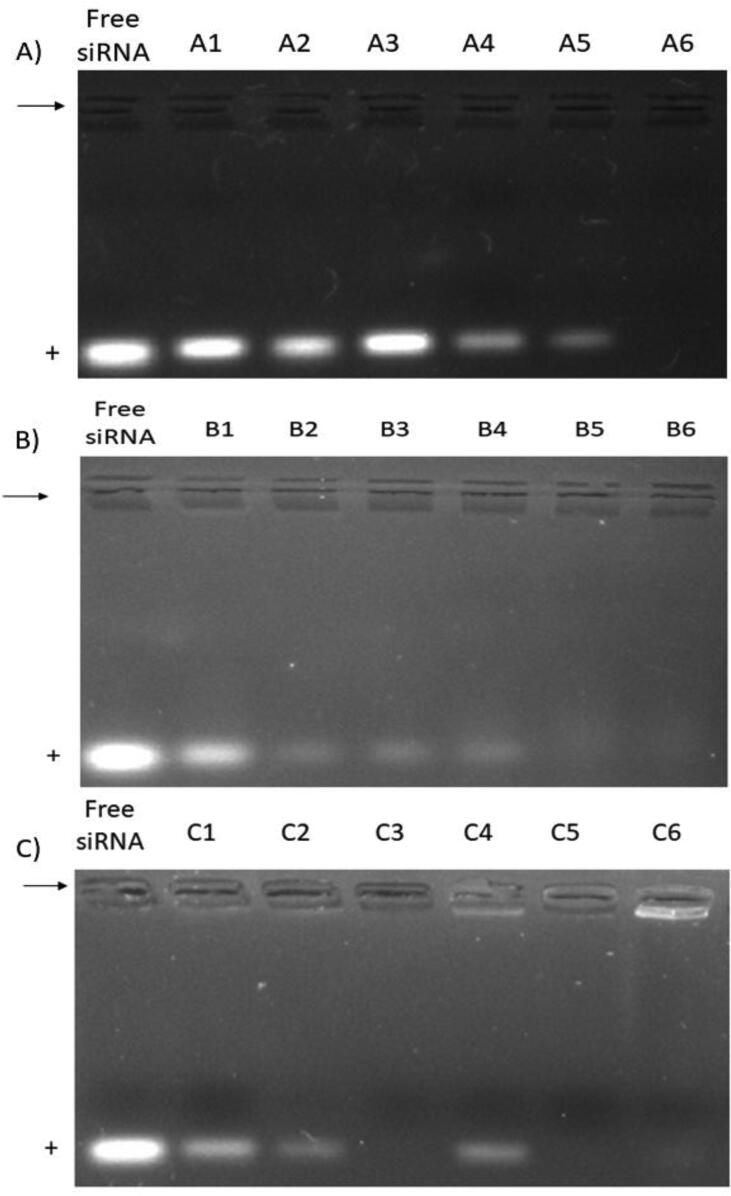
The expected encapsulation and the comparison between the encapsulation efficiency of each molar ratio will be assessed by gel retardation assay. The synthesized formulations were loaded into agarose gels and then transferred to electrophoresis. Fig. 1 (A) represents the UV image of the agarose gel loaded with a 1:1 M ratio formulation. It was observed that the band intensity of the free siRNA, which migrated toward the anode, decreased by increasing the ratio of GL67, and the band completely disappeared from the gel at the highest percentage of GL67 lipid in the formulation (A6), indicating the complete encapsulation of siRNA. Increasing the ratio to 2:1 Fig. 1 (B) showed improvement in siRNA's encapsulation and binding efficiency. The band intensity was decreased by increasing the content of GL67 lipid in the formulation and disappeared from the B5 formulation, which could indicate its complete encapsulation of siRNA. In Fig. 1 (C), the molar ratio of 3:1 showed a band of the free siRNA disappearing from the lower content of GL67 lipid (C3), except for C4, which unexpectedly showed a low band.
Fig. 1.
Encapsulation of siRNA in cationic liposome at 1:1 (A), 2:1 (B), and 3:1 (C) molar ratio formulations. Arrows indicate the site of wells. The anode site is marked with a positive sign.
3.3. In vitro cytotoxicity assessment of lipoplexes
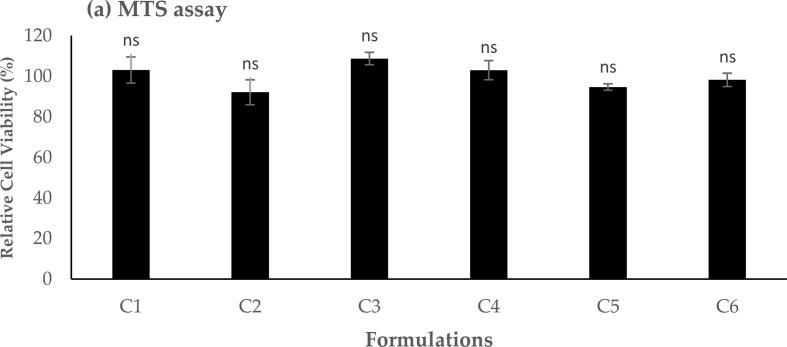
MTS assay can be applied to define the optimal safe dose for further studies and biomedical applications. Here, six different lipoplex formulations encapsulated with a molar ratio of 3:1 were evaluated against A549, and the results are presented in Fig. 2, Fig. 3. The effect of different formulations on the cellular metabolic activity of A549 cells after a 24 h cellular exposure exhibited that all tested nanoparticles have not significantly reduced the relative metabolic activity (p > 0.05) of the living cellular model in comparison to the positive control cells with 100 % cellular viability (Fig. 2). The application of lipoplexes demonstrated a high cellular viability of A549 (i.e., > 85 %) for all formulations over 24 h, which could be relatively safe at different contents of GL67 lipid.
Fig. 2.
MTS assay result showed the relative cell viability of different lipoplex formulations upon 24 h A549 cells exposure. Results are presented as the mean ± SD (n = 3), and ‘ns’ indicates no significant difference (p > 0.05).
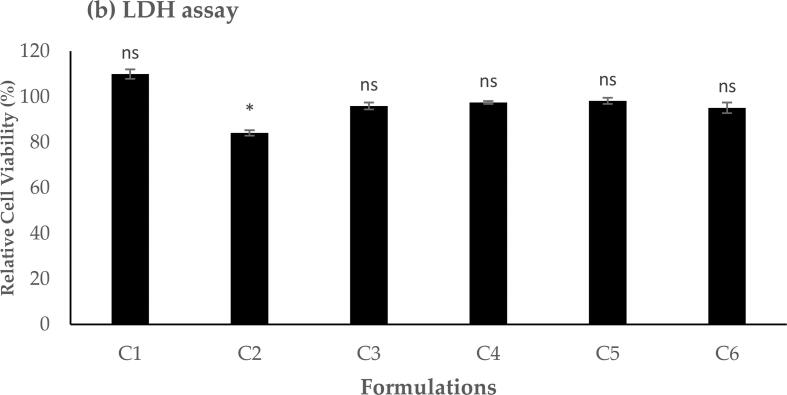
Fig. 3.
LDH assay result showed the relative cell viability of different lipoplex formulations upon 24 h A549 cell exposure. Results are presented as the mean ± SD (n = 3). * indicates a significant difference (p < 0.05), whereas ‘ns’ indicates no significant difference (p > 0.05).
In addition to evaluating A549 cells' metabolic activity using the MTS assay, the effect of the applied liposomal formulations on the membrane integrity of the cells was assessed using LDH assay, and the results are presented in Fig. 3. LDH is a cytoplasmic enzyme that can be released and quantified in a cell culture medium when the treated cellular membrane is disrupted (Alkahtani et al., 2021a, Alkahtani et al., 2021b). The results demonstrated a high cell viability and membrane integrity (>80 %) following 24 h cellular exposure to the lipoplex formulations at different contents of GL67 lipid with no significant difference (p > 0.05). However, the formulation of C2 shows a significant decrease in membrane integrity and cell viability (p < 0.05).
The overall in vitro cytotoxicity results (in Fig. 2, Fig. 3) demonstrated a high safety profile of A549 cells treated with liposomes with different contents of GL67 lipid.
3.4. Cellular uptake assessment of Cy3-lipoplexes
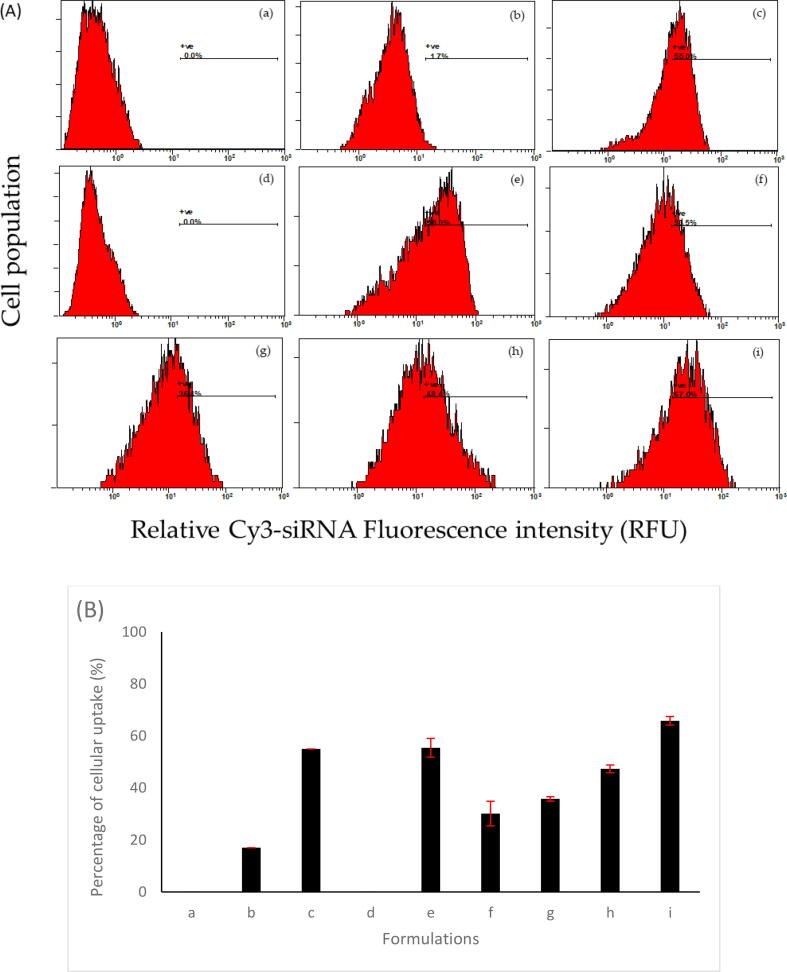
The previous physicochemical characterizations, which include the positive surface charge of the formulation and disappearance of the free siRNA band in the gel retardation assay that indicates full encapsulation of siRNA, do not guarantee that the optimized formulations can be engulfed by the cells. Therefore, the cellular uptake of the cy3-labelled lipoplexes was assessed using flow cytometry by investigating the cells that exhibited an elevated level of cy3-siRNA fluorescence. The results are summarized in Fig. 4. Trypan blue was utilized in the uptake experiment to quench the fluorescent dye only associated with the A549 cell membrane but did not reach the cytoplasmic compartments. Untreated cells (without siRNA), cells incubated with free cy3-siRNA molecules and cells treated with cy3-siRNA complexed with the golden standard transfection reagent (Lipofectamine®) were considered as blank, negative and positive controls, respectively. The use of Lipofectamine® as a positive control was based on its ability to transfect a wide range of cell types, including A549 cells (Chong et al., 2021). The data determined in this study are relative to the cells not treated with cy3-lipoplexes (Fig. 4a).
Fig. 4.
(A) Cellular uptake histograms and (B) chart of cy3-lipoplex formulations into A549 cells. Flow cytometry results show the percentage of cellular uptake after applying different liposomal nanocarriers to A549 cells at a molar ratio of 3:1, the molar ratio of cationic lipid to the zwitterionic lipid of 1:1, and gradually increased content of GL67 to DC-Chol. (a) Untreated cells with no cy3-siRNA exposure as blank, (b) cells incubated with free cy3-siRNA as a negative control, and (c) cells incubated with cy3-siRNA Lipofectamine nanocomplex as a positive control. The other histograms are shown for the different ratios of GL67:DC-Chol as (d) C1, (e) C2, (f) C3, (g) C4, (h) C5 and (i) C6, respectively.
The obtained histograms in Fig. 4 exhibited an A549 cell population with associated cy3-siRNA following 24 h exposure. GL67 cationic lipids in the liposomal formulations demonstrated a significant proportion of nanoparticle internalization across the cellular membrane. Data showed that increased cationic lipid content (i.e., GL67) in the formulations considerably induced the internalization of cy3-siRNA across the A549 cell membrane. Increasing the content of GL67 lipid in the total cationic lipids from 0 % to 100 % raised the successful internalization of liposomes-loaded cy3-siRNA from 0 % (C1, Fig. 4d) to approximately 67 % of the cell population (C6, Fig. 4i). Moreover, the achieved cellular uptake of C6 was even more than the one obtained following the exposure of A549 cells with the positive control, i.e., Lipofectamine®, which was around 55 % of the cell population (Fig. 4c).
4. Discussion
In this work, we compare the differences between the DC-Chol and GL67 cationic lipids and prove the superiority of GL67 to DC-Chol in terms of physicochemical characterizations and cellular uptake. Firstly, we compared the particle size, surface charge, and encapsulation efficiency of different formulations containing different molar ratios and composition ratios of the two cationic lipids, GL67 and DC-Chol. The small molecules, such as the free siRNA, can be excreted through the kidneys. In contrast, larger particles can be detected and removed from the blood circulation by the mononuclear phagocyte system (MPS) (Wang et al., 2016). The zeta potential is considered an indicator of the surface charge, and the more positive values will prevent the aggregation of the formulation and enhance its stability (Subhan and Torchilin 2019). Therefore, assessing the physicochemical properties of the prepared nanoparticles is crucial and essential in understanding the interactions of nanoparticles with plasma proteins (Clogston 2021).
In Table 2, the lowest molar ratio was used. It can be observed from the result that the particle sizes are small in general except in formulation A6 which can be attributed to the low concentration of lipid composition of the formulations in comparison to the 2:1 and 3:1 M ratios. It has been reported that the light scatter analysis of siRNA-liposome nanoparticles based on GL67 lipid demonstrated a positively charged formulation with a particle size of 294 nm (Griesenbach et al., 2006). The controlling of liposomes particle size is an essential parameter since its effect on the successful delivery of loaded molecules to the targeted cells and the circulation half-life of the prepared nanoparticles (Nsairat et al., 2022, van der Koog et al., 2022). The reason behind the achieved small particle size of prepared liposomal nanoparticles is that the collected liposomes, following the hydration of lipids thin film, usually passed through an extruder equipped with a small pore size membrane to reduce the particle size of liposome nanoparticles.
The net surface charge obtained at a 1:1 M ratio (Table 2) is highly negative, which causes a repulsion force between the particles except in A6, which has the higher size among them, which can be caused by the low negative charge that is close to neutral charge which can cause aggregation of the formulation. In Table 3, the zeta potential is still highly negative in B1, B2, and B3, and the particle sizes are relatively small. In B4, B5, and B6, the negative charge intensity of the formulations become lower to be closer to the neutral charge, which can cause the particle sizes to increase, which can be due to the aggregation of the formulations. In Table 4, the formulations' sizes are generally lower than that observed in Table 3. The zeta potential is positively charged and highly positive in formulation C6 which contains GL67 and does not contain DC-Chol. Therefore, it can be observed from the results in general that the formulations whose surface charges are closer to the neutral tend to aggregate and have larger particle sizes compared to the formulations with high negative or positive surface charges, as observed in A6 and B4 formulations.
Table 3.
Particle size and zeta potential of the formulations prepared at a 2:1 M ratio.
| Formulation | Particle size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|
| B1 | 307 ± 11 | 0.271 ± 0.004 | −21 ± 3 |
| B2 | 240 ± 5 | 0.289 ± 0.016 | −24 ± 2 |
| B3 | 333 ± 55 | 0.384 ± 0.094 | –22 ± 1 |
| B4 | 728 ± 48 | 0.494 ± 0.004 | −8 ± 3 |
| B5 | 443 ± 23 | 0.403 ± 0.029 | −11 ± 1 |
| B6 | 367 ± 35 | 0.384 ± 0.055 | −2 ± 3 |
Different factors could attribute the preparation of small-sized liposomes with low PDI and minimal aggregation. For instance, the extrusion of prepared nanoparticles through a polycarbonate membrane with a small pore size to formulate nanosized liposomes (Guo et al., 2018). Moreover, sonication is one of the conventional methods that can be implemented to convert the multilamellar liposomes to unilamellar liposomes with smaller particles size (Lombardo and Kiselev, 2022). Additionally, zeta potential is considered a key parameter to determine the surface charge and understand the physical stability of nanoparticles. The sufficient repulsive force between individual particles is crucial to attaining optimal physical colloidal stability of nanoparticles. Zeta potential with more positive than + 30 mV or more negative than −30 mV usually is considered stable. However, the aggregation and flocculation of nanoparticles can result from a small zeta potential value, hence low physical stability (Joseph and Singhvi 2019). It can be predicted from the zeta potential the stability of lipoplex in which the neutral charge formulation can aggregate because there is no repulsive force that prevents the lipoplex from flocculating.
In contrast, lipoplexes' highly positive or negative surface charge is more stable due to the repulsive force between nanoparticles in the medium that prevent their aggregation (Laouini et al., 2012, Guimarães et al., 2021). The negatively charged formulations are not suitable for cellular uptake because the negative charge can indicate that the siRNA was not encapsulated well inside the liposome. In addition, the mammalian cell membrane contains anionic proteoglycans that will not favor the negatively charged formulations for cellular uptake (Wang et al., 2010). It can be concluded from finding a result that the higher ratio of GL67 can enhance the encapsulation of siRNA by decreasing the negative surface charge of the formulation or increasing the positive surface charge since it can be electrostatically bound to the negative charge phosphate backbone of siRNA which is observed in Table 4 (3:1 M ratio). The PDI indicates the homogeneity of the formulation, which ranges from 0 to 1, in which the lower PDI means a more homogenous formulation. At the same time, PDI indicates that the liposomes in the population vary widely in size, which can affect their stability and efficacy as drug-delivery vehicles (Guimarães et al., 2021). A value of 0.4 and below is acceptable for drug delivery (Rahat et al., 2021). In this work, the PDI of C1, C2, C3, and C4 were higher than 0.41, indicating that they are less homogenous, whereas C5 and C6 have PDI lower than 0.36, showing that their homogeneity is acceptable.
It should be noted that the last two formulations (C5 and C6) that have acceptable PDI exhibited zeta potential values of 29 and 47 mV, respectively. This may lead to high repulsive force between particles, good physical stability and low aggregation; hence PDI values in the acceptable range have been achieved. However, the other formulations demonstrated lower zeta potential and higher PDI values. Several methods can be implemented in future work to enhance the lipoplexes homogeneity, improve physical stability and reduce the PDI value, such as adjusting the number of extrusion cycles, temperature, and pressure used. In addition, size exclusion chromatography (SEC) could help to eliminate smaller or larger lipoplexes populations and improve PDI.
To evaluate the ability of the liposomal formulation to encapsulate the siRNA, we used the gel retardation assay technique. From the gel retardation assay figure (Fig. 1), there was no observed band for the formulations expected to have a complete encapsulation of siRNA, which were probably entrapped inside the aqueous core of the liposomes or were strongly bound to the outer cationic surface of the liposomes. The latter might be because the SYBRTM Safe could not be attached to the siRNA; and, therefore, did not show any fluorescence under the UV light. Since the complete encapsulation of siRNA has been observed at the 3:1 M ratio, thus, no need to assess the effect of a further increase in the cationic lipid content in the formulation. This could interfere with siRNA release in the cytoplasmic compartment and induce cytotoxic effects. From the results in 3.1, 3.2, the 3:1 M ratio was the most suitable for further evaluation owing to the positively charged zeta potential, < 320 nm particle size and high encapsulation efficiency. MTS evaluated the cytotoxicity of the 3:1 M ratio formulation for metabolic activity and LDH for cell membrane integrity.
The cytotoxicity of the liposomal formulations was evaluated in the A549 cell line, which is human cancerous alveolar basal epithelial cells responsible for the diffusion of water and electrolytes across the alveoli has a fast doubling time which is 21 h (A549 | ATCC). It was selected because it is a great respiratory disorder research model, including gene therapy such as siRNA transfection (Dua et al., 2019). The cytotoxic effect that the application of cationic liposomes can raise might be due to the cationic nature of its hydrophilic head structure and the functional groups, which mainly contain primary, secondary, tertiary or quaternary amines (Lv et al., 2006). It was reported that the cationic derivative of cholesterol could be cytotoxic to the cells because of its interaction with protein kinase C (Rietwyk and Peer 2017). Moreover, the quaternary amine headgroup of cholesterol was demonstrated to be more cytotoxic than the tertiary amine headgroup of cholesterol (Rietwyk and Peer 2017). The results showed no toxic effect in all applied formulations, mainly due to using the lowest possible amount of cationic liposome in the prepared nanoparticles. Moreover, the efficient binding of cationic lipids to siRNA is assumed to minimize the free cationic liposome in the solution that is not bound to siRNA, decreasing the cytotoxic effect.
One of the major barriers that could hinder the efficient translation of siRNA therapeutics into clinical settings is the successful delivery of siRNA molecules into the targeted cells (Shi and Abrams, 2013). It was reported that the positively charged lipid-based nanoparticles could be used as an efficient siRNA delivery system, overcoming these gene therapy limitations and improving its transfection efficiency and targeted gene silencing (Balazs and Godbey 2011). Cationic liposomes are known to promote cellular internalization across the targeted cell membrane via the electrostatic interaction with the negatively charged cellular membrane; and, therefore, improve their endocytic mechanisms (Bozzuto and Molinari 2015). This study used a novel cationic lipid GL67 alone or in combination with other cationic lipids commonly used as in vitro siRNA transfection vectors, namely DC-Chol (Zhang et al., 2010), and different formulations were successfully prepared, as explained earlier. Therefore, assessing diverse lipid compositions and the various lipid ratios and their impact on transfection efficiency is crucial.
The efficient uptake of lipoplexes across the A549 cellular membrane is an important step to reach the site of siRNA action in the cytoplasmic compartments and initiate the gene silencing effect. Despite that, the GL67 lipid is well studied for DNA delivery in vitro and in vivo (Zabner et al., 1997, Alton et al., 2015); however, the use of GL67 as a nanocarrier for siRNA has yet to be fully explored. The siRNA and pDNA are different in size and electrostatic charge. In addition, the pDNA required access to the nucleus, while the siRNA acted in the cell cytosol (Lu et al., 2009). Griesenbach and her colleagues found the most efficient siRNA: GL67 ratio was 0.25:1, causing almost 75 % siRNA complexation.
Furthermore, the transfection using the above formulation on M1 cells showed almost 80 % uptake, which was evaluated by confocal imaging (Griesenbach et al., 2006). In our work, different formulations of lipoplexes with increased contents of GL67 lipid to the other cationic lipids were prepared. The optimum molar ratio of 3:1 was chosen for further testing due to its better physiochemical properties and higher encapsulation efficiency compared to the other ratios (1:1 and 2:1). It has been reported that the cellular internalization of cy3-siRNA across the A549 cell membrane could be achieved using a liposomal delivery system composed of DC-Chol and DOPE lipids (Alshehri et al., 2018). The presence of GL67 lipid in the liposomes of this current study enhanced the cellular uptake of the cy3-siRNA, hence, improving the transfection efficiency. Moreover, uptake of the formulation that contains 100 % GL67 (C6) was better than the known transfection reagent Lipofectamine® following the exposure of A549 cells, which was 67 % of the cell population (Fig. 4i), whereas Lipofectamine® was around 55 % of the cell population (Fig. 4c).
5. Conclusions
This study showed a successful in vitro transfection of A549 cells using lipid-based nanoparticles encapsulating siRNA molecules and containing a novel cationic lipid, GL67. Several parameters were demonstrated to be vital for the efficient designing of the liposomal delivery system, such as the molar ratio between cationic lipid content to the helper lipid, the molar ratio, and the ratio between the cationic lipids used, GL67 and DC-Chol. It should be noted that the formulations prepared at a molar ratio of 3:1 showed well-characterized physicochemical properties in terms of particle size (ranging from 144 nm to 332 nm) and zeta potential (ranging from (-9 mV to 47 mV) and also high encapsulation efficiency as shown by gel retardation assay. MTS and LDH assays demonstrated high metabolic activity of A549 cells after incubating formulations for 24 h. The cy3-siRNA liposomes cellular uptake results exhibited a substantial relationship between increasing the content of GL67 lipid and cellular internalization, as the highest uptake was achieved with the 100 % of GL67 lipid in the formulation. The obtained data provide a potential insight toward the delivery of siRNA to human epithelial cells as one step forward to exploiting this formulation in gene silencing and treating gene-based diseases. Further investigation is required to target such overexpressed proteins and quantify their effects using the qPCR technique.
Funding
This work is funded by King Abdulaziz City for Science and Technology through the Innovation Projects Initiative, 2022.
Availability of the Data: The authors confirm that the data supporting the findings of this study are available within the article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Somayah J. Jarallah, Email: sjarallah@kacst.edu.sa.
Ahmad M. Aldossary, Email: aaldossary@kacst.edu.sa.
Essam A. Tawfik, Email: etawfik@kacst.edu.sa.
Reem M. Altamimi, Email: raltamimi@kacst.edu.sa.
Wijdan K. Alsharif, Email: walsharif@kacst.edu.sa.
Nouf M. Alzahrani, Email: nmalzahrani@kacst.edu.sa.
Homood M. As Sobeai, Email: hassobeai@ksu.edu.sa.
Wajhul Qamar, Email: wqidris@ksu.edu.sa.
Ahmed J. Alfahad, Email: ajlfahad@kacst.edu.sa.
Manal A. Alshabibi, Email: malshabibi@kacst.edu.sa.
Sarah H. Alqahtani, Email: shalqahtani@kacst.edu.sa.
Abdullah A. Alshehri, Email: abdualshehri@kacst.edu.sa.
Fahad A. Almughem, Email: falmughem@kacst.edu.sa.
References
- A549 | ATCC, Available at: https://www.atcc.org/products/ccl-185. Accessed April 04, 2023.
- Alkahtani M.H., Almuqhim A.A., Alshehri A.A., et al. Fluorescent ruby nanocrystals for biocompatible applications. Appl. Phys. Lett. 2021;118 doi: 10.1063/5.0054775. [DOI] [Google Scholar]
- Alkahtani M., Alsofyani N., Alfahd A., et al. Engineering Red-Enhanced and Biocompatible Upconversion Nanoparticles. Nanomaterials (Basel, Switzerland). 2021;11 doi: 10.3390/nano11020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri A., Grabowska A., Stolnik S. Pathways of cellular internalization of liposomes delivered siRNA and effects on siRNA engagement with target mRNA and silencing in cancer cells. Sci. Rep. 2018;8:3748. doi: 10.1038/s41598-018-22166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton E., Armstrong D.K., Ashby D., et al. Repeated nebulization of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomized, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2015;3:684–691. doi: 10.1016/s2213-2600(15)00245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyami M.H., Alyami H.S., Alshehri A.A., et al. Tamoxifen Citrate Containing Topical Nanoemulgel Prepared by Ultrasonication Technique: Formulation Design and In Vitro Evaluation. Gels (Basel, Switzerland). 2022;8 doi: 10.3390/gels8070456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzahrani N.M., Booq R.Y., Aldossary A.M., et al. Liposome-Encapsulated Tobramycin and IDR-1018 Peptide Mediated Biofilm Disruption and Enhanced Antimicrobial Activity against Pseudomonas aeruginosa. Pharmaceutics. 2022;14 doi: 10.3390/pharmaceutics14050960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs D.A., Godbey W. Liposomes for use in gene delivery. J. Drug Delivery. 2011;2011 doi: 10.1155/2011/326497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett D.W., Davis M.E. Effect of siRNA nuclease stability on the in vitro and in vivo kinetics of siRNA-mediated gene silencing. Biotechnol. Bioeng. 2007;97:909–921. doi: 10.1002/bit.21285. [DOI] [PubMed] [Google Scholar]
- Batty P., Lillicrap D. Advances and challenges for hemophilia gene therapy. Hum. Mol. Genet. 2019;28:R95–r101. doi: 10.1093/hmg/ddz157. [DOI] [PubMed] [Google Scholar]
- Bennett C.F., Swayze E.E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 2010;50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- Bozzuto G., Molinari A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015;10:975–999. doi: 10.2147/ijn.s68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplen N.J., Alton E.W., Middleton P.G., et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat. Med. 1995;1:39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- Chong Z.X., Yeap S.K., Ho W.Y. Transfection types, methods and strategies: a technical review. PeerJ. 2021;9:e11165. doi: 10.7717/peerj.11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clogston J.D. The importance of nanoparticle physicochemical characterization for immunology research: What we learned and what we still need to understand. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113897. [DOI] [PubMed] [Google Scholar]
- Dua K., Wadhwa R., Singhvi G., et al. The potential of siRNA based drug delivery in respiratory disorders: Recent advances and progress. Drug Dev. Res. 2019;80:714–730. doi: 10.1002/ddr.21571. [DOI] [PubMed] [Google Scholar]
- Fire A.Z. Gene silencing by double-stranded RNA (Nobel Lecture) Angew. Chem. Int. Ed. Engl. 2007;46:6966–6984. doi: 10.1002/anie.200701979. [DOI] [PubMed] [Google Scholar]
- Fire A., Xu S., Montgomery M.K., et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Gao S., Dagnaes-Hansen F., Nielsen E.J., et al. The effect of chemical modification and nanoparticle formulation on stability and biodistribution of siRNA in mice. Mol. Therapy: J. Am. Soc. Gene Therapy. 2009;17:1225–1233. doi: 10.1038/mt.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillmore J.D., Gane E., Taubel J., et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021;385:493–502. doi: 10.1056/NEJMoa2107454. [DOI] [PubMed] [Google Scholar]
- Griesenbach U., Kitson C., Escudero Garcia S., et al. Inefficient cationic lipid-mediated siRNA and antisense oligonucleotide transfer to airway epithelial cells in vivo. Respir. Res. 2006;7:26. doi: 10.1186/1465-9921-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishok A., Pasquinelli A.E., Conte D., et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell. 2001;106:23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- Guimarães D., Cavaco-Paulo A., Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021;601 doi: 10.1016/j.ijpharm.2021.120571. [DOI] [PubMed] [Google Scholar]
- Guo P., Huang J., Zhao Y., et al. Nanomaterial Preparation by Extrusion through Nanoporous Membranes. Small (Weinheim an der Bergstrasse, Germany). 2018;14:e1703493. doi: 10.1002/smll.201703493. [DOI] [PubMed] [Google Scholar]
- Joseph E., Singhvi G. In: Nanomaterials for Drug Delivery and Therapy. Grumezescu A.M., editor. William Andrew Publishing; 2019. Chapter 4 - Multifunctional nanocrystals for cancer therapy: a potential nanocarrier; pp. 91–116. [Google Scholar]
- Kristen A.V., Ajroud-Driss S., Conceição I., et al. Patisiran, an RNAi therapeutic for the treatment of hereditary transthyretin-mediated amyloidosis. Neurodegen. Dis. Manage. 2019;9:5–23. doi: 10.2217/nmt-2018-0033. [DOI] [PubMed] [Google Scholar]
- Landen C.N., Jr., Chavez-Reyes A., Bucana C., et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–6918. doi: 10.1158/0008-5472.Can-05-0530. [DOI] [PubMed] [Google Scholar]
- Laouini A., Jaafar-Maalej C., Limayem-Blouza I., et al. Preparation, characterization and applications of liposomes: state of the art. J. Colloid Sci. Biotechnol. 2012;1:147–168. doi: 10.1166/jcsb.2012.1020. [DOI] [Google Scholar]
- Lee E.R., Marshall J., Siegel C.S., et al. Detailed analysis of structures and formulations of cationic lipids for efficient gene transfer to the lung. Hum. Gene Ther. 1996;7:1701–1717. doi: 10.1089/hum.1996.7.14-1701. [DOI] [PubMed] [Google Scholar]
- Levine B.L., Miskin J., Wonnacott K., et al. Global Manufacturing of CAR T Cell Therapy. Mol. Therapy. Methods Clin. Dev. 2017;4:92–101. doi: 10.1016/j.omtm.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo D., Kiselev M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics. 2022;14 doi: 10.3390/pharmaceutics14030543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.J., Langer R., Chen J. A novel mechanism is involved in cationic lipid-mediated functional siRNA delivery. Mol. Pharm. 2009;6:763–771. doi: 10.1021/mp900023v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H., Zhang S., Wang B., et al. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Maurer M.S., Schwartz J.H., Gundapaneni B., et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- McLachlan G., Davidson H., Holder E., et al. Preclinical evaluation of three non-viral gene transfer agents for cystic fibrosis after aerosol delivery to the ovine lung. Gene Ther. 2011;18:996–1005. doi: 10.1038/gt.2011.55. [DOI] [PubMed] [Google Scholar]
- Navarro S.A., Carrillo E., Griñán-Lisón C., et al. Cancer suicide gene therapy: a patent review. Expert Opin. Ther. Pat. 2016;26:1095–1104. doi: 10.1080/13543776.2016.1211640. [DOI] [PubMed] [Google Scholar]
- Nsairat H., Khater D., Sayed U., et al. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022;8:e09394. doi: 10.1016/j.heliyon.2022.e09394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozpolat B., Sood A.K., Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv. Drug Deliv. Rev. 2014;66:110–116. doi: 10.1016/j.addr.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A., Muralidharan A., Biswas A., et al. siRNA therapeutics and its challenges: Recent advances in effective delivery for cancer therapy. OpenNano. 2022;100063 doi: 10.1016/j.onano.2022.100063. [DOI] [Google Scholar]
- Persengiev S.P., Zhu X., Green M.R. Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs) RNA. 2004;10:12–18. doi: 10.1261/rna5160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahat I., Imam S.S., Rizwanullah M., et al. Thymoquinone-entrapped chitosan-modified nanoparticles: formulation optimization to preclinical bioavailability assessments. Drug Deliv. 2021;28:973–984. doi: 10.1080/10717544.2021.1927245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietwyk S., Peer D. Next-Generation Lipids in RNA Interference Therapeutics. ACS nano. 2017;11:7572–7586. doi: 10.1021/acsnano.7b04734. [DOI] [PubMed] [Google Scholar]
- Shi B., Abrams M. Technologies for investigating the physiological barriers to efficient lipid nanoparticle-siRNA delivery. J. Histochem. Cytochem.: Off. J. Histochem. Soc. 2013;61:407–420. doi: 10.1369/0022155413484152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subhan M.A., Torchilin V.P. Efficient nanocarriers of siRNA therapeutics for cancer treatment. Transl. Res. 2019;214:62–91. doi: 10.1016/j.trsl.2019.07.006. [DOI] [PubMed] [Google Scholar]
- van der Koog L., Gandek T.B., Nagelkerke A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc. Mater. 2022;11:e2100639. doi: 10.1002/adhm.202100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Kievit F.M., Zhang M. Nanoparticles for cancer gene therapy: Recent advances, challenges, and strategies. Pharmacol. Res. 2016;114:56–66. doi: 10.1016/j.phrs.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Wang J., Lu Z., Wientjes M.G., et al. Delivery of siRNA therapeutics: barriers and carriers. Aaps j. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Tian J., Chen X. Effect of surface properties on liposomal siRNA delivery. Biomaterials. 2016;79:56–68. doi: 10.1016/j.biomaterials.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J., Cheng S.H., Meeker D., et al. Comparison of DNA-lipid complexes and DNA alone for gene transfer to cystic fibrosis airway epithelia in vivo. J. Clin. Invest. 1997;100:1529–1537. doi: 10.1172/jci119676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li H., Sun J., et al. DC-Chol/DOPE cationic liposomes: a comparative study of the influence factors on plasmid pDNA and siRNA gene delivery. Int. J. Pharm. 2010;390:198–207. doi: 10.1016/j.ijpharm.2010.01.035. [DOI] [PubMed] [Google Scholar]