Abstract
Introduction:
There is a lack of effective therapeutic interventions for preeclampsia. A central factor in the etiology of the disease is the development of placental hypoxia due to abnormal vascular remodeling. However, methods to assess the impact of potential therapies on placental growth and remodeling are currently lacking. Here, we develop and validate ultrasound-guided photoacoustic imaging methods to monitor the placental response to therapeutic intervention. Establishing non-invasive tools to image placental function opens up previously unachievable understandings of placental therapeutic response.
Methods:
Studies were performed in the reduced uterine perfusion pressure (RUPP) rat model of preeclampsia. Preclinical research has identified tempol, a superoxide dismutase mimetic, and the phosphodiesterase inhibitor sildenafil as potential therapeutics for preeclampsia, as both improve in vivo maternal outcomes. PA images of the placental environment were acquired in RUPP rats receiving tempol (n = 8) or sildenafil (n = 8) to assess the longitudinal effects of treatment on placental oxygenation and vascular remodeling. Imaging measurements were validated with ex vivo histological analysis.
Results:
Spectral photoacoustic imaging non-invasively measured placental hypoxia and impaired vascular growth two days after the RUPP procedure was implemented. Sildenafil significantly improved (p < 0.05) placental oxygenation and promoted vascular remodeling in RUPP animals, while RUPP animals treated with tempol had a diminished placental therapeutic response.
Discussion:
We demonstrate that photoacoustic imaging provides in vivo measures of placental oxygenation and vascular remodeling, a previously unobtainable assessment of preeclamptic therapeutic response. These imaging tools have tremendous potential to accelerate the search for effective therapies for preeclampsia.
Keywords: Preeclampsia, Ultrasound imaging, Photoacoustic imaging, Placental hypoxia
1. Introduction
There is a significant need for noninvasive imaging methods to assess the therapeutic response of the preeclamptic placenta. Preeclampsia is a hypertensive disease that is estimated to affect up to 8% of pregnancies [1]. Despite being a leading cause of maternal and fetal mortality, there are no effective treatments for preeclampsia. Preeclampsia is clinically defined by the new onset of maternal hypertension and proteinuria which typically present during the second half of pregnancy [2]. However, the pathogenesis of the disease involves reduced placental perfusion due to abnormal remodeling of maternal vasculature during early development [3]. The resulting ischemia in the placenta increases oxidative stress and creates a hypoxic environment, upregulating the production of soluble antiangiogenic factors. In maternal circulation, these factors create the systemic endothelial dysfunction associated with the later onset of hypertension and proteinuria [4,5]. This placental-driven onset of preeclampsia suggests placental function is a critical indicator of disease progression and therapeutic response (Fig. 1).
Fig. 1. Photoacoustic imaging of placental therapeutic response.
The progression of preeclampsia from abnormal placental growth and development to the onset of maternal symptoms is shown in green. Tempol and sildenafil, two potential therapeutics for preeclampsia, have both been shown to reduce maternal hypertension through different mechanistic pathways (left). However, the in vivo effects on placental function, critical to maternal and fetal outcome, have not been demonstrated. (right) Here we demonstrate the ability of ultrasound-guided photoacoustic imaging to detect the in vivo effects of therapeutic intervention on placental oxygenation (top) and vascular growth (bottom) independent from maternal symptom management.
Existing placental imaging methods, such as ultrasound imaging and Doppler ultrasound, are insufficient for the assessment of placental function [6,7]. Ultrasound-based imaging techniques are extensively used in obstetrics for the assessment of placental size and flow since ultrasound is safe, noninvasive, and real-time. Ultrasound imaging of fetal biometrics such as abdominal circumference and Doppler ultrasound measurements of blood flow in maternal and fetal vasculature are currently used to assess placental function in high-risk pregnancies. However, these indirect measures do not adequately capture the altered placental function that occurs in preeclampsia, since ultrasound imaging cannot detect ischemia.
Photoacoustic imaging is a noninvasive imaging modality that can produce functional tissue information to supplement ultrasound [8]. Photoacoustic imaging uses nanosecond-pulsed laser light to irradiate chromophores in the tissue, such as endogenous hemoglobin. The optical energy absorbed by the chromophores causes a thermoelastic expansion, producing broadband acoustic ultrasound waves detectable with an ultrasound transducer. Spectral photoacoustic imaging exploits the wavelength-dependent optical absorption properties of chromophores to characterize the molar composition of the tissue. Hemoglobin is a unique photoabsorber because it has separate optical absorption peaks in the near-infrared tissue optical window (650–950 nm) for its oxygenated and deoxygenated states [9]. By varying the wavelength of the incident light and acquiring the resulting photoacoustic signal, spectral photoacoustic imaging can provide in vivo images of both blood oxygen saturation and total hemoglobin concentration.
The ability to use photoacoustic imaging to visualize placental structure and function in vivo has been demonstrated in several preclinical studies. Bayer et al. developed photoacoustic imaging methods for longitudinal oxygen saturation estimates in pregnant mice [10]. Yamaleyeva et al. used photoacoustic imaging to assess oxygen saturation in different zones of the placenta in a mouse model of preeclampsia [11]. Arthuis et al. investigated the effect of maternal hypoxia on placental oxygenation [12]. Similarly, Huda et al. evaluated placental oxygenation during maternal hypoxia using a spherical-view photoacoustic tomography system [13]. Additionally, photoacoustic imaging has been utilized in preclinical applications in oncology to evaluate the effects of therapeutic intervention on physiologic measures such as tissue oxygenation and vascular remodeling [14-16]. However, the common mechanism of cancer treatments reduces vascularization and blood flow to the tumor. In contrast, the placenta is a complex vascular network of maternal and fetal circulations that is continuously growing and remodeling during pregnancy to meet the needs of the developing fetus. Further, potential treatments for preeclampsia aim to increase placental perfusion and oxygenation by increasing blood flow and vascular supply. Because of these significant differences in tissue structure, function, and expected therapeutic response, we sought to validate photoacoustic imaging to evaluate expected placental therapeutic effects.
Previously, we demonstrated spectral photoacoustic imaging methods to monitor longitudinal changes in placental oxygenation in the reduced uterine perfusion pressure (RUPP) rat model of preeclampsia [17]. The RUPP surgery is a well-characterized procedure that models several pathological and molecular features of the human condition, including hypertension, proteinuria, oxidative stress, and maternal endothelial dysfunction [18-22]. Because of these similarities, the RUPP model has been used to improve understanding of the etiology of preeclampsia and to investigate potential therapeutic interventions.
The purpose of this study was to demonstrate that spectral photoacoustic imaging can detect the in vivo placental response to tempol or sildenafil treatment in the RUPP model of preeclampsia. Tempol and sildenafil are potential therapies for preeclampsia that target different mechanistic pathways in the RUPP rat model [19,23]. Tempol, a superoxide dismutase mimetic, is suggested to improve endothelial function and downregulate the placental production of antiangiogenic factors by reducing the damage caused by oxidative stress. The phosphodiesterase type 5 (PDE5) inhibitor, sildenafil, should improve placental perfusion and endothelial function by lengthening nitric oxide-mediated vasodilation. While both compounds improved maternal outcomes in the RUPP, the longitudinal effects on in vivo placental function have not been determined [19,23-25]. Our goal was to validate the use of photoacoustic imaging as a tool to assess placental growth and function predictive of therapeutic outcomes, independent from maternal symptom management and monitoring.
2. Methods
2.1. Animal studies
Timed-pregnant Sprague Dawley rats, approximately 8 weeks of age, were purchased from a commercial vendor (Charles River Laboratories, Boston, MA) and randomly assigned to six experimental groups (n = 8): normal pregnant (NP), normal pregnant + sildenafil treatment (NP + S), normal pregnant + tempol treatment (NP + T), RUPP (R), RUPP + sildenafil treatment (R + S), and RUPP + tempol treatment (R + T). Beginning on gestational day (GD) 12, tempol or sildenafil (Sigma-Aldrich, St. Louis, MO) was dissolved in the drinking water of treatment groups at a dose of 30 and 45 mg/kg, respectively, chosen to be within therapeutic ranges previously determined experimentally in rodents [19,23]. Maternal body weight and water intake were monitored daily to ensure adequate dosing. All animal studies were approved by the Institutional Animal Care and Use Committee at Tulane University.
After acquiring images on GD14, the RUPP surgical procedure was implemented following established techniques [26,27]. Briefly, animals in the RUPP experimental groups were anesthetized with isoflurane and the abdominal cavity was opened via a midline incision. Both uterine horns were exteriorized, and a silver clip (0.203 mm ID) was placed around the abdominal aorta between the renal arteries and iliac bifurcation. Secondary clips (0.100 mm ID) were also placed on the ovarian arteries proximal to the branching to the first pup to prevent a potential compensatory increase in uteroplacental blood flow. The RUPP surgical procedure reduces uteroplacental perfusion by approximately 40% resulting in maternal hypertension and proteinuria by late gestation [28]. Following all surgical procedures, animals received 1 mg/kg carprofen daily for postoperative pain management.
2.2. Ultrasound and photoacoustic imaging
All imaging was performed on a Vevo 2100 (FUJIFILM VisualSonics, Toronto, Canada) custom-integrated with an Opotek Phocus HE Benchtop laser (Opotek, Carlsbad, CA). Co-registered ultrasound and photoacoustic images were acquired using a 20 MHz ultrasound transducer (256 elements, 13–24 MHz broadband frequency). To reduce artifacts in the photoacoustic images caused by light reflections from the skin, a small piece of aluminum foil was coupled to the surface of the transducer with ultrasound gel [29]. An integrated fiberoptic bundle delivered an average fluence of 25 mJ/cm2 over the wavelengths imaged, measured at the beginning of each imaging session with a laser energy meter (Ophir Photonics Group, North Logan, UT). The laser light delivered by the fiberoptic bundle is emitted from either side of the transducer and intersects at a distance of 9 mm away from the surface of the transducer. During imaging, the surface of the skin was kept at a depth of 9 mm away from the transducer for optimal and consistent light delivery to the placenta.
Beginning on GD14, animals were anesthetized with isoflurane and abdominal hair was removed with depilatory cream. Animals were then transferred to a heated physiologic platform (FUJIFILM VisualSonics) for imaging. Heart rate, respiration rate, and body temperature were monitored and maintained during imaging by adjusting the temperature of the physiological platform and level of isoflurane in the anesthesia gas. B-mode ultrasound imaging was used to scan both sides of the abdominal cavity to locate placentas in each animal for imaging. The criteria for inclusion of placental images for analysis were placentas with clearly defined borders on the ultrasound image, and placentas unobstructed by maternal intestines, skin artifacts, or the developing fetus.
After locating a placenta meeting these criteria, Color Doppler ultrasound was used to locate the origin of the umbilical cord, defined as the placental midline, as a landmark for determining the imaging plane. Fifty frames of photoacoustic images with co-registered B-mode ultrasound images of anatomy were acquired at 690 and 950 nm (the relative optical absorption peaks of Hb and HbO2, respectively, in the near infrared tissue optical window), as well as the isosbestic point at 808 nm. The laser pulse energy was measured for each frame of the photoacoustic images using a power meter incorporated in the laser system. These energy values were used to correct for pulse-to-pulse variations in laser fluence before linear spectral unmixing. Ultrasound and photoacoustic images of five placentas from each animal were acquired on gestational days 14, 16, and 18 of pregnancy. In this work, photoacoustic imaging is used to describe all photoacoustic imaging and image processing techniques while spectral photoacoustic imaging refers to the multiwavelength photoacoustic imaging methods used to assess in vivo placental oxygenation.
All ultrasound and photoacoustic images were exported to Matlab (MathWorks, Natick, MA) for image processing. Methods detailing the placental oxygenation and vascular remodeling assessment are provided in the Supplement [30].
2.3. Mean arterial pressure measurements
Immediately following the RUPP procedure on GD14, a saline-filled catheter was surgically placed in the left common carotid artery of NP and RUPP animals for mean arterial pressure (MAP) measurements. The catheter was inserted in the artery until the tip was located near the left ventricle of the heart, then tunneled to the nape of the neck and exteriorized. A heparinized saline flush was then administered to maintain patency.
On GD18, animals were anesthetized and transferred to the heated physiologic platform. The catheter was then connected to a pressure transducer and computer data acquisition system (PowerLab, ADInstruments, Colorado Springs, CO) for MAP measurements. To reduce the effects of isoflurane on cardiovascular function, blood pressure measurements were always recorded for 10 min immediately following the induction of anesthesia, before ultrasound and spectral photoacoustic imaging [31]. Heart rate and respiration rate were continuously monitored and maintained by adjusting the level of isoflurane in the breathing gas.
2.4. Urinary protein excretion analysis
Animals were housed in metabolic cages from GD17 to 18 for urine collection. After 24 h, all urine was collected and stored at −20 °C until processing. Urinary protein excretion was measured using a Micro BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA) following manufacturer instructions. Optical absorbance was read at 500 nm using a plate reader (SpectraMax i3x, Molecular Devices, Sunnyvale, CA). The protein concentration in each sample was then calculated from a standard curve and multiplied by the volume of urine collected to determine the total protein excreted in 24-h.
2.5. Immunohistochemistry
Post-imaging on GD18, animals were euthanized with an overdose of CO2, and one placenta from each animal was collected for immunohistochemistry. The placentas were fixed in formalin, embedded in paraffin blocks, and cut into 4 μm sections. Immunohistochemical staining was performed using the Avidin Biotin Complex (ABC) method on placental sections closest to the placental midpoint – i.e. approximately the same region of photoacoustic image acquisition [32]. Placental sections were incubated with mouse monoclonal HIF-1α antibody (1:200 dilution) (abcam, Cambridge, MA) or mouse monoclonal CD31 (1:200 dilution) (Biocare Medical, Pacheco, CA) and secondary biotinylated universal pan-specific anti-mouse antibody (ABC Quick Kit, Vector Laboratories, Burlingame, CA). Slides were then incubated with 3,3′-diaminobenzidine (DAB) for 10-min, rinsed in a running water bath, and counterstained with hematoxylin. Placental sections were also stained with Hematoxylin and Eosin (H&E) via standard protocols.
Slides were scanned using an Olympus BX51 microscope (Center Valley, PA) and images were exported to Fiji image analysis software for processing [33]. The placental border was first manually segmented from the HIF-1α or CD31 stained images and a color deconvolution was applied to separate the DAB and hematoxylin signals. The expression of HIF-1α or CD31 was quantified as the percent area fraction of DAB staining compared to the total area of the placental section. All representative images of HIF-1α, CD31, and H&E-stained placentas show a section of the placental labyrinth.
To confirm our photoacoustic imaging measures of placental vascular remodeling, the diameters of maternal vascular spaces in the placenta were measured from the H&E-stained images. Fifteen maternal vascular spaces evenly distributed throughout the placental section were randomly selected and the diameter at each location was measured using Fiji. Maternal vascular spaces were identified in the placental section as blood-filled sinuses surrounding endothelial-lined fetal blood vessels. All histological image analysis was performed by users blinded to the study.
2.6. Statistical analysis
Sample sizes were determined from an a priori power analysis performed in G*Power Software (Heinrich-Heine-Universität, Dusseldorf, Germany). All post hoc analysis was performed in R software (R Foundation for Statistical Computing, Vienna, Austria). The five placental sO2 estimates from each animal were averaged for each gestational day and a two-way repeated measures ANOVA with a p-value of 0.05 was used to determine statistical significance. A pairwise t-test with Bonferroni correction (α < 0.05) was also performed to investigate significant changes in placental oxygenation during gestation.
Pearson’s correlation analysis was performed for all correlation plots. In normal pregnant litters, all viable fetal and corresponding placental weights (n = 72) were correlated to determine the impact of placental growth on fetal outcome. An interquartile range outlier analysis was performed prior to the correlation test and 6 fetal and corresponding placental weights were removed. The spectral photoacoustic imaging measures of placental oxygenation and vascular remodeling were also correlated with immunohistochemical measures of hypoxia and vascular growth to determine the physiological relevance of our imaging measures. For these analyses, the average imaging measurement in each animal was correlated with the average immunohistochemical analysis of the placental section harvested from that animal. All data are reported as mean ± standard error (SEM).
3. Results
3.1. Placental growth predicts fetal outcome
To demonstrate that placental growth predicts fetal outcome, we correlated placental and fetal weight in normal pregnant litters (Fig. S1). We found that higher fetal weights are associated with increased placental weight (r = 0.56, p < 0.05), consistent with previously reported data in humans and rodents [34-37]. This relationship between placental and fetal weight confirms that fetal outcome is improved by increasing placental growth.
A similar trend was observed in the RUPP; however, this trend was not statistically significant. This may be due to the high number of fetal resorptions following the RUPP surgery, which can lead to highly variable pup and placental weights. However, placental and fetal weight were significantly correlated in RUPP animals receiving tempol (r = 0.25, p < 0.05) or sildenafil (r = 0.26, p < 0.05) indicating improved fetal outcome following therapeutic intervention. Assessing the effects of treatment on placental growth and remodeling in vivo with photoacoustic imaging therefore could provide a critical predictor of fetal outcome.
3.2. In vivo photoacoustic imaging measures placental growth, remodeling, and function
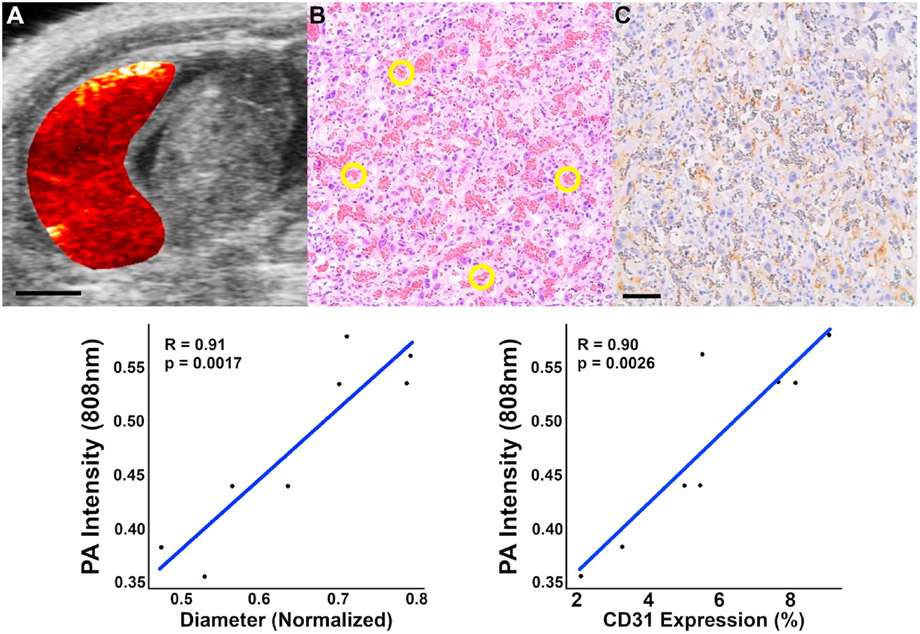
To validate our photoacoustic imaging measures of placental growth, we correlated vascular remodeling measurements to immunohistochemical analysis of placental sections at the same GD18 timepoint (Fig. 2). Placental vascular remodeling was evaluated using the 808 nm photoacoustic images of total hemoglobin concentration. At this wavelength, oxy- and deoxyhemoglobin absorb light equally, and therefore the measured photoacoustic signal intensity is proportional to the local blood volume. Changes in vasodilation and angiogenesis should therefore be reflected by photoacoustic signal intensity changes at this wavelength. In normal pregnant placentas, the photoacoustic signal intensity is positively correlated with vascular diameter (r = 0.91, p < 0.05) and vascular growth (r = 0.9, p < 0.05). The maternal vascular diameter in the placenta (Fig. 2b) was measured on H&E-stained placental sections. Vascular growth was determined from the expression of CD31, a cellular marker of angiogenesis.
Fig. 2. Immunohistochemical confirmation of placental vascular remodeling.
The PA images acquired at 808 nm were used to assess placental vascular growth and remodeling. Oxyhemoglobin and deoxyhemoglobin absorb light equally at this wavelength therefore the measured PA signal intensity is proportional to the local blood volume. Changes in placental blood volume caused by vasodilation or angiogenesis should result in a proportional increase in the acquired PA signal. In normal pregnant animals (n = 8), the average 808 nm PA signal intensity in GD18 placentas is positively correlated (p < 0.05) with placental vascular space diameter and vascular growth. (a) Photoacoustic signal generated in a normal pregnant placenta overlaid on the B-mode US image of the anatomy. (b) Placental vascular diameter was measured in 15 randomly selected locations (yellow ROI) evenly distributed in the maternal placenta on H&E-stained placental sections. (c) Angiogenesis and vascular growth were determined by the expression of CD31 (brown coloring) in the placenta. Scale bars = 3 mm for US and PA images, 0.1 mm immunohistochemistry.
Fig. 3 shows representative images of NP and RUPP placentas on GD18. Compared to normal pregnancy, oxygen saturation in the RUPP is decreased four days after surgical intervention indicated by the prominent blue coloring in the RUPP placenta (Fig. 3b, e). After imaging, placental sections were harvested and immunohistochemical staining for HIF-1α confirmed the hypoxic placental environment in the RUPP. The expression of HIF-1α in the placenta is negatively correlated with oxygen saturation (r = −0.67, p < 0.05) demonstrating the ability of spectral photoacoustic imaging to detect in vivo placental oxygenation. The results show that our photoacoustic imaging measures of placental function and vascular remodeling are confirmed by histological assessment of markers of ischemia and vascular development.
Fig. 3. sPA imaging can detect placental hypoxia in vivo.
To confirm the sPA imaging measures of placental hypoxia, the GD18 placental oxygenation measurements were correlated with the expression of HIF-1α in normal pregnant (n = 8) and RUPP (n = 8) placentas. Placental oxygenation was calculated by using the co-registered B-mode US images (a, d) to manually segment the placenta and a linear spectral unmixing algorithm was applied to the sPA images. (b, e) The custom oxygenation colormap overlay shows that placental oxygenation is decreased four days after the RUPP model was implemented. (c, f) Post-imaging on gestational day 18, placentas were harvested and immunostained for hypoxia-inducible factor-1α (HIF-1α). The expression of HIF-1α (brown coloring) was quantified as the percentage of stain area compared to the area of the placental section. Lower levels of placental oxygenation are associated with increased HIF-1α expression (p < 0.05), indicating the ability of sPA imaging to assess placental hypoxia in vivo in the RUPP model. Scale bars = 3 mm for US and sPA images, 0.1 mm for HIF-1α sections.
3.3. In vivo photoacoustic imaging of placental therapeutic effects
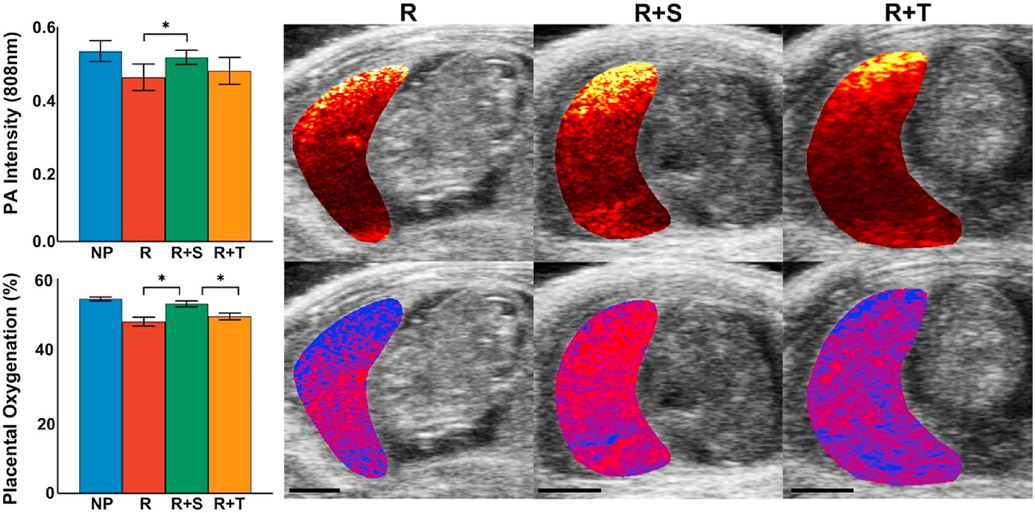
The effects of tempol or sildenafil treatment on placental oxygenation and vascular remodeling in the RUPP model of preeclampsia are shown in Fig. 4. RUPP animals exhibit placental hypoxia and impaired vascular remodeling in comparison with the normal pregnant group, similar to human preeclampsia, two days after the RUPP procedure was implemented. RUPP animals receiving sildenafil had an average placental oxygen saturation of 52.9 ± 0.8% significantly higher (p < 0.05) in comparison with the average 47.9 ± 1.2% in untreated RUPP. The average 808 nm photoacoustic signal intensity was also significantly increased (p < 0.05) in sildenafil treated RUPP placentas. While not significant, a trend towards improved placental oxygenation was observed in the R + T group suggesting tempol may have some therapeutic effect on the placenta. Placental oxygenation and vascular remodeling in the NP + treatment groups were not significantly different compared to the untreated normal pregnant group (Fig. S3).
Fig. 4. Sildenafil improves placental hypoxia and promotes vascular growth.
Two days after the RUPP, PA imaging measures of vascular remodeling (top) and sPA imaging of placental oxygenation (bottom) show the impaired placental function induced by the RUPP surgical procedure in comparison with normal pregnant animals. Treatment with sildenafil significantly improved (p < 0.05) oxygen saturation in the RUPP (n = 8) and promoted vascular growth indicated by the increase in 808 nm PA signal intensity in the placenta. While not significant, a trend towards improved placental oxygenation and vascular remodeling was observed in RUPP animals receiving tempol treatment. Data = mean ± SEM. Scale bars = 5 mm.
3.4. Validation of response to therapy
Fig. 5 illustrates the maternal response to tempol or sildenafil treatment. By late gestation, MAP and urinary protein excretion were significantly elevated (p < 0.05) in the RUPP compared with NP, indicating the RUPP exhibits hypertension and proteinuria characteristic of human preeclampsia. Administration of tempol or sildenafil significantly lowered (p < 0.05) MAP in the RUPP, consistent with previously reported findings [19,23]. Urinary protein excretion was also significantly lower in RUPP animals receiving tempol or sildenafil compared with the untreated RUPP group. No significant differences in urinary protein excretion or MAP were found between the normal pregnant treatment groups and untreated NP animals (Fig. S4).
Fig. 5. Maternal hypertension and proteinuria in the RUPP are restored by tempol or sildenafil treatment.
Mean arterial pressure (MAP) and urinary protein excretion are elevated in RUPP animals by late gestation compared to normal pregnancy indicating that the RUPP exhibits hypertension and proteinuria characteristic of human preeclampsia. Both MAP and urinary protein excretion were significantly reduced (p < 0.05) in RUPP animals receiving tempol (n = 8) or sildenafil (n = 8) treatment, consistent with previously reported data in the RUPP. Data = mean ± SEM.
Fig. 6 shows the immunohistochemical staining for HIF-1α and CD31 in the placenta. HIF-1α is a cellular protein regulator of oxygen homeostasis while CD31 selective stains vascular endothelial cells as a measure of vascular remodeling and blood vessel density [38,39]. RUPP placentas harvested on GD18 have elevated levels of HIF-1α and decreased CD31 expression compared to normal pregnant placental sections confirming the photoacoustic imaging measures of placental hypoxia and impaired vascular remodeling. Treatment with tempol or sildenafil significantly improved (p < 0.05) HIF-1α and CD31 expression in the RUPP. Placental expression of HIF-1α and CD31 was not significantly different in normal pregnant and normal pregnant animals receiving tempol or sildenafil (Fig. S5).
Fig. 6. Immunohistochemical analysis.
RUPP animals (n = 8) have increased placental HIF-1α and decreased CD31 expression compared to normal pregnancy confirming the PA imaging measures of placental hypoxia and impaired vascular remodeling. Treatment with tempol or sildenafil in the RUPP (n = 8) significantly reduced (p < 0.05) the expression of HIF-1α and increased (p < 0.05) placental CD31 expression. Data = mean ± SEM.
4. Discussion
In this study, we demonstrate spectral photoacoustic imaging methods to monitor the in vivo placental response to therapeutic intervention for preeclampsia. We validated that photoacoustic imaging measures of placental blood volume correlate to vasodilation and angiogenic markers on histology and demonstrate that a linear regression fit of spectral photoacoustic images to the optical absorption spectra of oxy- and deoxyhemoglobin is correlated to expression of HIF-1α, a marker of tissue ischemia. We have shown that photoacoustic imaging can detect the altered placental oxygenation and vascular development, two indicators of placental function, induced by the RUPP model before the onset of maternal symptoms and can be used to evaluate the effects of potential therapies on placental oxygenation and vascular growth.
While other imaging techniques such as Doppler ultrasound and blood oxygen level-dependent magnetic resonance imaging (BOLD MRI) have been investigated for placental imaging, they lack the sensitivity and throughput necessary for assessing potential therapies for preeclampsia [40-43]. In the present study, we have shown that ultrasound-guided photoacoustic imaging measures of placental oxygenation and vascular development are consistent with immunohistochemical staining of indicators of placental hypoxia and vascular remodeling. Our work shows that photoacoustic imaging can provide in vivo information about how preclinical therapeutics for preeclampsia affect placental growth and function, which is critical for improving fetal outcome. To our knowledge, the in vivo placental response to therapeutic intervention for preeclampsia has not previously been evaluated using photoacoustic imaging.
We performed photoacoustic imaging of placental oxygenation and vascular remodeling to determine the impact of tempol or sildenafil treatment on placental function independent of maternal symptom management. Given the role of the placenta in the pathology of preeclampsia, a therapeutic approach that restores placental function is an innovative, and previously unachievable, approach to improving maternal and fetal outcomes. Tempol has previously been shown to reduce oxidative stress-induced hypertension in RUPP rats and improve uterine artery blood flow velocity in a mouse model of preeclampsia [19,44]. Sildenafil reduces maternal hypertension in RUPP rats through PDE-5 inhibition and has been shown to improve uterine blood flow in preeclamptic mice [23,45]. Here, we have demonstrated that improved placental function is a key outcome of these treatments.
Placental oxygenation and vascular development were both impaired following the RUPP procedure which resulted in the onset of hypertension and proteinuria by late gestation. While tempol-treated RUPP animals showed a trend towards improved placental function, sildenafil alone restored placental oxygenation and vascular growth. However, both tempol and sildenafil improved the maternal symptoms of preeclampsia in RUPP animals. These findings suggest that tempol treatment may not have reached therapeutic levels in the placenta despite having a therapeutic effect in the maternal system. Further, therapeutic interventions that increase blood flow to the placenta, such as sildenafil, may have a greater impact on preeclampsia management than reducing oxidative stress. Noninvasive imaging will be a valuable tool to assess therapeutic dosage and confirm placental therapeutic response in future studies.
The developed techniques represent innovative and novel methods to assess therapeutic efficacy. There are hundreds of potential therapeutics for preeclampsia, including tempol and sildenafil, that have been reported in preclinical studies to reduce maternal mean arterial pressure. However, sildenafil treatment resulted in adverse fetal outcomes during clinical trials. Since insufficient placental growth, invasion and remodeling underlie the development of the disease, strategies to detect the placental therapeutic response, such as those presented here, could provide a revolutionary way to assess potential therapies for preeclampsia.
We evaluated the longitudinal effects of tempol or sildenafil treatment on placental function using photoacoustic imaging. We found that while both treatments improved the maternal symptoms of preeclampsia, sildenafil demonstrated improvements in restored placental function in RUPP animals. Our work shows that photoacoustic imaging is a powerful tool for evaluating the effects of novel pregnancy therapeutics on in vivo placental function.
Supplementary Material
Funding
This work was supported by the National Institutes of Health [NIH/NICHD R01HD097466].
Abbreviations
- RUPP
reduced uterine perfusion pressure
- Hb
deoxyhemoglobin
- HbO2
oxyhemoglobin
- sO2
oxygen saturation
- HIF-1α
hypoxia-inducible factor 1α
- MAP
mean arterial pressure
- NP
normal pregnant
- PDE5
inhibitor phosphodiesterase type 5 inhibitor
Footnotes
Declaration of competing interests
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.placenta.2022.06.006.
References
- [1].Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, Gulmezoglu AM, Temmerman M, Alkema L, Global causes of maternal death: a WHO systematic analysis, Lancet Global Health 2 (6) (2014) E323–E333. [DOI] [PubMed] [Google Scholar]
- [2].A.C.o.O.a. Gynecologists, Gestational hypertension and preeclampsia. ACOG practice bulletin No. 222, Obstet. Gynecol 135 (6) (2020) 237–260. [DOI] [PubMed] [Google Scholar]
- [3].Brosens I, Dixon HG, Robertson WB, The role of the spiral arteries in the pathogenesis of preeclampsia, Obstet. Gynecol. Annu 1 (1972) 177–191. [PubMed] [Google Scholar]
- [4].Bosio PM, McKenna PJ, Conroy R, O’Herlihy C, Maternal central hemodynamics in hypertensive disorders of pregnancy, Obstet. Gynecol 94 (6) (1999) 978–984. [DOI] [PubMed] [Google Scholar]
- [5].Zhao S, Gu Y, Fan R, Groome LJ, Cooper D, Wang Y, Proteases and sFlt-1 release in the human placenta, Placenta 31 (6) (2010) 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schild RL, Maringa M, Siemer J, Meurer B, Hart N, Goecke TW, Schmid M, Hothorn T, Hansmann ME, Weight estimation by three-dimensional ultrasound imaging in the small fetus, Ultrasound Obstet. Gynecol 32 (2) (2008) 168–175. [DOI] [PubMed] [Google Scholar]
- [7].Fraser KH, Poelma C, Zhou B, Bazigou E, Tang MX, Weinberg PD, Ultrasound imaging velocimetry with interleaved images for improved pulsatile arterial flow measurements: a new correction method, experimental and in vivo validation, J. R. Soc. Interface 14 (127) (2017) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Luke GP, Emelianov SY, Label-free detection of lymph node metastases with US-guided functional photoacoustic imaging, Radiology 277 (2) (2015) 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Laufer J, Delpy D, Elwell C, Beard P, Quantitative spatially resolved measurement of tissue chromophore concentrations using photoacoustic spectroscopy: application to the measurement of blood oxygenation and haemoglobin concentration, Phys. Med. Biol 52 (1) (2007) 141–168. [DOI] [PubMed] [Google Scholar]
- [10].Bayer CL, Wlodarczyk BJ, Finnell RH, Emelianov SY, Ultrasound-guided spectral photoacoustic imaging of hemoglobin oxygenation during development, Biomed. Opt Express 8 (2) (2017) 757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yamaleyeva LM, Sun Y, Bledsoe T, Hoke A, Gurley SB, Brosnihan KB, Photoacoustic imaging for in vivo quantification of placental oxygenation in mice, Faseb. J 31 (12) (2017) 5520–5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Arthuis CJ, Novell A, Raes F, Escoffre J-M, Lerondel S, Le Pape A, Bouakaz A, Perrotin F, Real-time monitoring of placental oxygenation during maternal hypoxia and hyperoxygenation using photoacoustic imaging, PLoS One 12 (1) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huda K, Wu C, Sider JG, Bayer CL, Spherical-view photoacoustic tomography for monitoring in vivo placental function, Photoacoustics (2020), 100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Johnson SP, Ogunlade O, Lythgoe MF, Beard P, Pedley RB, Longitudinal photoacoustic imaging of the pharmacodynamic effect of vascular targeted therapy on tumors, Clin. Cancer Res 25 (24) (2019) 7436–7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rich LJ, Miller A, Singh AK, Seshadri M, Photoacoustic imaging as an early biomarker of radio therapeutic efficacy in head and neck cancer, Theranostics 8 (8) (2018) 2064–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Laufer J, Johnson P, Zhang E, Treeby B, Cox B, Pedley B, Beard P, In vivo preclinical photoacoustic imaging of tumor vasculature development and therapy, 17, 2012, 056016, 5. [DOI] [PubMed] [Google Scholar]
- [17].Lawrence DJ, Escott ME, Myers L, Intapad S, Lindsey SH, Bayer CL, Spectral photoacoustic imaging to estimate in vivo placental oxygenation during preeclampsia, Sci. Rep 9 (2019) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP, Systemic Hemodynamic and Regional Blood Flow Changes in Response to Chronic Reductions in Uterine Perfusion Pressure in Pregnant Rats, 2007. [DOI] [PubMed] [Google Scholar]
- [19].Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP, Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats, Am. J. Hypertens 21 (10) (2008) 1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gilbert JS, Babcock SA, Granger JP, Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression, Hypertension 50 (6) (2007) 1142–1147. [DOI] [PubMed] [Google Scholar]
- [21].Gilbert JS, Gilbert SAB, Arany M, Granger JP, Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression, Hypertension 53 (2) (2008) 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gilbert JS, Ryan MJ, Lamarca BB, Sedeek M, Murphy SR, Granger JP, Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction, Am. J. Physiol. Heart Circ. Physiol 294 (2) (2008) H541–H550. [DOI] [PubMed] [Google Scholar]
- [23].George EM, Palei AC, Dent EA, Granger JP, Sildenafil attenuates placental ischemia-induced hypertension, Am. J. Physiol. Regul. Integr. Comp. Physiol 305 (4) (2013) R397–R403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Soobryan N, Murugesan S, Phoswa W, Gathiram P, Moodley J, Mackraj I, The effects of sildenafil citrate on uterine angiogenic status and serum inflammatory markers in an L-NAME rat model of pre-eclampsia, Eur. J. Pharmacol 795 (2017) 101–107. [DOI] [PubMed] [Google Scholar]
- [25].Parrish MR, Wallace K, Tam KBT, Herse F, Weimer A, Wenzel K, Wallukat G, Ray LF, Arany M, Cockrell K, Martin JN, Dechend R, LaMarca B, Hypertension in response to AT1-AA: role of reactive oxygen species in pregnancy-induced hypertension, Am. J. Hypertens 24 (7) (2011) 835–840. [DOI] [PubMed] [Google Scholar]
- [26].Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W, Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia, Methods Mol. Med 122 (2006) 383–392. [DOI] [PubMed] [Google Scholar]
- [27].Crews JK, Herrington JN, Granger JP, Khalil RA, Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat, Hypertension 35 (1) (2000) 367–372. [DOI] [PubMed] [Google Scholar]
- [28].Gilbert J, Dukes M, LaMarca B, Cockrell K, Babcock S, Granger J, Effects of reduced uterine perfusion pressure on blood pressure and metabolic factors in pregnant rats, Am. J. Hypertens 20 (6) (2007) 686–691. [DOI] [PubMed] [Google Scholar]
- [29].Preisser S, Held G, Akarcay HG, Jaeger M, Frenz M, Study of clutter origin in in-vivo epi-optoacoustic imaging of human forearms, J. Opt 18 (9) (2016) 9. [Google Scholar]
- [30].Kim S, Chen Y-S, Luke GP, Emelianov SY, In vivo three-dimensional spectroscopic photoacoustic imaging for monitoring nanoparticle delivery, Biomed. Opt Express 2 (9) (2011) 2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Albrecht M, Henke J, Tacke S, Markert M, Guth B, Effects of isoflurane, ketamine-xylazine and a combination of medetomidine, midazolam and fentanyl on physiological variables continuously measured by telemetry in Wistar rats, BMC Vet. Res 10 (2014) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cardiff RD, Miller CH, Munn RJ, Manual Immunohistochemistry Staining of Mouse Tissues Using the Avidin-Biotin Complex (ABC) Technique, 2014. [DOI] [PubMed] [Google Scholar]
- [33].Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, Fiji: an open-source platform for biological-image analysis, Nat. Methods 9 (7) (2012) 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hayward CE, Lean S, Sibley CP, Jones RL, Wareing M, Greenwood SL, Dilworth MR, Placental adaptation: what can we learn from birthweight:placental weight ratio? Front. Physiol 7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sanin LH, López SR, Olivares ET, Terrazas MC, Silva MA, Carrillo ML, Relation between birth weight and placenta weight, Biol. Neonate 80 (2) (2001) 113–117. [DOI] [PubMed] [Google Scholar]
- [36].Alexander BT, Placental insufficiency leads to development of hypertension in growth-restricted offspring, Hypertension 41 (3) (2003) 457–462. [DOI] [PubMed] [Google Scholar]
- [37].Dilworth MR, Andersson I, Renshall LJ, Cowley E, Baker P, Greenwood S, Sibley CP, Wareing M, Sildenafil citrate increases fetal weight in a mouse model of fetal growth restriction with a normal vascular phenotype, PLoS One 8 (10) (2013), e77748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Alexander-Sefre F, Detection of tumour lymphovascular space invasion using dual cytokeratin and CD31 immunohistochemistry, J. Clin. Pathol 56 (10) (2003) 786–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].El-Gohary YM, Metwally G, Saad RS, Robinson MJ, Mesko T, Poppiti RJ, Prognostic significance of intratumoral and peritumoral lymphatic density and blood vessel density in invasive breast carcinomas, Am. J. Clin. Pathol 129 (4) (2008) 578–586. [DOI] [PubMed] [Google Scholar]
- [40].Li L, Zheng Y, Zhu Y, Li J, Serum biomarkers combined with uterine artery doppler in prediction of preeclampsia, Exp. Therapeut. Med (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sorensen A, Sinding M, Peters DA, Petersen A, Frokjr JB, Christiansen OB, Uldbjerg N, Placental oxygen transport estimated by the hyperoxic placental BOLD MRI response, Phys. Rep 3 (10) (2015) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gómez O, Martínez JM, Figueras F, Del Río M, Borobio V, Puerto B, Coll O, Cararach V, Vanrell JA, Uterine artery Doppler at 11-14 weeks of gestation to screen for hypertensive disorders and associated complications in an unselected population, Ultrasound Obstet. Gynecol 26 (5) (2005) 490–494. [DOI] [PubMed] [Google Scholar]
- [43].Chien PFW, Arnott N, Gordon A, Owen P, Khan KS, How useful is uterine artery Doppler flow velocimetry in the prediction of pre-eclampsia, intrauterine growth retardation and perinatal death? An overview, BJOG: Int. J. Obstet. Gynaecol 107 (2) (2000) 196–208. [DOI] [PubMed] [Google Scholar]
- [44].Stanley JL, Andersson IJ, Hirt CJ, Moore L, Dilworth MR, Chade AR, Sibley CP, Davidge ST, Baker PN, Effect of the anti-oxidant tempol on fetal growth in a mouse model of fetal growth restriction, Biol. Reprod 87 (1) (2012), 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Burke SD, Zsengellér ZK, Khankin EV, Lo AS, Rajakumar A, Dupont JJ, McCurley A, Moss ME, Zhang D, Clark CD, Wang A, Seely EW, Kang PM, Stillman IE, Jaffe IZ, Karumanchi SA, Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia, J. Clin. Investig 126 (7) (2016) 2561–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.