Abstract

Ultramicroporous materials can be highly effective at trace gas separations when they offer a high density of selective binding sites. Herein, we report that sql-NbOFFIVE-bpe-Cu, a new variant of a previously reported ultramicroporous square lattice, sql, topology material, sql-SIFSIX-bpe-Zn, can exist in two polymorphs. These polymorphs, sql-NbOFFIVE-bpe-Cu-AA (AA) and sql-NbOFFIVE-bpe-Cu-AB (AB), exhibit AAAA and ABAB packing of the sql layers, respectively. Whereas NbOFFIVE-bpe-Cu-AA (AA) is isostructural with sql-SIFSIX-bpe-Zn, each exhibiting intrinsic 1D channels, sql-NbOFFIVE-bpe-Cu-AB (AB) has two types of channels, the intrinsic channels and extrinsic channels between the sql networks. Gas and temperature induced transformations of the two polymorphs of sql-NbOFFIVE-bpe-Cu were investigated by pure gas sorption, single-crystal X-ray diffraction (SCXRD), variable temperature powder X-ray diffraction (VT-PXRD), and synchrotron PXRD. We observed that the extrinsic pore structure of AB resulted in properties with potential for selective C3H4/C3H6 separation. Subsequent dynamic gas breakthrough measurements revealed exceptional experimental C3H4/C3H6 selectivity (270) and a new benchmark for productivity (118 mmol g–1) of polymer grade C3H6 (purity >99.99%) from a 1:99 C3H4/C3H6 mixture. Structural analysis, gas sorption studies, and gas adsorption kinetics enabled us to determine that a binding “sweet spot” for C3H4 in the extrinsic pores is behind the benchmark separation performance. Density-functional theory (DFT) calculations and Canonical Monte Carlo (CMC) simulations provided further insight into the binding sites of C3H4 and C3H6 molecules within these two hybrid ultramicroporous materials, HUMs. These results highlight, to our knowledge for the first time, how pore engineering through the study of packing polymorphism in layered materials can dramatically change the separation performance of a physisorbent.
Introduction
Metal–organic materials (MOMs)1 such as metal–organic frameworks (MOFs)2−4 and porous coordination polymers (PCPs)5 are of topical interest because of their potential utility in, for example, gas storage, catalysis, biochemical imaging, and drug delivery.6−9 With respect to design, the diversity of their structures and compositions makes MOMs amenable to crystal engineering,10 which can enable systematic tuning of composition to control pore size, shape, and chemistry, i.e. “pore engineering”.11−15 Established approaches to pore engineering include interpenetration, flexibility, open metal sites (OMSs), functionalized ligands, counterion substitution, and pore space partition, which tend to lower pore volume.15−17 To our knowledge, pore engineering by different packing of adjacent layers, i.e. polymorphism, has not yet been reported.
Hybrid ultramicroporous materials (HUMs), a subclass of MOMs, are based on inorganic pillars such as MFSIX (e.g., GeF62–, TiF62–, SiF62–, SnF62–), FOXY (e.g., NbOF52–), and M’FFIVE (e.g., AlF52–, FeF52–). HUMs are of topical interest thanks to their strong and selective binding interactions with small gas molecules including CO2 and hydrocarbons (HCs).18−26,11,20,27−37 Most HUMs are based on rigid organic linkers (e.g., pyrazine, 4,4′-bipyridine) and pillared by inorganic anions to afford three-dimensional rigid networks. Whether interpenetration of the networks occurs depends mainly on the length and rigidity of linker ligands (Table S2).18,19,21−23,38−40 Flexible linker ligands, e.g. 4,4′-dipyridylsulfide (dps), 4,4′-dipyridylsulfone, 4,4′-dipyridylsulfoxide, 1,2-bis(4-pyridyl)ethane (bpe), and 1,3-bis(4-pyridyl)propane (bpp), have the potential to form spiro-linked 1D coordination polymers that, when pillared by inorganic anions, afford 2D square lattice (sql) coordination networks (Scheme 1 and Table S3).24,27,41−44sql-SIFSIX-bpe-Zn, SIFSIX = SiF62–, exemplifies such structures and was reported to exhibit high binding affinity for C2H2 via an induced fit mechanism enabled by flexibility.27
Scheme 1. Rigid Linker Ligands Tend to Generate sql Topology Coordination Networks (Above) Whereas Flexible Linkers Can Afford Either sql Networks or Spiro-Linked 1D Coordination Polymers (Below).

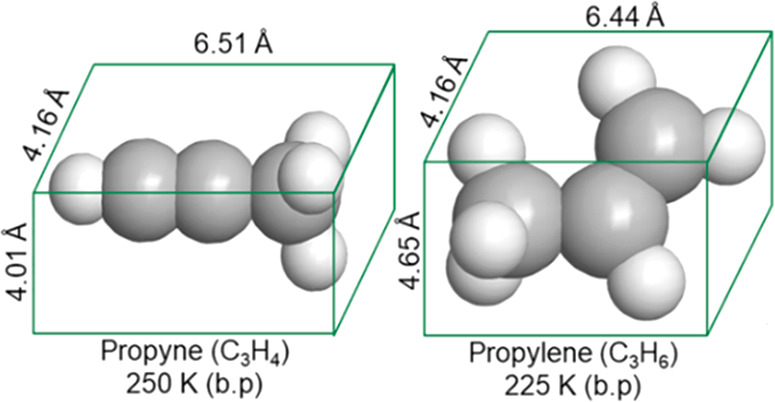
Herein, we report that solvent-mediated crystallization can result in packing polymorphs of the related material sql-NbOFFIVE-bpe-Cu, sql-NbOFFIVE-bpe-Cu-AA, AA, and sql-NbOFFIVE-bpe-Cu-AB, AB, which exhibit AAAA and ABAB packing of their sql layers, respectively, and study the effect of crystal packing upon the C3 sorption properties of sql-NbOFFIVE-bpe-Cu. C3 sorption is relevant because propylene (C3H6) is a feedstock for the production of commodity chemicals such as acrylonitrile, propylene oxide, and polypropylene.45−47 Worldwide propylene production capacity was as high as 140 million tons in 2020, second only to that of ethylene among chemical building blocks.13 Further purification of C3H6 is needed because trace amounts (∼1%) of propyne (C3H4) must be removed to afford polymer-grade (≥99.95%) C3H6 for downstream applications.38,48 That C3H4 and C3H6 exhibit similar physicochemical properties (Scheme 2 and Table S1)49 makes it a challenge for porous materials to produce polymer-grade C3H6. The C3 sorption properties of AA and AB are addressed through a series of experimental and computational studies.
Scheme 2. Comparison of the Molecular Structures and Physical Properties of C3H4 and C3H6.
Experimental Section
All reagents and solvents were purchased commercially and used as received without further purification, except the precursor CuNbOF5·4H2O, which was prepared by adapting a reported procedure.50
Synthesis of pcu-NbOFFIVE-bpe-Cu ([Cu(NbOF5)(bpe)2]n)
In a typical reaction, bpe (6.3 mg, 0.035 mmol) in 2 mL of methanol was carefully layered onto CuNbOF5·4H2O (7 mg, 0.026 mmol) in 2 mL of water. Blue block single crystals were obtained after 4 days in quantitative yield, collected by filtration and washed with methanol three times.
Synthesis of sql-NbOFFIVE-bpe-Cu-AA-α, AA-α, ([Cu(NbOF5)(bpe)2]n)
In a typical reaction, CuNbOF5·4H2O (0.0345 g, 0.13 mmol) and bpe (0.0276 g, 0.15 mmol) were added to 11.0 mL of H2O/CH3OH (v/v = 9:2). The solution was then sealed in a 14.5 mL vial and settled for 1 h. A light blue powder was obtained. This reaction can be readily scaled. When the reaction was conducted at room temperature or 60 °C for 2 months, blue block crystals of AA-α were obtained which were suitable for single-crystal X-ray diffraction (SCXRD) testing.
Preparation of sql-NbOFFIVE-bpe-Cu-AA-β, AA-β
A single crystal of the methanol exchanged phase of AA-α was activated at 333 K in situ on the goniometer of an SCXRD instrument. After 10 min SCXRD data showed that AA-α had transformed to AA-β. Bulk samples were prepared through activation at 333 K under vacuum.
Synthesis of sql-NbOFFIVE-bpe-Cu-AB-α, AB-α, ([Cu(NbOF5)(bpe)2]n)
In a typical reaction, a solution of CuNbOF5·4H2O (7 mg, 0.026 mmol) in 1 mL of water was carefully layered onto bpe (6.3 mg, 0.035 mmol) in 4 mL of 1,2-dichlorobenzene. Block shaped dark blue single crystals of AB-α were obtained after 3 days. The crystals were collected by filtration and washed with methanol three times, yield 85%.
Preparation of sql-NbOFFIVE-bpe-Cu-AB-β1, AB-β1
Methanol exchanged AB-α was activated by heating at 333 K under vacuum for 12 h and then exposed to air or soaked in water to yield AB-β1.
Results and Discussion
Solvent diffusion of CuNbOF5·4H2O and bpe in various organic solvents and water at room temperature afforded single crystals of three polymorphs of [Cu(NbOF5)(bpe)2]n: a 3D pcu network; two 2D sql networks. When using 1:1 H2O/CH3OH (v/v), single crystals of the 3D pcu network, pcu-NbOFFIVE-bpe-Cu, were obtained with bulk purity (Figures 1 and S12). SCXRD revealed that pcu-NbOFFIVE-bpe-Cu had crystallized in the tetragonal space group P4/n (Table S4). The noninterpenetrated network exhibits ∼9 × 9 Å pores, but crystals of pcu-NbOFFIVE-bpe-Cu did not survive guest removal, making it a first generation porous coordination polymer as classified by Kitagawa and co-workers (Figure S3).5 When the ratio of H2O/CH3OH was changed to 9:2 (v/v), single crystals of a 2D sql network variant, AA-α, were isolated (Figures 1, S1 and S5 upper). SCXRD analysis revealed that AA-α had crystallized in the monoclinic space group C2/c (Table S4). A 4:1 ratio of 1,2-dichlorobenzene and water (v/v) afforded single crystals of a polymorph of the same sql network, AB-α (Figures 1, S2, and S5 lower). SCXRD analysis revealed that AB-α had crystallized in tetragonal space group P42/mmc (Table S5). Bulk purities of AA-α and AB-α were confirmed by powder X-ray diffraction (PXRD, Figure S13).
Figure 1.

Synthetic conditions used and crystal structures of pcu-NbOFFIVE-bpe-Cu, sql-NbOFFIVE-bpe-Cu-AA-α, and sql-NbOFFIVE-bpe-Cu-AB-α. Color code: turquoise, Cu; green, Nb; red, O; bright green, F; blue, N; gray, C. Hydrogen atoms are omitted for clarity.
Each Cu2+ cation in AA-α and AB-α is six-coordinate, coordinated by four N atoms from bpe ligands as well as one O atom and one F atom from two NbOF52– anions (Figure S4). The pyridyl moieties are oriented in a gauche conformation about the C–C single bond backbone, resulting in a V-shaped bis(monodentate) linking mode. The layers in AB-α and AA-α stack differently. AA-α formed an AAAA layer arrangement similar to other sql topology HUMs (Figures 2, S5, and S6, some presented using simplified structures).11,24,27,41−44,51 Adjacent layers form H-bonds C(bpe ligand)–H···F(NbOF5) of 2.40 and 2.46 Å that result in an arrangement reminiscent of a zipper. With respect to AB-α, NbOF52– anion pillared Cu(bpe)2 chains along the a-axis and b-axis lie in the ab-plane (Figures S5 and S7). AB-α was found to exhibit ABAB stacking of layers, resulting in a pore structure distinct from that of AA-α and related materials.27 The resulting ultramicroporous channels (3.96 × 5.56 Å2, after subtracting the van der Waals radii, Figure 2f) represent 29.9% of the unit cell volume as calculated by PLATON.52 The 1D channels in AA-α resulted in a solvent-accessible space of 24.7% of the unit cell volume (Figure 2c). We anticipated that the inherent flexibility of bpe might enable induced fit or preferential binding toward C3H4 over C3H610 and that the electronegative NbOF52– anions lining the channels might preferentially bind alkynes vs alkenes (Figure 2c and 2f).24,42,53−56 We therefore undertook a study of the separation performance of these polymorphs toward C3H4 and C3H6.
Figure 2.
Comparisons of sql-NbOFFIVE-bpe-Cu-AA-α (a, b, c) and sql-NbOFFIVE-bpe-Cu-AB-α (d, e, f). Simplified crystal structure of sql-NbOFFIVE-bpe-Cu-AA-α along the b (a) and a axes (b); (c) 1D channels in sql-NbOFFIVE-bpe-Cu-AA-α; Simplified crystal structures of sql-NbOFFIVE-bpe-Cu-AB-α along the a (d) and c axes (e); (f) 3D channels in sql-NbOFFIVE-bpe-Cu-AB-α. Color code: blue = NbOF5; black = hydrogen bonds; Adjacent layers colored orange, green, and blue. Hydrogen atoms omitted for clarity.
As-synthesized AB-α transformed to a narrower-pore phase, AB-β, after methanol exchange at 333 K under vacuum for 12 h. We were unable to directly determine the crystal structure of activated (anhydrate) AB-β, as it captured water from air at low relative humidity (RH), as revealed by dynamic vapor sorption (DVS) (Figure S26). Figures S27 and S28 reveal that water vapor was adsorbed within minutes at 30% RH, 298 K. The SCXRD structure determined in air, AB-β1, was found to be a hydrate with twisted pores, twisted NbOF52– anions, and undulating pillars, unlike AB-α (Figure S9). TGA data collected after holding at 80 °C for 2 h revealed no weight loss, further indicating that AB-β is fully activated and that water vapor was captured from the laboratory atmosphere (Figure S18). AB-β1 crystallized in the tetragonal space group P42/mnm (Table S5). The distance between F atoms (dF···F) in adjacent pillars changed from 6.33 Å in AB-α to 6.91 and 5.37 Å in AB-β1 (Figure S10). Activation of AA-α resulted in AA-β. Heating at 333 K in situ on the SCXRD goniometer enabled structural determination of AA-β (Figure S8), which had crystallized in the monoclinic space group I2/m (Table S4). The dF···F value decreased from 7.1104 Å in AA-α to 6.9260 Å in AA-β (Figure S11).
These transformations were also investigated by variable-temperature PXRD (VT-PXRD). AA-α converted to AA-β by heating at 333 K under N2, the PXRD pattern matching that calculated from the SCXRD structure (Figure S16a). Methanol exchanged AB-α transformed to desolvated AB-β after heating at 393 K under N2 (Figure S16b). Methanol exchanged AB-α can also transform to AB-β by heating at 333 K under vacuum for 6 h (Figure S16c). The partially loaded AB-β1 phase was also observed by VT-PXRD at 333 K, its PXRD diffractogram matching the calculated PXRD pattern of AB-β1. AB-β was observed to transform to AA-β after heating at 473 K under N2 (Figure S16b).
To investigate the porosity of AA and AB, gas sorption isotherms of CO2, at 195 K, and N2, at 77 K, were collected (Figure S19). Prior to collection of sorption data, methanol exchanged AA and AB were activated at 333 K for 12 h under vacuum to generate their respective β forms. CO2 adsorption by AB revealed a stepped isotherm profile with an inflection at low pressure (ca. 0.024 bar) and an uptake of ca. 1.5 mmol g–1 after the first step.57 A saturated CO2 uptake of ∼2.9 mmol g–1 corresponds to almost 4 CO2 molecules per unit cell. In the case of N2 adsorption, an uptake of ∼2.0 mmol g–1 was observed. The corresponding values for AA revealed CO2 and N2 uptakes of ∼3.5 mmol g–1 and ∼1.0 mmol g–1, respectively. Langmuir surface areas of 356 m2 g–1 for AA and 295 m2 g–1 for AB were calculated from 195 K CO2 isotherms. The maximum pore size distributions derived from 195 K CO2 data were determined to be 3.55 for AA and 6.04 Å for AB, matching the pore sizes derived from SCXRD data (Figure S19). For a larger probe size, such as N2 at 77 K (3.6 Å for N2 vs 3.3 Å for CO2), AA shows lower uptake than that of AB because of its narrower pore.
Next, we studied the C3H4 and C3H6 adsorption properties of AB and AA at 273 and 298 K (Figures 3a, 3d, S20, and S21). The C3H4 sorption isotherm of AB revealed steep uptake at low pressure and an uptake of 3.04 mmol g–1 at 1 bar and 298 K, significantly higher than its C3H6 uptake (2.10 mmol g–1) under the same conditions. We note that the uptake of C3H6 was negligible at low pressure (0.01 mmol g–1 at 0.001 bar; 0.1 mmol g–1 at 0.01 bar; 0.23 mmol g–1 at 0.1 bar), reflecting the stepped sorption isotherm.57 The corresponding C3H4 uptakes were higher (1.20 mmol g–1 at 0.001 bar; 1.93 mmol g–1 at 0.01 bar; 2.40 mmol g–1 at 0.1 bar, Figure S21). Similar stepped isotherms were reported for GEFSIX-dps-Cu, ELM-12, and ZU-13.11,58,59 The uptake ratio of C3H4/C3H6 for AB at 1 mbar is higher than that of NKMOF-11.12 Sample regeneration was realized by exposure to vacuum at 333 K for as little as 10 min. Multiple sorption tests were performed, and similar sorption isotherms were observed, indicating good recyclability (Figure S22).
Figure 3.
(a) C3H4 and C3H6 adsorption isotherms of sql-NbOFFIVE-bpe-Cu-AB collected at 298 K; (b) S-PXRD (λ = 0.35424308 Å) patterns of C3H4-loaded and C3H6-loaded sql-NbOFFIVE-bpe-Cu-AB-α compared with the PXRD patterns calculated from SCXRD and modeling data; (c) Pawley profile fit for sql-NbOFFIVE-bpe-Cu-AB-β. The experimental S-PXRD data are presented in black, calculated in red, and the difference between experimental and calculated in blue. Bragg reflections are shown as green bars. Crystal system = Tetragonal, Space group = P42/mnm, a = b = 12.4888(4) Å, c = 18.8761(6) Å, V = 2944.1(2) Å3, r_wp = 3.216%, r_exp = 1.821%, r_p = 3.127%, GOF = 1.766; (d) C3H4 and C3H6 adsorption isotherms of sql-NbOFFIVE-bpe-Cu-AA at 298 K; (e) S-PXRD (λ = 0.35424308 Å) patterns of C3H4-loaded and C3H6-loaded sql-NbOFFIVE-bpe-Cu-AA-α compared with their corresponding PXRD patterns calculated from SCXRD and modeling data; (f) S-PXRD patterns of experimental sql-NbOFFIVE-bpe-Cu-AA-β compared with calculated PXRD of sql-NbOFFIVE-bpe-Cu-AA-β from SCXRD.
The differences between the single-component isotherms of C3H4 and C3H6 are indicative of potential utility for separation of C3H4/C3H6 binary mixtures. In the case of AA, the uptake ratio of C3H4/C3H6 at 1 mbar (∼3.8) is much lower than that of AB (∼120), although AA shows a similar uptake of C3H4 at 1 bar and lower uptake of C3H6 than AB (Figure S21). These results indicate that AB offers stronger potential for separation of C3H4/C3H6 than AA.
Synchrotron PXRD (S-PXRD) data were collected for activated as well as C3H4 and C3H6-loaded samples to study the guest-induced structural change. As shown in Figure 3b and 3e, S-PXRD diffractograms of C3H4 and C3H6-loaded AA and AB support the presence of the corresponding α phases. The S-PXRD pattern of activated AA-β obtained by heating at 333 K under vacuum is consistent with the calculated pattern (Figure 3f). Pawley fitting for AB-β revealed tetragonal space group P42/mnm (Figure 3c) and a unit cell volume of AB-β (2944.1(2) Å3) slightly smaller than that of AB-β1 (2954.6(4) Å3). These results indicate that both C3H4 and C3H6 can induce phase changes from the narrow-pore phases (AA-β and AB-β) to the respective open phases (AA-α and AB-α).
To quantify the potential of AB for separation of the challenging C3H4/C3H6 binary mixtures, ideal adsorption solution theory (IAST) calculations were conducted using Dual-site Langmuir–Freundlich (DSLF) and 3-site Langmuir–Freundlich isotherm shape models60−62 (Figures S23 and S24, Tables S6 and S7). The calculated adsorption selectivity values for 1:99 and 1:1 C3H4/C3H6 binary mixtures are up to 220 and over 180 at 298 K and 1 bar, respectively. Simulated breakthrough data using a methodology described previously predicts excellent separation performance for C3H4/C3H6 (Figure 4a).63−67
Figure 4.
(a) Simulated breakthrough curves of sql-NbOFFIVE-bpe-Cu-AB for separation of C3H4/C3H6 (1/99) mixture at 298 K; (b) Experimental breakthrough separation of sql-NbOFFIVE-bpe-Cu-AB for C3H4/C3H6 (1/99) at 298 K (gas velocity: 1.0 cm3 min–1); (c) Experimental breakthrough separation of sql-NbOFFIVE-bpe-Cu-AA for C3H4/C3H6 (1/99) at 298 K (gas velocity: 1.0 cm3 min–1); (d) Gravimetric kinetics of sql-NbOFFIVE-bpe-Cu-AB for C3H4 and C3H6 uptake (0–1.0 bar) at 303 K; (e) Gravimetric kinetics of sql-NbOFFIVE-bpe-Cu-AA for C3H4 and C3H6 uptake (1.0 bar) at 303 K; (f) Comparison of C3H6 productivity in representative benchmark materials for separation of 1/99 C3H4/C3H6 mixture.
In order to experimentally evaluate the C3H4/C3H6 separation performance of AA and AB, we conducted dynamic column breakthrough (DCB) experiments that mimic typical process conditions with an inlet gas mixture composition of 1:99 (v/v) C3H4/C3H6.12,22 This C3H4/C3H6 gas mixture with a flow rate of 1.0 cm3 min–1 was passed through a fixed bed column (8 mm diameter) packed with sorbent at 1 bar and 298 K. The fixed beds of methanol exchanged samples were first activated by heating at 353 K in a 20 cm3 min–1 flow of Helium for about 6 h. DCB experiments were commenced after samples were cooled to room temperature. Gas chromatography (GC) was used to monitor eluted components quantitatively at short sampling intervals (Figure S29; see Supporting Information for the experimental setup). As expected (Figure 4b and 4c), AB was indeed found to be more effective for C3H4/C3H6 separation than AA. For AB, C3H6 breakthrough occurred at 10 min g–1, well before C3H4 (2710 min g–1). This represents a C3H4 uptake capacity (1.2 mmol g–1, Table S8) comparable to that of the previous benchmark sorbent, NKMOF-1-Ni (1.21 mmol g–1) and Ni@FAU (1.59 mmol g–1).49,68 During the time lag of 2710 min g–1 before breakthrough, GC data showed that the concentration of C3H4 in the effluent gas stream was <1 ppm (Table S8). According to the DCB profile obtained from a 1/99 mixture, the polymer-grade C3H6 productivity (>99.99% purity) of AB sets a new benchmark value of 118 mmol g–1, beyond that of previous benchmark materials (NKMOF-11, 74.4 mmol g–1; UTSA-200, 62.0 mmol g–1; ZNU-2-Si, 52.9 mmol g–1; ZNU-2, 42 mmol g–1; SIFSIX-3-Ni, 20.05 mmol g–1 and SIFSIX-2-Cu-i, 26.64 mmol g–1, Figure 4f).12,13,38,48,69 Separation selectivity (αAC) was calculated to be 270, exceeding that of reported HUMs with sql topology GeFSIX-dps-Cu (82.1), GeFSIX-dps-Zn (65.6).11 That AB outperformed previous benchmark materials in terms of C3H6 productivity demonstrates that HUMs can offer both high selectivity and high uptake for challenging gas separations.
We also studied the pure gas adsorption kinetics for C3H4 and C3H6 using methanol exchanged samples, whereby activated samples of AA and AB were exposed to a constant flow of 10 cm3 min–1 C3H4 and C3H6 at 303 K and 1.0 bar. As presented in Figure 4d, the slope of the kinetic curve for AB is much steeper for C3H4 than that of C3H6, indicating faster adsorption kinetics for C3H4. The kinetic curves of C3H4 and C3H6 level off at 6.8 wt % (1.7 mmol g–1) after ca. 10 min and 2.5 wt % (1.2 mmol g–1) after ca. 60 min, respectively. Regeneration tests were performed by heating the samples at 353 K under N2 flow for ca. 1 h (flow rate: 60 cm3 min–1), and no changes in uptake were observed after successive cycles (Figure S25). With respect to AA, the slope of the kinetic curve is almost the same for C3H4 and C3H6, indicating similar adsorption kinetics for C3H4 and C3H6 (Figures 4e and S25). That gas adsorption kinetics in AB favors C3H4 over C3H6 is desirable for efficient gas separation during dynamic DCB tests.
Hydrolytic stability of a sorbent is a prerequisite for utility, prompting us to soak crystals of AB in water and perform water vapor sorption experiments using DVS. The water sorption experiment revealed a type I isotherm with approximately 15 wt % uptake at about 90% RH, which is consistent with the weight loss observed in the TGA curve (∼12 wt %, Figures S17 and S26). Cycling tests were performed 10 times and revealed that the sample retained stability when exposed to humidity (Figure S28). Crystals soaked in water for 5 days retained crystallinity (Figure S14).
Insight into the distinct sorption properties of AA and AB was gained through DFT calculations, which revealed that AA and AB have similar lattice energies, the AB to AA transformation being predicted to be exothermic by −13.2 kJ mol–1 per Cu2Nb2O2F10(bpe)4 formula unit. DFT was also used to identify the most plausible binding sites and their adsorption enthalpies whereas Canonical Monte Carlo (CMC) simulations were conducted to obtain adsorbate occupancy or the density map comprising the binding site regions (see Supporting Information for further details on the computational methodology). For each framework, the energetically most plausible orientations for C3H4 and C3H6 are represented in Figure 5. The binding sites were identified using DFT calculations in which atomic positions were optimized so that the binding pockets can adapt to each adsorbate.
Figure 5.

Binding sites of (a) C3H4 and (b) C3H6 in sql-NbOFFIVE-bpe-Cu-AB (a, b) and sql-NbOFFIVE-bpe-Cu-AA (c, d). The closest contacts (Å) between framework atoms and adsorbates are highlighted in orange for C–H···F, green for C–H···π, and red for C–H (with another framework)···π.
The resulting adsorption enthalpies C3H4 and C3H6 in AA were calculated to be −62.7 (C3H4) and −65.2 (C3H6) kJ mol–1, respectively. These similar adsorption enthalpies and Gibbs free energy differences are indicative of poor selectivity for C3 hydrocarbons. These values are also in line with experimental data (Figure 4c). In contrast, the adsorption enthalpies of −69.0 (C3H4) and −53.0 (C3H6) kJ mol–1 calculated for AB suggest enhanced binding of C3H4 and weaker C3H6 binding compared to AA, also in line with experimental observations (Figure 4b). The 16 kJ mol–1 difference in adsorption enthalpy for AB is also found in the adsorption Gibbs free energy differences ΔGads of −24.4 (C3H4) and −8.5 (C3H6) kJ mol–1, where a difference of only 4.4 kJ mol–1 was calculated for AA, with ΔGads values of −19.6 (C3H4) and −15.0 (C3H6) kJ mol–1, respectively. For both adsorbates, there are hydrogen bonds between H atoms of the adsorbates and framework F atoms (Figure 5), as is typical for HUMs.70−73 A detailed analysis of these binding sites, including adsorption energy and distances, is summarized in Table S9. Binding site isosurfaces from CMC simulations are visualized in Tables S11 and S12 and reveal that the density fields, comprising adsorbate mass-middle point occupancies of successful insertion moves, of C3H4 are larger than those of C3H6 for all frameworks. This can be attributed to the more linear geometry of C3H4 resulting in a larger binding area. Furthermore, the CMC results validate the preferred positions of the adsorbates in the framework and its channels in the vicinity of framework F atoms, which can be observed by merging the binding site outcomes from DFT and CMC (Figures S31 and S32). The four modeled crystal structures were used to calculate PXRD patterns, which are a good match for both the experimental S-PXRD data and the PXRD patterns calculated from SCXRD data (Figure 3b, 3e).
Conclusions
In summary, two packing polymorphs of an sql network with intrinsic ultramicropores, AA and AB, exhibit AAAA and ABAB packing of the sql layers, respectively. AA is isostructural with sql-SIFSIX-bpe-Zn and exhibits intrinsic 1D channels within each sql network. AB exhibits both intrinsic channels and extrinsic channels between the sql networks that arise from the different crystal packing of adjacent layers. Both polymorphs were found to display phase transformations induced by pressure and temperature. AB was found to be the most interesting sorbent in terms of sorption properties, as it exhibits excellent C3H4/C3H6 separation performance as indicated by a new high for C3H4/C3H6 experimental selectivity (270) and a new benchmark for polymer-grade C3H6 productivity (118 mmol g–1) from a 1:99 C3H4/C3H6 binary mixture. Modeling studies provided insight into the selective binding sites for C3H4. This work has not only resulted in a new benchmark for C3H4 separation from C3H6 but also brings a new approach to pore engineering. Specifically, whereas the packing polymorphs exhibit similar surface areas, their pore chemistry, size, and shape are distinctly different.
Acknowledgments
M.J.Z. acknowledges the support of the Science Foundation Ireland (16/IA/4624), the Irish Research Council (IRCLA/2019/167), and the European Research Council (ADG 885695). S.J.N. and M.V. acknowledge the Irish Centre for High-End Computing (ICHEC) for the provision of computational facilities and support. S.J.N. is grateful for the support by Enterprise Ireland and the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie (grant agreement no. 847402, project ID: MF20210297). We are especially grateful to ESRF for access to beamline ID22 and the Diamond Light Source for access to beamline I19, as well as the beamline scientists for their support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c03505.
Experimental and characterization details; additional figures and images; PXRD patterns; VT-PXRD patterns; TGA analysis, DVS analysis, and Sorption isotherms. (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Perry J. J.; Perman J. A.; Zaworotko M. J. Design and synthesis of metal-organic frameworks using metal-organic polyhedra as supermolecular building blocks. Chem. Soc. Rev. 2009, 38, 1400–1417. 10.1039/b807086p. [DOI] [PubMed] [Google Scholar]
- Li J. R.; Sculley J.; Zhou H. C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. 10.1021/cr200190s. [DOI] [PubMed] [Google Scholar]
- Yaghi O. M.; O’Keeffe M.; Ockwig N. W.; Chae H. K.; Eddaoudi M.; Kim J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]
- Furukawa H.; Cordova K. E.; O’Keeffe M.; Yaghi O. M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- Kitagawa S.; Kitaura R.; Noro S. Functional porous coordination polymers. Angew. Chem., Int. Ed. 2004, 43, 2334–2375. 10.1002/anie.200300610. [DOI] [PubMed] [Google Scholar]
- Sui J.; Liu H.; Hu S.; Sun K.; Wan G.; Zhou H.; Zheng X.; Jiang H. L. A General Strategy to Immobilize Single-Atom Catalysts in Metal-Organic Frameworks for Enhanced Photocatalysis. Adv. Mater. 2022, 34, 2109203. 10.1002/adma.202109203. [DOI] [PubMed] [Google Scholar]
- Moussa Z.; Hmadeh M.; Abiad M. G.; Dib O. H.; Patra D. Encapsulation of curcumin in cyclodextrin-metal organic frameworks: Dissociation of loaded CD-MOFs enhances stability of curcumin. Food Chem. 2016, 212, 485–494. 10.1016/j.foodchem.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Mortada B.; Matar T. A.; Sakaya A.; Atallah H.; Kara Ali Z.; Karam P.; Hmadeh M. Postmetalated Zirconium Metal Organic Frameworks as a Highly Potent Bactericide. Inorg. Chem. 2017, 56, 4739–4745. 10.1021/acs.inorgchem.7b00429. [DOI] [PubMed] [Google Scholar]
- Das M. C.; Xiang S.; Zhang Z.; Chen B. Functional mixed metal-organic frameworks with metalloligands. Angew. Chem., Int. Ed. 2011, 50, 10510–10520. 10.1002/anie.201101534. [DOI] [PubMed] [Google Scholar]
- Zhu A. X.; Yang Q. Y.; Mukherjee S.; Kumar A.; Deng C. H.; Bezrukov A. A.; Shivanna M.; Zaworotko M. J. Tuning the Gate-Opening Pressure in a Switching pcu Coordination Network, X-pcu-5-Zn, by Pillar-Ligand Substitution. Angew. Chem., Int. Ed. 2019, 58, 18212–18217. 10.1002/anie.201909977. [DOI] [PubMed] [Google Scholar]
- Ke T.; Wang Q.; Shen J.; Zhou J.; Bao Z.; Yang Q.; Ren Q. Molecular Sieving of C2 -C3 Alkene from Alkyne with Tuned Threshold Pressure in Robust Layered Metal-Organic Frameworks. Angew. Chem., Int. Ed. 2020, 59, 12725–12730. 10.1002/anie.202003421. [DOI] [PubMed] [Google Scholar]
- Peng Y. L.; Wang T.; Jin C.; Li P.; Suepaul S.; Beemer G.; Chen Y.; Krishna R.; Cheng P.; Pham T.; Space B.; Zaworotko M. J.; Zhang Z. A robust heterometallic ultramicroporous MOF with ultrahigh selectivity for propyne/propylene separation. J. Mater. Chem. A 2021, 9, 2850–2856. 10.1039/D0TA08498K. [DOI] [Google Scholar]
- Jiang Y.; Hu J.; Wang L.; Sun W.; Xu N.; Krishna R.; Duttwyler S.; Cui X.; Xing H.; Zhang Y. Comprehensive Pore Tuning in an Ultrastable Fluorinated Anion Cross-Linked Cage-Like MOF for Simultaneous Benchmark Propyne Recovery and Propylene Purification. Angew. Chem., Int. Ed. 2022, 61, e202200947. 10.1002/anie.202200947. [DOI] [PubMed] [Google Scholar]
- Guo L.; Savage M.; Carter J. H.; Han X.; da Silva I.; Manuel P.; Rudic S.; Tang C. C.; Yang S.; Schroder M. Direct Visualization of Supramolecular Binding and Separation of Light Hydrocarbons in MFM-300(In). Chem. Mater. 2022, 34, 5698–5705. 10.1021/acs.chemmater.2c01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R. B.; Zhang Z.; Chen B. Achieving High Performance Metal-Organic Framework Materials through Pore Engineering. Acc. Chem. Res. 2021, 54, 3362–3376. 10.1021/acs.accounts.1c00328. [DOI] [PubMed] [Google Scholar]
- Hong A. N.; Yang H.; Bu X.; Feng P. Pore space partition of metal-organic frameworks for gas storage and separation. EnergyChem. 2022, 4, 100080. 10.1016/j.enchem.2022.100080. [DOI] [Google Scholar]
- Kokcam-Demir U.; Goldman A.; Esrafili L.; Gharib M.; Morsali A.; Weingart O.; Janiak C. Coordinatively unsaturated metal sites (open metal sites) in metal-organic frameworks: design and applications. Chem. Soc. Rev. 2020, 49, 2751–2798. 10.1039/C9CS00609E. [DOI] [PubMed] [Google Scholar]
- Subramanian S.; Zaworotko M. J. Porous Solids by Design: [Zn(4,4’-bpy)2(SiF6)]n·xDMF, a Single Framework Octahedral Coordination Polymer with Large Square Channel. Angew. Chem., Int. Ed. 1995, 34, 2127–2128. 10.1002/anie.199521271. [DOI] [Google Scholar]
- Cadiau A.; Belmabkhout Y.; Adil K.; Bhatt P. M.; Pillai R. S.; Shkurenko A.; Martineau-Corcos C.; Maurin G.; Eddaoudi M. Hydrolytically stable fluorinated metal-organic frameworks for energy-efficient dehydration. Science 2017, 356, 731–735. 10.1126/science.aam8310. [DOI] [PubMed] [Google Scholar]
- Song B. Q.; Yang Q. Y.; Wang S. Q.; Vandichel M.; Kumar A.; Crowley C.; Kumar N.; Deng C. H.; GasconPerez V.; Lusi M.; Wu H.; Zhou W.; Zaworotko M. J. Reversible Switching between Nonporous and Porous Phases of a New SIFSIX Coordination Network Induced by a Flexible Linker Ligand. J. Am. Chem. Soc. 2020, 142, 6896–6901. 10.1021/jacs.0c01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent P.; Belmabkhout Y.; Burd S. D.; Cairns A. J.; Luebke R.; Forrest K.; Pham T.; Ma S.; Space B.; Wojtas L.; Eddaoudi M.; Zaworotko M. J. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. 10.1038/nature11893. [DOI] [PubMed] [Google Scholar]
- Chen K. J.; Madden D. G.; Mukherjee S.; Pham T.; Forrest K. A.; Kumar A.; Space B.; Kong J.; Zhang Q. Y.; Zaworotko M. J. Synergistic sorbent separation for one-step ethylene purification from a four-component mixture. Science 2019, 366, 241–246. 10.1126/science.aax8666. [DOI] [PubMed] [Google Scholar]
- Cui X.; Chen K.; Xing H.; Yang Q.; Krishna R.; Bao Z.; Wu H.; Zhou W.; Dong X.; Han Y.; Li B.; Ren Q.; Zaworotko M. J.; Chen B. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 2016, 353, 141–144. 10.1126/science.aaf2458. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhang Y.; Su Y.; Liu X.; Zhang P.; Lin R. B.; Chen S.; Deng Q.; Zeng Z.; Deng S.; Chen B. Fine pore engineering in a series of isoreticular metal-organic frameworks for efficient C2H2/CO2 separation. Nat. Commun. 2022, 13, 200. 10.1038/s41467-021-27929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensharma D.; O’Hearn D. J.; Koochaki A.; Bezrukov A. A.; Kumar N.; Wilson B. H.; Vandichel M.; Zaworotko M. J. The First Sulfate-Pillared Hybrid Ultramicroporous Material, SOFOUR-1-Zn, and Its Acetylene Capture Properties. Angew. Chem., Int. Ed. 2022, 61, e202116145. 10.1002/anie.202116145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H. S.; Shivanna M.; Bajpai A.; Chen K.-J.; Madden D. G.; Perry J. J. IV; Zaworotko M. J. Enhanced Stability toward Humidity in a Family of Hybrid Ultramicroporous Materials Incorporating Cr2O72– Pillars. Cryst. Growth Des. 2017, 17, 1933–1937. 10.1021/acs.cgd.6b01881. [DOI] [Google Scholar]
- Shivanna M.; Otake K. I.; Song B. Q.; van Wyk L. M.; Yang Q. Y.; Kumar N.; Feldmann W. K.; Pham T.; Suepaul S.; Space B.; Barbour L. J.; Kitagawa S.; Zaworotko M. Benchmark acetylene binding affinity and separation through induced fit in a flexible hybrid ultramicroporous material. Angew. Chem., Int. Ed. 2021, 60, 20383–20390. 10.1002/anie.202106263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R. B.; Xiang S.; Xing H.; Zhou W.; Chen B. Exploration of porous metal-organic frameworks for gas separation and purification. Coord. Chem. Rev. 2019, 378, 87–103. 10.1016/j.ccr.2017.09.027. [DOI] [Google Scholar]
- Zhao X.; Wang Y.; Li D. S.; Bu X.; Feng P. Metal-Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. 10.1002/adma.201705189. [DOI] [PubMed] [Google Scholar]
- Barnett B. R.; Gonzalez M. I.; Long J. R. Recent Progress Towards Light Hydrocarbon Separations Using Metal-Organic Frameworks. Trends Chem. 2019, 1, 159–171. 10.1016/j.trechm.2019.02.012. [DOI] [Google Scholar]
- Lan T.; Li L.; Chen Y.; Wang X.; Yang J.; Li J. Opportunities and critical factors of porous metal-organic frameworks for industrial light olefins separation. Mater. Chem. Front. 2020, 4, 1954–1984. 10.1039/D0QM00186D. [DOI] [Google Scholar]
- Wang H.; Liu Y.; Li J. Designer Metal-Organic Frameworks for Size-Exclusion-Based Hydrocarbon Separations: Progress and Challenges. Adv. Mater. 2020, 32, 2002603. 10.1002/adma.202002603. [DOI] [PubMed] [Google Scholar]
- Pei J.; Shao K.; Zhang L.; Wen H. M.; Li B.; Qian G. Current Status of Microporous Metal-Organic Frameworks for Hydrocarbon Separations. Top. Curr. Chem. 2019, 377, 33. 10.1007/s41061-019-0257-0. [DOI] [PubMed] [Google Scholar]
- Li H.; Li L.; Lin R.-B.; Zhou W.; Zhang Z.; Xiang S.; Chen B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem. 2019, 1, 100006. 10.1016/j.enchem.2019.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R. B.; Xiang S.; Zhou W.; Chen B. Microporous Metal-Organic Framework Materials for Gas Separation. Chem. 2020, 6, 337. 10.1016/j.chempr.2019.10.012. [DOI] [Google Scholar]
- Wang B.; Xie L. H.; Wang X.; Liu X. M.; Li J.; Li J. R. Applications of metal-organic frameworks for green energy and environment: New advances in adsorptive gas separation, storage and removal. Green Energy Environ. 2018, 3, 191–228. 10.1016/j.gee.2018.03.001. [DOI] [Google Scholar]
- Wang S. Q.; Mukherjee S.; Zaworotko M. J. Spiers Memorial Lecture: Coordination networks that switch between nonporous and porous structures: an emerging class of soft porous crystals. Faraday Discuss. 2021, 231, 9–50. 10.1039/D1FD00037C. [DOI] [PubMed] [Google Scholar]
- Li L.; Wen H. M.; He C.; Lin R. B.; Krishna R.; Wu H.; Zhou W.; Li J.; Li B.; Chen B. A Metal-Organic Framework with Suitable Pore Size and Specific Functional Sites for the Removal of Trace Propyne from Propylene. Angew. Chem., Int. Ed. 2018, 57, 15183–15188. 10.1002/anie.201809869. [DOI] [PubMed] [Google Scholar]
- Shekhah O.; Belmabkhout Y.; Chen Z.; Guillerm V.; Cairns A.; Adil K.; Eddaoudi M. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture. Nat. Commun. 2014, 5, 4228. 10.1038/ncomms5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhah O.; Belmabkhout Y.; Adil K.; Bhatt P. M.; Cairns A. J.; Eddaoudi M. A facile solvent-free synthesis route for the assembly of a highly CO2 selective and H2S tolerant NiSIFSIX metal-organic framework. Chem. Commun. 2015, 51, 13595–13598. 10.1039/C5CC04487A. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhang Y.; Zhang P.; Hu J.; Lin R. B.; Deng Q.; Zeng Z.; Xing H.; Deng S.; Chen B. Optimizing Pore Space for Flexible-Robust Metal-Organic Framework to Boost Trace Acetylene Removal. J. Am. Chem. Soc. 2020, 142, 9744–9751. 10.1021/jacs.0c02594. [DOI] [PubMed] [Google Scholar]
- Shen J.; He X.; Ke T.; Krishna R.; van Baten J. M.; Chen R.; Bao Z.; Xing H.; Dinca M.; Zhang Z.; Yang Q.; Ren Q. Simultaneous interlayer and intralayer space control in two-dimensional metal-organic frameworks for acetylene/ethylene separation. Nat. Commun. 2020, 11, 6259. 10.1038/s41467-020-20101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R. B.; Li L. B.; Wu H.; Arman H.; Li B.; Lin R. G.; Zhou W.; Chen B. L. Optimized Separation of Acetylene from Carbon Dioxide and Ethylene in a Microporous Material. J. Am. Chem. Soc. 2017, 139, 8022–8028. 10.1021/jacs.7b03850. [DOI] [PubMed] [Google Scholar]
- Suen M. C.; Chan Z. K.; Chen J. D.; Wang J. C.; Hung C. H. Syntheses and structures of three new coordination polymers generated from the flexible 1,3-bis(4-pyridyl)propane ligand and zinc salts. Polyhedron 2006, 25, 2325–2332. 10.1016/j.poly.2006.01.031. [DOI] [Google Scholar]
- Cadiau A.; Adil K.; Bhatt P. M.; Belmabkhout Y.; Eddaoudi M. A metal-organic framework-based splitter for separating propylene from propane. Science 2016, 353, 137–140. 10.1126/science.aaf6323. [DOI] [PubMed] [Google Scholar]
- Zeng H.; Xie M.; Wang T.; Wei R.-J.; Xie X.-J.; Zhao Y.; Lu W.; Li D. Orthogonal-array dynamic molecular sieving of propylene/propane mixtures. Nature 2021, 595, 542–548. 10.1038/s41586-021-03627-8. [DOI] [PubMed] [Google Scholar]
- Gao M. Y.; Song B. Q.; Sensharma D.; Zaworotko M. J. Crystal engineering of porous coordination networks for C3 hydrocarbon separation. SmartMat 2021, 2, 38–55. 10.1002/smm2.1016. [DOI] [Google Scholar]
- Yang L.; Cui X.; Yang Q.; Qian S.; Wu H.; Bao Z.; Zhang Z.; Ren Q.; Zhou W.; Chen B.; Xing H. A Single-Molecule Propyne Trap: Highly Efficient Removal of Propyne from Propylene with Anion-Pillared Ultramicroporous Materials. Adv. Mater. 2018, 30, 1705374. 10.1002/adma.201705374. [DOI] [PubMed] [Google Scholar]
- Peng Y. L.; He C.; Pham T.; Wang T.; Li P.; Krishna R.; Forrest K. A.; Hogan A.; Suepaul S.; Space B.; Fang M.; Chen Y.; Zaworotko M. J.; Li J.; Li L.; Zhang Z.; Cheng P.; Chen B. Robust Microporous Metal-Organic Frameworks for Highly Efficient and Simultaneous Removal of Propyne and Propadiene from Propylene. Angew. Chem., Int. Ed. 2019, 58, 10209–10214. 10.1002/anie.201904312. [DOI] [PubMed] [Google Scholar]
- Heier K. R.; Poeppelmeier K. R. Reinvestigation of CuNbOF5·4H2O. J. Solid State Chem. 1997, 133, 576–579. 10.1006/jssc.1997.7529. [DOI] [Google Scholar]
- Lin M. J.; Jouaiti A.; Pocic D.; Kyritsakas N.; Planeix J. M.; Hosseini M. W. Molecular tectonics: tubular crystals with controllable channel size and orientation. Chem. Commun. 2010, 46, 112–114. 10.1039/B915665H. [DOI] [PubMed] [Google Scholar]
- Spek A. L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. 10.1107/S0021889802022112. [DOI] [Google Scholar]
- Yang L.; Cui X.; Zhang Z.; Yang Q.; Bao Z.; Ren Q.; Xing H. An Asymmetric Anion-Pillared Metal-Organic Framework as a Multisite Adsorbent Enables Simultaneous Removal of Propyne and Propadiene from Propylene. Angew. Chem., Int. Ed. 2018, 57, 13145–13149. 10.1002/anie.201807652. [DOI] [PubMed] [Google Scholar]
- Li J.; Bhatt P. M.; Li J.; Eddaoudi M.; Liu Y. Recent Progress on Microfine Design of Metal-Organic Frameworks: Structure Regulation and Gas Sorption and Separation. Adv. Mater. 2020, 32, 2002563. 10.1002/adma.202002563. [DOI] [PubMed] [Google Scholar]
- Cui W. G.; Hu T. L.; Bu X. H. Metal-Organic Framework Materials for the Separation and Purification of Light Hydrocarbons. Adv. Mater. 2020, 32, 1806445. 10.1002/adma.201806445. [DOI] [PubMed] [Google Scholar]
- Qian S.; Hu J.; Wang X.; Yang L.; Suo X.; Wang Z.; Cui X.; Xing H. Anion-pillared microporous material incorporated mixed-matrix fiber adsorbents for removal of trace propyne from propylene. AIChE J. 2023, e18091. 10.1002/aic.18091. [DOI] [Google Scholar]
- Yang Q. Y.; Lama P.; Sen S.; Lusi M.; Chen K. J.; Gao W. Y.; Shivanna M.; Pham T.; Hosono N.; Kusaka S.; Perry J. J. t.; Ma S.; Space B.; Barbour L. J.; Kitagawa S.; Zaworotko M. J. Reversible Switching between Highly Porous and Nonporous Phases of an Interpenetrated Diamondoid Coordination Network That Exhibits Gate-Opening at Methane Storage Pressures. Angew. Chem., Int. Ed. 2018, 57, 5684–5689. 10.1002/anie.201800820. [DOI] [PubMed] [Google Scholar]
- Li L.; Lin R. B.; Krishna R.; Wang X.; Li B.; Wu H.; Li J.; Zhou W.; Chen B. Flexible-Robust Metal-Organic Framework for Efficient Removal of Propyne from Propylene. J. Am. Chem. Soc. 2017, 139, 7733–7736. 10.1021/jacs.7b04268. [DOI] [PubMed] [Google Scholar]
- Yang L.; Cui X.; Zhang Y.; Yang Q.; Xing H. A highly sensitive flexible metal-organic framework sets a new benchmark for separating propyne from propylene. J. Mater. Chem. A 2018, 6, 24452–24458. 10.1039/C8TA08198K. [DOI] [Google Scholar]
- Gutierrez-Sevillano J. J.; Calero S.; Krishna R. Separation of benzene from mixtures with water, methanol, ethanol, and acetone: highlighting hydrogen bonding and molecular clustering influences in CuBTC. Phys. Chem. Chem. Phys. 2015, 17, 20114–20124. 10.1039/C5CP02726H. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Sevillano J. J.; Calero S.; Krishna R. Selective Adsorption of Water from Mixtures with 1-Alcohols by Exploitation of Molecular Packing Effects in CuBTC. J. Phys. Chem. C 2015, 119, 3658–3666. 10.1021/jp512853w. [DOI] [Google Scholar]
- Pan H.; Ritter J. A.; Balbuena P. B. Examination of the Approximations Used in Determining the Isosteric Heat of Adsorption from the Clausius-Clapeyron Equation. Langmuir 1998, 14, 6323–6327. 10.1021/la9803373. [DOI] [Google Scholar]
- Krishna R. Metrics for Evaluation and Screening of Metal-Organic Frameworks for Applications in Mixture Separations. ACS Omega 2020, 5, 16987–17004. 10.1021/acsomega.0c02218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna R. Methodologies for screening and selection of crystalline microporous materials in mixture separations. Sep. Purif. Technol. 2018, 194, 281–300. 10.1016/j.seppur.2017.11.056. [DOI] [Google Scholar]
- Krishna R. The Maxwell-Stefan description of mixture diffusion in nanoporous crystalline materials. Microporous Mesoporous Mater. 2014, 185, 30–50. 10.1016/j.micromeso.2013.10.026. [DOI] [Google Scholar]
- Krishna R. Methodologies for evaluation of metal-organic frameworks in separation applications. RSC Adv. 2015, 5, 52269–52295. 10.1039/C5RA07830J. [DOI] [Google Scholar]
- Krishna R. Screening metal-organic frameworks for mixture separations in fixed-bed adsorbers using a combined selectivity/capacity metric. RSC Adv. 2017, 7, 35724–35737. 10.1039/C7RA07363A. [DOI] [Google Scholar]
- Chai Y.; Han X.; Li W.; Liu S.; Yao S.; Wang C.; Shi W.; da-Silva I.; Manuel P.; Cheng Y.; Daemen L. D.; Ramirez-Cuesta A. J.; Tang C. C.; Jiang L.; Yang S.; Guan N.; Li L. Control of zeolite pore interior for chemoselective alkyne/olefin separations. Science 2020, 368, 1002–1006. 10.1126/science.aay8447. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; Wang L.; Yan T.; Hu J.; Sun W.; Krishna R.; Wang D.; Gu Z.; Liu D.; Cui X.; Xing H.; Zhang Y. Insights into the thermodynamic-kinetic synergistic separation of propyne/propylene in anion pillared cage MOFs with entropy-enthalpy balanced adsorption sites. Chem. Sci. 2023, 14, 298–309. 10.1039/D2SC05742E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M. Y.; Sensharma D.; Bezrukov A. A.; Andaloussi Y. H.; Darwish S.; Deng C.; Vandichel M.; Zhang J.; Zaworotko M. J. A Robust Molecular Porous Material for C2H2/CO2 Separation. Small 2023, 19, e2206945. 10.1002/smll.202206945. [DOI] [PubMed] [Google Scholar]
- Wang J. X.; Liang C.-C.; Gu X.-W.; Wen H.-M.; Jiang C.; Li B.; Qian G.; Chen B. Recent advances in microporous metal-organic frameworks as promising adsorbents for gas separation. J. Mater. Chem. A 2022, 10, 17878–17916. 10.1039/D2TA04835C. [DOI] [Google Scholar]
- Li X.; Bian H.; Huang W.; Yan B.; Wang X.; Zhu B. A review on anion-pillared metal-organic frameworks (APMOFs) and their composites with the balance of adsorption capacity and separation selectivity for efficient gas separation. Coord. Chem. Rev. 2022, 470, 214714. 10.1016/j.ccr.2022.214714. [DOI] [Google Scholar]
- Ebadi Amooghin A.; Sanaeepur H.; Luque R.; Garcia H.; Chen B. Fluorinated metal-organic frameworks for gas separation. Chem. Soc. Rev. 2022, 51, 7427–7508. 10.1039/D2CS00442A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





