Abstract
Aims
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a key regulator of plasma low-density lipoprotein cholesterol (LDL-C) concentration, and its inhibition reduces the risk of atherosclerotic cardiovascular disease (ASCVD). We aimed to assess the sex-differential effect of either pharmacological or genetic inhibition of PCSK9 on LDL-C levels.
Methods and results
We meta-analyzed six real-life studies (1216 men and 641 women) that investigated the effects of PCSK9 monoclonal antibodies (mAbs) on LDL-C reduction in men and women. Despite higher LDL-C levels in women at baseline [mean difference (MD) = 17.4 mg/dL, P < 0.0001, women = 175 mg/dL vs. men = 152 mg/dL], the LDL-C reduction under PCSK9 mAb treatment was significantly greater in men (MD = 7.6 mg/dL, 95% confidence interval: 2.7–12.4, P = 0.002) than in women.
We tested the sex-related association of the loss-of-function variant PCSK9-R46L with LDL-C plasma levels in 382 813 individuals (219 301 women and 163 512 men) free of lipid-lowering drugs from the UK Biobank general population cohort. The magnitude of LDL-C reduction was larger in men than in women (mean LDL-C difference: –35 mg/dL vs. –26 mg/dL, when comparing homozygous carriers with non-carriers in men and women, respectively). The relationship between PCSK9-R46L and LDL-C was significantly dependent on sex (P for interaction = 7.2e–04).
Conclusion
These results demonstrate by complementary approaches that the decrease in LDL-C mediated by PCSK9 inhibition is slightly, but significantly, less marked in women than in men. These data reinforce the need for specific studies to develop sex-specific recommendations for the management of ASCVD in women.
Keywords: PCSK9 inhibitors, Sex difference, LDL reduction, Genetics
Introduction
High concentrations of low-density lipoprotein cholesterol (LDL-C) are recognized as the main driving factors behind the development of atherosclerotic cardiovascular disease (ASCVD) and its major clinical consequences.1 The proprotein convertase subtilisin/kexin type 9 (PCSK9) is a natural inhibitor of the LDL receptor (LDLR) and a key regulator of LDL-C plasma levels.2 Briefly, after an intracellular autocleavage, the mature form of PCSK9 is secreted by the liver in the circulation, where it binds to the extracellular domain of the LDLR at the cell surface of hepatocytes, promoting its endocytosis and lysosomal degradation.
Several studies have suggested a potential sex difference in plasma PCSK9 concentrations. Plasma PCSK9 levels were found to be slightly higher in pre-menopausal women than in men.3,4 Menopausal status alters plasma PCSK9 concentrations with post-menopausal women exhibiting higher values than pre-menopausal ones.3 High endogenous estrogen treatment has been shown to decrease hepatic PCSK9 expression in rats5 and plasma PCSK9 levels in women,6 while the latter observation was not consistently retrieved across studies.4 Interestingly, a recent study in a large European cohort demonstrated that the independent association between plasma PCSK9 and cholesterol concentrations is only observed in men.7 Altogether, these data suggest a sex-specific effect on the regulation of LDL-C metabolism by PCSK9.
Nevertheless, PCSK9 inhibition with pharmacological agents [i.e. specific monoclonal antibodies (mAbs)] or ‘natural’ genetic factors decreases circulating lipid levels (mostly LDL-C) and reduces the risk of ASCVD in both sexes.8,9
Notably, the FOURIER randomized clinical trial showed a beneficial effect of evolocumab in both sexes but surprisingly with a stronger LDL-C reduction in men than in women.10 Also, recent data from a real-life patient registry (the LIPID-REAL Registry) have shown a significant sex-specific difference in the intensity of LDL-C reduction under PCSK9 mAbs.11
To get more insights into the sex-specific effects of PCSK9 inhibition on LDL-C reduction, we studied (i) the pharmacological inhibition of PCSK9 on LDL-C by PCSK9 mAbs in real-life studies and (ii) the natural genetic inhibition of PCSK9 loss-of-function variant (R46L) in the general population.
Methods
Meta-analysis of real-life studies with PCSK9 mAbs
Data sources and searching
We searched for real-life observational studies published until August 2022, reporting data in men and women on LDL-C levels at baseline and during the follow-up. A systematic screening was performed in electronic databases (PubMed, Web of Science, and Scopus) according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12 The following search string was applied: [(real-world) OR (real-life)] AND [(PCSK9) OR (proprotein convertase subtilisin/kexin type 9)] AND [(LDL-C) OR (low-density lipoprotein cholesterol)]. We retained only studies in which LDL-C concentrations were measured separately in men and women. We also manually consulted the reference lists of all included articles and collected data from published randomized clinical trials. Two authors (V.A.M. and P.P.) analyzed each article and extracted the data separately. In the case of disagreement, a third investigator was consulted (M.C.), and discrepancies were resolved by consensus.
Study selection, data extraction, and quality assessment
According to the established protocol, all studies reporting data about the association of the PCSK9 inhibitors’ effect on LDL-C reduction in men and women were included, while case reports and studies on animal models were excluded. Data related to clinical and demographic characteristics of patients treated with PCSK9 mAbs were extracted in each study. Methodological quality evaluation for each study was performed according to the Newcastle–Ottawa Scale (Supplementary material online, Table S1).
Data synthesis and analysis
Statistical analyses of real-life studies with PCSK9 mAbs were performed using Comprehensive Meta-Analysis version 3 (Biostat). The LDL-C levels were collected from the above-reported studies, and the data were transformed into a format needed for analyses, e.g. from the reported median and 95% confidence interval (CI) to mean and standard deviation (SD).13 The overall effect was tested using Z scores and significance was set at P < 0.05. Statistical heterogeneity was assessed with chi-square Cochran's Q test and with the I2 statistic (I2 values of 0% indicate no heterogeneity, 25% low, 25–50% moderate, and 50% or more high heterogeneity). To explore the effect of clinically relevant baseline covariates on the association between LDL-C reduction differences in men and women, we performed a study-level random effect model meta-regression analysis. Publication bias was assessed by Egger's test. In the case of significant publication bias, Duval and Tweedie's trim and fill method was used to estimate the adjusted effect size.14
Genetic analysis
Study population
The UK Biobank (UKBB) study is a population-based prospective cohort in the United Kingdom in which approximately 500 000 individuals aged between 40 and 69 years were recruited from 2006 through 2010.15 All participants have given informed consent. The UKBB has ethical approval from the North West–Haydock Research Ethics Committee (REC reference: 16/NW/0274). The present research has been conducted using the UKBB resource under the application number 49823. The records of 77 individuals (last updated on 22 February 2022) who have withdrawn from UKBB were removed from the analyses. LDL-C was measured using an enzymatic selective protection method.
Genetic analysis, data processing, and plotting
Variant filtering (GRCh38) was performed using bgen and vcf files (format VCFv4.2) with plink216 and BCFtools (v1.14).17 Genetic and phenotypic data were combined and processed using RStudio (v.2022.02.1). Plots were generated using RStudio using the ggplot218 R library, and statistical comparisons between groups were tested with Wilcoxon and two-way ANOVA tests using the R software.
Results
Effect of pharmacological PCSK9 inhibition on LDL-C reduction in men and women in a real-life setting
We identified six real-life studies,11,19–23 including 1216 men and 641 women (overall mean age of 60 ± 4 years) with follow-ups ranging from 2 to 47 months (Supplementary material online, Figure S1). The presence of cardiovascular risk factors, such as hypertension, occurred in 32–83%, diabetes in 16–28%, previous stroke in 5–12%, and coronary artery disease (CAD) was present in 66–83% of patients (Supplementary material online, Table S2). All patients were under lipid-lowering therapy (LLT) with statin and/or ezetimibe. Total statin intolerance was observed from 18 to 50% of the studied subjects, while partial statin intolerance was presented from 10 to 27% of the patients.
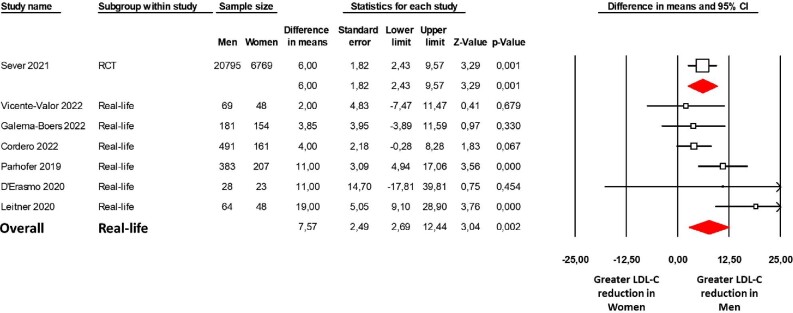
The meta-analysis showed that despite higher LDL-C levels in women at baseline [mean difference (MD) = 17.4 mg/dL; P < 0.0001, women = 175 mg/dL vs. men = 152 mg/dL; Supplementary material online, Figure S2 and Table S3], the LDL-C reduction was significantly greater in men (MD = 7.6 mg/dL, 95% CI: 2.7–12.4; P = 0.002; Figure 1) than in women under PCSK9 mAbs treatment. The heterogeneity among the studies was moderate (I2 = 54%; P = 0.052), and no differences between PCSK9 mAbs (evolocumab or alirocumab) or study type (prospective or retrospective) were observed. Moreover, the examination of the funnel plot showed no publication bias (Egger's test P = 0.511; Supplementary material online, Figure S3).
Figure 1.
Sex differences in LDL-C reduction induced by PCSK9 monoclonal antibodies in a real-life setting. Forest plot of the difference in LDL-C reduction between men and women. LDL-C reduction was evaluated with a mean difference expressed in mg/dL. The diamond represents the estimated overall effect, while the squares represent each study with 95% CI. LDL-C, low-density lipoprotein cholesterol; RCT, randomized clinical trial; CI, confidence interval.
Effect of genetic PCSK9 inhibition on LDL-C reduction in men and women in the general population
To investigate a potential sex effect on the reduction of LDL-C induced by genetic inhibition of PCSK9, we made use of the loss-of-function variant PCSK9-R46L as a genetic instrument. We first selected participants from the UKBB for whom we had genotyping data available (n = 487 418) (Supplementary material online, Figure S4). We excluded individuals under lipid-lowering therapies (LLT, n = 71 805) and participants who retracted consent (n = 77). A total of 382 813 individuals (219 301 women and 163 512 men) with genetic information for PCSK9-R46L (rs11591147) and plasma LDL-C levels were included in the final analysis.
We compared non-carriers of PCSK9-R46L (0/0) with heterozygous carriers (0/1) and homozygous carriers (1/1) (Figure 2). We first observed that the magnitude of the LDL-C difference between homozygous R46L carriers, when compared with controls, is larger in men than in women {mean LDL-C reduction: 35 mg/dL [143(±30)–108(±27)] vs. 26 mg/dL [145(±33)–119(±32)], respectively (P = 7.0E−18 vs. 4.6E−10, respectively) (Table 1 and Figure2)}. This difference was even more significant when correcting LDL-C for age (P = 1.2E−18 vs. 4.8E−11, respectively).
Figure 2.
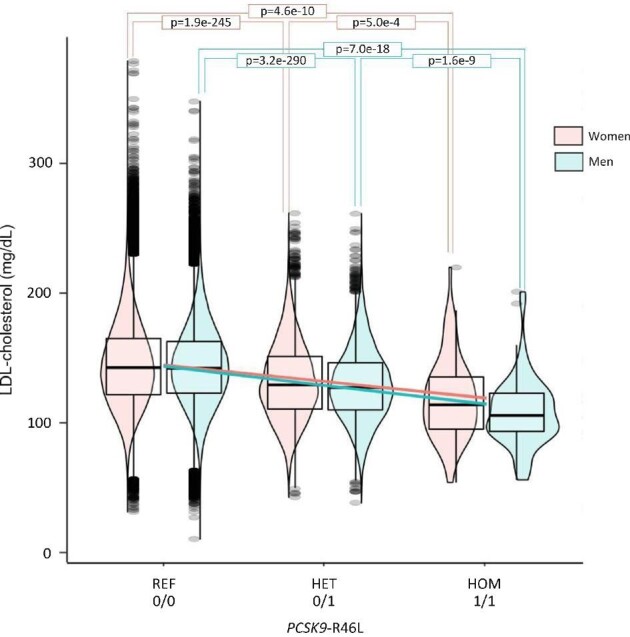
Plasma LDL-cholesterol levels according to the genetic inhibition of PCSK9 (as a function of PCSK9-R46L variant) in the UK Biobank. The plot depicts data from 382 813 individuals from the UK Biobank [219 301 women (pink symbols), 163 512 men (blue symbols)] free for lipid-lowering therapies. The y-axis showed LDL-C plasma levels (mmol/L) of individuals and the x-axis show groups of individuals based on their sex and genetic status. PCSK9, proprotein convertase subtilisin/kexin type 9; REF (0/0), non-carriers of the PCSK9-R46L variant; HET (0/1), heterozygous carriers of the PCSK9-R46L variant; HOM (1/1), homozygous carriers of the PCSK9-R46L variant; LDL-C, low-density lipoprotein cholesterol; SD, standard deviation to the mean.
Table 1.
Plasma LDL-cholesterol levels according to the PCSK9-R46L variant in the UKBB
| PCSK9-R46L | UKBB carriers (n) | LDL-C (mg/dL) (mean ± SD) |
|
|---|---|---|---|
| REF (0/0) | 211 742 | 145 (±33) | |
| Women | HET (0/1) | 7494 | 132 (±30) |
| HOM (1/1) | 65 | 119 (±32) | |
| REF (0/0) | 157 610 | 143 (±30) | |
| Men | HET (0/1) | 5839 | 129 (±28) |
| HOM (1/1) | 63 | 108 (±27) |
PCSK9, proprotein convertase subtilisin/kexin type 9; REF (0/0), non-carriers of the PCSK9-R46L variant; HET (0/1), heterozygous carriers of the PCSK9-R46L variant; HOM (1/1), homozygous carriers of the PCSK9-R46L variant; LDL-C, low-density lipoprotein cholesterol; SD, standard deviation to the mean; UKBB, UK Biobank.
We further tested the interaction of PCSK9-R46L and sex using a two-way ANOVA. We found that the relationship between PCSK9-R46L and LDL-C concentrations was significantly associated with sex (P for interaction = 3.3e–3). This interaction was even more significant when adjusted for age (P for interaction = 7.2e–4).
Discussion
The present work aimed to determine the existence of a sex-dependent regulation of LDL-C metabolism by PCSK9 in humans. As a first approach, a meta-analysis of real-life studies showed a slight, but significantly smaller decrease in LDL-C, under PCSK9 mAbs in women than in men. As a next step, in order to eliminate potential environmental or psychosocial confounding factors, we compared the intensity of LDL-C decrease in relation to genetic inhibition of PCSK9 in both sexes. By using the PCSK9-R46L loss-of-function variant as a tool to study the ‘genetic’ inhibition of PCSK9 in the UKBB population cohort, we confirmed that the decrease in LDL-C, associated with PCSK9-R46L, is less marked in women than in men with a significant interaction with sex. Taken together, these data support the hypothesis of differential regulation of LDL-C metabolism by PCSK9 according to sex, in agreement with some pre-clinical studies.24,25
The FOURIER and ODYSSEY clinical trials demonstrated the powerful effect of PCSK9 mAbs (evolocumab and alirocumab, respectively) on LDL-C reduction in both sexes.10,26 However, in the former trial, a significantly greater LDL-C reduction was observed in men compared with women (−58% vs. −52%, respectively; P < 0.001) in the evolocumab-treated group. Similarly, in the pooled analysis of 10 ODYSSEY phase three clinical trials, women had lower LDL-C reduction compared with men (−48.3% and −60.0%, respectively) under alirocumab. Of note, in the ODYSSEY trials, fewer women reached the optimal LDL-C goal <50 mg/dL than men (36.5% vs. 58.7%, respectively; P < 0.0001).26 In addition, several real-life studies shed light on differences between men and women regarding the magnitude of LDL-C reduction in patients treated with PCSK9 inhibitors.11,19
Overall, our meta-analysis of real-life cohorts confirmed significant differences in LDL-C reduction between men and women under treatment with evolocumab or alirocumab. Furthermore, the results of two11,23 out of six real-life studies confirmed the data on the number of patients that achieved the on-treatment target. In particular, only 25% of women and 50% of men reached the treatment goal (<55 mg/dL as per 2019 ESC/EAS guidelines).27
It is known that women are less likely to be prescribed LLT and are more susceptible to discontinuing treatment; thus, they could have greater difficulty reaching LDL-C targets.28,29 The possible reasons for such non-adherence to LLT in women could lie in gender-specific factors, such as satisfaction, health beliefs, naïve illness theories, preferences for health care, and fear of side effects.29 Nevertheless, recent results from ODYSSEY APPRISE demonstrated a high patient adherence to both background statin therapy and a new LLT administration (i.e. alirocumab) in real-life settings.30 This may suggest that, in this particular study, physicians paid specific attention to sex-related adherence or that patients followed in clinical trials are more adherent than those in real-life cohorts.
Another potential explanation for the smaller decrease in LDL-C in response to PCSK9 mAbs in women could be related to differences in background LLT. Indeed, Cordero et al.11 suggested that LDL-C reduction was lower in women than in men who were not taking high-dose statins but were on ezetimibe treatment. Interestingly, the same authors also highlighted the potential involvement of body weight in the sex difference in LDL-C reduction; in particular, women with body mass index (BMI) > 25 kg/m2 showed significantly lower LDL-C reduction than men.
Based on the slightly lower efficacy of PCSK9 mAbs on LDL-C reduction, one could expect that women are at higher risk of cardiovascular outcomes than men. However, recent clinical trials, in which target LDL-C levels were only partially achieved for both sexes, revealed no sex-specific differences in cardiovascular risk reduction in secondary prevention patients.10,26,31 As for the real-life context, no studies are available, and therefore we cannot completely rule out any sex differences in PCSK9 inhibition treatments on cardiovascular outcomes.
In addition to a lower efficacy of PCSK9 mAbs in women, our complementary genetic study strongly suggests the existence of sex-specific biological mechanisms of PCSK9, which cannot be only attributed to treatment adherence. Moreover, pre-clinical studies in mice support the hypothesis of a sex-specific effect of PCSK9 on LDL-C metabolism. Indeed, the regulation of hepatic LDLR expression in response to genetic deficiency (using PCSK9 knockout mouse models) or pharmacological inhibition (with PCSK9 mAbs) of PCSK9 differs between male and female mice.24,25 As a possible molecular mechanism, a sex-specific shedding of excess hepatic LDLR in female mice following PCSK9 inhibition has recently been described.24 Even though further investigations are needed to get more insights into these putative mechanisms in humans, these findings will have a direct impact on the treatment of hypercholesterolaemia in clinical practice, with a particular focus on the achievement of the LDL-C target in women.
Limitations
The present study has limitations that should be acknowledged. First, the difference in LDL-C reduction between sexes at the individual study level is variable, ranging from 2 to 19 mg/dL, with a moderate heterogeneity among the real-life studies considered. However, the genetic analysis confirmed the effect size (i.e. LDL-C reduction). Thus, we are confident that women taking mAbs have a decreased LDL-C reduction compared with men. Second, it was not possible to consider the rate of non-adherent patients in real-life studies. Indeed, as discussed, women are known to be less adherent to therapy than men, which could have influenced our meta-analysis's results. Nevertheless, the genetic data confirm the sex difference with no bias in adherence since PCSK9-R46L is independent of personal behaviour. Third, both the meta-analysis and the genetic data showed a small but significant difference in LDL-C reduction between men and women. Still, recent findings unveil sex-specific shedding of excess hepatic LDLR in an animal model, indicating a potentially relevant biological mechanism.24 Fourth, some parameters, such as hypertension, BMI, diabetes, presence of CAD, or stroke, were collected in our study, but unfortunately, the number was not sufficient to perform a meta-regression analysis. Finally, we cannot exclude that other confounding factors, such as underlying LLT or other genetic variants, might have influenced our analysis.
Conclusions
High LDL-C is the main modifiable ASCVD risk factor, and PCSK9 inhibitors are the most potent drugs to achieve the LDL-C target. Based on complementary evidence, we showed here that PCSK9 inhibition is slightly less effective on LDL-C reduction in women than in men. These findings reinforce the need for dedicated studies to develop sex-specific recommendations for the management of ASCVD. In addition, the underlying molecular mechanism(s) sustaining this sex difference requires further studies.
Supplementary Material
Acknowledgements
The present research has been conducted using the UKBB resource under the application number 49823. We are most grateful to the Bioinformatics Core Facility of Nantes BiRD, member of Biogenouest, Institut Français de Bioinformatique (IFB) (ANR-11-INBS-0013) for the use of its resources and its technical support.
Contributor Information
Veronika A Myasoedova, Centro Cardiologico Monzino IRCCS, Via Carlo Parea 4, 20138, Milan, Italy.
Antoine Rimbert, Nantes Université, CHU Nantes, CNRS, INSERM, l'institut du thorax, F-44000 Nantes, France.
Marina Camera, Centro Cardiologico Monzino IRCCS, Via Carlo Parea 4, 20138, Milan, Italy; Department of Pharmaceutical Sciences, Università degli Studi di Milano, Milan, Italy.
Cedric Le May, Nantes Université, CHU Nantes, CNRS, INSERM, l'institut du thorax, F-44000 Nantes, France.
Romain Capoulade, Nantes Université, CHU Nantes, CNRS, INSERM, l'institut du thorax, F-44000 Nantes, France.
Bertrand Cariou, Nantes Université, CHU Nantes, CNRS, INSERM, l'institut du thorax, F-44000 Nantes, France.
Paolo Poggio, Centro Cardiologico Monzino IRCCS, Via Carlo Parea 4, 20138, Milan, Italy.
Funding
This work was supported by the INSTINCTIVE research programme funded by the Fondation pour la Recherche Médicale (FRM: EQU201903007846); the GENESIS project, funded by the Agence Nationale de la Recherche (ANR-21-CE14-0051); the Italian Ministry of Health funds (Ricerca Finalizzata: GR-2019-12370560 and GR-2018-12366423, and Ricerca Corrente); and the European Research Area Network on Cardiovascular Disease (ERA-CVD) Joint Transnational Call 2018 (PICASSO JTC2018-042). A.R. is supported by a post-doctoral fellowship grant from the ‘Fondation Recherche Médicale’. R.C. is supported by a ‘Connect Talent’ research chair from Région Pays de la Loire and Nantes Métropole. P.P. is supported by Fondazione Gigi e Pupa Ferrari ONLUS (FPF-14).
Role of the funder/sponsor
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
Concept and design: V.A.M., B.C., and P.P.
Acquisition, analysis, or interpretation of data: V.A.M., A.R., M.C., C.L.M., R.C., B.C., and P.P.
Drafting of the manuscript: V.A.M., A.R., M.C., and P.P.
Critical revision of the manuscript for important intellectual content: C.L.M., R.C., and B.C.
Statistical analysis: A.R. and P.P.
Obtained funding: V.A.M., A.R., R.C., B.C., and P.P.
Supervision: B.C. and P.P.
Conflict of interest
B.C. reports grants and/or personal fees from Akcea, Amgen, AstraZeneca, Bristol Myers Squibb, Gilead, Eli Lilly, Novartis, Novo Nordisk, Sanofi, and Regeneron, outside the submitted work. V.A.M., A.R., M.C., C.L., R.C., and P.P. have nothing to declare.
Data availability statement
The meta-analysis data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, Daemen MJ, Demer LL, Hegele RA, Nicholls SJ, Nordestgaard BG, Watts GF, Bruckert E, Fazio S, Ference BA, Graham I, Horton JD, Landmesser U, Laufs U, Masana L, Pasterkamp G, Raal FJ, Ray KK, Schunkert H, Taskinen M-R, Van De Sluis B, Wiklund O, Tokgozoglu L, Catapano AL, Ginsberg HN. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2020;41:2313–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stoekenbroek RM, Lambert G, Cariou B, Hovingh GK. Inhibiting PCSK9—biology beyond LDL control. Nat Rev Endocrinol 2018;15:52–62. [DOI] [PubMed] [Google Scholar]
- 3. Cui Q, Ju X, Yang T, Zhang M, Tang W, Chen Qi, Hu Y, Haas JV, Troutt JS, Pickard RT, Darling R, Konrad RJ, Zhou H, Cao G. Serum PCSK9 is associated with multiple metabolic factors in a large Han Chinese population. Atherosclerosis 2010;213:632–636. [DOI] [PubMed] [Google Scholar]
- 4. Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab 2009;94:2537–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Persson L, Gälman C, Angelin Bo, Rudling M. Importance of proprotein convertase subtilisin/kexin type 9 in the hormonal and dietary regulation of rat liver low-density lipoprotein receptors. Endocrinology 2009;150:1140–1146. [DOI] [PubMed] [Google Scholar]
- 6. Persson L, Henriksson P, Westerlund E, Hovatta O, Angelin Bo, Rudling M. Endogenous estrogens lower plasma PCSK9 and LDL cholesterol but not Lp(a) or bile acid synthesis in women. Arterioscler Thromb Vasc Biol 2012;32:810–814. [DOI] [PubMed] [Google Scholar]
- 7. Ferri N, Ruscica M, Coggi D, Bonomi A, Amato M, Frigerio B, Sansaro D, Ravani A, Veglia F, Capra N, Lupo MG, Macchi C, Castelnuovo S, Savonen K, Silveira A, Kurl S, Giral P, Pirro M, Strawbridge RJ, Gigante B, Smit AJ, Tremoli E, Colombo GI, Baldassarre D.. Sex-specific predictors of PCSK9 levels in a European population: the IMPROVE study. Atherosclerosis 2020;309:39–46. [DOI] [PubMed] [Google Scholar]
- 8. Cordero A, Rodríguez-Mañero M, Fácila L, Fernández-Olmo MR, Gómez-Martínez MJ, Valle A, Castellano JMª, Toro MM, Seijas-Amigo J, Vicedo A, González-Juanatey JR. Prevention of myocardial infarction and stroke with PCSK9 inhibitors treatment: a metanalysis of recent randomized clinical trials. J Diabetes Metab Disord 2020;19:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cupido AJ, Reeskamp LF, Hingorani AD, Finan C, Asselbergs FW, Hovingh GK, Schmidt AF. Joint genetic inhibition of PCSK9 and CETP and the association with coronary artery disease: a factorial mendelian randomization study. JAMA Cardiol 2022;7:955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sever P, Gouni-Berthold I, Keech A, Giugliano R, Pedersen TR, Im K, Wang H, Knusel B, Sabatine MS, O'donoghue ML.. LDL-cholesterol lowering with evolocumab, and outcomes according to age and sex in patients in the FOURIER Trial. Eur J Prev Cardiol 2021;28:805–812. [DOI] [PubMed] [Google Scholar]
- 11. Cordero A, Fernández Del Olmo MR, Cortez Quiroga GA, Romero-Menor C, Fácila L, Seijas-Amigo J, Fornovi A, Murillo JR, Rodríguez-Mañero M, Bello Mora MC, Valle A, Miriam S, Pamias RF, Bañeras J, García PB, Clemente Lorenzo MM, Sánchez-Alvarez S, López-Rodríguez L, González-Juanatey JR. Sex differences in low-density lipoprotein cholesterol reduction with PCSK9 inhibitors in real-world patients: the LIPID-REAL registry. J Cardiovasc Pharmacol 2022;79:523–529. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sterne JAC, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 2001;323:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, Mccarthy SA, Davies RM, Li H.. Twelve years of SAMtools and BCFtools. Gigascience 2021;10:giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wickham H. ggplot2 Data Analysis. Elegant Graphics for Data Analysis. 2nd ed.Berlin: Springer; 2016. 189–201. [Google Scholar]
- 19. Galema-Boers AMH, Steward K, Mulder JWCM, Roeters Van Lennep JE. Sex differences in efficacy and side effects of proprotein convertase subtilisin /kexin 9 (PCSK9) inhibitors in real world data. Atherosclerosis 2022;355:155. [Google Scholar]
- 20. D'erasmo L, Commodari D, Di Costanzo A, Minicocci I, Polito L, Ceci F, Montali A, Maranghi M, Arca M.. Evolving trend in the management of heterozygous familial hypercholesterolemia in Italy: a retrospective, single center, observational study. Nutr Metab Cardiovasc Dis 2020;30:2027–2035. [DOI] [PubMed] [Google Scholar]
- 21. Leitner DR, Toplak H, Kedenko L, Steinmaurer T, Gräff V, Metzner T, Schwaiger EM, Prager R.. Efficacy and tolerability of alirocumab in Austrian clinical practice—results of the non-interventional PEARL-AT study. Curr Med Res Opin 2020;36:1419–1425. [DOI] [PubMed] [Google Scholar]
- 22. Parhofer KG, Von Stritzky B, Pietschmann N, Dorn C, Paar WD. PEARL: a non-interventional study of real-world alirocumab use in German clinical practice. Drugs Real World Outcomes 2019;6:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vicente-Valor J, García-González X, Ibáñez-García S, Durán-García ME, De Lorenzo-Pinto A, Rodríguez-González C, Méndez-Fernández I, Percovich-Hualpa JC, Herranz-Alonso A, Sanjurjo-Sáez M. PCSK9 inhibitors revisited: effectiveness and safety of PCSK9 inhibitors in a real-life Spanish cohort. Biomed Pharmacother 2022;146:112519. [DOI] [PubMed] [Google Scholar]
- 24. Roubtsova A, Garçon D, Lacoste S, Chamberland A, Marcinkiewicz J, Métivier R, Sotin T, Paquette M, Bernard S, Cariou B, Le May C, Koschinsky ML, Seidah NG, Prat A. PCSK9 deficiency results in a specific shedding of excess LDLR in female mice only: role of hepatic cholesterol. Biochim Biophys Acta Mol Cell Biol Lipids 2022:159217. [DOI] [PubMed] [Google Scholar]
- 25. Roubtsova A, Chamberland A, Marcinkiewicz J, Essalmani R, Fazel A, Bergeron JJ, Seidah NG, Prat A. PCSK9 deficiency unmasks a sex- and tissue-specific subcellular distribution of the LDL and VLDL receptors in mice. J Lipid Res 2015;56:2133–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vallejo‐Vaz AJ, Ginsberg HN, Davidson MH, Eckel RH, Cannon CP, Lee LV, Bessac L, Pordy R, Letierce A, Ray KK.. Lower on-treatment low-density lipoprotein cholesterol and major adverse cardiovascular events in women and men: pooled analysis of 10 ODYSSEY phase 3 alirocumab trials. J Am Heart Assoc 2018;7:e009221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, Mueller C, Drexel H, Aboyans V, Corsini A, Doehner W, Farnier M, Gigante B, Kayikcioglu M, Krstacic G, Lambrinou E, Lewis BS, Masip J, Moulin P, Petersen S, Petronio AS, Piepoli MF, Pintó X, Räber L, Ray KK, Reiner Ž, Riesen WF, Roffi M, Schmid J-P, Shlyakhto E, Simpson IA, Stroes E, Sudano I, Tselepis AD, Viigimaa M, Vindis C, Vonbank A, Vrablik M, Vrsalovic M, Zamorano JL, Collet J-P, Koskinas KC, Casula M, Badimon L, John Chapman M, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, Windecker S, Aboyans V, Baigent C, Collet J-P, Dean V, Delgado V, Fitzsimons D, Gale CP, Grobbee D, Halvorsen S, Hindricks G, Iung B, Jüni P, Katus HA, Landmesser U, Leclercq C, Lettino M, Lewis BS, Merkely B, Mueller C, Petersen S, Petronio AS, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Touyz RM, Nibouche D, Zelveian PH, Siostrzonek P, Najafov R, Van De Borne P, Pojskic B, Postadzhiyan A, Kypris L, Špinar J, Larsen ML, Eldin HS, Viigimaa M, Strandberg TE, Ferrières J, Agladze R, Laufs U, Rallidis L, Bajnok L, Gudjónsson T, Maher V, Henkin Y, Gulizia MM, Mussagaliyeva A, Bajraktari G, Kerimkulova A, Latkovskis G, Hamoui O, Slapikas R, Visser L, Dingli P, Ivanov V, Boskovic A, Nazzi M, Visseren F, Mitevska I, Retterstøl K, Jankowski P, Fontes-Carvalho R, Gaita D, Ezhov M, Foscoli M, Giga V, Pella D, Fras Z, De Isla LP, Hagström E, Lehmann R, Abid L, Ozdogan O, Mitchenko O, Patel RS. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 28. Amrock SM, Duell PB, Knickelbine T, Martin SS, O'brien EC, Watson KE, Mitri J, Kindt I, Shrader P, Baum SJ, Hemphill LC, Ahmed CD, Andersen RL, Kullo IJ, Mccann D, Larry JA, Murray MF, Fishberg R, Guyton JR, Wilemon K, Roe MT, Rader DJ, Ballantyne CM, Underberg JA, Thompson P, Duffy D, Linton MF, Shapiro MD, Moriarty PM, Knowles JW, Ahmad ZS. Health disparities among adult patients with a phenotypic diagnosis of familial hypercholesterolemia in the CASCADE-FH patient registry. Atherosclerosis 2017;267:19–26. [DOI] [PubMed] [Google Scholar]
- 29. Olmastroni E, Boccalari MT, Tragni E, Rea F, Merlino L, Corrao G, Catapano AL, Casula M. Sex-differences in factors and outcomes associated with adherence to statin therapy in primary care: need for customisation strategies. Pharmacol Res 2020;155:104514. [DOI] [PubMed] [Google Scholar]
- 30. Banach M, López-Sendon JL, Averna M, Cariou B, Loy M, Manvelian G, Batsu I, Poulouin Y, Gaudet D. Treatment adherence and effect of concurrent statin intensity on the efficacy and safety of alirocumab in a real-life setting: results from ODYSSEY APPRISE. Arch Med Sci 2022;18:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Das A, Roy B, Bandyopadhyay D, Dasgupta S, Chakraborty S, Soudant C, Gulati M, Ray KK, Lavie CJ. Non-statin interventions in the prevention of cardiovascular events: sex-based meta-analysis. Prog Cardiovasc Dis 2020;63:228–232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The meta-analysis data underlying this article will be shared on reasonable request to the corresponding author.