Abstract
Concerns about the duration of protection conferred by coronavirus disease 2019 (COVID-19) vaccines have arisen in postlicensure evaluations. “Depletion of susceptibles,” a bias driven by differential accrual of infection among vaccinated and unvaccinated individuals, may obscure vaccine effectiveness (VE) estimates, hindering interpretation. We enrolled California residents who received molecular SARS-CoV-2 tests in a matched, test-negative design, case-control study to estimate VE of mRNA-based COVID-19 vaccines between February 23 and December 5, 2021. We analyzed waning protection following 2 vaccine doses using conditional logistic regression models. Additionally, we used data from a population-based serological study to adjust for “depletion-of-susceptibles” bias and estimated VE for 3 doses, by time since second dose receipt. Pooled VE of BNT162b2 and mRNA-1273 against symptomatic SARS-CoV-2 infection was 91.3% (95% confidence interval (CI): 83.8, 95.4) at 14 days after second-dose receipt and declined to 50.8% (95% CI: 19.7, 69.8) at 7 months. Adjusting for depletion-of-susceptibles bias, we estimated VE of 53.2% (95% CI: 23.6, 71.2) at 7 months after primary mRNA vaccination series. A booster dose of BN162b2 or mRNA-1273 increased VE to 95.0% (95% CI: 82.8, 98.6). These findings confirm that observed waning of protection is not attributable to epidemiologic bias and support ongoing efforts to administer additional vaccine doses to mitigate burden of COVID-19.
Keywords: bias, COVID-19, depletion of susceptibles, SARS-CoV-2, vaccination, vaccine effectiveness
Abbreviations
- aOR
adjusted odds ratio
- CDPH
California Department of Public Health
- CI
confidence interval
- COVID-19
coronavirus disease 2019
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- VE
vaccine effectiveness
Vaccination has been critical for mitigation of the ongoing coronavirus disease 2019 (COVID-19) pandemic (1). Vaccines currently available in the United States provide robust protection against severe COVID-19 outcomes including hospitalization and death (2–4). However, suboptimal vaccine uptake in various settings (5), continued public health guidance for isolation of infected and exposed individuals, and lower vaccine effectiveness (VE) among clinically vulnerable patient populations (6–8) highlight the need for robust immunity within the general population to suppress severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission (9). Postlicensure observational studies (10–12) have estimated declining VE over time following immunization for individuals who received 2 doses of mRNA-based vaccines. Whereas studies analyzing variant-specific protection have identified only modest differences in VE against Delta (B.1.617.2), Alpha (B.1.1.7), and earlier SARS-CoV-2 lineages among individuals who completed their primary series (13–16), the real-world durability of protection for SARS-CoV-2 infection remains of concern. These considerations have been amplified following the emergence of the Omicron (B.1.1.529) variant, which is associated with additional immune escape (17–19).
In epidemiologic studies, it is critical to distinguish waning of VE from time-varying confounders in the association between vaccination and disease. Under both observational and randomized studies, differential accrual of immunity through natural exposure to SARS-CoV-2 among vaccinated and unvaccinated persons may gradually erode vaccine-associated differences in disease incidence (20–23). Termed “depletion-of-susceptibles bias” (24), this phenomenon may merit consideration in studies of COVID-19 vaccines due to the substantial likelihood for cases to evade detection due to asymptomatic or mild clinical presentation (24), particularly in the context of high rates of transmission that have persisted following COVID-19 vaccine rollout in the United States. The potential contribution of such biases to reported waning of COVID-19 VE remains unclear (25), along with the role of other factors including patients’ age and clinical risk profile, vaccine product received (26), and spacing of vaccine doses (27).
We analyzed data from a test-negative design, case-control study to characterize differences in VE associated with time since receipt of mRNA-based COVID-19 vaccines, to assess the extent of waning that may be attributable to depletion-of-susceptibles bias, and to determine the effectiveness of administering a third mRNA vaccine doses under real-world conditions.
METHODS
Recruitment
As part of an ongoing study (28–30), we enrolled California residents with SARS-CoV-2 molecular diagnostic test results reported to the California Department of Public Health (CDPH) between February 24 and December 5, 2021. Analyses were limited to this time window to exclude cases with Omicron variant infection, which accounted for the majority of new-onset SARS-CoV-2 infections in California by late December 2021 and is associated with reduced VE (19, 31). Trained interviewers administered a telephone questionnaire in English and Spanish to individuals whose test result was reported to CDPH in the preceding 48 hours (Web Appendix 1, available at https://doi.org/10.1093/aje/kwad017). Cases and control participants were defined as individuals testing positive and negative for SARS-CoV-2, respectively. We sampled cases across 9 regions of California (Web Table 1; Web Figure 1). For each enrolled case, interviewers attempted to enroll 1 control, matched by age category, sex, and geographic region, from a random sample of individuals testing negative for SARS-CoV-2 whose results were reported to CDPH within the same week.
Individuals were eligible to participate if they had not previously received confirmation of SARS-CoV-2 infection, based on their recollection of any previous positive test result (e.g., molecular, antigen, or serological) or clinical diagnosis. This analysis excludes data from participants aged ≤12 years, who were ineligible to receive vaccination until late in the study period, as well as participants who reported receiving vaccines other than BNT162b2 (Pfizer/BioNTech, New York, New York) or mRNA-1273 (Moderna, Cambridge, Massachusetts), due to limited observations.
The study protocol was approved as public health surveillance by the State of California Health and Human Services Agency Committee for the Protection of Human Subjects (Project Number: 2021-034).
Exposures
The primary exposure of interest was each participant’s self-reported COVID-19 vaccination status. Participants who indicated receipt of a COVID-19 vaccine were asked to identify the vaccine manufacturer and dates of receipt for each dose. Participants were asked to reference their physical vaccination card or another recall aid (e.g., California Digital COVID-19 Vaccine Record (https://myvaccinerecord.cdph.ca.gov), calendar reminder, or e-mail confirmation of vaccine appointment, etc.) to confirm their vaccination history during the interview. Participants who completed their primary series of 2 doses of BNT162b2 or mRNA-1273 more than 14 days before SARS-CoV-2 testing were considered fully vaccinated. Other participants who reported receipt of BNT162b2 or mRNA-1273 at the time of testing, and who had not completed their primary series ≥14 days before testing, were considered partially vaccinated. Participants who reported receipt of no COVID-19 vaccine doses at the time of testing were considered unvaccinated. Individuals who reported receiving a third dose prior to their testing date were considered as a separate group.
All participants were further asked to indicate their reasons for seeking a SARS-CoV-2 test and to list all potential symptoms of COVID-19 they had experienced in the 14 days prior to testing; participants who did not indicate experiencing symptoms were prompted with a list of common nonsevere symptoms (fever, chills, myalgia, loss of appetite, cough, shortness of breath) to verify their asymptomatic status. We also asked whether participants sought health care in association with their SARS-CoV-2 diagnosis, including telehealth consultations and outpatient care (at physician offices, urgent care, or retail pharmacy locations), whether they presented to an emergency room, and whether they were hospitalized. Participants also self-reported whether they had any preexisting or immunocompromising conditions that placed them at higher risk for SARS-CoV-2 infection or COVID-19. We categorized chronic conditions reported by participants using broad classes previously reported by the US Centers for Disease Control and Prevention (32); within our sample, these categories included conditions associated with weakened immune systems, respiratory disorders, cardiovascular or metabolic disorders, and disorders of the liver and/or kidneys (Web Table 2).
Outcomes
The primary endpoint for our analyses was symptomatic SARS-CoV-2 infection, defined as a positive SARS-CoV-2 test result with ≥1 symptom reported up to 14 days before testing. This symptomatic infection endpoint was the preferred study endpoint because asymptomatic infections were underrepresented among cases in this sample, who self-referred for testing; prospective testing strategies would be needed to recruit cases with a representative distribution of infection severity including asymptomatic infections (33, 34). As secondary endpoints, we further considered any SARS-CoV-2 infection (regardless of symptoms), infection with fever and ≥1 respiratory symptom reported, and infection for which participants sought or received medical care or advice (beyond testing) in any care setting (virtual, outpatient, emergency department, or inpatient).
Time-varying protection after receipt of second dose
Our primary analysis estimated VE against symptomatic SARS-CoV-2 infection among fully vaccinated participants, in relation to time since receipt of the second dose. Power requirements for determining VE at differing effectiveness thresholds under our study design have been described previously (26). To assess waning of VE, we estimated the adjusted odds ratio (aORs) of prior vaccination among cases versus controls using conditional logistic regression, including an interaction between vaccination status and time from full vaccination (14 days after second dose receipt) to participants’ test date to allow for changes in protection over time. This approach allowed the relationship between infection risk and vaccination status to vary as a function of time since vaccination. We used the Bayesian information criterion to compare fit of alternative models formulated with linear, square-root, or logarithmic functions of time since second dose receipt, thus allowing for differing patterns of change in vaccine protection over time. We defined 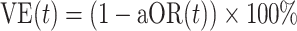 to describe the level of protection experienced t days after participants were considered fully vaccinated. We defined conditional logistic regression strata according to participants’ age group, sex, week of study enrollment, and geographic region to account for potential confounding in the relationship between vaccination status and infection outcomes.
to describe the level of protection experienced t days after participants were considered fully vaccinated. We defined conditional logistic regression strata according to participants’ age group, sex, week of study enrollment, and geographic region to account for potential confounding in the relationship between vaccination status and infection outcomes.
We repeated these analyses for alternative SARS-CoV-2 infection and symptomatic disease endpoints, redefining the control group as individuals that tested negative for SARS-CoV-2 and met the same thresholds for symptoms as case participants to mitigate potential confounding from association between test-seeking and willingness to be vaccinated (29, 30). We also repeated analyses restricting the sample to participants who reported referencing their vaccination records during telephone interviews to verify robustness of our primary analyses to exposure misclassification.
We undertook the same analyses in subgroups within which we hypothesized that initial VE or risk of waning protection could differ. These included groups defined by product received (BNT162b2 or mRNA-1273), participant age (<50 vs. ≥50 years old), and presence of self-reported preexisting conditions (individuals reporting immunocompetency without any chronic underlying conditions vs. those reporting chronic conditions or weakened immune status). We also undertook subgroup analyses for immunocompetent participants who reported only cardiovascular disease (including hypertension) or obesity as comorbid conditions, as these were the most prevalent conditions reported among all participants.
Second-dose protection associated with varying interdose intervals
Based on previous evidence that spacing between COVID-19 vaccine doses impacts immunogenicity (35), we also assessed whether 2-dose VE and subsequent waning varied in association with the time interval between receipt of the first and second dose (interdose interval). We hypothesized that the length of the interdose interval (between the first and second doses) could influence the initial strength of protection conferred by 2 doses or the persistence of protection over time after receipt of a second dose. We extended the conditional logistic regression frameworks described above to test these hypotheses, assessing improvements in fit of alternative model formulations using the Bayesian information criterion, described in Web Table 3.
Risk-of-bias analysis
We next sought to determine whether inferences of time-varying VE were robust to depletion-of-susceptibles bias resulting from the differential acquisition rates of natural (infection-derived) immunity among vaccinated and unvaccinated persons within the population (21). While we attempted to reduce such bias in our study design by excluding participants who reported a history of SARS-CoV-2 infection prior to their test, such exclusions are imperfect since a substantial proportion of infections go undiagnosed, especially if symptoms did not occur (36, 37). Following previous work (20, 22, 33), we considered that the aOR of prior vaccination t days before testing, comparing cases and controls, would measure the quantity:
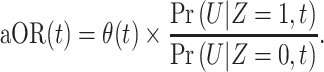 |
Here we defined 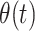 as the relative susceptibility of fully vaccinated individuals, compared with unvaccinated individuals, t days after vaccination, owing only to vaccine-derived protection, such that
as the relative susceptibility of fully vaccinated individuals, compared with unvaccinated individuals, t days after vaccination, owing only to vaccine-derived protection, such that 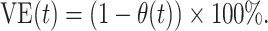 We defined
We defined 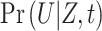 as the probability that individuals remained uninfected (U), given their vaccination status (considering
as the probability that individuals remained uninfected (U), given their vaccination status (considering 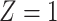 to indicate fully vaccinated status and
to indicate fully vaccinated status and 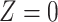 to indicate unvaccinated status, for individuals offered vaccination t days previously). Thus, aOR at t days after vaccination was considered to represent differences in infection outcomes under prevailing levels of naturally acquired immunity in the vaccinated and unvaccinated populations. For simplicity, this formulation did not account for recurrent infections; substantial protection was associated with naturally acquired immunity for both vaccinated and unvaccinated individuals prior to emergence of the Omicron variant (38), resulting in low risk of reinfection over the time span of several months.
to indicate unvaccinated status, for individuals offered vaccination t days previously). Thus, aOR at t days after vaccination was considered to represent differences in infection outcomes under prevailing levels of naturally acquired immunity in the vaccinated and unvaccinated populations. For simplicity, this formulation did not account for recurrent infections; substantial protection was associated with naturally acquired immunity for both vaccinated and unvaccinated individuals prior to emergence of the Omicron variant (38), resulting in low risk of reinfection over the time span of several months.
We used the product limit formula to estimate the proportion of fully vaccinated and unvaccinated Californians remaining uninfected by time t:
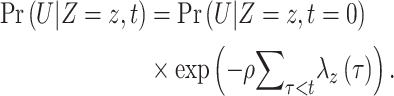 |
Here, 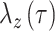 indicated daily incidence rates of SARS-CoV-2 infection among California residents with vaccination status
indicated daily incidence rates of SARS-CoV-2 infection among California residents with vaccination status 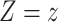 , computed as a 7-day moving average around each day
, computed as a 7-day moving average around each day 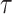 between February and December 2021 (39). To account for the underreporting of cases, the multiplier
between February and December 2021 (39). To account for the underreporting of cases, the multiplier 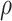 conveyed the ratio of total SARS-CoV-2 infections to reported cases over the period of interest. We used estimates of
conveyed the ratio of total SARS-CoV-2 infections to reported cases over the period of interest. We used estimates of 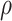 (mean = 2.6, 95% confidence interval: 2.3, 2.9) from a statewide serological study conducted between April and June 2021 (40), and we conducted sensitivity analyses allowing for differing values of
(mean = 2.6, 95% confidence interval: 2.3, 2.9) from a statewide serological study conducted between April and June 2021 (40), and we conducted sensitivity analyses allowing for differing values of 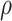 among vaccinated and unvaccinated persons, which may have arisen due to differences in the likelihood of symptoms or test-seeking between these groups (33). Last, the term
among vaccinated and unvaccinated persons, which may have arisen due to differences in the likelihood of symptoms or test-seeking between these groups (33). Last, the term  indicated the proportion of individuals who remained uninfected at the time of receiving vaccination, or (for those who remained unvaccinated) at the time vaccination became available to them.
indicated the proportion of individuals who remained uninfected at the time of receiving vaccination, or (for those who remained unvaccinated) at the time vaccination became available to them.
We assumed that prevalence of prior infection could differ among vaccine recipients and nonrecipients for various reasons, including the initial prioritization of COVID-19 vaccines for populations at high risk of exposure (e.g., essential workers) and, conversely, because willingness to receive vaccination could be correlated with other risk-mitigating behaviors. We conducted sensitivity analyses allowing for  equal to 100%, 90%, and 80%, for both the vaccinated and unvaccinated, based on estimates of statewide population seroprevalence as of November 2020 (41).
equal to 100%, 90%, and 80%, for both the vaccinated and unvaccinated, based on estimates of statewide population seroprevalence as of November 2020 (41).
To estimate bias-corrected VE, we solved for 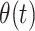 according to
according to
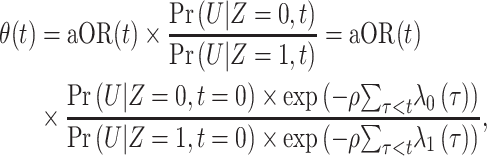 |
for 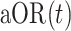 estimated via the conditional logistic regression framework described above in our primary analyses.
estimated via the conditional logistic regression framework described above in our primary analyses.
Thus, naive (bias-uncorrected) VE measured as 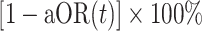 would be interpreted to represent differences in risk of symptomatic SARS-CoV-2 infection among unvaccinated individuals and those vaccinated t days previously, owing to levels of both vaccine-derived and naturally acquired protection within the vaccinated and unvaccinated populations. In contrast, bias-corrected VE, measured as
would be interpreted to represent differences in risk of symptomatic SARS-CoV-2 infection among unvaccinated individuals and those vaccinated t days previously, owing to levels of both vaccine-derived and naturally acquired protection within the vaccinated and unvaccinated populations. In contrast, bias-corrected VE, measured as 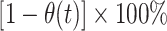 , represented the degree of vaccine-conferred protection against symptomatic infection t days after vaccination, adjusting for effects of differential prevalence of naturally acquired protection within the vaccinated and unvaccinated populations.
, represented the degree of vaccine-conferred protection against symptomatic infection t days after vaccination, adjusting for effects of differential prevalence of naturally acquired protection within the vaccinated and unvaccinated populations.
Protection after third dose receipt
Last, we sought to estimate VE for 3 doses of BNT162b2 or mRNA-1273 against symptomatic SARS-CoV-2 infection, ≥7 days after receipt of the third dose. We defined 3-dose VE relative to receipt of zero doses and 2 doses at varying time intervals after the second dose (defined categorically by months since individuals were considered fully vaccinated with 2 doses). Assuming 90% VE of ≥3 doses, we determined that analyses with 85 cases and 85 controls, where 10% of controls had received ≥3 doses, would provide 80% power to infer VE > 0% at 2-sided P < 0.05.
We estimated the aOR for prior receipt of 3 doses, relative to zero doses and 2 doses, using logistic regression models that included participants’ age group, sex, region, presence of immunocompromising or comorbid conditions, and the week of study enrollment as categorical covariates. We defined third-dose VE as 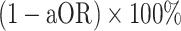 . As our study period encompassed only a narrow window of time after individuals were recommended to receive booster doses, analyses did not distinguish protection as a function of time since receipt of the third dose.
. As our study period encompassed only a narrow window of time after individuals were recommended to receive booster doses, analyses did not distinguish protection as a function of time since receipt of the third dose.
RESULTS
Enrollment and descriptive analyses
Between February 24 and December 5, 2021, we enrolled 2,238 participants, including 1,052 cases (testing positive for SARS-CoV-2) and 1,186 controls (testing negative for SARS-CoV-2; Table 1). In total, 862 participants, including 250 cases (24% of 1,052) and 612 controls (52% of 1,186), reported receiving any mRNA vaccine doses prior to SARS-CoV-2 testing.
Table 1.
Descriptive Attributes of Participants Included in the Analysis of Waning Vaccine Effectiveness Against Symptomatic Infection in California, February to November 2021
| All Participants (n = 2,238) | Cases (n = 1,052) | Controls (n = 1,186) | ||||
|---|---|---|---|---|---|---|
| Variable | No. | % | No. | % | No. | % |
| Age, years | ||||||
| 13–17 | 123 | 5.5 | 53 | 5.0 | 70 | 5.9 |
| 18–29 | 742 | 33.2 | 342 | 32.5 | 400 | 33.7 |
| 30–49 | 853 | 38.1 | 400 | 38.0 | 453 | 38.2 |
| 50–64 | 370 | 16.5 | 186 | 17.7 | 184 | 15.5 |
| ≥65 | 150 | 6.7 | 71 | 6.7 | 79 | 6.7 |
| Sexa | ||||||
| Male | 1,047 | 46.8 | 486 | 46.2 | 561 | 47.3 |
| Female | 1,190 | 53.2 | 566 | 53.8 | 624 | 52.6 |
| Household income, $ | ||||||
| <50,000 | 565 | 25.2 | 292 | 27.8 | 273 | 23.0 |
| 50,000–99,999 | 512 | 22.9 | 253 | 24.0 | 259 | 21.8 |
| 100,000–149,999 | 286 | 12.8 | 104 | 9.9 | 182 | 15.3 |
| ≥150,000 | 307 | 13.7 | 119 | 11.3 | 188 | 15.9 |
| Refuse | 334 | 14.9 | 172 | 16.3 | 162 | 13.7 |
| Not sure | 234 | 10.5 | 112 | 10.6 | 122 | 10.3 |
| Race/ethnicity | ||||||
| Non-Hispanic White | 969 | 43.3 | 448 | 42.6 | 521 | 43.9 |
| Non-Hispanic Black | 107 | 4.8 | 63 | 6.0 | 44 | 3.7 |
| Hispanic (any race) | 631 | 28.2 | 321 | 30.5 | 310 | 26.1 |
| Asian | 208 | 9.3 | 84 | 8.0 | 124 | 10.5 |
| Native American | 27 | 1.2 | 16 | 1.5 | 11 | 0.9 |
| Native Hawaiian | 15 | 0.7 | 6 | 0.6 | 9 | 0.8 |
| Middle Eastern | 11 | 0.5 | 7 | 0.7 | 4 | 0.3 |
| More than 1 race | 212 | 9.5 | 76 | 7.2 | 136 | 11.5 |
| Refuse | 58 | 2.6 | 31 | 2.9 | 27 | 2.3 |
| Region of residenceb | ||||||
| Predominantly urban regions | ||||||
| San Francisco Bay Area | 223 | 10.0 | 102 | 9.7 | 121 | 10.2 |
| Greater Los Angeles Area | 241 | 10.8 | 123 | 11.7 | 118 | 9.9 |
| Greater Sacramento Area | 250 | 11.2 | 110 | 10.5 | 140 | 11.8 |
| San Diego and southern border | 253 | 11.3 | 119 | 11.3 | 134 | 11.3 |
| Predominantly rural regions | ||||||
| Central Coast | 276 | 12.3 | 130 | 12.4 | 146 | 12.3 |
| Northern Sacramento Valley | 260 | 11.6 | 126 | 12.0 | 134 | 11.3 |
| San Joaquin Valley | 246 | 11.0 | 109 | 10.4 | 137 | 11.6 |
| Northwestern California | 250 | 11.2 | 116 | 11.0 | 134 | 11.3 |
| Sierras Region | 239 | 10.7 | 117 | 11.1 | 122 | 10.3 |
| Preexisting conditions | ||||||
| No preexisting conditions | 1,679 | 75.5 | 807 | 77.4 | 872 | 73.8 |
| Any preexisting conditionsc | 545 | 24.5 | 235 | 22.6 | 310 | 26.2 |
| Cardiovascular disease and/or obesity | 329 | 14.7 | 154 | 14.6 | 175 | 14.8 |
| Immunocompromising conditions | 92 | 4.1 | 25 | 2.4 | 67 | 5.6 |
| Vaccination | ||||||
| Vaccination statusd | ||||||
| Unvaccinated | 1,366 | 61.3 | 800 | 76.2 | 566 | 48.0 |
| Partially vaccinated | 222 | 10.0 | 65 | 6.2 | 157 | 13.3 |
| Fully vaccinated | 640 | 28.7 | 185 | 17.6 | 455 | 38.6 |
| Product received | ||||||
| BNT162b2 | 497 | 22.3 | 150 | 14.3 | 347 | 29.5 |
| mRNA-1273 | 365 | 16.4 | 100 | 9.5 | 265 | 22.5 |
| Dosing intervale | ||||||
| Below recommended interval | 192 | 22.0 | 74 | 29.4 | 118 | 19.0 |
| At recommended interval | 434 | 49.8 | 115 | 45.6 | 319 | 51.5 |
| Above recommended interval | 246 | 28.2 | 63 | 25.0 | 183 | 29.5 |
a One case-participant identified as nonbinary but was excluded from analysis due to small strata.
b Counties grouped into each region in Web Table 1.
c Specific conditions reported by participants enumerated in Web Table 2. Numbers may not sum to the total due to missing responses from some participants.
d Participants defined as fully vaccinated at the time of testing if >14 days had passed following receipt of a second dose of BNT162b2 (Pfizer/BioNTech, New York, New York) or mRNA-1273 (Moderna, Cambridge, Massachusetts). Participants who received JNJ-78436735 (Janssen, Beerse, Belgium) were excluded from this analysis. Participants who had received at least 1 dose of any coronavirus disease 2019 (COVID-19) vaccine but did not meet these criteria for fully vaccinated status were considered partially vaccinated. Participants who had not received any COVID-19 vaccine doses were considered unvaccinated. Total may sum to less than the total number of participants due to missing responses from some participants.
e Dosing interval calculated by days elapsed between doses. Participants who received the second dose 21 days (BNT162b2) or 28 days (mRNA-1273) after the first dose were classified “at recommended interval.”
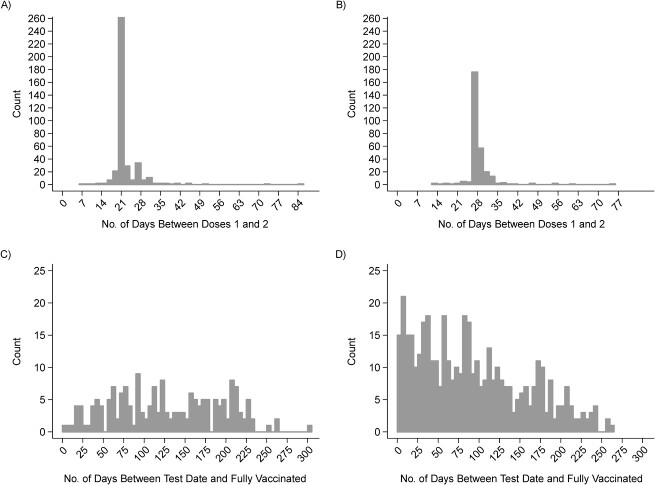
Among these 862 vaccinated participants, 497 (58%) and 365 (42%) reported receipt of BNT162b2 and mRNA-1273, respectively. In total, 640 participants were fully vaccinated at the time of their SARS-CoV-2 test. While participants received second doses as few as 14 days after their primary doses, the majority received their second dose at or after the recommended interval (21 days for BNT162b2 and 28 days for mRNA-1273; Figure 1A–B). Positive tests among vaccinated cases occurred between zero and 251 days after they were considered fully vaccinated (Figure 1C). In contrast, the majority of controls were enrolled within the first 100 days after being considered fully vaccinated (Figure 1D).
Figure 1.
Descriptive attributes of California residents seeking testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, enrolled between February to December 2021. Distribution of the days elapsed between vaccination and SARS-CoV-2 testing among individuals who received 2 doses of either BNT162b2 (Pfizer/BioNTech, New York, New York) or mRNA-1273 (Moderna, Cambridge, Massachusetts). Plots show the distribution of BNT162b2 (A) and mRNA-1273 (B), for which second doses are recommended ≥21 and ≥28 days after first dose, respectively, and distribution of the days elapsed between participants’ date of full vaccination for cases testing positive for SARS-CoV-2 (C) and controls testing negative (D). Participants were considered fully vaccinated if they were tested ≥14 days after their second dose of mRNA-1273 or BNT162b2.
Time-varying protection after receipt of second dose
We estimated the pooled VE for 2 doses of BNT162b2 or mRNA-1273 against symptomatic SARS-CoV-2 infection to be 91.3% (95% confidence interval (CI): 83.8, 95.4) at 14 days after second-dose receipt but declined thereafter (Figure 2). We estimated that VE for 2 doses of BNT162b2 or mRNA-1273 reached 50.8% (95% CI: 19.7, 69.8) 7 months after individuals were considered fully vaccinated (absolute difference in VE: 40.2%, 95% CI: 17.5, 73.7). Lower bounds of the 95% CI crossed zero at 8 months after participants were considered fully vaccinated with 2 doses (VE = 42.9%, 95% CI: −0.1, 67.1; Web Table 4). We obtained similar findings in analyses excluding individuals (n = 114) who reported receipt of BNT162b2 or mRNA-1273 but did not reference their vaccination record during the interview (Web Figure 1). Estimates were near identical in analyses restricting the control sample to participants who tested negative for SARS-CoV-2 and reported at least 1 symptom (Web Figure 2). Waning was likewise evident in analyses considering endpoints of any SARS-CoV-2 infection, infection with fever and ≥1 respiratory symptom, and infection for which participants received care in any clinical setting.
Figure 2.
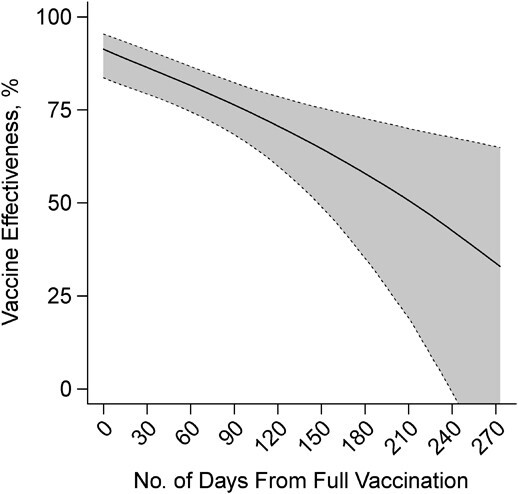
Two-dose vaccine effectiveness against symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection over time, California, February to December 2021. Estimates from a conditional logistic regression model matching cases and controls on age group, sex, region, and week of SARS-CoV-2 testing are shown for vaccine effectiveness of 2 doses of BNT162b2 (Pfizer/BioNTech, New York, New York) or mRNA-1273 (Moderna, Cambridge, Massachusetts) by days since participants were fully vaccinated. Shaded areas denote 95% confidence intervals around point estimates. Numerical estimates plotted in this figure are presented in Web Table 4. Model estimates a 40.2% (95% confidence interval: 17.5, 73.7) reduction in vaccine effectiveness over 7 months.
Waning effects did not vary by vaccine manufacturer (Figure 3A–B). Over a 7-month interval from the date participants were considered fully vaccinated, we estimated 40.5% (95% CI: 17.6, 73.2) and 40.4% (95% CI: 17.7, 74.2) absolute reductions in VE against symptomatic infection for recipients of BNT162b2 and mRNA-1273, respectively. Over the same interval, participants aged ≥50 years (n = 520) experienced a 42.6% (95% CI: 10.2, 103.3) absolute reduction in VE, compared with 34.9% (95% CI: 14.4, 64.0) absolute reduction in VE among participants aged 13–49 years (n = 1,718; Figure 3C–D). Among 545 participants who reported having any preexisting conditions, the reduction in estimated VE over a 7-month interval was 53.0% (95% CI: −4.1, 143.4; Figure 3F). However, the estimated reduction in VE was 33.1% (95% CI: 10.3, 69.1; Figure 3E) among individuals who did not report any preexisting conditions.
Figure 3.
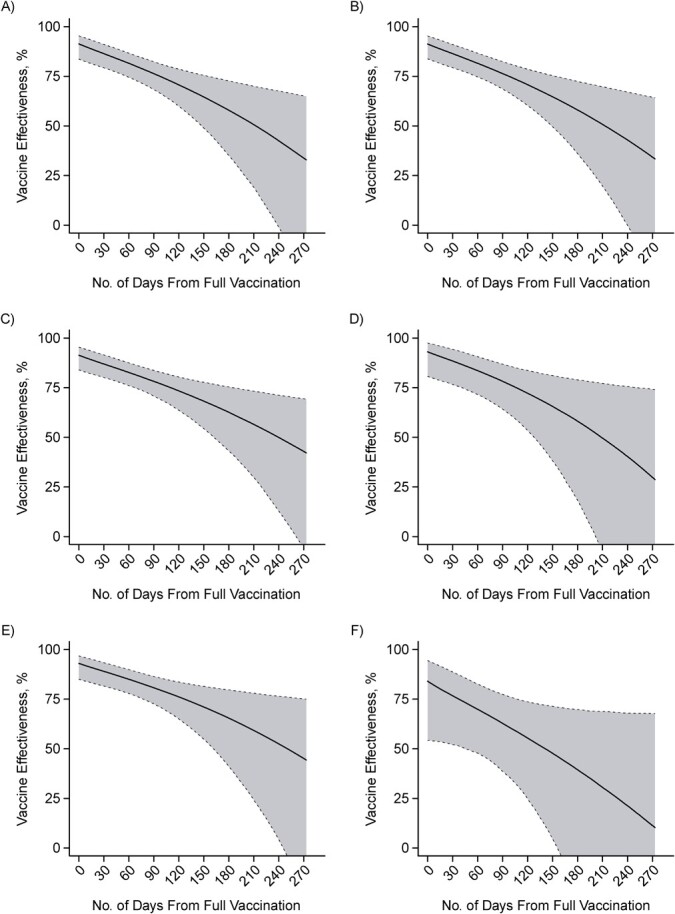
Two-dose vaccine effectiveness against symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection over time within distinct participant strata, California, February to December 2021. Vaccine effectiveness estimates are partitioned for 2 doses by time since participants were considered fully vaccinated, for BNT162b2 (Pfizer/BioNTech, New York, New York) recipients (A), mRNA-1273 (Moderna, Cambridge, Massachusetts) recipients (B), participants aged 13–49 years (C), participants aged ≥50 years (D), participants with no self-reported preexisting conditions (E), and participants who self-reported any preexisting conditions (F). Comorbid conditions within the participant sample are enumerated in Web Table 2. Estimates obtained via conditional logistic regression models matching cases and controls on age group, sex, region, and week of SARS-CoV-2 testing. Shaded areas denote 95% confidence intervals (CIs) around point estimates. Model estimates a 7-month reduction of 40.5% (95% CI: 17.6, 73.2) for BNT-162b2 (A); 40.4% (95% CI: 17.7, 74.2) for mRNA-1723 (B); 34.9% (95% CI: 14.4, 64.0) for participants under 50 years of age (C); 42.6% (95% CI: 10.2, 103.3) for participants over 50 years of age (D); 33.1% (95% CI: 10.3, 69.1) for those who are healthy with no preexisting conditions (E); and 53.0% (95% CI: −4.1, 143.4) for those with preexisting conditions (F).
Lengthening the dosing interval from 21 days to 51 days for either BNT162b2 or mRNA-1273 was associated with an absolute increase in VE of 16.3% (95% CI: −2.4, 28.7) at 14 days after receipt of the second dose (VE = 75.6% (95% CI: 66.0, 82.4) vs. VE = 92.1% (95% CI: 75.8, 97.4); Figure 4). We did not identify evidence for differences in the degree of vaccine effectiveness waning for participants who received second doses at longer time intervals after their first dose, although these analyses were limited by the low degree of variability in second-dose timing (Web Figure 3; Web Table 3).
Figure 4.
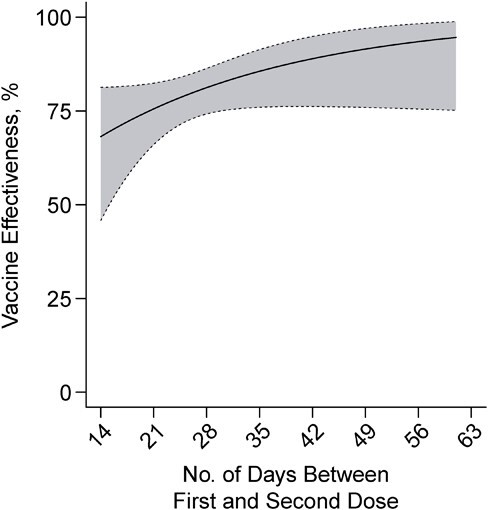
Two-dose vaccine effectiveness against symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection by interval between receipt of first and second doses, California, February to December 2021. Two-dose vaccine effectiveness estimates presented as of 14 days after second dose receipt, according to the length of the interval between receipt of first and second doses. Estimates were obtained via conditional logistic regression models matching cases and controls on age group, sex, region, and week of SARS-CoV-2 testing. Shaded areas denote 95% confidence intervals around point estimates. Longer-term vaccine effectiveness for 2 doses plotted according to the length of the interdose interval in Web Figure 3.
Risk-of-bias analysis
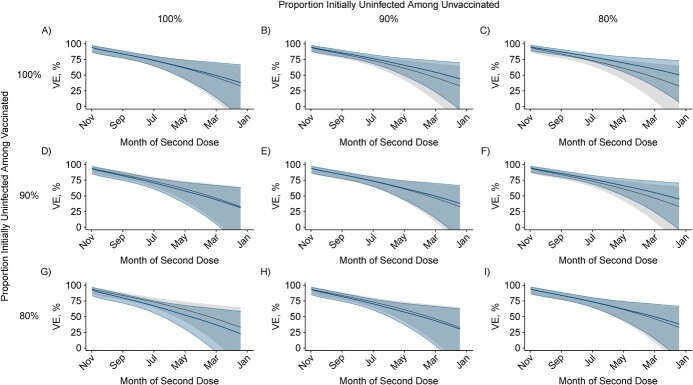
Throughout the study period, incidence rates of reported COVID-19 among unvaccinated persons exceeded those among the vaccinated (Web Figure 4). Incidence rates among the unvaccinated and vaccinated peaked at 90 cases per day per 100,000 and 15 cases per day per 100,000 respectively, in late July 2021. For a hypothetical cohort that became fully vaccinated as of May 2021, assuming 20% of the population had been infected by this time, bias-corrected VE as of December 2021 (7 months after participants were considered fully vaccinated) was 53.2% (95% CI: 23.6, 71.2), compared with 50.8% (95% CI: 19.7, 69.8) without bias correction (Figure 5I). The direction and magnitude of bias varied according to whether prevaccination prevalence of naturally acquired immunity was higher among individuals who opted to receive or not to receive vaccination (Web Table 5). For example, when assuming 0% initial prevalence of naturally acquired immunity among the vaccinated and 20% initial prevalence of naturally acquired immunity among the unvaccinated, bias-corrected VE after 7 months was 62.5% (95% CI: 38.9, 77.0). This scenario corresponds to bias driven by enhanced naturally acquired immunity among the unvaccinated, which masks differences in susceptibility attributable to vaccination. Under a reverse scenario of 20% initial prevalence of naturally acquired immunity among the vaccinated, and 0% initial prevalence of naturally acquired immunity among the unvaccinated, bias-corrected VE was 41.5% (95% CI: 4.5, 64.1). This scenario corresponds to bias driven by low susceptibility among the vaccinated as a result of naturally acquired immunity in conjunction with vaccine-derived protection.
Figure 5.
Two-dose vaccine effectiveness against symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection as of December 2021 by time since second dose receipt, correcting for depletion-of-susceptibles bias, in a hypothetical cohort. “Naive” (bias-uncorrected) estimates of 2-dose vaccine effectiveness from Figure 2 (gray) were overlaid with bias-corrected estimates (blue) accounting for unreported infections among vaccinated and unvaccinated persons, as listed in Web Table 5, for hypothetical cohorts of individuals offered vaccination 1–7 months prior to December 2021. Rows and columns correspond to differing assumptions about the proportion of individuals who remained uninfected at the time vaccination was offered to them, among the vaccinated and unvaccinated, respectively. Scenarios considered include: 100% uninfected among the vaccinated and the unvaccinated when offered vaccination (A), 90% uninfected among the unvaccinated and 100% uninfected among the vaccinated (B), 80% uninfected among the unvaccinated and 100% uninfected among the vaccinated (C), 100% uninfected among the unvaccinated and 90% uninfected among the vaccinated (D), 90% uninfected among the unvaccinated and the vaccinated (E), 80% uninfected among the unvaccinated and 90% uninfected among the vaccinated (F), 100% uninfected among the vaccinated and 80% uninfected among the unvaccinated (G), 90% uninfected among the vaccinated and 80% uninfected among the unvaccinated (H), and 80% uninfected among the vaccinated and the unvaccinated (I). Corresponding numerical estimates are presented in Web Table 5.
Protection after receipt of third dose
We enrolled 3 cases and 22 controls who reported receiving a third mRNA vaccine dose ≥7 days before their SARS-CoV-2 test (Table 1, Web Table 6); these participants received their third dose within 7–76 days before testing. Among all 3-dose recipients, 22 (88.0% of 25) received 3 doses of a single product, while 3 (12.0% of 25) received mixed series. The median time between second and third dose receipt was 213 days. Participants who had received 3 doses of BNT162b2 or mRNA-1273 experienced 95.0% (95% CI: 82.8, 98.6) protection against symptomatic SARS-CoV-2 infection relative to unvaccinated participants (Table 2). The relative effectiveness of 3 doses versus 2 doses 1 month after participants had completed their primary series was 71.6% (95% CI: −45.4, 94.3). The relative effectiveness of 3 doses versus 2 doses at 8 months after participants had completed their primary series was 87.9% (95% CI: 51.9, 97.0).
Table 2.
Effectivenessa of Third Doses of BNT162b2 or mRNA-1273b Against Symptomatic SARS-CoV-2 Infection in California, February to November 2021
| Cases | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposure | No. | % | No. | % | Absolute VE vs. 0 Doses | 95% CI | Relative VE of 3 Doses vs. Indicated Exposure | 95% CI |
| 0 doses | 942 | 83.6 | 842 | 64.9 | 1.0 | Referent | 95.0 | 82.8, 98.6 |
| 2 doses (fully vaccinated) | ||||||||
| Fully vaccinated 1 month prior | 12 | 6.5 | 88 | 19.3 | 89.2 | 80.7, 94.4 | 71.6 | −45.4, 94.3 |
| Fully vaccinated 2 months prior | 19 | 10.3 | 82 | 18.0 | 82.4 | 71.2, 89.8 | 81.1 | 11.7, 96.0 |
| Fully vaccinated 3 months prior | 27 | 14.6 | 74 | 16.2 | 75.4 | 61.2, 84.8 | 84.1 | 33.6, 96.4 |
| Fully vaccinated 4 months prior | 28 | 15.1 | 56 | 12.3 | 72.3 | 55.5, 83.2 | 85.9 | 42.8, 96.6 |
| Fully vaccinated 5 months prior | 22 | 11.9 | 39 | 8.6 | 71.3 | 50.0, 83.9 | 84.4 | 37.1, 96.3 |
| Fully vaccinated 6 months prior | 27 | 14.6 | 45 | 9.9 | 70.1 | 49.7, 82.5 | 85.0 | 40.5, 96.4 |
| Fully vaccinated 7 months prior | 24 | 13.0 | 28 | 6.1 | 65.0 | 36.0, 81.0 | 85.3 | 41.1, 96.4 |
| Fully vaccinated 8 months prior | 23 | 12.4 | 22 | 4.8 | 58.4 | 19.9, 78.3 | 87.9 | 51.9, 97.0 |
| 3 doses | ||||||||
| 3 doses ≥7 days before testing | 3 | 0.3 | 22 | 1.7 | 95.0 | 82.8, 98.6 | ||
Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
a Logistic regression model adjusting for age, sex, region, week of testing, and presence of immunocompromising or comorbid conditions used to assess the adjusted odds ratio of each vaccine exposure category (defined categorically by months since individuals were considered fully vaccinated), defining the reference exposure as receipt of 0 doses (for absolute VE) or as receipt of 2 doses at each indicated time interval (for recipients of 3 doses). Descriptive characteristics of 3 dose recipients are listed in Web Table 6.
b BNT162b2 (Pfizer/BioNTech, New York, New York) or mRNA-1273 (Moderna, Cambridge, Massachusetts).
DISCUSSION
Our study demonstrates waning protection against symptomatic SARS-CoV-2 infection with increasing time from receipt of 2 doses of BNT162b2 or mRNA-1273. Depletion-of-susceptibles bias resulting from acquisition of immunity through natural infection did not account for the observed waning of VE with increasing time from receipt of the second dose; estimates correcting for depletion-of-susceptibles bias differed minimally from primary regression-based VE estimates accounting for time-varying protection unless analyses assumed substantial baseline differences in prevalence of naturally acquired immunity among individuals who received or did not receive vaccination. These results substantiate previous reports of waning protection up to 5 months after individuals completed their primary vaccination series with BNT162b2 (10–12, 42–44), demonstrating the persistence of statistically significant protection against symptomatic infection through 7 months. Our study builds on other findings from the United States that third doses of BNT162b2 or mRNA-1273 restore waning VE to levels resembling observations 14 days after receipt of a second dose, thus conferring substantial protection against pre-Omicron variants (45). This study’s findings that waning of 2-dose VE is not driven by epidemiologic bias, and that VE is restored after a third dose, provide evidence in support of the decision by CDPH, and subsequently the Centers for Disease Control and Prevention, to recommend booster doses for all adults ≥6 months after primary series completion.
Point estimates from our study suggested earlier declines in immunity among older adults (aged ≥50 years) than among younger adults (aged <50 years). Although these results should be interpreted cautiously given our limited sample size in older age groups, they are consistent with knowledge that functional changes in innate and adaptive immunity associated with immunosenescence at older ages may reduce the effectiveness of vaccines among older adults (46). Prior studies with greater enrollment of older adults have reported similar findings (9, 10). Our analyses also suggested that, compared with immunocompetent individuals without comorbidities, individuals with weakened immune function or comorbid conditions experienced greater reductions in VE over time after receipt of their second doses (47). Although our analyses address third doses only, our findings are in conceptual agreement with current recommendations prioritizing older and immunocompromised individuals for additional booster doses (48).
Extended spacing between the first and second dose of mRNA vaccines was employed in the United Kingdom, Canada, and other countries as a dose-sparing strategy to maximize coverage of first doses. Our findings suggest that longer (>21 or >28 days) intervals between the first and second doses of BNT162b2 or mRNA-1273 may be associated with enhanced clinical protection. In the United Kingdom, individuals receiving second doses 6–12 weeks after their first dose experienced enhanced immunogenicity as well as increased VE against SARS-CoV-2 infection for BNT162b2, and enhanced immunogenicity for ChAdOx1 (Oxford/AstraZeneca, Cambridge, United Kingdom) (49). Consideration of extended spacing of doses may thus be appropriate in settings where supply and/or access remains limited, although benefits should be weighed against additional risk of infection during longer intervals between doses.
Our analysis has limitations. The study is observational in nature, and while we attempted to mitigate confounding through matching on test-seeking (by design) as well as age, sex, region, and time, unmeasured confounding in the association between COVID-19 vaccination and individuals’ likelihood of a positive test result may persist. Because we enrolled participants and administered questionnaires via telephone interviews, our study generally did not enroll cases experiencing severe disease. The symptomatic SARS-CoV-2 infection endpoint we monitored should thus be considered to encompass mild or moderate infections, for which most individuals had not been hospitalized by the time of their interviews. Collection of isolates for sequencing was not feasible under our retrospective study design; thus, it was not possible to identify differences in VE across SARS-CoV-2 variants. Data were collected in the study prior to, during, and after the surge of the Delta variant in California, and before emergence of Omicron as a prominent circulating lineage. However, previous analyses distinguishing protection against Delta, Mu, Alpha, and other SARS-CoV-2 variants have suggested that reductions in VE over time are likely due to waning protection over time rather than variant-specific differences in protection (10). While we provide point estimates for waning protection within differing subgroups and against differing endpoints, analyses are underpowered for determining whether durations of protection differ significantly across subgroups. Point estimates from our study suggesting longer durations of protection among younger individuals and those without medically significant comorbid conditions (Figure 3) resemble findings from large-scale studies linking participants’ SARS-CoV-2 testing results to comprehensive medical record data sets (9, 40). However, our finding of faster waning of protection against SARS-CoV-2 infection with fever and ≥1 respiratory symptom (Web Figure 2) is counterintuitive. This finding may be an artifact of low statistical power or endpoint misclassification due to imperfect reporting of symptoms, or may be explained by the occurrence of more severe breakthrough infections among individuals with poorer health status, in the event of insufficient statistical adjustment. Finally, while misclassification of self-reported vaccination status is possible, our VE estimates were robust in analyses excluding participants who did not reference a vaccination card during the interview. Moreover, individuals’ familiarity with referencing their vaccination records to enter restaurants, bars, workplaces, and other public spaces during the COVID-19 pandemic likely reduces the risk for inaccurate reporting of COVID-19 vaccination status relative to other vaccines.
Given the limited duration of follow-up in early COVID-19 vaccine trials, our results demonstrate the value of observational studies to monitor longer-term VE under real-world circumstances. Our findings indicate that waning of VE for BNT162b2 and mRNA-1273 is unlikely to be an artifact of epidemiologic bias, despite such concerns often being raised in studies stratifying VE by age or time since vaccination (17, 18). These findings are in agreement with prior theoretical studies demonstrating that risk of bias in VE estimates due to differential depletion of susceptibles is low if vaccination confers substantial protection (25); during our study period, point estimates of initial 2-dose protection exceeded 90%. As bias is expected to be greater when vaccines offer lower degrees of protection, our findings may not hold for the Omicron variant, which is associated with greater degrees of vaccine escape than prior variants (31). Similar analyses integrating cumulative infection data from serological studies should be prioritized to monitor the contributions of naturally acquired and vaccine-derived immunity to protection against SARS-CoV-2 as prevalence of naturally acquired immunity increases within the population (50).
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: California Department of Public Health, Richmond, California, United States (Kristin L. Andrejko, Jake M. Pry, Jennifer F. Myers, Megha Mehrotra, Katherine Lamba, Esther Lim, Nozomi Fukui, Jennifer L. DeGuzman, John Openshaw, James Watt, Seema Jain); Division of Epidemiology and Biostatistics, School of Public Health, University of California, Berkeley, Berkeley, California, United States (Kristin L. Andrejko, Joseph A. Lewnard); Division of Infectious Diseases and Vaccinology, School of Public Health, University of California, Berkeley, Berkeley, California, United States (Joseph A. Lewnard); and Center for Computational Biology, College of Engineering, University of California, Berkeley, Berkeley, California, United States (Joseph A. Lewnard).
K.L.A. and J.M.P contributed equally to this work as first authors.
This work was supported by the California Department of Public Health. J.M.P., J.O., and J.F.M. were supported by the Epidemiology & Laboratory Capacity for Infectious Diseases Program (grant 5-NU50CK000539) of the US Centers for Disease Control and Prevention (program 0187.0150). J.A.L. was supported by the National Institute of Allergy and Infectious Diseases (grant R01-AI14812701).
Please contact authors regarding requests to review data.
We thank all of the study participants who made this work possible.
This work was presented at the annual meeting of the Society for Epidemiologic Research, June 14–17, 2022, Chicago, Illinois.
A preprint of this article has been published online. Andrejko KL, Pry J, Myers JF, et al. Waning of two-dose BNT162b2 and mRNA-1273 vaccine effectiveness against symptomatic SARS-CoV-2 infection is robust to depletion-of-susceptibles bias. medRxiv. 2022. https://www.medrxiv.org/content/10.1101/2022.06.03.22275958v1.
The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views or opinions of the California Department of Public Health or the California Health and Human Services Agency.
Conflict of interest: J.A.L. discloses receipt of grants and honoraria from Pfizer, Inc., unrelated to this work. The other authors report no conflicts.
REFERENCES
- 1. Sandmann FG, Jit M. Rapid COVID-19 vaccine rollout: immense success but challenges ahead. Lancet Infect Dis. 2022;22(3):302–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . COVIDVaxView. 2021; https://www.cdc.gov/vaccines/imz-managers/coverage/covidvaxview/index.html. Accessed November 30, 2021.
- 6. Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021;27(11):1652–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines for preventing coronavirus disease 2019 hospitalizations in the United States. Clin Infect Dis. 2022;74(9):1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention . Quarantine & isolation. 2022. https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html. Accessed June 3, 2022.
- 9. Saad-Roy CM, Wagner CE, Baker RE, et al. Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years. Science. 2020;370(6518):811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385(24): e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seppälä E, Veneti L, Starrfelt J, et al. Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Eurosurveillance. 2021;26(35):2100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nasreen S, Chung H, He S, et al. Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. Nat Microbiol. 2022;7(3):379–385. [DOI] [PubMed] [Google Scholar]
- 16. Bruxvoort KJ, Sy LS, Qian L, et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ. 2021;375:e068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scott L, Hsiao N, Moyo S, et al. Track Omicron’s spread with molecular data. Science. 2021;374(6574):1454–1455. [DOI] [PubMed] [Google Scholar]
- 18. Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. Science. 2022;376(6593):science.abn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewnard J, Hong V, Patel M, et al. Clinical outcomes associated with Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in southern California. Nat Med. 2022;28(9):1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith P, Rodrigues L, Fine P. Assessment of the protective efficacy of vaccines against common diseases using case-control and cohort studies. Int J Epidemiol. 1984;13(1):87–93. [DOI] [PubMed] [Google Scholar]
- 21. Lipsitch M. Challenges of vaccine effectiveness and waning studies. Clin Infect Dis. 2019;68(10):1631–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lewnard JA, Tedijanto C, Cowling BJ, et al. Measurement of vaccine direct effects under the test-negative design. Am J Epidemiol. 2018;187(12):2686–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. León TM. COVID-19 cases and hospitalizations by COVID-19 vaccination status and previous COVID-19 diagnosis—California and New York, May–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71(4):125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yola M, Lucien A. Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. J Clin Epidemiol. 1994;47(7):731–737. [DOI] [PubMed] [Google Scholar]
- 25. Kahn R, Schrag SJ, Verani JR, et al. Identifying and alleviating bias due to differential depletion of susceptible people in Postmarketing evaluations of COVID-19 vaccines. Am J Epidemiol. 2022;191(5):800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Self WH. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions—United States, March–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(38):1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iacobucci G, Mahase E. Covid-19 vaccination: what’s the evidence for extending the dosing interval? BMJ. 2021;372:n18. [DOI] [PubMed] [Google Scholar]
- 28. Andrejko KL, Pry J, Myers JF, et al. Prevention of coronavirus disease 2019 (COVID-19) by mRNA-based vaccines within the general population of California. Clin Infect Dis. 2022;74(8):1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Andrejko KL, Pry J, Myers JF, et al. Predictors of severe acute respiratory syndrome coronavirus 2 infection following high-risk exposure. Clin Infect Dis. 2022;75(1):e276–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andrejko KL, Pry JM, Myers JF, et al. Effectiveness of face mask or respirator use in indoor public settings for prevention of SARS-CoV-2 infection—California, February–December 2021. MMWR Morb Mortal Wkly Rep. 2022;71(6):212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tartof SY, Slezak JM, Puzniak L, et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: a test-negative case–control study. Lancet Respir Med. 2022;10(7):689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention . People with certain medical conditions. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed November 9, 2021.
- 33. Lewnard JA, Patel MM, Jewell NP, et al. Theoretical framework for retrospective studies of the effectiveness of SARS-CoV-2 vaccines. Epidemiology. 2021;32(4):508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dean NE, Hogan JW, Schnitzer ME. Covid-19 vaccine effectiveness and the test-negative design. N Engl J Med. 2021;385(15):1431–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Payne RP, Longet S, Austin JA, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184(23):5699–5714.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu SL, Mertens AN, Crider YS, et al. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat Commun. 2020;11(1):4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oran DP, Topol EJ. The proportion of SARS-CoV-2 infections that are asymptomatic. Ann Intern Med. 2021;174(5):655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shenai MB, Rahme R, Noorchashm H. Equivalency of protection from natural immunity in COVID-19 recovered versus fully vaccinated persons: a systematic review and pooled analysis. Cureus. 2021;13(10):e19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. California Health and Human Services Open Data Portal . COVID-19 post-vaccination infection data. https://data.chhs.ca.gov/dataset/covid-19-post-vaccination-infection-data. Accessed October 28, 2021.
- 40. Mehrotra ML, Lim E, Lamba K, et al. CalScope: monitoring SARS-CoV-2 seroprevalence from vaccination and prior infection in adults and children in California May 2021–July 2021. Open Forum Infect Dis. 2022;9(7):ofac246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lamba K, Bradley H, Shioda K, et al. SARS-CoV-2 cumulative incidence and period seroprevalence: results from a statewide population-based serosurvey in California. Open forum. Infect Dis. 2021;8(8):ofab379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Israel A, Merzon E, Schäffer AA, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test negative design study. BMJ. 2021;375:e067873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2 breakthrough infections to time-from-vaccine; preliminary study [preprint]. medRxiv; 2021. 10.1101/2021.07.29.21261317. Accessed October 19, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Andrews N, Tessier E, Stowe J, et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med. 2022;386(4):340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ferdinands JM. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cox LS, Bellantuono I, Lord JM, et al. Tackling immunosenescence to improve COVID-19 outcomes and vaccine response in older adults. Lancet Healthy Longev. 2020;1(2):e55–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anand S, Montez-Rath ME, Han J, et al. SARS-CoV-2 vaccine antibody response and breakthrough infection in patients receiving dialysis. Ann Intern Med. 2021;175(3):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Centers for Disease Control and Prevention . CDC strengthens recommendations and expands eligibility for COVID-19 booster shots. 2022. https://www.cdc.gov/media/releases/2022/s0519-covid-booster-acip.html. Accessed June 3, 2022.
- 49. Amirthalingam G, Bernal JL, Andrews NJ, et al. Serological responses and vaccine effectiveness for extended COVID-19 vaccine schedules in England. Nat Commun. 2021;12(1):7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Clarke KEN. Seroprevalence of infection-induced SARS-CoV-2 antibodies—United States, September 2021–February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(17):606–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.