Abstract
Purpose:
We sought to describe coping strategies reported by young breast cancer survivors and evaluate the relationship between utilization of specific coping strategies and anxiety in survivorship.
Methods:
Participants enrolled in The Young Women’s Breast Cancer Study, a multi-center, cohort of women diagnosed with breast cancer at age ≤40 years, completed surveys that assessed demographics, coping strategies (reported at 6 months post-enrollment and 18 months post-diagnosis), and anxiety (2 years post-diagnosis). We used univariable and multivariable logistic regression to examine the relationship between coping strategies and anxiety.
Results:
833 women with stage 0–3 breast cancer were included in the analysis; median age at diagnosis was 37 (range: 17–40) years. Social supports were the most commonly reported coping strategies, with the majority reporting moderate or greater use of emotional support from a partner (90%), parents (78%), other family (79%), as well as reliance on friends (88%) at both 6 and 18 months. In multivariable analyses, those with moderate or greater reliance on emotional support from other family (odds ratio (OR): 0.37, 95% confidence ratio (CI): 0.22–0.63) at 18 months were less likely to have anxiety at 2 years, while those with moderate or greater reliance on alcohol/drug use (OR: 1.83, 95%CI: 1.12–3.00) and taking care of others (OR: 1.90, 95%CI: 1.04–3.45) to cope were more likely to have anxiety.
Conclusion:
Young breast cancer survivors rely heavily on support from family and friends. Our findings underscore the importance of considering patients’ social networks when developing interventions targeting coping in survivorship.
Clinical Trial Registration Number:
NCT01468246 (first posted November 9, 2011)
Keywords: coping, young breast cancer survivors, social support, anxiety
BACKGROUND
In the United States, breast cancer is the most common cancer among young adults, with greater than 12,000 new diagnoses each year among women younger than 40 [1,2]. Young adult breast cancer survivors may face specific issues related to their age and life stage. These include interruptions to educational or career trajectories and experiencing financial hardship [3]. Commonly used therapies may result in premature ovarian failure, sexual dysfunction and infertility, all of which can impact quality of life [1,4]. Additionally, development and progression of their peer and romantic relationships might be affected. They may be pregnant or parenting young children, which is difficult to navigate with a cancer diagnosis [5]. Further, young women are prone to feeling isolated from other breast cancer patients due to their relative young age, which can exacerbate their psychosocial difficulties [6].
Consequently, young women are at increased risk of emotional and psychological sequelae both during and after treatment compared to other age groups, with one study suggesting adolescent and young adult (AYA) cancer survivors are nearly twice as likely (11.5% vs. 5.8%) to have psychological distress than adults without cancer history [7]. Anxiety is a common psychiatric sequelae in young breast cancer patients [8]. In addition to negatively impacting quality of life and resumption of one’s normal activities, anxiety can be a barrier to engagement in survivorship care [9].
Coping has been described as emotional, behavioral, and cognitive reactions to manage event-related distress [10]. While multiple studies have examined coping strategies used by women with breast cancer, including a recent study that shown an inverse association between social support and anxiety [11], most have had limited sample sizes [10,12,13] and have included largely post-menopausal populations. With few studies exploring coping strategies employed by young breast cancer survivors, we sought to describe coping strategies reported by young women diagnosed with breast cancer at age 40 and younger in the first two years following diagnosis and to evaluate the relationship between coping strategies and anxiety.
METHODS
Participants
Helping Ourselves, Helping Others: The Young Women’s Breast Cancer Study (YWS) is a longitudinal cohort study of women 40 years old or younger when diagnosed with breast cancer (ClinicalTrials.gov Identifier: NCT01468246). It was established to examine biological, medical, and quality of life issues specific to young women with breast cancer. From 2006 to 2016, women with newly diagnosed breast cancer were systematically identified at several community and academic hospital sites in Massachusetts as well as academic sites in Colorado, Minnesota, and Toronto, Canada. After informed consent and enrollment, women were sent surveys every 6 months for the first 3 years after diagnosis. Participants completed the baseline survey a median of 5 months after their breast cancer diagnosis. This study was approved by Institutional Review Boards at the Dana-Farber/Harvard Cancer Center and other study sites.
In total, 1,302 women enrolled in the cohort. For the current analysis, we excluded women deemed ineligible post-enrollment (n=4) and one woman who withdrew consent. We also excluded women who completed modified versions of the surveys (n=91), including all women enrolled at the Toronto site, women who did not complete the 6- and 18-month surveys, which included measures of coping (n=328), and women with metastatic disease at diagnosis (n=45). The final analytic cohort included 833 YWS participants (Figure 1).
Figure 1.
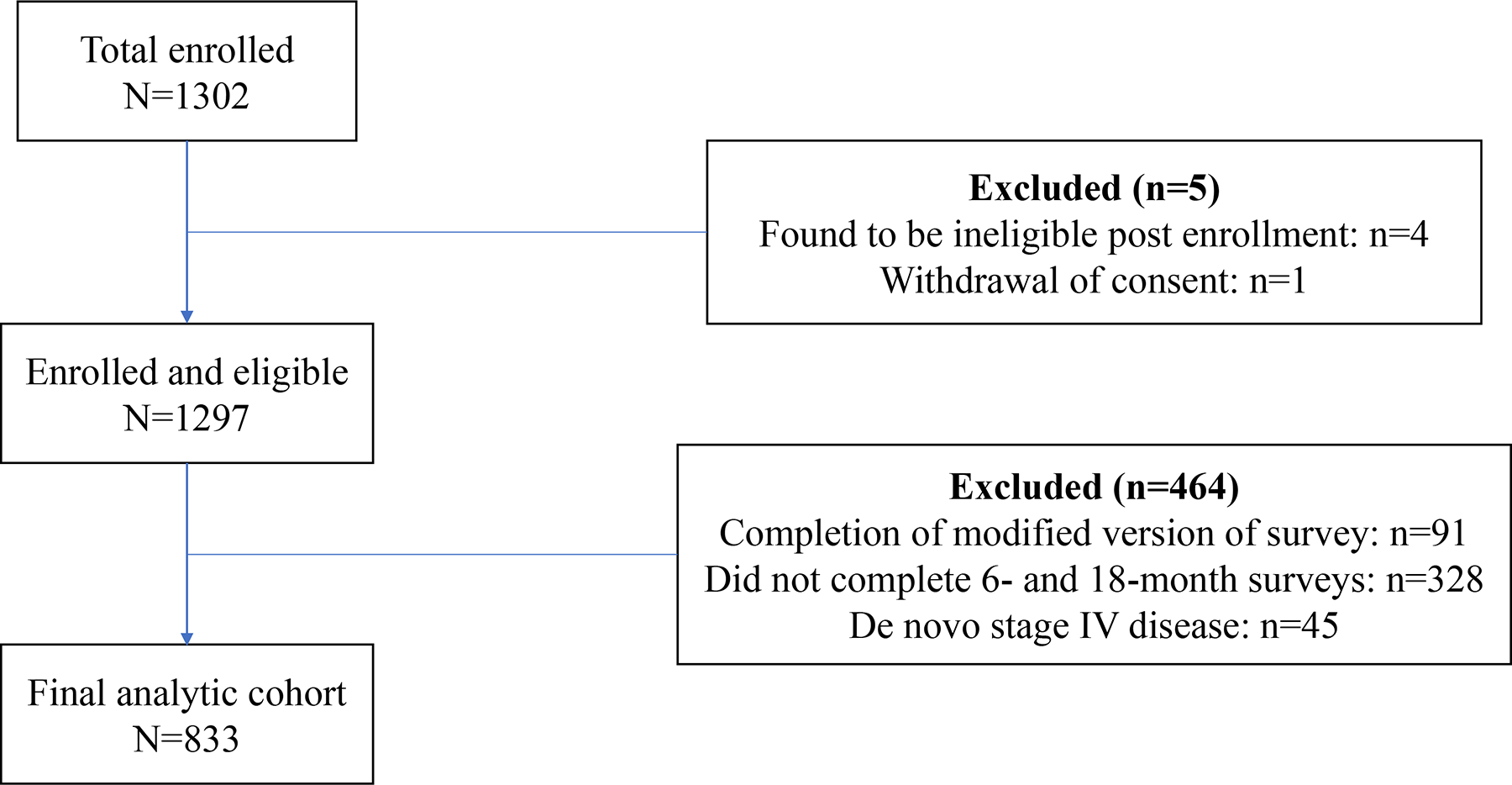
Study flow diagram
Sociodemographic, disease, and treatment characteristics
Race, ethnicity, college education, marital status, pre-diagnosis parity (dichotomized as any children vs. no children prior to diagnosis), a single item of financial stress (enough money for special things after paying bills vs. enough money to pay bills, but little spare money to buy extra or special things vs. enough money to pay bills but have to cut back on things/difficulty paying bills no matter what) [14,15], and employment status were self-reported by participants on the baseline survey. Race and ethnicity as recorded in the medical record were used, if not available via self-report. Medical record review was used to assess stage and receptor status. Treatment was ascertained by medical record review in combination with self-report on the baseline and 6-months surveys.
Anxiety
Anxiety was assessed yearly using the Hospital Anxiety and Depression Scale anxiety 7-item subscale (HADS-A), a reliable and valid scale that has been used in other studies of young women with breast cancer [16]. For the current analysis, HADS-A data 24-month survey was used. HADS-A scores range from 0 to 21, with higher scores indicating more symptoms. Scores are typically grouped into low (0–7), mild (8–10), and moderate to high (11–21), with a moderate to high score representative of clinically meaningful anxiety and suggestive of a need for intervention[17]. Cronbach’s alpha in the current study for the HADS-A was 0.87.
Coping
Coping was assessed at 18 and 24 months using 18 investigator-developed items (see Supplemental Table 1 in Online Resource 1) designed to assess factors that are unique to the experience of being a young cancer survivor. Items included ‘emotional support from partner/spouse/significant other’, ‘emotional support from parent(s)’, emotional support from other family members (e.g., children, siblings)’, ‘friends’, ‘co-workers’, ‘health care providers’, ‘work’, ‘taking care of children, family, friends’, ‘shopping’, ‘hobbies (e.g., reading, photography)’, ‘religious beliefs or activities’, spiritual practices (e.g. meditation)’, ‘exercise’, ‘dietary changes’, ‘vitamin or herbal supplements (e.g., gingko, vitamin C, St. John’s wort)’, ‘complementary therapies (e.g., acupuncture, massage)’, ‘drinking alcohol’, ‘using recreational drugs (e.g., marijuana, cocaine)’, and ‘other (please specify)’. Participants were asked, ‘To what extent have the following factors helped you cope with your breast cancer diagnosis and treatment?’ Participants responded on a 0 to 4 scale: 0 = ‘not at all’, 1 = ‘to a small extent’, 2 = ‘to a moderate amount’, 3 = ‘to a large extent’, and 4 = ‘it is the most important thing that keeps me going’, or ‘does not apply’. Responses to each coping item were dichotomized into ‘moderate or greater’ (response of 2, 3, or 4) vs. ‘small extent or less’ (response of 0 or 1).
Statistical Analysis
Frequencies and medians were reported for categorical and continuous variables, respectively. Responses of “does not apply” were tabulated for descriptive purposes but excluded from the dichotomized coping variable since this indicated a non-endorsement of any of the response options. McNemar tests were used to evaluate changes in the frequencies of coping strategies reported between 6 and 18 months.
Univariable and multivariable logistic regression models were fit to examine the relationship between specific coping strategies reported at 18 months and HADS-A at 24 months (HADS-A subscale score ≥ 11 vs. ≤10). The models included coping strategies reported at 18 months due to the temporal proximity to the HADS-A data collected at 24 months. Due to their known or hypothesized associations with psychosocial health, particularly anxiety [18–21], the following coping strategies were included in the model: support from family, support from healthcare providers, taking care of others, alcohol/drug use, exercise, and vitamin use. Coping strategies that were significant at the p ≤ 0.20 level in the univariable model were retained in the multivariable model, which also adjusted for age, stage, receipt of chemotherapy, and financial stress.
All analyses were conducted in SAS Studio Version 3.71 and SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Study population characteristics are summarized in Table 1. Median age at diagnosis was approximately 37 (range: 17–40) years old. Median age at the time of the baseline survey was 37 (range: 18–42). Most women were White (89.2%), 5% were Asian, and 2.9% Black, while 4.6% identified as Hispanic. Approximately 35% and 42% of women were diagnosed with stage I and II disease, respectively. Mean HADS-A subscale score was 7.51 (standard deviation (SD): 4.22) at study baseline and 6.87 (SD: 4.36) at 24 months.
Table 1.
Study Population Demographic, Disease, and Treatment Characteristics (n=833)
| Variable | Median (range) |
|---|---|
| Age at diagnosis | 36.9 (17 to 40) |
| Variable | N (%) |
| Race | |
| American Indian or Alaska Native | 3 (0.4) |
| Asian | 42 (5.0) |
| Black, Haitian, or African American | 24 (2.9) |
| White | 743 (89.2) |
| Multiracial | 10 (1.2) |
| Other/Unknown | 11 (1.3) |
| Ethnicity | |
| Hispanic | 38 (4.6) |
| Non-Hispanic | 775 (93.0) |
| Unknown | 20 (2.4) |
| College educated | |
| Yes | 694 (85.6) |
| No | 117 (14.4) |
| Missing/unknown | 22 |
| Married/living as married | |
| Yes | 632 (77.9) |
| No | 179 (22.1) |
| Missing/unknown | 22 |
| Children pre-diagnosis | |
| Any children | 524 (64.9) |
| No children | 283 (35.1) |
| Missing/unknown | 26 |
| Full-time employment | |
| Yes | 512 (63.4) |
| Other | 296 (36.6) |
| Missing | 25 |
| Financial situation | |
| Have enough money for special things that you want |
424 (52.7) |
| Little spare money to buy extra or special things |
229 (28.5) |
| Have money to pay bills because cut back on things or have difficulty paying bills no matter what |
151 (18.8) |
| Missing | 29 |
| Stage at diagnosis | |
| 0 | 69 (8.3) |
| I | 295 (35.4) |
| II | 352 (42.3) |
| III | 117 (14.1) |
| Estrogen receptor (ER) status | |
| ER-positive | 595 (71.5) |
| ER-negative | 237 (28.5) |
| Missing | 1 |
| Her-2 Neu status | |
| HER2-positive | 234 (29.4) |
| HER2-negative | 549 (69.0) |
| Indeterminate | 13 (1.6) |
| Missing | 37 |
| Surgery | |
| Lumpectomy | 242 (29.1) |
| Unilateral mastectomy | 206 (24.7) |
| Bilateral mastectomy | 385 (46.2) |
| Chemotherapy | |
| Yes | 632 (75.9) |
| No | 201 (24.1) |
| Radiation | |
| Yes | 514 (61.7) |
| No | 319 (38.3) |
ER, estrogen receptor; HER2, human epidermal growth factor receptor 2
Use of coping strategies at 6 and 18 months
Figure 2 depicts coping strategies utilized at 6 months post-enrollment and 18 months post-diagnosis (also see Supplemental Tables 2–4 in Online Resource 1). Greater than 80% of women reported at least moderate reliance on a partner, parents, other family members, friends, and health care providers at 6 and/or 18 months. Other commonly reported coping strategies (at least 65% reporting moderate or greater reliance) at one or both time points included taking care of others, hobbies, and exercise.
Figure 2.
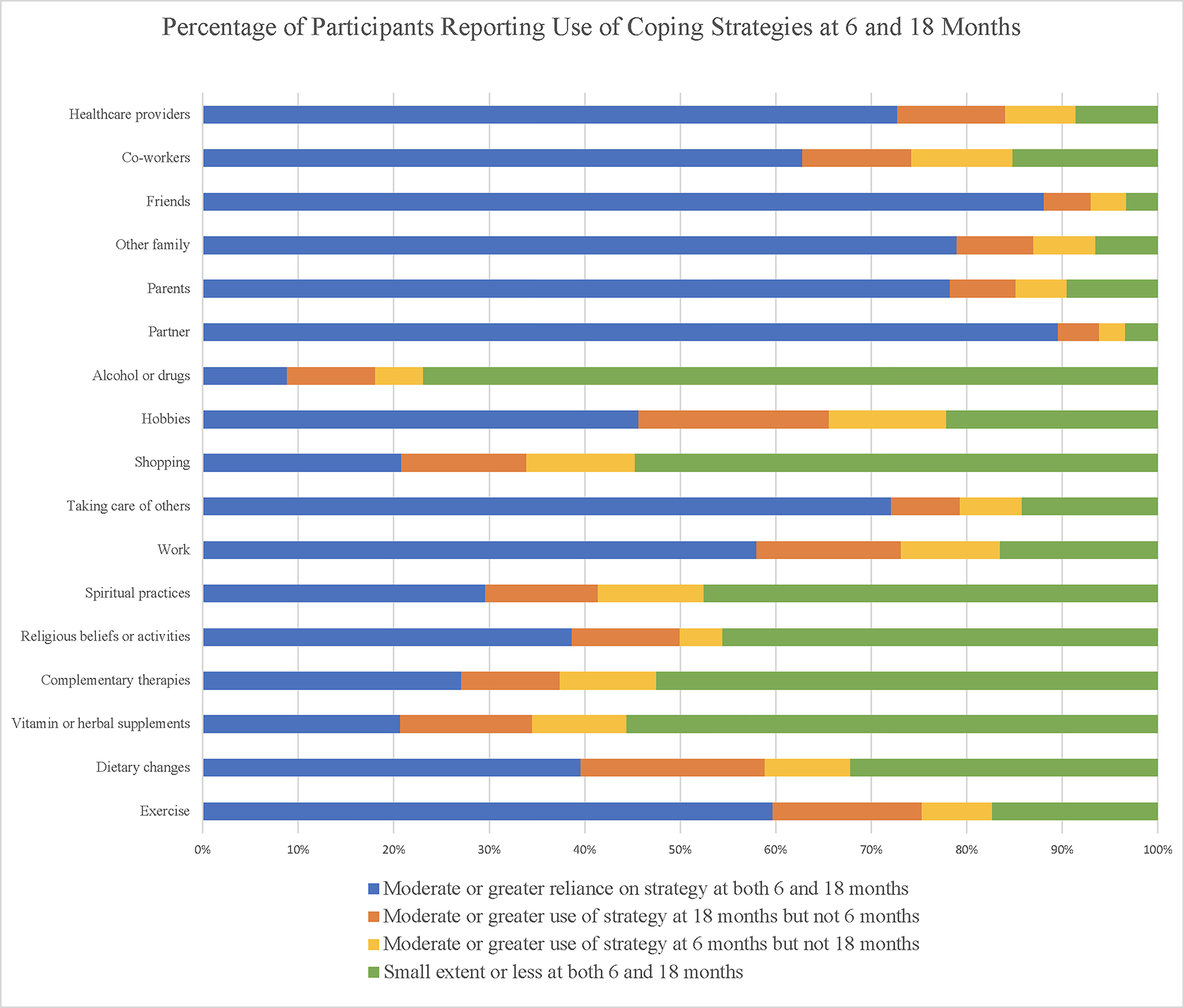
Coping strategies reported at 6 and 18 months
At 18 months, higher proportions of women reported moderate or greater reliance on specific coping strategies compared with at 6 months, including support from healthcare providers (84.0% vs. 80.1%, p=0.01), work (73.1% vs. 68.4%; p=0.019), hobbies (65.6% vs.58.1%; p = 0.0002), religion (49.9% vs. 43.1%; p <0.0001), alcohol/drug use (18.0% vs. 13.9%; p = 0.005), exercise (75.3% vs. 67.1%; p <0.0001), dietary change (58.9% vs. 48.5%; p <0.0001), and vitamins (34.5% vs. 30.6%; p=0.04). Moderate or greater reliance on all other coping strategies were not significantly different between the two time points.
Coping strategies at 18 months and anxiety at 24 months
Table 2 includes results from the univariable and multivariable logistic regression analyses. Compared to no reliance/reliance to a small extent, moderate or greater reliance on emotional support from a partner/spouse/significant other (odds ratio [OR]: 0.38, 95% confidence interval [CI]: 0.20–0.72) and moderate or greater reliance on emotional support from other family members (OR: 0.44, 95% CI: 0.28, 0.70) was associated with lower odds of anxiety while moderate or greater reliance on alcohol/drug use (vs. no reliance/reliance to a small extent) was associated with higher odds of anxiety (OR: 1.98, 95% CI: 1.25, 3.13) in univariable analyses. In the multivariable model, moderate or greater reliance on alcohol/drug use (vs. no reliance/reliance to small extent, OR: 1.83, 95% CI: 1.12, 3.00) and moderate or greater reliance on taking care of others (vs. no reliance/reliance to small extent, OR: 1.90, 95% CI: 1.04, 3.45) were significantly associated with higher odds of anxiety. Moderate or greater reliance on emotional support from other family (vs. no reliance/reliance to small extent, OR: 0.37, 95% CI: 0.22, 0.63) was associated with lower odds of anxiety.
Table 2.
Univariable and Multivariable Logistic Regression of Coping at 18 Months and Anxiety (HADS-A subscale score ≥ 11) at 24 Months (N=765)†
| Univariable models | Multivariable model‡ | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Emotional support from partner/spouse/significant other to at least a moderate extent | 0.38 | 0.20–0.72 | 0.003 | 0.58 | 0.28–1.18 | 0.13 |
| Emotional support from parents to at least a moderate extent | 0.73 | 0.46–1.19 | 0.207 | |||
| Emotional support from other family to at least a moderate extent§ | 0.44 | 0.28–0.70 | 0.0005 | 0.37 | 0.22–0.63 | 0.0002 |
| Healthcare providers to at least a moderate extent§ | 1.41 | 0.83–2.39 | 0.20 | 1.66 | 0.92–2.97 | 0.09 |
| Taking care of others to at least a moderate extent§ | 1.45 | 0.91–2.34 | 0.12 | 1.90 | 1.04–3.45 | 0.04 |
| Alcohol and/or drug use to at least a moderate extent§ (ref= Not at all/small extent) | 1.98 | 1.25–3.13 | 0.003 | 1.83 | 1.12–3.00 | 0.015 |
| Exercise to at least a moderate extent§ (ref= Not at all/small extent) | 0.70 | 0.47–1.04 | 0.08 | 0.72 | 0.46–1.11 | 0.13 |
| Vitamins‡ to at least a moderate extent§ (ref= Not at all/small extent) | 1.38 | 0.93–2.04 | 0.11 | 1.35 | 0.88–2.09 | 0.18 |
Excludes participants who did not have available HADS anxiety HADS-A subscale score ≥ 11 vs. ≤10). data at 2 years (n=68); missing indicator variables used for coping variables, pre-diagnosis parity, financial stress where this data was unavailable.
Adjusted for age, pre-diagnosis parity, financial stress, stage and chemotherapy
Moderate or greater report of this variable as a coping mechanism (ref = less than moderate report of this variable as a coping mechanism)
CI, confidence interval; HADS-A, Hospital Anxiety and Depression Scale anxiety subscale; OR, odds ratio
DISCUSSION
To the best of our knowledge, this analysis is the first to examine coping strategies used specifically by young adult breast cancer survivors. Social supports, particularly family, friends, and partner support, were the most commonly relied upon coping strategies at both 6 and 18 months. In addition, we also found that a significantly greater percentage of women reported exercise as a coping strategy at 18 months compared to 6 months, suggesting that women may increase their physical activity following the completion of active treatment.
Partner support was the most commonly reported coping strategy employed by the cohort, with nearly 90% of women reporting moderate or greater support from their partner at 6 and 18 months. Young breast cancer survivors may be particularly vulnerable to a perceived lack of partner support, in part due to less life experience with hardship, with a prior study from our team finding that among young women with breast cancer who were partnered, those who felt unsupported had higher odds of experiencing anxiety than those who felt supported [22]. Young couples often have competing demands on time and emotional energy, including financial issues, career trajectory and work productivity, fertility issues, and raising young children [1,3,4]. Distress screening and early intervention to address these challenges should include exploration of individuals’ sources of social support, particularly from their most important relationships.
In our study, moderate or greater reliance on alcohol and/or drugs was associated with anxiety. Alcohol use is a known coping strategy with significant morbidity. Alcohol use can co-occur with anxiety in the general population, with increased odds of anxiety ranging from 2.1 to 3.3 in several studies [23], similar to the magnitude of our findings. Further, alcohol may be a risk factor for cancer recurrence [24,25], underscoring the importance of addressing the potential negative effects of alcohol intake with cancer survivors.
We observed that moderate or greater reliance on taking care of others was associated with anxiety. Many young women have dependent children and they also may be caring for aging parents. Caretaker roles can increase feelings of stress and anxiety. [26] Breast cancer survivors with young children in particular can feel burdened by physical demands of that care as well as the need to explain their diagnosis to their children, potentially having to answer uncomfortable and upsetting questions about their mortality.[27] Additionally, this cohort of women, with a median age of 37, are in their prime earning years, with work and career potentially competing with other priorities. Given these challenges, interventions that aim to better support young patients should incorporate patients’ families, particularly their partners and children. Studies have shown that holistic interventions for cancer patients, their children and the rest of their family unit are beneficial for all [28].
Our study has some limitations, including limited generalizability, as YWS participants are largely White, college-educated, and financially secure. Further, the coping items were investigator-developed and are not part of a validated scale. Additionally, because women are enrolled into the YWS following diagnosis, we were unable to account for pre-existing psychological or physical co-morbidities, which may impact psychosocial health following diagnosis.
Young women with breast cancer are a uniquely vulnerable population, having to navigate the diagnosis of a serious illness and cancer treatment while confronting challenges specific to their stage of life. For both oncology and primary care providers who may be caring for this population, awareness of these issues is critical to improving survivorship care and outcomes. While young women in our study reported adopting a spectrum of coping strategies, identification of those patients who are not able to employ protective coping strategies on their own should be prioritized. Further, there have been interventions that have been developed to successfully target coping skills. A recent randomized study of a family-focused consult-based intervention in breast cancer patients demonstrated a positive impact on coping ability [29]. Additionally, a pilot trial of a positive affect skills intervention (online vs. in-person vs. online and in-person) for women with metastatic breast cancer reported a “marginally significant” improvement in utilization of positive affect skills, mindfulness and self-compassion, in the online and in-person group [30]. As new interventions to promote healthy coping continue to be developed, attention to the needs of young breast cancer survivors should be a consideration, with the goal of optimizing psychosocial health in this population.
Supplementary Material
Acknowledgements:
The authors wish to thank Valerie Hope Goldstein for editorial and submission assistance.
Funding:
This research was supported by Susan G. Komen (Partridge), the Breast Cancer Research Foundation (Partridge), the Agency for Healthcare Research and Quality K01HS023680 (Rosenberg), and the Harvard Kennedy School Center for Public Leadership’s Zuckerman Fellowship (Krasne). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Footnotes
Competing Interests: S.M.R. reports grants from the Agency for Healthcare Research and Quality, during the conduct of the study and current grant funding from Pfizer. R.M.T. reports grants from the NIH/NCI, during the conduct of the study. J.P. reports personal fees from GlaxosmithKline (Spouse, employment), grants from Outcomes4Me Inc, personal fees from Athenex, and personal fees from Abbott Labs, outside the submitted work. A.H.P. receives royalties for co-authoring the Breast Cancer Survivorship section of UpToDate. The remaining authors have no relevant financial or non-financial interests to disclose.
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the IRB. The study was approved by the IRBs of Dana-Farber Harvard Cancer Center, and other participating sites (Beth Israel Deaconess Medical Center, Brigham and Women’s Hospital, Brigham and Women’s Faulkner Hospital, Cape Cod Hospital, Lowell General Hospital, Massachusetts General Hospital, Mayo Clinic, Newton-Wellesley Hospital, North Shore Cancer Center Salem, South Shore Hospital, Sunnybrook Health Sciences Centre, and University of Colorado Hospital).
Consent to Participate: Informed consent was obtained from all individuals included in the study.
Availability of data and material:
The data are not publicly available as the Institutional Review Board Committee (IRB)-approved research protocol specified that all data must be collected, coded, and stored at the Dana-Farber Cancer Institute and be limited-access and password-protected, in order to protect the identity of respondents. Requests can be made to share data privately. However, any data sharing will require a formal data transfer agreement between the Dana-Farber Cancer Institute and the other party. Requests to this effect should be directed to the corresponding author.
REFERENCES:
- 1.Johnson RH, Anders CK, Litton JK, Ruddy KJ, Bleyer A (2018) Breast cancer in adolescents and young adults. Pediatr Blood Cancer 65 (12):e27397. doi: 10.1002/pbc.27397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL (2020) Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin 70 (6):443–459. doi: 10.3322/caac.21637 [DOI] [PubMed] [Google Scholar]
- 3.Stone DS, Ganz PA, Pavlish C, Robbins WA (2017) Young adult cancer survivors and work: a systematic review. Journal of cancer survivorship : research and practice 11 (6):765–781. doi: 10.1007/s11764-017-0614-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cathcart-Rake EJ, Ruddy KJ, Bleyer A, Johnson RH (2021) Breast Cancer in Adolescent and Young Adult Women Under the Age of 40 Years. JCO Oncol Pract 17 (6):305–313. doi: 10.1200/OP.20.00793 [DOI] [PubMed] [Google Scholar]
- 5.Northouse LL (1994) Breast cancer in younger women: effects on interpersonal and family relations. J Natl Cancer Inst Monogr (16):183–190 [PubMed] [Google Scholar]
- 6.Gould J, Grassau P, Manthorne J, Gray RE, Fitch MI (2006) ‘Nothing fit me’: nationwide consultations with young women with breast cancer. Health Expect 9 (2):158–173. doi: 10.1111/j.1369-7625.2006.00383.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelhadi OA, Pollock BH, Joseph JG, Keegan THM (2022) Psychological distress and associated additional medical expenditures in adolescent and young adult cancer survivors. Cancer 128 (7):1523–1531. doi: 10.1002/cncr.34064 [DOI] [PubMed] [Google Scholar]
- 8.Harris J, Cornelius V, Ream E, Cheevers K, Armes J (2017) Anxiety after completion of treatment for early-stage breast cancer: a systematic review to identify candidate predictors and evaluate multivariable model development. Support Care Cancer 25 (7):2321–2333. doi: 10.1007/s00520-017-3688-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg CJ, Stratton E, Esiashvili N, Mertens A (2016) Young Adult Cancer Survivors’ Experience with Cancer Treatment and Follow-Up Care and Perceptions of Barriers to Engaging in Recommended Care. J Cancer Educ 31 (3):430–442. doi: 10.1007/s13187-015-0853-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geyer S, Koch-Giesselmann H, Noeres D (2015) Coping with breast cancer and relapse: Stability of coping and long-term outcomes in an observational study over 10 years. Soc Sci Med 135:92–98. doi: 10.1016/j.socscimed.2015.04.027 [DOI] [PubMed] [Google Scholar]
- 11.Zamanian H, Amini-Tehrani M, Jalali Z, Daryaafzoon M, Ala S, Tabrizian S, Foroozanfar S (2021) Perceived social support, coping strategies, anxiety and depression among women with breast cancer: Evaluation of a mediation model. Eur J Oncol Nurs 50:101892. doi: 10.1016/j.ejon.2020.101892 [DOI] [PubMed] [Google Scholar]
- 12.Manuel JC, Burwell SR, Crawford SL, Lawrence RH, Farmer DF, Hege A, Phillips K, Avis NE (2007) Younger women’s perceptions of coping with breast cancer. Cancer Nurs 30 (2):85–94. doi: 10.1097/01.NCC.0000265001.72064.dd [DOI] [PubMed] [Google Scholar]
- 13.Lauver DR, Connolly-Nelson K, Vang P (2007) Stressors and coping strategies among female cancer survivors after treatments. Cancer Nurs 30 (2):101–111. doi: 10.1097/01.NCC.0000265003.56817.2c [DOI] [PubMed] [Google Scholar]
- 14.Gierisch JM, Earp JA, Brewer NT, Rimer BK (2010) Longitudinal predictors of nonadherence to maintenance of mammography. Cancer Epidemiol Biomarkers Prev 19 (4):1103–1111. doi: 10.1158/1055-9965.EPI-09-1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams RB, Barefoot JC, Califf RM, Haney TL, Saunders WB, Pryor DB, Hlatky MA, Siegler IC, Mark DB (1992) Prognostic importance of social and economic resources among medically treated patients with angiographically documented coronary artery disease. JAMA 267 (4):520–524 [PubMed] [Google Scholar]
- 16.Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67 (6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 17.Bjelland I, Dahl AA, Haug TT, Neckelmann D (2002) The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 52 (2):69–77. doi: 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 18.Lopez G, Liu W, Madden K, Fellman B, Li Y, Bruera E (2018) Adolescent-young adults (AYA) with cancer seeking integrative oncology consultations: demographics, characteristics, and self-reported outcomes. Support Care Cancer 26 (4):1161–1167. doi: 10.1007/s00520-017-3937-8 [DOI] [PubMed] [Google Scholar]
- 19.Burstein HJ, Gelber S, Guadagnoli E, Weeks JC (1999) Use of alternative medicine by women with early-stage breast cancer. N Engl J Med 340 (22):1733–1739. doi: 10.1056/NEJM199906033402206 [DOI] [PubMed] [Google Scholar]
- 20.Lee MK, Yun YH, Park HA, Lee ES, Jung KH, Noh DY (2014) A Web-based self-management exercise and diet intervention for breast cancer survivors: pilot randomized controlled trial. Int J Nurs Stud 51 (12):1557–1567. doi: 10.1016/j.ijnurstu.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 21.Kushner MG, Abrams K, Borchardt C (2000) The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev 20 (2):149–171. doi: 10.1016/s0272-7358(99)00027-6 [DOI] [PubMed] [Google Scholar]
- 22.Borstelmann NA, Rosenberg SM, Ruddy KJ, Tamimi RM, Gelber S, Schapira L, Come S, Borges V, Morgan E, Partridge AH (2015) Partner support and anxiety in young women with breast cancer. Psychooncology 24 (12):1679–1685. doi: 10.1002/pon.3780 [DOI] [PubMed] [Google Scholar]
- 23.Smith JP, Randall CL (2012) Anxiety and alcohol use disorders: comorbidity and treatment considerations. Alcohol Res 34 (4):414–431 [PMC free article] [PubMed] [Google Scholar]
- 24.Kwan ML, Kushi LH, Weltzien E, Tam EK, Castillo A, Sweeney C, Caan BJ (2010) Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: the life after cancer epidemiology study. J Clin Oncol 28 (29):4410–4416. doi: 10.1200/JCO.2010.29.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC (2011) Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 306 (17):1884–1890. doi: 10.1001/jama.2011.1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naerde A, Tambs K, Mathiesen KS, Dalgard OS, Samuelsen SO (2000) Symptoms of anxiety and depression among mothers of pre-school children: effect of chronic strain related to children and child care-taking. J Affect Disord 58 (3):181–199. doi: 10.1016/s0165-0327(99)00119-6 [DOI] [PubMed] [Google Scholar]
- 27.Barnes J, Kroll L, Burke O, Lee J, Jones A, Stein A (2000) Qualitative interview study of communication between parents and children about maternal breast cancer. BMJ 321 (7259):479–482. doi: 10.1136/bmj.321.7259.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyman GH, Greenlee H, Bohlke K, Bao T, DeMichele AM, Deng GE, Fouladbakhsh JM, Gil B, Hershman DL, Mansfield S, Mussallem DM, Mustian KM, Price E, Rafte S, Cohen L (2018) Integrative Therapies During and After Breast Cancer Treatment: ASCO Endorsement of the SIO Clinical Practice Guideline. J Clin Oncol 36 (25):2647–2655. doi: 10.1200/JCO.2018.79.2721 [DOI] [PubMed] [Google Scholar]
- 29.Moghaddam Tabrizi F, Alizadeh S (2018) Family Intervention Based on the FOCUS Program Effects on Cancer Coping in Iranian Breast Cancer Patients: a Randomized Control Trial. Asian Pac J Cancer Prev 19 (6):1523–1528. doi: 10.22034/APJCP.2018.19.6.1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung EO, Cohn MA, Dunn LB, Melisko ME, Morgan S, Penedo FJ, Salsman JM, Shumay DM, Moskowitz JT (2017) A randomized pilot trial of a positive affect skill intervention (lessons in linking affect and coping) for women with metastatic breast cancer. Psychooncology 26 (12):2101–2108. doi: 10.1002/pon.4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are not publicly available as the Institutional Review Board Committee (IRB)-approved research protocol specified that all data must be collected, coded, and stored at the Dana-Farber Cancer Institute and be limited-access and password-protected, in order to protect the identity of respondents. Requests can be made to share data privately. However, any data sharing will require a formal data transfer agreement between the Dana-Farber Cancer Institute and the other party. Requests to this effect should be directed to the corresponding author.


