Abstract
Objective
To retrieve evolving respiratory measures in the first minutes after birth in normal neonates born at term using a respiratory function monitor.
Study design
We evaluated newborn babies delivered at term via cesarean after uncomplicated pregnancies. Immediately after birth, a respiratory function monitor with an adapted flowmeter and a face mask were applied at 2, 5, and 10 minutes after birth for 90 seconds in each period. We analyzed expired and inspired tidal volume, respiratory rate (RR), percentage of leakage, and number of analyzed breaths in each individual infant's recording using a respiratory research software.
Results
A total of 243 infants completed the study. The final data set included 59 058 (48.35%) valid observations for each of the variables representing the analysis of 32 801 breaths. With these data, we constructed a reference range with 10th, 25th, 50th, 75th, and 90th percentiles for expired tidal volume and RR. Tidal volumes plateaued earlier in female than in male infants. No correlation with delayed cord clamping, gestational age, maternal morbidity, or indication for cesarean delivery were established.
Conclusions
We have constructed a reference range with percentiles for inspired and expired tidal volumes and RR in newborn babies born at term for the first 10 minutes after birth. Reference ranges can be employed for research and can be useful in the clinical setting to guide positive pressure ventilation in the delivery room.
Keywords: tidal volume, respiratory rate, respiratory function monitor, mask leakage, newborn
Approximately 130 million babies are born every year worldwide, of whom 5%-10% will require respiratory support in the immediate postnatal period.1,2 Cesarean delivery requires surgical intervention that increases the risk and severity of neonatal respiratory morbidity.3,4 The cesarean delivery rate, even in high-income countries, exceeds the World Health Organization recommendations.5,6 Consequently, postnatal care of babies born by cesarean delivery constitutes an important concern for neonatologist.3,4
Adequate lung ventilation is key to successful postnatal stabilization.2,7,8 In newly born infants who do not initiate effective breathing despite tactile stimulation, positive pressure ventilation (PPV) should be rapidly provided.9 The goal of PPV is to create a functional residual capacity (FRC), deliver an adequate tidal volume (VT), normalize blood gases, and stimulate breathing while minimizing lung injury.10 Optimal PPV should be guided by continuous monitoring of inspiratory and expiratory airway pressure, gas flow, VT, and mask leakage9,11; these goals can be facilitated using a respiratory function monitor (RFM) during PPV in the delivery room (DR).10,12
Effective ventilation requires the provision of peak inspiratory pressure (PIP) capable of generating sufficient VT to aerate the lungs and positive end-expiratory pressure that helps establish FRC sufficient to prevent alveolar collapse/atelectasis during expiration.2,13 To achieve these ventilatory goals, VT monitoring during PPV in the DR is more relevant than PIP.10,13,14 Kattwinkel et al demonstrated that monitoring VT better detected changes of lung compliance than monitoring pressure.14 Hernandez et al concluded that pulmonary overdistension due to high VT rather than high PIP caused lung damage.15 Thus, to minimize volutrauma, PIP should be adjusted to ensure the delivery of a VT within a safe range.13 Schmölzer et al showed that setting a PIP of 30 cm H2O could deliver VT that ranged from 0 to 30 mL/kg.12 Moreover, studies in animals revealed that VT between 8 and 15 mL/kg16,17 caused lung damage independent of PIP, suggesting that PIP varies according to the lung compliance.16
However, keeping the VT within an established range using a pre-established PIP limit is difficult. Even in expert hands, airway obstruction and/or mask leakage are extremely common.9 In addition, breathing efforts of the newborn infant will increase the inconsistency of volumes delivered with a fixed PIP.9,13 However, if VT is kept constant despite pressure changes, lung injury is likely to be minimized while ventilation remains adequate.9,14 Thus, measuring and adjusting VT during PPV may be particularly helpful during postnatal stabilization.10,12,18,19 Previous studies on spontaneous breathing in newborns born at term suggest that the normal VT ranges between 5 and 7 mL/kg.20,21 However, these studies include limitations such as sample size or do not incorporate recent changes in the DR management such as delayed cord clamping (DCC) in babies born by cesarean delivery.7
We aimed to assess the normal VT and respiratory rate (RR) in babies born at term via cesarean delivery in the first minutes after birth. We employed an RFM to retrieve changes in the expired tidal volume (VTe) and RR in the first 10 minutes of life in healthy infants born at term via elective cesarean delivery, spontaneously breathing, without respiratory distress, and not needing resuscitation. Thus, we were able to define a reference range and construct a percentile graph to guide PPV but also positive end-expiratory pressure/continuous positive airway pressure level in spontaneously breathing infants during postnatal stabilization in the DR.
Methods
We conducted a prospective, single-center observational study of newborn infants born in the Obstetric Area of the University and Polytechnic Hospital La Fe (Valencia, Spain). Eligible Infants were born at ≥37 weeks of gestational age by elective cesarean delivery under spinal anesthesia and did not need ventilation or oxygen during DR stabilization. The current guidelines of the Spanish Neonatal Society indicate that babies born after elective cesarean delivery should be observed over a period of 10 minutes in the resuscitator. Conversely, babies born by vaginal delivery should be placed immediately on their mother's chest in a prone position for skin-to-skin contact.22 Under these circumstances, the hospital ethics committee did not allow the use of RFM, which might interfere with maternal–infant bonding in vaginal deliveries. Thus, we excluded babies born by vaginal delivery, preterm, with congenital cardiac and respiratory malformations, or needing any type of intervention in the DR.
The study design was approved by the Ethics Committee of the University and Polytechnic Hospital La Fe, and informed consent was signed by parents of all the enrolled patients before birth.
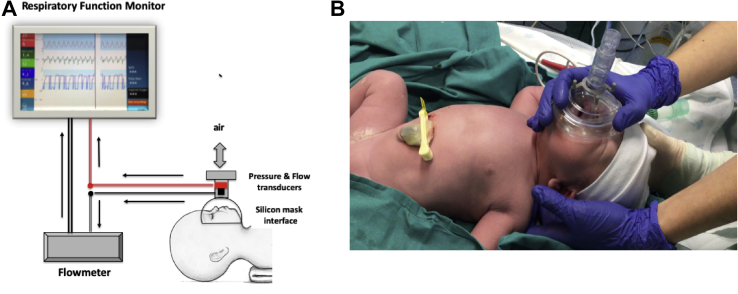
Lung function and gas flow were assessed using a noninvasive RFM New Life Box Neo-RSD (Advanced Life Diagnostics UG) and New Life Box-R (Advanced Life Diagnostics UG), respectively.23 An open 50-mm face mask (Fisher Paykel Healthcare) was placed upon the baby's face with the distal end open to the air, thus allowing the infant to breathe with minimal resistance.24,25 The RFM uses a variable orifice anemometer (Avea Varflex Flow Transducer; CareFusion) to measure circuit pressure and gas flow in and out of a T-piece (Figure 1, A).
Figure 1.
A, Scheme of the monitoring system. Face mask was applied to the infant's face covering nose and mouth. Sensors for monitoring respiratory parameters and flow were connected to the mask interface. Mask is open to air with neglectable expiratory resistance. B, Photograph depicting newborn's position and optimal mask holding during monitoring.
The dead space of the pneumotachograph is <0.7 mL25,26 and of the mask approximately 20 mL27; providing a good seal significantly (Figure 1, B) reduced the mask dead space by 30%-40%.28 The mask was applied at 2, 5, and 10 minutes after birth. Respiratory function was recorded for periods of 90 seconds in each period to avoid interfering with postnatal adaptation.25
The New Life Box Neo-RSD monitor exhibits graphical and numerical flow and volume information. All signals were digitized and recorded at 200 Hz using the Polybench neonatal physiological recording program (Applied Biosignals GmbH, edition 1.30.0.3505).23
In January 2019, the hospital guidelines started to recommend delaying cord clamping for 60 seconds, during which time the baby was dried and wrapped in warm clothes while the attending neonatologist supervised postnatal adaptation.2,8 Tactile stimulation was only performed if babies did not initiate spontaneous breathing efforts 30 seconds after birth. If the transition was adequate, the face mask was applied at 2, 5, and 10 minutes after birth for 90 seconds. For this purpose, the baby was placed in a supine position and the head in a neutral position to keep the airway open.8 The mask completely covered the nose and mouth area; the newborn's chin was held firmly in place to prevent leakage25 (Figure 1, B). After 12 minutes, researchers stopped monitoring the infant. If there were signs of respiratory compromise, recruitment was suspended and ventilatory support was given according to neonatal resuscitation guidelines.2,8 Researchers collecting respiratory data were independent of the attending clinicians and did not interfere with their decisions. A standardized protocol for the procedure and RFM data collection was established and measurements were performed by trained members of the research team.
A breath-by-breath analysis of each infant's recording was performed employing a data analysis software for respiratory research (Pulmochart; Advance Life Diagnostics).29 This allowed for reproduction of the waveforms and breaths of each infant and eliminated possible noise and sensor interferences. Breath-by-breath analysis and breath elimination conditions are described in (Table I).25,30, 31, 32
Table I.
Description of the circumstances that interfered with the assessment of physiologic breaths in the respiratory registries
| Variables | Description |
|---|---|
| Flow and VTe | Airway obstruction25 coinciding with
|
| RR | Reliable range between 15 and 150 bpm25 |
We only included data recordings if there was a clean flow and VT signal. For this purpose, we averaged the VTe of 6 breaths in a row and satisfied the requirements as shown in Table I.33 We retrieved the following respiratory parameters: VTe, inspired tidal volume (VTi), RR, number of analyzed breaths of each infant, and average percentage of leakage.
Statistical Analyses
Data were summarized using mean (SD) and median (first, third quartile) for continuous variables and relative and absolute frequencies for categorical variables. Bayesian spline percentile regression models were used to estimate the reference value curves of the parameters VTe and RR. For each parameter the 50th, 25th, 75th, 10th, and 90th percentiles were estimated over the first 12 minutes of life. Because the study design was of repeated measurements, a random intercept was added for everyone to consider the dependence relationship of the observations of the same individual. As the use of splines makes the interpretation of the models difficult, nomograms were generated to facilitate the practical consultation of the reference values estimated by the different models.34,35
The leakage estimation through the mask is not possible when using an open system because there is no pressure inside the mask. Thus, the most reliable method to detect leakage was to calculate VTi and VTe separately, omitting the use of automatic current correction algorithms, and comparing these values directly. The leakage was calculated as the percentage of gas volume that did not return through the flow sensor during expiration using the following formula: percentage of leakage = VTi–VTe/VTi × 100.36
In addition, we studied the possible association of VTe values with sex, DCC, indication for cesarean delivery, maternal morbidity, and gestational age. For this purpose, we averaged VTe in the 30-second period that included the most representative values for each individual. The association between VTe and each factor was analyzed using the Wilcoxon test for dichotomous factors and Kruskal–Wallis test in the case of factors with more than 2 categories. P values <.05 were statistically significant. Credibility or 95% CIs was obtained for all estimates. All analyses were performed using the software R (version 4.0; The R Foundation for Statistical Computing) and the packages click R (version 0.4.47; The R Foundation for Statistical Computing), brms (version 2.12), and splines (version 4.0).
Results
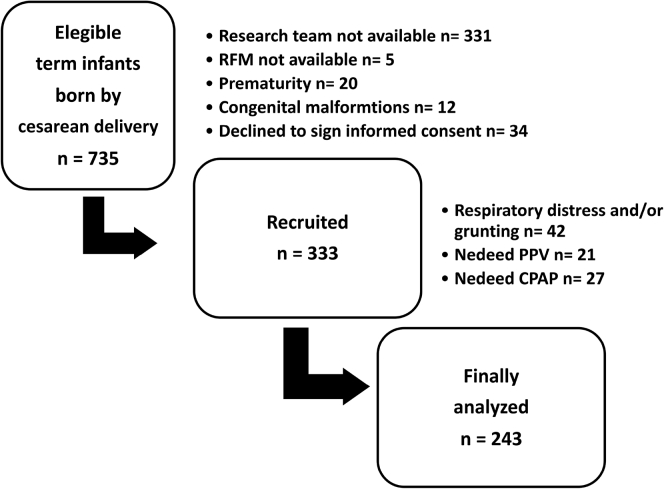
Between January 2018 and August 2019, a total of 243 infants completed the study. The final data set included 59 058 (48.35%) valid observations for each of the variables, which represented the analysis of 32 801 breaths (Figure 2). The characteristics of the infants and mothers are described in Table II.
Figure 2.
Flow diagram describing the recruitment process during the study. CPAP, continuous positive airway pressure.
Table II.
Characteristics of mothers and infants included the study
| Characteristics | Values N = 243 |
|---|---|
| Mother's age, y (IQR) | 36 (32, 39) |
| Maternal morbidities, no. (%) | 52 (21.4%) |
| Elective cesarean delivery, no. (%) | 222 (91.7%) |
| Failed induction/stopped birth, no. (%) | 21 (8.2%) |
| Prenatal corticosteroids, no. (%) | 13 (4.6%) |
| Gestational ages, wk, median (IQR) | 39 (38.2, 39.2) |
| Birth weight, g, mean (SD) | 3267 (571.85) |
| Female sex, no. (%) | 125 (51.4%) |
| Apgar score at 1 min, median (IQR) | 9 (9, 10) |
| Apgar score at 5 min, median (IQR) | 10 (10, 10) |
| DCC (>30 s), no. (%) | 62 (25.51%) |
| Time of DCC, s, mean (SD) | 59.2 (12.14) |
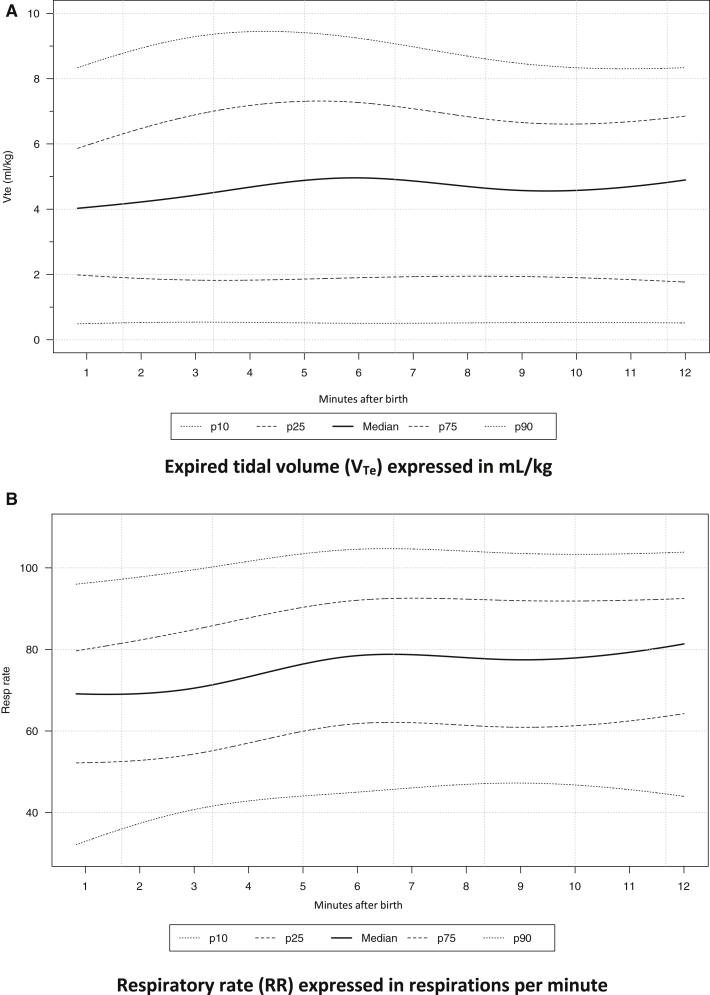
We determined 10th, 25th, 50th, 75th, and 90th percentiles for VTe and RR. Results are graphically depicted in Figure 3, A and B. Numerical values represented as a nomogram throughout the first 12 minutes of life are shown in Figures 4 and 5.
Figure 3.
A, The 10th, 25th, 50th, 75th, and 90th percentiles of VTe mL/Kg measured with an RFM in healthy newborns born at term via cesarean delivery and spontaneously breathing during the first 12 minutes after birth. B, The 10th, 25th, 50th, 75th, and 90th percentiles of RR bpm measured with an RFM in spontaneously breathing healthy term newborn infants born by cesarean delivery during the first 12 minutes after birth.
Figure 4.
Percentiles of VTe mL/kg during the first 12 minutes of life in healthy infants born full term via elective caesarean delivery, with spontaneous breathing.
Figure 5.
Percentiles of RR bpm during the first 12 minutes of life in healthy infants born full term via elective caesarean delivery, with spontaneous breathing.
The Bayesian percentile regression models approach showed an upward trajectory for VTe during the first 5 minutes of life that plateaued thereafter. For the 10th and 25th percentiles the trajectory remained constant throughout the period analyzed. The trajectory of the RR curves showed a similar tendency. The RR increased slightly until the sixth minute after birth and then plateaued.
Median and IQR of VTe at 2, 5, and 10 minutes after birth were 4.2 (1.9-6.5), 4.9 (1.9-7.3), and 4.6 (1.9-6.6) mL/kg, respectively. Median (IQR) of RR at 2, 5, and 10 minutes after birth were 69 (53-82), 76 (60-90), and 78 (61-92), beats per minute (bpm), respectively.
Both VTe and RR showed great variability throughout the first 12 minutes of life, ranging from 0.5 to 9.4 mL/kg for VTe and 33 to 105 bpm for RR. We also analyzed the influence of different factors upon VTe. Female infants obtained greater values of VTe than male infants (P = .022, P = .002, and P = .008, at 2, 5, and 10 minutes after birth, respectively). No statistically significant association was found for DCC, maternal morbidity, gestational age, or indication for cesarean delivery. We also calculated the correlation between evolving VTe with RR and oxygen saturation (SpO2) in the first 12 minutes after birth; both VTe/SpO2 (ρ = 0.96) and VTe/RR (ρ = 0.83) were significant.
Discussion
We assessed evolving values for VTe and RR in the first 10 minutes after birth in healthy infants born full term via elective cesarean delivery. Current evidence recommends that optimal ventilation should be guided by continuous visualization of airway pressure, gas flow, VT, and mask leak.9,11 Our data using a validated RFM provide reference ranges for VTe and RR presented as a percentile chart, which could serve as guide for clinicians when applying PPV in the DR.
We observed that percentile curves for both VTe and RR showed an increasing tendency immediately after birth (Figure 3, A and B) and plateaued at 5 and 6 minutes after birth, respectively. Of note, there was a highly significant correlation between VTe and RR (ρ = 0.83) in the first minutes after birth. The interpretation of VTe plateauing before RR may be because adequate VTe favors the establishment of FRC, which in turn enhances alveolar–capillary gas exchange and subsequently reduces the RR. Moreover, SpO2 in the first minutes after birth also significantly correlated with VTe progression (ρ = 0.96). Our results coincide with data reported in other studies both in newborns born at term20,21 and preterm.37 Blank et al described a plateau of 5.3 mL/kg (2.5-8.4 mL/kg) at 130 seconds after birth.21 In our study, we reached a plateau of 4.9 mL/kg (1.9-7.3) at 5 minutes of life. Notably, the study by Blank et al included both babies born by cesarean and vaginal delivery.21 Babies born by vaginal delivery attain a VTe plateau earlier than those born by cesarean delivery. Finn et al also showed a delay of VTe stabilization between 3 and 4 minutes after birth in babies born by cesarean delivery.20 This delay may be related with a slower extrusion of lung fluid to the interstitial space, or to an increased re-entry of lung fluid into the alveoli in the expiratory phase, thus hindering/delaying the establishment of an FRC.38,39
We established a median (IQR) for VTe of 4.2 (1.9-6.5), 4.9 (1.9-7.3), and 4.6 (1.9-6.6) mL/kg at 2, 5, and 10 minutes after birth, respectively. Both Blank et al and Finn et al reported VTe values similar to ours in the first minutes after birth.20,21 Blank et al reported a VTe of 5.3 mL/kg (2.5-8.4) and didn't find differences in VTe between babies born by cesarean and vaginal delivery.21 Finn et al registered a mean VTe at 2 and 5 minutes of 5.7 (2.2) and 6.05 (2.4) mL/kg.20 However, Schmölzer et al retrieved a mean VTe of 6.3 (±3) mL/kg at 2 minutes of age, which is substantially greater than ours.40 These differences in VTe may be due to the smaller sample size in the study by Schmölzer et al and/or to the fact that VTe was expressed as a mean, which is not as descriptive as the median.40 Consistent with these reports, we observed a VTe ranging between 4 and 6 mL/kg during the first minutes of life, indicating that babies born by cesarean delivery achieve similar VT to those of babies born by vaginal delivery.21,38
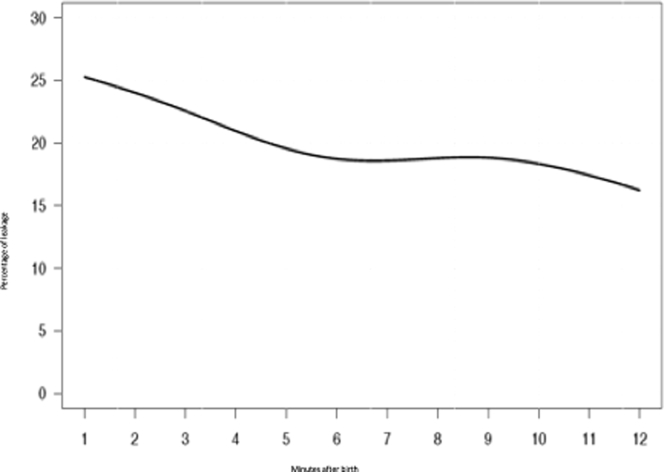
Previous studies also reported a great variability of VTe21,37,41; in our patients, VTe ranged from 0.5 to 9.4 mL/Kg (10th and 90th percentile predictions). Variability could be attributed to reflex movements of the lower pharynx, epiglottis, and glottis.41 These physiological adjustments are common during postnatal adaptation and are meant to provoke a positive end-expiratory pressure that contributes to the establishment of an FRC.25 It should be noted that mask leakage in our study fluctuated between 16% and 25% (Figure 6), which confirms that the VTe depicted in the nomogram was a good estimate of the volume that entered the lungs.42,43
Figure 6.
The graph shows the percentage of leakage (%) during the first 10 minutes after birth in heathy newborn babies born at term via cesarean delivery during the first 10 minutes after birth.
We also analyzed evolving RR. We reported a median and IQR of 69 (53-82), 76 (60-90), and 78 (61-92) bpm at 2, 5, and 10 minutes after birth. te Pas et al showed that babies during in the first minutes after birth presented a panting pattern with an average frequency of RR between 50 and 90 bpm.25 We confirmed an increased incidence of transient tachypnea in our babies born by cesarean delivery, as has been previously described.44 Although some previous studies found no difference in RR comparing mode of delivery,21,38 perhaps our large sample size made the tachypnea more evident.
VTe was influenced by the sex of the newborn infant: male newborn infants had significantly lower VTe than girls at the same postnatal timings. Clinical studies on respiratory morbidity in infants born at term via cesarean delivery report that male sex has an increased the probability of admission to neonatal intensive care unit and/or suffering from respiratory distress syndrome.44,45 We speculate this reflects a delay in lung maturation in male fetuses as compared with female fetuses.46
We acknowledge certain limitations to our study. The ethics committee didn't allow us to include babies born by vaginal delivery to avoid interfering with maternal–infant bonding and initiation of early breastfeeding. In addition, measuring respiratory changes with the RFM during the stabilization period in DR was technically very challenging.21,25,47 To minimize interference with breathing, the mask was applied only for short periods of 90 seconds, which reduced the data available for analysis. In addition, all recordings with artifacts were excluded, which could lead to bias. We could not capture the first breaths, so it is possible that some early changes in VTe and RR were not included.41 In a recent study, it has been shown that the energic application of the face mask upon the face can stimulate the trigeminal nucleus and subsequently a vagal reflex, which can interfere the breathing pattern.48 Notwithstanding, our results coincide with established ranges of VTe described in previous studies.9,13,20,21 Strengths of our study include a dedicated and trained team exclusively devoted to precisely make the measurements in the DR, the large number of analyzed registers, and the large sample size.
In conclusion, we present a reference range nomogram for VTe and RR during the immediate postnatal adaptation of healthy infants born full term via cesarean delivery. The nomogram may be used to guide PPV when its directed by volume rather than pressure. Moreover, we found infants born by cesarean delivery required a longer period to reach a stable VTe and RR than babies born by vaginal delivery, as deduced from published literature. We plan to enhance our studies by including infants born preterm.
Acknowledgments
We thank F. Escribá, MD, V. Modesto, MD, and A. J. Cañada-Martín, MSc, for their support in the data analysis. We also thank the pediatric nursing staff and pediatricians who helped with patient recruiting for the study, especially Begoña Torres, MD. Finally, we express our utmost gratitude to all the parents who generously agreed to allow their babies to participate in our study.
Footnotes
M.V. and A.S-G. acknowledge the Redes Tematicas de Investigación Cooperativa en Salud (RETICS) funded by the PN 2018-2021 (Spain), ISCIII- Sub-Directorate General for Research Assessment and Promotion and the European Regional Development Fund (FEDER), reference RD16/0022/0001. The authors declare no conflicts of interest.
References
- 1.GBD 2017 Population and Fertility Collaborators Population and fertility by age and sex for 195 countries and territories, 1950-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1995–2051. doi: 10.1016/S0140-6736(18)32278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perlman J.M., Wyllie J., Kattwinkel J., Wyckoff M.H., Aziz K., Guinsburg R., et al. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132(16 suppl 1):S204–S241. doi: 10.1161/CIR.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 3.De Luca R., Boulvain M., Irion O., Berner M., Pfister R.E. Incidence of early neonatal mortality and morbidity after late-preterm and term cesarean delivery. Pediatrics. 2009;123:e1064–e1071. doi: 10.1542/peds.2008-2407. [DOI] [PubMed] [Google Scholar]
- 4.Warren J.B., Anderson J.M. Newborn respiratory disorders. Pediatr Rev. 2010;31:487–495. doi: 10.1542/pir.31-12-487. [DOI] [PubMed] [Google Scholar]
- 5.Boerma T., RonsmansC, Melesse D.Y., Barros A.J.D., Barros F.C., Juan, et al. Global epidemiology of use of and disparities in caesarean sections. Lancet. 2018;392:1341–1348. doi: 10.1016/S0140-6736(18)31928-7. [DOI] [PubMed] [Google Scholar]
- 6.Belizán J.M., Althabe F., Cafferata M.L. Health consequences of the increasing caesarean section rates. Epidemiology. 2007;18:485–486. doi: 10.1097/EDE.0b013e318068646a. [DOI] [PubMed] [Google Scholar]
- 7.Foglia E.E., Te Pas A.B. Effective ventilation: The most critical intervention for successful delivery room resuscitation. Semin Fetal Neonatal Med. 2018;23:340–346. doi: 10.1016/j.siny.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyllie J., Bruinenberg J., Roehr C.C., Rüdiger M., Trevisanuto D., Urlesberger B. European Resuscitation Council Guidelines for Resuscitation 2015: Section 7. Resuscitation and support of transition of babies at birth. Resuscitation. 2015;95:249–263. doi: 10.1016/j.resuscitation.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Schmölzer G.M., Kamlin O.C., Dawson J.A., te Pas A.B., Morley C.J., Davis P.G. Respiratory monitoring of neonatal resuscitation. Arch Dis Child Fetal Neonatal Ed. 2010;95:F295–F303. doi: 10.1136/adc.2009.165878. [DOI] [PubMed] [Google Scholar]
- 10.Schmölzer G., Morley C., Wong C., Dawson J., Kamlin C.O., Donath S., et al. Respiratory function monitor guidance of mask ventilation in the delivery room: a feasibility study. J Pediatr. 2012;160:377–381.e2. doi: 10.1016/j.jpeds.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 11.van Vonderen J.J., Roest A.A.W., Siew M.L., Walther F.J., Hooper S.B., te Pas A.B. Measuring physiological changes during the transition to life after birth. Neonatology. 2014;105:230–242. doi: 10.1159/000356704. [DOI] [PubMed] [Google Scholar]
- 12.Schmölzer G.M., Kamlin O.C., O'Donnell C.P., Dawson J.A., Morley C.J., Davis P.G. Assessment of tidal volume and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal. 2010;95:F393–F397. doi: 10.1136/adc.2009.174003. [DOI] [PubMed] [Google Scholar]
- 13.Schmölzer G.M., Te Pas A.B., Davis P.G., Morley C.J. Reducing lung injury during neonatal resuscitation of preterm infants. J Pediatr. 2008;153:741–745. doi: 10.1016/j.jpeds.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Kattwinkel J., Stewart C., Walsh B., Gurka M., Paget-Brown A. Responding to compliance changes in a lung model during manual ventilation: perhaps volume, rather than pressure, should be displayed. Pediatrics. 2009;123:e465–e470. doi: 10.1542/peds.2008-2012. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez L.A., Peevy K.J., Moise A.A., Parker J.C. Chest wall restriction limits high airway pressure-induced lung injury in young rabbits. J Appl Physiol. 1989;66:2364–2368. doi: 10.1152/jappl.1989.66.5.2364. [DOI] [PubMed] [Google Scholar]
- 16.Hillman N.H., Moss T.J., Kallapur S.G., Bachurski C., Pillow J.J., Polglase G.R., et al. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med. 2007;176:575–581. doi: 10.1164/rccm.200701-051OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polglase G.R., Hillman N.H., Pillow J.J., Cheah F.C., Nitsos I., Moss T.J., et al. Positive end-expiratory pressure and tidal volume during initial ventilation of preterm lambs. Pediatr Res. 2008;64:517–522. doi: 10.1203/PDR.0b013e3181841363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mian Q., Cheung P.Y., O'Reilly M., Pichler G., van Os S., Kushniruk K., et al. Spontaneously breathing preterm infants change in tidal volume to improve lung aeration immediately after birth. J Pediatr. 2015;167:274–278.e1. doi: 10.1016/j.jpeds.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 19.Klingenberg C., Wheeler K.I., McCallion N., Morley C.J., Davis P.G. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst Rev. 2017;10:CD003666. doi: 10.1002/14651858.CD003666.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finn D., De Meulemeester J., Dann L., Herlihy I., Livingstone V., Boylan G.B., et al. Respiratory adaptation in term infants following elective caesarean section. Arch Dis Child Fetal Neonatal Ed. 2018;103:F417–F421. doi: 10.1136/archdischild-2017-312908. [DOI] [PubMed] [Google Scholar]
- 21.Blank D.A., Gaertner V.D., Kamlin C.O.F., Nyland K., Eckard N.O., Dawson J.A., et al. Respiratory changes in term infants immediately after birth. Resuscitation. 2018;130:105–110. doi: 10.1016/j.resuscitation.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Reanimación del Recién Nacido . Sociedad Española de Neonatología. 3rd ed. Ergón Editores; Madrid: 2015. Grupo de Reanimación. [Google Scholar]
- 23.Advanced Life Diagnostics Germany. http://www.lifediag.com/ Accessed August 1, 2020.
- 24.Mortola J.P., Fisher J.T., Smith J.B., Fox G.S., Weeks S., Willis D. Onset of respiration in infants delivered by cesarean section. J Appl Physiol Respir Environ Exerc Physiol. 1982;52:716–724. doi: 10.1152/jappl.1982.52.3.716. [DOI] [PubMed] [Google Scholar]
- 25.te Pas A.B., Wong C., Kamlin C.O.F., Dawson J.A., Morley C.J., Davis P.G. Breathing patterns in preterm and term infants immediately after birth. Pediatr Res. 2009;65:352–356. doi: 10.1203/PDR.0b013e318193f117. [DOI] [PubMed] [Google Scholar]
- 26.Marsh M.J., Ingram D., Milner A.D. The effect of instrumental dead space on measurement of breathing pattern and pulmonary mechanics in the newborn. Pediatr Pulmonol. 1993;16:316–322. doi: 10.1002/ppul.1950160508. [DOI] [PubMed] [Google Scholar]
- 27.Amirav I., Luder A.S., Halamish A., Marzuk C., Daitzchman M., Newhouse M.T. Computerized dead-space volume measurement of face masks applied to simulated faces. Respir Care. 2015;60:1247–1251. doi: 10.4187/respcare.03813. [DOI] [PubMed] [Google Scholar]
- 28.Shah S.A., Berlinski A.B., Rubin B.K. Force-dependent static dead space of masks used with holding chambers. Respir Care. 2006;51:140–144. [PubMed] [Google Scholar]
- 29.Advanced Life Diagnostics Germany Pulmochart—Scientific Respiratory Data Analysis. http://www.pulmochart.net/index.php Accessed September 11, 2020.
- 30.Dassios T., Kaltsogianni O., Greenough A. Determinants of pulmonary dead space in ventilated newborn infants. Early Hum Dev. 2017;108:29–32. doi: 10.1016/j.earlhumdev.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Dassios T., Dixon P., Hickey A., Fouzas S., Greenough A. Physiological and anatomical dead space in mechanically ventilated newborn infants. Pediatr Pulmonol. 2018;53:57–63. doi: 10.1002/ppul.23918. [DOI] [PubMed] [Google Scholar]
- 32.Vilstrup C.T., Bjorklund L.J., Werner O., Larsson A. Lung volumes and pressure-volume relations of the respiratory system in small ventilated neonates with severe respiratory distress syndrome. Pediatr Res. 1996;39:127–133. doi: 10.1203/00006450-199601000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Quanjer P.H., Tammeling G.J., Cotes J.E., Pedersen O.F., Peslin R., Yernault J. Lung volumes and forced ventilatory flows. Eur Respir J Suppl. 1993;16:5–40. doi: 10.1183/09041950.005s1693. [DOI] [PubMed] [Google Scholar]
- 34.Koenker R. Quantile regression for longitudinal data. J Multivariate Anal. 2004;91:74–89. [Google Scholar]
- 35.Bürkner P.C. bmrs. An R package for Bayesian multilevel models using stan. J Stat Soft. 2017;80:1. [Google Scholar]
- 36.Kondo T., Matsumoto I., Lanteri C.J., Sly P.D. Respiratory mechanics during mechanical ventilation: a model study on the effects of leak around a tracheal tube. Pediatr Pulmonol. 1997;24:423–428. doi: 10.1002/(sici)1099-0496(199712)24:6<423::aid-ppul7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 37.Mian Q.N., Pichler G., Binder C., O'Reilly M., Aziz K., Urlesberger B., et al. Tidal volumes in spontaneously breathing preterm infants supported with continuous positive airway pressure. J Pediatr. 2014;165:702–706.e1. doi: 10.1016/j.jpeds.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 38.Milner A.D., Saunders R.A., Hopkin I.E. Effects of delivery by caesarean section on lung mechanics and lung volume in the human neonate. Arch Dis Child. 1978;53:545–548. doi: 10.1136/adc.53.7.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vyas H., Milner A.D., Hopkin I.E. Intrathoracic pressure and volume changes during the spontaneous onset of respiration in babies born by cesarean section and by vaginal delivery. J Pediatr. 1981;99:787–791. doi: 10.1016/s0022-3476(81)80412-x. [DOI] [PubMed] [Google Scholar]
- 40.Schmölzer G.M., Hooper S.B., Wong C., Kamlin C.O., Davis P.G. Exhaled carbon dioxide in healthy term infants immediately after birth. J Pediatr. 2015;166:844–849.e3. doi: 10.1016/j.jpeds.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Karlberg P. The adaptive changes in the immediate postnatal period, with particular reference to respiration. J Pediatr. 1960;56:585–604. doi: 10.1016/s0022-3476(60)80332-0. [DOI] [PubMed] [Google Scholar]
- 42.Herber-Jonat S., von Bismarck P., Freitag-Wolf S., Nikischin W. Limitation of measurements of expiratory tidal volume and expiratory compliance under conditions of endotracheal tube leaks. Pediatr Crit Care Med. 2008;9:69–75. doi: 10.1097/01.PCC.0000298660.16328.BA. [DOI] [PubMed] [Google Scholar]
- 43.O’Donnell C.P.F., Kamlin C.O.F., Davis P.G., Morley C.J. Neonatal resuscitation 1: a model to measure inspired and expired tidal volumes and assess leakage at the face mask. Arch Dis Child Fetal Neonatal Ed. 2005;90:F388–F391. doi: 10.1136/adc.2004.064683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silasi M., Coonrod D.V., Kim M., Drachman D. Transient tachypnea of the newborn: is labor prior to cesarean delivery protective? Am J Perinatol. 2010;27:797–802. doi: 10.1055/s-0030-1254549. [DOI] [PubMed] [Google Scholar]
- 45.Alderdice F., McCall E., Bailie C., Craig S., Dornan J., McMillen R., et al. Admission to neonatal intensive care with respiratory morbidity following 'term' elective caesarean section. Ir Med J. 2005;98:170–172. [PubMed] [Google Scholar]
- 46.Seaborn T., Simard M., Provost P.R., Piedboeuf B., Tremblay Y. Sex hormone metabolism in lung development and maturation. Trends Endocrinol Metab. 2010;21:729–738. doi: 10.1016/j.tem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 47.te Pas A.B., Davis P.G., Hooper S.B., Morley C.J. From liquid to air: breathing after birth. J Pediatr. 2008;152:607–611. doi: 10.1016/j.jpeds.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 48.Kuypers K.L.A.M., Lamberska T., Martherus T., Dekker J., Böhringer S., Hooper S.B., et al. The effect of a face mask for respiratory support on breathing in preterm infants at birth. Resuscitation. 2019;144:178–184. doi: 10.1016/j.resuscitation.2019.08.043. [DOI] [PubMed] [Google Scholar]