Abstract
Background
Radix Paeoniae Alba is a traditional Chinese herbal medicine. It can accelerate salivary secretion and alleviate the dry mouth of patients with Sjogren’s syndrome (SS). Although it is widely used in clinical treatment, its target and mechanism remain unclear.
Objective
This study aims to analyze the main components of Radix Paeoniae Alba, explore the target genes, and propose the possible mechanism for Radix Paeoniae Alba’s acceleration of salivary secretion.
Methods
The main active components and potential targets of Radix Paeoniae Alba were searched through the TCMSP database. Efforts were made to search for the related genes of Sjogren’s syndrome in OMIM and GeneCards databases. Cytoscape v3.8.0 software was used to link target genes of active components and key genes of the disease. The software Autodock vina1.1.2. was adopted to simulate the interaction between active components and target genes. Human submandibular gland (HSG) cells were used in vitro experiments to verify the results of our analysis.
Results
β-Sitosterol, the main component of Radix Paeoniae Alba, may intervene in the disease through CHRM3. Molecular docking shows β-Sitosterol has a high affinity with CHRM3, and the interaction between CHRM3 and β-Sitosterol is the basis of biological activity. The in vitro experiments showed that β-Sitosterol could significantly up-regulate the mRNA and protein expression levels of both CHRM3 and secretion-related genes in HSG cells.
Conclusion
Our study shows that the chemical components of Radix Paeoniae Alba have a positive effect on the related mechanism of salivary secretion. We found that β-Sitosterol can promote the expression of CHRM3, stimulate salivary secretion, treat Sjogren’s syndrome and potentially improve its prognosis.
Keywords: Sjogren’s syndrome, radix paeoniae alba, β-Sitosterol, CHRM3, network pharmacology, xerostomia
1. INTRODUCTION
Sjogren’s syndrome (SS) is a chronic autoimmune disease. The patient's exocrine gland is infiltrated by immune cells, resulting in the destruction of the secretory function of the gland. The clinical manifestations are mainly xerostomia and xerophthalmia [1]. The incidence rate of SS is 0.3% ~ 3%, leading it to be the second-highest disease incidence rate among rheumatic immune diseases [2].
As a Traditional Chinese Medicine, Radix Paeoniae Alb has been used to treat inflammation and immune disorders for more than 1,000 years in China [3]. Research has found that Radix Paeoniae Alb alleviates SS-like symptoms by ameliorating inflammatory infiltration and cytokine production in vivo [4]. Total glucosides of Radix Paeoniae Alb (TGP) have been proven effective in treating inflammatory and autoimmune diseases [5]. In previous studies, we found that TGP can increase intestinal probiotics and inhibit the growth of pathogenic bacteria [6]. We also demonstrated that TGP could increase AQP5 expression in the submandibular gland of non-obese diabetic (NOD) mice, regulate the aquaporin pathway, increase salivary secretion and relieve dry mouth symptoms [7].
So far, there has been no specific drug available to treat SS patients. Although hydroxychloroquine is commonly used to treat SS, there is no direct evidence to prove its effectiveness [8]. In clinical treatment, we found that Radix Paeoniae Alba can effectively alleviate the symptoms of SS patients. The efficacy of Radix Paeoniae Alba is equivalent to that of hydroxychloroquine in treating SS patients but with a preferred safety profile. Clinical trials have confirmed that TGP can reduce serum gamma globulin and increase salivary and tear secretion [9]. A randomized, double-blinded, placebo-controlled clinical trial was conducted on 45 patients with pSS. The mean EULAR SS patient reported index (ESSPRI) in the dry-mouth portion of the questionnaire in SS patients was significantly reduced following TGP treatment. Furthermore, the concentrations of TNF-α, IFN-γ, and erythrocyte sedimentation rate (ESR) decreased in the TGP group [10]. The researchers synthesized the results of seven clinical randomized controlled trials and showed that TGP combined with immunosuppressant showed greater efficacy for improving the saliva flow test of pSS compared to immunosuppressant alone [11].
Based on the previous research, our group considered whether other active components of Radix Paeoniae Alba beyond TGP could promote saliva secretion and potentially treat SS. This study aimed at screening common target genes of Radix Paeoniae Alba and SS through the database and excavate and elucidate the components and possible mechanism of Radix Paeoniae Alba in the treatment of SS. Furthermore, we aimed to carry out experimental verification to provide a new basis and possibility for the treatment of SS.
2. MATERIALS AND METHODS
2.1. Network Pharmacology Analysis
2.1.1. Screening the Main Components of Radix Paeoniae Alba
OB (oral bioavailability) ≥30 and DL (drug symbol) ≥0.18 were used as screening conditions to obtain the main components of Radix Paeoniae Alba in the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP http://tcmspw.com/tcmsp.php) [12].
2.1.2. Obtaining and Analyzing the Target Genes of the Main Components
We searched the target genes of the main components of Radix Paeoniae Alba in two databases and summarized these genes. The databases retrieved include: Swiss Target Prediction (http://www.swisstargetprediction.ch/) [13] PubChem (https://pubchem.ncbi.nlm.nih.gov/) [14] and TCMSP (https://tcmspw.com/tcmsp.php) [12]. Targets were converted into gene symbols by Uniprot (http://www.uniprot.org/) [15].
2.1.3. Calculating and Screening Important Active Components
The resulting file of Ingredients-Targets was imported into Cytoscape v3.8.0 software [16, 17], and the plugin cytoNCA was used to calculate the degree centrality (DC). The active components with a high correlation with target genes were screened according to their DC value.
2.1.4. Searching Target Genes of SS
“Sjogren’s syndrome” was used as a keyword to search for relevant SS targets in OMIM (https://omim.org/) [18] and GeneCards databases (https://www.genecards.org/) [19].
2.1.5. D-I-G-D Network Construction
Related files were established of ”drug ingredients,” “ingredients targets,” and “disease targets.” Files were imported into Cytoscape v3.8.0 to build a “drug-ingredients-gene symbols-disease” network.
2.2. Molecular Docking
2.2.1. Main Components of Radix Paeoniae Alba: Construction of the Small Molecular Model
According to the CAS number of the small molecule, we downloaded the 3D structure of small molecules in mol2 format from ZINC (chttp://zinc.docking.org/) [20]. We then imported it into ChemBio3D ultra 14.0 for energy minimization, set the minimum RMS gradient to 0.001, and saved the small molecules in “mol2” format. The optimized small molecules were imported into AutodockTools v1.5.6 for hydrogenation, charged calculation, charged distribution, set the rotatable key, and saved in “pdbqt” format.
2.2.2. Common Target Genes of Diseases and Traditional Chinese Medicine Components: Construction of the Protein Model
We downloaded the key target proteins from PDB (http://www.rcsb.org/) [21]. We used PyMOL 2.3.0 to remove protein crystal water, original ligands, etc. Then, imported the protein structure into Autodock tools v1.5.6 for hydrogenation, charged calculation, charged distribution, and atom assignment and saved it in ”pdbqt” format.
2.2.3. Computational Validation
The two common targets we screened belong to the same Ingredients. To explore and determine the interaction between the active Ingredient and the protein target, we established molecular docking for them by CB-Dock (http://clab.labshare.cn/cb-dock/) [22, 23] and AutoDock Vina1.1.2 [24].
3. EXPERIMENTAL
3.1. Cell Culture
HSG cells (TongPai, Shanghai, China) were cultured in DMEM/F12 medium (11320033, Thermo, USA) containing 10% FBS and 1% double-antibody and placed in a 37°C and 5% CO2 cell incubator. The solution was changed every 2-3 days and then digested and subcultured when the cells grew to 80% confluency.
3.2. CCK-8 Screening Drug Concentration
HSG cells were divided into groups according to different drug concentrations: 0µM, 5µM, 10µM, 20µM, 40µM, 80µM, 120µM, 160µM. After treatment with different concentrations for 24 hours and 48 hours, cell proliferation was detected using the CCK8 method, and the absorbance was measured at 450nm to calculate the cell survival rate.
3.3. qRT-PCR
Total RNA was extracted and isolated using Trizol reagent (Invitrogen, Thermo Fisher Scientific, Braunschweig, Germany) according to the manufacturer’s instructions. RNA was converted into cDNA using the FastKing gDNA Dispelling RT SuperMix (K2118-02, Tiangen Biotech, Beijing, China). qRT-PCR was accomplished using SYBR Green PCR Master Mix according (A4004M, Lifeint, China) to the manufacturer’s instructions. GADPH was used as an internal reference. The relative expression level was computed using the 2-∆∆Ct method. Gene-specific primers are given as follows in Table 1.
Table 1.
Target gene primers.
| GENE | Primer | Sequence (5’- -3) |
|---|---|---|
| CHRM3 | Forward | TGCTACATCAACAGCACCGT |
| Reverse | TGCGCGCTTGTGAAAAATGA | |
| Gq | Forward | GATCAACGACGAGATCGAGCG |
| Reverse | GACCTTTGGCCCCCTACATC | |
| AMY1 | Forward | TGGTTGCTTTTCACCATTGGG |
| Reverse | AACCCCTCCAAATCCCTTGG | |
| PRB1 | Forward | GGAGGCAACAAGCCCC |
| Reverse | GGGGAGGTCTGGAAGGTCT | |
| AQP5 | Forward | TCATGAATCGGTTCAGCCCC |
| Reverse | CCTTTGATGATGGCCACACG | |
| GAPDH | Forward | GACCCGTGCTGCTTTCTTGA |
| Reverse | ATGGGTGGAGTCGTACTGGA |
3.4. Western Blotting
Western blotting was conducted under standard procedures. HSG cells were lysed to obtain proteins using RIPA (P0010S, Beyotime, CHINA). Proteins were loaded onto SDS-PAGE (10-15%) (P0015A, Beyotime, CHINA) gels, separated electrophoretically, and transferred to PVDF (FFP24, Beyotime, CHINA) membranes. After blocking with 5% dried skim milk (P0216, Beyotime, CHINA) for 3h at room temperature, the membranes were incubated overnight at 4°C with primary antibodies against anti-muscarinic acetylcholine receptor M3 (CHRM3) and aquaporin related genes (both from Abcam, USA). After primary incubation, the membranes were washed, then incubated with goat anti-rabbit IgG(H+L) labeled secondary antibodies (ab205718, Abcam, USA). The immunoreactive protein bands were visualized using an ECL kit. The images were acquired using a Bio-Spectrum Gel Imaging System (UVP, USA), and the intensity of the protein bands was quantified and normalized to that of GAPDH.
3.5. Data Processing
All data were presented in the form of mean ± SD. One-way ANOVA compared the differences between groups. All statistical analysis was completed using the GraphPad 7.0 software. A p-value less than 0.05 denotes a statistical difference between the groups.
4. RESULTS
4.1. Network Pharmacology and Molecular Docking
4.1.1. Active Components and Targets of Radix Paeoniae Alba
Thirteen active components and 123 predicted targets were obtained from the TCMSP database, as shown in Table 2. According to the topological analysis of IngredientsTargets, the degree of Ingredients was obtained. Among them, BS6 (kaempferol) and BS7 (β-Sitosterol) had the largest degree and were the most associated genes, as shown in Fig. (1a and Table 3).
Table 2.
Main Components of Radix Paeoniae Alba.
| Molecule Name | OB (%) | DL |
|---|---|---|
| paeoniflorgenone | 87.59 | 0.37 |
| paeoniflorin_qt | 68.18 | 0.4 |
| albiflorin_qt | 66.64 | 0.33 |
| 11alpha,12alpha-epoxy-3beta-23-dihydroxy-30-norolean-20-en-28,12beta-olide | 64.77 | 0.38 |
| Mairin | 55.38 | 0.78 |
| (+)-catechin | 54.83 | 0.24 |
| paeoniflorin | 53.87 | 0.79 |
| Lactiflorin | 49.12 | 0.8 |
| (3S,5R,8R,9R,10S,14S)-3,17-dihydroxy-4,4,8,10,14-pentamethyl-2,3,5,6,7,9-hexahydro-1H-cyclopenta[a]phenanthrene-15,16-dione | 43.56 | 0.53 |
| kaempferol | 41.88 | 0.24 |
| β-Sitosterol | 36.91 | 0.75 |
| sitosterol | 36.91 | 0.75 |
| benzoyl paeoniflorin | 31.27 | 0.75 |
Fig. (1).
The results of network pharmacology prediction. (a) Topological analysis of target genes of active components of Radix Paeoniae Alba. Radix Paeoniae Alba was expressed by BS. The 8 active components of Radix Paeoniae Alba were expressed in BS1-BS8 (Table 2). The deeper the color, the higher the correlation between active components and target genes. (b) Venn diagram of SS-related genes. The blue and purple sets are SS-related genes obtained in GeneCards and OMIM databases, respectively. (c) D- I-G-D Network Analysis. Purple represents the target gene of the active ingredient of Radix Paeoniae Alba, blue represents the SS-related gene, and red represents the common gene of SS and the active ingredient of Radix Paeoniae Alba.
Table 3.
Ingredients-targets topological analysis.
| No. | Type | Molecule Name | Degree |
|---|---|---|---|
| 1 | BS6 | kaempferol | 63 |
| 2 | BS7 | β-Sitosterol | 38 |
| 3 | BS3 | (+)-catechin | 12 |
| 4 | BS4 | paeoniflorin | 5 |
| 5 | BS8 | sitosterol | 4 |
| 6 | BS5 | (3S,5R,8R,9R,10S,14S)-3,17-dihydroxy-4,4,8,10,14-pentamethyl-2,3,5,6,7,9-hexahydro-1H-cyclopenta[a]phenanthrene-15,16-dione | 3 |
| 7 | BS2 | Mairin | 2 |
| 8 | BS1 | paeoniflorgenone | 2 |
4.1.2. Target Gene of Sjogren’s syndrome
1038 and 630 target genes were obtained from genecards and omim databases. Moreover, after the intersection, a total of 14 disease targets were obtained and are shown in Fig. (1b): ATP13A2, CASP8, CD46, CFH, CHRM3, COL4A3, CTLA4, FASLG, HADHA, HOXD13, HSPG2, LBR, LMNA, NOTCH2.
4.1.3. D-I-G-D Network Analysis
There are two common target genes of the main components of Radix Paeoniae Alba and SS, namely CHRM3 and CASP8, as shown in Fig. (1c). Both belong to β-Sitosterol. Combined with the analysis results, we speculate that βSitosterol, the main component of Radix Paeoniae Alba, may be an essential part of the treatment of SS.
4.1.4. Ingredient-Targets Interactions
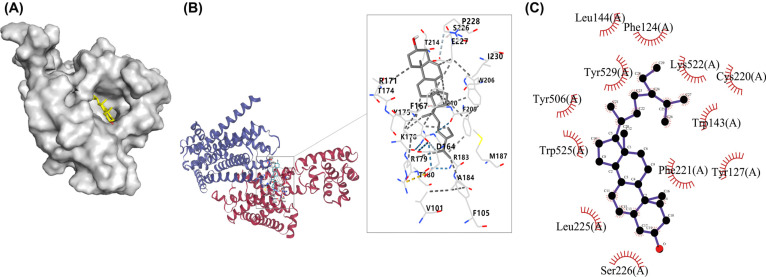
There are 5 docking structures between β-Sitosterol and CHRM3. The structure with the highest score of Vina was selected for visualization by CB-Dock, as shown in Table (4 and Fig 2b). The results of AutoDock Vina1.1.2. showed that β-Sitosterol had a good affinity with CHRM3, as shown in Figs. (2a and c). Based on the docking results, we suggest that the interaction between CHRM3 and β-Sitosterol is the basis of biologic activity.
Table 4.
Vina score of β-Sitosterol and CHRM3.
| No. | Vina Score | Cavity Size | Center | Center | ||||
| x | y | z | x | y | z | |||
| 1 | -9.5 | 1259 | 30 | 74 | 49 | 25 | 25 | 25 |
Fig. (2).
The binding modes of β-Sitosterol and CHRM3. a) Spatial structure of protein and small molecule binding. b) The structure with the highest score of Vina. c) The bond between protein and small molecules.
4.2. Experimental Verification In Vitro
4.2.1. Cell Viability
The absorbance of cells at 450mm was used to judge the effect of different concentrations of β-sitosterol on cell activity, as shown in Figs. (3a and b). Under the condition of 24 hours of administration, the cell survival rate of the administration group was greater than 90%, as shown in Fig. (3c). When administered for 48 hours, the survival rate of cells decreased significantly, as shown in Fig. (3d). Therefore, three groups of cells with concentrations of 5µM, 10µM, and 20µM were treated for 24 hours as the administration time.
Fig. (3).
Experimental results of CCK-8 screening drug concentration. Absorbance of cells at 450nm under different administration concentrations: (a) 24 hours, (b) 48 hours; Cell viability under different administration concentrations: (c) 24 hours, (d) 48 hours; * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001.
4.2.2. The Effect of βate-Sitosterol on mRNA Expression
Through PCR experiments, we measured the mRNA expression of water channel-related genes in HSG cells under the intervention of β-sitosterol. Compared with the normal group, the expression of CHRM3, Gq, AMY1, PRB1, and AQP5 increased (P<0.05). The mRNA of the CHRM3 gene was also up-regulated with an increase in dosage, but there was no significant increase in group 20 μM compared to the 10μM group, as shown in Fig. (4a). The mRNA of AMY1 and AQP5 were positively correlated with the dose, and the increase in each group was statistically significant(P<0.01, as shown in Figs. (4b and c). Furthermore, the increasing trend of Gq gene mRNA expression gradually weakened with the increased dosage. There was no significant increase in group 20μM compared to the 10μM group Fig. (4d). Likewise, the mRNA expression of the PRB1 gene in each group was not statistically significant except compared with the 5μM Fig. (4e).
Fig.(4).
The effect of β-Sitosterol on mRNA expression of the target gene. a) CHRM3; b) AQP5; c) AMY1; d) Gq; e) PRB1. Compared with control group: * P<0.05, ** P<0.01; Compared with group 5µM: # P<0.05, ## P<0.01; Compared with group 10µM: ∆ P<0.05.
4.2.3. The Effect of Bate-Sitosterol on Protein Expression
We next conducted WBs to explore the protein production of channel-related genes in HSG cells with β-sitosterol treatment. Protein expression was calculated according to the protein development concentration on the band, as shown in Fig (4f). Compared with the control group, the protein expression of CHRM3, Gq, AMY1, PRB1, and AQP5 were significantly increased (P<0.01). In contrast, the CHRM3 gene was
up-regulated with an increased dose, but no significant increase in protein expression was observed in the 20μM group compared to the 10μM group (Fig. 5a). The protein of AMY1 and AQP5 were positively correlated with the dose, and the increase in each group was statistically significant, as shown in Figs. (5b and c). The increasing trend of Gq gene protein expression gradually weakened with an increased dose. Additionally, there was no significant increase in the 20 μM group compared to the 10μM group, as shown in Fig. (5d). The protein expression of the PRB1 gene in each group was not statistically significant except compared with the 5μM groups, as shown in Fig. (5e).
Fig. (5).
The effect of β-Sitosterol on protein expression of the target gene. (a) CHRM3; (b) AQP5; (c) AMY1; (d) Gq; (e) PRB1. Compared with control group: * P<0.05, ** P<0.01,*** P<0.001; Compared with group 5µM: # P<0.05, ## P<0.01; Compared with group 10µM: ∆P<0.05.
5. DISCUSSION
SS is an autoimmune disease, in which antibodies, such as SS-A and SS-B, can be detected. Likewise, studies have found that patients with SS carry inhibitory autoantibodies, such as anti-CHRM3, which targets the CHRM3 protein [25]. Notably, the CHRM3 antibody has been found to decrease the salivary secretions of severe combined immunodeficient (SCID) mice lacking adaptive immunity and may play a role in SS [26]. This study's network pharmacological analysis results showed that CHRM3 is a common target gene of SS and Radix Paeoniae Alba. Likewise, several studies have shown that the expression of CHRM3 increased in the salivary glands of patients with SS or the submandibular glands of NOD mice, relating to compensatory up-regulation due to the increase of CHRM3 antibodies [27]. Muscarinic cholinergic receptors belong to the G protein-coupled receptor family and have five subtypes of membrane proteins. The intracellular part binds to GTP to regulate, interact, and activate proteins (G-proteins). CHRM3 interacts with Gq type G protein, and the activated form of the G protein induces many intracellular signal transduction systems [28]. The extracellular part of the Muscarinic cholinergic receptors binds to acetylcholine (ACh). The binding of CHRM3 to acetylcholine forms different muscarinic types, which determines the diversity of its functions. CHRM3 can affect many functions of acetylcholine in the central and peripheral nervous system [29]. The combination of CHRM3 and acetylcholine causes the increase of intracellular calcium (Ca2+), and the high intracellular calcium concentration stimulates the transport of AQP5 to the cell membrane to improve cell permeability to water and promote salivary secretions [30]. Importantly, AQP5 is a well-understood protein that plays a crucial role in salivary secretion. Animal experiments show that under the intervention of pilocarpine, saliva hypertonic and salivary viscosity increased in AQP5-null mice, which showed a decrease in salivary secretion [31]. Additionally, studies have shown that salivary secretion is closely related to the water permeability of acinar cells. In AQP5-null mice, the abnormal water permeability of acinar cells induced a significant decrease in salivary secretion (more than 60%), suggesting that AQP5 plays an important role in regulating water permeability and salivary secretion [30]. Furthermore, AMY1 and PRB1 have been proved to be important proteins in saliva. AMY1 is the most important enzyme secreted by the salivary gland [32]. It helps break down carbohydrates to produce monosaccharides such as dextrin and maltose, which are further broken down into simpler sugars in the small intestine for absorption into the blood. PRB1 is the most heterogeneous proline-rich protein in human salivary proteins, and previous studies have shown that their expression increases during salivary gland secretion [33]. Therefore, this study used them to verify the secretion and physiological function of saliva.
According to the results of network pharmacology, β- Sitosterol is the component associated with the SS disease-related protein, CHRM3. We previously confirmed that Radix Paeoniae Alba could increase salivary secretion in NOD mice [7]. In this investigation, we verified that β-Sitosterol could significantly increase the protein and mRNA expression of CHRM3, AQP5, Gq, AMY1, and PRB1. Under the condition of cell survival rate>90%, the expression of CHRM3 may be dose-dependent, which indicates that the β-Sitosterol can promote salivary secretion. Compared with other genes, AMY1 and AQP5 are more sensitive to dose. Thus, we consider that β-Sitosterol binds to CHRM3 and activates the regulation of CHRM3 on Ca2+. The increase of intracellular Ca2+ leads to AQP5 transport to the cell membrane and promotes the transport of water molecules to increase salivary secretion.
Extensive in vitro studies have illustrated that β-sitosterol exerts cholesterol-lowering [34], antioxidant [35], anti-inflammatory [36, 37], and anticancer [38] effects. Recent studies have found that the pro-inflammatory mediators together with cardiovascular disease are more prevalent in pSS patients [39], and patients with SS have a higher incidence rate of lipid metabolism dysfunction [40]. Research has suggested that serum cholesterol is closely related to salivary cholesterol level, and a high salivary cholesterol level will affect salivary secretions and oral health [41]. Importantly, It has been confirmed that β- Sitosterol can reduce serum cholesterol levels and control inflammatory mediators [42]. In addition to regulating water channel-related genes,β- sitosterol may also inhibit salivary gland inflammation and treat SS by reducing salivary cholesterol levels. However, this inference needs further experimental verification.
Notably, investigators have identified multiple residues: Gln207, Gly211, Arg213, Gly218, Ile222, Phe224, Leu225, and Pro228 in the second extracellular structure of the CHRM3, which may play an important role in the activation of the protein [43]. Leu225, located in the A chain, is connected with β-Sitosterol. It is hypothesized that the hydrophobic force between β-Sitosterol may activate receptors by binding residues of CHRM3 to stimulate AQP5 transport, promote salivary secretion, and improve the symptoms of dry mouth. However, this hypothesis needs further experimental verification.
CONCLUSION
Our network pharmacology and experimental verification findings suggest that β-Sitosterol, an active component of Radix Paeoniae Alba, likely stimulates AQP5 transport to the cell membrane by binding CHRM3 residue activating proteins, increasing water permeability of acinar cells, and promoting water penetration and salivary secretions. Therefore, β-Sitosterol can increase the salivary secretion of patients with SS, improve the physiological function of saliva, and potentially play a therapeutic effect on the typical symptoms of dry mouth associated with the disease.
ACKNOWLEDGEMENTS
Special thanks to Geshuo Wang for providing guidance on article typesetting. The authors thank AiMi Academic Services (www.aimieditor.com) for the English language editing and review services.
LIST OF ABBREVIATIONS
- NOD
Non-Obese Diabetic
- TGP
Totalglucosides of Radix Paeoniae Alb
- SS
Sjogren’s Syndrome
- HSG
Human Submandibular Gland
- SGECs
Salivary Gland Epithelial Cells
- Ach
Acetylcholin
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
All data used to support the findings of this study are available from the corresponding author upon request. The datasets generated and analysed during the current study are available in several databases, the web link to the datasets was shown in the manuscript. TCMSP (http://tcmspw.com/tcmsp.php); Swiss Target Prediction (http://www.swisstarget prediction.ch/); PubChem (https://pubchem.ncbi.nlm.nih.gov/); Uniprot (http://www.uniprot.org/); OMIM (https://omim.org/); GeneCards (https://www.genecards.org/); ZINC (chttp://zinc.docking.org/); PDB(http://www.rcsb.org/); CB-Dock (http://clab.labshare.cn/cb-dock/);
FUNDING
The study was supported by National Natural Science Foundation of China (No. 81473604) and Natural Science Foundation of Zhejiang Province, China (No. LY19H270013).
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
- 1.Parisis D., Chivasso C., Perret J., Soyfoo M.S., Delporte C. Current state of knowledge on primary Sjögren’s syndrome, an autoimmune exocrinopathy. J. Clin. Med. 2020;9(7):2299. doi: 10.3390/jcm9072299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivino F.B., Carsons S.E., Foulks G., Daniels T.E., Parke A., Brennan M.T., Forstot S.L., Scofield R.H., Hammitt K.M. New treatment guidelines for Sjögren’s disease. Rheum. Dis. Clin. North Am. 2016;42(3):531–551. doi: 10.1016/j.rdc.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L., Wei W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2020;207:107452. doi: 10.1016/j.pharmthera.2019.107452. [DOI] [PubMed] [Google Scholar]
- 4.Li B., Liu G., Liu R., He S., Li X., Huang L., Wang Z., Li Y., Chen Y., Yin H., Fang W. Total glucosides of paeony (TGP) alleviates Sjogren’s syndrome through inhibiting inflammatory responses in mice. Phytomedicine. 2020;71:153203. doi: 10.1016/j.phymed.2020.153203. [DOI] [PubMed] [Google Scholar]
- 5.Yang X.Z., Wei W. CP-25, a compound derived from paeoniflorin: research advance on its pharmacological actions and mechanisms in the treatment of inflammation and immune diseases. Acta Pharmacol. Sin. 2020;41(11):1387–1394. doi: 10.1038/s41401-020-00510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu W.W., Fu T.X., Wang Q., Chen Y.L., Li T.Y., Wu G.L. The effect of total glucoside of paeony on gut microbiota in NOD mice with Sjögren’s syndrome based on high-throughput sequencing of 16SrRNA gene. Chin. Med. 2020;15:61. doi: 10.1186/s13020-020-00342-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu G.L., Pu X.H., Yu G.Y., Li T.Y. Effects of total glucosides of peony on AQP-5 and its mRNA expression in submandibular glands of NOD mice with Sjogren’s syndrome. Eur. Rev. Med. Pharmacol. Sci. 2015;19(1):173–178. [PubMed] [Google Scholar]
- 8.Gottenberg J.E., Ravaud P., Puéchal X., Le Guern V., Sibilia J., Goeb V., Larroche C., Dubost J.J., Rist S., Saraux A., Devauchelle-Pensec V., Morel J., Hayem G., Hatron P., Perdriger A., Sene D., Zarnitsky C., Batouche D., Furlan V., Benessiano J., Perrodeau E., Seror R., Mariette X. Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome: The JOQUER randomized clinical trial. JAMA. 2014;312(3):249–258. doi: 10.1001/jama.2014.7682. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H.F., Hou P., Xiao W.G. Clinical observation on effect of total glucosides of paeony in treating patients with non-systemic involved Sjögren syndrome. Chung Kuo Chung Hsi I Chieh Ho Tsa Chih. 2007;27(7):596–598. [PubMed] [Google Scholar]
- 10.Zhou Y., Jin L., Kong F., Zhang H., Fang X., Chen Z., Wang G., Li X., Li X. Clinical and immunological consequences of total glucosides of paeony treatment in Sjögren’s syndrome: A randomized controlled pilot trial. Int. Immunopharmacol. 2016;39:314–319. doi: 10.1016/j.intimp.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Jin L., Li C., Li Y., Wu B. Clinical efficacy and safety of total glucosides of paeony for primary Sjögren’s syndrome: A systematic review. Evid. Based Complement. Alternat. Med. 2017;2017:3242301. doi: 10.1155/2017/3242301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ru J., Li P., Wang J., Zhou W., Li B., Huang C., Li P., Guo Z., Tao W., Yang Y., Xu X., Li Y., Wang Y., Yang L. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daina A., Michielin O., Zoete V. Swiss Target Prediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47(W1):W357–W364. doi: 10.1093/nar/gkz382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu G., Batchelor C., Dumontier M., Hastings J., Willighagen E., Bolton E. PubChemRDF: Towards the semantic annotation of PubChem compound and substance databases. J. Cheminform. 2015;7:34. doi: 10.1186/s13321-015-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UniProt Consortium. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47(D1):D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otasek D., Morris J.H., Bouças J., Pico A.R., Demchak B. Cytoscape automation: Empowering workflow-based network analysis. Genome Biol. 2019;20(1):185. doi: 10.1186/s13059-019-1758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amberger J.S., Bocchini C.A., Scott A.F., Hamosh A. OMIM.org: Leveraging knowledge across phenotype-gene relationships. Nucleic Acids Res. 2019;47(D1):D1038–D1043. doi: 10.1093/nar/gky1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safran M., Dalah I., Alexander J., Rosen N., Iny Stein T., Shmoish M., Nativ N., Bahir I., Doniger T., Krug H., Sirota-Madi A., Olender T., Golan Y., Stelzer G., Harel A., Lancet D. Gene cards version 3: The human gene integrator. Database (Oxford) 2010;2010:baq020. doi: 10.1093/database/baq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterling T., Irwin J.J. ZINC 15 - Ligand discovery for everyone. J. Chem. Inf. Model. 2015;55(11):2324–2337. doi: 10.1021/acs.jcim.5b00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.wwPDB consortium. Protein data bank: The single global archive for 3D macromolecular structure data. Nucleic Acids Res. 2019;47(D1):D520–D528. doi: 10.1093/nar/gky949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y., Grimm M., Dai W.T., Hou M.C., Xiao Z.X., Cao Y. CB-Dock: A web server for cavity detection-guided protein-ligand blind docking. Acta Pharmacol. Sin. 2020;41(1):138–144. doi: 10.1038/s41401-019-0228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trott O., Olson A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawson L., Tobin A., Smith P., Gordon T. Antimuscarinic antibodies in Sjögren’s syndrome: Where are we, and where are we going? Arthritis Rheum. 2005;52(10):2984–2995. doi: 10.1002/art.21347. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen K.H., Brayer J., Cha S., Diggs S., Yasunari U., Hilal G., Peck A.B., Humphreys-Beher M.G. Evidence for antimuscarinic acetylcholine receptor antibody-mediated secretory dysfunction in nod mice. Arthritis Rheum. 2000;43(10):2297–2306. doi: 10.1002/1529-0131(200010)43:10<2297:AID-ANR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 27.Gresz V., Horvath A., Gera I., Nielsen S., Zelles T. Immunolocalization of AQP5 in resting and stimulated normal labial glands and in Sjögren’s syndrome. Oral Dis. 2015;21(1):e114–e120. doi: 10.1111/odi.12239. [DOI] [PubMed] [Google Scholar]
- 28.Haga T. Molecular properties of muscarinic acetylcholine receptors. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 2013;89(6):226–256. doi: 10.2183/pjab.89.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J., Ha Y.M., Kü N.Y., Choi S.Y., Lee S.J., Oh S.B., Kim J.S., Lee J.H., Lee E.B., Song Y.W., Park K. Inhibitory effects of autoantibodies on the muscarinic receptors in Sjögren’s syndrome. Lab. Invest. 2004;84(11):1430–1438. doi: 10.1038/labinvest.3700173. [DOI] [PubMed] [Google Scholar]
- 30.Krane C.M., Melvin J.E., Nguyen H.V., Richardson L., Towne J.E., Doetschman T., Menon A.G. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J. Biol. Chem. 2001;276(26):23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- 31.Ma T., Song Y., Gillespie A., Carlson E.J., Epstein C.J., Verkman A.S. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J. Biol. Chem. 1999;274(29):20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 32.Janeček Š., Svensson B., MacGregor E.A. α-Amylase: An enzyme specificity found in various families of glycoside hydrolases. Cell. Mol. Life Sci. 2014;71(7):1149–1170. doi: 10.1007/s00018-013-1388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manconi B., Castagnola M., Cabras T., Olianas A., Vitali A., Desiderio C., Sanna M.T., Messana I. The intriguing heterogeneity of human salivary proline-rich proteins: Short title: Salivary proline-rich protein species. J. Proteomics. 2016;134:47–56. doi: 10.1016/j.jprot.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Hwang S.L., Kim H.N., Jung H.H., Kim J.E., Choi D.K., Hur J.M., Lee J.Y., Song H., Song K.S., Huh T.L. Beneficial effects of β-sitosterol on glucose and lipid metabolism in L6 myotube cells are mediated by AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2008;377(4):1253–1258. doi: 10.1016/j.bbrc.2008.10.136. [DOI] [PubMed] [Google Scholar]
- 35.Wong H.S., Chen N., Leong P.K., Ko K.M. β-sitosterol enhances cellular glutathione redox cycling by reactive oxygen species generated from mitochondrial respiration: Protection against oxidant injury in H9c2 cells and rat hearts. Phytother. Res. 2014;28(7):999–1006. doi: 10.1002/ptr.5087. [DOI] [PubMed] [Google Scholar]
- 36.Shi C., Luo X., Wang J., Long D. Incorporation of β-sitosterol into the membrane prevents tumor necrosis factor-α-induced nuclear factor-κB activation and gonadotropin-releasing hormone decline. Steroids. 2015;96:1–6. doi: 10.1016/j.steroids.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Lampronti I., Dechecchi M.C., Rimessi A., Bezzerri V., Nicolis E., Guerrini A., Tacchini M., Tamanini A., Munari S., D’Aversa E., Santangelo A., Lippi G., Sacchetti G., Pinton P., Gambari R., Agostini M., Cabrini G. β-sitosterol reduces the expression of chemotactic cytokine genes in cystic fibrosis bronchial epithelial cells. Front. Pharmacol. 2017;8:236. doi: 10.3389/fphar.2017.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajavel T., Packiyaraj P., Suryanarayanan V., Singh S.K., Ruckmani K., Pandima Devi K. β-sitosterol targets Trx/Trx1 reductase to induce apoptosis in A549 cells via ROS mediated mitochondrial dysregulation and p53 activation. Sci. Rep. 2018;8(1):2071. doi: 10.1038/s41598-018-20311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berardicurti O., Ruscitti P., Cipriani P., Ciccia F., Liakouli V., Guggino G., Carubbi F., Di Benedetto P., Triolo G., Giacomelli R. Cardiovascular disease in primary Sjögren’s syndrome. Rev. Recent Clin. Trials. 2018;13(3):164–169. doi: 10.2174/1574887113666180315130336. [DOI] [PubMed] [Google Scholar]
- 40.Lodde B.M., Sankar V., Kok M.R., Leakan R.A., Tak P.P., Pillemer S.R. Serum lipid levels in Sjögren’s syndrome. Rheumatology (Oxford) 2006;45(4):481–484. doi: 10.1093/rheumatology/kei190. [DOI] [PubMed] [Google Scholar]
- 41.Karjalainen S., Sewón L., Söderling E., Larsson B., Johansson I., Simell O., Lapinleimu H., Seppänen R. Salivary cholesterol of healthy adults in relation to serum cholesterol concentration and oral health. J. Dent. Res. 1997;76(10):1637–1643. doi: 10.1177/00220345970760100401. [DOI] [PubMed] [Google Scholar]
- 42.Cheng Y., Chen Y., Li J., Qu H., Zhao Y., Wen C., Zhou Y. Dietary β-sitosterol regulates serum lipid level and improves immune function, antioxidant status, and intestinal morphology in broilers. Poult. Sci. 2020;99(3):1400–1408. doi: 10.1016/j.psj.2019.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scarselli M., Li B., Kim S.K., Wess J. Multiple residues in the second extracellular loop are critical for M3 muscarinic acetylcholine receptor activation. J. Biol. Chem. 2007;282(10):7385–7396. doi: 10.1074/jbc.M610394200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support the findings of this study are available from the corresponding author upon request. The datasets generated and analysed during the current study are available in several databases, the web link to the datasets was shown in the manuscript. TCMSP (http://tcmspw.com/tcmsp.php); Swiss Target Prediction (http://www.swisstarget prediction.ch/); PubChem (https://pubchem.ncbi.nlm.nih.gov/); Uniprot (http://www.uniprot.org/); OMIM (https://omim.org/); GeneCards (https://www.genecards.org/); ZINC (chttp://zinc.docking.org/); PDB(http://www.rcsb.org/); CB-Dock (http://clab.labshare.cn/cb-dock/);