Abstract
Background
There are only a few epidemiological reports available for reference. The clinicopathological features are not clear, so there is no consensus on treating rectal multiple neuroendocrine neoplasms. This study aims to summarize the clinicopathological characteristics and preliminarily discuss the clinical diagnosis and treatment of rectal multiple neuroendocrine neoplasms.
Methods
This study retrospectively analyzed rectal neuroendocrine neoplasm patients diagnosed and treated at the Fourth Hospital of Hebei Medical University from February 2007 to May 2021. The clinicopathological characteristics of rectal multiple neuroendocrine neoplasms were summarized and analyzed in combination with 14 studies on rectal multiple neuroendocrine neoplasms.
Results
The incidence of RM-NENs accounted for 3.8% of all R-NENs in this study. The number of tumors varied to some extent, the size of tumors was basically no more than 10 mm, and there were more G1 grade tumors. In the analysis of 46 cases with known lymph node metastasis, the difference in lymph node metastasis rate between the number of tumors < 8 and ≥ 8 was statistically significant (p = 0.002).
Conclusions
The incidence of rectal multiple neuroendocrine neoplasms accounted for 3.8% of all rectal neuroendocrine neoplasms. For rectal multiple neuroendocrine neoplasms, the lymph node metastasis rate was higher when the number of tumors was ≥ 8. The influence of the number of tumors on lymph node metastasis should be considered in the selection of treatment.
Keywords: Rectal neuroendocrine neoplasm, Rectal multiple neuroendocrine neoplasms, Lymph node metastasis
Introduction
In recent years, the incidence of rectal neuroendocrine neoplasms (R-NENs) has shown an obvious increasing trend, which is probably related to the progress of clinical medicine, the improvement of health awareness and the popularity of colonoscopy [1]. Meanwhile, the disease has gradually gained people’s attention. It has been reported that R-NENs are more common in Asian populations [2] and have become the second-most common neuroendocrine tumors in China [3].
R-NENs usually present as submucosal lesions and yellow mucosa [4, 5]. The treatment and prognosis of rectal single neuroendocrine neoplasm (RS-NEN) are relatively well known [6]. The prognosis of patients with small RS-NEN without lymph node metastasis or distant metastasis is favorable. Although the tumors in the majority of R-NEN cases are single focal tumors, there have been reports of multiple focal tumors [7–11]. According to the existing statistics, the incidence of rectal multiple neuroendocrine neoplasms (RM-NENs) is 2% ~ 5.7% [8, 12], indicating the rarity of the disease. Unlike RS-NEN, there are no standard guidelines for the treatment of RM-NENs. Furthermore, the prognosis of patients with RM-NENs is still uncertain. Several studies have reported favorable short-term results after the endoscopic resection of RM-NENs smaller than 10 mm [13, 14]. Due to the small number of cases, only a few epidemiological reports are available for reference, and the clinicopathological features are not clear. It is also difficult to conclude whether the presence of multiple tumors is associated with lymph node metastasis. In summary, there is no consensus on the treatment of RM-NENs.
This study summarized the clinicopathological characteristics of RM-NENs in patients at our center, reviewed literature reports, and preliminarily discussed the clinical diagnosis and treatment of RM-NENs.
Methods
Clinical data collection
This study retrospectively analyzed R-NEN patients diagnosed and treated at the Fourth Hospital of Hebei Medical University from February 2007 to May 2021. The exclusion criteria were as follows: 1) R-NENs combined with other types of colorectal cancer and 2) R-NENs combined with other malignant tumors. This project was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (ID: 2021KS002).
Literature search
A comprehensive search strategy was adopted to include as many relevant studies as possible. The PubMed and Chinese academic publication websites were searched for articles published from inception until May 2021. The search term combinations were Medical Subject Heading (MeSH) terms, text words, and variants of neuroendocrine tumors, neuroendocrine neoplasms, carcinoid, rectal, and multiple. The reference lists of all retrieved articles were searched manually for other possible studies. In the retrieved articles, the cases of RM-NENs were recorded, and duplicate cases were excluded.
The clinicopathological characteristics of RM-NENs were summarized and analyzed in combination with 14 studies on RM-NENs.
Data analysis
Statistical analysis was performed using IBM SPSS Statistics V. 25.0.0 (IBM Corp, New York). Continuous variables are expressed as the mean ± standard deviation (SD) or median and interquartile range, and statistical analysis was performed using a t test. Other data are expressed as numbers and percentages and were analyzed by the chi-square test. Two-tailed p values were used for all statistical tests, and P values < 0.05 were considered statistically significant.
Results
In total, 183 patients with R-NENs were diagnosed between February 2007 and May 2021, including 176 patients (96.2%) with RS-NEN and 7 patients (3.8%) with RM-NENs.
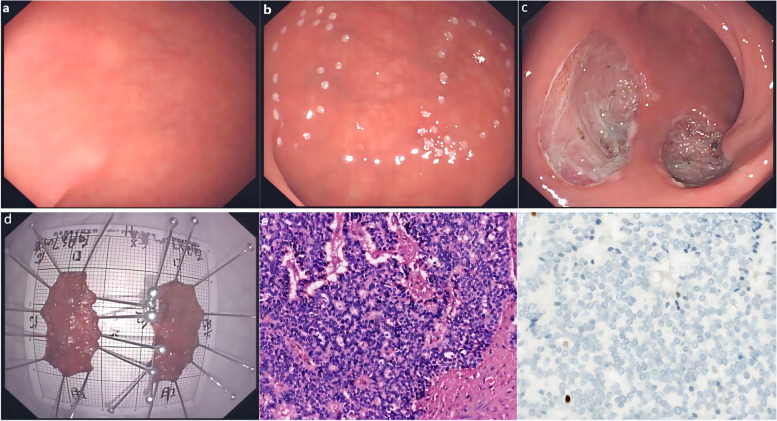
Among the patients with RM-NENs, there were 4 males (57.1%) and 3 females (42.9%), with a median age of 49 (46–69) years; there was a total of 17 tumors, including 2 tumors in 6 cases and 5 tumors in 1 case, all of which were less than 10 mm in size. All tumors invaded the submucosa. No lymph node metastasis or distant metastasis was found in the preoperative examination of any patient. All tumors were completely resected by endoscopic resection (Fig. 1). Thirteen tumors were grade G1, 2 were G2, and 2 was ungraded. The median follow-up was 92 (32 ~ 132) months. No tumor recurrence or metastasis was discovered on follow-up (follow-up ended in May 2021).
Fig. 1.
Endoscopic resection of RM-NENs: a Five tumors found in the rectum. b Marked preresection area. c Wound after tumor resection. d Fixed specimens. e HE staining (10×). f Ki-67 index of 1% (20×)
Combined with cases in the 14 reviewed studies, there were 47 cases of RM-NENs (Table 1). Among these patients (Table 2), 29 were males (61.7%), and 18 were females (38.3%), aged between 32 and 81 years. The tumor size was generally less than 10 mm, and the number of tumors ranged from 2 to 69. Thirty-four patients (72.3%) had fewer than 8 tumors, and 13 patients (27.7%) had more than 8 tumors. In 45 cases (95.7%), tumors invaded the submucosa, and in 2 cases (4.3%), tumors were confined to the mucosa. There were 29 (61.7%) patients with grade G1 tumors, 3 (6.4%) patients with grade G2 tumors, and 15 (31.9%) patients with ungraded tumors. There were 8 patients (17.0%) with lymph node metastasis, 38 patients (80.9%) without lymph node metastasis, and 1 patient (2.1%) with an unclear lymph node metastasis status. There were 44 patients (93.6%) without distant metastasis and 3 (6.4%) with an unclear distant metastasis status. In terms of the tumor treatment plan, follow-up was performed in 1 case (2.1%), the treatment was unspecified in 1 case (2.1%), and surgical excision (the specific method could not be determined) was performed in the remaining cases (95.8%).
Table 1.
A bibliographic listing of RM-NEN reports
| NO. | Cases | Gender | Age | Tumor Number | Size (mm) | Depth | Histology | Lymph node metastasis | Distant metastasis | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
MICHAEL KANTER et al. [9] (1987, USA) |
M | 50 | 17 | < 10 | SM | carcinoid | Yes | No | Surgery |
| 2 |
Sung Sil PARK et al. [10] (2018, Korea) |
M | 57 | 12 | 1–10 | SM | G1 | Yes | No | Surgery |
| 3 |
Masashi Haraguchi et al. [15] (2007, Japan) |
M | 69 | 30 | < 10 | SM | carcinoid | No | No | Surgery |
| 4 |
Kevin A. Ghassemi et al. [16] (2009, USA) |
F | 53 | 6 | 2–3 | SM | carcinoid | No | No | Follow-up |
| 5 |
Shunichi Sasou et al. [7] (2012, Japan) |
M | 51 | 69 | < 8 | SM | G1 | Yes | No | Surgery |
| 6 |
Shunichi Sasou et al. [7] (2012, Japan) |
M | 58 | 62 | < 7 | MP | G2 | Yes | No | Surgery |
| 7 |
Chan Seo Park et al. [17] (2014, Korea) |
M | 52 | 4 | 4 | SM | G1 | No | No | EMR-L |
| 8 |
Chan Seo Park et al. [17] (2014, Korea) |
M | 32 | 3 | 5–7 | SM | G1 | No | No | EMR-L |
| 9 |
Chan Seo Park et al. [17] (2014, Korea) |
F | 65 | 3 | 5–7 | SM | NET | No | No | EMR |
| 10 |
Chan Seo Park et al. [17] (2014, Korea) |
M | 62 | 2 | 5 | SM | G1 | No | No | EMR-L |
| 11 |
Chan Seo Park et al. [17] (2014, Korea) |
F | 48 | 2 | - | SM | G1 | No | No | EMR-L |
| 12 |
Jiao-Lin Zhou et al. [18] (2015, China) |
M | 47 | 3 | 5–8 | SM | G1 | No | No | TEM |
| 13 |
Bai Hua et al. [19] (2016, China) |
F | 61 | > 10 | 3–10 | SM | G1 | No | No | Surgery |
| 14 |
Momoko Doi et al. [20] (2016, Japan) |
M | 61 | 42 | 1–6 | SM | G1 | No | No | Surgery |
| 15 |
Momoko Doi et al. [20] (2016, Japan) |
M | 61 | 36 | 1–5 | SM | G1 | No | No | Surgery |
| 16 |
Rui Xie et al. [21] (2018, China) |
F | 39 | dense | 3–25 | SM | G1 | Yes | No | Follow-up |
| 17 |
M Kato et al. [22] (1986, Japan) |
M | 61 | 52 | 1–6 | SM | - | - | - | - |
| 18 |
Maruyama M et al. [8] (1988) |
M | 53 | 5 | 4–10 | MP | - | No | - | Surgery |
| 19 |
Okamoto Y et al. [23] (2004, Japan) |
M | 54 | 4 | < 6 | SM | - | No | - | EMR-L |
| 20 |
Mine (2019, China) |
M | 46 | 2 | 5–6 | SM | G1 | No | No | ESD |
| 21 |
Mine (2011, China) |
F | 49 | 2 | 4–8 | SM | NET | No | No | EMR-C |
| 22 |
Mine (2012, China) |
F | 50 | 2 | 6–10 | SM | G1 | No | No | EMR-C |
| 23 |
Mine (2014, China) |
F | 48 | 2 | 4–10 | SM | G1 | No | No | EMR-C |
| 24 |
Mine (2010, China) |
F | 48 | 2 | 6–8 | SM | G2 | No | No | EMR-C |
| 25 |
Mine (2018, China) |
M | 69 | 2 | 4–8 | SM | G1 | No | No | ESD |
| 26 |
Mine (2014, China) |
M | 53 | 5 | 3–8 | SM | G1 | No | No | ESD |
| 27–47 |
Yusuke Nishikawa et al. [12] (2019, Japan) |
M = 12 F = 9 |
59 (42–81) |
2–27 | < 10 | SM |
G1 = 13 G2 = 1 |
Yes = 3 No = 18 |
No | ER\Surgery |
Table 2.
Clinicopathological features of 47 patients with RM-NENs
| Factors | Cases (n = 47) |
|---|---|
| Gender | |
| Male | 29 (61.7%) |
| Female | 18 (38.3%) |
| Age, years, range | 32–81 |
| Tumor number, range | 2–69 |
| < 8 | 34 (72.3%) |
| ≥ 8 | 13 (27.7%) |
| Tumor size, mm | ≤ 10 |
| Infiltration depth | |
| SM | 45 (95.7%) |
| Muscularis propria | 2 (4.3%) |
| Histology | |
| Unknown | 15 (31.9%) |
| G1 | 29 (61.7%) |
| G2 | 3 (6.4%) |
| Lymph node metastasis | |
| Unknown | 1 (2.1%) |
| No | 38 (80.9%) |
| Yes | 8 (17.0%) |
| Distant metastasis | |
| Unknown | 3 (6.4%) |
| No | 44 (93.6%) |
| Yes | 0 (0) |
In the analysis of 46 cases of known lymph node metastasis (Table 3), there was a significant difference in the lymph node metastasis rate between those with < 8 and ≥ 8 tumors (p = 0.002).
Table 3.
Risk factors for lymph node metastasis in RM-NENs
| Factors | Cases (n = 46) | Lymph node metastasis (n = 8) | p-value |
|---|---|---|---|
| Gender | 0.453 | ||
| Male | 28 | 6 (21.4%) | |
| Female | 18 | 2 (11.1%) | |
| Tumor number | 0.002 | ||
| < 8 | 34 | 2 (5.9%) | |
| ≥ 8 | 12 | 6 (50%) | |
| Infiltration depth | 0.321 | ||
| SM | 44 | 7 (15.9%) | |
| Muscularis propria | 2 | 1(50%) | |
| Histology | 0.099 | ||
| Unknown | 14 | 1 (7.1%) | |
| G1 | 29 | 5 (17.2%) | |
| G2 | 3 | 2 (66.7%) |
Discussion
R-NENs are hindgut tumors. The pathogenesis of hindgut NENs has not been elucidated, especially at the molecular level. Studies suggest that endocrine cells in the crypt proliferate, infiltrate or migrate to the lamina propria, muscularis mucosae and submucosa and may develop into carcinoid cells, which may be multipotent [7, 8]. Pathological examination of R-NENs has shown that they are not multipotent. In addition, patients with rectal neuroendocrine tumors usually only have one, and only 2%-5.7% of patients have multiple tumors [8, 12]. In our study, the incidence of RM-NENs among R-NEN patients was 3.8%, which is consistent with literature reports. Previous studies have suggested that the MEN1 gene and PI3-K/AKT, Raf/MEK/ERK, Notch, GSK-3β and other signaling pathways may be involved in the occurrence and metastasis of multiple rectal tumors [24]. It has also been reported that RM-NENs may be associated with inflammatory bowel disease [25]. Hiripi et al. [26] reported a significantly increased risk of NENs in individuals with a parental history of such tumors. Momoko Do et al. [20] reported multiple neuroendocrine tumors in identical twins at the same location (rectum), suggesting that the occurrence of tumors is related to genetic background.
Clinical evidence [27, 28] has shown that when the size of an RS-NEN is less than 10 mm, lymph node metastasis is rarely observed, and the metastasis rate is less than 10%. However, when the R-NEN diameter is larger than 20 mm, the lymph node metastasis rate can be as high as 60%-80%, and the distant metastasis rate can be up to 40%. Surgical excision is considered the most appropriate treatment. Standard surgical methods [14, 29–37] include endoscopic submucosal dissection (ESD), endoscopic mucosal resection (EMR), transanal resection of the mass, and surgical resection. The choice of surgical method depends on the tumor size, depth of invasion, regional lymph nodes, distant metastasis, and malignancy. Meanwhile, endoscopic ultrasonography (EUS) [38–40] can be used to determine the size, depth of invasion and metastasis status of adjacent lymph nodes and detect whether a submucosal mass is separate from the muscularis propria, which is crucial in deciding the treatment plan and evaluating the stability of endoscopic resection. However, since EUS cannot be used to determine the distant lymph node or liver metastasis status, abdominal CT, MRI, and PET-CT examinations are required to evaluate distant metastasis.
Due to the rarity of RM-NENs compared to RS-NEN, there are no standard treatment guidelines. In addition, the long-term prognosis of RM-NENs patients after endoscopic resection remains uncertain. However, treatment can be performed based on the size and depth of infiltration of each R-NEN. Multiple tumors were successfully resected by endoscopy in 7 patients, with an excellent short-term prognosis and no local recurrence. However, due to the slow growth of R-NENs, it is difficult to evaluate the long-term efficacy of or prognosis after endoscopic resection [17].
In small intestinal carcinoid tumors, polycentricity is a poor prognostic factor [41, 42]. However, its prognostic effect in R-NENs is unclear. It has been reported that the incidence of lymph node metastasis is very high in patients with multiple tumors, regardless of tumor size and pathological grade [20]. Yusuke Nishikawa et al. [12] showed in a single-center retrospective analysis that the overall lymph node metastasis rate of RM-NENs was 14.3% and increased to 33.3% when the number of tumors was ≥ 8. In our study, lymph node metastasis occurred in 17% of patients, but no distant metastasis was observed. This is also an extremely rare situation; due to the extensive proliferation of neuroendocrine cells in the rectum, the number of tumor lesions could not be estimated because they could not be seen by the naked eye. Therefore, surgical resection was required [7, 8]. However, how can the number of tumors in RM-NENs patients be determined? There is no size criterion to distinguish neuroendocrine tumors from endocrine cell micronests (ECMs). According to the judgment of senior pathology teachers, 7 cases in our center did not have ECMs. Based on the description and picture information in the literature reviewed, we conclude that there may be 10 cases of suspected ECMs, which needs to be carefully judged. In upper gastrointestinal neuroendocrine tumors, lesions larger than 0.5 mm meeting the immunohistochemical criteria can be diagnosed as tumors. In gastric neuroendocrine tumors, tumor cells mainly develop from gastric mucosa intestinal chromaffin cells [43]. Gastrinemia induces the proliferation of intestinal chromaffin cells, leading to type I gastric neuroendocrine tumors, which are prone to be accompanied by ECMs [44–48]. Because cases of R-NENs with ECMs are so rare, it is not clear whether treatment for these ECMs is needed. Maruyama et al. [8, 48] described the possible origin of ECMs for R-NENs; however, it is not clear whether glandular endocrine cells are derived from the neuroectoderm along the nerve fibers or endoderm stem cells. It has also been speculated that ECMs can be regarded as the initial or intermediate stage of carcinoid development [8]. In addition, it has previously been reported that ECMs might be a marker of the presence of multiple carcinoid and lymph node metastases [7, 10]. In contrast, Wong et al. [25] argued that ECMs do not appear to develop into neuroendocrine tumors and may not require further clinical examination or invasive procedures, including endoscopic resection, during surveillance and noted that inflammatory bowel disease is prone to cause neuroendocrine cell proliferation. Sho Suzuki et al. [44] reported that multiple ECMs existed around an RS-NEN lesion. No lymph node metastasis or distant metastasis was found on CT examination 6 years after endoscopic resection. Because case reports of multiple ECMs are very rare, many aspects of ECMs remain unclear to date, and the significance of malignancy is unclear; thus, further studies are needed to confirm the role of ECMs.
It is unclear whether the biology and behavior of RM-NENs are consistent with those of the largest lesion, as a cumulative tumor burden, or whether the number of tumors affects prognosis. The treatment plan should be individualized in each case, and careful follow-up is needed. Clinicians should aim to improve the understanding of this disease, which requires early detection and treatment.
Of course, there are some limitations to our study. First, this was a single-center retrospective study, and there may have been bias in the selection of cases. Second, the sample size was relatively small. Therefore, a large prospective randomized controlled trial is needed to investigate RM-NENs.
In conclusion, RM-NENs accounted for 3.8% of all R-NENs in this study. The number of tumors varied to some extent, most tumors were no more than 10 mm in size, and there were more grade G1 tumors. For RM-NENs, the lymph node metastasis rate was higher when the number of tumors was ≥ 8. The influence of the number of tumors on lymph node metastasis should be considered in the selection of treatment.
Acknowledgements
We thank all the subjects of this study for their participation.
Abbreviations
- R-NEN
Rectal neuroendocrine neoplasm
- RM-NENs
Rectal multiple neuroendocrine neoplasms
- RS-NEN
Rectal single neuroendocrine neoplasm
- EMR
Endoscopic mucosal resection
- ESD
Endoscopic submucosal dissection
- EUS
Endoscopic ultrasonography
- ECMs
Endocrine cell micronests
Authors’ contributions
Xiuli Zheng, Mingli Wu, Shengmian Li designed the study; Xiuli Zheng, Limian Er, Shuo Guo, Huiyan Deng and Zhihuan Liu collected and analyzed the data; Xiuli Zheng wrote the main manuscript. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Funding
There was no funding for this study.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request without breaching participant confidentiality.
Declarations
Ethics approval and consent to participate
Our study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (ID: 2021KS002). All methods were carried out in accordance with relevant guidelines and regulations. Written informed consent was obtained from all patients before performing any endoscopic procedures. Informed consent was obtained from all participants in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hu P, Bai JA, Liu M, Xue J, Chen T, Li R, Kuai X, Zhao H, Li X, Tian Y, et al. Trends of incidence and prognosis of gastric neuroendocrine neoplasms: a study based on seer and our multicenter research. Gastric Cancer. 2020;23(4):591–599. doi: 10.1007/s10120-020-01046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konishi T, Watanabe T, Muto T, Kotake K, Nagawa H. Site distribution of gastrointestinal carcinoids differs between races. Gut. 2006;55(7):1051–1052. [PMC free article] [PubMed] [Google Scholar]
- 3.Fan JH, Zhang YQ, Shi SS, Chen YJ, Yuan XH, Jiang LM, Wang SM, Ma L, He YT, Feng CY, et al. A nation-wide retrospective epidemiological study of gastroenteropancreatic neuroendocrine neoplasms in china. Oncotarget. 2017;8(42):71699–71708. doi: 10.18632/oncotarget.17599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jetmore AB, Ray JE, Gathright JJ, Mcmullen KM, Hicks TC, Timmcke AE. Rectal carcinoids: the most frequent carcinoid tumor. Dis Colon Rectum. 1992;35(8):717–725. doi: 10.1007/BF02050318. [DOI] [PubMed] [Google Scholar]
- 5.Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128(6):1717–1751. doi: 10.1053/j.gastro.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 6.Ramage JK, De Herder WW, Delle Fave G, Ferolla P, Ferone D, Ito T, Ruszniewski P, Sundin A, Weber W, Zheng-Pei Z, et al. Enets consensus guidelines update for colorectal neuroendocrine neoplasms. Neuroendocrinology. 2016;103(2):139–143. doi: 10.1159/000443166. [DOI] [PubMed] [Google Scholar]
- 7.Sasou S, Suto T, Satoh T, Tamura G, Kudara N. Multiple carcinoid tumors of the rectum: report of two cases suggesting the origin of carcinoid tumors. Pathol Int. 2012;62(10):699–703. doi: 10.1111/j.1440-1827.2012.02852.x. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama M, Fukayama M, Koike M. A case of multiple carcinoid tumors of the rectum with extraglandular endocrine cell proliferation. Cancer. 1988;61(1):131–136. doi: 10.1002/1097-0142(19880101)61:1<131::AID-CNCR2820610123>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 9.Kanter M, Lechago J. Multiple malignant rectal carcinoid tumors with immunocytochemical demonstration of multiple hormonal substances. Cancer. 1987;60(8):1782–1786. doi: 10.1002/1097-0142(19871015)60:8<1782::AID-CNCR2820600819>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 10.Sung Sil Park NHJL Multiple small rectal neuroendocrine tumors with numerous micronests. J Dig Dis. 2018;19(9):572–575. doi: 10.1111/1751-2980.12645. [DOI] [PubMed] [Google Scholar]
- 11.Winburn GB. 1998–01–01. Atlanta: Southeastern Surgical Congress; 1998. Multiple rectal carcinoids: a case report; pp. 1200–1203. [PubMed] [Google Scholar]
- 12.Nishikawa Y, Chino A, Ide D, Saito S, Igarashi M, Takamatsu M, Fujisaki J, Igarashi Y. Clinicopathological characteristics and frequency of multiple rectal neuroendocrine tumors: a single-center retrospective study. Int J Colorectal Dis. 2019;34(11):1887–1894. doi: 10.1007/s00384-019-03405-z. [DOI] [PubMed] [Google Scholar]
- 13.Toriyama K, Yamamura T, Nakamura M, Maeda K, Sawada T, Mizutani Y, Ishikawa E, Furukawa K, Ishikawa T, Ohno E, et al. An evaluation of resectability among endoscopic treatment methods for rectal neuroendocrine tumors <10 mm. Arab J Gastroenterol. 2021;22(2):104–110. doi: 10.1016/j.ajg.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Zhao S, Zhang C, Ji K, Wu W, Yin L, Yan H, Zhou J, Tang R, Miao L. Endoscopic and surgical treatment of t1n0m0 colorectal neuroendocrine tumors: a population-based comparative study. Surg Endosc. 2022;36(4):2488–2498. doi: 10.1007/s00464-021-08535-6. [DOI] [PubMed] [Google Scholar]
- 15.Haraguchi M, Kinoshita H, Koori M, Tsuneoka N, Kosaka T, Ito Y, Furui J, Kanematsu T. Multiple rectal carcinoids with diffuse ganglioneuromatosis. World J Surg Oncol. 2007;5:19. doi: 10.1186/1477-7819-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghassemi KA, Ou H, Roth BE. Multiple rectal carcinoids in a patient with neurofibromatosis. Gastrointest Endosc. 2010;71(1):216–218. doi: 10.1016/j.gie.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Park CS, Lee SH, Kim SB, Kim KO, Jang BI. Multiple rectal neuroendocrine tumors: report of five cases. Korean J Gastroenterol. 2014;64(2):103. doi: 10.4166/kjg.2014.64.2.103. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Lin G, Zhao D, Zhong G, Qiu H. Resection of multiple rectal carcinoids with transanal endoscopic microsurgery: case report. World J Gastroenterol. 2015;21(7):2220–2224. doi: 10.3748/wjg.v21.i7.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua B, Fandong M, Xun Y. Multiple carcinoid of rectum: a case report and literature review. Zhong Guo Yi Yao Dao Kan. 2016;18(12):1282–1283. [Google Scholar]
- 20.Doi M, Ikawa O, Taniguchi H, Kawamura T, Katsura K. Multiple rectal carcinoid tumors in monozygotic twins. Clin J Gastroenterol. 2016;9(4):215–221. doi: 10.1007/s12328-016-0662-7. [DOI] [PubMed] [Google Scholar]
- 21.Xie R, Fu K, Chen S, Tuo B, Wu H. Neurofibromatosis type 1-associated multiple rectal neuroendocrine tumors: a case report and review of the literature. World J Gastroenterol. 2018;24(33):3806–3812. doi: 10.3748/wjg.v24.i33.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato M, Yonemura Y, Sugiyama K, Hashimoto T, Shima Y, Miyazaki I, Sugiura H, Kurumaya H, Hoso M, Yao T. Multiple rectal carcinoids–with special reference to the histogenesis of these lesions. Gan No Rinsho. 1986;32(14):1894. [PubMed] [Google Scholar]
- 23.Okamoto Y, Fujii M, Tateiwa S, Sakai T, Ochi F, Sugano M, Oshiro K, Masai K, Okabayashi Y. Treatment of multiple rectal carcinoids by endoscopic mucosal resection using a device for esophageal variceal ligation. Endoscopy. 2004;36(5):469–470. doi: 10.1055/s-2004-814386. [DOI] [PubMed] [Google Scholar]
- 24.Ning L, Chen H, Kunnimalaiyaan M. Focal adhesion kinase, a downstream mediator of raf-1 signaling, suppresses cellular adhesion, migration, and neuroendocrine markers in bon carcinoid cells. Mol Cancer Res. 2010;8(5):775–782. doi: 10.1158/1541-7786.MCR-09-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong M, Larson BK, Dhall D. Neuroendocrine proliferations in inflammatory bowel disease: differentiating neuroendocrine tumours from neuroendocrine cell micronests. Histopathology. 2019;74(3):415–423. doi: 10.1111/his.13769. [DOI] [PubMed] [Google Scholar]
- 26.Hiripi E, Bermejo JL, Sundquist J, Hemminki K. Familial gastrointestinal carcinoid tumours and associated cancers. Ann Oncol. 2009;20(5):950–954. doi: 10.1093/annonc/mdn706. [DOI] [PubMed] [Google Scholar]
- 27.Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, Kelestimur F, Plöckinger U, Papotti M, Salazar R, Pascher A. Enets consensus guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95(2):88–97. doi: 10.1159/000335594. [DOI] [PubMed] [Google Scholar]
- 28.Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, Nagawa H. Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut. 2007;56(6):863–868. doi: 10.1136/gut.2006.109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Park YE, Choi JH, Heo NY, Park J, Park SH, et al. Comparison between cap-assisted and ligation-assisted endoscopic mucosal resection for rectal neuroendocrine tumors. Ann Gastroenterol. 2020;33(4):385–90. [DOI] [PMC free article] [PubMed]
- 30.Park SB. Advantage of endoscopic mucosal resection with a cap for rectal neuroendocrine tumors. World J Gastroenterol. 2015;21(31):9387. doi: 10.3748/wjg.v21.i31.9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SJ. Tips and tricks for better endoscopic treatment of colorectal tumors: usefulness of cap and band in colorectal endoscopic mucosal resection. Clin Endosc. 2013;46(5):492. doi: 10.5946/ce.2013.46.5.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SS, Kim BC, Lee DE, Han KS, Kim B, Hong CW, Sohn DK. Comparison of endoscopic submucosal dissection and transanal endoscopic microsurgery for t1 rectal neuroendocrine tumors: a propensity score-matched study. Gastrointest Endosc. 2021;94(2):408–415.e2. doi: 10.1016/j.gie.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 33.He L, Wen W, Ye L, Liao K, Hu B. Endoscopic mucosal resection with double band ligation for small rectal neuroendocrine tumors. Am J Gastroenterol. 2021;116(9):1827–1828. doi: 10.14309/ajg.0000000000001302. [DOI] [PubMed] [Google Scholar]
- 34.Sun P, Zheng T, Hu C, Gao T, Ding X. Comparison of endoscopic therapies for rectal neuroendocrine tumors: endoscopic submucosal dissection with myectomy versus endoscopic submucosal dissection. Surg Endosc. 2021;35(11):6374–6378. doi: 10.1007/s00464-021-08622-8. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Chai N, Linghu E, Li H, Zhai Y, Feng X, Zhang W, Zou J, Li L, Xiang J. Efficacy and safety of hybrid endoscopic submucosal dissection compared with endoscopic submucosal dissection for rectal neuroendocrine tumors and risk factors associated with incomplete endoscopic resection. Ann Transl Med. 2020;8(6):368–368. doi: 10.21037/atm.2020.02.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Chai N, Linghu E, Qiu S, Li L, Zou J, Xiang J, Li X. The outcomes of modified endoscopic mucosal resection and endoscopic submucosal dissection for the treatment of rectal neuroendocrine tumors and the value of endoscopic morphology classification in endoscopic resection. BMC Gastroenterol. 2020;20(1):1–2. doi: 10.1186/s12876-020-01340-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed Y, Othman M. Emr/esd: techniques, complications, and evidence. Curr Gastroenterol Rep. 2020;22(8):1–2. doi: 10.1007/s11894-020-00777-z. [DOI] [PubMed] [Google Scholar]
- 38.Kim MK. Endoscopic ultrasound in gastroenteropancreatic neuroendocrine tumors. Gut Liver. 2012;6(4):405–410. doi: 10.5009/gnl.2012.6.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zilli A, Arcidiacono PG, Conte D, Massironi S. Clinical impact of endoscopic ultrasonography on the management of neuroendocrine tumors: lights and shadows. Dig Liver Dis. 2018;50(1):6–14. doi: 10.1016/j.dld.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Gu Q, Lin Y, Cen L, Xu M, Li H, Lin X, Lu C. Endoscopic ultrasonography is useful in the diagnosis and treatment of rectal neuroendocrine neoplasms: a case series. J Zhejiang Univ Sci B. 2019;20(10):861–864. doi: 10.1631/jzus.B1900168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burke AP, Thomas RM, Elsayed AM, Sobin LH. Carcinoids of the jejunum and ileum: an immunohistochemical and clinicopathologic study of 167 cases. Cancer. 1997;79(6):1086–1093. doi: 10.1002/(SICI)1097-0142(19970315)79:6<1086::AID-CNCR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 42.Lundqvist M, Wilander E. A study of the histopathogenesis of carcinoid tumors of the small intestine and appendix. Cancer. 1987;60(2):201–206. doi: 10.1002/1097-0142(19870715)60:2<201::AID-CNCR2820600214>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 43.Iwai K, Yao T, Nakamura S, Matsumoto T, Nishiyama K, Iida M, Tsuneyoshi M. Multiple gastric carcinoids and endocrine cell micronests in type a gastritis: nuclear morphometric and immunohistochemical analysis. Oncol Rep. 2005;13(3):397. [PubMed] [Google Scholar]
- 44.Suzuki S, Kawakami H, Miike T, Yamamoto S, Abe H, Shimoda K, Ashizuka S, Inatsu H, Kubota Y, Ban T, et al. Single rectal neuroendocrine tumor associated with multiple endocrine cell micronests. Intern Med. 2020;59(5):619–623. doi: 10.2169/internalmedicine.3582-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mastracci L, Rindi G, Grillo F, Solcia E, Campora M, Fassan M, Parente P, Vanoli A, La Rosa S. Neuroendocrine neoplasms of the esophagus and stomach. Pathologica. 2021;113(1):5–11. doi: 10.32074/1591-951X-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishioka M, Hirasawa T, Kawachi H, Nakano K, Kunieda J, Yoshimizu S, Horiuchi Y, Ishiyama A, Yoshio T, Tsuchida T, et al. Enterochromaffin-like cell neuroendocrine tumor associated with parietal cell dysfunction. Gastrointest Endosc. 2019;90(5):841–845.e1. doi: 10.1016/j.gie.2019.06.029. [DOI] [PubMed] [Google Scholar]
- 47.Tsolakis AV, Ragkousi A, Vujasinovic M, Kaltsas G, Daskalakis K. Gastric neuroendocrine neoplasms type 1: a systematic review and meta-analysis. World J Gastroenterol. 2019;25(35):5376–5387. doi: 10.3748/wjg.v25.i35.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itsuno MWHIM. Multiple carcinoids and endocrine cell micronests in type a gastritis. Cancer. 1989;63(5):881–890. doi: 10.1002/1097-0142(19890301)63:5<881::AID-CNCR2820630515>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request without breaching participant confidentiality.