Abstract
Tumor‐associated macrophages (TAMs) are one of the most abundant immunosuppressive cells in the tumor microenvironment and possess crucial functions in facilitating tumor progression. Emerging evidence indicates that altered metabolic properties in cancer cells support the tumorigenic functions of TAMs. However, the mechanisms and mediators the underly the cross‐talk between cancer cells and TAMs remain largely unknown. In the present study, we revealed that high solute carrier family 3 member 2 (SLC3A2) expression in lung cancer patients was associated with TAMs and poor prognosis. Knockdown of SLC3A2 in lung adenocarcinoma cells impaired M2 polarization of macrophages in a coculture system. Using metabolome analysis, we identified that SLC3A2 knockdown altered the metabolism of lung cancer cells and changed multiple metabolites, including arachidonic acid, in the tumor microenvironment. More importantly, we showed that arachidonic acid was responsible for SLC3A2‐mediated macrophage polarization in the tumor microenvironment to differentiate into M2 type both in vitro and in vivo. Our data illustrate previously undescribed mechanisms responsible for TAM polarization and suggest that SLC3A2 acts as a metabolic switch on lung adenocarcinoma cells to induce macrophage phenotypic reprogramming through arachidonic acid.
Keywords: arachidonic acid, lung adenocarcinoma, macrophage polarization, SLC3A2, tumor‐associated macrophage
High SLC3A2 expression in lung adenocarcinoma (LUAD) patients were associated with tumor‐associated macrophages and poor prognosis. SLC3A2 in LUAD cells was essential for macrophage M2 polarization. SLC3A2 regulated metabolic properties in LUAD cells. SLC3A2‐mediated arachidonic acid secretion promoted macrophage M2 polarization.
Abbreviations
- AA
arachidonic acid
- ARG‐1
arginase‐1
- FAO
fatty acid oxidation
- IHC
immunohistochemical
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LUAD
lung adenocarcinoma
- PGE2
prostaglandin E2
- SLC
solute carrier
- SLC3A2
solute carrier family 3 member 2
- TAM
tumor‐associated macrophage
- TCGA
The Cancer Genome Atlas
- TME
tumor microenvironment
1. INTRODUCTION
Macrophages have an essential and indispensable role in homeostasis and immunity, but in the context of cancer they lose their protective functions and become TAMs. 1 Tumor‐associated macrophages are one of the most abundant immunosuppressive innate immune cell populations in the TME. 2 It is well documented that TAMs play a tumor‐promoting role in the growth and invasion of cancer primary and metastatic sites and high TAM levels correlate with aggressive cancer phenotype and disease progression in various types of human cancer. 3 Tumor‐associated macrophages interact with tumor cells and facilitate proliferation, chemoresistance, distance metastasis, and survival of tumor cells. 3 In addition, TAMs contribute to the development of immunosuppressive TME by producing cytokines and chemokines and triggering the exhaustion of T cells. 4 Emerging evidence indicates that altered signaling pathways and metabolic properties support the tumorigenic functions of TAMs. 5 Reprogramming or switching functions from tumorigenic to antitumorigenic TAMs serve as novel therapeutic options of precision medicine in recent years.
Macrophages are highly heterogeneous. Their plasticity enables them to develop distinct adaptation to slight alteration of tissue environments, including nutrients, metabolites, and oxygen, which results in significant diversity of TAMs among different cancer types even within the same tumor. The diversity of macrophages was previously streamlined as the simplified concept of pro‐inflammatory (M1) and alternative (M2) phenotypes. 6 During the early period of inflammation, macrophages transit to the pro‐inflammatory (M1) type through the classical activation pathway to defend against intracellular bacteria or viruses, while in late inflammatory or TME, macrophages polarize to the alternative (M2) type through the alternative activation pathway to help tissue healing and tolerate self‐antigens. 7 Unlike IL‐4/IL‐13‐stimulated M2 macrophages, TAMs are mainly induced by Toll‐like receptor ligands and A2 adenosine receptor agonists or IL‐6. 8 In addition, some noncytokines such as lactic acid, glutamine, and other metabolites also participate in the induction of TAMs. 9 As the metabolic characteristics of TAMs largely depend on the surrounding microenvironment, the mechanisms underly TAM polarization in different tissues are highly contextual and cell‐type dependent. For example, in the early stage of tumor development, TAMs rely on glycolysis to meet their energy demands and produce high concentrations of lactic acid, which further enhances glycolysis by activating the Akt/mTOR pathway. 10 This is very similar to the characteristics of M1 type macrophages that are highly dependent on glycolysis. 8 , 11 However, with the development of tumors, TAMs show low glycolytic rates because of hypoxia induces REDD1 upregulation, which further inhibits mTOR, glucose uptake, and glycolysis, which is similar to M2 type macrophages. In addition, TAMs rely on the uptake and oxidation of fatty acids, with α‐ketoglutarate derived from glutamine as a positive metabolic regulator of FAO. 11 , 12 Further understanding of the mechanisms by which metabolic changes influence TAMs' function is an essential step in developing novel therapeutic approaches for human cancer.
The SLC family is the second largest family of membrane proteins in the human genome. 13 SLC3A2 is the most typical member of this family, encoding CD98hc protein, which forms heterodimeric complexes with light subunits LAT1 and LAT2 to function as an amino acid transporter. 14 SLC3A2 regulates LAT1/LAT2 stability and plasma membrane localization to uptake glutamine and leucine, which regulates the mTORC1 pathway and cellular metabolism. 15 , 16 SLC3A2 is highly expressed in many malignant tumor cells, such as lung cancer, breast cancer, prostate cancer, colorectal cancer, and glioma. 17 , 18 , 19 SLC3A2 is overexpressed in 62.5% of large cell neuroendocrine carcinoma and 50% of small‐cell lung cancer and serves as an independent prognostic factor for thymic epithelial tumors and non‐small‐cell lung cancer. 20 Overexpression of SLC3A2 leads to radiotherapy resistance of tumors, indicating SLC3A2 could become a promising clinical target for tumor therapy. 21 However, whether and how SLC3A2 influences macrophage polarization in TME remains elusive.
In this study, we aimed to elucidate the role of SLC3A2‐mediated metabolism in regulating macrophage polarization in lung cancer. Through the comprehensive analysis of transcriptome and metabolome characteristics, our data showed that SLC3A2 plays an important role in the alternative activation of macrophages through the metabolic reprogramming of lung cancer cells. The current findings present a novel mechanism that controls TAM polarization and reveals SLC3A2 as a pivotal regulatory factor in determining TAM polarization in human lung cancer.
2. MATERIALS AND METHODS
All methods and key reagents can be found in Appendix S1 and Table S1.
3. RESULTS
3.1. Elevated expression of SLC3A2 is associated with poor prognosis in LUAD
To find aberrantly expressed SLC family genes in human cancer, we compared the expression of 422 SLC family genes in tumors and adjacent normal tissues in 17 tumor types in the TCGA database (Figure 1A). According to the expression pattern, SLC genes were divided into three types. Remarkably, type III SLC genes were highly expressed in tumors when compared to adjacent normal tissues, especially in LUAD (Figure 1A). To further screen the prognosis‐related SLC genes, we undertook multivariate Cox survival analysis on type III SLC genes in lung cancer and determined that SLC3A2 was the most relevant risk factor for LUAD prognosis (Figure 1B). Furthermore, high level of SLC3A2 was associated with poor prognosis in lung cancer (Figure 1C,D). In order to verify the clinicopathological relevance of SLC3A2 in LUAD, we performed IHC staining in 51 LUAD patients. As expected, the expression of SLC3A2 in lung cancer was significantly higher than that in paired normal tissues, and SLC3A2 was negatively correlated with the overall survival rate of LUAD (Figure 1E,F). In addition, we found that a few macrophages are also expressed SLC3A2 in tumor adjacent tissues (Figure 1E(b)). In order to further clarify the expression of SLC3A2 in the TME, we undertook multifluorescent microscopy analysis of SLC3A2 and markers of various types of cells in the TME (including the myofibroblast marker α‐SMA, the T cell marker CD3, the B cell marker CD20, and the macrophages marker CD68). The results showed that SLC3A2 was mainly expressed in tumor cells, and rarely expressed in a minority of macrophages (Figure S1).
FIGURE 1.
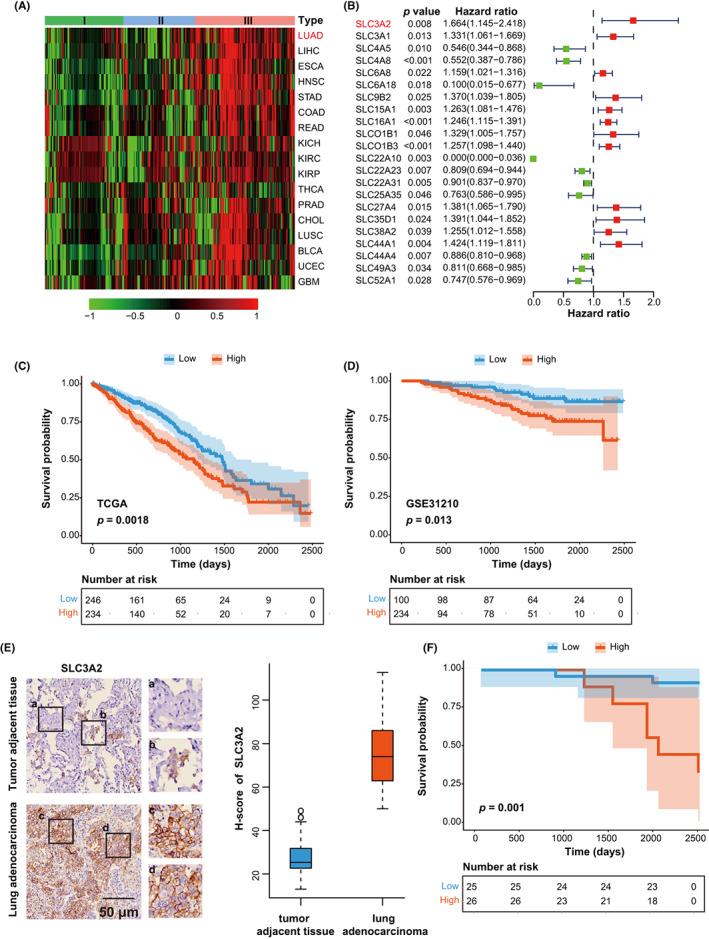
SLC3A2 is significantly highly expressed and associated with poor prognosis in lung adenocarcinoma (LUAD). (A) Expression heatmap of 422 SLC family genes in 17 human cancers. Each grid in the heatmap represents the log2 logarithm of the ratio of the expression of this gene in the tumor and the adjacent normal tissue. SLC family genes are classified into types I–III according to the ratio from low to high. (B) Multivariate Cox analysis results of type III SLC family genes in LUAD. Hazard ratio greater than 1 indicates a risk factor and is associated with poor prognosis. (C, D) Kaplan Meier survival curve of The Cancer Genome Atlas (TCGA) (C) and GSE31210 (D). Patients are divided into high and low expression groups according to the median expression of SLC3A2. (E) Immunohistochemical staining and quantification of SLC3A2 in LUAD and adjacent tissues. Representative images are enlarged in the right panel. (a) Normal lung epithelial cells. (b) Macrophages. (c,d) LUAD cells. Scale bar, 50 μm; n = 51. (F) Kaplan–Meier survival curve of 51 LUAD patients. Patients were divided into high and low expression groups according to the median expression of SLC3A2 based on immunohistochemical staining
3.2. SLC3A2 is essential for proliferation, migration, and invasion of LUAD cells
To investigate the role of SLC3A2 on LUAD cells, we knocked down SLC3A2 in A549 and H1299 cells by lentiviral transduction of shRNA (Figure 2A). SLC3A2 knockdown markedly impaired LUAD cell proliferation (Figure 2B). Wound healing assay showed that the knockout of SLC3A2 resulted in a significant reduction in cell migration ability (Figure 2C). Moreover, the Transwell assay indicated that the downregulation of SLC3A2 significantly repressed the invasion of LUAD cells (Figure 2D). Overall, the results indicated that SLC3A2 is critical for LUAD proliferation and migration.
FIGURE 2.
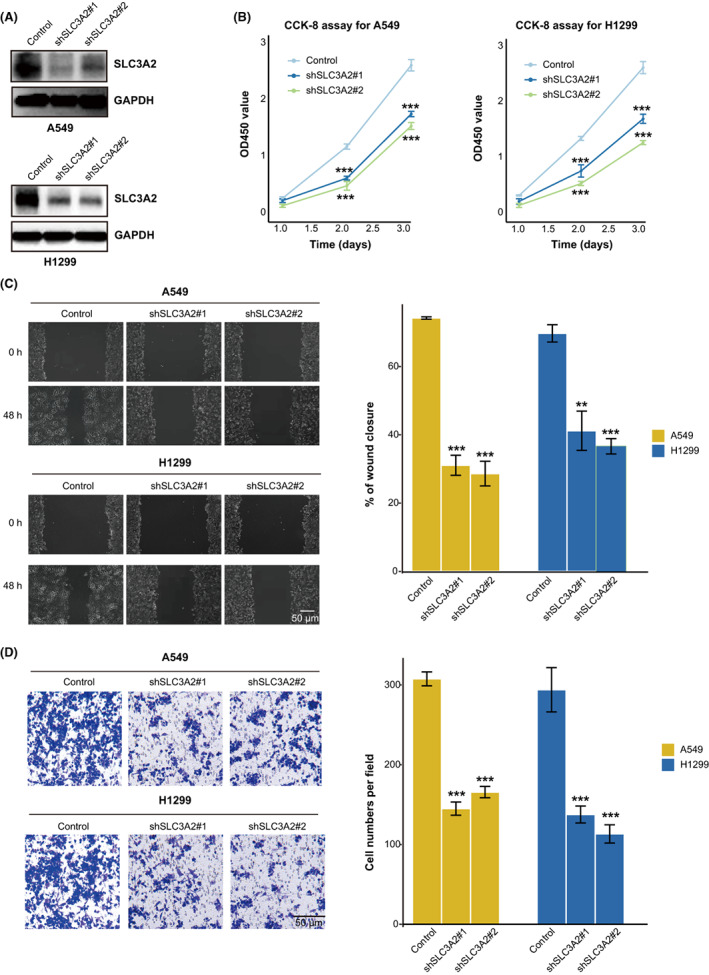
SLC3A2 inhibits lung adenocarcinoma cell proliferation, migration, and invasion. (A) Establishment of A549 and H1299 cells with stably knocked down SLC3A2. (B) CCK‐8 assay to check the effect of SLC3A2 on cell proliferation. (C) Wound healing experiments of WT and SLC3A2 knockdown cells; quantification data is shown in the right panel. (D) Transwell migration experiments of WT and SLC3A2 knockdown cells; quantification data is shown in the right panel. Graphs show the mean ± SEM of at least three independent experiments. ***p < 0.001, one‐way ANOVA
3.3. SLC3A2 in LUAD is critical for M2 macrophage polarization in TME
To further explore the relevance of SLC3A2 in the LUAD immune microenvironment, we analyzed the infiltration of 22 types of immue cells in TCGA (Figure 3A). We found high expression of SLC3A2 was associated with increased infiltration of M2 macrophages and decreased infiltration of M1 macrophages (Figure 3A). By immunohistochemistry analysis in 51 LUAD patients, we confirmed that SLC3A2 expression is positively or negatively correlated with infiltration of M2 or M1 type macrophages, respectively (Figure 3B,C). To investigate whether SLC3A2 in lung cancer cells affects the polarization of macrophages in TME, we cocultured human monocyte THP‐1 cells with either normal or SLC3A2 knockdown A549 cells and measured macrophage markers by using quantitative RT‐PCR (Figure 3D). Interestingly, we found that THP‐1 cells cocultured with conditional medium from SLC3A2 knockdown A549 cells showed increased expression of M1 macrophage markers iNOS, IL‐6, and IL‐12 and reduced expression of M2 macrophages marker ARG‐1, IL‐10, and CD163 (Figure 3E). These data suggested that SLC3A2 in LUAD cells is critical in maintaining M2 phenotype of macrophages in TME.
FIGURE 3.
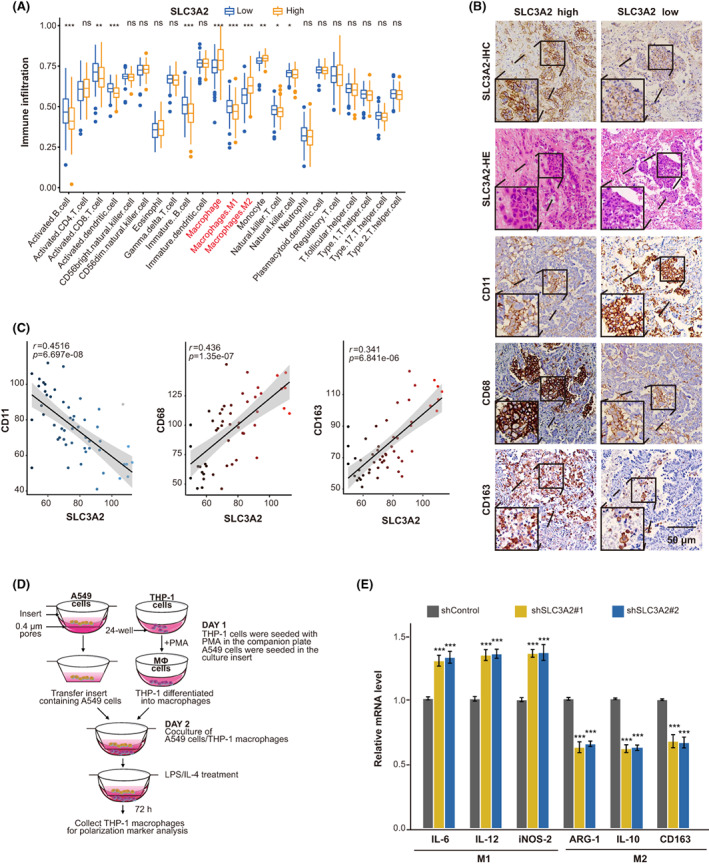
Tumor SLC3A2 expression is positively correlated with M2 macrophage infiltration. (A) Correlation between immune cell infiltration and SLC3A2 in The Cancer Genome Atlas. Patients were divided into high and low expression groups, and the proportion of immune cells in the microenvironment is analyzed. (B) Immunohistochemical (IHC) and H&E staining of SLC3A2, IHC staining of CD163 (M2 marker), CD68 (macrophage marker), and CD11c (M1 marker) was carried out on lung adenocarcinoma samples. Scale bar, 50 μm; n = 51. (C) The H‐score of each marker was calculated, and the linear correlation scatter plots of SLC3A2 and three macrophage markers are plotted respectively. (D) Schematic diagram of coculture of macrophages and A549 cells. (E) Quantitative RT‐PCR is used to detect polarization‐related markers in cells cocultured cells. Interleukin (IL)‐6, IL‐12, and inducible nitric oxide synthase (iNOS)‐2 are used as M1 type macrophage markers, while Arginase‐1 (ARG‐1), IL‐10, and CD163 are M2 type macrophage markers. Graphs show the mean ± SEM of at least three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, one‐way ANOVA. LPS, lipopolysaccharide; ns, no significance
3.4. Arachidonic acid is responsible for SLC3A2‐mediated M2 macrophage polarization
Due to the critical role of SLC3A2 in cell metabolism, we speculated that SLC3A knockdown in lung cancer cells might alter the secretion of metabolites to TME, which led to the impairment of M2 polarization. To test this hypothesis, we first carried out metabolome analysis in control and SLC3A2 knockdown A549 cells (Table S2). A total of 813 differential metabolites were identified in SLC3A2 KO cells, 380 of which were downregulated metabolites (Figure 4A). We next performed enrichment of these downregulated metabolites based on the Kyoto Encyclopedia of Genes and Genomes and Human Metabolome Database (Figure 4B). The results indicated that lipid metabolism and lipids were the most significantly different pathways (Figure 4B); AA and PGE2 were the top two downregulated metabolites in SLC3A2 knockdown cells (Figure 4C). To further investigate the impact of AA and PGE2 on M2 polarization, we treated cells with those metabolites and measured macrophage marker of polarization. We found that all those metabolites were sufficient to increase the expression of surface markers of M2 macrophages (ARG‐1, IL‐10, and CD163) (Figure 4D). We then tested whether AA is responsible for LUAD cells maintaining the M2 phenotype of macrophages by addition of AA to the coculture system of THP‐1 cells and SLC3A2 knockdown A549 cells (Figure 4E). The results indicated that addition of AA partially rescues the defect of polarization to M2 macrophages cocultured with SLC3A2 knockdown A549 cells (Figure 4E,F). In addition, we confirmed the content of AA decreased significantly in knockdown SLC3A2 lung cancer cell lines by ELISA (Figure 4G).
FIGURE 4.
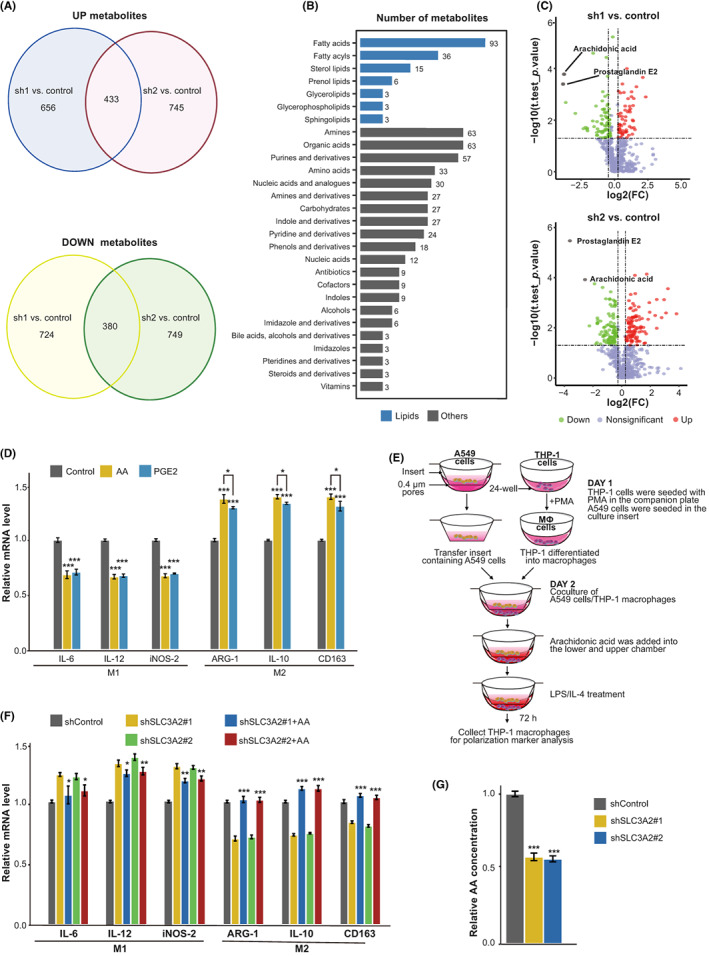
SLC3A2 induces macrophage differentiation into M2 type by arachidonic acid (AA). (A) Wayne diagram of metabolites whose contents are significantly downregulated in cells with stable KO of SLC3A2. (B) Pathway enrichment map of metabolomic differential metabolites in cells with stable KO of SLC3A2. (C) Differential metabolite volcano map of cells with stable KO of SLC3A2. (D) AA and prostaglandin E2 (PGE2) induced macrophage phenotype. PBS was used as the control reagent for AA, and ethanol was used as the control reagent for PGE2. Interleukin (IL)‐6, IL‐12, and inducible nitric oxide synthase (iNOS)‐2 are M1 type macrophage markers, whereas Arginase‐1 (ARG‐1), IL‐10, and CD163 are M2 type macrophage markers. (E) Schematic diagram of the polarization effect of AA on macrophages cocultured with A549 cells. (F) Quantitative RT‐PCR detected polarization‐related markers in cocultured cells. (G) Concentration of AA in the extracellular fluid of cells with stable KO of SLC3A2 was determined by ELISA. Graphs show the mean ± SEM of at least three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, one‐way ANOVA
3.5. SLC3A2 in LUAD cells promotes M2 polarization of TAMs in vivo
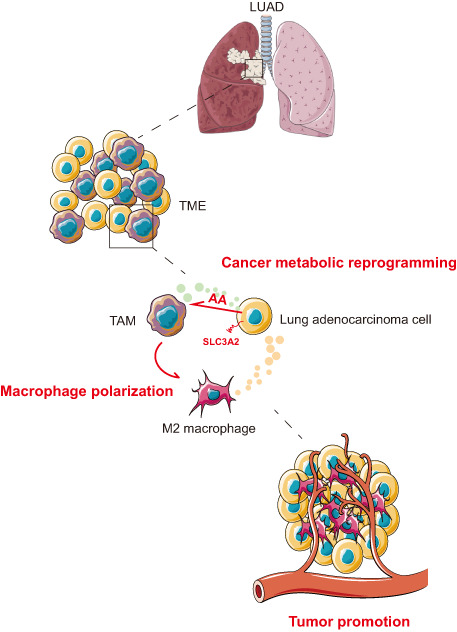
To further investigate the role of SLC3A2 in macrophage polarization in vivo, we generated SLC3A2 KO Lewis lung cancer (LLC) cells using the CRISPR/CAS9 method and implanted them into C57 mice (Figure 5A,B). Compared with WT tumors, the xenograft tumor growth rate and tumor volume were significantly decreased in SLC3A2 tumors (Figure 5C–E). Interestingly, the injection of AA into tumors could significantly rescue the inhibition of tumor growth in SLC3A2 KO tumors (Figure 5C–E). By measuring the AA content in the tumors of each group, the result showed that the AA content in SLC3A2 KO tumors was significantly lower than that in the WT tumors (Figure 5F). Subsequently, the results of IHC staining showed decreased M2 macrophages (CD163+) and increased M1 macrophages (CD11c+) in SLC3A2 KO tumors, while the SLC3A2 KO tumors injected with AA contained M2 macrophages comparable to WT tumors (Figure 5G,H). In conclusion, the data indicated that SLC3A2 acted as a metabolic switch on LUAD cells and induces macrophage phenotype reprogramming through AA (Figure 6).
FIGURE 5.
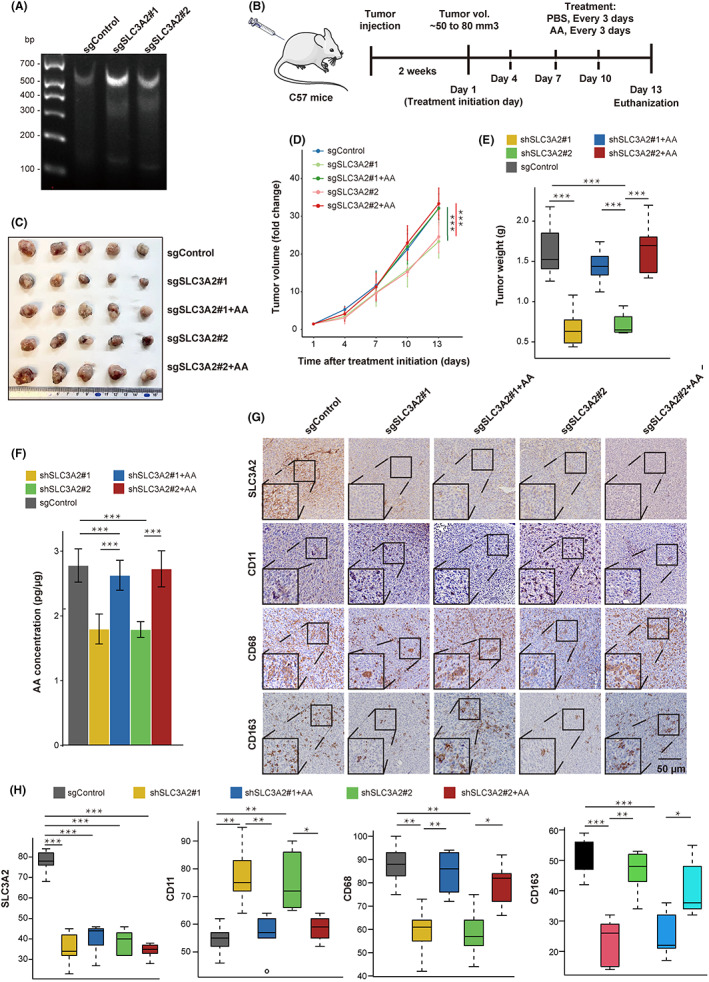
SLC3A2 on lung adenocarcinoma (LUAD) promotes tumor growth and M2 macrophage polarization in vivo. (A) Established Lewis lung cancer (LLC) cell lines with stable KO of SLC3A2. (B) Schematic diagram of LUAD tumor model with stable KO of SLC3A2. LLC cells with stable knockout of SLC3A2 and LLC cells expressing empty vector (1 × 106) were subcutaneously implanted into the flank of female C57 mice. When the average tumor size was 50–80 mm3, mice were randomly divided into a control group or arachidonic acid (AA) treatment group, and the tumor tissue injected every 3 days. Mice were killed after three treatments, and tumor tissues were taken for further examination. (C) Gross images of tumors after tumor resection at the endpoint of the experiment. (D) Tumor volumes were measured every third day and represented as fold change over tumor volume at day 1. (E) Tumor weight (g) was measured after tumor resection at the experimental endpoint and plotted as a boxplot. (F) Measurement of AA content in control and SLC3A2 KO tumors. (G) Immunohistochemical (IHC) staining for SLC3A2, CD163 (M2 marker), CD68 (macrophage marker), and CD11c (M1 marker) in mouse tumors. Scale bar, 50 μm; n = 5 for each group. (H) Analysis of CD163, CD68, and CD11c in different groups based on IHC staining. Graphs show mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, one‐way ANOVA
FIGURE 6.
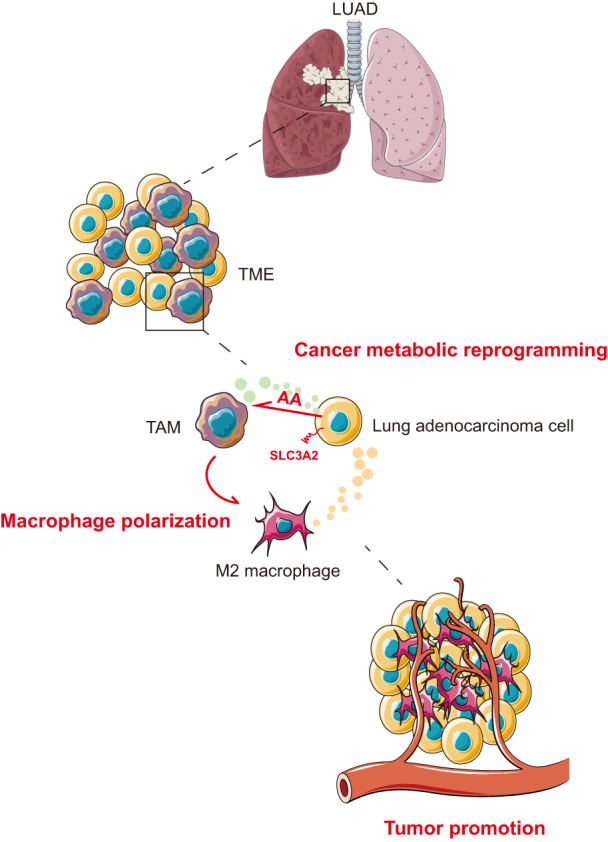
Schematic model of the polarization mechanism of tumor‐associated macrophages (TAMs). SLC3A2, as a metabolic switch on lung adenocarcinoma (LUAD) cells, induces macrophage phenotype reprogramming to M2 type through arachidonic acid (AA), and promotes the progress of lung adenocarcinoma. TME, tumor microenvironment
4. DISCUSSION
In this study, we identified SLC3A2 as the most relevant risk factor for LUAD, and knockdown of SLC3A2 showed that SLC3A2 not only affected the proliferation and migration of LUAD cells but also indirectly affected the polarization of macrophages. By conducting metabolomic analyses, we revealed that SLC3A2 mainly affected the contents of LUAD metabolites in the microenvironment: AA and PGE2. We further confirmed that AA was responsible for SLC3A2‐mediated TAM polarization both in vitro and in vivo. Our data thus provided new mechanisms responsible for TAM polarization in lung cancer and suggested that SLC3A2 acts as a metabolic switch to induce macrophage phenotypic reprogramming by modulating AA secretion.
The exact mechanism by which SLC3A2 regulates AA secretion in LUAD remains to be further investigated. As a polyunsaturated fatty acid, AA is the direct precursor of many bioactive substances of circulating eicosanoic acid derivatives, such as PGE2, prostacyclin (PGI2), thromboxane A2 (TXA2), and leukotriene and C4 (LTC4). 22 These bioactive substances have important regulatory effects on lipid protein metabolism, leukocyte function, and platelet activation. 23 , 24 Previous studies have shown that SLC3A2 regulates the activation of the mTORC1 pathway by regulating leucine transport. Mammalian TORC1 regulates a variety of metabolic processes, including protein synthesis, lipogenesis, energy expenditure, and autophagy, and is highly active in cancer tissues. In fat metabolism, the mTORC1 signal is a key regulator of the cyclooxygenase‐2/PGE2 pathway and plays a key role in regulating fat inflammation. 25 , 26 , 27 Cyclooxygenase‐2 oxidizes AA and converts it into PGE2 and other prostaglandins. 28 It would be interesting to further address whether the regulatory effects of SLC3A2 on AA are mTORC1 pathway‐ related.
Although AA and PGE2 were both altered by SLC3A2, AA showed a more profound effect than PGE2 on macrophage polarization. As one of the metabolites of AA, PGE2 is the most common prostaglandin found in different human cancers, including colon cancer, lung cancer, breast cancer, and head and neck cancer. 29 , 30 Studies have shown that PGE2 can promote cell proliferation, migration, and invasion in an autocrine manner. 31 Notably, PGE2 can induce the transformation of M1 to M2 macrophages by inhibiting the formation of inflammatory body complex (ASC/Cas‐1/NLRP3) in THP‐1 cells. 32 In contrast, the role of AA in cancer seems more complex and context‐dependent. 33 , 34 It has been found that AA can enhance the autophagy of tumor cells by inducing caspase‐3/7 activation and enhancing oxidative DNA and protein damage in cells. 35 Although the xenograft model in this study cannot completely exclude the possibility that AA itself plays a role in LUAD proliferation regardless of macrophages, previous studies suggest that AA does not promote tumor proliferation. In both lung cancer and melanoma cell lines, AA showed inhibition of tumor proliferation. 36 Some studies have shown that AA promotes M2 polarization through its derived metabolite PGE2 in esophageal squamous cell carcinoma and does not have the effect of inducing polarization itself; AA inhibits the viability of macrophages by inducing cell cycle arrest in S phase. 36 , 37 Other studies suggest that AA can directly promote the M2 polarization of macrophages and regulate pyruvate kinase M2 (PKM2) and hypoxia inducible factor‐1α (HIF‐1α) as well as iNOS against acute myocardial infarction. 38 , 39
Tumor development is not only dependent on genetic changes of cancer cells but also affected by interactions with the ECM and other cell subsets in the TME. Macrophages, one of the most abundant cell types in the TME, are immune cells with reversible activation states, including tumor suppressing (M1) and tumor promoting (M2) states. 40 Tumor‐associated macrophages usually have similar phenotypes and functions to M2‐like macrophages, and participate in tumor immunosuppression, angiogenesis, and tumor metastasis. 1 , 41 Deciphering how macrophages regulate the transition from one state to another is the key to a deeper understanding of tumor development and related therapies.
The polarization of macrophages is regulated by the metabolic microenvironment, and macrophages with different phenotypes have different metabolic levels. 42 M1 type macrophages utilize glycolysis and pentose phosphate pathways to meet energy supply, while the metabolic activity of M2 macrophages is characterized by enhanced FAO and oxidative phosphorylation. 2 , 43 , 44 As the functional characteristics of macrophages are mainly phagocytosis, lipid metabolism is particularly important as the basis of plasma membrane replacement. 45 , 46 Macrophages ingest different forms of lipids from phagocytic dying cells and microenvironment, and free fatty acids are converted into different products in mitochondria through the FAO pathway. 47 , 48 Studies have shown that M2 macrophages can be reprogrammed to M1 by inhibiting FAO. 48 , 49 In addition, amino acid catabolism is also associated with macrophage polarization. 50 Considering that the metabolism of a tumor is significantly different from that of normal tissue, and the level of metabolites in the microenvironment at different stages of tumor development is also different, the metabolic level of tumor cells could affect the induction of macrophage phenotype by changing the microenvironment. Therefore, we speculate that M2‐like macrophages might be reprogrammed into M1‐like macrophages by silencing the metabolic switch on tumor cells, to achieve the therapeutic purpose of tumor suppression. However, due to the limitation of experimental methods, we are currently unable to remove AA or PGE2 from the conditioned medium of the coculture system. We hope that this experiment can be further improved by constructing stable cell lines knocking out AA or PGE2‐related synthetases in the future.
In conclusion, our data illustrate molecular mechanisms responsible for TAM polarization and suggest that SLC3A2 acts as a metabolic switch on LUAD cells to induce macrophage phenotypic reprogramming through AA. Our findings thus highlight the importance of SLC3A2 in lung cancer progression and provide a useful target for the development of mechanism‐based cancer therapeutic strategies.
AUTHOR CONTRIBUTIONS
L.W., Z.L., and L.S. designed the research. Z.L., S.C., X.H., and S.G. performed the research. L.W., Z.L., S.C., and X.H. analyzed data. L.W., Z.L., and S.C. wrote the paper.
FUNDING INFORMATION
This work was funded by the following grants and associations: The National Multidisciplinary Cooperative Diagnosis and Treatment Capacity Building Project for Major Diseases (Lung Cancer, z027002), National Natural Science Foundations of China (81974465 and 81900199), and the recruitment program for Huxiang Talents (2019RS1009) to L.W., National Natural Science Foundation of China (81974458, 82170607), grant 2021JJ30463, 2022JJ10037 from Hunan Provincial Natural Science Foundation (2021JJ30463, 2022JJ10037), and Hunan Normal University Grant (2022XKQ0205, KF2022001) to Z.L.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an institutional review board: This study has been approved by the Medical Ethics Committee of Xiangya Hospital of Central South University (No. 2019030387).
Informed consent: N/A.
Registry and registration no. of the study/trial: N/A.
Animal studies: The animal experiments were approved by the Experimental Animal Welfare Ethics Committee of Central South University (No. 202009476). This study was performed in accordance with the Declaration of Helsinki.
Supporting information
Appendix S1
Figure S1
Table S1
Table S2
ACKNOWLEDGMENTS
National Natural Science Foundation of China (Grant/Award Nos. 81974458, 82170607, 81974465, and 81900199).
Li Z, Chen S, He X, Gong S, Sun L, Weng L. SLC3A2 promotes tumor‐associated macrophage polarization through metabolic reprogramming in lung cancer. Cancer Sci. 2023;114:2306‐2317. doi: 10.1111/cas.15760
Zhuan Li, Songming Chen, and Xiang He contributed equally to this work
REFERENCES
- 1. Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis V. The complex role of tumor‐infiltrating macrophages. Nat Immunol. 2022;23:1148‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bai R, Li Y, Jian L, Yang Y, Zhao L, Wei M. The hypoxia‐driven crosstalk between tumor and tumor‐associated macrophages: mechanisms and clinical treatment strategies. Mol Cancer. 2022;21:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erbani J, Boon M, Akkari L. Therapy‐induced shaping of the glioblastoma microenvironment: macrophages at play. Semin Cancer Biol. 2022;86:41‐56. [DOI] [PubMed] [Google Scholar]
- 4. Revel M, Sautès‐Fridman C, Fridman W, Roumenina L. C1q+ macrophages: passengers or drivers of cancer progression. Trends Cancer. 2022;8:517‐526. [DOI] [PubMed] [Google Scholar]
- 5. Pittet M, Michielin O, Migliorini D. Clinical relevance of tumour‐associated macrophages. Nat Rev Clin Oncol. 2022;19:402‐421. [DOI] [PubMed] [Google Scholar]
- 6. Robertson S, Best S. The domiNO effect turns macrophage activation deadly. Immunity. 2022;55:382‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mouton A, Li X, Hall M, Hall J. Obesity, hypertension, and cardiac dysfunction: novel roles of Immunometabolism in macrophage activation and inflammation. Circ Res. 2020;126:789‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehla K, Singh P. Metabolic regulation of macrophage polarization in cancer. Trends Cancer. 2019;5:822‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi Q, Shen Q, Liu Y, et al. Increased glucose metabolism in TAMs fuels O‐GlcNAcylation of lysosomal Cathepsin B to promote cancer metastasis and chemoresistance. Cancer Cell. 2022;40:1207‐1222. [DOI] [PubMed] [Google Scholar]
- 11. Yan J, Horng T. Lipid metabolism in regulation of macrophage functions. Trends Cell Biol. 2020;30:979‐989. [DOI] [PubMed] [Google Scholar]
- 12. Ryan D, O'Neill L. Krebs cycle reborn in macrophage Immunometabolism. Annu Rev Immunol. 2020;38:289‐313. [DOI] [PubMed] [Google Scholar]
- 13. Lin L, Yee S, Kim R, Giacomini K. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov. 2015;14:543‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan R, Zhao X, Lei J, Zhou Q. Structure of the human LAT1‐4F2hc heteromeric amino acid transporter complex. Nature. 2019;568:127‐130. [DOI] [PubMed] [Google Scholar]
- 15. Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hansen C, Ng Y, Lam W, Plouffe S, Guan K. The hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res. 2015;25:1299‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hasegawa K, Ikeda S, Yaga M, et al. Selective targeting of multiple myeloma cells with a monoclonal antibody recognizing the ubiquitous protein CD98 heavy chain. Sci Transl Med. 2022;14:eaax7706. [DOI] [PubMed] [Google Scholar]
- 18. Digomann D, Kurth I, Tyutyunnykova A, et al. The CD98 heavy chain is a marker and regulator of head and neck squamous cell carcinoma Radiosensitivity. Clin Cancer Res. 2019;25:3152‐3163. [DOI] [PubMed] [Google Scholar]
- 19. Poettler M, Unseld M, Braemswig K, Haitel A, Zielinski C, Prager G. CD98hc (SLC3A2) drives integrin‐dependent renal cancer cell behavior. Mol Cancer. 2013;12:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanai Y. Amino acid transporter LAT1 (SLC7A5) as a molecular target for cancer diagnosis and therapeutics. Pharmacol Ther. 2022;230:107964. [DOI] [PubMed] [Google Scholar]
- 21. Bajaj J, Konuma T, Lytle N, et al. CD98‐mediated adhesive signaling enables the establishment and propagation of acute Myelogenous leukemia. Cancer Cell. 2016;30:792‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bazinet R, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771‐785. [DOI] [PubMed] [Google Scholar]
- 23. Vanni S, Riccardi L, Palermo G, De Vivo M. Structure and dynamics of the acyl chains in the membrane trafficking and enzymatic processing of lipids. Acc Chem Res. 2019;52:3087‐3096. [DOI] [PubMed] [Google Scholar]
- 24. Bäck M, Yurdagul A, Tabas I, Öörni K, Kovanen P. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16:389‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Menon D, Salloum D, Bernfeld E, et al. de novoLipid sensing by mTOR complexes via synthesis of phosphatidic acid. J Biol Chem. 2017;292:6303‐6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ladu S, Calvisi D, Conner E, Farina M, Factor V, Thorgeirsson S. E2F1 inhibits c‐Myc‐driven apoptosis via PIK3CA/Akt/mTOR and COX‐2 in a mouse model of human liver cancer. Gastroenterology. 2008;135:1322‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li H, Jin F, Jiang K, et al. mTORC1‐mediated downregulation of COX2 restrains tumor growth caused by TSC2 deficiency. Oncotarget. 2016;7:28435‐28447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mitchell J, Shala F, Elghazouli Y, et al. Cell‐specific gene deletion reveals the antithrombotic function of COX1 and explains the vascular COX1/prostacyclin paradox. Circ Res. 2019;125:847‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pelly V, Moeini A, Roelofsen L, et al. Anti‐inflammatory drugs remodel the tumor immune environment to enhance immune checkpoint blockade efficacy. Cancer Discov. 2021;11:2602‐2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hou W, Sampath P, Rojas J, Thorne S. Oncolytic virus‐mediated targeting of PGE2 in the tumor alters the immune status and sensitizes established and resistant tumors to immunotherapy. Cancer Cell. 2016;30:108‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cilenti F, Barbiera G, Caronni N, et al. A PGE‐MEF2A axis enables context‐dependent control of inflammatory gene expression. Immunity. 2021;54:1665‐1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park H, Kim J, Saima F, et al. Adipose‐derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1‐macrophage population through prostaglandin E2. Biochem Biophys Res Commun. 2018;498:988‐995. [DOI] [PubMed] [Google Scholar]
- 33. Zajdel A, Wilczok A, Tarkowski M. Toxic effects of n‐3 polyunsaturated fatty acids in human lung A549 cells. Toxicol In Vitro. 2015;30:486‐491. [DOI] [PubMed] [Google Scholar]
- 34. Hammoud M, Dietze R, Pesek J, et al. Arachidonic acid, a clinically adverse mediator in the ovarian cancer microenvironment, impairs JAK‐STAT signaling in macrophages by perturbing lipid raft structures. Mol Oncol. 2022;16:3146‐3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu Z, Wang J, Liu L, et al. Chronic ethanol consumption and HBV induce abnormal lipid metabolism through HBx/SWELL1/arachidonic acid signaling and activate Tregs in HBV‐Tg mice. Theranostics. 2020;10:9249‐9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zajdel A, Wilczok A, Chodurek E, Gruchlik A, Dzierzewicz Z. Polyunsaturated fatty acids inhibit melanoma cell growth in vitro. Acta Pol Pharm. 2013;70:365‐369. [PubMed] [Google Scholar]
- 37. Shen Z, Ma Y, Ji Z, et al. Arachidonic acid induces macrophage cell cycle arrest through the JNK signaling pathway. Lipids Health Dis. 2018;17:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sorgi C, Zarini S, Martin S, et al. Dormant 5‐lipoxygenase in inflammatory macrophages is triggered by exogenous arachidonic acid. Sci Rep. 2017;7:10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheng Y, Feng Y, Xia Z, Li X, Rong J. ω‐Alkynyl arachidonic acid promotes anti‐inflammatory macrophage M2 polarization against acute myocardial infarction via regulating the cross‐talk between PKM2, HIF‐1α and iNOS. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:1595‐1605. [DOI] [PubMed] [Google Scholar]
- 40. Feng Q, Ma X, Cheng K, et al. Engineered bacterial outer membrane vesicles as controllable two‐way adaptors to activate macrophage phagocytosis for improved tumor immunotherapy. Adv Mater. 2022;34:e2206200. [DOI] [PubMed] [Google Scholar]
- 41. Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21:799‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wei Z, Oh J, Flavell R, Crawford J. LACC1 bridges NOS2 and polyamine metabolism in inflammatory macrophages. Nature. 2022;609:348‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pan X, Zhu Q, Pan L, Sun J. Macrophage immunometabolism in inflammatory bowel diseases: from pathogenesis to therapy. Pharmacol Ther. 2022;238:108176. [DOI] [PubMed] [Google Scholar]
- 44. Seim G, Fan J. A matter of time: temporal structure and functional relevance of macrophage metabolic rewiring. Trends Endocrinol Metab. 2022;33:345‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. di Conza G, Tsai C, Gallart‐Ayala H, et al. Tumor‐induced reshuffling of lipid composition on the endoplasmic reticulum membrane sustains macrophage survival and pro‐tumorigenic activity. Nat Immunol. 2021;22:1403‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Antonyak M, Lukey M, Cerione R. Lipid‐filled vesicles modulate macrophages. Science. 2019;363:931‐932. [DOI] [PubMed] [Google Scholar]
- 47. Tumor‐secreted lipids induce ER stress response to polarize macrophages. Cancer Discov. 2022; 12:OF5. [DOI] [PubMed] [Google Scholar]
- 48. Raines L, Zhao H, Wang Y, et al. PERK is a critical metabolic hub for immunosuppressive function in macrophages. Nat Immunol. 2022;23:431‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu J, Wei Y, Jia W, et al. Chenodeoxycholic acid suppresses AML progression through promoting lipid peroxidation via ROS/p38 MAPK/DGAT1 pathway and inhibiting M2 macrophage polarization. Redox Biol. 2022;56:102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lin S, Zhang A, Yuan L, et al. Targeting parvalbumin promotes M2 macrophage polarization and energy expenditure in mice. Nat Commun. 2022;13:3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Figure S1
Table S1
Table S2


