Abstract
Tumor‐associated macrophages (TAMs) are the most prominent immune cells in the breast cancer microenvironment, and the protumor functions of TAMs are thought to affect cancer progression and resistance to anticancer therapy. Numerous studies using human breast cancer samples, cell lines, and murine breast cancer models have revealed details of the mechanisms by which the protumor functions of TAMs are activated. Recent advances have highlighted the significant involvement of TAMs in the resistance of breast cancer cells to immunotherapy. Tumor‐associated macrophages express a number of immunosuppressive genes, and single‐cell sequence analyses of human and murine cancer samples have helped elucidate the mechanism of TAM‐induced immunosuppression. As TAMs are considered suitable targets for anticancer therapies, we summarized the protumor functions of TAMs and the potential of anticancer therapies targeting TAMs, with a focus on breast cancer research.
Keywords: breast cancer, CD163, CD204, macrophage, PD‐L1
Tumor‐associated macrophages (TAMs) are the most prominent immune cells in the breast cancer microenvironment, and the protumor functions of TAMs are thought to affect cancer progression and resistance to anti‐cancer therapy. Recent advances have highlighted the significant involvement of TAMs in the resistance of breast cancer cells to immunotherapy.
Abbreviations
- APOE
apolipoprotein E
- ARID5A
AT‐rich interaction domain 5A
- C1QA
complement C1q A chain
- CADM1
cell adhesion molecule 1
- CCL
C‐C motif chemokine ligand
- CCR
C‐C motif chemokine receptor
- CSF‐1
colony‐stimulating factor 1
- EGF
epidermal growth factor
- FOLR2
folate receptor 2
- FOSL2
FOS‐like 2
- GM‐CSF
granulocyte/macrophage colony‐stimulating factor
- HER2
human epidermal growth factor receptor 2
- IFN
interferon
- IHC
immunohistochemistry
- IKKβ
inhibitor of nuclear factor kappa B kinase subunit beta
- IL
interleukin
- IRF5
interferon regulatory factor 5
- PD1
programmed death 1
- PD‐L1
programmed death 1 ligand 1
- RNA‐seq
RNA sequencing
- SPP1
secreted phosphoprotein 1
- STAT
signal transducer and activator of transcription
- TAM
tumor‐associated macrophage
- TLS
tertiary lymphoid structure
- TNBC
triple‐negative breast cancer
- TREM2
triggering receptor expressed on myeloid cells 2
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
Breast cancer is the most frequent malignant tumor in women, and its incidence has increased in recent decades worldwide. 1 Breast cancer is pathologically classified into four molecular subtypes depending on the expression of hormone receptors, Ki‐67, and HER2. Neoadjuvant chemotherapy is the standard treatment for patients with breast cancer. Triple‐negative breast cancer subtypes account for 10%–20% of breast cancers and are more aggressive than other subtypes due to resistance to hormone therapy and anti‐HER2 Ab therapy. An immunotherapy targeting PD‐L1 combined with chemotherapy is effective against early and advanced TNBC subtypes, 2 , 3 and it has been approved in several countries for treating PD‐L1‐positive TNBC. Several studies have reported that PD‐L1 expression in cancer tissues is predictive of beneficial clinical response to anti‐PD‐L1 therapy.
Infiltrating macrophages in cancer/tumor microenvironment are known as TAMs. 4 Accumulating evidence suggests that the immune microenvironment also contributes to the efficacy of anticancer immunotherapy, and TAMs are the primary immune cells within the immune microenvironment of cancer tissues (Figure 1A). Immunohistochemistry using human breast cancer specimens revealed that PD‐L1 is primarily expressed on immune cells, especially TAMs (Figure 1B,C). Single‐cell sequence analyses suggested that PD‐L1 and PD‐L2 expression by myeloid cells in breast cancer is associated with immune regulation. 5 Thus, macrophages are thought to play a role in immunosuppression in the tumor microenvironment of breast cancer.
FIGURE 1.
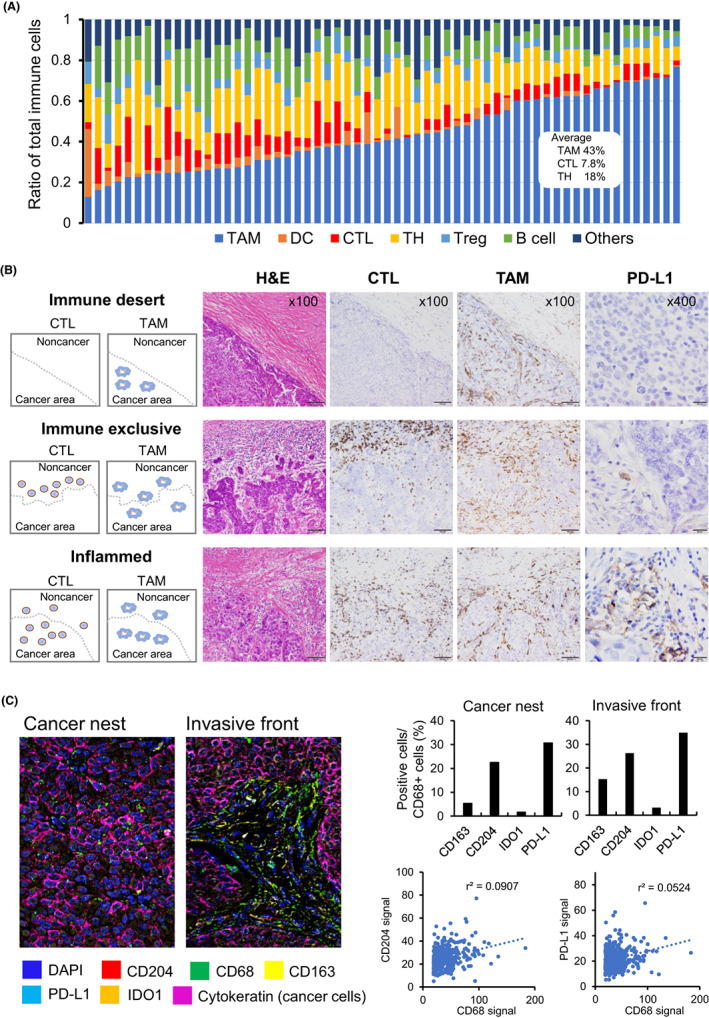
Immune microenvironment of breast cancer. (A) The proportions of immune cells in triple‐negative breast cancers (TNBCs) are presented. Sixty randomly selected cases from The Cancer Genome Atlas data were analyzed using CIBERSORTx, and the proportions of each type of immune cell relative to the total number of immune cells was calculated. (B) Cases were classified into three categories based on immune cell infiltration. Programmed death 1 ligand 1 (PD‐L1) expression is predominantly observed in “inflamed” cases, and cell–cell interactions between CTLs and TAMs are suggested to be involved in PD‐L1 overexpression. (C) Multiplex immunohistochemical analysis of an inflamed breast cancer specimen. Cancer cells were positive for cytokeratin but negative for PD‐L1, and 30%–35% of CD68+ TAMs were positive for PD‐L1 in this case. The signal values of CD68 in TAMs were positively correlated with the signal values of CD204 and PD‐L1. As CD68 expression is a marker of tumor‐associated macrophage (TAM) maturation, the expression of CD204 and PD‐L1 depends on the maturation of TAMs. DC, dendritic cell; TH, helper T cell; Treg, regulatory T cell.
Macrophages are immune cells that play critical roles in many diseases, and it is important to note that many researchers have focused on the functions of macrophages in cancer biology in recent years. In this review, we discuss how TAMs contribute to cancer progression, focusing on breast cancer, and we summarize the development of anticancer therapies targeting TAMs. Macrophage activation is a heterogeneous and complex process. In the late 1990s, two hypotheses of macrophage activation (classical/alternative activation and M1/M2 classification) based on the activating condition of monocyte‐derived macrophages were proposed by Gordon et al. and Mills et al., respectively. 6 , 7 M1 macrophages are considered pro‐inflammatory cells that play a role in antimicrobial host defense, whereas M2 macrophages generally counteract inflammatory responses and promote tissue repair. 8 Although the M1/M2 classification has been widely accepted, it is now considered an oversimplified concept. The M1/M2 concept first emerged from studies using mouse bone marrow‐derived macrophages, and it was unclear whether this concept would be applicable to humans. 9 , 10 In addition, the M1/M2 concept is considered inadequate for the classification of resident macrophages. 10 Therefore, terms such as “M1‐like” and “M2‐like”, rather than “M1” and “M2”, should be used based on the relative expression levels of each marker.
2. TUMOR‐ASSOCIATED MACROPHAGES IN HUMAN BREAST CANCER: STUDIES USING A PAN‐MACROPHAGE MARKER
Tumor‐associated macrophages can be detected in almost all cancer tissues, and recent studies indicated that TAMs play a protumor role in many kinds of cancers. 11 , 12 Tumor‐associated macrophages have been shown to secrete growth, angiogenic, tissue remodeling, and immunosuppressive factors, and a high density of TAMs is thought to be associated with worse clinical course or higher histological grade of malignancy in many cancers. As it is difficult to detect TAMs in routine microscopic pathology sections; descriptions of TAMs in cancer tissues were scarce before 1990. However, the development of new IHC methods for identifying TAMs in tumor tissue has advanced TAM research. Beginning in the 1990s, numerous studies on human samples were carried out using CD68 as a macrophage marker. 13 The first study using IHC to examine TAMs in breast cancer was published in 1992. 14 That study, which combined IHC and in situ hybridization suggested that CSF‐1, which is secreted by breast cancer cells, mediates the chemotaxis of CSF‐1R‐positive TAMs. After CD68 was found to be a marker for macrophages, 15 EGF was shown to be secreted by CD68+ TAMs in breast cancer, but not by cancer cells per se. 16 It was also shown that the density of CD68+ TAMs is associated with high blood vessel density and poor clinical course. 17 A significant positive correlation was observed between the density of TAMs and either VEGF expression or vessel density, with increased TAM density predicting a poor clinical course. 18 An increase in the density of CD68+ TAMs infiltrating the stromal area was shown to be associated with estrogen receptor and progesterone receptor negativity, HER2 positivity, and worse clinical course. 19 Thus, many studies on CD68+ TAMs showed that these cells contribute to cancer progression.
3. TUMOR‐ASSOCATED MACROPHAGES IN HUMAN BREAST CANCER: STUDIES FOCUSING ON PHENOTYPIC HETEROGENEITY
Many studies using surgically resected human cancer samples have been carried out using CD68 as a total macrophage marker, whereas CD163, CD204, and CD206 have been used as markers of M2‐like macrophages in recent studies. CD163 and CD204 were first identified as hemoglobin scavenger receptor and modified low‐density lipoprotein scavenger receptor, respectively, specifically expressed on macrophages. 20 , 21 CD163 and CD204 were then shown to be expressed in protumor phenotype TAMs and related to protumor activation signals. 22 , 23 Depletion of TAMs expressing these molecules stopped cancer growth or progression in preclinical models. 24 , 25
A study using CD68 and CD163 to detect TAMs showed a high density of CD163+ TAMs rather than CD68+ TAMs, and this was associated with worse clinical course. 26 In that in vitro study, conditioned medium of MDA‐MB231 cells stimulated macrophage differentiation into the CD163+ M2 phenotype. As MDA‐MB231 cells highly produce CSF‐1, it was suggested that CSF‐1 induces M2 polarization. 26 In our previous study, CD204 expression in cultured macrophages was upregulated by exposure to conditioned medium from cultures of three human breast cancer cell lines (MCF7, MDA‐MB453, and OCUB‐M), but expression of CD163 and CD206 was not affected. 27 We next evaluated CD68+, CD163+, and CD204+ TAMs in serial sections of breast cancer tissues and found that only the density of CD204+ TAMs was a prognostic factor in breast cancer. 27 Kuroda et al. evaluated immune cells by monitoring the expression of CD68, CD163, CD8 (CTL marker), CD4 (helper T lymphocyte marker), and CD20 (B cell marker) in TNBC cases. They indicated that cases involving a high density of CD68/CD163+ TAMs and a low density of T and B lymphocytes showed the worst clinical course. 28 Strack et al. reported an increase in CD206− TAMs in invasive breast cancer as compared with noninvasive breast cancer, and the increased density of CD206−/MHC‐II‐high TAMs was associated with poor clinical course. 29 A study of TNBC reported that a high number of CD206+ TAMs is associated with a high density of CTLs and better clinical course. 30 These studies indicated that CD68+ TAMs can be divided into various subpopulations based on M2‐related markers; however, it is unclear whether there is a subpopulation of TAMs showing strong protumor functions in breast cancer.
A recent single‐cell RNA‐seq analysis examined myeloid cell infiltration of breast cancer tissues. 31 The authors proposed that TAMs in breast cancer can be categorized into several subpopulations. In particular, FOLR2+ macrophages localize in perivascular areas of breast cancer tissues, where they interact with CD8+ T cells, thereby possibly inducing tumor immunity. To determine which genes are expressed in TAM subpopulations, we reanalyzed the single‐cell RNA‐seq data reported by that study (Figure 2). Although the FOLR2+ cluster was unclear, it was found that TAMs were broadly divided into two subtypes (Group 1: high expression of CD204, APOE, C1QA, TREM2, CADM1, and SPP1; Group 2: strong expression of CD206, S100A9, and FCN1). As FOLR2+ clustering was reported to be positive for CD206, 31 the Group 2 cluster might express low levels of FOLR2. Considering these results together with those of previous reports described above, CD204‐expressing TAMs appear to support the progression of breast cancer, whereas CD206‐expressing TAMs potentially have unknown anticancer activities. CD204+ cells express APOE, and APOE expression reportedly plays a significant role in the protumor function of TAMs in other cancers. 32 It was shown that CD163+ and CD204+ TAMs are not the same (Figure 2). Our previous study also revealed a discrepancy in the numbers between CD163+ and CD204+ cells in breast cancer samples. 27 Multiplex IHC using a CODEX system suggested that CD204 was strongly expressed in TAMs, and CD163 signals were less intense than CD204 signals (Figure 1C). It is considered that CD204 is a better marker for predicting the protumor functions of TAMs in breast cancer than several other markers. such as CD163.
FIGURE 2.
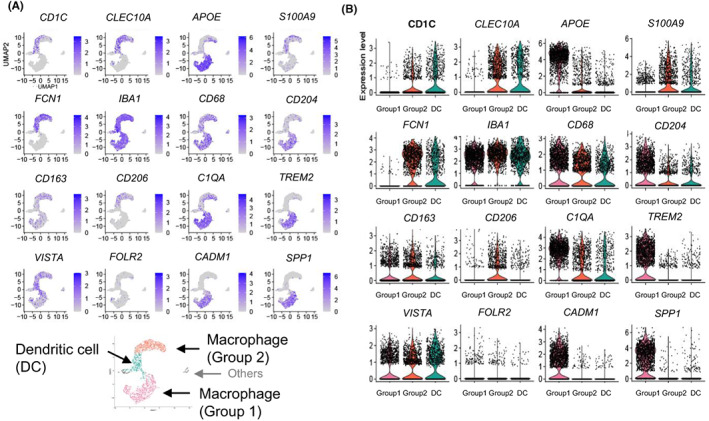
Heterogeneity of tumor‐associated macrophages in breast cancer patients. Single‐cell RNA sequencing (RNA‐seq) data of CD11C+ HLA‐DR+ myeloid cells infiltrating breast cancer tumor tissues were reanalyzed. (A) CD11C+ HLA‐DR+ myeloid cells were subdivided into three main subpopulations: macrophages (Group 1 and Group 2) and dendritic cells (DCs). (B) Macrophage (Group 1) specifically express APOE, MSR1 (CD204), C1QA, TREM2, CADM1, and SPP1, whereas macrophage (Group 2) strongly express S100A9, FCN1, and MRC1 (CD206). Cluster expressing CD1C and CLEC10A was classified as DC. The single‐cell RNA‐seq data were retrieved from the NCBI Gene Expression Omnibus database (accession code GSE192935). The RNA count data were obtained from Fastq files using Cellranger software (version 7.0.1). Cluster analysis and visualization by UMAP and violin plots were undertaken using Seurat (version 4.2.0).
CD206‐expressing TAMs have immune‐stimulatory functions in the early stages of lung cancer. 33 CD206‐expressing macrophages were suggested to be resident cells in the breast. 30 The Group 2 cluster was FCN1high and CD204low, and it is known that FCN1 was highly expressed in monocytes, but expression was low in macrophages. 34 In contrast, CD204 expression was low in monocytes, but high in macrophages. 35 Therefore, the Group 2 cluster in Figure 2 was suggested to include resident macrophages and immature monocytic cells.
When the density of TAMs in the tumor nest and tumor stroma were evaluated, the density of TAMs in the stromal area of tumors is reportedly associated with cancer cell proliferation, whereas the density of TAMs in the cancer nest is linked to vessel density. 36 A high density of CD68+ and CD163+ TAMs in the tumor stroma, but not in the tumor nest, is associated with worse clinical course. 37 Thus, the distribution of TAMs appears to determine their function. However, it was often difficult to distinguish between stromal TAMs and nest TAMs in our microscopic observations, especially in scirrhous‐type cancers, as the cancer nest cannot be detected in approximately 40% of breast cancer cases. 38
4. FUNCTIONS OF TAMs IN BREAST CANCER PROGRESSION: INSIGHTS FROM HUMAN STUDIES
Tumor‐associated macrophages reportedly secrete several factors in human breast cancer tissue. Expression of VEGF in TAMs has been linked to angiogenesis, as described above. Heparin‐binding EGF‐like growth factor and oncostatin‐M synergistically stimulate cancer growth by activating STAT3 signaling. 39 Tumor‐associated macrophage‐derived IL‐6, IL‐1β, prostaglandin E2, and osteopontin are also reportedly involved in cancer growth and progression. 40 , 41 , 42 Thus, many protumor factors derived from human TAMs are thought to be involved in progression, metastasis, invasion, chemoresistance, and angiogenesis in breast cancer (Figure 3).
FIGURE 3.
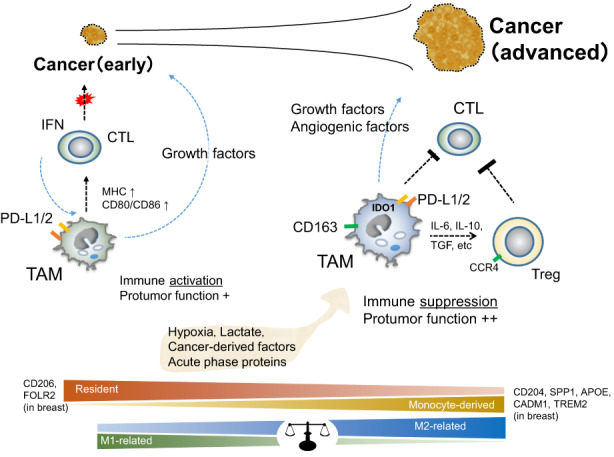
Relationship between macrophages and antitumor immunity in the tumor microenvironment. The functions, origins, and polarization of tumor‐associated macrophages (TAMs) are thought to change depending on cancer stage. In early‐stage cancers, TAMs are mainly composed of resident macrophages with both M2‐like and M1‐like properties and can express growth factors for cancer cells while also inducing an immune response. In advanced cancers, TAMs are replaced by monocyte‐derived cells polarized into the M2‐like phenotype depending on the status of the tumor microenvironment and express not only growth factors but also factors related to angiogenesis, infiltration, metastasis, and immune suppression. APOE, apolipoprotein E; CADM1, cell adhesion molecule 1; CCR4, C‐C motif chemokine receptor 4; FOLR2, folate receptor 2; IDO1, Indoleamine 2,3‐dioxygenase 1; IFN, interferon; PD‐L1/2, programmed death 1 ligand 1/2; SPP1, secreted phosphoprotein 1; TGF, transforming growth factor; Treg, regulatory T cell; TREM2, triggering receptor expressed on myeloid cells 2.
Some studies have reported a relationship between TAMs and anticancer immunity. A high density of TAMs has been linked to poor clinical course, whereas a high density of CD8+ CTLs is associated with a favorable course in breast cancer. 43 Additionally, PD‐1 and related signals are important TAM‐derived factors, as immunotherapies targeting PD‐L1 have been approved in several countries for the treatment of PD‐L1‐positive TNBC. 44 Recent studies using human breast cancer samples have indicated that PD‐L1 is mainly expressed on immune cells, especially on TAMs. 45 Expression of PD‐L1 was reportedly detected in cancer cells (cut‐off value, 1%) in 12% of cases and in immune cells (cut‐off value, 10%) in 28% of cases, and high PD‐L1 expression in immune cells was associated with a favorable clinical course. 46 Spatial gene expression analyses indicated that a high IFN signature was linked to increased PD‐L1 expression, suggesting that PD‐L1 expression reflects the anticancer immune response in TNBC tissues. 47 A high density of PD‐L1+ and CD163− TAMs in the cancer nest is predictive of a better clinical course. 48 Another study reported a positive correlation between CTL infiltration and PD‐L1 expression, 49 indicating that lymphocyte‐derived factors mediate PD‐L1 overexpression in TAMs. In addition to IFN signals, GM‐CSF derived from activating lymphocytes synergistically enhances PD‐L1 expression in TAMs through STAT3 signaling activation. 50 It has been suggested that STAT3 signaling enhances PD‐L1 expression mediated by IFN‐STAT1 signaling.
5. PROTUMOR FUNCTIONS OF TAMs AND TAM‐TARGETING THERAPIES: INSIGHTS FROM ANIMAL STUDIES
Animal studies have provided many insights into the functions of TAMs. The anticancer functions of TAMs have been examined using animal models since the 1990s. However, following a 2001 study examining the protumor function of TAMs using an MMTV‐PyMT mouse breast cancer model, 51 many studies have focused on the protumor functions of TAMs. Cancer cell‐derived CSF‐1 stimulates protumor TAMs, which in turn secrete EGF and promote cancer cell migration, 52 and that study also suggested that cell–cell communication between cancer cells and TAMs plays an important role in breast cancer. Cancer cell‐derived CCL2 (MCP1) induces the recruitment of Gr1+/Ly6C+ inflammatory monocytes into metastatic sites, whereas Ab blockade of CCL2 suppresses lung metastasis, and VEGF derived from myeloid cells plays a role in the extravasation of cancer cells. 53 High CCL2 production has been reported in TNBC cells, activating cancer cell invasion and tumor progression through the MAPK signaling pathway in an autocrine manner. 54 Similar mechanisms were reported in studies of the CCL5–CCR5 axis. Breast cancer cells express CCL5, which potentially predicts a worse clinical course, 55 and blockade of CCR5 was shown to suppress cancer cell invasion and metastasis while enhancing chemosensitivity. 56 Production of CCL2 and CCL5 by cancer cells is elevated by IFN, and the inhibitor FROUNT (a coactivator of CCR2 and CCR5) was shown to abrogate breast cancer cell growth by inhibiting TAM recruitment. 40
Breast cancer cells also secrete CSF‐1 and IL‐34, which are ligands of CSF‐1R and recruit TAMs into cancer tissues. An antagonist of CSF‐1R suppressed cancer cell growth in combination with cytotoxic therapy by enhancing the anticancer immune responses. 57 Inhibition of CSF‐1R signaling was shown to decrease TAM infiltration and increase the numbers of central and effector memory T cells; cell–cell communication between T cells and TAMs may suppress the response to anticancer agents. 58 Thus, inhibiting the migration of TAMs into cancer tissues is an additional promising approach for anticancer therapy.
Reprogramming the protumor phenotype of TAMs to a tumoricidal phenotype, a process known as macrophage conversion therapy, has been reported to be an effective anticancer approach. 59 Inhibition of PI3Kγ was shown to enhance the anticancer effect of several therapies by inducing TAM polarization into an M1‐like phenotype. 60 Peroxisome proliferator‐activated receptor‐γ and retinoic acid signaling mediated by MAPK and histone deacetylase was shown to be involved in M2‐like polarization, and thus, inhibitors of these pathways are potentially useful for anticancer therapy. 61 Cyclic sulfur compounds that suppress the phenotypic change of TAMs to the M2‐like/protumor phenotype exert anticancer effects by inducing anticancer T cell responses. 62 Among the many signaling pathways related to M1/M2 balance, STAT3 signaling is one of the most critical with regard to change of TAMs to the M2‐like/protumor phenotype. Selective STAT3 inhibition in TAMs is thus suggested to be an effective anticancer therapy modulating the M1/M2 balance of TAMs. 63
Other research is focusing on the phagocytic activity of TAMs. CD47 is a “don't eat me signal” expressed by red blood cells that has also been detected in cancer cells. Blocking the interaction between CD47 and signal regulatory protein‐α (SIRPα) using an anti‐CD47 Ab increased the phagocytosis of cancer cells by TAMs; this anticancer effect of the anti‐CD47 Ab in combination with an anti‐CD20 Ab was first reported against B‐cell lymphoma. 64 Breast cancer cells express CD47, and CD47 positivity has been linked to a poor clinical course. Animal studies showed that treatment with an anti‐CD47 Ab enhances STING‐stimulating immunotherapy. 65 STING is a critical molecule involved in the conversion of macrophages to the M1‐like phenotype. 66
6. MACROPHAGES AS CRITICAL DETERMINANTS FOR THE EFFICACY OF CANCER IMMUNOTHERAPY: BEYOND THE CLASSICAL TAM‐TARGETING STRATEGY
Macrophages are critical cells that direct the immune response against tumors as well as the responsiveness to anti‐PD1/PD‐L1 therapy because of their immunosuppressive and protumorigenic effects that impede the activation and effector function of tumor‐specific T cells. 67 Tumor‐associated macrophages contribute to resistance to anti‐PD1/PD‐L1 therapy through several mechanisms. The simplest interpretation is that anti‐PD1/PD‐L1 therapy is not sufficient to reactivate tumor‐specific T cells due to the strong effects of TAM‐derived immunosuppressive factors such as transforming growth factor‐β, IL‐6, and IL‐10, other than PD‐L1/L2. 67 An alternative mechanism underlying resistance to therapy is that Fc receptors expressed by TAMs strip the anti‐PD‐1 Abs bound to T cells, which reactivates the PD1/PD‐L1 signaling and prevents T cell activation. 68 Furthermore, it has been suggested that modulation of PD1 signaling in PD1‐expressing TAMs by anti‐PD1/PD‐L1 therapy increases IL‐6 production, leading to immunosuppressive effects against antitumor T cell responses, 69 or conversely, polarization of TAMs into an immunostimulatory phenotype, 70 the effects of which could vary depending on the type of tumor. Based on these findings, promising research has focused on treatment strategies combining targeting of macrophages and the PD1/PD‐L1 interaction. Indeed, a preclinical mouse model study showed that targeting TAMs increases the responsiveness to anti‐PD1/PD‐L1 therapy. 71 , 72 However, for such treatments, heterogeneity in terms of functional differences must be considered, 67 in addition to the source or ontogenetic origin (monocytic vs. embryonic progenitors), 73 and subsequent movement or niches in the tumor microenvironment. 74
Single‐cell RNA sequencing analyses have documented the heterogeneity of TAMs in human breast cancer, suggesting that TAM subpopulations coexisting within the same tumor tissue play both immunostimulatory and immunosuppressive roles. A recent study showed that clonal expansion of CD8+ T cells responsible for tumor immunosurveillance is inversely correlated with the presence of CX3CR1+ rather than CCR2+ cells among TAM subpopulations in breast cancer tissue, 75 suggesting that certain types of TAMs enriched within tumor tissues have a negative impact on T cell‐mediated antitumor immunity. On the other hand, two distinct subpopulations, FOLR2+ and TREM2/CADM1+ TAMs, have been identified in breast tumors. 31 , 76 , 77 Folate receptor 2‐positive macrophages are also found in healthy breast tissue as tissue‐resident macrophages, but they are largely eliminated during tumor progression. A beneficial role of FOLR2+ resident macrophages in tumor control is suggested by observations that a high frequency of these cells is associated with signature antitumor immune responses, such as higher infiltration of CD8+ T cells and dendritic cells, with better outcomes for patients with breast cancer. 31 Interestingly, these TAMs preferentially localize in the tumor stroma, especially within the TLS in tumor tissues. 76 This phenomenon is consistent with the hypothesis that the presence of TLS in the tumor microenvironment is associated with better response to the anti‐PD1/PD‐L1 therapy. 78 However, this assumption regarding tissue‐resident macrophages is not consistent with the results of other studies showing that CX3CR1+CCR2low‐mid tissue‐resident TAMs exert tumor‐promoting functions in the TME of particular organs in cancers such as pancreatic ductal adenocarcinoma, colon adenocarcinoma, and brain tumors. 73 , 74 , 79
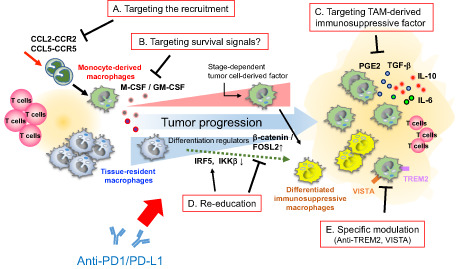
In contrast to FOLR2+ macrophages, TREM2+ macrophages in breast cancer have been correlated with poor prognosis and induced T cell exhaustion associated with resistance to anti‐PD1/PD‐L1 therapy. 31 , 71 This immunosuppressive subpopulation of TAMs tends to reside close to the tumor nest. 31 Therefore, in pathological diagnosis, such spatial distribution of specific macrophages could be of particular interest in determining whether the tumor microenvironment is susceptible to anti‐PD1/PD‐L1 therapy. Meanwhile, the complex and diverse heterogeneity and differential functions of TAMs comprising a mixture of both monocyte‐derived and tissue‐resident macrophages likely vary depending on the stage of cancer progression and the characteristics of the tissue in which the tumors are located. 80 Typically, the effect of tumor‐related factors that regulate TAM survival/differentiation, such as GM‐CSF and CSF‐1, changes with tumor progression and disease stage. 81 , 82 , 83 Macrophage‐targeting therapeutic approaches have traditionally focused on reducing the infiltration of macrophages into tumor tissues by inhibiting relevant signals. 67 Modulation of CCL2–CCR2 axis‐mediated recruitment and enrichment of monocyte‐derived immunosuppressive macrophages has also been proposed as a combined strategy in conjunction with anti‐PD1/PD‐L1 therapy. 72 However, such approaches are likely to provide less improvement of antitumor immunity for cancers with tissue‐resident immunosuppressive TAMs. Approaches targeting the whole TAM population might fail to take advantage of the potential antitumor activity of immunostimulatory macrophages. Therefore, tissue‐ or subpopulation‐specific TAM‐targeting strategies such as anti‐TREM2+ cell‐specific targeting are promising cancer type‐tailored immunotherapeutic approaches. 67 It is also important to consider the re‐education of macrophage functions through modification of master regulators such as the IRF5/IKKβ and β‐catenin/FOSL2/ARID5A pathways to direct the differentiation into immunostimulatory or immunosuppressive macrophages (Figure 4). 84 , 85
FIGURE 4.
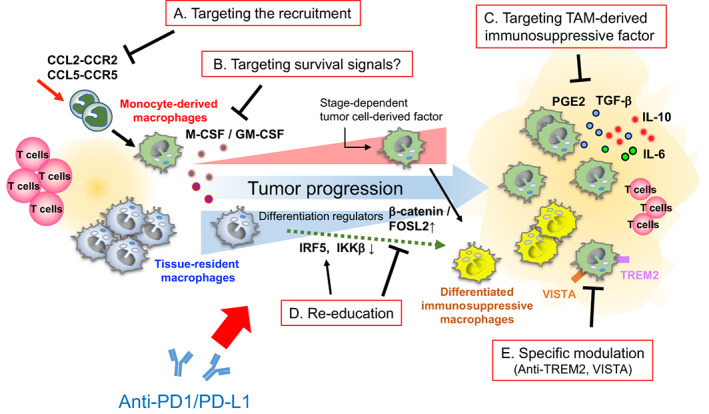
Treatments targeting tumor‐associated macrophages (TAMs) to improve the therapeutic efficacy of anti‐programmed death 1 (PD1)/PD1 ligand 1 (PD‐L1) therapy. Multiple modes of targeting TAMs to restore resistance to anti‐PD1/PD‐L1 therapy. (A) Anti‐PD1/PD‐L1 therapy combined with inhibiting the recruitment and infiltration of macrophage precursors (monocytes) in the tumor microenvironment (TME). (B) Ameliorating the survival signals responsible for the maintenance of immunosuppressive TAMs can effectively prevent the infiltration of TAMs in the TME. (C) Specific inhibition of TAM‐derived immunosuppressive factors. (D) Re‐education of immunosuppressive TAMs to the immunostimulatory phenotype by modifying the expression of differentiation regulators. (E) Combination of precise and specific targeting of immunosuppressive TAMs in the TME together with anti‐PD1/PD‐L1 therapy improves the responsiveness to treatment. CCL, C‐C motif chemokine ligand; CCR, C‐C motif chemokine receptor; FOSL2, FOS‐like antigen 2; GM‐CSF, granulocyte/macrophage colony‐stimulating factor; IKKβ, inhibitor of nuclear factor kappa‐B kinase subunit β; IL, interleukin; IRF5, interferon regulatory factor 5; M‐CSF, macrophage colony‐stimulating factor; PGE2, prostaglandin E2; TGF‐β, transforming growth factor‐β; TREM2, triggering receptor expressed on myeloid cells 2.
FUNDING INFORMATION
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (20H03459).
CONFLICT OF INTEREST STATEMENT
Y.K. is an editorial board member of Cancer Science. The other authors have no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an institutional review board: The study design was approved by the Institutional Review Board of Kumamoto University (#2059) in accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki.
Informed consent: The need for individual patient consent for inclusion in the study was waived by the Institutional Review Board of Kumamoto University (#2059) since the present study was a retrospective analysis using previously published data 27 ; however, although all of the retrospective patient data were automatically included in the study, the patients were given the opportunity to refuse participation by opting out of the study.
Registry and registration no. of the study/trial; N/A.
Approval for animal experiments: N/A.
ACKNOWLEDGMENTS
We thank Mr Takenobu Nakagawa, Ms Kumiko Imabayashi, Ms Mitsuko Haruki, and Mr Shingo Usuki for technical assistance. This work was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (20H03459).
Komohara Y, Kurotaki D, Tsukamoto H, et al. Involvement of protumor macrophages in breast cancer progression and characterization of macrophage phenotypes. Cancer Sci. 2023;114:2220‐2229. doi: 10.1111/cas.15751
Yoshihiro Komohara, Daisuke Kurotaki, and Hirotake Tsukamoto contributed equally to this work.
REFERENCES
- 1. Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39:1485‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134‐1150. [DOI] [PubMed] [Google Scholar]
- 3. Cortes J, Rugo HS, Cescon DW, et al. Pembrolizumab plus chemotherapy in advanced triple‐negative breast cancer. N Engl J Med. 2022;387:217‐226. [DOI] [PubMed] [Google Scholar]
- 4. Kreutz M, Fritsche J, Andreesen R. Macrophages in tumor biology. In: Burke B, Lewis CE, eds. The Macrophage. 2nd ed. Oxford Univ. Press; 2002:458‐489. [Google Scholar]
- 5. Wu SZ, Al‐Eryani G, Roden DL, et al. A single‐cell and spatially resolved atlas of human breast cancers. Nat Genet. 2021;53:1334‐1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23‐35. [DOI] [PubMed] [Google Scholar]
- 7. Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32:463‐488. [DOI] [PubMed] [Google Scholar]
- 8. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsukamoto H, Komohara Y, Oshiumi H. The role of macrophages in anti‐tumor immune responses: pathological significance and potential as therapeutic targets. Hum Cell. 2021;34:1031‐1039. [DOI] [PubMed] [Google Scholar]
- 10. Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17:887‐904. [DOI] [PubMed] [Google Scholar]
- 11. Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor‐associated macrophages: potential therapeutic targets for anti‐cancer therapy. Adv Drug Deliv Rev. 2016;99:180‐185. [DOI] [PubMed] [Google Scholar]
- 12. Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bingle L, Brown NJ, Lewis CE. The role of tumour‐associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254‐265. [DOI] [PubMed] [Google Scholar]
- 14. Tang R, Beuvon F, Ojeda M, Mosseri V, Pouillart P, Scholl S. M‐CSF (monocyte colony stimulating factor) and M‐CSF receptor expression by breast tumour cells: M‐CSF mediated recruitment of tumour infiltrating monocytes? J Cell Biochem. 1992;50:350‐356. [DOI] [PubMed] [Google Scholar]
- 15. Pulford KA, Sipos A, Cordell JL, Stross WP, Mason DY. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990;2:973‐980. [DOI] [PubMed] [Google Scholar]
- 16. O'Sullivan C, Lewis CE, Harris AL, McGee JO. Secretion of epidermal growth factor by macrophages associated with breast carcinoma. Lancet. 1993;342:148‐149. [DOI] [PubMed] [Google Scholar]
- 17. Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625‐4629. [PubMed] [Google Scholar]
- 18. Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14:425‐431. [PubMed] [Google Scholar]
- 19. Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour‐infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65:159‐163. [DOI] [PubMed] [Google Scholar]
- 20. Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198‐201. [DOI] [PubMed] [Google Scholar]
- 21. Suzuki H, Kurihara Y, Takeya M, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292‐296. [DOI] [PubMed] [Google Scholar]
- 22. Shiraishi D, Fujiwara Y, Horlad H, et al. CD163 is required for Protumoral activation of macrophages in human and murine sarcoma. Cancer Res. 2018;78:3255‐3266. [DOI] [PubMed] [Google Scholar]
- 23. Komohara Y, Takemura K, Lei XF, et al. Delayed growth of EL4 lymphoma in SR‐A‐deficient mice is due to upregulation of nitric oxide and interferon‐gamma production by tumor‐associated macrophages. Cancer Sci. 2009;100:2160‐2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Linde N, Casanova‐Acebes M, Sosa MS, et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat Commun. 2018;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Etzerodt A, Tsalkitzi K, Maniecki M, et al. Specific targeting of CD163+ TAMs mobilizes inflammatory monocytes and promotes T cell‐mediated tumor regression. J Exp Med. 2019;216:2394‐2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sousa S, Brion R, Lintunen M, et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015;17:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miyasato Y, Shiota T, Ohnishi K, et al. High density of CD204‐positive macrophages predicts worse clinical prognosis in patients with breast cancer. Cancer Sci. 2017;108:1693‐1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuroda H, Jamiyan T, Yamaguchi R, et al. Tumor microenvironment in triple‐negative breast cancer: the correlation of tumor‐associated macrophages and tumor‐infiltrating lymphocytes. Clin Transl Oncol. 2021;23:2513‐2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strack E, Rolfe PA, Fink AF, et al. Identification of tumor‐associated macrophage subsets that are associated with breast cancer prognosis. Clin Transl Med. 2020;10:e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bobrie A, Massol O, Ramos J, et al. Association of CD206 protein expression with immune infiltration and prognosis in patients with triple‐negative breast cancer. Cancers (Basel). 2022;14:4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nalio Ramos R, Missolo‐Koussou Y, Gerber‐Ferder Y, et al. Tissue‐resident FOLR2+ macrophages associate with CD8+ T cell infiltration in human breast cancer. Cell. 2022;185:1189‐1207.e25. [DOI] [PubMed] [Google Scholar]
- 32. Obradovic A, Chowdhury N, Haake SM, et al. Single‐cell protein activity analysis identifies recurrence‐associated renal tumor macrophages. Cell. 2021;184:2988‐3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singhal S, Stadanlick J, Annunziata MJ, et al. Human tumor‐associated monocytes/macrophages and their regulation of T cell responses in early‐stage lung cancer. Sci Transl Med. 2019;11:eaat1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frankenberger M, Schwaeble W, Ziegler‐Heitbrock L. Expression of M‐ficolin in human monocytes and macrophages. Mol Immunol. 2008;45:1424‐1430. [DOI] [PubMed] [Google Scholar]
- 35. Matsubara E, Komohara Y, Esumi S, et al. SPP1 derived from macrophages is associated with a worse clinical course and chemo‐resistance in lung adenocarcinoma. Cancers (Basel). 2022;14:4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ch'ng ES, Tuan Sharif SE, Jaafar H. In human invasive breast ductal carcinoma, tumor stromal macrophages and tumor nest macrophages have distinct relationships with clinicopathological parameters and tumor angiogenesis. Virchows Arch. 2013;462:257‐267. [DOI] [PubMed] [Google Scholar]
- 37. Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nozoe T, Oyama T, Mori E, et al. Clinicopathologic significance of an immunohistochemical expression of p27 in scirrhous carcinoma of the breast. Breast Cancer. 2007;14:277‐280. [DOI] [PubMed] [Google Scholar]
- 39. Vlaicu P, Mertins P, Mayr T, et al. Monocytes/macrophages support mammary tumor invasivity by co‐secreting lineage‐specific EGFR ligands and a STAT3 activator. BMC Cancer. 2013;13:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yonemitsu K, Miyasato Y, Shiota T, et al. Soluble factors involved in cancer cell‐macrophage interaction promote breast cancer growth. Anticancer Res. 2021;41:4249‐4258. [DOI] [PubMed] [Google Scholar]
- 41. Asanprakit W, Lobo DN, Eremin O, Bennett AJ. M1 macrophages evoke an increase in polymeric immunoglobulin receptor (PIGR) expression in MDA‐MB468 breast cancer cells through secretion of interleukin‐1β. Sci Rep. 2022;12:16842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qin Q, Ji H, Li D, Zhang H, Zhang Z, Zhang Q. Tumor‐associated macrophages increase COX‐2 expression promoting endocrine resistance in breast cancer via the PI3K/Akt/mTOR pathway. Neoplasma. 2021;68:938‐946. [DOI] [PubMed] [Google Scholar]
- 43. Zheng S, Zou Y, Xie X, et al. Development and validation of a stromal immune phenotype classifier for predicting immune activity and prognosis in triple‐negative breast cancer. Int J Cancer. 2020;147:542‐553. [DOI] [PubMed] [Google Scholar]
- 44. Keenan TE, Tolaney SM. Role of immunotherapy in triple‐negative breast cancer. J Natl Compr Canc Netw. 2020;18:479‐489. [DOI] [PubMed] [Google Scholar]
- 45. Wang J, Browne L, Slapetova I, et al. Multiplexed immunofluorescence identifies high stromal CD68+PD‐L1+ macrophages as a predictor of improved survival in triple negative breast cancer. Sci Rep. 2021;11:21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hong M, Kim JW, Kim MK, Chung BW, Ahn SK. Programmed cell death‐ligand 1 expression in stromal immune cells is a marker of breast cancer outcome. J Cancer. 2020;11:7246‐7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gruosso T, Gigoux M, Manem VSK, et al. Spatially distinct tumor immune microenvironments stratify triple‐negative breast cancers. J Clin Invest. 2019;129:1785‐1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shinohara H, Kobayashi M, Hayashi K, et al. Spatial and quantitative analysis of tumor‐associated macrophages: Intratumoral CD163‐/PD‐L1+ TAMs as a marker of favorable clinical outcomes in triple‐negative breast cancer. Int J Mol Sci. 2022;23:13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nagano M, Saito K, Kozuka Y, et al. CD204‐positive macrophages accumulate in breast cancer tumors with high levels of infiltrating lymphocytes and programmed death ligand‐1 expression. Oncol Lett. 2021;21:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yonemitsu K, Pan C, Fujiwara Y, et al. GM‐CSF derived from the inflammatory microenvironment potentially enhanced PD‐L1 expression on tumor‐associated macrophages in human breast cancer. Sci Rep. 2022;12:12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony‐stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wyckoff J, Wang W, Lin EY, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022‐7029. [DOI] [PubMed] [Google Scholar]
- 53. Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast‐tumour metastasis. Nature. 2011;475:222‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dutta P, Sarkissyan M, Paico K, Wu Y, Vadgama JV. MCP‐1 is overexpressed in triple‐negative breast cancers and drives cancer invasiveness and metastasis. Breast Cancer Res Treat. 2018;170:477‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yaal‐Hahoshen N, Shina S, Leider‐Trejo L, et al. The chemokine CCL5 as a potential prognostic factor predicting disease progression in stage II breast cancer patients. Clin Cancer Res. 2006;12:4474‐4480. [DOI] [PubMed] [Google Scholar]
- 56. Jiao X, Wang M, Zhang Z, et al. Leronlimab, a humanized monoclonal antibody to CCR5, blocks breast cancer cellular metastasis and enhances cell death induced by DNA damaging chemotherapy. Breast Cancer Res. 2021;23:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Singh S, Lee N, Pedroza DA, et al. Chemotherapy coupled to macrophage inhibition induces T‐cell and B‐cell infiltration and durable regression in triple‐negative breast cancer. Cancer Res. 2022;82:2281‐2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mills CD, Lenz LL, Harris RA. A breakthrough: macrophage‐directed cancer immunotherapy. Cancer Res. 2016;76:513‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. De Henau O, Rausch M, Winkler D, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature. 2016;539:443‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. He L, Jhong JH, Chen Q, et al. Global characterization of macrophage polarization mechanisms and identification of M2‐type polarization inhibitors. Cell Rep. 2021;37:109955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pan C, Fujiwara Y, Horlad H, Iriki T, Shiraishi D, Komohara Y. Cyclic sulfur compounds targeting macrophage polarization into M2/protumor phenotype and their anti‐tumor effects. Cancer Immunol Immunother. 2022;71:1331‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Andersen MN, Etzerodt A, Graversen JH, et al. STAT3 inhibition specifically in human monocytes and macrophages by CD163‐targeted corosolic acid‐containing liposomes. Cancer Immunol Immunother. 2019;68:489‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Advani R, Flinn I, Popplewell L, et al. CD47 blockade by Hu5F9‐G4 and rituximab in non‐Hodgkin's lymphoma. N Engl J Med. 2018;379:1711‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kosaka A, Ishibashi K, Nagato T, et al. CD47 blockade enhances the efficacy of intratumoral STING‐targeting therapy by activating phagocytes. J Exp Med. 2021;218:e20200792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ohkuri T, Ghosh A, Kosaka A, et al. STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol Res. 2014;2:1199‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Arlauckas SP, Garris CS, Kohler RH, et al. In vivo imaging reveals a tumor‐associated macrophage‐mediated resistance pathway in anti‐PD‐1 therapy. Sci Transl Med. 2017;9:eaal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tsukamoto H, Fujieda K, Miyashita A, et al. Combined blockade of IL6 and PD‐1/PD‐L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78:5011‐5022. [DOI] [PubMed] [Google Scholar]
- 70. Xiong H, Mittman S, Rodriguez R, et al. Anti‐PD‐L1 treatment results in functional remodeling of the macrophage compartment. Cancer Res. 2019;79:1493‐1506. [DOI] [PubMed] [Google Scholar]
- 71. Binnewies M, Pollack JL, Rudolph J, et al. Targeting TREM2 on tumor‐associated macrophages enhances immunotherapy. Cell Rep. 2021;37:109844. [DOI] [PubMed] [Google Scholar]
- 72. Flores‐Toro JA, Luo D, Gopinath A, et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc Natl Acad Sci U S A. 2020;117:1129‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhu Y, Herndon JM, Sojka DK, et al. Tissue‐resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity. 2017;47:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Soncin I, Sheng J, Chen Q, et al. The tumour microenvironment creates a niche for the self‐renewal of tumour‐promoting macrophages in colon adenoma. Nat Commun. 2018;9:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bassez A, Vos H, Van Dyck L, et al. A single‐cell map of intratumoral changes during anti‐PD1 treatment of patients with breast cancer. Nat Med. 2021;27:820‐832. [DOI] [PubMed] [Google Scholar]
- 76. Bugatti M, Bergamini M, Missale F, et al. A population of TIM4+FOLR2+ macrophages localized in tertiary lymphoid structures correlates to an active immune infiltrate across several cancer types. Cancer Immunol Res. 2022;10:1340‐1353. [DOI] [PubMed] [Google Scholar]
- 77. Molgora M, Esaulova E, Vermi W, et al. TREM2 modulation remodels the tumor myeloid landscape enhancing anti‐PD‐1 immunotherapy. Cell. 2020;182:886‐900.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561‐565. [DOI] [PubMed] [Google Scholar]
- 79. Friebel E, Kapolou K, Unger S, et al. Single‐cell mapping of human brain cancer reveals tumor‐specific instruction of tissue‐invading leukocytes. Cell. 2020;181:1626‐1642.e20. [DOI] [PubMed] [Google Scholar]
- 80. Braun DA, Street K, Burke KP, et al. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell. 2021;39:632‐648.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schulz M, Michels B, Niesel K, et al. Cellular and molecular changes of brain metastases‐associated myeloid cells during disease progression and therapeutic response. iScience. 2020;23:101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hamidzadeh K, Belew AT, El‐Sayed NM, Mosser DM. The transition of M‐CSF‐derived human macrophages to a growth‐promoting phenotype. Blood Adv. 2020;4:5460‐5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shinchi Y, Ishizuka S, Komohara Y, et al. The expression of PD‐1 ligand 1 on macrophages and its clinical impacts and mechanisms in lung adenocarcinoma. Cancer Immunol Immunother. 2022;71:2645‐2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang F, Parayath NN, Ene CI, et al. Genetic programming of macrophages to perform anti‐tumor functions using targeted mRNA nanocarriers. Nat Commun. 2019;10:3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sarode P, Zheng X, Giotopoulou GA, et al. Reprogramming of tumor‐associated macrophages by targeting β‐catenin/FOSL2/ARID5A signaling: a potential treatment of lung cancer. Sci Adv. 2020;6:eaaz6105. [DOI] [PMC free article] [PubMed] [Google Scholar]


