Abstract
Background
There is an incomplete understanding of the predictors of morbidity and mortality in patients with severe tricuspid regurgitation (TR). This study sought to identify key risk factors for all-cause mortality and heart failure (HF) hospitalization among patients with severe TR.
Methods
Patients with severe TR were identified from 2 centers, Oregon Health & Science University and Abrazo Health, from January 01, 2016 to December 31, 2018. Patients with any concomitant severe valvular diseases or prior valvular intervention were excluded. Multivariable regression was utilized to identify demographic, clinical, and echocardiographic variables independently associated with all-cause mortality or HF hospitalization.
Results
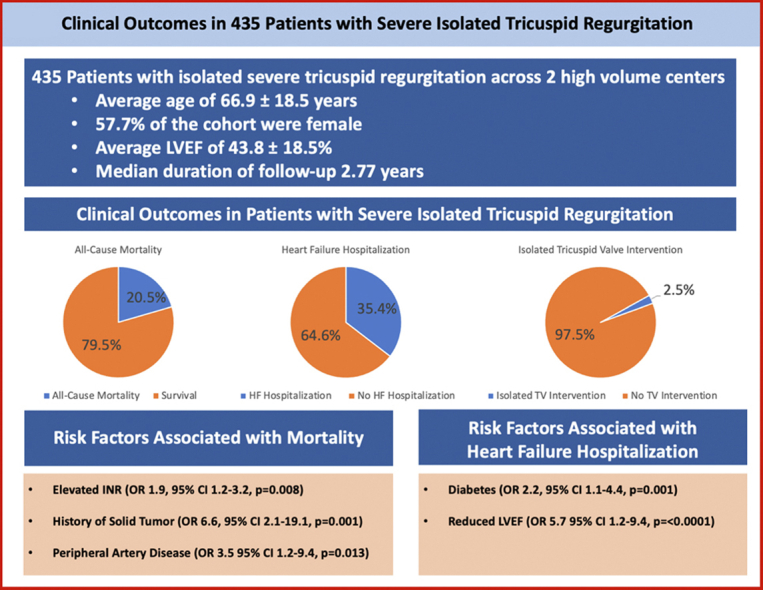
435 patients with severe TR were followed for a median of 2.8 years. The mean age of the population was 66.9 ± 18.5 years and 58% were female. All-cause mortality was identified in 20.5% of the population. Of the cohort, 35.4% of patients were hospitalized for HF. Isolated tricuspid valve intervention was performed in 2.5% of patients. Independent predictors of all-cause mortality included history of solid tumor (odds ratio [OR] 6.6, 95% confidence interval [CI] 2.1-19.1, p = 0.001), history of peripheral artery disease (OR 3.5, 95% CI 1.2-9.4, p = 0.013), and elevated international normalized ratio in the absence of anticoagulation (OR 1.9, 95% CI 1.2-3.2, p = 0.008). Predictors of HF hospitalization included history of diabetes mellitus (OR 2.2, 95% CI 1.1-4.0, p = 0.014) and history of reduced left ventricular ejection fraction (OR 5.7, 95% CI 2.9-11.7, p < 0.0001).
Conclusions
Severe untreated TR is associated with high mortality and frequent HF hospitalizations. Understanding predictors of these outcomes is important to identify patients who may benefit from early tricuspid valve intervention to help improve outcomes in this patient population.
Keywords: Heart failure hospitalization, Mortality, Outcomes, Tricuspid regurgitation
Graphical abstract
Introduction
Tricuspid regurgitation (TR) is a common form of valvular heart disease, with over 80% of patients in the Framingham Heart Study having detectable TR and up to 15% of male patients and 18% of female patients in the community setting having at least mild TR.1,2 TR poses significant limitations in functional status of patients due to symptoms of right-sided heart failure (HF) which may include exertional dyspnea, fatigue, and lower extremity edema.3 Symptoms due to TR are due in part to reduced cardiac output reserve, elevated systemic and pulmonary venous filling pressures, and elevated left ventricular pressures due to enhanced ventricular interaction which over time may contribute to subsequent right ventricle (RV) failure.4,5
Severe TR is associated with increased mortality,2 even in the absence of RV or left ventricle (LV) dysfunction and/or elevated pulmonary artery (PA) pressures.6 Increasing TR severity in the natural history of tricuspid valvular disease demonstrates poorer prognosis in patients with TR, independent of patient demographic characteristics,7, 8, 9 as well as in patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension.10
Current medical therapy for the treatment of severe TR remains limited and is aimed at relieving symptoms of right-sided HF through diuretics or aldosterone antagonists, and reducing PA pressures and pulmonary vascular resistance through the use of vasodilators in patients with precapillary pulmonary hypertension. Surgical intervention for isolated severe primary or secondary TR with HF that is unresponsive to medical therapy remains a Class IIA indication, with Level of Evidence C,11,12 though often RV remodeling and dysfunction has already occurred and medical therapy remains the most commonly utilized therapeutic option.13 Surgical intervention for isolated severe TR may be associated with improved survival compared with medical therapy alone, albeit associated with high procedural morbidity and mortality.14
Due to the high rates of morbidity/mortality in this cohort related to surgical therapies, a new era of transcatheter therapeutics has emerged as an area of interest to provide another therapeutic modality in the treatment of this complex population to improve symptoms, reduce hospitalizations, and potentially survival. This is accomplished through either coaptation of the leaflets using off-label MitraClip (Abbott Vascular, Menlo Park, CA), annuloplasty rings or spacer devices, valve-in-valve transcatheter replacement,15, 16, 17 or dedicated transcatheter valve systems and coaptation devices designed specifically for the tricuspid valve (TV) currently under investigation such as the EVOQUE (Edwards Lifesciences, Irvine, CA) transcatheter TV replacement device,18 TriClip (Abbott Vascular, Menlo Park, CA),19 and PASCAL (Edwards Lifesciences, Irvine, CA) repair devices.20
Despite evidence that TR progression and severe TR is associated with a higher risk for death, poorer prognosis, and increased morbidity and mortality, predictors of mortality in this patient population are not well-defined. Previous studies have demonstrated that echocardiographic variables such as estimated right ventricular systolic pressure greater than 40 mmHg as measured by transthoracic echocardiogram (TTE) is a predictor of lower event-free survival in patients with moderate TR, but not severe TR,21 while elevated central venous pressure via TTE is a predictor of adverse outcomes in isolated TR.22
Therefore, we aimed to identify clinical, demographic, and echocardiographic predictors of mortality and HF hospitalization in a population of patients with severe TR, so that this subset of patients at higher risk for death can be identified earlier and treated with medical, surgical, or advanced transcatheter therapeutic options.
Methods
Study Population and Design
We performed a retrospective cohort study of consecutive patients with severe TR on echocardiogram from 2 centers: Oregon Health and Science University and Abrazo Health between January 01, 2016 and December 31, 2018. Patients with severe TR were identified via retrospective review using Mpirik Cardiac Intelligence (Milwaukee, WI) software and subsequently confirmed by authors (KK, TS) to verify accuracy. TR severity was assessed via a combination of quantitative measures (regurgitant volume >45 mL, effective regurgitant orifice area >40 mm2), semiquantitative measures (hepatic vein flow reversal, proximal isovelocity surface area >0.9 cm, vena contracta width >7 mm), and qualitative assessments by color Doppler echocardiography as trace, mild, moderate, or severe as per the American Society of Echocardiography criteria.23 Subjects with severe TR at the time of index TTE were included for analysis. Patients with a history of complex congenital heart disease; previous aortic, mitral, or TV intervention; concomitant severe aortic, mitral, or pulmonic valve disease; and those with incomplete follow-up data were excluded from the analysis.
Demographics, baseline characteristics, comorbid conditions, laboratory data, medications, and therapies were extracted closest to the date of the index TTE demonstrating severe TR. The primary outcome was all-cause mortality during the follow-up period. Secondary outcomes were HF hospitalization and TV intervention (isolated tricuspid surgery and/or heart transplant) during the follow-up period. Time to primary and secondary outcomes was defined as the time from the index TTE to the event. Clinical outcomes were subsequently reviewed via retrospective chart review through the date of the last known follow-up. All extracted variables were reviewed by authors (KK, TS) to verify accuracy.
This study was approved by the institutional review board of both institutions. Requirement for written informed consent was waived given the retrospective nature of the study and analysis.
Statistical Analysis
Patient characteristics are reported as frequencies and percentages for categorical variables and means with standard deviations for continuous variables. Clinical variables were assessed for the probability of the primary and secondary outcomes via univariable logistic regression analysis to identify statistically significant predictors. The final analysis combined the statistically significant univariable covariates in a multivariable model. Multivariable logistic regression was then utilized to identify the predictors of mortality and HF hospitalization in patients with severe TR. All analysis was performed via R stan software (rstanarm R package, v.2.21.1, 2020) and p values < 0.05 were considered statistically significant.
Results
Demographics and Clinical Characteristics
A total of 435 patients were identified as having severe TR at index TTE following exclusion for other severe valvular disease and complex congenital heart disease, and were included in the analysis cohort. Baseline characteristics of the cohort are reported in Table 1. The mean age was 66.9 ± 18.5 years, and 57.7% were female. Patients were followed for both the primary and secondary endpoints for a median follow-up time of 2.77 years. The average Society of Thoracic Surgeons (STS) score of the cohort was 6.7%. Patients with liver disease had a mean STS score of 8.8% and those with peripheral artery disease (PAD) had a mean STS score of 7.6%.
Table 1.
Baseline characteristics of patients with severe tricuspid regurgitation
| Baseline characteristics | Total cohort (n = 435) |
|---|---|
| Demographics | |
| Age, years | 66.9 ± 18.5 |
| Female, n (%) | 251 (57.7%) |
| STS score for mortality, (%) | 6.7% |
| Comorbid conditions | |
| Atrial fibrillation/flutter, n (%) | 229 (52.6%) |
| Coronary artery disease, n (%) | 158 (36.3%) |
| History of CABG, n (%) | 51 (11.7%) |
| History of PCI, n (%) | 45 (10.3%) |
| Diabetes mellitus, n (%) | 126 (28.9%) |
| Chronic kidney disease, n (%) | 94 (21.6%) |
| Dialysis, n (%) | 34 (7.8%) |
| Hypertension, n (%) | 276 (63.4%) |
| Heart failure, n (%) | 268 (61.6%) |
| Peripheral artery disease, n (%) | 45 (10.3%) |
| Former or current smoker, n (%) | 137 (31.5%) |
| History of solid tumor, n (%) | 39 (8.9%) |
| History of stroke or TIA, n (%) | 79 (18.2%) |
| COPD, n (%) | 81 (18.6%) |
| Obstructive sleep apnea, n (%) | 43 (9.8%) |
| Interstitial lung disease, n (%) | 9 (2.0%) |
| Connective tissue disease, n (%) | 6 (1.3%) |
| Liver disease, n (%) | 63 (14.5%) |
| Laboratory data | |
| Hemoglobin, g/dL | 11.3 ± 2.5 |
| Creatinine, mg/dL | 1.58 ± 1.58 |
| INR of cohort | 1.63 |
| INR on AC | 1.89 |
| INR not on AC | 1.35 |
| AST/ALT of cohort | 52/40 U/L |
| AST/ALT on AC | 57/38 U/L |
| AST/ALT not on AC | 46/44 U/L |
| Medical therapy | |
| ACEi/ARB, n (%) | 105 (24.1%) |
| Beta blocker, n (%) | 173 (39.7%) |
| Calcium channel blocker, n (%) | 43 (9.8%) |
| Loop diuretic, n (%) | 176 (40.5%) |
| Thiazide diuretic, n (%) | 45 (10.3%) |
| Aldosterone antagonist, n (%) | 49 (11.2%) |
| Anticoagulation, n (%) | 129 (29.6%) |
| Antiarrhythmic, n (%) | 65 (14.9%) |
| Statin, n (%) | 133 (30.5%) |
AC, anticoagulation; ACEi, angiotensin-converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate transaminase; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; INR, international normalized ratio; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons; TIA, transient ischemic attack.
Reference ranges: aspartate transaminase (AST) and alanine aminotransferase (ALT) – <60 U/L and <41 U/L, respectively, creatinine – 0.7 to 1.3 mg/dL, hemoglobin – 13.5 to 17.5 g/dL, international normalized ratio (INR) – <1.2.
Patients had a high burden of comorbid disease including hypertension (63.4%), atrial fibrillation (52.6%), and a history of HF with reduced ejection fraction (61.6%). 40.5% of the cohort were on loop diuretic therapy at the time of the index TTE, while the use of thiazide diuretics accounted for 10.3% of the study population.
Within the cohort, the mean international normalized ratio (INR) was 1.63 (reference range <1.2) in all patients; and was 1.35 in patients not on anticoagulation and 1.89 in patients on anticoagulation. Mean aspartate transaminase and alanine aminotransferase were 52 U/L and 40 U/L, respectively (reference range <60 U/L and <41 U/L) in the full cohort, and 57 U/L and 38 U/L in patients not on anticoagulation versus 46 U/L and 44 U/L in patients on anticoagulation.
Mean echocardiographic indices at baseline included left ventricular ejection fraction (LVEF) of 43 ± 19%, TR velocity of 3.26 ± 0.82 m/s, and estimated right ventricular systolic pressure of 58.4 ± 23.2 mmHg (Table 2). Mean tricuspid annular plane systolic excursion was 1.63 ± 0.76 cm with mean S′ of 10.08 ± 4.47 cm/s. Estimated right atrial pressure estimated via TTE was 12.9 ± 5.9 mmHg.
Table 2.
Index echocardiogram and right heart catheterization data of patients with severe tricuspid regurgitation
| Total cohort (n = 435) | |
|---|---|
| Index echocardiogram data | n = 435 |
| Ejection fraction, % | 43.8 ± 18.5 |
| TR peak velocity, m/s | 3.26 ± 0.82 |
| Estimated RVSP, mmHg | 58.4 ± 23.2 |
| TAPSE, cm | 1.63 ± 0.76 |
| S′, cm/s | 10.08 ± 4.4 |
| Estimated RA pressure, mmHg | 12.9 ± 5.91 |
| Right heart catheterization | n = 40 |
| RA mean pressure, mmHg | 13.0 ± 5.6 |
| RVSP mean, mmHg | 51.5 ± 21.4 |
| PA mean, mmHg | 32.9 ± 12.9 |
| PCWP mean, mmHg | 17.7 ± 7.2 |
| Cardiac output, L/min | 3.48 ± 1.49 |
| Cardiac index, L/min/m2 | 1.85 ± 0.90 |
| PVR, Wood's Units | 5.81 ± 6.6 |
cm, centimeters; L/min, liters per minute; L/min/m2, liters per minute per meters squared; mmHg, millimeters mercury; m/s, meters per second; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; PVR, peripheral vascular resistance; RA, right atrial; RVSP, right ventricular systolic pressure; sec, seconds; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Medical therapy at the time of enrollment included angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, beta blockade, and aldosterone antagonists use in 24.1%, 39.7%, and 11.2% of patients, respectively.
Of the cohort, 40 patients (9.1% of the study population) underwent right heart catheterization (RHC) at the time of index TTE. There was no relationship between RHC at the time of index TTE and mortality within the cohort; however, 28% of patients who were admitted for HF underwent RHC, accounting for 45% of all invasive hemodynamic assessment. Echocardiographic parameters and RHC parameters are listed in Table 2.
Clinical Outcomes
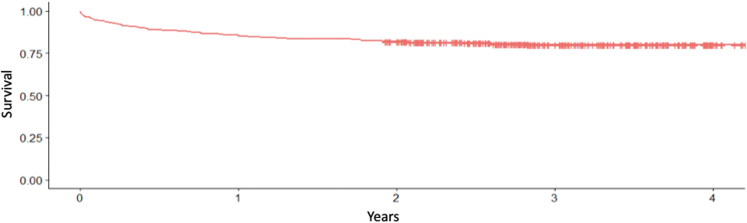
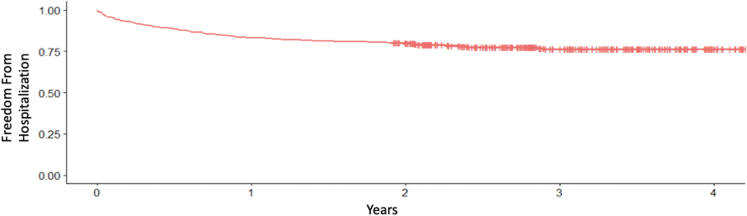
The primary endpoint of all-cause mortality occurred in 89 (20.5%) patients during the median follow-up period of 2.77 years (Figure 1). A total of 153 patients (35.2%) were admitted for HF exacerbation (Figure 2). Thirteen (3%) patients underwent TV intervention including isolated TV surgery in 11 (2.5%) and heart transplant in 2 (0.46%) patients. Clinical outcomes are detailed in Table 3.
Figure 1.
Kaplan-Meier survival curve for mortality in patients with severe tricuspid regurgitation.
Figure 2.
Kaplan-Meier survival curve for heart failure hospitalization in patients with severe tricuspid regurgitation.
Table 3.
Clinical outcomes of patients with severe tricuspid regurgitation
| Clinical outcome | Total population (n = 435) |
|---|---|
| All-cause mortality, n (%) | 89 (20.5%) |
| Heart failure hospitalization, n (%) | 153 (35.4%) |
| Major intervention, n (%) | 17 (3.9%) |
| Heart transplant, n (%) | 2 (0.46%) |
| Left ventricular assist device, n (%) | 1 (0.23%) |
| Tricuspid valve replacement, n (%) | 11 (2.5%) |
| Biventricular pacemaker, n (%) | 3 (0.69%) |
Predictors of All-Cause Mortality and HF Hospitalization
Via multivariable logistic regression analysis, independent predictors of all-cause mortality included history of solid tumor (odds ratio [OR] 6.6, 95% CI 2.1-19.1, p = 0.001), history of PAD (OR 3.5 95% CI 1.2-9.4, p = 0.013), and elevated INR greater than 1.2 in the absence of oral anticoagulation (OR 1.9, 95% CI 1.2-3.2, p = 0.008). RV dilation was associated with a trend toward higher odds of mortality; however, this did not reach statistical significance (OR, 2.4, 95% CI 1.0-6.2, p = 0.055). The risk of mortality was 1.4 times greater in those with HF hospitalization, though this did not reach statistical significance (OR 1.4, 95% CI 0.77-2.4, p = 0.32). Predictors of HF hospitalization included history of diabetes mellitus (OR 2.2, 95% CI 1.1-4.0, p = 0.014) and a history of reduced LVEF (OR 5.7, 95% CI 2.9-11.7, p < 0.0001). Additional predictors of mortality and HF hospitalization are listed in Table 4.
Table 4.
Clinical predictors of mortality and heart failure hospitalization
| Variable | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| Predictors of mortality | |||
| History of solid tumor | 6.6 | 2.1-19.1 | 0.001 |
| Peripheral artery disease | 3.5 | 1.2-9.4 | 0.013 |
| Elevated INR | 1.9 | 1.2-3.2 | 0.008 |
| Predictors of heart failure hospitalization | |||
| Diabetes mellitus | 2.2 | 1.1-4.0 | 0.014 |
| Reduced left ventricular systolic function | 5.7 | 2.9-11 | <0.0001 |
INR, international normalized ratio.
Discussion
In this multicenter analysis of patients with isolated severe TR, we identified via multivariable logistic regression, several clinical variables that increased the risk for mortality and HF hospitalization. The overall mortality within our cohort at a median of 2.7 years of follow-up was 20.5%, with 1-year survival in patients with severe TR of 78.9%. These mortality data are in line with previously published analysis of severe TR survival rates,13 though our mortality within the median study period is likely related to a longer median follow-up time than prior reports.
Predictors of All-Cause Mortality and HF Hospitalization
Elevated INR in the absence of anticoagulation suggests a degree of hepatic synthetic dysfunction, likely related to severe TR with regurgitant flow within the hepatic vasculature.24 Such hemodynamic changes over time may induce hepatic remodeling and in turn increase the risk of developing congestive hepatopathy, liver dysfunction, and potentially cirrhosis. Patients with liver disease have been shown to have a higher risk of mortality when undergoing cardiac surgery.25 Identification of this subgroup of patients can identify patients at risk for mortality, with referral for early intervention to help identify patients who potentially may have higher surgical morbidity and mortality and potentially benefit from transcatheter interventions.
Our analysis also demonstrated that a history of solid tumor and PAD increased risk for mortality in patients with severe TR. While these 2 clinical variables comprised 8.9% and 10.3%, respectively, of the study cohort, these patients were identified as having a statistically higher risk of mortality in the setting of severe TR. Several mechanisms may contribute to the excess mortality in these patients. History of solid tumor leading to recurrence, metastasis, or past/future treatment of tumor via chemotherapy or radiation therapy may lead to excess mortality in these patients both independent of cardiovascular disease and due to direct cardiotoxic effects of therapeutic agents and potential immunosuppression.26 The most common type of tumor was breast cancer in 8 patients, 50% of whom were treated with radiation and the remaining with surgical mastectomy. The remaining most common tumors included prostate cancer (3 patients), lung cancer (2 patients), and cervical/uterine cancer (2 patients). Of the patients with solid tumors, 5 patients received chest radiation therapy (23%), while 17 patients underwent surgical resection (77%). In addition, in patients with PAD, the vasculopathy is likely not limited to just peripheral vasculature, and can often affect cerebral, renal, and cardiovascular systems as well, increasing the mortality in these patients. Of the patients with solid tumor and PAD, the increased STS risk for surgical/transcatheter intervention contributed to a lower rate of intervention within this subgroup.
The population of patients with severe TR and concurrent RHC data was 40 patients. While the pulmonary vascular resistance and estimated PA systolic pressures approached severe pulmonary hypertension, subgroup analysis of these patients did not identify any independent predictor of mortality or HF hospitalization that was statistically significant; thus, no RHC measurements were included in our multivariable modeling. While invasive hemodynamics are important in this population to assess severity of volume/pressure overload, not all patients underwent invasive evaluation which may have reduced the power needed to show statistically significant predictors of mortality or HF hospitalization.
The comparably high rates of all-cause mortality and HF hospitalization within this population may, inpart, be attributed to a relatively low rate of the use of guideline-directed medical therapy for LV systolic dysfunction in patients with mild-moderately reduced LVEF, as in our population. In addition, close to 60% of patients were not prescribed loop diuretics at the timepoint of index TTE identifying severe TR, which potentially can lead to a volume overload state and subsequent hospitalization. This suggests patients with severe isolated TR may be undertreated in contemporary practice and could benefit from more optimal utilization of both medical and TV interventions to improve symptoms and outcomes.
Tricuspid Valve Interventions
Our study demonstrated that while subjects with severe TR suffered significant burden of HF hospitalization and subsequently all-cause mortality, the rate of isolated TV intervention remained low. Only 2.5% of the cohort underwent TV intervention via a surgical approach, and none via a transcatheter approach. This is likely influenced by both the high-risk population as well as the study period between 2016 and 2018 as these transcatheter interventions were off-label compassionate use and not a routine therapeutic intervention offered at the time within our institutions. In addition, 2 patients were referred for heart transplant during the follow-up period.
Our study describes a population of patients with severe TR and high comorbid disease burden with intermediate to high-risk STS profiles that likely influenced the low rate of intervention within this population. The study elucidates key issues that can help improve patient outcomes in the future. First is the need for early identification of patients with severe TR and longitudinal follow-up. This will help clinicians to assess for changes in medical treatment as well as early identification of patients with candidacy for catheter-based or surgical-based interventions to reduce the high rate of mortality and HF hospitalizations in this population. Data from Olmsted County revealed a significant burden of TR in this population with a statistically higher rate of mortality as compared to matched controls with trace/trivial TR.27 Thus, our study has many important prognostic and clinical implications that can help assist clinicians in shared decision-making with patients to reduce mortality and HF hospitalizations in patients with severe TR. While mortality and HF hospitalization approach 20% and 35%, respectively, in our study, there remains a large unmet need for potential medical and surgical or transcatheter therapies to help this group of patients.
Recent advances in transcatheter therapy28 have demonstrated early favorable outcomes both in terms of procedural success and in reducing symptoms related to severe TR. Identification of patients who are at risk of mortality and HF hospitalization based on clinical predictors may help clinicians to better risk stratify and prognosticate and predict which select patients may benefit the most from surgical or transcatheter intervention versus continued medical management. Thus, as the interventional options for TV interventions expand from first-in-human to early feasibility trials, clinicians can be better equipped to offer patients referral for a heart team approach to TV management and consideration for advanced therapeutic interventions.
Study Limitations
While this was a multicenter study, there is much heterogeneity within the world population as it relates to the burden of comorbid disease, medical and interventional therapy, and therefore extrapolation to other populations may be limited. While this limitation exists, our analysis was in concordance with prior studies with regard to the degree of mortality and HF hospitalizations that exist within this patient population. In addition, there is a large degree of variation in patient symptomatology as well as adherence and dosages of medical management of severe TR. This study, due to its retrospective nature, identified patients who obtained index TTE for potentially a non-HF indication, which may introduce bias into the cohort. Furthermore, our study included patients from 2016 onward prior to the 5-grade TR classification system. Evaluation of TR at our institutions during this time period demonstrated that TR classification was based upon either quantitative or qualitative measurements or a combination of both. In the patients where the 5-grade TR classification was utilized following 2017, the numbers of patients with torrential TR was too small to utilize for analysis of predictors of mortality or HF hospitalization. In addition, newer markers of RV dysfunction such as RV strain were not included as this was not routinely a part of our echocardiogram protocol.
Our analysis, however, shows that current treatment strategies heavily favor medical therapy versus interventional therapies at this point in time, and this may contribute to the degree of mortality seen in this population across multiple centers. Lastly, as this was a 2 center, retrospective observational study, further subgroup analysis of the predictors of mortality in primary TR versus functional TR was not undertaken.
Conclusions
In conclusion, in our multicenter experience of patients with severe TR, we have identified history of solid tumor, PAD, and presence of elevated INR as predictors of mortality and diabetes mellitus and reduced LVEF as predictors of HF hospitalization. Understanding the predictors of mortality and HF hospitalization in patients with severe TR is important for prompt initiation of medical therapy aimed reducing symptoms, treatment of the underlying factors that contribute to morbidity and mortality, and for early referral of patients for either surgical or transcatheter intervention via a heart team approach to help alleviate symptoms and improve event-free survival rates.
Ethics Statement
Research contained within this manuscript adhered to relevant ethical guidelines. The study was approved by the institutional review board of both institutions and requirement for written informed consent was waived given the retrospective nature of the study.
Funding
The authors have no funding to report.
Disclosure Statement
Dr. Zahr reports research grants from Edwards Lifesciences, Medtronic and Siemens. Dr. Zahr is also an Associate Editor of Structural Heart Journal. Dr. Golwala reports serves as an advisory consultant for Medtronic. Dr. Song reports serves as a consultant for Medtronic and Edwards Lifesciences. Dr. Chadderdon reports is an educational consultant for Medtronic and Edwards Lifesciences. Dr. Byrne reports is an educational consultant and proctor for Medtronic and is a proctor for Abbott Vascular. The other authors had no conflicts to declare.
Review Statement
Given his role as an editor, Firas Zahr, MD, had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Randy Martin, MD.
Guest Editor: Randy Martin, MD
References
- 1.Singh J., Evans J., Levy D. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation the Framingham heart study. Am J Cardiol. 1999;83(6):897–902. doi: 10.1016/s0002-9149(98)01064-9. [DOI] [PubMed] [Google Scholar]
- 2.Topilsky Y., Nkomo V.T., Vatury O., Michelena H.I., Letourneau T., Suri R.M., et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging. 2014;7(12):1185–1194. doi: 10.1016/j.jcmg.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Fender E.A., Zack C.J., Nishimura R.A. Isolated tricuspid regurgitation: outcomes and therapeutic interventions. Heart. 2018;104(10):798–806. doi: 10.1136/heartjnl-2017-311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen M.J., Nishimura R.A., Borlaug B.A. The hemodynamic basis of exercise intolerance in tricuspid regurgitation. Circ Heart Fail. 2014;7(6):911–917. doi: 10.1161/CIRCHEARTFAILURE.114.001575. [DOI] [PubMed] [Google Scholar]
- 5.Badano L.P., Muraru D., Enriquez-Sarano M. Assessment of functional tricuspid regurgitation. Eur Heart J. 2013;34(25):1875–1885. doi: 10.1093/eurheartj/ehs474. [DOI] [PubMed] [Google Scholar]
- 6.Nath J., Foster E., Heidenreich P.A. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43(3):405–409. doi: 10.1016/j.jacc.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Benfari G., Antoine C., Miller W.L., Thapa P., Topilsky Y., Rossi A., et al. Excess mortality associated with functional tricuspid regurgitation complicating heart failure with reduced ejection fraction. Circulation. 2019;140(3):196–206. doi: 10.1161/CIRCULATIONAHA.118.038946. [DOI] [PubMed] [Google Scholar]
- 8.Koelling T.M., Aaronson K.D., Cody R.J., Bach D.S., Armstrong W.F. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J. 2002;144(3):524–529. doi: 10.1067/mhj.2002.123575. [DOI] [PubMed] [Google Scholar]
- 9.Neuhold S., Huelsmann M., Pernicka E., Graf A., Bonderman D., Adlbrecht C., et al. Impact of tricuspid regurgitation on survival in patients with chronic heart failure: unexpected findings of a long-term observational study. Eur Heart J. 2013;34(11):844–852. doi: 10.1093/eurheartj/ehs465. [DOI] [PubMed] [Google Scholar]
- 10.Grapsa J., Pereira Nunes M.C., Tan T.C., Cabrita I.Z., Coulter T., Smith B.C., et al. Echocardiographic and hemodynamic predictors of survival in precapillary pulmonary hypertension: Seven-year follow-up. Circ Cardiovasc Imaging. 2015;8(6) doi: 10.1161/CIRCIMAGING.114.002107. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., 3rd, Fleisher L.A., et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American heart association Task Force on clinical practice guidelines. Circulation. 2017;135(25):e1159–e1195. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., 3rd, Guyton R.A., et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):e57–e185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 13.Ingraham B., Pislaru S. Characteristics and treatment strategies for severe tricuspid regurgitation. Heart. 2019;105(16):1244–1250. doi: 10.1136/heartjnl-2019-314741. [DOI] [PubMed] [Google Scholar]
- 14.Lee J.W., Song J.M., Park J.P., Lee J.W., Kang D.H., Song J.K. Long-term prognosis of isolated significant tricuspid regurgitation. Circ J. 2010;74(2):375–380. doi: 10.1253/circj.cj-09-0679. [DOI] [PubMed] [Google Scholar]
- 15.Hahn R.T., Meduri C.U., Davidson C.J., Lim S., Nazif T.M., Ricciardi M.J., et al. Early feasibility study of a transcatheter tricuspid valve annuloplasty: SCOUT trial 30-day results. J Am Coll Cardiol. 2017;69(14):1795–1806. doi: 10.1016/j.jacc.2017.01.054. [DOI] [PubMed] [Google Scholar]
- 16.Perlman G., Praz F., Puri R., Ofek H., Ye J., Philippon F., et al. Transcatheter tricuspid valve repair with a new transcatheter coaptation system for the treatment of severe tricuspid regurgitation: 1-year clinical and echocardiographic results. JACC Cardiovasc Interv. 2017;10(19):1994–2003. doi: 10.1016/j.jcin.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 17.Regazzoli D., Ielasi A., Lanzillo G., Ancona M.B., Agricola E., Giannini F., et al. Sustained reduction of tricuspid regurgitation after percutaneous repair with the MitraClip system in a patient with a dual Chamber Pacemaker. JACC Cardiovasc Interv. 2017;10(16):e147–e149. doi: 10.1016/j.jcin.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Kodali S., Hahn R.T., George I., Davidson C.J., Narang A., Zahr F., et al. Transfemoral tricuspid valve replacement in patients with tricuspid regurgitation: TRISCEND study 30-day results. JACC Cardiovasc Interv. 2022;15(5):471–480. doi: 10.1016/j.jcin.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Lurz P., Stephan von Bardeleben R., Weber M., Sitges M., Sorajja P., Hausleiter J., et al. Transcatheter Edge-to-Edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol. 2021;77(3):229–239. doi: 10.1016/j.jacc.2020.11.038. [DOI] [PubMed] [Google Scholar]
- 20.Szerlip M., Spargias K.S., Makkar R., Kar S., Kipperman R.M., O’Neill W.W., et al. 2-Year outcomes for transcatheter repair in patients with mitral regurgitation from the CLASP study. JACC Cardiovasc Interv. 2021;14(14):1538–1548. doi: 10.1016/j.jcin.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Saeed S., Smith J., Grigoryan K., Urheim S., Chambers J.B., Rajani R. Impact of pulmonary hypertension on outcome in patients with moderate or severe tricuspid regurgitation. Open Heart. 2019;6(2) doi: 10.1136/openhrt-2019-001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fender E.A., Petrescu I., Ionescu F., Zack C.J., Pislaru S.V., Nkomo V.T., et al. Prognostic importance and predictors of survival in isolated tricuspid regurgitation: a Growing Problem. Mayo Clin Proc. 2019;94(10):2032–2039. doi: 10.1016/j.mayocp.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Zoghbi W. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 24.Xanthopoulos A., Starling R.C., Kitai T., Triposkiadis F. Heart failure and liver disease: Cardiohepatic interactions. JACC Heart Fail. 2019;7(2):87–97. doi: 10.1016/j.jchf.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Hawkins R.B., Young B.A.C., Mehaffey J.H., Speir A.M., Quader M.A., Rich J.B., et al. Model for End-Stage liver disease score independently predicts mortality in cardiac surgery. Ann Thorac Surg. 2019;107(6):1713–1719. doi: 10.1016/j.athoracsur.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topilsky Y., Maltais S., Medina Inojosa J., Oguz D., Michelena H., Maalouf J., et al. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging. 2019;12(3):433–442. doi: 10.1016/j.jcmg.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Fam N.P., von Bardeleben R.S., Hensey M., Kodali S.K., Smith R.L., Hausleiter J., et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE system: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14(5):501–511. doi: 10.1016/j.jcin.2020.11.045. [DOI] [PubMed] [Google Scholar]