Abstract
Background
Sex-specific thresholds of computed tomography (CT)–derived aortic valve calcification (AVC) or AVC density (AVCd) to identify severe aortic stenosis (AS) have been established in populations that consisted mainly of Caucasians with a tricuspid aortic valve. The objective of this study was to evaluate the accuracy (i.e., sensitivity and specificity) of previously established thresholds to identify severe AS in patients with bicuspid aortic valve (BAV) and according to ethnicity: Caucasian vs. Asian.
Methods
We built a multicenter registry of echocardiographic and CT data collected in BAV patients with at least mild AS and preserved left ventricular ejection fraction from 7 different centers. Anatomic severity of AS obtained by CT-derived AVC and AVCd was compared to hemodynamic severity of AS obtained by echocardiography.
Results
Among 485 BAV patients (60% men, 73% Asians), the best thresholds of AVC and AVCd to identify severe AS in BAV patients were 2315 arbitrary units (AU) (sensitivity [Se]/specificity [Spe] = 82/78%) in men, 1103 AU (Se/Spe = 80/82%) in women, and 561 AU/cm2 (Se/Spe = 86/91%) in men, and 301 AU/cm2 (Se/Spe = 83/82%) in women, respectively. According to ethnicity, thresholds for severe AS in Caucasian patients were, respectively, in men and women: 2208 AU (Se/Spe = 83/83%) and 1230 AU (Se/Spe = 87/82%) for AVC and 474 AU/cm2 (Se/Spe = 88/83%) and 358 AU/cm2 (Se/Spe = 80/82%) for AVCd. In Asian patients, they were 2582 AU (Se/Spe = 76/78%) and 924 AU (Se/Spe = 84/80%) for AVC and 640 AU/cm2 (Se/Spe = 82/89%) and 255 AU/cm2 (Se/Spe = 86/80%) for AVCd.
Conclusions
The optimal thresholds to identify hemodynamically severe AS in BAV patients are similar in Caucasians but appear to be higher in Asian men, compared with thresholds previously reported in tricuspid aortic valve patients. Nonetheless, the thresholds currently proposed in the guidelines have good accuracy and can be applied in BAV patients to confirm AS severity.
Keywords: Aortic stenosis, Aortic valve calcification, Bicuspid aortic valve, Ethnicity, Severity
Introduction
Patients with a bicuspid aortic valve (BAV), the most frequent cardiac congenital anomaly, are at a higher risk of developing aortic stenosis (AS). They generally develop AS earlier in life than subjects with a tricuspid aortic valve (TAV).1, 2, 3, 4 AS severity is generally established by echocardiography and is crucial for determining the need and timing for aortic valve replacement. AS is considered severe when peak aortic jet velocity is ≥4 m/s, mean transvalvular gradient is ≥40 mmHg, and indexed aortic valve area is ≤0.6 cm2/m2.5,6 However, approximately 30% of patients with AS show discordant grading parameters, and thus, AS severity is inconclusive with echocardiographic assessment alone.7
The aortic valve calcification (AVC) score by computed tomography (CT) has emerged in recent years as a valuable imaging modality to confirm AS severity in patients with discordant or inconclusive gradients by echocardiography. Previous studies revealed the presence of sex-related differences with regard to AVC; thus, sex-specific AVC thresholds for severe AS were proposed and validated to confirm AS severity.8, 9, 10 However, those studies were mainly conducted in patients with TAV AS and might not necessarily apply to patients with BAV AS. Indeed, correlations between hemodynamic severity and anatomic severity (i.e., AVC) in patients with a BAV are weaker than those of patients with a TAV, especially in younger women with a BAV.11 Moreover, previous studies predominantly included patients of Caucasian ethnicity, which might also not apply to other ethnicities, such as Asians.
Thus, the objectives of this study were to evaluate the accuracy, i.e., the sensitivity, specificity, and percentage of overall correct classification (CC), of previously established AVC and AVC density (AVCd) thresholds to identify severe AS in patients with a BAV and according to ethnicity: Caucasian vs. Asian.
Materials and Methods
Study Population
This study included 485 patients with a BAV from 7 different centers and countries: Quebec Heart and Lung Institute [Canada], Asan Medical Center Heart Institute [Korea], Leiden University Medical Center [The Netherlands], British Heart Foundation Centre for Cardiovascular Science in Edinburgh [United Kingdom], Beaumont Health-Royal Oak [United States of America], Italian Hospital of Buenos Aires [Argentina], and National University Heart Centre of Singapore [Singapore]. Each center was invited to include clinical, Doppler echocardiography, and CT data of the patients into a multicenter registry. The inclusion criteria for this registry were as follows: (1) age ≥18 years, (2) at least mild AS defined by a peak aortic jet velocity ≥2 m/s, (3) preserved left ventricular ejection fraction (LVEF ≥50%), and (4) Doppler echocardiography and CT exams performed within 4 months of each other. The exclusion criteria were patients with an ethnicity other than Caucasian or Asian, aortic regurgitation ≥ moderate, mitral stenosis or regurgitation ≥ moderate, history of aortic valve endocarditis, previous aortic valve intervention (surgical or transcatheter aortic valve replacement (TAVR) and/or valvuloplasty), rheumatic valve disease, and previous chest radiotherapy.
Patients from the Quebec Heart and Lung Institute and British Heart Foundation Centre for Cardiovascular Science were recruited via prospective studies approved by their respective institutional review board, and all patients signed an informed consent form. Patients from the 5 other centers were retrospectively included in this study, and informed consent was waived. These patients had an echocardiogram and CT done as part of their clinical follow-up or prior to an aortic valve intervention to replace their valve. The Quebec Heart and Lung Institute was the coordinating center.
Clinical Data
Patients’ anthropometric measurements, risk factors, and medical history were retrieved from medical records by each center. These clinical data included body surface area (BSA), body mass index, and history of hypertension, dyslipidemia, diabetes, smoking, chronic kidney disease, and coronary artery disease.
Doppler Echocardiography
All Doppler echocardiographic examinations were acquired using a commercially available ultrasound machine, and measurements were done according to current recommendations.5,12,13 Left ventricular outflow tract diameter was measured at the insertion of the aortic valve leaflets in a parasternal long-axis zoom view, and LVEF was assessed by the biplane Simpson method by measuring end-systolic and end-diastolic volumes on the apical 2- and 4-chamber views. Stroke volume was calculated by multiplying the left ventricular outflow tract area by the velocity-time integral obtained by pulsed wave Doppler in the left ventricular outflow tract and indexed by the BSA to have the stroke volume index. Hemodynamic parameters were used to assess AS hemodynamic severity: peak aortic jet velocity measured by continuous wave Doppler, mean transvalvular gradient derived from the modified Bernoulli formula, and aortic valve area calculated by the continuity equation and indexed by the BSA to obtain the indexed aortic valve area.
Doppler-echocardiography was used as the gold standard for determining AS hemodynamic severity in this study. To avoid equivocal cases, only patients with a preserved LVEF (≥50%) and concordant grading with a mean transvalvular gradient and indexed aortic valve area were included. Severe AS was thus defined as a mean transvalvular gradient ≥40 mmHg and an indexed aortic valve area ≤0.6 cm2/m2, whereas nonsevere AS (mild or moderate) was defined as a mean transvalvular gradient <40 mmHg and an indexed aortic valve area >0.6 cm2/m2.
Computed Tomography
All participating centers performed noncontrast CT scans and measured AVC using commercially available scanners and analysis software (Supplemental Table 1). Two (Leiden University Medical Center and Italian Hospital of Buenos Aires) of the 7 centers used Toshiba scanners, while the 5 other centers (Quebec Heart and Lung Institute, Asan Medical Center Heart Institute, British Heart Foundation Centre for Cardiovascular Science in Edinburgh, Beaumont Health-Royal Oak, and National University Heart Centre of Singapore) used Siemens scanners. AVC was measured in each center according to the Agatston method following current recommendations and expressed in arbitrary units (AU).14 AVC was obtained by the sum of AVC values obtained from each contiguous 3-mm axial slices, with special care taken to exclude calcium originating from adjacent structures, such as the mitral valve annulus, ascending aorta, and coronary arteries.14 To take into account the variability in aortic annulus of the patients, AVC was indexed to the left ventricular outflow tract area measured by echocardiography to obtain the AVCd expressed in AU/cm2. AVCd thus allows comparing calcification scores in patients with different body size and, thus, different aortic annulus area.
Statistical Analyses
Continuous variables were expressed as medians [25th-75th percentiles]. Categorical variables were expressed as frequencies (%). Comparisons were performed with Student t tests if the continuous variables followed a normal distribution (according to the Shapiro-Wilk test) and with Wilcoxon’s tests if they did not. Categorical variables were compared with χ2 tests or with Fisher’s exact tests, as appropriate. AVC and AVCd values were transformed with the use of a square root for normalization. The accuracy of the previously validated, sex-specific AVC (2065 AU in men and 1274 AU in women) and AVCd (476 AU/cm2 in men and 292 AU/cm2 in women) thresholds by Clavel et al.8 was evaluated in the subset of BAV patients with concordant grading of AS severity (using Doppler-echocardiography). The following accuracy parameters were calculated: sensitivity, specificity, and percentage of overall CC, and good accuracy was considered when the percentage of overall CC was >80%. The accuracy to correctly classify AS severity as severe or nonsevere was evaluated in the whole cohort of BAV patients and its different subgroups (men, women, Caucasians, Asians, Caucasian men, Caucasian women, Asian men, and Asian women). Comparisons of accuracy between the different subgroups of patients were performed with χ2 tests, and comparisons between AVC and AVCd were performed with McNemar’s tests. Furthermore, sex-specific AVC and AVCd thresholds defining severe AS were determined with receiver operating characteristic (ROC) curves in these different subgroups of BAV patients with concordant grading of AS: (i) all men, (ii) all women, (iii) Caucasian men, (iv) Caucasian women, (v) Asian men, and (vi) Asian women. We reported thresholds that showed the best sensitivity (Se) and specificity (Spe) balance to define severe AS. The percentage of CC achieved by these thresholds was also reported. The best thresholds of AVC and AVCd obtained in the present study were compared with those previously reported by Clavel et al.8 and were considered similar when values were within 15% relative difference. Statistical analyses were done with JMP and SPSS software, and a 2-tailed p value <0.05 was considered statistically significant.
Results
Characteristics of the Study Population
Clinical, Doppler echocardiographic, and CT characteristics are presented in Table 1. There were 485 patients with a median age of 63 [55-70] years, most of whom were men (n = 289, 60%). There were 132 (27%) Caucasians and 353 (73%) Asians. Among the 485 patients, there were 391 (81%) patients with concordant grading of AS severity by the mean transvalvular gradient and indexed aortic valve area, of whom, 337 (86%) had hemodynamically severe AS, while 54 (14%) had a nonsevere AS (Supplemental Figure 1). Finally, 321 (66%) patients had severe AVC [>2065 AU in men and >1274 AU in women], while 345 (71%) patients had severe AVCd [>476 AU/cm2 in men and >292 AU/cm2 in women].
Table 1.
Clinical, Doppler echocardiography, and CT characteristics according to ethnicity
| Variables | All BAV N = 485 | Caucasians N = 132, 27% | Asians N = 353, 73% | p value |
|---|---|---|---|---|
| Clinical data | ||||
| Age, y | 63 [55-70] | 59 [49-66] | 64 [58-71] | <0.0001 |
| Male, n (%) | 289 (60) | 79 (60) | 210 (59) | 0.94 |
| Body surface area, m2 | 1.72 [1.60; 1.86] | 1.89 [1.77-2.04] | 1.67 [1.56-1.78] | <0.0001 |
| Body mass index (BMI), kg/m2 | 25 [23-28] | 27 [25-31] | 24 [22-26] | <0.0001 |
| Obesity [BMI >30], n (%) | 51 (11) | 38 (29) | 13 (4) | <0.0001 |
| Hypertension, n (%) | 212 (44) | 62 (47) | 150 (42) | 0.38 |
| Dyslipidemia, n (%) [n = 452] | 75 (17) | 35 (35) | 40 (11) | <0.0001 |
| Diabetes, n (%) | 69 (14) | 15 (11) | 54 (15) | 0.27 |
| History of smoking, n (%) [n = 447] | 191 (43) | 52 (54) | 139 (40) | 0.01 |
| CKD, n (%) [n = 472] | 17 (4) | 12 (10) | 5 (1) | <0.0001 |
| Coronary artery disease, n (%) | 42 (9) | 24 (18) | 18 (5) | <0.0001 |
| Echocardiography data | ||||
| LVOT diameter, mm | 21.6 [20.5-23.0] | 22.3 [21.0-24.0] | 21.3 [20.5-22.4] | <0.0001 |
| Vpeak, cm/s | 460 [390-520] | 347 [287-417] | 483 [430-538] | <0.0001 |
| MG, mmHg | 51 [37-66] | 30 [19-43] | 57 [44-71] | <0.0001 |
| AVA, cm2 | 0.71 [0.58-0.90] | 0.97 [0.77-1.21] | 0.65 [0.55-0.80] | <0.0001 |
| AVAi, cm2/m2 | 0.42 [0.34-0.52] | 0.52 [0.41-0.64] | 0.40 [0.33-0.47] | <0.0001 |
| Stroke volume (SV), mL | 76 [66-86] | 80 [65-91] | 75 [67-84] | 0.08 |
| SV index (SVi), mL/m2 | 44 [39-50] | 41 [37-46] | 45 [40-51] | <0.0001 |
| LVEF, % [n = 474] | 64 [60-67] | 63 [59-67] | 64 [61-67] | 0.13 |
| Concordant MG-AVAi, n (%) | 391 (81) | 79 (60) | 312 (88) | <0.0001 |
| Severe AS | 337 (86) | 39 (49) | 298 (96) | |
| Mild/moderate AS | 54 (14) | 40 (51) | 14 (4) | |
| Computed tomography data | ||||
| AVC, AU | 2392 [1227-3842] | 1659 [412-2966] | 2695 [1569-4105] | <0.0001 |
| Men | 3229 [1878-4817] | 2183 [764-3840] | 3374 [2214-5252] | <0.0001 |
| Women | 1644 [786-2590] | 866 [66-1863] | 1865 [939-2757] | <0.0001 |
| AVCd, AU/cm2 | 637 [333-1046] | 395 [116-742] | 737 [449-1146] | <0.0001 |
| Men | 810 [468-1300] | 483 [198-947] | 925 [614-1396] | <0.0001 |
| Women | 471 [191-734] | 236 [18-466] | 535 [296-760] | <0.0001 |
| Severe AVC, n (%) | 321 (66) | 63 (48) | 258 (73) | <0.0001 |
| Severe AVCd, n (%) | 345 (71) | 63 (48) | 282 (80) | <0.0001 |
Notes. Values are median [25th-75th percentiles]. Bold values indicate statistically significant (p < 0.05).
Abbreviations: AS, aortic stenosis; AVA, aortic valve area; AVAi, aortic valve area indexed by the body surface area; AVC, aortic valve calcification; AVCd, aortic valve calcification density; BAV, bicuspid aortic valve; CKD, chronic kidney disease; CT, computed tomography; LVEF, left ventricular ejection fraction; LVOT, left ventricle outflow tract; MG, mean transvalvular gradient; Vpeak, peak aortic jet velocity.
Comparisons between Caucasians and Asians showed that Caucasian patients had more comorbidities and risk factors. Caucasians were younger (59 [49-66] vs. 64 [58-71] years old, p < 0.0001), had a larger BSA (1.89 [1.77-2.04] vs. 1.67 [1.56-1.78] m2, p < 0.0001), and had higher prevalence of obesity (29 vs. 4%, p < 0.0001), dyslipidemia (35 vs. 11%, p < 0.0001), history of smoking (54 vs. 40%, p = 0.01), chronic kidney disease (10 vs. 1%, p < 0.0001), and coronary artery disease (18 vs. 5%, p < 0.0001). Regarding Doppler echocardiographic data, Caucasian patients had a larger left ventricular outflow tract diameter (22.3 [21.0-24.0] vs. 21.3 [20.5-22.4] mm, p < 0.0001) and lower proportions of concordant grading of AS (60 vs. 88%, p < 0.0001) and severe AS (49 vs. 96% severe AS, p < 0.0001) than Asian patients. Caucasians also had lower AVC (men: 2183 [764-3840] vs. 3374 [2214-5252] AU and women: 866 [66-1863] vs. 1865 [939-2757] AU, all p < 0.0001) and AVCd (men: 483 [198-947] vs. 925 [614-1396] AU/cm2 and women: 236 [18-466] vs. 535 [296-760] AU/cm2, all p < 0.0001) than Asians. The proportion of patients with severe AVC (>2065 AU in men and >1274 AU in women) or severe AVCd (>476 AU/cm2 in men and >292 AU/cm2 in women) was lower in Caucasian vs. Asian patients (AVC: 48 vs. 73%, p < 0.0001 and AVCd: 48 vs. 80%, p < 0.0001).
Accuracy of AVC and AVCd to Identify Severe AS in Patients With a BAV
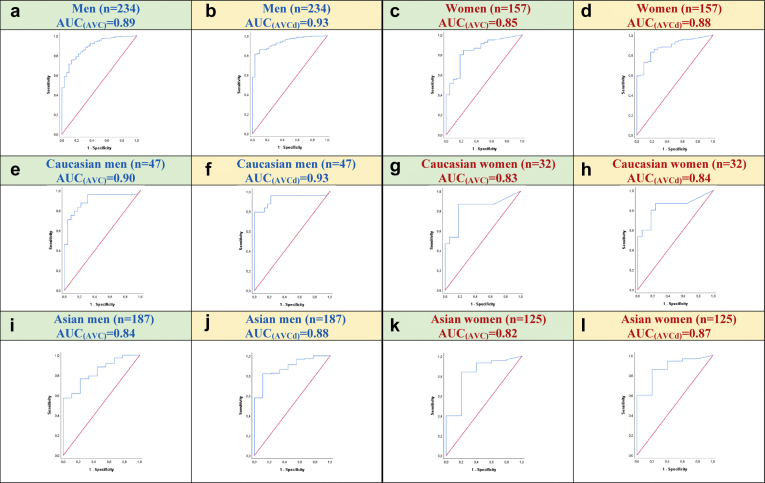
ROC curves were used in BAV patients with concordant grading of AS severity (concordant mean transvalvular gradient and indexed aortic valve area) to establish sex- and ethnicity-specific AVC and AVCd thresholds for severe AS and to compare with thresholds previously proposed and validated for patients with a TAV (Figure 1, panels a-l).
Figure 1.
Receiver operating characteristic (ROC) curves and areas under the curve (AUC) according to aortic valve calcification (AVC) and AVC density (AVCd) in the different subgroups of patients with bicuspid aortic valves (BAVs). ROC curves for AVC are presented in panels a (all men), c (all women), e (Caucasian men), g (Caucasian women), i (Asian men), and k (Asian women), while for AVCd, they are presented in panels b (all men), d (all women), f (Caucasian men), h (Caucasian women), j (Asian men), and l (Asian women).
The area under the curve (AUC) in all subgroups of patients was ≥0.82. In the entire cohort, AUC(AVC) and AUC(AVCd) were 0.89 and 0.93, respectively, in men vs. 0.85 and 0.88 in women (Figure 1, panels a-d). There were no significant differences in terms of AUC between men and women regarding AVC (0.89 vs. 0.85, p = 0.38) or AVCd (0.93 vs. 0.88, p = 0.17). However, AUC(AVCd) was significantly larger than AUC(AVC) in both men and women (both p = 0.01). The AUC(AVC) and AUC(AVCd) were 0.90 and 0.93, respectively, in Caucasian men vs. 0.83 and 0.84 in Caucasian women, with no significant difference in AUC between men vs. women or AVC vs. AVCd (Figure 1, panels e-h).
The AUC(AVC) and AUC(AVCd) were 0.84 and 0.88 in Asian men, respectively, vs. 0.82 and 0.87 in Asian women, with no significant difference in AUC between groups (Figure 1, panels i-l).
There were no significant differences in AUC between Caucasian men vs. Asian men (p = 0.39 and p = 0.46, respectively, for AUC(AVC) and AUC(AVCd)) (Figure 1, panel e vs. i and f vs. j) or between Caucasian women vs. Asian women (p = 0.74 and p = 0.97, respectively, for AUC(AVC) and AUC(AVCd)) (Figure 1, panel g vs. k and h vs. l).
Sex- and Ethnic-Specific Thresholds of AVC and AVCd in Patients With a BAV
Sex-specific thresholds for severe AS derived from ROC curves are presented in Table 2. In this cohort of BAV patients, without taking into account the ethnicity, the best thresholds were, respectively, in men and women, 2315 AU (Se/Spe = 82/78%) and 1103 AU (Se/Spe = 80/82%) for AVC and 561 AU/cm2 (Se/Spe = 86/91%) and 301 AU/cm2 (Se/Spe = 83/82%) for AVCd. Compared to thresholds previously proposed by Clavel et al.8 in a cohort of mostly TAV patients, we found that women with a BAV had similar thresholds for AVC (1103 vs. 1274 AU) or AVCd (301 vs. 292 AU/cm2). However, in men with a BAV, we found that the optimal thresholds were slightly higher vs. those proposed by Clavel et al.8 (AVC: 2315 vs. 2065 AU and AVCd: 561 vs. 476 AU/cm2).
Table 2.
Sex-specific thresholds for severe AVC and AVCd in patients with a BAV and in subgroups according to ethnicity
| Ethnicity | Sex | AVC |
Sex | AVCd |
||||
|---|---|---|---|---|---|---|---|---|
| Clavel et al.8 (mainly TAV cohort) | Present study (BAV cohort) | Clavel et al.8 (mainly TAV cohort) | Present study (BAV cohort) | |||||
| All | Men (n = 234) | AUC | 0.90 | 0.89 | Men (n = 234) | AUC | 0.92 | 0.93 |
| Threshold | 2065 | 2315 | Threshold | 476 | 561 | |||
| Se/Spe/CC (%) | 89/80/83 | 82/78/81 | Se/Spe/CC (%) | 90/80/87 | 86/91/86 | |||
| Women (n = 157) | AUC | 0.91 | 0.85 | Women (n = 157) | AUC | 0.93 | 0.88 | |
| Threshold | 1274 | 1103 | Threshold | 292 | 301 | |||
| Se/Spe/CC (%) | 86/89/75 | 80/82/80 | Se/Spe/CC (%) | 92/81/83 | 83/82/83 | |||
| Caucasians | Men (n = 47) | AUC | 0.90 | 0.90 | Men (n = 47) | AUC | 0.92 | 0.93 |
| Threshold | 2065 | 2208 | Threshold | 476 | 474 | |||
| Se/Spe/CC (%) | 89/80/81 | 83/83/83 | Se/Spe/CC (%) | 90/80/83 | 88/83/85 | |||
| Women (n = 32) | AUC | 0.91 | 0.83 | Women (n = 32) | AUC | 0.93 | 0.84 | |
| Threshold | 1274 | 1230 | Threshold | 292 | 358 | |||
| Se/Spe/CC (%) | 86/89/75 | 87/82/84 | Se/Spe/CC (%) | 92/81/78 | 80/82/81 | |||
| Asians | Men (n = 187) | AUC | 0.90 | 0.84 | Men (n = 187) | AUC | 0.92 | 0.88 |
| Threshold | 2065 | 2582 | Threshold | 476 | 640 | |||
| Se/Spe/CC (%) | 89/80/84 | 76/78/77 | Se/Spe/CC (%) | 90/80/88 | 82/89/82 | |||
| Women (n = 125) | AUC | 0.91 | 0.82 | Women (n = 125) | AUC | 0.93 | 0.87 | |
| Threshold | 1274 | 924 | Threshold | 292 | 255 | |||
| Se/Spe/CC (%) | 86/89/75 | 84/80/83 | Se/Spe/CC (%) | 92/81/84 | 86/80/86 | |||
Abbreviations: AUC, area under the curve; AVC, aortic valve calcification; AVCd, aortic valve calcification density; BAV, bicuspid aortic valve; CC, overall correct classification; Se, sensitivity; Spe, specificity; TAV, tricuspid aortic valve.
When analyses were stratified according to ethnicity, thresholds for severe AS in Caucasian patients were, respectively, in men and women, 2208 AU (Se/Spe = 83/83%) and 1230 AU (Se/Spe = 87/82%) for AVC and 474 AU/cm2 (Se/Sp = 88/83%) and 358 AU/cm2 (Se/Spe = 80/82%) for AVCd. In Asian patients, thresholds for men and women were, respectively, 2582 AU (Se/Spe = 76/78%) and 924 AU (Se/Spe = 84/80%) for AVC and 640 AU/cm2 (Se/Spe = 82/89%) and 255 AU/cm2 (Se/Spe = 86/80%) for AVCd. Compared to thresholds found by Clavel et al.8 in a cohort of mostly TAV Caucasian patients, our study shows that Caucasian patients with a BAV had similar AVC (2208 vs. 2065 AU in men and 1230 vs. 1274 AU in women) and AVCd thresholds (474 vs. 476 AU/cm2 in men and 358 vs. 292 AU/cm2 in women). In Asian patients, men with a BAV had higher AVC (2582 vs. 2065 AU) and AVCd (640 vs. 476 AU/cm2) thresholds vs. those reported by Clavel et al.8 On the other hand, in Asian women with a BAV, the threshold for severe AVC was lower (924 vs. 1274 AU) than the one from the study by Clavel et al.8 However, the AVCd threshold was similar (255 vs. 292 AU/cm2).
Prevalence of Severe AVC and Severe AVCd According to Optimal Thresholds in Case of Concordant or Discordant AS Grading
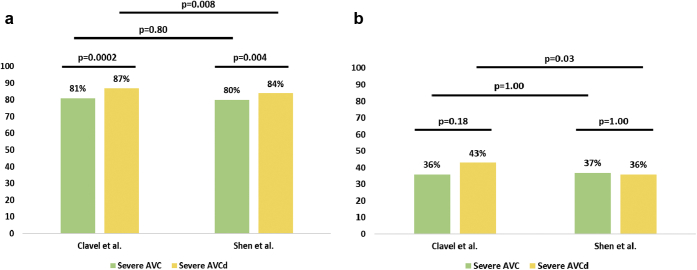
In the 391 patients with concordant grading of AS severity, 337 patients had hemodynamically severe AS. In those 337 patients, AVC and AVCd thresholds by Clavel et al.8 (AVC: 2065 AU in men and 1274 AU in women; AVCd: 476 AU/cm2 in men and 292 AU/cm2 in women) classified, respectively, 81% and 87% of patients as severe (AVC vs. AVCd, p = 0.0002), whereas those of the present study (AVC: 2315 AU in men and 1103 AU in women; AVCd: 561 AU/cm2 in men and 301 AU/cm2 in women) identified 80% and 84% of patients as severe (AVC vs. AVCd, p = 0.004) (Figure 2, panel a). The prevalence of severe AS was higher with AVCd than with AVC.
Figure 2.
Prevalence of severe AVC and severe AVCd according to optimal thresholds by Clavel et al.8and by Shen et al. [present study] in patients with concordant grading severe AS (panel a, n = 337) or discordant grading of AS severity (panel b, n = 94) at echocardiography. AVC and AVCd thresholds by Clavel et al.8 were, respectively, 2065 AU and 476 AU/cm2 in men and 1274 AU and 292 AU/cm2 in women. AVC and AVCd thresholds found in the present study in BAV patients were, respectively, of 2315 AU and 561 AU/cm2 in men and 1103 AU and 301 AU/cm2 in women.
Abbreviations: AVC, aortic valve calcification; AVCd, aortic valve calcification density.
In the subset of 94 patients with discordant grading of AS, AVC identified 36% and 37% (p = 1.00) of patients with severe AS using thresholds proposed by Clavel et al.8 vs. those proposed in the present study, while AVCd identified 43% vs. 36% (p = 0.03) patients with severe AS (Figure 2, panel b).
Discussion
The main findings of this study were as follows: (1) Sex-specific thresholds of AVC and AVCd previously established by Clavel et al.8 in a population predominantly composed of Caucasian patients with a TAV showed good accuracy (i.e., >80% overall CC) to identify hemodynamically severe AS in patients with a BAV, (2) Optimal thresholds of AVC and AVCd obtained in the present study were overall similar in Caucasian men and women, but higher in Asian men compared to thresholds previously reported by Clavel et al.8 for TAV patients, (3) Asian women had lower AVC but similar AVCd compared to thresholds reported by Clavel et al.,8 and (4) Diagnostic accuracy was slightly better for AVCd vs. AVC in the present cohort of BAV patients.
AVC and AVCd by CT as a Tool to Confirm AS Severity
AS severity is routinely assessed by echocardiography in clinical practice, and accurately assessing the true severity of AS is of utmost importance to determine the need for aortic valve replacement.6 However, discordant grading of AS severity on echocardiographic exam is found in approximately 30% of patients with AS and, in the absence of measurement errors, is related to a low flow state (stroke volume index <35 mL/m2) or inherent inconsistencies between Doppler echocardiography hemodynamic parameters.7,15 Measurement of AVC by CT has emerged in the recent years as a simple and accurate method to confirm AS severity and has since been included in the European guidelines for the management of AS.16 Sex-specific AVC and AVCd thresholds for severe AS have been developed, validated, and found to be strongly associated with clinical outcomes.8,10,17
However, previous studies, in which thresholds have been established and validated, consisted mainly of patients with a TAV and mainly of Caucasians.8,10 Our study extends the thresholds previously reported by Clavel et al.,8 to identify severe AS in patients with a BAV. This analysis is important and relevant because subjects with a BAV generally present AVC at a younger age and with more severe AVC than those with a TAV.4 Also, some patients with a BAV might present with significant AS but with no or few calcifications, such as younger women with a BAV.11
In this study, we found that AVC and AVCd thresholds proposed by Clavel et al.8 and included in the European guidelines showed reasonably good accuracy (>80%) to identify severe AS in patients with a BAV, suggesting that these thresholds can also be applied clinically in the BAV population. In Caucasian women, the AVCd threshold appeared to be higher than the one reported by Clavel et al.,8 but this result should be interpreted with caution because this subset includes a small number (n = 32) of patients.
Compared to absolute AVC, AVCd generally provided slightly better CC of AS severity in this BAV cohort and, especially, in Asian women. This superiority of AVCd vs. AVC may be related to the fact that AVCd takes into account the interindividual variability in the aortic annulus area, which may be particularly important between women vs. men, Asians vs. Caucasians, and BAV vs. TAV. It thus appears preferable to use the AVCd rather than AVC in the BAV population as well as in the Asian population, which presents with important interindividual variability in the aortic annulus area and thus of calcium density for a given absolute calcium score.
Severe AVC and AVCd Thresholds According to Valve Phenotype, Sex, and Ethnicity
Few studies have examined the accuracy and thresholds of AVC and AVCd to identify severe AS in the BAV population. In a Korean population with BAV, Choi et al.18 found a threshold of 2573 AU (AUC = 0.80, Se/Spe = 73/82%) in men and 1423 AU (AUC = 0.80, Se/Spe = 68/91%) in women to define severe AS in BAV patients. These thresholds are consistent with what we found in Asian men but are higher than what we found in Asian women. The difference in our findings vs. those reported by Choi et al.18 may be related to the fact that they included patients with mixed aortic valve disease, whereas we only included patients with AS. In a Chinese population with a BAV, Ren et al.19 found a threshold of 897 AU to be associated with severe AS (AUC = 0.86, Se/Spe = 87/72%). However, they did not differentiate between the sexes, they did not provide thresholds for AVCd, and they included patients with aortic regurgitation and with mixed aortic valve disease. Potential mechanisms that might explain the higher degree of calcification in the BAV vs. TAV, and particularly in men, are underlying genetic mutations, such as those found in the NOTCH1 gene. Indeed, NOTCH1 has been associated not only with the development of BAV, but also with AS and accelerated calcification of the aortic valves.20 Also, mechanical stresses imposed on the aortic valve leaflets are more important in the BAV than in the TAV due to eccentricity of the aortic valve jet, which might contribute to accelerate the calcification process.21,22
Ethnic-Related Differences in Patients With AS and/or With a BAV
Jilaihawi et al.23 reported that among patients undergoing TAVR, Chinese patients had a higher degree of calcification than Caucasian patients. The reason explaining this finding is still unclear as the Chinese patients in this previous study were younger and had fewer risk factors and comorbidities than the Caucasians. Interestingly, they also found that a BAV was more frequent in Chinese patients undergoing TAVR (almost 50% in their TAVR series) than in Caucasian TAVR series (<2-10%). Another study by Liu et al.24 reported a higher prevalence of BAV in Chinese people, where among 14,530 Chinese children, there was a prevalence of 7.9% for BAV. In the present study, we found that the optimal thresholds of both AVC and AVCd to identify hemodynamically severe AS were higher in Asian men with a BAV than those in Caucasian men with a BAV and those previously reported by Clavel et al.8 for Caucasian men. On the other hand, in Asian women, the AVC threshold was lower than in Caucasian women with a BAV or with a TAV, but this difference was no longer present when using AVCd. Taken together, these findings suggest that there may be ethnic differences in the occurrence of BAV as well as in the pathways leading to the development of calcification, especially in men. Further studies are needed to investigate these potential differences in the aortic valve calcific processes between Asian vs. Caucasian populations.
Study Limitations
First, the vast majority of patients included in this study, especially the Asian patients, presented with severe AS. Second, several subgroup analyses were based on a small number of patients. Also, this study only included patients of Caucasian or Asian ethnicity. Our findings can thus not be extended to patients of Hispanic or African-American ethnicity, and further studies focused on these ethnicities are needed.
Not all 7 centers used the same CT scanners to measure AVC, although the majority of them used scanners from Siemens, and all centers measured AVC using the Agatston method. However, according to a multicenter study, the type of scanner used has no or minimal effect on the results of AVC.10
The threshold values of AVC and AVCd were established in the subset of patients with concordant grading of AS severity at echocardiography. There is no way to ascertain that these thresholds established in concordant grading cases are also valid in the patients with discordant grading cases. However, discordant grading is, in the vast majority of cases, related to the presence of low-flow state, and the main advantage of AVC and AVCd is precisely that these parameters are independent of flow and other hemodynamic conditions. Therefore, it is unlikely that AVC and AVCd would perform differently or that their threshold values would differ in concordant vs. discordant grading cases.
Conclusion and Clinical Implications
The optimal thresholds of AVC and AVCd to identify hemodynamically severe AS obtained in BAV patients are similar in Caucasian men and women but higher in Asian men compared to those previously reported by Clavel et al.8 in a population predominantly composed of Caucasian patients with a TAV. Furthermore, the overall accuracy obtained with AVCd was better than the one with AVC in this BAV population.
Although this study suggests that Asian men with a BAV may have a higher AVC burden for a given degree of AS hemodynamic severity, the AVC and AVCd thresholds currently proposed in the guidelines nonetheless appear to have overall good accuracy (>80% CC) to identify severe AS in the BAV population. Furthermore, given the important interindividual variability in aortic annulus size, it may be preferable to use the AVCd rather than the AVC to confirm AS severity in the BAV population and, particularly, in Asian women. The application of CT-derived AVC and AVCd may be particularly useful in patients with a BAV who present with discordant grading of AS hemodynamic severity on echocardiography.
Ethics Statement
The research reported in this article has adhered to the relevant ethical guidelines. Patients from the Quebec Heart and Lung Institute and British Heart Foundation Centre for Cardiovascular Science were recruited via prospective studies approved by their respective institutional review board, and all patients signed an informed consent form. Patients from the 5 other centers were retrospectively included in this study, and informed consent was waived.
Funding
M.S. was supported by a PhD grant from the Fonds de Recherche Québec-Santé (FRQS), E.G. was supported by a research grant from the Quebec Heart & Lung Institute Foundation, L.T. was supported by a PhD grant from the Fonds de Recherche Québec-Santé (FRQS), M.R.D. was supported by the British Heart Foundation (FS/14/78/31020) and is the recipient of the Sir Jules Thorn Award for Biomedical Research 2015 (15/JTA), M-A.C. holds a New National Investigator award from the Heart and Stroke Foundation (NNI-2019-2020) of Canada and an early career investigator award from the Canadian Institutes of Health Research (EIA-CFBA-179680), and P.P. holds the Canada Research Chair in Valvular Heart Diseases and a Foundation Scheme Grant (FDN-143225 from the Canadian Institutes of Health Research).
Disclosure statement
The department of Cardiology of the Leiden University Medical Center received unrestricted research grants from Abbott Vascular, Bayer, Biotronik, Bioventrix, Boston Scientific, Edwards Lifesciences, GE Healthcare, and Medtronic. Jeroen J. Bax received speaker fees from Abbott Vascular. Victoria Delgado received speaker fees from Abbott Vascular, MSD, Medtronic, Edwards Lifesciences, and GE Healthcare.
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
Supplementary Material
References
- 1.Roberts C. The congenitally bicuspid aortic valve: a study of 85 autopsy cases. Am J Cardiol. 1970;26:72–83. doi: 10.1016/0002-9149(70)90761-7. [DOI] [PubMed] [Google Scholar]
- 2.Fedak P.W., Verma S., David T.E., Leask R.L., Weisel R.D., Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. 2002;106:900–904. doi: 10.1161/01.cir.0000027905.26586.e8. [DOI] [PubMed] [Google Scholar]
- 3.Edwards J.E. The congenital bicuspid aortic valve. Circulation. 1961;23:485–488. doi: 10.1161/01.cir.23.4.485. [DOI] [PubMed] [Google Scholar]
- 4.Hope M.D., Urbania T.H., Yu J.P., Chitsaz S., Tseng E. Incidental aortic valve calcification on CT scans: significance for bicuspid and tricuspid valve disease. Acad Radiol. 2012;19:542–547. doi: 10.1016/j.acra.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner H., Hung J., Bermejo J., et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–392. doi: 10.1016/j.echo.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura R.A., Otto C.M., Bonow R.O., et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–e185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 7.Minners J., Allgeier M., Gohlke-Baerwolf C., Kienzle R.P., Neumann F.J., Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J. 2008;29:1043–1048. doi: 10.1093/eurheartj/ehm543. [DOI] [PubMed] [Google Scholar]
- 8.Clavel M.A., Messika-Zeitoun D., Pibarot P., et al. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler-echocardiographic and computed tomographic study. J Am Coll Cardiol. 2013;62:2329–2338. doi: 10.1016/j.jacc.2013.08.1621. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal S.R., Clavel M.A., Messika-Zeitoun D., et al. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ Cardiovasc Imaging. 2013;6:40–47. doi: 10.1161/CIRCIMAGING.112.980052. [DOI] [PubMed] [Google Scholar]
- 10.Pawade T., Clavel M.A., Tribouilloy C., et al. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ Cardiovasc Imaging. 2018;11 doi: 10.1161/CIRCIMAGING.117.007146. [DOI] [PubMed] [Google Scholar]
- 11.Shen M., Tastet L., Capoulade R., et al. Effect of age and aortic valve anatomy on calcification and haemodynamic severity of aortic stenosis. Heart. 2017;103:32–39. doi: 10.1136/heartjnl-2016-309665. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell C., Rahko P.S., Blauwet L.A., et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Pawade T., Sheth T., Guzzetti E., Dweck M.R., Clavel M.A. Why and how to measure aortic valve calcification in patients with aortic stenosis. JACC Cardiovasc Imaging. 2019;12:1835–1848. doi: 10.1016/j.jcmg.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 15.Lindman B.R., Clavel M.A., Mathieu P., et al. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. doi: 10.1038/nrdp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumgartner H., Falk V., Bax J.J., et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease: the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2017;38:2739–2791. [Google Scholar]
- 17.Clavel M.A., Pibarot P., Messika-Zeitoun D., et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an International Registry Study. J Am Coll Cardiol. 2014;64:1202–1213. doi: 10.1016/j.jacc.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi B.H., Ko S.M., Shin J.K., Chee H.K., Kim J.S., Kim J. Association between aortic valvular calcification and characteristics of the aortic valve in patients with bicuspid aortic valve stenosis. Acta Radiol. 2019;60:468–477. doi: 10.1177/0284185118787359. [DOI] [PubMed] [Google Scholar]
- 19.Ren X., Zhang M., Liu K., et al. The significance of aortic valve calcification in patients with bicuspid aortic valve disease. Int J Cardiovasc Imaging. 2016;32:471–478. doi: 10.1007/s10554-015-0783-y. [DOI] [PubMed] [Google Scholar]
- 20.Garg V., Muth A.N., Ransom J.F., et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 21.Bouchareb R., Boulanger M., Pépin A., Pibarot P., Mathieu P. Mechanical stress enhances aortic valve calcification: implication for bicuspid aortic valve mineralization. Can J Cardiol. 2012;28:S214. Abstract #317. [Google Scholar]
- 22.Saikrishnan N., Mirabella L., Yoganathan A.P. Bicuspid aortic valves are associated with increased wall and turbulence shear stress levels compared to trileaflet aortic valves. Biomech Model Mechanobiol. 2015;14:577–588. doi: 10.1007/s10237-014-0623-3. [DOI] [PubMed] [Google Scholar]
- 23.Jilaihawi H., Wu Y., Yang Y., et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv. 2015;85 Suppl 1:752–761. doi: 10.1002/ccd.25863. [DOI] [PubMed] [Google Scholar]
- 24.Liu F., Yang Y.N., Xie X., et al. Prevalence of congenital heart disease in Xinjiang multi-ethnic region of China. PLoS One. 2015;10:e0133961. doi: 10.1371/journal.pone.0133961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.