Abstract
Transcatheter aortic valve replacement has emerged as the preferred treatment modality in most patients with severe aortic stenosis. With its global adoption and broader application in younger and healthier patients, the issue of transcatheter bioprosthetic valve degeneration and its impact on valve durability continues to earn clinical relevance. Differences in the pathophysiologic processes that separate native from transcatheter heart valve deterioration remain poorly understood. When compared to surgical aortic bioprostheses, the mechanisms of valve degeneration are similar in transcatheter heart valves, with meaningful differences most noticeably found between the individual constructs of their design. Recognizing the clinical and hemodynamic presentation of structural valve degeneration remains paramount. The recently revised consensus guidelines that incorporate the integration of advanced multimodality imaging with invasive hemodynamics represent a major step forward in our ability to accurately diagnose bioprosthetic valve degeneration, and to identify differences in durability patterns, and to establish treatment recommendations for the lifetime management of patients with aortic stenosis. Parallel efforts to unmask the biomolecular differences in atherosclerotic plaque burden, valve calcification, and thrombotic diathesis, including host immunocompetence, between the different available bioprostheses, will further advance the role of emerging valve tissue technologies to improve durability. As with surgical heart valves, the optimal treatment options for redo-transcatheter aortic valve replacement and surgical explant remain poorly understood. Ongoing translational research in bench testing coupled with prospectively designed core lab-adjudicated clinical trials are much needed. This report provides a contemporary overview of transcatheter structural valve degeneration, including evolving concepts in its pathogenesis, diagnosis, and treatment.
Keywords: TAVR, Aortic valve, Transcatheter interventions, Aortic stenosis, Echocardiography
Introduction
Celebrating 20 years since its advent, transcatheter aortic valve replacement (TAVR) has become the preferred treatment option for most patients with severe aortic stenosis.1, 2, 3 With respect to clinical outcomes, TAVR has been shown to be superior or equivalent to surgical aortic valve replacement (SAVR) across multiple major randomized clinical trials encompassing all surgical risk profiles. Controversial data persists as to the durability of transcatheter heart valves (THV) vs. surgical heart valves (SHV) beyond 3 to 5 years. While several posthoc analyses suggest THV durability to be equal to or superior to SHV, other studies have demonstrated SHV durability to be superior.4,5 This has been partly explained by the absence of well-defined rigorous diagnostic criteria and long-term adjudicated cardiac imaging follow-up. Understanding the mechanisms through which THV degeneration occurs remains central to the development of emerging technologies that incorporate durable valve tissue solutions. Ongoing efforts to understand the management of structural valve degeneration (SVD) from bench testing to prospectively designed clinical trials with redo-TAVR and surgical explant are underway. This document provides a contemporary overview of SVD in THV and its implications in the lifetime management of patients with aortic stenosis.
Defining Structural Valve Degeneration in Transcatheter Heart Valves
The definition of SVD and our understanding of this condition have evolved over the years (Figure 1). In 2017, the European Association of Percutaneous Cardiovascular Interventions (EAPCI) published its standardized definition of SVD, which was later endorsed by the European Society of Cardiology and European Association for Cardiothoracic Surgery.6 Through their document, Capodanno et al. expand the definition of SVD to range from morphological SVD to bioprosthetic valve failure (BVF) (Table 1, EAPCI definition of SVD). Furthermore, Dvir et al.7 in the Valve In Valve International Database registry outlines a concise definition of SVD that excludes infective endocarditis and valvular thrombosis and incorporates quantitative assessment of effective orifice area (EOA) and doppler velocity index (DVI) (Table 1, VIVID definition of SVD).
Figure 1.
Latest definitions of THV durability.
Abbreviations: BVD, bioprosthetic valve dysfunction; BVF, bioprosthetic valve failure; EAPCI, European Association of Percutaneous Cardiovascular Interventions; NSVD, non-structural valve deterioration; SVD, structural valve deterioration; VARC-3, Valve Academic Research Consortium 3; VIVID, Valve In Valve International Database.
Table 1.
EAPCI and VIVID definitions of structural valve deterioration
| EAPCI definition of SVD (2017 Capodanno et al.) | VIVID definition of SVD (2018 Dvir et al.) |
|---|---|
| SVD Stage 0 = no chance from immediate post-implant | |
Morphological SVD–
|
SVD Stage 1 = morphological leaflet abnormality w/o hemodynamic change. Visualized leaflet calcification, sclerosis, thickening or newfound motion dysfunction. Excludes infective endocarditis, valve thrombosis, isolated PPM without valvular deterioration, isolated PVL within SVD definition but these are included within Stage 1 definition as these bioprostheses can be prone to early SVD. |
Moderate SVD → includes ANY of the following:
|
SVD Stage 2S – moderate stenosis
|
SVD Stage 2R – moderate regurgitation.
| |
| SVD Stage 2RS – moderate stenosis AND regurgitation | |
Severe SVD → includes ANY of the following
|
SVD Stage 3 – severe stenosis and/or regurgitation
|
BVF – findings at autopsy of SVD → valve-related death
|
Not defined |
AR, aortic regurgitation; BVF, bioprosthetic valve failure; DVI, Doppler velocity index; EAPCI, European Association of Percutaneous Coronary Interventions; EOA, effective orifice area; PPM, patient-prosthesis mismatch; SVD, structural valve deterioration; VIVID, Valve In Valve International Database.
The advent and integration of invasive hemodynamics and multimodality imaging have further refined our understanding of SVD as revised by the most recent Valve Academic Research Consortium (VARC) 3. In this contemporary consensus statement, the authors go on to provide additional clarity as to the differences between SVD and non-SVD, and also stratify BVF by severity8 (Table 2, VARC-3 definitions of SVD vs non-SVD and BVF). According to the VARC-3 definitions, a patient with Stage 3 hemodynamic SVD is defined as someone with intrinsic permanent changes to the prosthetic valve (which may include wear/tear, leaflet disruption, flail leaflet, fibrotic leaflet/calcification, or strut fracture) with an increase in transvalvular gradient ≥20 mmHg resulting in a mean gradient ≥30 mmHg with a concomitant decrease in EOA ≥0.6 cm2 (or ≥50% of prior EOA) and/or decrease in DVI ≥0.2 (or ≥40% of prior DVI); compared with echocardiographic assessment performed 1 to 3 months postprocedure; or with a new occurrence or increase by ≥2 grades of intraprosthetic aortic regurgitation resulting in severe aortic regurgitation. Through this rigorous consensus document, a new standard definition for SVD provides a foundation for future clinical research in both surgical and transcatheter valves while emphasizing the role of noninvasive imaging, including echocardiography and computed tomography angiography, as well as invasive hemodynamics.
Table 2.
VARC-3 definition of SVD, non-SVD, thrombosis, and endocarditis for bioprosthetic aortic valves
| Bioprosthetic valve degeneration categories | Clinical presentation | Stages of deterioration |
|---|---|---|
| SVD – intrinsic permanent changes to prosthetic valve; includes wear/tear, leaflet disruption, flail leaflet, fibrotic leaflet/calcification, strut fracture | Subclinical → any bioprosthetic dysfunction associated without hemodynamic changes, ABSENT symptoms |
Stage 1: Morphological degeneration →
|
| Non-SVD – any abnormality, non-intrinsic → valve dysfunction. i.e., residual intra-/para-prosthetic AR; pannus/suture entrapping of leaflets; inappropriate positioning/sizing; aortic root dilatation; PPM; embolization |
Bioprosthetic valve failure stage 1: Any bioprosthetic valve dysfunction associated with clinically expressive criteria (new/worsening symptoms, LV dilation/hypertrophy/dysfunction, or pulmonary hypertension) OR irreversible stage 3 hemodynamic valve deterioration Bioprosthetic Valve failure stage 2: Aortic valve reoperation or re-intervention Bioprosthetic Valve failure stage 3: Valve-related death. |
Stage 2: Moderate hemodynamic valve deterioration →
|
Thrombosis – defined as clinical sequelae of a thromboembolic event or worsening AS/AR and
|
Stage 3: Severe hemodynamic valve deterioration →
|
|
| Endocarditis – (1) Fulfilling Duke endocarditis criteria; (2) evidence of abscess/pus/vegetation confirmed on histological/microbiological studies during re-op; (3) Criteria in (2) confirmed during autopsy |
VARC-3, Valve Academic Research Consortium 3; SVD, structural valve deterioration; AR, aortic regurgitation; PPM, patient-prosthesis mismatch; AS, aortic stenosis; PVL, paravalvular leak; EOA, effective orifice area; EAPCI, European Association of Percutaneous Cardiovascular Interventions.
Pathogenesis of Transcatheter Structural Valve Deterioration
Leaflet degeneration is the primary mechanism through which THV degeneration occurs. Akin to native aortic valves, progressive leaflet calcification resulting in stenosis or regurgitation is believed to be the principal mechanism through which bioprosthetic valve degeneration occurs.9 In the case of THV vs. SHV degeneration, several differences have been demonstrated. Acute intraprocedural degeneration has been shown to occur more commonly with THV. It is attributed to the trauma to leaflet structures that may occur during valve crimping, loading and/or balloon deployment/valve recapture, and instances of post-balloon dilation.10 Other proposed mechanisms have been related to the presence of turbulent blood flow persisting across the aortic root during THV deployment, unlike cardioplegic arrest during SHV replacement.11,12 Another important consideration, perhaps more applicable to self-expandable THV, may relate to the higher tendency of THV to adopt a final elliptical shape, thus potentially impairing normal valvular function and predisposing patients to premature degeneration.13 Studies evaluating the impact of under- or asymmetric THV expansion suggest a correlation with early thrombosis, impaired leaflet motion, and possible premature degeneration.14 While not well understood, the burden and persistence of progressive underlying native valvular calcification processes may also impact long-term THV durability.12 Higher residual transvalvular gradients post-THV deployment in subjects with either small annuli and/or patient-prosthesis mismatch have also been associated with premature valve deterioration.13,15
While not technically denoted as true SVD per VARC3, infective endocarditis and bioprosthetic leaflet thrombus may result in hemodynamic deterioration or BVF. Similarly, paravalvular regurgitation is considered a non-SVD type of BVF. In the early TAVR experience, paravalvular leak (PVL) was considered a precipitator of premature SVD due to increased transvalvular flow and leaflet shear stress.16 With the advent of improved THV designs to minimize PVL, the potential for PVL-mediated SVD is less likely.
Proposed Molecular Mechanisms of Transcatheter Heart Valve Degeneration
Numerous efforts have been made to understand the biomolecular principles involved in the pathogenesis of native and bioprosthetic valve degeneration, with long-term immune rejection and atherosclerotic tissue remodeling both playing an important role.17 Important biomolecular differences have been revealed when comparing SVD in bioprosthetic THV to those of native human valves. Most THVs use xenografts from porcine or bovine pericardial tissue with either a nitinol or cobalt chromium frame.18,19 Native aortic valves are comprised of 3 extracellular matrix coatings, labelled fibrosa, spongiosa, and ventricularis, with each layer mechanically responsible for load dampening, elasticity, and stress response.20 Xenografts, on the other hand, are made from bovine or porcine pericardium that lack these distinct layers. The absence of layered diversity in xeno-pericardial valves promotes the process of accelerated delamination with premature collagen destruction when compared to native valves. The presence of valve-interstitial cells in native human valves is another important difference. The cell lining continuously remodels the extracellular matrix in a compensatory biomolecular adaptive response to shear stress. The absence of interstitial cells in THVs also contributes to early degeneration in these valves.21 Several other cellular and biomolecular factors have been linked to the disruptive process of valvular membranes in native aortic stenosis (Figure 2); a similar molecular pathogenesis contributing to transcatheter SVD has been proposed.22 Finally, well-known, host-mediated factors have been previously recognized to impact the rate at which SVD occurs,21 with younger age at the time of bioprosthetic valve implantation being one of the most important risk factors for predicting early-onset SVD.23 Other risk factors that have been implicated in early SVD include arterial hypertension, patient-prosthesis mismatch, end-stage kidney disease, diabetes mellitus, and hyperparathyroidism.21
Figure 2.
Top panel showing normal aortic valve leaflet. Bottom panel showing biomolecular pathogenesis imposed in a severely stenotic aortic valve (Adapted from Dadlani et al. Calcific Aortic Valve Disease and Hypertension, 2008).
TAVR Durability
Although recent analyses from landmark trials have provided further insight into the durability of THV’s, longer-term data are needed. Tam et al.24 recently demonstrated through simulation modeling sensitivity analysis that TAVR valve durability must be <70% shorter than SAVR durability in order to result in reduced life expectancy in patients with similar demographics as those in the major TAVR trials. This analysis yielded optimism for TAVR in an older patient profile; however, it raised concern for TAVR if considered in a younger, lower-risk cohort. Multiple other trials have demonstrated comparability between SHV and THV durability. Table 3 provides a summary of the major studies surrounding THV degeneration reported to date.
Table 3.
Summary of TAVR durability data
| Contributor | Follow-up | Sample size | Major findings | |
|---|---|---|---|---|
| Jergesen, T.H.25 (NOTION 8y data) | 8y | 145 TAVR | 135 SAVR |
|
| Murray, MI26 | 7y | 103 TAVR | BVF (3.8%) Severe SVD (1.3%) Moderate SVD (9%) Thrombosis (1.3%) Endocarditis (1.3%) |
|
| Didier, R.27 | 5y | 4187 TAVR (2774 BEV/1413 SEV) | Mod SVD (13.3%) Severe SVD (2.5%) |
|
| Holy, EW28 | 8y | 152 TAVR (SEV) | BVF 4.5% Mod-Sev. SVD 0% |
|
| Deutsch, M-A29 | 7y | 300 TAVR | SVD 14.9% BVF (n = 10) |
|
| Aldalati, O.30 | 6.5y | 269 TAVR | 174 SAVR | Mod SVD (TAVR 11.5% vs. SAVR 20.7%, p = 0.007) |
| Gleason T.G.31 | 5y | 391 TAVR | 359 SAVR | Mod SVD (TAVR 9.5% vs. SAVR 26.6%, p < 0.001) Severe SVD (TAVR 0.8% vs. SAVR 1.7%, p = 0.32) |
| Testa, L.32 | 8y | 990 TAVR | Mod SVD 3% Severe SVD 1.6% Late BVF 2.5% |
|
| Durand E.33 | 7y | 1403 TAVR | Mod SVD 7% Severe SVD 4.2% BVF 1.9% |
|
| Blackman, D.J34 | 5.8y | 241 TAVR | Severe SVD <0.5% Moderate SVD 8.7% |
|
| Sathananthan, J.35 | 10y | 235 TAVR | SVD 6.5% BVF 2.5% |
|
| Barbanti, M.36 | 8y | 286 TAVR | Severe SVD 2.3% Moderate SVD 5.9% BVF 4.5% Thrombosis 0% |
|
| Eltchaninoff, H.37 | 8y | 378 TAVR | SVD 3.2% Late BVF 0.6% |
|
| Panico, R.A.38 | 7y | 278 TAVR | SVD3.6% BVF 2.5% Thrombosis 0% |
|
| Orvin, K.39 | 5y | 450 TAVR | SVD 12.3% BVF 0.6% annualized incidence BVD 1.8% annualized incidence |
|
| Pibarot P.,40 | 5y | Sapien XT TAVR (n = 774) Sapien 3 TAVR (n = 891) SAVR (n = 664) |
Sapien XT vs. SAVR: SVD (1.6% vs. 0.6%, p < 0.01)
|
|
| Reardon, MJ41 | 5y | TAVR-SEV (n = 1128) SAVR (n = 971) |
SVD 4.4% (SAVR) vs. 2.6% (SEV-TAVR) p = 0.0095 SVD in smaller (<23 mm) annular diam. SAVR vs. TAVR:
|
|
TAVR, transcatheter aortic valve replacement; SEV, self-expanding valves; SAVR, surgical aortic valve replacement; SVD, structural valve deterioration; NSVD, non-structural valve deterioration; BVF, bioprosthetic valve failure; BVD, bioprosthetic valve dysfunction; BEV, balloon-expandable valves; VIVID, Valve In Valve International Database; VARC-3, Valve Academic Research Consortium 3.
Given the advanced age of TAVR recipients and the high prevalence of multiple comorbidities at baseline, very little data exist regarding valve durability beyond 10 years. Sathananthan et al.35 recently showed, in a longitudinal 10-year follow-up analysis involving 235 patients, that the rate of structural valve deterioration at 10 years was only 6.5%; however, there was a high rate of mortality from other causes unrelated to SVD. The results, while encouraging, may be subject to a selection bias. The NOTION trial, on the other hand, after 8-year follow-up reported higher rates of SVD post-SAVR (28.5%) when compared to TAVR (14.1%).25 These findings were consistent with those reported by Reardon et al., in the 5-year SURTAVI follow-up displaying a lower rate of SVD in the self-expanding TAVR prosthesis when compared to SAVR.41 While we await the 10-year findings from this dataset, the intermediate-term results are encouraging.
Managing Aortic Transcatheter SVD
Similar to degenerative bioprosthetic surgical valves, transcatheter valve-in-valve (THV-in-THV) and surgical explants have been proposed to treat patients with THV deterioration. In contrast to SHVs, there is a scarcity of clinical data to responsibly advocate and determine the safety and efficacy of either option for THV, including important technical differences and recommendations. With the anticipated rise in number of patients with transcatheter SVD in the coming years, early recognition and careful examination of available treatment options is crucial in the lifetime management of aortic stenosis.
THV-in-THV vs. Surgical Explant
Little data exists regarding the safety and efficacy of redo-THV or surgical explant for the treatment of transcatheter SVD. Most involve small registries of retrospective design with nonadjudicated clinical and echocardiographic outcomes. In a few multicenter studies evaluating the role of surgical explant for the treatment of degenerative THV, the surgical strategy resulted in a significant risk of 30-day and 1-year mortality.42,43 Selection bias may have contributed to the results (e.g., patients with endocarditis were more likely to undergo surgery).
The proposed role and feasibility of THV-in-THV in patients with SVD derives from the historical outcomes of patients who, during their index TAVR procedure, developed acute severe bioprosthetic valve malfunction requiring redo-TAVR. In these patients, THV-in-THV was associated with satisfactory 1-year clinical and echocardiographic outcomes.44,45
Efforts to understand the safety and efficacy of redo-TAVR in patients with true SVD years after their index procedure are underway. In the study of Landes et al.,46 the authors sought to examine outcomes following redo-TAVR and demonstrated excellent safety with only a 1.4% 30-day mortality in patients who received redo-TAVR for the indication of a failed THV. Nonetheless, it is important to highlight the several limitations, including the observational, nonadjudicated study design and lack of core lab imaging analysis. Furthermore, while the authors report a relatively low risk of coronary obstruction of 0.7%, it is possible that the analysis may have suffered from ascertainment bias. In other words, patients who had a perceived prohibitive risk of coronary obstruction were likely referred for surgical explant, and thus excluded from the analysis. Current studies do not provide distinct clarity or insight as to the technical considerations that should be employed at the time of THV-in-THV, including annular sizing, valve size, implant depth, and commissural alignment.
Further efforts to compare explant vs. THV-in-THV were recently presented this year. In the explant-or-redo-TAVR international registry, Zaid et al. provide important insights into the contemporary short- and long-term prognosis comparing transcatheter and surgical reintervention. In their multicenter analysis involving over 350 patients, Zaid et al. demonstrated that surgical explant was shown to have a significantly higher in-hospital mortality rate of 11.8% compared to 2.3% in redo-TAVR. The 30-day mortality rate with surgical explant was 14.2% compared to 3.5% in the redo-TAVR cohort, and the 1-year mortality rate was 35.5% compared to 14.6% in the redo-TAVR.47 However, in the landmark analysis conducted by the study investigators, it was observed that mortality beyond 30 days was not significantly different between THV-in-THV and surgical explant. Given the reassuring data that THV-in-THV is safe and effective compared to surgical reintervention, understanding the technical feasibility of performing THV-in-THV is essential as most patients will not undergo surgical intervention, if they are a candidate for redo-TAVR.
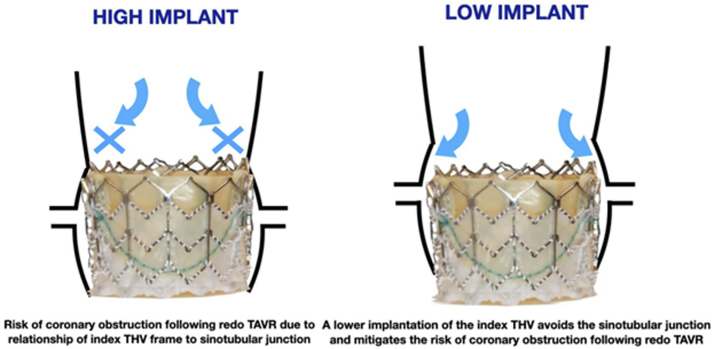
Much of the existing data for preprocedural planning for THV-in-SHV to eliminate coronary obstruction may be applicable in redo-TAVR as well48 (Figure 3). With an anticipated rise in the demand of redo-TAVR on the horizon, novel terminology has been introduced by Sathananthan et al.49 from benchtop hydrodynamic assessment of redo-TAVR. Terms such as neo-skirt height, sinus sequestration, leaflet modification, commissural alignment, and leaflet overhang are imperative to understand when implanting THV-in-THVs to ensure feasible coronary access and optimal performance.50 Tall-frame THVs pose a challenge of ensuring cell alignment, if there is planning for placement of a tall-frame THV within another tall-frame THV (Figure 4a). Given these valves already have a high skirt, the “neo-skirt” of a potential tall-frame THV-in-THV can pose significant challenges when it comes to coronary access (Figure 4b).51 Avoidance of this challenge entails implementing a low-implant depth deployment of the index TAVR, a concept highlighted in Figure 4, to ensure coronary obstruction does not occur in future THV-in-THV procedures.
Figure 3.
Index THV implant depth matters; high implant has been recommended to avoid conduction system disturbance but the suggested benefit of this may be offset by increased risk of coronary obstruction during potential THV-in-THV.
THV, transcatheter heart valve; TAVR, transcatheter aortic valve replacement.
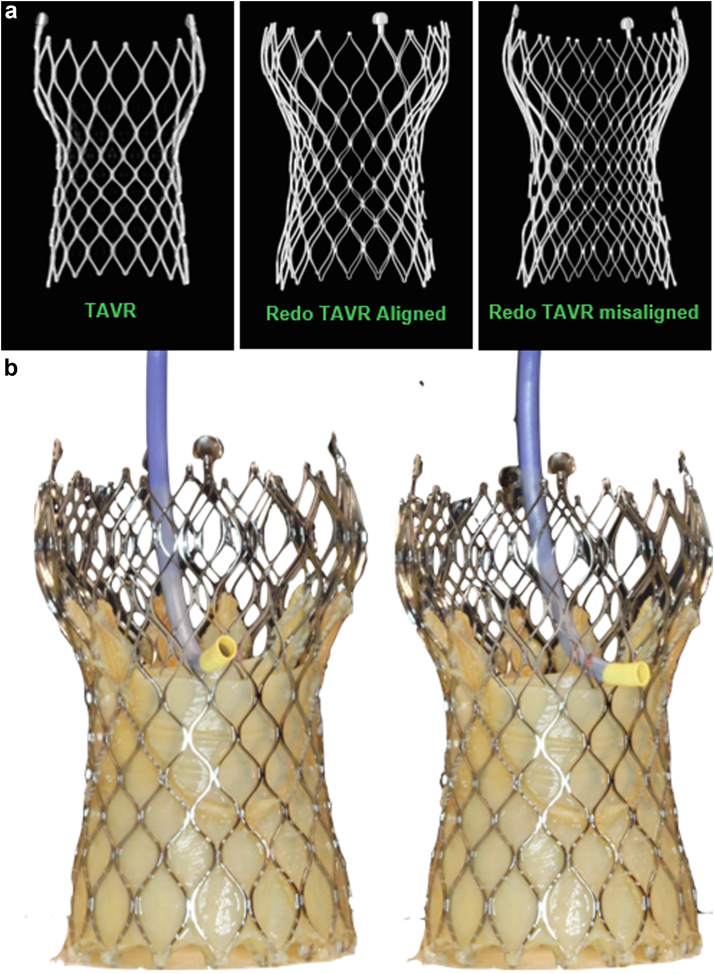
Figure 4.
(a) Cell misalignment of the redo-THV within the index THV when performing THV-in-THV with 2 tall-frame SEV’s can pose a significant challenge for future coronary access. (b) Tall-frame THV’s pose a challenge for coronary access alone, thus optimal commissural alignment of the index THV is essential to facilitate future coronary access.
THV, transcatheter heart valve; SEV, self-expanding valve; TAVR, transcatheter aortic valve replacement.
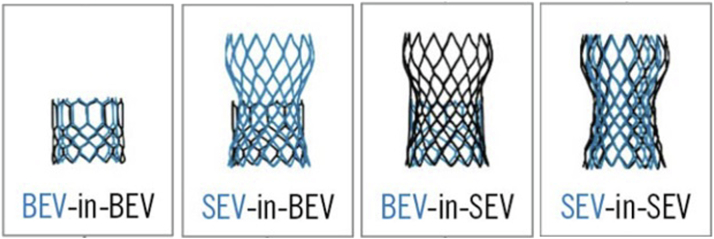
Nonetheless, too low of an implant depth during a redo-THV could potentially lead to a phenomenon described as “leaflet overhang” that may result in leaflet pin-wheeling with mal-coaptation and aortic insufficiency. Balloon-expandable valves have been demonstrated to theoretically impose less risk of coronary obstruction through computational simulation data.52, 53 Though this may apply to risk of sinus sequestration and coronary access feasibility, implantation of a short-frame balloon-expandable THV within a tall-frame self-expandable THV can lead to leaflet overhang of the index tall-frames’ leaflets layering over the redo-THVs’ leaflet, theoretically compromising valve function.50 Though numerous permutations of THV-in-THV exist, these can be simplified to those illustrated in Figure 5. Lastly, novel technical concepts must be taken into consideration to optimize redo-TAVR performance. Predilation of balloon-expandable THVs has been hypothesized through hydrodynamic bench testing to prevent expansion of the underlying index THV, thereby mitigating the risk for potential sinus sequestration.51,54 Infinite possibilities remain on the horizon for the foreseeable future to optimize THV-in-THV therapies. Further incorporating advanced imaging-based modeling techniques using cardiac computed tomography is paramount and will provide important insights as to the risk of coronary obstruction and valve selection.55 In a cardiac computed tomography-based modeling study from Rogers et al., the risk of sinus sequestration was lower with balloon-expandable valves compared to self-expanding valves and thus may inform the need for leaflet modification techniques, such as BASILICA52 or a ShortCut (Pi-Cardia) valve leaflet splitting catheter, when deemed necessary.56
Figure 5.
Combinations for THV-in-THV.
Considerations for optimization include factoring in 1) Index THV design, 2) Implant depth of index valve, 3) Redo-TAVR THV design, and 4) Implant depth of redo-TAVR THV. Fostering a strong understanding of the risks and benefits associated with each combination is crucial given the anticipated rise in future THV-in-THVs.
THV, transcatheter heart valve; TAVR, transcatheter aortic valve replacement; BEV, balloon-expandable valves; SEV, self-expanding valve.
The technical considerations and challenges of redo-TAVR as summarized above underscore the importance of encouraging operators performing every index TAVR procedure to consider all factors that may potentially influence the safety and feasibility of a future THV-in-THV (Figure 4). Finally, clinical and anatomical factors must be considered when deciding between THV-in-THV vs. surgical explant including patient’s age, anatomy, preference, THV-type, implant depth, baseline annular size, and risk of patient-prosthesis mismatch, among others.
Future Directions
Prospective core lab-adjudicated clinical trials of THV-in-THV that incorporate advanced imaging, technical consideration, and rigorous follow-up are much needed and are soon to come. The RENEWAL trial presented at the 2022 TVT meeting by Cubeddu et al. and the Edwards Lifesciences Alliance AVIV may be the first of its kind. Additionally, promising advancements of valve design using novel biopolymers may also lead to prolonged durability, as proposed by the SAPIEN X4-RESILIA (Edwards, Inc), DurAVR (Anteris, Inc) and Tria valve systems (Foldax, Inc). The SAPIEN-X4 RESILIA adds enhanced anti-calcification technology with hopes of decreasing SVD rates, similar to the technology already incorporated in the Inspiris-RESILIA SHV.57 With dynamic advancements in breakthrough technology suggestive of longer THV durability, illustrative data surrounding redo-TAVR, SVD, and THV failure will continue to emerge.
Funding
The authors have no funding to report.
Disclosure Statement
The authors report no conflict of interest.
References
- 1.Smith C.R., Leon M.B., Mack M.J., et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 2.Leon M.B., Smith C.R., Mack M.J., et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 3.Mack M.J., Leon M.B., Thourani V.H., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 4.Van Mieghem N.M. Presented at: TCT 2021. November 5, 2021. 5-Year clinician and echocardiographic outcomes from the randomized SURTAVI trial. Orlando, FL. [Google Scholar]
- 5.Ler A., Ying Y.J., Sazzad F., Choong A.M.T.L., Kofidis T. Structural durability of early-generation transcatheter aortic valve replacement valves compared with surgical aortic valve replacement valves in heart valve surgery: a systematic review and meta-analysis. J Cardiothorac Surg. 2020;15(1):127. doi: 10.1186/s13019-020-01170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capodanno D., Petronio A.S., Prendergast B., et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2017;38(45):3382–3390. doi: 10.1093/eurheartj/ehx303. [DOI] [PubMed] [Google Scholar]
- 7.Dvir D., Bourguignon T., Otto C.M., et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018;137(4):388–399. doi: 10.1161/CIRCULATIONAHA.117.030729. [DOI] [PubMed] [Google Scholar]
- 8.VARC-3 WRITING COMMITTEE. Généreux P., Piazza N., et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. 2021;42(19):1825–1857. doi: 10.1093/eurheartj/ehaa799. [DOI] [PubMed] [Google Scholar]
- 9.Dvir D., Barbanti M., Tan J., Webb J.G. Transcatheter aortic valve-in-valve implantation for patients with degenerative surgical bioprosthetic valves. Curr Probl Cardiol. 2014;39(1):7–27. doi: 10.1016/j.cpcardiol.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Mylotte D., Andalib A., Thériault-Lauzier P., et al. Transcatheter heart valve failure: a systematic review. Eur Heart J. 2015;36(21):1306–1327. doi: 10.1093/eurheartj/ehu388. [DOI] [PubMed] [Google Scholar]
- 11.Costa G., Criscione E., Todaro D., Tamburino C., Barbanti M. Long-term transcatheter aortic valve durability. Interv Cardiol. 2019;14(2):62–69. doi: 10.15420/icr.2019.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbanti M., Tamburino C. Late degeneration of transcatheter aortic valves: pathogenesis and management. EuroIntervention. 2016;12(Y):Y33–Y36. doi: 10.4244/EIJV12SYA8. [DOI] [PubMed] [Google Scholar]
- 13.Didier R., Benic C., Nasr B., et al. High post-procedural transvalvular gradient or delayed mean gradient increase after transcatheter aortic valve implantation: incidence, prognosis and associated variables. The FRANCE-2 Registry. J Clin Med. 2021;10(15):3221. doi: 10.3390/jcm10153221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia S., Fukui M., Dworak M.W., et al. Clinical impact of hypoattenuating leaflet thickening after transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2022;15(3) doi: 10.1161/CIRCINTERVENTIONS.121.011480. [DOI] [PubMed] [Google Scholar]
- 15.Jin R., Cox E.J., Reynolds B.R., et al. Comparison of 26-mm Evolut and 23-mm sapien 3 valves in TAVR for small aortic annulus. J Invasive Cardiol. 2022;34(6):E433–E441. doi: 10.25270/jic/21.00260. [DOI] [PubMed] [Google Scholar]
- 16.Généreux P., Head S.J., Hahn R., et al. Paravalvular leak after transcatheter aortic valve replacement: the new Achilles’ heel? A comprehensive review of the literature. J Am Coll Cardiol. 2013;61:1125–1136. doi: 10.1016/j.jacc.2012.08.1039. [DOI] [PubMed] [Google Scholar]
- 17.Cote N., Pibarot P., Clavel M.A. Incidence, risk factors, clinical impact, and management of bioprosthesis structural valve degeneration. Curr Opin Cardiol. 2017;32:123–129. doi: 10.1097/HCO.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 18.Choudhury T., Solomonica A., Bagur R. The Evolut R and Evolut PRO transcatheter aortic valve systems. Expert Rev Med Devices. 2019;16(1):3–9. doi: 10.1080/17434440.2019.1557045. [DOI] [PubMed] [Google Scholar]
- 19.Holoshitz N., Kavinsky C.J., Hijazi Z.M. The Edwards SAPIEN transcatheter heart valve for calcific aortic stenosis: a review of the valve, procedure, and current literature. Cardiol Ther. 2012;1(1):6. doi: 10.1007/s40119-012-0006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arjunon S., Rathan S., Jo H., Yoganathan A.P. Aortic valve: mechanical environment and mechanobiology. Ann Biomed Eng. 2013;41:1331–1346. doi: 10.1007/s10439-013-0785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kostyunin A.E., Yuzhalin A.E., Rezvova M.A., Ovcharenko E.A., Glushkova T.V., Kutikhin A.G. Degeneration of bioprosthetic heart valves: update 2020. J Am Heart Assoc. 2020;9(19) doi: 10.1161/JAHA.120.018506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostyunin A.E., Yuzhalin A.E., Ovcharenko E.A., Kutikhin A.G. Development of calcific aortic valve disease: do we know enough for new clinical trials? J Mol Cell Cardiol. 2019;132:189–209. doi: 10.1016/j.yjmcc.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Katsi V., Georgiopoulos G., Oikonomou D., et al. Aortic stenosis, aortic regurgitation and arterial hypertension. Curr Vasc Pharmacol. 2019;17(2):180–190. doi: 10.2174/1570161116666180101165306. [DOI] [PubMed] [Google Scholar]
- 24.Tam D.Y., Wijeysundera H.C., Naimark D., et al. Impact of transcatheter aortic valve durability on life expectancy in low-risk patients with severe aortic stenosis. Circulation. 2020;142(4):354–364. doi: 10.1161/CIRCULATIONAHA.119.044559. [DOI] [PubMed] [Google Scholar]
- 25.Jørgensen T.H., Thyregod H.G.H., Ihlemann N., et al. Eight-year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transcatheter vs. surgical aortic valve replacement. Eur Heart J. 2021;42(30):2912–2919. doi: 10.1093/eurheartj/ehab375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray M.I., Hofmann E., De Rosa R., et al. Hemodynamic outcome and valve durability beyond five years after transcatheter aortic valve replacement. J Invasive Cardiol. 2020;32(3):82–87. doi: 10.25270/jic/19.00346. [DOI] [PubMed] [Google Scholar]
- 27.Didier R., Eltchaninoff H., Donzeau-Gouge P., et al. Five-year clinical outcome and valve durability after transcatheter aortic valve replacement in high-risk patients. Circulation. 2018;138(23):2597–2607. doi: 10.1161/CIRCULATIONAHA.118.036866. [DOI] [PubMed] [Google Scholar]
- 28.Holy E.W., Kebernik J., Abdelghani M., et al. Long-term durability and haemodynamic performance of a self-expanding transcatheter heart valve beyond five years after implantation: a prospective observational study applying the standardised definitions of structural deterioration and valve failure. EuroIntervention. 2018;14:e390–e396. doi: 10.4244/EIJ-D-18-00041. [DOI] [PubMed] [Google Scholar]
- 29.Deutsch M.-A., Erlebach M., Burri M., et al. Beyond the five-year horizon: long-term outcome of high-risk and inoperable patients undergoing TAVR with first-generation devices. EuroIntervention. 2018;14:41–49. doi: 10.4244/EIJ-D-17-00603. [DOI] [PubMed] [Google Scholar]
- 30.Aldalati O., Kaura A., Khan H., et al. Bioprosthetic structural valve deterioration: how do TAVR and SAVR prostheses compare? Int J Cardiol. 2018;268:170–175. doi: 10.1016/j.ijcard.2018.04.091. [DOI] [PubMed] [Google Scholar]
- 31.Gleason T.G., Reardon M.J., Popma J.J., et al. 5-Year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol. 2018;72:2687–2696. doi: 10.1016/j.jacc.2018.08.2146. [DOI] [PubMed] [Google Scholar]
- 32.Testa L., Latib A., Brambilla N., et al. Long-term clinical outcome and performance of transcatheter aortic valve replacement with a self-expandable bioprosthesis. Eur Heart J. 2020;41:1876–1886. doi: 10.1093/eurheartj/ehz925. [DOI] [PubMed] [Google Scholar]
- 33.Durand E., Sokoloff A., Urena-Alcazar M., et al. Assessment of long-term structural deterioration of transcatheter aortic bioprosthetic valves using the new European definition. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.118.007597. [DOI] [PubMed] [Google Scholar]
- 34.Blackman D.J., Saraf S., MacCarthy P.A., et al. Long-term durability of transcatheter aortic valve prostheses. J Am Coll Cardiol. 2019;73:537–545. doi: 10.1016/j.jacc.2018.10.078. [DOI] [PubMed] [Google Scholar]
- 35.Sathananthan J., Lauck S., Polderman J., et al. Ten year follow-up of high-risk patients treated during the early experience with transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2021;97(3):E431–E437. doi: 10.1002/ccd.29124. [DOI] [PubMed] [Google Scholar]
- 36.Barbanti M., Costa G., Zappulla P., et al. Incidence of long-term structural valve dysfunction and bioprosthetic valve failure after transcatheter aortic valve replacement. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eltchaninoff H., Durand E., Avinée G., et al. Assessment of structural valve deterioration of transcatheter aortic bioprosthetic balloon-expandable valves using the new European consensus definition. EuroIntervention. 2018;14:e264–e271. doi: 10.4244/EIJ-D-18-00015. [DOI] [PubMed] [Google Scholar]
- 38.Panico R.A., Giannini C., De Carlo M., et al. Long-term results and durability of the CoreValve transcatheter aortic bioprosthesis: outcomes beyond five years. EuroIntervention. 2019;14:1639–1647. doi: 10.4244/EIJ-D-18-00779. [DOI] [PubMed] [Google Scholar]
- 39.Orvin K., Zekry S.B., Morelli O., et al. Long-term functional and structural durability of bioprosthetic valves placed in the aortic valve position via percutaneous Rout in Israel. Am J Cardiol. 2019;124:1748–1756. doi: 10.1016/j.amjcard.2019.08.043. [DOI] [PubMed] [Google Scholar]
- 40.Pibarot P., Ternacle J., Jaber W.A., et al. Structural deterioration of transcatheter versus surgical aortic valve bioprostheses in the PARTNER-2 trial. J Am Coll Cardiol. 2020;76:1830–1843. doi: 10.1016/j.jacc.2020.08.049. [DOI] [PubMed] [Google Scholar]
- 41.Bioprosthetic valve failure and durability – does TAVR really outperform SAVR; Presented at: TCT 2022; September 17, 2022; Boston, MA by Michael J. Reardon, et al.
- 42.Hirji S.A., Percy E.D., McGurk S., et al. Incidence, characteristics, predictors, and outcomes of surgical explantation after transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76(16):1848–1859. doi: 10.1016/j.jacc.2020.08.048. [DOI] [PubMed] [Google Scholar]
- 43.Bapat V.N., Zaid S., Fukuhara S., et al. Surgical explantation after TAVR failure: mid-term outcomes from the EXPLANT-TAVR International Registry. JACC Cardiovasc Interv. 2021;14(18):1978–1991. doi: 10.1016/j.jcin.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Barbanti M., Tamburino C. Late degeneration of transcatheter aortic valves: pathogenesis and management. EuroIntervention. 2016;12(Y):Y33–Y36. doi: 10.4244/EIJV12SYA8. [DOI] [PubMed] [Google Scholar]
- 45.Barbanti M., Webb J.G., Tamburino C., et al. Outcomes of redo transcatheter aortic valve replacement for the treatment of postprocedural and late occurrence of paravalvular regurgitation and transcatheter valve failure. Circ Cardiovasc Interv. 2016;9(9) doi: 10.1161/CIRCINTERVENTIONS.116.003930. [DOI] [PubMed] [Google Scholar]
- 46.Landes U., Webb J.G., De Backer O., et al. Repeat transcatheter aortic valve replacement for transcatheter prosthesis dysfunction. J Am Coll Cardiol. 2020;75(16):1882–1893. doi: 10.1016/j.jacc.2020.02.051. [DOI] [PubMed] [Google Scholar]
- 47.Explant versus Redo TAVR after TAVR failure: outcomes from the EXPLANTORREDO-TAVR International Registry; Presented at TVT 2022; June 7th, 2022, Chicago, IL by Syed Zaid, GH Tang et al.
- 48.Landes U., Sathananthan J., Witberg G., et al. Transcatheter replacement of transcatheter versus surgically implanted aortic valve bioprostheses. J Am Coll Cardiol. 2021;77(1):1–14. doi: 10.1016/j.jacc.2020.10.053. [DOI] [PubMed] [Google Scholar]
- 49.Sathananthan J., Fraser R., Landes U., et al. Repeat transcatheter aortic valve implantation and implications for transcatheter heart valve performance: insights from bench testing. EuroIntervention. 2021;17(10):856–864. doi: 10.4244/eij-d-20-00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akodad M., Sellers S., Landes U., et al. Balloon-expandable valve for treatment of Evolut valve failure: implications on Neoskirt height and leaflet overhang. JACC Cardiovasc Interv. 2022;15(4):368–377. doi: 10.1016/j.jcin.2021.12.021. [DOI] [PubMed] [Google Scholar]
- 51.Technical lessons from TAVI valve-in-valve: contrasting TAV in SAVR and TAV in TAV; Presented at TVT 2022; June 8th, 2022; Chicago, IL by Janarthanan Sathananthan.
- 52.Forrestal B.J., Case B.C., Yerasi C., et al. Risk of coronary obstruction and feasibility of coronary access after repeat transcatheter aortic valve replacement with the self-expanding Evolut valve: a computed tomography simulation study. Circ Cardiovasc Interv. 2020;13(12) doi: 10.1161/CIRCINTERVENTIONS.120.009496. [DOI] [PubMed] [Google Scholar]
- 53.Tarantini G., Nai Fovino L., Scotti A., et al. Coronary access after transcatheter aortic valve replacement with commissural alignment: the ALIGN-ACCESS study. Circ Cardiovasc Interv. 2022;15(2) doi: 10.1161/CIRCINTERVENTIONS.121.011045. [DOI] [PubMed] [Google Scholar]
- 54.Blanke P., Soon J., Dvir D., et al. Computed tomography assessment for transcatheter aortic valve in valve implantation: the Vancouver approach to predict anatomical risk for coronary obstruction and other considerations. J Cardiovasc Comput Tomogr. 2016;10:491–499. doi: 10.1016/j.jcct.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Ochiai T., Oakley L., Sekhon N., et al. Risk of coronary obstruction due to sinus sequestration in redo transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2020;13(22):2617–2627. doi: 10.1016/j.jcin.2020.09.022. [DOI] [PubMed] [Google Scholar]
- 56.Tchétché D., Kodali S.K., Dvir D. First dedicated transcatheter leaflet splitting device: the ShortCut device. EuroIntervention. 2022;18(5):e428–e429. doi: 10.4244/EIJ-D-22-00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bavaria J.E., Griffith B., Heimansohn D.A., et al. Five-year outcomes of the COMMENCE trial investigating aortic valve replacement with RESILIA tissue. Ann Thorac Surg. 2022 doi: 10.1016/j.athoracsur.2021.12.058. [DOI] [PubMed] [Google Scholar]