Abstract
Valvular heart disease can develop as a long-term side effect of radiation therapy, along with other cardiovascular complications. The timeline until clinically significant disease can be on the order of decades, with left-sided valves most commonly affected. Only a few retrospective studies have characterized the incidence and findings associated with radiation therapy associated valvular heart disease. Furthermore, treatment can be difficult in this population given certain factors that can contribute to high surgical risk. In recent years, however, the development of transcatheter options for valvular disease has led to expanded eligibility criteria for cancer patients who may receive radiation therapy as part of their treatment course. We therefore sought to review the literature on radiation-induced valvular heart disease and provide an overview of recent studies on post-intervention outcomes in these patients.
Keywords: Cancer, Radiation therapy, Valvular heart disease
Introduction
Radiation therapy has long been associated with an increased risk of cardiovascular disease.1,2 The effects of radiation on cardiovascular tissue can result in a wide range of diseases including coronary artery disease, cardiomyopathy, conduction system abnormalities, pericardial disease, and valvular heart disease. Notably, valvular heart disease and coronary artery disease can manifest decades after therapy,3,4 and be confused with degenerative changes associated with aging, making it difficult to detect subclinical dysfunction.5 Because of the long latency period, it is important that clinicians are aware of the risk factors associated with radiation valvulopathy and the need for serial, often multi-modality, imaging. The current manuscript will review the prevalence and risk factors associated with the disease, the complex presentations of radiation valvulopathy, and the possible treatment options for severe, symptomatic disease (Figure 1).
Figure 1.
Overview diagnosis and management of valvular heart disease associated with radiation therapy.
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Prevalence of Radiation Valvulopathy
Cancer treatment that typically includes radiation therapy involving the heart includes Hodgkin lymphoma, non-Hodgkin lymphoma, breast cancer, and thoracic malignancies (typically lung and esophageal cancer).2 Notably, in Hodgkin lymphoma patients, cardiovascular complications are the second most common cause of treatment-related morbidity.6 Several retrospective studies have attempted to characterize the prevalence of valvular heart disease in survivors of these cancers although at different time points since radiation therapy.3,6,7 Valvular regurgitation is more commonly seen than stenosis, which when it occurs, is most likely to involve the aortic valve.8,9 In a review by Heidenreich et al, at 10 years following radiation, the prevalence of mild or greater valvular disease was as follows: aortic regurgitation was 5%, mitral regurgitation (MR) was 26%, tricuspid regurgitation was 9%, and pulmonic regurgitation was 2%. By greater than 20 years following radiation, however, the prevalence of valvular disease had increased with aortic regurgitation prevalence of 60%, MR 52%, tricuspid regurgitation 26%, pulmonic regurgitation 12% and aortic stenosis (AS) 16%.5 The odds of AS increased by close to 14-fold for every 10-year latency period since radiation therapy after adjusting for age and gender.5 Most studies support the finding of a higher prevalence of left-sided valvular disease despite the anterior position of the right heart structures, suggesting that factors other than radiation dose may play a role in the pathogenesis of radiation valvulopathy.10 Furthermore, the time elapsed since radiation is an important parameter determining severity of disease. Among 5-year Hodgkin lymphoma survivors, one study found an incidence of 1.6% of valvular disorders rising to 4% at 30 years compared to siblings.11 In a retrospective cohort of 20-year Hodgkin lymphoma survivors, 42% were found to have hemodynamically significant valvular dysfunction by cardiac magnetic resonance imaging.12 Another study of Hodgkin lymphoma survivors found that clinically important valvular dysfunction occurred at a median of 22 years after radiation therapy with an incidence of 1% at 10 years increased to 6% at 20 years; as previously noted, left-sided valvular disease, namely AS and mitral insufficiency, were found to be the most common valvulopathies.7 Specifically, a study of 89 Hodgkin lymphoma survivors found that the aortic valve and mitral valve were affected in 51% and 25% of patients, respectively; 18% had both valves affected.13
Mechanisms and Risk Factors
Radiation-induced valvular disease involves a degenerative process thought to begin with early valve retraction resulting initially in regurgitation and ultimately, thickening and calcification of valves leading to stenosis. Proposed mechanistic pathways for degenerative valve calcification include the activation of valvular interstitial cells with osteoblast-like differentiation.14 Once this occurs, calcium may be actively deposited into the valve interstitium. The pathogenesis of radiation-induced valvulopathy may be a result of specific cellular and extracellular matrix responses to ionizing radiation with activation of stromal fibroblasts and transforming growth factor-β leading to collagen deposition. A recent in vitro study demonstrated that remodeling of the extracellular matrix through upregulation of matrix metalloproteinases via valvular interstitial cells is an important pathway.15 Surrounding structures including the annulus, subvalvular apparatus, and the aorto-mitral curtain can often be concurrently calcified.16 Furthermore, as mentioned previously, left-sided valves—aortic and mitral valves—have been found to be more commonly affected than the pulmonary and tricuspid valves suggesting a role of higher pressure flows as an additional stimulus.6,17 The pulmonary valve is rarely affected.
Prior studies have posited several risk factors for radiation-induced valvular heart disease; these include dosage of radiation,13 time elapsed since therapy,5 baseline cardiovascular risk factors,13 and concurrent anthracycline-based chemotherapy which has also been independently associated with valvular dysfunction.3,18,19 Other risk factors in general for radiation-induced heart disease include anterior or left chest irradiation location, younger patients (<50 years), high dose of either cumulative dosage of radiation or radiation fractions, lack of shielding or cobalt as the radiation source, tumor neighboring the heart or within the heart, cardiovascular risk factors (such as diabetes, smoking) and preexisting cardiovascular disease8,9 (Table 1). In one study, the 30-year cumulative risk of valvular heart disease ranged from 3% for patients receiving a total radiation dose of <30 Gy to 12.4% for those receiving >40 Gy,13 with another study finding that >30 Gy as an independent risk factor for valvular disease with a 45% prevalence of valvular disease in 15-year lymphoma survivors treated with >30 Gy.20 Although a recent review found an almost four-fold increase in radiation-induced coronary artery disease related cardiovascular events and mortality in women compared to men who had received radiation therapy for Hodgkin lymphoma,21 future studies will be needed on whether there are sex differences in the valvular degeneration process.
Table 1.
Risk factors for radiation-induced heart disease
| Location |
| • Radiation in the left chest and/or anterior chest |
| • Tumor located within or neighboring the heart |
| • Lack of shielding |
| Cancer treatment |
| • Concurrent chemotherapy (such as anthracyclines)∗ |
| Dosage features |
| • High cumulative dosage (>30 Gy)∗ |
| • High dose of radiation fraction (>2Gy/d) |
| Patient factors |
| • Age <50 y |
| • Time elapsed since radiation therapy∗ |
| • Cardiovascular risk factors∗ |
| • Baseline cardiovascular disease |
Risk factors specifically associated with valvular disease.
Given the close relationship between radiation dosage and size of field with the risks of radiation-induced cardiovascular disease including valvular disease, techniques have evolved over time to minimize these risks while still delivering effective therapy. In terms of lymphoma, radiation was historically delivered at a high dose (≥40 Gy) using an extended field approach commonly given to the mantle field causing the heart to receive significantly high doses of radiation.9 Modern approaches over time have led to smaller field sizes focusing on involved sites and involved lymph nodes as well as the use of lower radiation dosages to 20 to 30 Gy. In one study comparing involved node radiotherapy to mantle field radiation for Hodgkin lymphoma, the use of involved node radiotherapy led to significantly decreased mean doses of radiation to all cardiac substructures including all 4 valves although the most cephalad structures including the aortic and pulmonic valves received relatively higher doses.22 Other techniques such as proton therapy and using a deep inspiratory breath hold—which moves the heart more inferiorly—or prone positioning have also minimized radiation exposure to cardiac structures.9,23
The aforementioned techniques have similarly been applied to breast cancer patients9 for whom the degree of radiation received by cardiac structures also depends on the laterality—with left-sided lesions more affected—as well as the stage of the breast cancer.24 For example, regimens that include irradiation of the internal mammary chain deliver higher mean doses than regimens without, with one study finding a doubled average mean dose to the heart.25 In terms of the type of radiation, proton radiation therapy rather than traditional photon therapy typically spares the distal organs including cardiac structures and has been found to deliver the lowest average dose, although long-term follow-up data are needed and cost and accessibility limit practicality.26,27 To this end, a randomized clinical trial of proton vs. photon therapy on patients with nonmetastatic breast cancer (RADCOMP, clinical trial number NCT02603341) is currently underway to assess longitudinal impact on cardiovascular morbidity and mortality. Intensity-modulated radiation therapy has also been found to deliver significantly reduced cardiac doses although a systemic review of different radiation regimens in breast cancer still found this technique to deliver a relatively higher mean dose to the heart.28 Overall, however, the radiation doses delivered to the total heart in left-sided breast cancer have decreased over the past decades from 5.4 Gy from the years 2003 to 2013 to 3.6 Gy over a time period from 2014 to 2017; when breathing techniques were used, the average dose decreased even further to 1.7 Gy from 2014 to 2017.29
Diagnostic Testing
Radiation-induced valvular disease diagnosis and surveillance commonly involves using transthoracic echocardiography (TTE), but other imaging techniques are also helpful in evaluation including transesophageal echocardiography (TEE), 3-dimensional echocardiography, computed tomography, and cardiac magnetic resonance imaging2 (Table 2). A representative patient example is shown in Figure 2. TEE and 3-dimensional echocardiography are helpful in further evaluating valvular regurgitation or stenosis especially when there are difficult windows on TTE or significant shadowing from calcification and/or fibrotic tissue or when lesions are complex.17 Cardiac magnetic resonance imaging can additionally help in further evaluating valvular function along with left ventricular dysfunction with less interobserver variability.12 Given reduced spatial resolution, nuclear cardiology is less favored in evaluating for valvular disease.17
Table 2.
Multi-modality imaging characteristics of radiation-associated valvular heart disease
| Modality | Features | Caveats |
|---|---|---|
| TTE |
|
|
| TEE |
|
|
| Cardiac MRI |
|
|
| Cardiac CT |
|
|
CT, computed tomography; MRI, magnetic resonance imaging; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Figure 2.
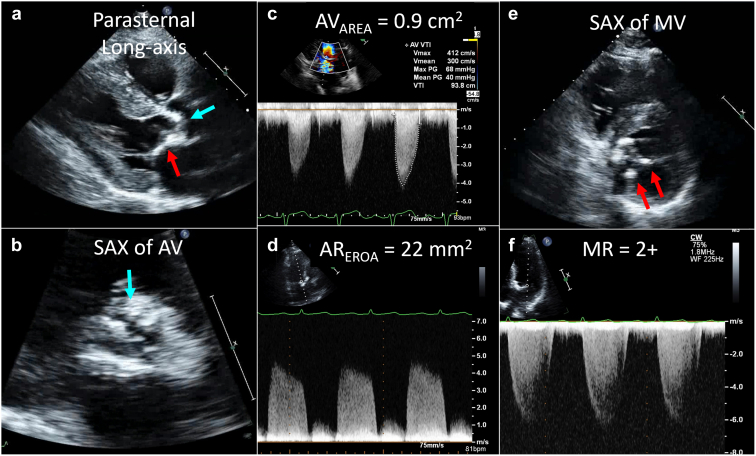
Transthoracic echocardiography in radiation valvular disease. The patient shown has a history of Hodgkin lymphoma treated with mantle radiation 33 years prior to presentation. The echocardiogram was performed for progressive shortness of breath. Panel a shows the parasternal long-axis view with calcification of the aortic valve (blue arrow) as well as calcification of the mitral-aortic curtain (red arrow). The short-axis view of the aortic valve (AV) in systole (panel b) confirms bulky calcification with reduced valve opening. Continuous wave Doppler across the AV (panel c) showed increased systolic velocity (4.1 m/s) and mean gradient (40 mmHg) with calculated AV area of 0.9 cm2 but also moderate aortic regurgitation (panel d). Evaluation of the mitral valve (MV) showed focal calcification (panel e, red arrows) with mild-moderate mitral regurgitation (MR) (panel f).
Abbreviations: EROA, effective regurgitant orifice area; SAX, short-axis.
Guidelines recommend TTE as the first imaging modality for valvular heart disease.30,31 Radiation-induced valvular damage characteristically leads to diffuse valvular thickening and calcification and fibrosis of the aortic apparatus (root, annulus, leaflets) as well as the inter-valvular fibrosa. It also affects the mitral valve annulus and leaflets, although characteristically includes the mid and base rather than the tips and commissures.17 The thickness of the aorto-mitral curtain on TTE, potentially suggestive of increased fibrosis, has also been found to correlate with increased mortality from cardiothoracic surgery in a study of approximately 170 patients with radiation associated cardiac disease.32 In contrast, the mitral valve tips are commonly involved in rheumatic heart disease, another similar entity, in which there is also a characteristic loss of the commissural fissure which is not a feature of radiation induced valvular disease.17 When evaluating these patients, it is also important to keep in mind the differential diagnosis for valvulopathies that can be drug-induced such as the by use of fenfluramine-phentermine, as well as valvular disease secondary to chronic kidney disease.8 Concurrent findings of other radiation therapy induced cardiovascular toxicities can also be present including pericarditis and cardiomyopathy.
The American Society of Echocardiography/European Society of Cardiovascular Imaging guidelines for cardiovascular considerations after radiotherapy17 additionally provide guidance specific to this patient population and based on the above echocardiographic features. For example, severe calcification can limit the ability to calculate planimetry. In terms of the aortic valve, concurrent left ventricular systolic dysfunction may warrant additional testing to differentiate between pseudo-obstruction from a fixed severe lesion. With regards to the mitral valve, given that the mitral valve leaflet tips are spared without commissural fusion, this can underestimate the severity of stenosis. As reported above, since radiation therapy can affect other cardiovascular structures, the presence of a restrictive cardiomyopathy may lead to diastolic dysfunction that precludes accurate assessment of the mitral valve area by affecting the pressure half-time calculation.17 Diastolic dysfunction itself may also contribute significantly to pulmonary hypertension that may not be solely from elevated pressures secondary to mitral valve pathology. Furthermore, the presence of significant mitral annular calcification can lead to difficulties in assessing the true severity of valvular pathology.
In general, based on the timeline of various types of cardiovascular disease after radiation therapy, consensus guidelines have made similar recommendations on time intervals for screening. The American Society of Echocardiography and European Society of Cardiovascular Imaging have recommended an echocardiogram 10 years postradiation therapy and every 5 years thereafter.17 Baseline cardiovascular risk factors should be aggressively optimized as well, although further studies are needed on the impact of cardiovascular risk factors specifically on valvular disease as opposed to general radiation-induced heart disease. A recent multidisciplinary expert consensus statement from the International Cardio-Oncology Society this past year recommends evaluation for subclinical valvular heart disease with a TTE 5 years postradiation therapy and every 5 years thereafter.33 A best practices guideline document by Chang et al34 on cardiovascular complications also recommends an echocardiogram 5 years postradiation therapy.
Management and Outcomes
Management of patients who have received radiation therapy with concurrent valvulopathy can be difficult. Current management options include both surgical approaches and/or transcatheter approaches. Notably, a surgical approach can be particularly difficult in this population due to the presence of chest wall scarring and concern for delayed wound healing from radiation, the presence of prior cardiac surgeries, a very calcified aorta, the presence of circumferential mitral annular calcification, and significant valvular calcification, leading to increased risk of paravalvular leak.35 Of note, the 2020 American Heart Association/American College of Cardiology guidelines on patients with valvular heart disease include prior radiation therapy as a factor associated with prohibitive surgical risk in patients with severe symptomatic AS as well as a patient factor to consider for high-risk for reintervention and thereby favoring mechanical over bioprosthetic valve.30,36 Concomitant pulmonary disease, such as pulmonary fibrosis, has also been shown to affect surgical outcomes in patients with radiation-associated cardiac disease and complications, such as restrictive lung disease and recurrent pleural effusion are commonly observed.9,37
Surgical Outcomes
Several studies have characterized the perioperative and postoperative outcomes in patients with a history of mediastinal irradiation who have undergone cardiac surgery, including valvular surgery. These studies have found that patients with a history of mediastinal radiation have worse short- and long-term outcomes, likely due to a combination of need for concurrent cardiac procedures, higher rates of conduction disturbances requiring device therapy and postoperative ventricular dysfunction.38,39 Reoperation in these patients is also associated with high risk, with one study demonstrating the reoperative mortality to be 17.4% compared to 2.3% in those without prior radiation.40 Importantly, traditional risk calculators do not fully capture the significantly increased risk in this population.39, 40, 41 In a retrospective observational cohort study of patients undergoing cardiothoracic surgery, postradiation therapy patients had a 45% survival rate compared to 72% in the age and sex-matched comparison group during a mean follow-up time of 7.6 ± 3 years.38 In a patient population with severe AS undergoing surgical aortic valve replacement, radiation therapy was a significant predictor of long-term mortality; the postradiation therapy patients had worse outcomes even when both the radiation and control groups were stratified by Society of Thoracic Surgeons score and even when patients undergoing concurrent coronary artery bypass graft were excluded.42 Of note, all patients in the radiation therapy group were noted to have mediastinal adhesions compared to adhesions being found only in the redo surgery patients in the control group. In terms of mitral valve surgery, the same group again demonstrated decreased long-term mortality for postradiation therapy patients with mitral valve disease undergoing either replacement or repair with a 5-year survival rate of 55% compared to ≥80% for either mitral valve repair or replacement at the same institution.43
In terms of risk factors, those with more extensive radiation have been found to have worse time-related survival and hospital death.44 Certain risk factors may also be associated with early and late mortality. A retrospective study of 60 patients with a history of mediastinal radiation followed over a 23-year period after valvular surgery found that increased early mortality was associated with left ventricular dysfunction, the presence of constrictive pericarditis (40% vs. 6%) and longer time on cardiopulmonary bypass. Factors associated with late mortality were the following: New York Heart Association class IV symptoms, presence of atrial fibrillation, congestive heart failure, and left ventricular dysfunction.45 A follow-up observational study from the same group studied 22 postradiation therapy patients who underwent mitral and/or tricuspid valve repair—of which over half had concurrent coronary artery disease—found that valve dysfunction occurred in 32% of early survivors with half of these patients requiring further surgery. Given this, the authors also suggest a need to critically evaluate those who will most benefit from a valve repair rather than a replacement given the potential decreased durability.46
Transcatheter Approaches to the Aortic Valve
The recent 2020 American Heart Association/American College of Cardiology guidelines on valvular heart disease presented expanded eligibility criteria for transcatheter aortic valve replacement (TAVR) in which TAVR has now become a reasonable alternative in high-, intermediate- and low-risk patients for surgical aortic valve replacement (SAVR).47 Previously, cancer patients had been excluded from clinical trials on TAVR, but these therapies have now been offered much more routinely in this population following commercialization. In a recent meta-analysis of cancer patients who had underwent TAVR placement, the authors found improved rates of stroke and acute kidney injury with comparable bleeding risk between cancer and non-cancer patients.35 This is particularly exciting in light of specific considerations in this population that may limit SAVR, as described previously. Several studies, especially in recent years, have sought to characterize the outcomes in these patients as outlined in Table 3.48, 49, 50, 51, 52, 53, 54, 55, 56, 57 These have compared outcomes among patients receiving a TAVR with or without a history of mediastinal radiation, as well as surgical vs. TAVR outcomes in patients postradiation therapy. Bouleti et al56 compared post-TAVR outcomes in patients with severe symptomatic AS in 262 patients without prior radiation to 26 matched controls and found comparable mortality rates at 5 years. However, in a more recent meta-analysis of 4 studies on outcomes post-TAVR in patients with and without a history of chest radiation, the authors concluded that TAVR had similar short-term all-cause mortality in both groups as well as safety and efficacy, but had an increased 1-year all-cause mortality. In terms of TAVR vs. SAVR outcomes, Zhang et al55 conducted an observational study of patients with severe AS and a history of chest irradiation, and found that patients who underwent TAVR had lower 30-day and 1-year mortality with TAVR than with SAVR after adjusting for intergroup baseline Society of Thoracic Surgeons score differences. TAVR was found to be more favorable compared to SAVR in subsequent studies as well.48,50 Of note, in a subgroup analysis from Nauffal et al regarding TAVR vs. SAVR outcomes during an earlier time period of the years 2011 to 2014 compared to 2015 to 2018, the authors found that 30-day mortality was decreased in patients with a history of mediastinal radiation who underwent TAVR in the later vs. the earlier period suggesting improved outcomes over time.50 On a procedural level, it is also worth mentioning unique considerations and potential complications in TAVR patients including injuries such as dissection or perforation to the aorta, aortic valve annulus or ventricle from guide wire placement and the valve delivery device.58 Although data are currently limited on the frequency of these complications, given potential concurrent myocardial disease in postradiation therapy patients, additional care is needed on wire placement and manipulation to avoid such complications. Overall, further prospective, multicenter studies with both short-term and long-term outcomes data are needed in the TAVR population to elucidate factors that may contribute to increased risk in those who have received radiation therapy.
Table 3.
Studies on TAVR and/or SAVR outcomes in postradiation therapy patients
| Year | Authors | Study design | Study population (N) | Follow-up | Outcome |
|---|---|---|---|---|---|
| Sep 2021 | Yazdchi et al48 | Retrospective, single-center | TAVR (69) vs SAVR (117) in patients with a history of AS and chest-directed radiation therapy | 37 mo (median) | TAVR was associated with better expected mortality compared to intermediate-/high-risk SAVR patients and similar outcomes compared to low-risk SAVR patients; TAVR patients had shorter ICU time, length of stay, and required less postoperative blood transfusions |
| April 2021 | Kherallah et al49 | Retrospective, single-center | TAVR patients (N = 1341) of whom a subset49 had prior chest radiation therapy | 24 mo (median) | TAVR patients with a prior history of chest radiation had significant increased rates of postprocedural respiratory failure and need for a permanent, but no difference in overall mortality, 30-d mortality, 30-d readmission rate compared to patients without a prior history of chest radiation |
| Feb 2021 | Nauffal et al50 | Retrospective, multi-center (Society of Thoracic Surgeons’ database) | TAVR (1668) vs SAVR (2611) in patients with severe symptomatic AS and a prior history of mediastinal radiation | Database from 2011 to 2018 and studied 30-d outcomes | TAVR was associated with significantly ↓ 30-d mortality and postoperative complications compared to SAVR |
| 2020 | Elbadawi et al51 | Retrospective, multi-center (National Inpatient Sample Database) | TAVR (2170) vs SAVR (1505) in patients with severe AS and prior mediastinal radiation | Database from 2012 to 2017 and studied in-hospital mortality | TAVR compared to SAVR was associated with lower in-hospital mortality as well as decreased rates of acute kidney injury, use of mechanical circulatory support, bleeding and respiratory complications, and shorter length of hospital stay, but higher rates of pacemaker insertion |
| 2020 | Zafar et al52 | Systemic review and meta-analysis (Dijos et al, Bouleti et al, Agrawal et al, and Gajanana et al, see below) | TAVR outcomes in patients with severe AS with a history of chest radiation therapy (164) vs. those with severe AS without a history of chest radiation therapy (1846) | 1 y | TAVR produced similar short-term all-cause mortality, safety and efficacy in patients with prior radiation vs. those without, but 1-y all-cause mortality and postprocedural heart failure exacerbation increased in group with prior radiation |
| 2019 | Agrawal et al53 | Observational cohort | TAVR in patients with symptomatic severe AS (610) comparing a subset with a prior history of chest radiation therapy (75) to the remainder of the cohort | 17.1 mo (mean) | TAVR patients with prior radiation therapy had a significantly higher incidence of all-cause mortality (nearly 2-fold increase in mortality) and major adverse cardiovascular event |
| 2019 | Gajanana et al54 | Single-center, prospective | TAVR in patients with a history of prior chest radiation44 compared to those without a prior history of chest radiation (1106) | 1 y | No significant difference in 30-d or 1-y mortality in TAVR patients with vs. without prior radiation therapy |
| 2019 | Zhang et al55 | Single-center, retrospective | TAVR54 vs. SAVR54 in patients with severe AS with a history of mediastinal radiation | 1 y | TAVR patients with native severe AS and prior chest radiation had a shorter hospital stay and decreased incidence of postprocedural atrial fibrillation, as well as lower adjusted 30-d and 1-y all-cause mortality |
| 2016 | Bouleti et al56 | Single-center, prospective | TAVR in patients with symptomatic severe AS (288) comparing a subset with a prior history of chest radiation26 to matched controls | 5-y | TAVR patients with a prior history of radiation experienced improved functional status after TAVR and comparable mortality rates as those without; the most common cause of death was respiratory failure in the group postradiation therapy |
| 2015 | Dijos et al57 | Single-center, prospective | TAVR in patients with severe symptomatic AS divided into radiation-induced valvular disease cases19 and suspected degenerative cases (179) | 6 mo | TAVR patients with radiation-induced valvular heart disease did not have a significantly different 30-d mortality from the degenerative valvular disease group and had a comparably lower mortality at 6-mo follow-up |
AS, aortic stenosis; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Transcatheter Approaches to the Mitral Valve
There are limited studies on the effects of mitral valve intervention on outcomes of patients after radiation therapy. A case report of a 61-year-old female with both severe AS and severe mitral stenosis 40 years after radiation therapy for lymphoma underwent both a successful transcatheter aortic and transcatheter mitral valve replacement with good functional status at her 7-month follow-up.59 A small study including 15 patients receiving mitral transcatheter edge-to-edge repair for MR found that there was sustained improvement in 6- and 12-month follow-up and a reduction in both MR and tricuspid regurgitation,60 suggesting that transcatheter options are feasible and potentially beneficial in a patient population that may not be ideal surgical candidates. However, hemodynamic mitral stenosis developed in 27% of patients at follow-up reminding clinicians that radiation valvulopathy is a progressive disease and mitral transcatheter edge-to-edge repair in this patient population may not be a long-term solution.
Other Considerations
Given the concurrent risk of coronary artery disease, myocardial disease, pericardial disease, and conduction system abnormalities in patients after radiation therapy, those who have been deemed to have significant valvular disease may need even closer monitoring for these other cardiovascular complications. Especially at the time of requiring valvular intervention, patients may already have preexisting conduction abnormalities which may lead to progressive disturbances after intervention; for example, right bundle branch block and infra-nodal blocks have been found to be commonly associated with radiation therapy.16 Preintervention cardiac catheterizations are routine and can identify lesions that can be intervened upon prior to valvular intervention and may factor into the consideration for a surgical or percutaneous approach.
Future Directions
Given the decades in which radiation therapy was used in larger doses as well as current cancer treatment protocols which continue to include adjuvant radiation therapy, and the improved longevity of cancer patients, the presence of radiation-induced valvular heart disease will likely only grow over the next few decades. Screening protocols for these patients both at baseline and also with ongoing surveillance are needed. Current advances in transcatheter approaches will likely be options for patients who develop clinical disease; however, long-term cardiovascular and non-cardiovascular outcomes data after transcatheter interventions are needed to better understand the optimal management of valvular heart disease postradiation therapy.
Funding
The authors have no funding to report.
Disclosure Statement
Dr. Hahn reports speaker fees from Abbott Vascular, Baylis Medical, and Edwards Lifesciences; institutional consulting for Abbott Structural, Edwards Lifesciences, Medtronic; equity with Navigate; and is Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored trials, for which she receives no direct industry compensation. The other author had no conflicts to declare.
References
- 1.Groarke J.D., Nguyen P.L., Nohria A., Ferrari R., Cheng S., Moslehi J. Cardiovascular complications of radiation therapy for thoracic malignancies: the role for non-invasive imaging for detection of cardiovascular disease. Eur Heart J. 2014;35:612–623. doi: 10.1093/eurheartj/eht114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergom C., Bradley J.A., Ng A.K., et al. Past, present, and future of radiation-induced cardiotoxicity: refinements in targeting, surveillance, and risk Stratification. JACC CardioOncol. 2021;3:343–359. doi: 10.1016/j.jaccao.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gujral D.M., Lloyd G., Bhattacharyya S. Radiation-induced valvular heart disease. Heart. 2016;102:269–276. doi: 10.1136/heartjnl-2015-308765. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen K.M., Offersen B.V., Nielsen H.M., Vaage-Nilsen M., Yusuf S.W. Short and long term radiation induced cardiovascular disease in patients with cancer. Clin Cardiol. 2017;40:255–261. doi: 10.1002/clc.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidenreich P.A., Hancock S.L., Lee B.K., Mariscal C.S., Schnittger I. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003;42:743–749. doi: 10.1016/s0735-1097(03)00759-9. [DOI] [PubMed] [Google Scholar]
- 6.Jaworski C., Mariani J.A., Wheeler G., Kaye D.M. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;61:2319–2328. doi: 10.1016/j.jacc.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 7.Hull M.C., Morris C.G., Pepine C.J., Mendenhall N.P. Valvular dysfunction and Carotid, Subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–2837. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- 8.Lancellotti P., Nkomo V.T., Badano L.P., et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:721–740. doi: 10.1093/ehjci/jet123. [DOI] [PubMed] [Google Scholar]
- 9.Desai M.Y., Windecker S., Lancellotti P., et al. Prevention, diagnosis, and management of radiation-associated cardiac disease: JACC Scientific expert panel. J Am Coll Cardiol. 2019;74:905–927. doi: 10.1016/j.jacc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Lenneman C.G., Sawyer D.B. Cardio-oncology: an update on cardiotoxicity of cancer-related treatment. Circ Res. 2016;118:1008–1020. doi: 10.1161/CIRCRESAHA.115.303633. [DOI] [PubMed] [Google Scholar]
- 11.Mulrooney D.A., Armstrong G.T., Huang S., et al. Cardiac outcomes in adult survivors of childhood cancer exposed to Cardiotoxic therapy: a cross-sectional study. Ann Intern Med. 2016;164:93–101. doi: 10.7326/M15-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machann W., Beer M., Breunig M., et al. Cardiac magnetic resonance imaging findings in 20-year survivors of mediastinal radiotherapy for Hodgkin's disease. Int J Radiat Oncol Biol Phys. 2011;79:1117–1123. doi: 10.1016/j.ijrobp.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 13.Cutter D.J., Schaapveld M., Darby S.C., et al. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst. 2015;107:djv008. doi: 10.1093/jnci/djv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutkovskiy A., Malashicheva A., Sullivan G., et al. Valve interstitial cells: the key to understanding the pathophysiology of heart valve calcification. J Am Heart Assoc. 2017;6:e006339. doi: 10.1161/JAHA.117.006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meerman M., Driessen R., van Engeland N.C.A., et al. Radiation Induces valvular interstitial cell calcific response in an in vitro model of calcific aortic valve disease. Front Cardiovascular Medicine. 2021;8:687885. doi: 10.3389/fcvm.2021.687885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koutroumpakis E., Deswal A., Yusuf S.W., et al. Radiation-induced cardiovascular disease: Mechanisms, Prevention, and treatment. Curr Oncol Rep. 2022;24:543–553. doi: 10.1007/s11912-022-01238-8. [DOI] [PubMed] [Google Scholar]
- 17.Lancellotti P., Nkomo V.T., Badano L.P., et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013–1032. doi: 10.1016/j.echo.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Aleman B.M.P., van den Belt-Dusebout A.W., De Bruin M.L., et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 19.van Nimwegen F.A., Schaapveld M., Janus C.P.M., et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Internal Medicine. 2015;175:1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 20.Murbraech K., Wethal T., Smeland K.B., et al. Valvular dysfunction in lymphoma survivors treated with autologous stem cell transplantation: a national cross-sectional study. JACC Cardiovasc Imaging. 2016;9:230–239. doi: 10.1016/j.jcmg.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Khalid Y., Fradley M., Dasu N., Dasu K., Shah A., Levine A. Gender disparity in cardiovascular mortality following radiation therapy for Hodgkin’s lymphoma: a systematic review. Cardiooncology. 2020;6:12. doi: 10.1186/s40959-020-00067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maraldo M.V., Brodin N.P., Vogelius I.R., et al. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2012;83:1232–1237. doi: 10.1016/j.ijrobp.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Dabaja B.S., Rebueno N.C., Mazloom A., et al. Radiation for Hodgkin's lymphoma in young female patients: a new technique to avoid the breasts and decrease the dose to the heart. Int J Radiat Oncol Biol Phys. 2011;79:503–507. doi: 10.1016/j.ijrobp.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Haque R., Yood M.U., Geiger A.M., et al. Long-term safety of radiotherapy and breast cancer laterality in older survivors. Cancer Epidemiol Biomarkers Prev. 2011;20:2120–2126. doi: 10.1158/1055-9965.EPI-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor C.W., Wang Z., Macaulay E., Jagsi R., Duane F., Darby S.C. Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses Published during 2003 to 2013. Int J Radiat Oncol Biol Phys. 2015;93:845–853. doi: 10.1016/j.ijrobp.2015.07.2292. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald S.M., Patel S.A., Hickey S., et al. Proton therapy for breast cancer after mastectomy: early outcomes of a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2013;86:484–490. doi: 10.1016/j.ijrobp.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 27.Luo L., Cuaron J., Braunstein L., et al. Early outcomes of breast cancer patients treated with post-mastectomy uniform scanning proton therapy. Radiother Oncol. 2019;132:250–256. doi: 10.1016/j.radonc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Mahdavi H. Radiation oncologists' perspectives on reducing radiation-induced heart disease in early breast cancer. Curr Probl Cancer. 2020;44:100509. doi: 10.1016/j.currproblcancer.2019.100509. [DOI] [PubMed] [Google Scholar]
- 29.Drost L., Yee C., Lam H., et al. A systematic review of heart dose in breast radiotherapy. Clin Breast Cancer. 2018;18:e819–e824. doi: 10.1016/j.clbc.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 32.Desai M.Y., Wu W., Masri A., et al. Increased aorto-mitral curtain thickness independently predicts mortality in patients with radiation-associated cardiac disease undergoing cardiac surgery. Ann Thorac Surg. 2014;97:1348–1355. doi: 10.1016/j.athoracsur.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell J.D., Cehic D.A., Morgia M., et al. Cardiovascular Manifestations from Therapeutic radiation. JACC CardioOncol. 2021;3:360–380. doi: 10.1016/j.jaccao.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang H.M., Okwuosa T.M., Scarabelli T., Moudgil R., Yeh E.T.H. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 2. J Am Coll Cardiol. 2017;70:2552–2565. doi: 10.1016/j.jacc.2017.09.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marmagkiolis K., Monlezun D.J., Cilingiroglu M., et al. TAVR in cancer patients: comprehensive review, meta-analysis, and meta-regression. Front Cardiovasc Med. 2021;8:641268. doi: 10.3389/fcvm.2021.641268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura R.A., Otto C.M., Bonow R.O., et al. 2017 AHA/ACC focused Update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 37.Desai M.Y., Karunakaravel K., Wu W., et al. Pulmonary fibrosis on multidetector computed tomography and mortality in patients with radiation-associated cardiac disease undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2014;148:475–481.e3. doi: 10.1016/j.jtcvs.2013.08.087. [DOI] [PubMed] [Google Scholar]
- 38.Wu W., Masri A., Popovic Z.B., et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation. 2013;127:1476–1485. doi: 10.1161/CIRCULATIONAHA.113.001435. [DOI] [PubMed] [Google Scholar]
- 39.Dolmaci O.B., Farag E.S., Boekholdt S.M., van Boven W.J.P., Kaya A. Outcomes of cardiac surgery after mediastinal radiation therapy: a single-center experience. J Card Surg. 2020;35:612–619. doi: 10.1111/jocs.14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ejiofor J.I., Ramirez-Del Val F., Nohria A., et al. The risk of reoperative cardiac surgery in radiation-induced valvular disease. J Thorac Cardiovasc Surg. 2017;154:1883–1895. doi: 10.1016/j.jtcvs.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 41.Ghoneim A., Bouhout I., Perrault L.P., et al. Reexamining the role of surgical aortic valve replacement after mediastinal radiation therapy. Ann Thorac Surg. 2017;104:485–492. doi: 10.1016/j.athoracsur.2017.01.097. [DOI] [PubMed] [Google Scholar]
- 42.Donnellan E., Masri A., Johnston D.R., et al. Long-term outcomes of patients with mediastinal radiation-associated severe aortic stenosis and subsequent surgical aortic valve replacement: a matched cohort study. J Am Heart Assoc. 2017;6:e005396. doi: 10.1161/JAHA.116.005396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donnellan E., Alashi A., Johnston D.R., et al. Outcomes of patients with mediastinal radiation-associated mitral valve disease undergoing cardiac surgery. Circulation. 2019;140:1288–1290. doi: 10.1161/CIRCULATIONAHA.119.040546. [DOI] [PubMed] [Google Scholar]
- 44.Chang A.S., Smedira N.G., Chang C.L., et al. Cardiac surgery after mediastinal radiation: extent of exposure influences outcome. J Thorac Cardiovasc Surg. 2007;133:404–413. doi: 10.1016/j.jtcvs.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 45.Handa N., McGregor C.G., Danielson G., et al. Valvular heart operation in patients with previous mediastinal radiation therapy. Ann Thorac Surg. 2001;71:1880–1884. doi: 10.1016/s0003-4975(01)02588-7. [DOI] [PubMed] [Google Scholar]
- 46.Crestanello J.A., McGregor C.G., Danielson G.K., et al. Mitral and tricuspid valve repair in patients with previous mediastinal radiation therapy. Ann Thorac Surg. 2004;78:826–831. doi: 10.1016/j.athoracsur.2004.04.008. discussion 826-31. [DOI] [PubMed] [Google Scholar]
- 47.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2021;143:e35–e71. doi: 10.1161/CIR.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 48.Yazdchi F., Hirji S.A., Nohria A., et al. Transcatheter compared with surgical aortic valve replacement in patients with previous chest-directed radiation therapy. JACC CardioOncol. 2021;3:397–407. doi: 10.1016/j.jaccao.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kherallah R.Y., Harrison D., Preventza O., et al. Transcatheter aortic valve replacement after chest radiation: a propensity-matched analysis. Int J Cardiol. 2021;329:50–55. doi: 10.1016/j.ijcard.2020.12.054. [DOI] [PubMed] [Google Scholar]
- 50.Nauffal V., Bay C., Shah P.B., et al. Short-term outcomes of transcatheter versus Isolated surgical aortic valve replacement for mediastinal radiation-associated severe aortic stenosis. Circ Cardiovasc Interv. 2021;14:e010009. doi: 10.1161/CIRCINTERVENTIONS.120.010009. [DOI] [PubMed] [Google Scholar]
- 51.Elbadawi A., Albaeni A., Elgendy I.Y., et al. Transcatheter versus surgical aortic valve replacement in patients with prior mediastinal radiation. JACC Cardiovasc Interv. 2020;13:2658–2666. doi: 10.1016/j.jcin.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Zafar M.R., Mustafa S.F., Miller T.W., Alkhawlani T., Sharma U.C. Outcomes after transcatheter aortic valve replacement in cancer survivors with prior chest radiation therapy: a systematic review and meta-analysis. Cardiooncology. 2020;6:8. doi: 10.1186/s40959-020-00062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agrawal N., Kattel S., Waheed S., et al. Clinical outcomes after transcatheter aortic valve replacement in cancer survivors treated with ionizing radiation. Cardiooncology. 2019;5:8. doi: 10.1186/s40959-019-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gajanana D., Rogers T., Attaran S., et al. Transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis and prior External chest radiation. Cardiovasc Revasc Med. 2019;20:376–380. doi: 10.1016/j.carrev.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Zhang D., Guo W., Al-Hijji M.A., et al. Outcomes of patients with severe symptomatic aortic valve stenosis after chest radiation: transcatheter versus surgical aortic valve replacement. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. 2019;8:e012110. doi: 10.1161/JAHA.119.012110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouleti C., Amsallem M., Touati A., et al. Early and late outcomes after trans-catheter aortic valve implantation in patients with previous chest radiation. Heart. 2016;102:1044–1051. doi: 10.1136/heartjnl-2015-309101. [DOI] [PubMed] [Google Scholar]
- 57.Dijos M., Reynaud A., Leroux L., et al. Efficacy and follow-up of transcatheter aortic valve implantation in patients with radiation-induced aortic stenosis. Open Heart. 2015;2:e000252. doi: 10.1136/openhrt-2015-000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langer N.B., Hamid N.B., Nazif T.M., et al. Injuries to the aorta, aortic annulus, and left ventricle during transcatheter aortic valve replacement: management and outcomes. Circ Cardiovasc Interv. 2017;10:e004735. doi: 10.1161/CIRCINTERVENTIONS.116.004735. [DOI] [PubMed] [Google Scholar]
- 59.Ali K., Lee D.J., Adamson D.L., Khan J.N. Radiation-induced dystrophic calcification and severe valvular stenosis: the central role of multimodality 3D cardiac imaging in disease assessment and planning of combined transcatheter aortic and mitral valve replacement. BMJ Case Rep. 2020;13:e239368. doi: 10.1136/bcr-2020-239368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scarfò I., Denti P., Citro R., Buzzatti N., Alfieri O., La Canna G. MitraClip for radiotherapy-related mitral valve regurgitation. Hellenic J Cardiol. 2019;60:232–238. doi: 10.1016/j.hjc.2018.07.006. [DOI] [PubMed] [Google Scholar]