Abstract
Background
In specific patients with severe mitral regurgitation (MR), mitral valve (MV) pathology is unique and requires creative transcatheter repair techniques. This study aimed to evaluate the feasibility and safety of a new transcatheter MV repair technique, using occluder devices in symptomatic high-surgical-risk patients with severe MR, either due to MV leaflet (MVL) perforations or due to post-clips residual MR, and to report on their 6-month outcomes.
Methods
The study enrolled all high-risk patients with severe MR due to MVL perforations and post-clips residual MR who underwent transcatheter MV repair using occluder devices, from November 2016 to August 2019.
Results
The study enrolled 16 patients; 9 (56.25%) with MVL perforations and 7 (43.75%) with post-MitraClip (Abbott Laboratories, Abbott Park, Illinois) residual MR, with a mean age of 55.75 ± 16.69 years. Mean perforation/jet diameters were 5.75 ± 1.67 and 6.5 ± 1.93 mm, and the mean 3D-vena contracta area was 0.54 ± 0.14 cm2. Perforations were crossed retrograde (transaortic in 7 [43.75%] patients and transapical in 2 [12.5%] patients), and post-MitraClip devices residual jets were crossed antegrade (transvenous/transseptal). Six (37.5%) patients required arteriovenous loop formation for device deployment, that was antegrade transvenous/transseptal in 13 (81.25%) patients and retrograde transapical in 3 (18.75%) patients. Devices used were Amplatzer-ASO in 10 (62.5%) patients and Amplatzer-VP-II in 6 (37.5%) patients. Mean procedural and fluoroscopy times were 55.13 ± 16.24 and 16.25 ± 4.03 minutes, respectively. Patients passed successfully, without MV gradient change or device-related complications.
Conclusions
Transcatheter MV repair of MVL perforations/post-clips residual MR is a new, feasible, and safe technique for high-surgical-risk patients.
Keywords: Mitral valve leaflet perforation, Post-clips residual mitral regurge, Transcatheter mitral valve regurgitation repair
Introduction
The traditional treatment for patients with severe symptomatic mitral regurgitation (MR) due to primary degenerative mitral valve (MV) disease or due to secondary functional MR is the MV surgery. However, almost half of the patients with significant MR are not candidates for surgery due to prohibitive surgical risk.1 As a result, the transcatheter MV clip repair has emerged as a treatment option for these patients. However, in certain specific patients with severe MR, the pathology of the MV lesion is rare and extremely unique that requires other creative transcatheter MV repair techniques. These pathologies include MV leaflet (MVL) perforations and post-clips residual MR.
MVL perforations have been reported to be iatrogenic in patients following cardiac surgeries such as MV repair, aortic valve (AV) surgeries, or congenital heart disease operations2, 3, 4 and in patients who experienced transcatheter AV replacement.5 Also, infective endocarditis or autoimmune diseases were considered causes of MVL perforations.6,7 But spontaneous MVL perforations were very rarely observed.4 Alhough surgery is the traditional treatment option for severe MR due to MVL perforations, it is accompanied by increased morbidity and mortality.8 Few sporadic cases of anterior mitral leaflet (AML) perforations either after surgery or after endocarditis have been reported for percutaneous repair.9, 10, 11, 12
Percutaneous MitraClip (Abbott Laboratories, Abbott Park, Illinois) implantation appears to be a safe treatment option for severe degenerative and functional MR.13, 14, 15, 16, 17 It may, however, be complicated by post-clips severe residual MR, either commissural between the clips and the commissures or inter-clip between the clips themselves. In such patients with a more challenging MV anatomy, this residual MR cannot be treated completely using multiple clips. So, transcatheter deployment of occluder devices may be an attractive solution for these significant residual jets.
Percutaneous closure of MVL perforations and post-clips residual MR are not guideline-based, with minimal evidence on long-term outcomes. It should be reserved for high-surgical risk patients or as a rescue protocol for serious complications during transcatheter interventions. In this study, we aimed to evaluate the feasibility and safety of a new transcatheter MV repair technique, using occluder devices in symptomatic high-surgical risk patients with severe MR, either due to MVL perforations or due to post-clips residual MR, and to report on their midterm (6-month) outcomes.
Materials and Methods
Patients
This study enrolled all patients with severe MR due to either MVL perforations or post-clips residual MR, from November 2016 to August 2019. All the studied patients were discussed by the institute’s advanced intervention heart team which included cardiac surgeons, interventional cardiologists, and anesthesiologists and were considered at high or prohibitive Society of Thoracic Surgeons risk for MV surgery. Active infective endocarditis was excluded in all patients. After optimizing guideline-directed medical therapy as the first step, all patients underwent transcatheter MV repair, using occluder devices for occlusion of MVL perforations/post-clips residual jets. This study complied with the Declaration of Helsinki ethical guidelines (as revised in 2013) and was approved by the institutional review board committee. Informed written consent was obtained from all patients before the procedure.
The Procedural Technique, Tips, and Tricks
Preprocedural antibiotic prophylaxis was given to all patients. The procedure was performed under general anesthesia with fluoroscopic and three-dimensional (3D) transesophageal echocardiographic (TEE) guidance using PHILIPS-iE33 and PHILIPS-EPIQ-CVx ultrasound (Philips Healthcare, Cambridge, Massachusetts).
Accesses
Groin Accesses
Percutaneous common femoral vein access was obtained using the modified Seldinger technique, and common femoral artery access was obtained using the Perclose ProGlide sutures (Abbott Vascular, Abbott Park, Illinois). Left ventriculography was performed, utilizing a 5F pigtail (PG) catheter to demarcate the regurgitant jet through the MV.
Septal Access
The septal puncture was achieved, either to secure the antegrade device deployment or was already performed in patients who underwent MitraClip device insertion. Safe septal access was confirmed by TEE and by fluoroscopic visualization of both the needle and the bubbles in the left atrium (LA). Sometimes, septal balloon dilatation was achieved for a thick septum.
Apical Access
The apical puncture was achieved when transapical wire crossing or transapical device deployment was required when the antegrade and retrograde transaortic crossing attempts could not be achieved in medially located perforations/jets. Apical closure was achieved by using an Amplatzer vascular plug (VP)-II 10 mm (Abbott Vascular, Abbott Park, Illinois).
Heparin
At this point, 100-IU/kg unfractionated heparin was administered with booster doses to maintain an activated clotting time >250 seconds.
Hemodynamic Assessment
The left atrial pressure (LAP) was recorded before and immediately after the procedure.
Crossing Approach
As the crossing of the MVL perforation/post-clips residual MR was challenging, the crossing approach was chosen individually based on the anatomical location of the perforation/jet.
Principally, the antegrade transvenous/transseptal crossing was the safest approach for navigation to the LA. Also, crossing through a mechanical AV, with the risk of hemodynamic instability or mechanical valve disruption, favored starting with trials of the antegrade crossing. It was also the most appropriate approach in the crossing of the post-clips residual MR. An 8.5F-Agilis NxT steerable sheath (St. Jude Medical, Inc, St Paul, Minnesota) was advanced to the LA, and then it was flexed and directed toward the MV. Sometimes, a 5F multipurpose catheter was telescoped through the Agilis NxT sheath and used to navigate a 0.035-inch/260 curved guidewire (Terumo Medical Corporation, Tokyo, Japan) from the LA aspect through the targeted perforation/jet to the left ventricle (LV) and then to the aorta.
The retrograde crossing approach was used when the antegrade crossing attempts were unlikely to be achieved, with medially located perforations near the A3 or P3 scallops, or when the regurgitant jet faced the crossing wire. The wire crossed easily from the LV, as the systolic regurgitant flow guided the wire across the perforation to the LA. In the right anterior oblique and lateral projections, retrograde crossing was done either by the transaortic approach using a suitable curve catheter (5F-Judkin right, 5F-PG, or 5F-cut PG] or by the transapical approach using a 5F multipurpose catheter. The catheter and the floppy guidewire were carefully pulled to the level of the MV after crossing the AV or the LV cavity. In patients with mechanical AV, an internal mammary catheter was used for negotiating the perforation from the aorta in the lateral slit of the mechanical AV, to avoid tension on both discs simultaneously (Supplemental Video 1).
In some patients with MVL perforations, an arteriovenous loop (AV loop) formation was achieved using an Amplatzer Gooseneck Snare (Abbott Vascular, Abbott Park, Illinois) to snare a 0.035-inch/260 stiffer guidewire within a 6F-Judkin right guiding-catheter (Cordis, Milpitas, California) that was guided from the LA side.
Device Selection
Device selection was determined individually based on 3D-TEE and fluoroscopic findings of the site and the size of the MVL perforation/post-clips residual MR. The chosen device was small enough to be distant from the MV closure line, to avoid affecting the leaflet mobility or creating any new commissural MR. The device waist was selected 1-2 mm larger than the perforation/jet size.
-
-In MVL perforations,
-
•If the perforation was in the body of the MVL, the Amplatzer atrial septal occluder (ASO) device was the best choice.
-
•If the perforation was annularly located at the base of the MVL, the Amplatzer VP-II was the preferred device.
-
•
-
-In post-clips residual MR,
-
•If the residual jet was commissural between the MitraClip devices and one of the MV commissures, the Amplatzer VP-II was utilized (TAIBA technique).18
-
•If the residual jet was inter-MitraClip between the clips themselves, either an Amplatzer ASO device or a VP-II was used.
-
•
Device Deployment
An Amplatzer TorqVue delivery sheath (St. Jude Amplatzer) was crossed either antegrade from the atrial septum or retrograde from the LV side. The device was partially opened, the whole system was pulled back toward the MV, and then the rest of the device was fully unsheathed to close the targeted perforation/jet.
Transesophageal Echocardiography and 3D TEE
Preprocedural TEE was done and evaluated MR etiology, site of the MVL perforation/post-clips residual MR, its diameters, its 3D-vena contracta area (effective regurgitant orifice area), mean MV pressure gradient, tricuspid regurgitation (TR) severity, estimated systolic pulmonary artery pressure (eSPAP), left ventricular outflow tract (LVOT) gradient, and LV ejection fraction. The pulmonary venous flow (PVF) was also estimated to assess MR severity.
The whole procedure was TEE- and 3D-TEE-guided. The device was released once extensive TEE assessment revealed no interference with MV closure mechanism, MV and LVOT gradients, and with the absence of residual MR.
Follow-up and Outcome
New York Heart Association functional class assessment, transthoracic echocardiography parameters, and any postoperative complications were recorded before discharge and at 3-month and 6-month follow-up. Hemolysis was tracked using hemoglobin and lactate dehydrogenase at baseline and during follow-up.
Statistical Analysis
Statistical analysis was performed using the SPSS statistical package (Version 25; SPSS Inc, Chicago, Illinois). Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as numbers with relative percentages. Differences between baseline and follow-up data were analyzed by the paired sample t-test. A p-value <0.05 was considered statistically significant at a confidence interval of 95%.
Results
Demographic and Clinical Characteristics
In this study, we report on 16 patients who underwent a recent transcatheter MV repair technique for the management of severe MR as a result of MVL perforations or post-MitraClip residual MR. The mean age of the studied patients was 55.75 ± 16.69 years, and the mean weight was 69.13 ± 9.40 Kg (50% were males). In the MV position, 2 (25%) patients had previously implanted rings, and 2 (12.5%) patients had previously implanted MitraClip devices. The mean Society of Thoracic Surgeons risk score for morbidity and mortality was 12.35 ± 8.62%. The demographic characteristics and clinical presentations of the studied patients are illustrated in Table 1.
Table 1.
Demographic characteristics, clinical presentations, echocardiographic data, and procedural safety and quality measures of the studied patients
| Characteristics | N = 16 |
|---|---|
| Demographic characteristics | |
| Age (y) | 55.75 ± 16.69 |
| Male gender | 8 (50%) |
| Weight (Kg) | 69.13 ± 9.40 |
| Height (m) | 1.59 ± 0.09 |
| BSA (m2) | 1.71 ± 0.17 |
| Medical comorbidities | |
| Hypertension | 8 (50%) |
| Diabetes mellitus II | 4 (25%) |
| Chronic kidney disease | 5 (31.25%) |
| Previous cancer/chemotherapy | 2 (12.5%) |
| Liver cirrhosis | 2 (12.5%) |
| Bronchial asthma | 1 (6.25%) |
| Peripheral vascular disease | 2 (12.5%) |
| Hypothyroidism | 3 (18.75%) |
| Original diagnosis | |
| CAD | 4 (25%) |
| DCM | 7 (43.75%) |
| Myxomatous MV | 2 (12.5%) |
| AV lesions | 2 (12.5%) |
| No cardiac history | 1 (6.25%) |
| Previous interventions | |
| CABG | 3 (18.75%) |
| Valve surgery | 5 (31.25%) |
| MitraClip devices | 2 (12.5%) |
| No previous interventions | 6 (37.5%) |
| Previous rings/clips in MV position | |
| No | 12 (75%) |
| Rings | 2 (12.5%) |
| Clips | 2 (12.5%) |
| Time since last intervention (mo) | 22.83 ± 20.44 |
| Previous cardiac medications | |
| Yes | 15 (93.75%) |
| No | 1 (6.25%) |
| STS score (%) | 12.35 ± 8.62 |
| Clinical presentations | |
| NYHA-FC | |
| III | 10 (62.5%) |
| IV | 6 (37.5%) |
| HF hospitalization within the last 12 mo | 2.86 ± 1.07 |
| Auscultation | |
| Pansystolic murmur | 15 (93.75%) |
| Midsystolic murmur | 1 (6.25%) |
| Echocardiographic data | |
| Etiology and site of MR MVL perforations: | 9 (56.25%) |
| Iatrogenic AML perforation | 4 (25%) |
| Ischemic AML perforation | 2 (12.5%) |
| Spontaneously ruptured aneurysm with a perforation in AML | 1 (6.25%) |
| Postinfective para-ring perforation/leak | 2 (12.5%) |
| Post-MitraClip residual MR jets | 7 (43.75%) |
| Commissural residual MR | 3 (18.75%) |
| Inter-MitraClip residual MR | 4 (25%) |
| MR severity | |
| Moderate (II) | 0 (0%) |
| Severe (III) | 16 (100%) |
| Perforation/jet diameters (mm) | 5.75 ± 1.67 × 6.5 ± 1.93 |
| 3D-VCA (EROA) (cm2) | 0.54 ± 0.14 |
| MV Pg (mmHg) | 3.42 ± 0.84 |
| Procedural safety and quality measures | |
| General anesthesia | 16 (100%) |
| Septal puncture | 16 (100%) |
| Apical puncture | 3 (18.75%) |
| Invasive LAP (mmHg) | 44.38 ± 6.26 |
| Crossing approach | |
| Antegrade transvenous/transeptal | 7 (43.75%) |
| Retrograde transaortic | 7 (43.75%) |
| Retrograde transapical | 2 (12.5%) |
| Crossing catheters | |
| JR | 1 (6.25%) |
| PG | 1 (6.25%) |
| IM | 2 (12.5%) |
| MP | 5 (31.25%) |
| Agilis | 7 (43.75%) |
| Arteriovenous loop | |
| Yes | 6 (37.5%) |
| No | 10 (62.5%) |
| Deployment approach | |
| Antegrade transvenous/transeptal | 13 (81.25%) |
| Retrograde transapical | 3 (18.75%) |
| Device type | |
| Amplatzer ASO | 10 (62.5%) |
| Amplatzer VP-II | 6 (37.5%) |
| Number of devices used | |
| One | 15 (93.75%) |
| Two | 1 (6.25%) |
| Procedure type | |
| Elective | 9 (56.25%) |
| Urgent | 5 (31.25%) |
| Emergency/salvage | 2 (12.5%) |
| Procedural time (min) | 55.13 ± 16.24 |
| Fluoroscopy time (min) | 16.25 ± 4.03 |
| Contrast (mL) | 33.75 ± 10.94 |
| Radiation dose (μGym2) | 16.25 ± 4.41 |
| Technical success | |
| Yes | 16 (100%) |
| No | 0 (0%) |
| Complete closure | |
| Yes | 15 (93.75%) |
| Residual shunt | 1 (6.25%) |
| Procedural complications | |
| No | 13 (81.25%) |
| Access site complications | 1 (6.25%) |
| Arrhythmia/CHB/new pacemaker | 2 (12.5%) |
| Conversion to open heart surgery | |
| Yes | 0 (0%) |
| No | 16 (100%) |
| Procedure-related mortality | |
| Yes | 0 (0%) |
| No | 16 (100%) |
3D, 3-dimensional; AML, anterior mitral leaflet; ASO, atrial septal occluder; AV, aortic valve; BSA, body surface area; CABG, coronary artery bypass graft; CAD, coronary artery disease; CHB, complete heart block; DCM, dilated cardiomyopathy; EROA, effective regurgitant orifice area; HF, heart failure; IM, internal mammary; JR, Judkin right; LAP, left atrial pressure; MP, multipurpose; MR, mitral regurgitation; MV, mitral valve; MVL, mitral valve leaflet; NYHA-FC, New York Heart Association Functional Class; PG, pigtail; Pg, pressure gradient; STS, Society of Thoracic Surgeons; VCA, vena contracta area; VP, vascular plug.
Preprocedural Echocardiographic Assessment
All the studied patients had severe MR due to MVL perforation or post-clips residual MR. MVL perforation was reported in 9 (56.25%) patients; iatrogenic AML perforation in 4 (25%) patients, ischemic AML perforation in 2 (12.5%) patients, spontaneously ruptured AML aneurysm with a perforation into LA in 1 (6.25%) patient, and postinfective para-ring perforation/leak in 2 (12.5%) patients. The site of the perforation was in the A1 scallop in 2 (12.5%) patients, in the A2 scallop in 2 (12.5%) patients, and in the A3 scallop in 5 (31.25%) patients. Post-MitraClip residual MR was reported in 7 (43.75%) patients; commissural residual MR in 3 (18.75%) patients and inter-MitraClip residual MR in 4 (25%) patients. The mean perforation/jet diameters were 5.75 ± 1.67 and 6.5 ± 1.93 mm, and the mean 3D-vena contracta area (effective regurgitant orifice area) was 0.54 ± 0.14 cm2.
The preprocedural mean MV gradient was 3.42 ± 0.84 mmHg. The LVOT remained without obstruction with a mean gradient of 1.93 ± 1.33 mmHg, and with a left ventricular ejection fraction of 47.38 ± 10.97%. TR was mild in 6 (37.5%) patients, moderate in 8 (50%) patients, and severe in 2 (12.5%) patients, with a mean eSPAP of 41.0 ± 11.1 mmHg. PVF systolic/diastolic ratio was <1 in all patients. Preprocedural echocardiographic data are shown in Table 1.
Procedural Safety and Quality Measures
Immediately after the procedure, the invasively estimated LAP decreased significantly from 44.38 ± 6.26 mmHg to 18.25 ± 3.85 mmHg (p < 0.001). All patients underwent septal punctures, with 3 (18.75%) patients required to have further apical ones. All MVL perforations were crossed retrograde (transaortic in 7 [43.75%] patients and transapical in 2 [12.5%] patients). And all post-MitraClip residual MR were crossed antegrade (transvenous/transseptal). Ten (62.5%) patients underwent device deployment without AV loop formation; however, the other 6 (37.5%) patients required AV loop formation for better stabilization. The deployment approach was antegrade transvenous/transseptal in 13 (81.25%) patients and retrograde transapical in 3 (18.75%) patients, without the usage of the retrograde transaortic deployment approach.
Devices used were Amplatzer ASO in 10 (62.5%) patients and Amplatzer VP-II in 6 (37.5%) patients. Among the 9 (56.25%) patients with MVL perforations,
-
-
Seven (43.75%) patients had perforations in the AML body and were closed with Amplatzer ASO devices; an Amplatzer ASO 6-mm device was used in 6 (37.50%) patients (3 [18.75%] with iatrogenic perforations [Figure 1 & Supplemental Videos 2 and 3], 2 [12.5%] with ischemic perforations, and 1 [6.25%] with a spontaneously ruptured AML aneurysm with a perforation into the LA [Figure 2 & Supplemental Videos 4 and 5]), and an Amplatzer ASO 4-mm device was used in 1 (6.25%) patient with an iatrogenic perforation.
-
-
The other 2 (12.5%) patients had a para-ring perforation/leak at the base of the MVL close to the annulus and were closed with Amplatzer VP-II 10-mm devices (Supplemental Videos 6 and 7).
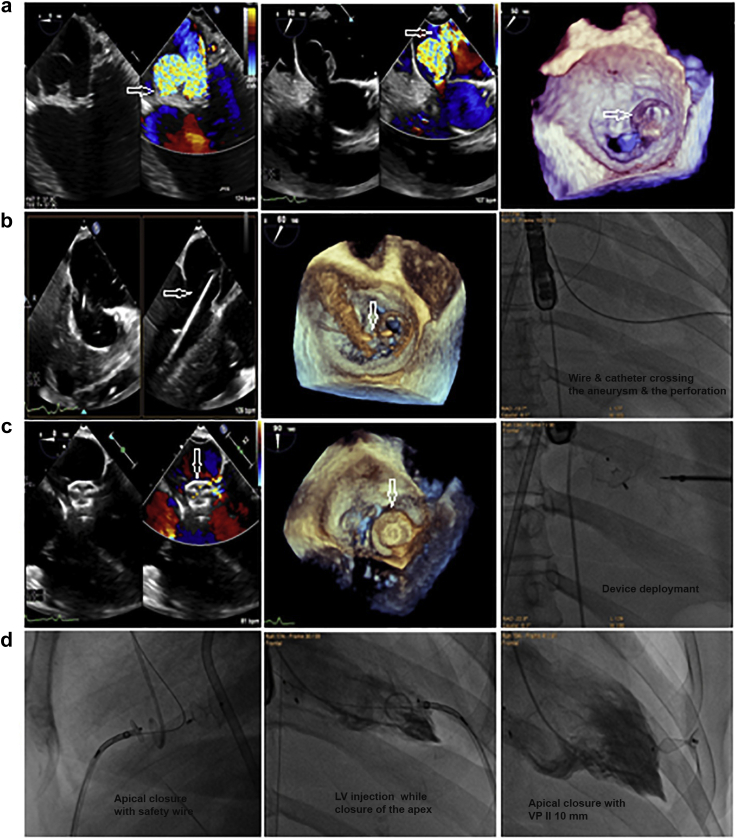
Figure 1.
Device closureof an iatrogenic perforation in the AML (A3 scallop). A 62-year-old male displays an iatrogenic perforation of the AML, 20 months after CABG. (a) TEE shows a severe MR across a perforation in the AML (A3 scallop) (white arrows), and 3D-TEE shows a 3D-VCA of 0.5 cm2 (black arrow). (b) TEE, 3D-TEE, and fluoroscopy show the septal puncture (white and black arrows) using an 8.5F SL sheath and a 0 BRK needle (St. Jude Medical). (c) 5F JR Catheter on a 0.035-inch/260 curved Terumo guidewire crossed the perforation retrogradely transaortic. The wire was snared transvenous/transseptal for an AV loop formation with the advancement of a 6F Amplatzer TorqVue delivery sheath. (d) TEE, 3D-TEE, and fluoroscopy show an Amplatzer ASO 6-mm device (white and black arrows) that was deployed antegrade transvenous/transeptal to completely occlude the perforation with a mean MV-pg of 3 mmHg.
Abbreviations: 3D, 3-dimensional; AML, anterior mitral leaflet; ASO, atrial septal occlude; AV, arteriovenous; BRK, Brockenbrough; CABG, coronary artery bypass graft; JR, Judkin right; MR, mitral regurge; MV, mitral valve; pg, pressure gradient; TEE, transesophageal echocardiography; VCA, vena contracta area.
Figure 2.
Device closure of a spontaneously ruptured AML aneurysm intheA3 scallop withaperforation into the LA. A 61-year-old female represents a spontaneously ruptured AML aneurysm and a perforation into the LA. (a) TEE and 3D-TEE show severe MR across an AML aneurysm in the A3 scallop measuring 2 × 1.7 cm, that bulges in the LA with a perforation (white arrows) with a 3D-VCA of 0.5 cm2. (b) A 5F MP-I catheter on a 0.035-inch/260 curved Terumo guidewire (white arrows) crossed the perforation retrogradely transapical. (c) TEE, 3D-TEE, and fluoroscopy show an Amplatzer ASO 6-mm device (white arrows) that was deployed retrogradely transapical through a 6F Amplatzer TorqVue delivery sheath to occlude the perforation and entangle the aneurysm completely by the left disc of the device with a mean MV-pg of 5 mmHg. (d) Apical access was closed using an Amplatzer VP-II-10 mm while keeping a safety wire.
Abbreviations: 3D, 3-dimensional; AML, anterior mitral leaflet; ASO, atrial septal occlude; LA, left atrium; LV, left ventricle; MP, multipurpose; MR, mitral regurge; pg, pressure gradient; TEE, transesophageal echocardiography; VCA, vena contracta area; VP, vascular plug.
Among the 7 (43.75%) patients with post-MitraClip residual MR (after implantation of 2 or more MitraClip devices),
-
-
Three (18.75%) patients had commissural residual MR between MitraClip devices and one of the commissures and were closed with Amplatzer VP-II (Figure 3 & Supplemental Video 8).
-
-
Four (25%) patients had inter-clip residual MR between the MitraClip itself; (3 [18.75%] of them were closed with Amplatzer ASO 6-mm devices [Figure 4 & Supplemental Videos 9 and 10], and 1 (6.25%) was closed with an Amplatzer VP-II 10-mm device [Supplemental Video 11]).
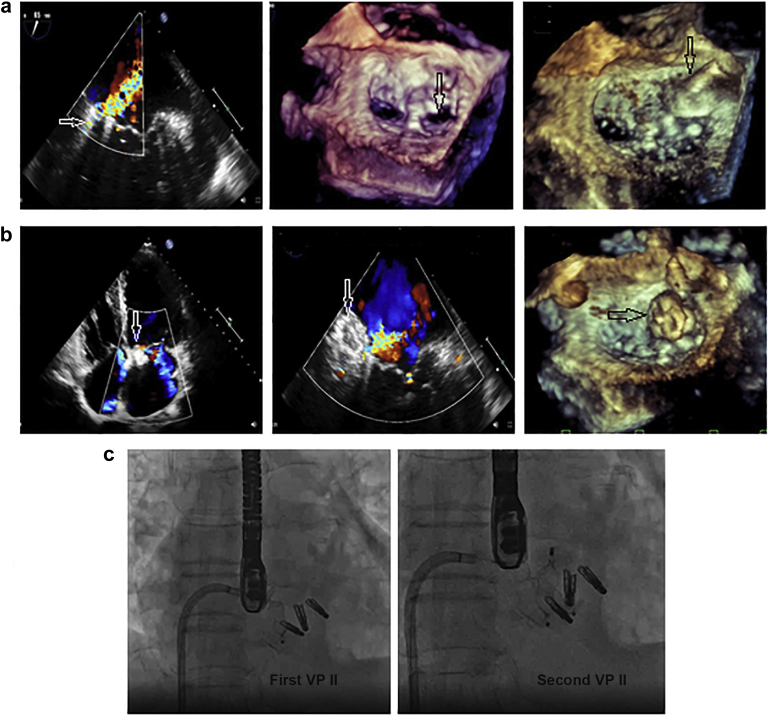
Figure 3.
Device closure ofpost-MitraClipsevereresidualcommissural MR between 3 MitraClip devices and the medial commissure attheP1scallop. A 54-year-old male shows post-MitraClip severe residual commissural MR. (a) TEE shows severe MR between the MitraClip and the medial commissure at the P1 scallop (white arrow); 3D-TEE shows a VCA of 0.7 cm2, then the defect was crossed with the Agilis catheter (black arrow) through the septal puncture. (b) TTE, TEE, and 3D-TEE show 2 Amplatzer VPs-II 16-mm and 12-mm devices were deployed antegrade transvenous/transeptal (white and black arrows). (c) Fluoroscopy shows the deployment of the first and the second VPs-II to occlude the medial commissure resting on the lateral MitraClip devices with mild residual commissural MR and with a mean MV-pg of 4 mmHg.
Abbreviations: 3D, 3-dimensional; MR, mitral regurge; MV-pg, mitral valve pressure gradient; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; VCA, vena contracta area; VP, vascular plug.
Figure 4.
Device closure ofaninter-MitraClip severeresidualMR jet. A 71-year-old female with DCM presents with inter-MitraClip severe residual MR, 48 months after MV clipping by 2 MitraClip devices and an ostium secundum ASD closure with an Amplatzer ASO 18-mm device. (a) TEE shows severe residual MR between 2 MitraClip devices (white arrow), 3D-TEE shows 3 commissural openings (white asterisks from the LA and LV aspects) with a central inter-clips 3D-VCA of 0.6 cm2. (b) Fluoroscopy shows the crossing of the wire with the advancement of the 6F Amplatzer TorqVue delivery sheath through the inter-clips residual jet antegrade transvenous/transeptal to deploy an Amplatzer ASO 6-mm device. (c) Color 3D-TEE, 3D-TEE, and fluoroscopy show the device (white arrows) completely occluded the inter-Clips residual MR with a mean MV-pg of 4 mmHg.
Abbreviations: 3D, 3-dimensional; ASD, atrial septal defect; ASO, atrial septal occlude; DCM, dilated cardiomyopathy; LA, left atrium; LV, left ventricle; MR, mitral regurge; MV, mitral valve; pg, pressure gradient; TEE, transesophageal echocardiography; VCA, vena contracta area.
Five (31.25%) patients had associated MitraClip insertion in the same set of device closure. The procedure was elective in 9 (56.25%) patients, urgent in 5 (31.25%) patients, and emergency/salvage in 2 (12.5%) patients. The mean procedural time was 55.13 ± 16.24 minutes, fluoroscopy time was 16.25 ± 4.03 minutes, radiation dose was 16.25 ± 4.41 μGym2, and the total contrast used was 33.75 ± 10.94 mL. All patients showed acute procedural success with no need for any mechanical assist devices. Most patients passed without procedural complications, except 1 (6.25%) who showed access site hematoma (managed conservatively) and 2 (12.5%) showed infrequent atrial ectopics (resolved spontaneously). No patient was converted to open heart surgery or exhibited device-related compilations or procedure-related mortality. Procedural safety and quality measures of the studied patients are summarized in Table 1.
Follow-up Functional and Echocardiographic Outcomes
By the end of the study, survival data were available for all patients. Functional status improved in all patients except for 1 (6.25%) who remained with New York Heart Association functional class III. All patients were successfully extubated on postoperative day 0, with a mean intensive care unit stay of 1.38 ± 0.52 days and a mean total in-hospital stay of 2.63 ± 1.06 days. No patients were hospitalized, and no patient required reintervention, displayed complications, or exhibited mortality.
Immediately after the procedure, all patients showed no residual MR from the targeted perforation/jet; however, 3 (18.75%) of the post-Mitraclip patients showed mild residual commissural MR away from the targeted MR. The device was stable with no device-related complications or change in the mean MV gradient (p < 0.05). TR severity decreased with a significant reduction in ESPAP immediately after the procedure and onwards (p < 0.05). Left ventricular ejection fraction improved significantly at 3 months and 6 months (p < 0.05), without a documented LVOT obstruction (p > 0.05). Functional and echocardiographic outcomes immediately after the procedure and throughout follow-up are summarized in Table 2.
Table 2.
Functional and echocardiographic outcomes immediately after procedure and throughout follow-up
| Patients, N = 16 | Preprocedural | Immediately/in-hospital | 3-Mo after procedure | 6-Mo after procedure |
|---|---|---|---|---|
| Functional outcome | ||||
| Invasive LAP (mmHg) | 44.38 ± 6.26 | 18.25 ± 3.85 (p < 0.001) | ||
| NYHA-FC III-IV | 16 (100%) | 1 (6.25%) | 1 (6.25%) | 1 (6.25%) |
| Extubation in d 0 | 16 (100%) | |||
| ICU stay (d) | ||||
| 1 d | 13 (81.25%) | |||
| 2 d | 3 (18.75%) | |||
| Total in-hospital stay (d) | ||||
| ≤2 d | 5 (31.25%) | |||
| >2 d | 11 (68.75%) | |||
| Reintervention | 0 (0%) | 0 (0%) | 0 (0%) | |
| Complications | 0 (0%) | 0 (0%) | 0 (0%) | |
| Mortality | 0 (0%) | 0 (0%) | 0 (0%) | |
| Medications | ||||
| Clopidogrel | 13 (81.25%) | 13 (81.25%) | 13 (81.25%) | |
| Anticoagulant (warfarin/apixaban) | 3 (18.75%) | 3 (18.75%) | 3 (18.75%) | |
| Echocardiographic outcome | ||||
| MR severity across the defect | ||||
| No/trivial | 16 (100%) | 16 (100%) | 16 (100%) | |
| Mild (I) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Moderate (II) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Severe (III) | 16 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| MV Pg (mmHg) | 3.42 ± 0.84 | 4.00 ± 1.07 (p = 0.17) | 4.38 ± 1.46 (p = 0.09) | 4.57 ± 1.41 (p = 0.07) |
| TR severity | ||||
| None/trivial | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Mild (I) | 6 (37.5%) | 10 (62.5%) | 10 (62.5%) | 10 (62.5%) |
| Moderate (II) | 8 (50%) | 6 (37.5%) | 6 (37.5%) | 6 (37.5%) |
| Sever (III) | 2 (12.5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| eSPAP (mmHg) | 41.0 ± 11.1 | 38.13 ± 9.8 (p = 0.006∗) | 32.88 ± 5.79 (p = 0.016∗) | 32.25 ± 5.12 (p = 0.015∗) |
| LVOT Pg (mmHg) | 1.93 ± 1.33 | 2.3 ± 1.8 (p = 0.2) | 2.13 ± 1.89 (p = 0.49) | 2.19 ± 1.77 (p = 0.45) |
| LVEF (%) | 47.38 ± 10.97 | 48.63 ± 10.93 (p = 0.06) | 51.13 ± 9.69 (p = 0.027) | 51.25 ± 10.01 (p = 0.054) |
| PVF (S/D ratio) | ||||
| Decreased S wave, S/D ratio <1 | 12 (75%) | |||
| Blunted S wave, S/D ratio <1 | 4 (25%) | |||
| Normal S/D ratio >1 | 0 (0%) | 16 (100%) | 16 (100%) | 16 (100%) |
eSPAP, estimated systolic pulmonary artery pressure; ICU, intensive care unit; LAP, left atrial pressure; LVEF, left ventricle ejection fraction; LVOT, left ventricular outflow tract; MR, mitral regurge; MV, mitral valve; NYHA-FC, New York Heart Association Functional Class; Pg, pressure gradient; PVF, pulmonary venous flow; S/D, pulmonary venous systolic/diastolic flow; TR, tricuspid regurge.
Discussion
The surgical repair procedure is the standard treatment in patients with valvular system backflow. Due to the surgical high-risk complications, percutaneous options are becoming more popular.19 Percutaneous procedures have many advantages including a relatively low risk and shorter hospitalization. They are especially recommended in patients with a high-surgical risk of morbidity and mortality and when the patient refuses cardiac surgery.20
MVL Perforations
As perforations in the MVLs are very rare, data in the literature are insufficient. Perforation of the AML is seldom caused by iatrogenic injuries during AV surgery; in a prior study of 475 patients with AV insufficiency surgical repair, only 2 patients had AML perforation as a postoperative complication.4 AML perforation can happen due to the fibrous continuity between the AML and the AV; the middle of the AML corresponds to the commissure between the left-coronary and the noncoronary AV sinuses. Due to this close anatomical proximity, either of the 2 valves may be injured during the intervention for the other.21 Another mechanism of MV injury is an AV regurgitant jet that is directed toward the AML, eroding the tissue and leaving the surface more liable to infection.21 Perforations in the AML may be the only mechanism of MR in some patients, and when large in size, can cause severe heart failure and thus deserve intervention.8,22
MVL perforations are usually managed by a surgical approach, with potentially severe complications for patients. Sareyyupoglu et al.8 reported 26 patients with AML perforation who underwent surgical MV repair with a patch in 11 (42%) patients and a primary suture closure in 15 (58%) patients, with patients’ survival of 95% at 1 year and 90% at 5 years. Due to the high-risk redo surgeries and patients’ preference, the percutaneous option was suggested to solve these challenges.
AML perforation can occur either in the AML body or the AML base close to the annulus. Here, we report on 9 (56.25%) MVL perforations due to different etiologies and scenarios; 7 (43.75%) AML body perforations, and 2 (12.5%) para-ring perforations/leaks. In all 7 (43.75%) patients with AML body perforations, Amplatzer ASO devices were used, as the distance between the ASO discs matched very well with the leaflet body thickness (1-3 mm), keeping the device discs aligned with the leaflet body. Despite the perforation being crossed retrogradely in all of them, the device was deployed antegrade (transvenous/transseptal) after AV loop formation, allowing the ASO large disc to face the high-pressure LV side for better closure and stability. In 1 (6.25%) patient with a spontaneously ruptured AML aneurysm, the device was deployed directly, retrogradely, transapical allowing the large ASO disc to capture the aneurysm completely. Our experience is in agreement with that of Javed et al.10 who was the first to describe a percutaneously closed AML perforation with severe MR in a 59-year-old male after CABG and mitral annuloplasty in whom an Amplatzer ASO 5-mm device was implanted antegrade transseptal in the perforation.
However, in the 2 (12.5%) patients with para-ring MVL perforations/leaks at the base of the MVL, it was better to choose a device with retention discs of soft and low-profile nature; an Amplatzer VP-II device was transapically deployed with successful results. In agreement with this principle, Raczkiewicz et al. described a 79-year-old woman with a significant backflow across an aortic-mitral perforation located in the basal AML, 4 years after surgical AV replacement. The procedure was carried out in a hybrid operating room with the implantation of a 6 × 3 mm rectangular paravalvular leak device (Occlutech GmbH, Jena, Thüringen, Germany) through a transseptal puncture.11 Goswami et al.23 also described perforation defect closure at the base of the posterior mitral leaflet through the anterograde approach, using an Amplatzer duct occluder (ADO)-II 6 × 4 mm in an adult patient with end-stage chronic kidney disease.
Post-MitraClip Residual MR Jets
The maneuver of clips around the MV commissure can increase the risk of complications due to degenerative anatomy, flail leaflets, restricted leaflets, calcification, and complex chordal structure. Careful patient selection is therefore mandatory to minimize risks such as clip dislodgement and failure, chordal entanglement and rupture, and MV destruction. Furthermore, the area of maximal MR, which matches the region of maximal pathology, is often difficult to grasp with additional clips.24 Thus, significant residual post-clips MR jets are a current challenge with limited treatment options.
In our study, we report on 7 (43.75%) patients with post-MitraClip severe residual MR jets; 3 (18.75%) commissural and 4 (25%) inter-clip. In such patients, the residual MR cannot be completely managed using multiple clips; any additional clip implantation could lead to chordal rupture or interference with the existing clip function. In our series, a deflectable Agilis sheath was used for residual jet crossing as it allows easier crossing within smaller jets, then a TorqVue delivery sheath was used for device deployment. In one recently reported patient, a 6F-guiding catheter was advanced through a deflectable MitraClip guiding catheter to insert an Amplatzer VP-II in the residual leak.25 Another case series used Agilis catheter not only in crossing the inter-clip leak but also in deploying Amplatzer ADO-II devices, for better deliverability and stress reduction on the implanted clips.26
In the current study, we used Amplatzer ASO devices in 3 (18.75%) patients with centrally located residual inter-clip jets, and we used Amplatzer VP-II in the other 4 (25%) patients with annularly located residual jets. The central soft waist of the VP-II made it easier to wedge in the annular jets and limit interaction with the implanted clips. To choose the smallest device, it was important to reduce the residual gap between the clips. Our data agrees with that of Taramasso et al.25 who established the first-in-man case report of a 64-year-old male patient with a significant residual MR 4 years after transcatheter MitraClip repair procedure. Two further MitraClip devices were placed, with residual inter-clip MR with no further space for additional clips. So, an 18-mm Amplatzer VP-II device was successfully deployed between the 2 clips with final mild residual MR and without mitral stenosis.25 Also, Czerny et al.26 implanted an 8-mm Amplatzer ASO device for sealing a severe MR after 2 MitraClip devices were implanted in an anterograde fashion in an adult patient. Other suggested devices were the Amplatzer ADO-II, which was evaluated by Kubo et al.27 who managed 9 patients with post-MitraClip MR (3 with residual commissural MR and 6 with residual inter-clips MR). They preferred the ADO-II device for the larger retention discs that held the device steady in place.27 The difference between the Amplatzer VP-II and ADO-II devices is the difference between the retention discs and the central waist diameters. The ADO-II has a 4- to 6-mm difference, while the AVP-II has no difference; which may contribute to the better anchoring of the AVP-II device. Device size was chosen according to TEE, 3D-TEE quantification, and fluoroscopic data findings.
Follow-up
In all our patients, device deployment completely occluded the targeted perforation/jet with normalization of LAP and PVF and without affecting the MV function. Although hemolysis was recognized with the Amplatzer occluders because of the high-velocity blood flow through them,28,29 in the current study, we did not observe device-related complications throughout the midterm 6-month follow-up. Therefore, device durability without complications may be expected, if the defect site and the occluder size were optimally estimated. In agreement, Kubo et al.27 showed a reduction in MR, decrease in LAP, and normalization of systolic PVF without an increase in the transmitral gradient or hemolysis in their patients. Only in 1 of their patients, an ADO-II device was retrieved because it was embolized to the ostial right coronary artery 9 hours after the procedure. They supposed either the device was underestimated or the leaflet integrity was suboptimal to tolerate the device.27
Transcatheter closure for such specific defects should be considered in anatomically challenging high-risk patients with severe MR and may provide acute hemodynamic improvement as a bridge for surgery. To our knowledge, this study enrolled the largest cohort for such specific lesions with severe MR, which were managed percutaneously with respectable immediate and 6-month outcomes.
Limitations of the Study
First, this was a single-center retrospective study with only a midterm follow-up period. Longer follow-up observation with a larger population would be necessary to confirm the long-term efficacy and safety. Second, this procedure was limited to highly selected patients with a very high or prohibitive cardiac risk for surgery; future studies should consider this technique in patients at lower risk.
Conclusion
Percutaneous transcatheter MV repair of MVL perforations or post-clips residual MR is a new, feasible, and effective technique. This technique is challenging; however, it is reserved for properly selected patients who have a high-risk surgery or in patients who refuse surgery. Among the challenges are the ideal and safe access, the appropriate and easier crossing approach, the feasibility of negotiating a mechanical AV, and the stability of the closure mechanism of the MV. Further research is needed to establish the long-term follow-up of this technique and to evaluate it in patients at lower risk. Meanwhile, it can motivate other physicians to perform a more reliable transcatheter MV repair of MVL perforations, post-clips residual commissural MR, and inter-clip MR.
Ethics Statement
The research reported has adhered to the relevant ethical guidelines. It has complied with the Declaration of Helsinki ethical guidelines (as revised in 2013) and was approved by the institutional review board committee. Informed written consent was obtained from all patients before the procedure.
Funding
The authors have no funding to report.
Disclosure statement
All authors declare that they have no conflicts of interest and that all illustrations and figures in the manuscript are entirely original and do not require reprint permission.
Acknowledgments
The authors are grateful to all the participating patients.
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
Supplementary Material
Retrograde crossing in a mechanical aortic valve
TEE and 3D-TEE of a patient with an iatrogenic perforation of AML (A3 scallop)
Fluoroscopic guidance of a patient with an iatrogenic perforation of AML (A3 scallop)
TEE and 3D-TEE of a patient with a spontaneously ruptured AML aneurysm with a perforation into the LA (A3 scallop)
Fluoroscopic guidance of a patient with a spontaneously ruptured AML aneurysm with a perforation into the LA (A3 scallop)
TEE and 3D-TEE guidance of a patient with a para-ring perforation/leak (A3 scallop)
Fluoroscopic guidance of a patient with a para-ring perforation/leak (A3 scallop)
Device closure of a post-MitraClip severe residual MR between 3 MitraClip and the medial commissure at the P1 scallop (TAIBA technique)
TEE and 3D-TEE guidance of a patient with an inter-MitraClip residual MR
Fluoroscopic guidance of a patient with an inter-MitraClip residual MR
Device closure of an inter-MitraClip severe residual MR
References
- 1.Estévez-Loureiro R., Settergren M., Pighi M., et al. Effect of advanced chronic kidney disease in clinical and echocardiographic outcomes of patients treated with MitraClip system. Int J Cardiol. 2015;198:75–80. doi: 10.1016/j.ijcard.2015.06.137. [DOI] [PubMed] [Google Scholar]
- 2.Abuelatta R., Naeim H., AlAhmadi A., et al. Transcatheter repair of anterior mitral leaflet perforation in a patient with mechanical aortic valve using antegrade and retrograde approaches. J Struct Heart Dis. 2018;4:234–239. doi: 10.12945/j.jshd.2018.002.18. [DOI] [Google Scholar]
- 3.Stout K.K., Verrier E.D. Valvular heart disease: changing concepts in disease management; acute valvular regurgitation. Circulation. 2009;119:3232–3241. doi: 10.1161/CIRAHA.108.782292. [DOI] [PubMed] [Google Scholar]
- 4.Van Dyck M., Glineur D., de Kerchove L., El Khoury G. Complications after aortic valve repair and valve sparing procedures. Ann Cardiothorac Surg. 2013;2:130–139. doi: 10.3978/j.issn.2225-319X.2012.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cozzarin A., Cianciulli T.F., Guidoin R., et al. CoreValve prosthesis causes anterior mitral leaflet perforation resulting in severe mitral regurgitation. Can J Cardiol. 2014;30:1108.e11–1108.e13. doi: 10.1016/j.cjca.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Bouma W., Klinkenberg T.J., van der Horst I.C., et al. Mitral valve surgery for mitral regurgitation caused by libman-sacks endocarditis: a report of four cases and a systematic review of the literature. J Cardiothorac Surg. 2010;5:13. doi: 10.1186/1749-8090-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arat-Ozkan A., Okcun B., Mert M., Baran T., Kucukoglu S. Tear in mitral anterior leaflet as a complication of Manouguian’s procedure in a woman with aortic valve prosthesis. J Heart Valve Dis. 2004;13:630–631. [PubMed] [Google Scholar]
- 8.Sareyyupoglu B., Schaff H.V., Suri R.M., Connolly H.M., Daly R.C., Orszulak T.A. Safety and durability of mitral valve repair for anterior leaflet perforation. J Thorac Cardiovasc Surg. 2010;139:1488–1493. doi: 10.1016/j.jtcvs.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Velasco S., Larman M., Eneriz M. Percutaneous closure of a native mitral valve perforation. Rev Esp Cardiol. 2010;63:597. doi: 10.1016/S1885-5857(10)70121-3. [DOI] [PubMed] [Google Scholar]
- 10.Javed U., Smith T.W., Rogers J.H. Percutaneous repair of anterior mitral leaflet perforation. J Invasive Cardiol. 2012;24:134–137. [PubMed] [Google Scholar]
- 11.Raczkiewicz S., Matejszczak-Wo S.M., Pysz P., Zaremba-Flis E., Smolka G., Kleinrok A. First report of percutaneous closure of anterior mitral leaflet perforation using a Paravalvular Leak Device (PLD) Postepy Kardiol Interwencyjnej. 2016;12:274–275. doi: 10.5114/aic.2016.61653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sengun B., Yildirim I., Yildiz O., Celiker A. Retrograde transcatheter closure of anterior mitral valve leaflet perforation. Ann Pediatr Cardiol. 2019;12:312–314. doi: 10.4103/apc.APC_162_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggebrecht H., Schelle S., Puls M., et al. Risk and outcomes of complications during and after MitraClip implantation: experience in 828 patients from the German TRAnscatheter mitral valve interventions (TRAMI) registry. Catheter Cardiovasc Interv. 2015;86:728–735. doi: 10.1002/ccd.25838. [DOI] [PubMed] [Google Scholar]
- 14.Van den Branden B.J.L., Post M.C., Swaans M.J., et al. Percutanous edge to edge mitral valve repair in high surgical risk patients: do we hit the target? JACC Cardiovasc Interv. 2012;5:105–111. doi: 10.1016/j.jcin.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Obadia J.F., Zeitoun D.M., Leurent G., et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379:2297–2306. doi: 10.1056/NEJMoa1805374. [DOI] [PubMed] [Google Scholar]
- 16.Stone G.W., Lindenfeld J., Abraham W.T., et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 17.Pibarot P., Delgado V., Bax J.J. MITRA-FR vs. COAPT: lessons from two trials with diametrically opposed results. Eur Heart J Cardiovasc Imaging. 2019;20:620–624. doi: 10.1093/ehjci/jez073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naeim H.A., Abuelatta R. Tear of posterior mitral valve leaflet during MitraClip, successful bailout using vascular plugs. JACC Case Rep. 2019;1(2):197–201. doi: 10.1016/j.jaccas.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smolka G., Ochała A., Jasiński M., Pysz P., Biernat J., Gasior Z. Percutaneous treatment of periprosthetic valve leak in patients not suitable for reoperation. Kardiol Pol. 2010;68:369–373. [PubMed] [Google Scholar]
- 20.Hein R., Lang K., Wenderlich N., Wilson N., Sievert H. Percutaneous closure of paravalvular leaks. J Interv Cardiol. 2006;19(5):73–77. doi: 10.1111/j.1540-8183.2006.00174. [DOI] [Google Scholar]
- 21.Veronesi F., Corsi C., Sugeng L., et al. A study of functional anatomy of aortic-mitral valve coupling using 3D matrix transesophageal echocardiography. Circ Cardiovasc Imaging. 2009;2:24–31. doi: 10.1161/CIRCIMAGING.108.78590. [DOI] [PubMed] [Google Scholar]
- 22.Nomeir A.M., Downes T.R., Cordell A.R. Perforation of the anterior mitral leaflet caused by aortic valve endocarditis: diagnosis by two-dimensional, transesophageal echocardiography and color flow Doppler. J Am Soc Echocardiogr. 1992;5(2):195–198. doi: 10.1016/S0894-7317(14)80553-1. [DOI] [PubMed] [Google Scholar]
- 23.Goswami R., Colin B., Jackson M., Kleiman N., Little S. Transesophageal echocardiography-guided percutaneous intervention for a mitral valve leaflet perforation. JACC Cardiovasc Interv. 2015;8:754–755. doi: 10.1016/j.jcin.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Van Mieghem N.M., Piazza N., Anderson R.H., et al. Anatomy of the mitral valvular complex and its implications for transcatheter interventions for mitral regurgitation. J Am Coll Cardiol. 2010;56:617–626. doi: 10.1016/j.jacc.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 25.Taramasso M., Zuber M., Gruner C., Gaemperli O., Nietlispach F., Maisano F. First-in-man report of residual “intra-clip” regurgitation between two MitraClip treated by Amplatzer Vascular Plug II. EuroIntervention. 2016;11:1537–1540. doi: 10.4244/EIJY14M12_04. [DOI] [PubMed] [Google Scholar]
- 26.Czerny M., Taramasso M., Guidotti A., Maisano F. A creative transcatheter approach to correct complex recurring mitral regurgitation after previous surgical repair. EuroIntervention. 2016;11:e1302–e1304. doi: 10.4244/EIJV11I11A252. [DOI] [PubMed] [Google Scholar]
- 27.Kubo S., Cox J.M., Mizutani Y., et al. Transcatheter procedure for residual mitral regurgitation after MitraClip implantation using Amplatzer Duct Occluder II. Jacc Cardiovasc Interv. 2016;9(12):1280–1288. doi: 10.1016/j.jcin.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Lambert V., Belli E., Piot J.D., Planche C., Losay J. Hemolysis, a rare complication after percutaneous closure of an atrial septal defect. Arch Mal Coeur Vaiss. 2000;93:623–625. [PubMed] [Google Scholar]
- 29.Spence M.S., Thomson J.D., Weber N., Qureshi S.A. Transient renal failure due to hemolysis following transcatheter closure of a muscular VSD using an Amplatzer muscular VSD occluder. Catheter Cardiovasc Interv. 2006;67:663–667. doi: 10.1002/ccd.20629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Retrograde crossing in a mechanical aortic valve
TEE and 3D-TEE of a patient with an iatrogenic perforation of AML (A3 scallop)
Fluoroscopic guidance of a patient with an iatrogenic perforation of AML (A3 scallop)
TEE and 3D-TEE of a patient with a spontaneously ruptured AML aneurysm with a perforation into the LA (A3 scallop)
Fluoroscopic guidance of a patient with a spontaneously ruptured AML aneurysm with a perforation into the LA (A3 scallop)
TEE and 3D-TEE guidance of a patient with a para-ring perforation/leak (A3 scallop)
Fluoroscopic guidance of a patient with a para-ring perforation/leak (A3 scallop)
Device closure of a post-MitraClip severe residual MR between 3 MitraClip and the medial commissure at the P1 scallop (TAIBA technique)
TEE and 3D-TEE guidance of a patient with an inter-MitraClip residual MR
Fluoroscopic guidance of a patient with an inter-MitraClip residual MR
Device closure of an inter-MitraClip severe residual MR