Abstract
Background:
Short-term exposure to high or low temperatures is associated with increased mortality and morbidity. Less is known about effects of long-term exposure to high or low temperatures. Prolonged exposure to high or low temperatures might contribute to pathophysiological mechanisms, thereby influencing the development of diseases. Our aim was to evaluate associations of long-term temperature exposure with cardiovascular disease (CVD) hospitalizations.
Methods:
We constructed an open cohort consisting of all fee-for-service Medicare beneficiaries, aged ≥65, living in the contiguous US from 2000 through 2016 (~61.6 million individuals). We used data from the 4 km Gridded Surface Meteorological dataset to assess the summer (June–August) and winter (December–February) average daily maximum temperature for each year for each zip code. Cox-equivalent Poisson models were used to estimate associations with first CVD hospitalization, after adjustment for potential confounders. We performed stratified analyses to assess potential effect modification by sex, age, race, Medicaid eligibility and relative humidity.
Results:
Higher summer average and lower winter average temperatures were associated with an increased risk of CVD hospitalization. We found a HR of 1.068 (95% CI: 1.063, 1.074) per IQR increase (5.2 °C) for summer average temperature and a HR of 1.022 (95% CI: 1.017, 1.028) per IQR decrease (11.7 °C) for winter average temperature. Positive associations of higher summer average temperatures were strongest for individuals aged <75 years, Medicaid eligible, and White individuals. Positive associations of lower winter average temperatures were strongest for individuals aged <75 years and Black individuals, and individuals living in low relative humidity areas.
Conclusions:
Living in areas with high summer average temperatures or low winter average temperatures could increase the risk of CVD hospitalizations. The magnitude of the associations of summer and winter average temperatures differs by demographics and relative humidity levels.
Keywords: Cardiovascular disease, Climate, Temperature
1. Introduction
Current climate change scenarios predict warmer meteorological conditions and more weather extremes in the future (IPCC. Global Warming of 1.5 °C, 2018). To assess potential health impacts of climate change scenarios, it is important to evaluate the health effects of exposure to temperature. Numerous epidemiological studies have shown that short-term exposure to extreme and moderately (temperatures above and below the optimum temperature) high or low temperatures are linked to increased mortality (Gasparrini et al., 2015; Chen et al., 2018; Yu et al., 2012) and morbidity (Schwartz et al., 2004; Ye et al., 2012).
Not much is known about health effects of long-term exposure to high or low temperatures. Few studies have reported positive associations of long-term summer average temperature exposure with mortality (Shi et al., 2015, 2016). Living in a warm or cold climate (prolonged exposure to high or low temperatures) might contribute to pathophysiological mechanisms, thereby influencing the development of diseases. For example, some studies reported higher hypertension prevalence in populations living closer to the poles (Li et al., 2015; Neufcourt et al., 2019). A meta-analysis reported that annual mean temperature is negatively associated with metabolic rate and could explain why populations living in warmer areas tend to have a lower metabolic rate than populations living in cooler areas (Froehle, 2008). Recent reviews showed that higher long-term (annual or seasonal) average temperature exposures were associated with a range of adverse health outcomes (Zanobetti and O’Neill, 2018; Zafeiratou et al., 2021). However, the number of studies that evaluated associations with cardiovascular diseases (CVD) was limited.
The aim of this paper was to study whether local temperatures were associated with CVD hospitalization in the contiguous US. We evaluated associations of long-term exposure to summer (June–August) and winter (December–February) average temperature with CVD hospitalizations in all fee-for-service Medicare beneficiaries aged ≥65 years from 2000 through 2016 (~61.6 million individuals). Our study thus differs from some long-term temperature studies that evaluated associations between annual mortality and annual/seasonal summaries of heat and cold to address the question of whether short-term effects represent “harvesting” (Rehill et al., 2015a; Armstrong et al., 2017; Goggins et al., 2015). This study evaluates the health impact of spatial contrasts in local long-term exposures, and is similar in design to long-term air pollution studies (Klompmaker et al., 2021; Shi et al., 2020).
2. Methods
2.1. Study population
We created an open cohort using data from Medicare (the US national health insurance program). Medicare provides health insurance for Americans aged 65 and older and for younger people with disability status. Our cohort included all 65+ years, fee-for-service (FFS) Medicare beneficiaries, living in the contiguous US from 2000 (January 1) through the end of 2016 (December 31). Follow-up started on January 1st, 2000 or January 1st of the year following Medicare enrolment. We followed each beneficiaries till the first CVD hospital admission, the end of the follow-up time, death or censoring. Beneficiaries under 65 years of age were excluded from analyses.
2.2. Outcome definition
Hospital admissions for all Medicare FFS beneficiaries were derived from the Medicare Provider Analysis and Review dataset. In this dataset, ICD-9 (2000 - the third quarter of 2015) and ICD-10 (third quarter of 2015–2016) codes were used to define hospital discharge diagnosis. We used first hospital admissions with a primary discharge diagnosis of CVD (ICD-9: 390–459, ICD-10: I00–I99). For secondary outcomes, we used first hospital admissions with a primary discharge diagnosis of coronary heart disease (ICD-9 code: 410–414, ICD-10 codes: I20–I25), and cerebrovascular disease (ICD-9 codes: 430–438, ICD-10 codes: I60–I69), hereafter referred to as CHD, and CBV, respectively. We created separate cohorts for each outcome.
2.3. Exposure assessment
We used data from the Gridded Surface Meteorological dataset (Abatzoglou, 2013) to assess the summer and winter average daily maximum air temperature. This dataset is based on a combination of data from the North American Land Data Assimilation System Phase 2 (Mitchell et al., 2004), and of the Parameter-elevation Regressions on Independent Slopes Model (Daly et al., 2008). Attributes of the NLDAS-2 and PRISM were blended to create a daily high-resolution meteorological dataset. Detailed information about the development and validation of the dataset can be found elsewhere (Abatzoglou, 2013).
The Gridded Surface Meteorological data provides daily surface fields of maximum air temperature at ~4 km spatial resolution covering the contiguous US from 1979 onwards. We used Google Earth Engine (Gorelick et al., 2017) to assess the spatially weighted daily maximum temperature for each zip code for each day. Next, we calculated spatially weighted summer (June–August) and winter (December–February) average daily maximum temperature for each zip code for each year of follow-up, hereafter referred to as summer and winter average temperature. Winter average temperature for a specific year was based on January and February of that year and December of the previous year. Summer and winter average temperatures are based on daily maximum temperature estimates, as the Gridded Surface Meteorological dataset does not contain information about daily average temperatures. We note that previous studies showed very strong correlations between daily maximum temperature and daily mean temperature (Metzger et al., 2010; Barnett et al., 2010). We also calculated spatially weighted summer and winter average daily minimum temperature for each zip code for each year.
Further, we calculated the spatially weighted annual (January–December) average daily maximum temperature for each zip code for each year of follow-up. We calculated the summer and winter average temperature for each zip code for the entire study period (2000–2016).
All temperature exposures were assigned to beneficiaries who lived within that zip code in a given calendar year. For example, 2002 summer (June–August 2002) and winter (December 2001, January–February 2002) average temperature estimates were assigned to beneficiaries for the year 2002.
2.4. Covariates
The Medicare beneficiary file contains information about sex, age (at year of Medicare enrolment), race, Medicaid eligibility, year of entry, and zip code of residence for all Medicare beneficiaries for each year. Medicaid is a federal health insurance program for people with limited income. We use Medicaid eligibility as it is a proxy for low socioeconomic status (SES). Information about Medicare beneficiary race and ethnicity is generally obtained from the Social Security Administration, which collects race and ethnicity data at the time of application for a Social Security Number (Filice and Joynt, 2017). Race was included in our models as it is documented that non-white individuals have higher prevalence of cardiovascular risk factors, such as hypertension and diabetes (Brewer and Cooper, 2014). As SES and race have been linked to CVD and CVD risk factors (Brewer and Cooper, 2014; de Mestral and Stringhini, 2017), we linked several zip code-level SES variables: population density, median household income, median home value, percent below the poverty level, percent of owner-occupied housing units percent Black, percent Hispanic and percent of the population with less than a high school degree. These variables were derived from the US Census and American Community Survey. From the nationwide Behavioral Risk Factor Surveillance System (BRFSS), we derived two county-level lifestyle variables (mean BMI and percent population that were ever smokers) as these are important risk factors for CVD. BRFSS is an annual national telephone and cellular surveillance survey that collects information about modifiable risk behaviours and chronic disease prevalence. Zip code-level SES (2000, 2009–2016) and county-level lifestyle (2000–2011) variables were available for some years but not all. We temporally interpolated data for years with missing information using a moving average algorithm within each zip code, as described previously (Di et al., 2017).
For each zip code for each year (2000–2016), daily ambient specific humidity, maximum relative humidity and daily total precipitation were estimated using data from the Gridded Surface Meteorological dataset (Abatzoglou, 2013). We calculated the spatially weighted summer and winter average specific humidity, relative humidity and precipitation for each zip code for each year. We also assessed seasonal temperature variability. Temperature variability was based on the standard deviation of the spatially weighted daily maximum temperature within the summer/winter for each zip code for each year. Further, we defined heat and cold waves as a minimum of two consecutive days with a maximum temperature above (heat waves) or below (cold waves) a threshold. For each zip code, the cold wave temperature threshold was defined as the 1st percentile of daily maximum temperature in that zip code for all days from 2000 to 2016. The heat wave temperature threshold was defined as the 99th percentile of daily maximum temperature in each zip code for all days from 2000 to 2016. The number of heat wave days and cold wave days was calculated for each zip code for each year. We also used zip code-level annual average particulate matter less than 2.5 μm (PM2.5) and ozone concentration estimates based on predictions from well-validated spatio-temporal ensemble models (Di et al., 2019; Requia et al., 2020). Annual average concentrations were estimated by averaging the predictions at grid cells whose centroids fall within the boundary of that ZIP code (Di et al., 2019; Requia et al., 2020). US climate regions were defined according to the National Oceanic and Atmospheric Administration (Fig. S1).
2.5. Statistical analysis
We applied a Cox-equivalent re-parameterized Poisson approach to overcome computational challenges caused by our large-scale cohort. Within this approach, individual-level records were collapsed to a high-dimensional space of features, while keeping the integrity of stratum units for analysis (Shi et al., 2020). We aggregated all beneficiaries included in our cohort with the same sex, race (White, Black, other/unknown), Medicaid eligibility, 2-year categories of age at study entry, year of follow-up, that live within the same zip code in a specific year, and treated them as one single grid cell, because they belonged to the same stratum and as such were treated as interchangeable in the analysis. Detailed information about this approach is presented elsewhere (Shi et al., 2020).
Briefly, a stratified quasi-Poisson model was used to evaluate associations of time-varying average temperature with the rate of first CVD hospitalizations. The dependent variable was the count of first CVD (or CHD, CBV) hospitalizations in each stratum (as described above), using the corresponding total person-time in each stratum as the offset. This model is mathematically equivalent to a time-varying Cox proportional hazard model under an Anderson-Gill representation. To calculate statistically robust 95% confidence intervals (95% CIs), we applied an m-out-n bootstrap method using zip code units to account for within zip code correlated observations across years. This bootstrap method is described in detail elsewhere (Shi et al., 2020).
Summer average and winter average temperature were included simultaneously in the model. The main model included calendar year, population density, median household income, median home value, percent below the poverty level, percent of owner-occupied housing units percent Black, percent Hispanic, percent of the population with less than a high school degree, mean BMI, percent population that were ever smokers, summer and winter relative humidity, an offset for total person-time, and strata for all possible combinations of sex, race, Medicaid Eligibility, age at study entry (2-year categories), and follow-up year. We evaluated the shape of the exposure-response curves for each exposure by adding natural splines (2 or 3 degrees of freedom) to the temperature terms. To evaluate whether the observed associations were modified by demographics, we performed stratified analyses to assess potential effect modification by sex (male, female), age in follow up year (<75 years, 75–84 years, 85+ years), race (White, Black), Medicaid eligibility (yes/no, as an indicator for SES). Further, we examined potential effect modification by tertiles of summer and winter average relative humidity. We did not test whether spline models were statistically significantly different from linear models or whether interaction terms were significant, as even very small differences would result in significant p-values in this large-scale cohort. Hence, we visually inspected exposure-response curves and focused on differences in strength of the effect estimates.
We assessed multiple sensitivity analyses to test whether associations were robust. To exclude potential prevalent CVD cases, we excluded individuals who had their first hospital admission within the first year of their follow-up and all records in the year 2000. We used summer and winter average temperature for the entire study period (2000–2016) instead of yearly estimates. We used summer and winter average temperature based on daily minimum temperature instead of daily maximum temperature. Further, we adjusted for summer and winter average specific humidity instead of relative humidity, to control for the air’s actual amount of moisture instead of the relative amount of moisture. We also additionally adjusted for summer and winter average precipitation, annual average PM2.5, annual average ozone, and the number of heat and cold wave days per year. The rationale to adjust for summer and winter average precipitation was to control for meteorological differences not adjusted for by the other covariates in the model. The rationale to control for heat and cold wave days was to control for peak temperature exposures and only capture effects of long-term average temperature. We also examined potential effect modification by climate regions. Further, we used annual average temperature and we evaluated associations of summer and winter average temperature with CHD and CBV hospitalization.
Beneficiaries with missing data in any of the variables included in the main model were removed from the cohort (~2%). Hazard ratios (HRs) and 95% confidence intervals (CI) were expressed per interquartile range (IQR) increase for summer average temperature and per IQR decrease for winter average temperature.
We conducted analyses on the Harvard Research Computing Environment, which is supported by the Institute for Quantitative Social Science at Harvard University. We used R software (R Project for Statistical Computing) version 3.6.1 for our analyses.
3. Results
The full cohort consisted of 61, 612, 471 Medicare beneficiaries aged ≥65 living in the contiguous US in 2000–2016. We observed 18, 130, 973 first CVD hospital admissions in 390, 753, 808 person years, the median follow-up period was 5 years. Most Medicare beneficiaries were white, not eligible for Medicaid and between 65 and 74 years of age at study entry (Table 1). The hospitalization rate (# hospitalizations/person years) was highest for males, individuals aged 85+ years, Black individuals and individuals eligible for Medicaid (Table S1). The median (IQR) summer and winter average temperature were 29.8 °C (5.2) and 8.4 °C (11.8), respectively (Table 1 and Table S2). As expected, summer and winter average temperature were higher in the South of the US (Fig. 1). Winter average temperature was moderately positively correlated with summer average temperature (Pearson r = 0.58, Fig. S1).
Table 1.
Descriptive statistics of all US Medicare fee-for-service beneficiaries aged >65 living in the contiguous US.
| Demographics at study entry | |
|---|---|
|
| |
| Individual-level covariates | N (%) |
|
| |
| Sex | |
| Female | 33,985,877 (55.2) |
| Male | 27,626,594 (44.8) |
| Age | |
| 65–74 years | 47,199,810 (76.6) |
| 75–84 years | 10,571,973 (17.2) |
| 85+ years | 3,840,688 (6.2) |
| Race | |
| White | 51,998,567 (84.4) |
| Black | 5,418,919 (8.8) |
| Other/unknown | 4,194,985 (6.8) |
| Medicaid Eligibility | |
| Not eligible | 53,950,444 (87.6) |
| Eligible | 7,662,027 (12.4) |
| Aggregated data (2000–2016) a | |
| Zip code-level covariates | Median (IQR) |
| Temperature | |
| Summer average temperature (◦ C) | 29.8 (5.2) |
| Winter average temperature (◦C) | 8.4 (11.8) |
| US census covariates | |
| Population density (persons/mile2) | 629.5 (3068.4) |
| Median home value ($1000) | 144.3 (148.4) |
| Median household income ($1000) | 46.6 (25.3) |
| % with less than a high school degree | 24.5 (21.4) |
| % below the poverty level | 8.5 (8.0) |
| % owner-occupied housing units | 71.4 (21.9) |
| % Black | 3.9 (13.7) |
| % Hispanic | 5.3 (14.3) |
| BRFSS covariates | |
| % ever smoked | 46.2 (9.1) |
| Average BMI | 27.4 (1.3) |
| Other environmental exposures | |
| Summer average max relative humidity (%) | 90.3 (10.9) |
| Winter average max relative humidity (%) | 86.0 (9.4) |
Descriptive statistics of the zip code level covariates are given for the strata (aggregated data based on zip code, year, sex, race, Medicaid eligibility, 2-year categories of age at study entry and year of follow-up).
Fig. 1.
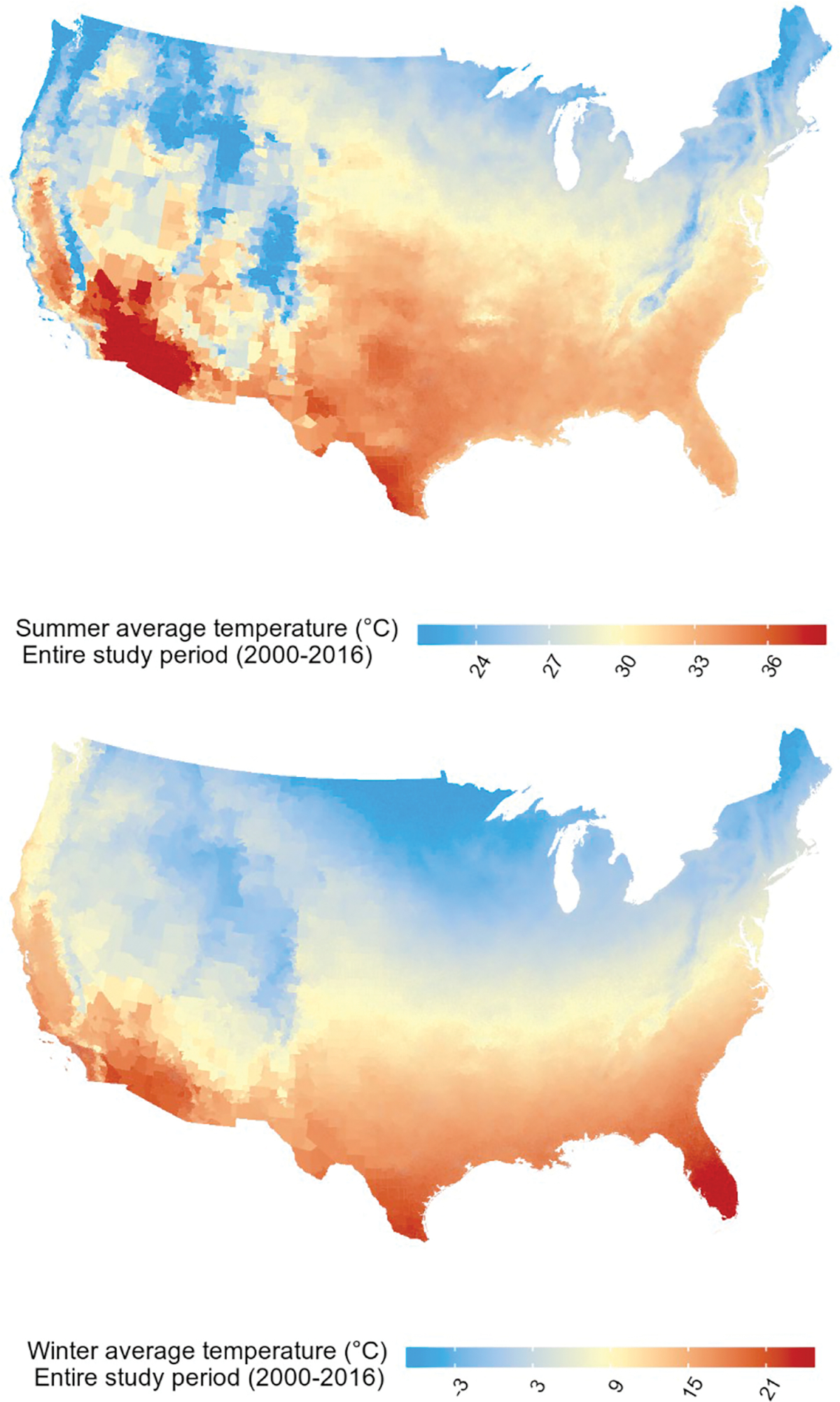
The spatial variation of summer and winter 2000–2016 average temperature per zip code in the contiguous US. Note: Summer 2000–2016 average temperature = spatially weighted summer (June–August) 2000–2016 average daily maximum temperature. Winter 2000–2016 average temperature = spatially weighted winter (December–February) 2000–2016 average daily maximum temperature.
Exposure-response curves showed no or small deviations from linearity for all exposures (Fig. S2). Higher summer average temperatures and lower winter average temperatures were associated with an increased risk of CVD hospitalization. We observed a HR of 1.068 (95% CI: 1.063, 1.074) per IQR (5.2 °C) increase in summer average temperature and a HR of 1.022 (95% CI: 1.017, 1.028) per IQR (11.7 °C) decrease in winter average temperature. We found slightly stronger positive associations of higher summer and lower winter average temperature for the 65–74 and 75–84 age groups than for 85 years of age or older (Fig. 2, Table S3). Positive associations of summer average temperature were stronger for Medicaid Eligible individuals than for individuals not eligible for Medicaid. For a decrease in winter average temperature, positive associations were stronger for Black individuals, while for an increase in summer average temperature, positive associations were stronger for White individuals. Positive associations of lower winter average temperatures were strongest in low humidity areas, and no associations were observed in high humidity areas (Fig. S3 shows the spatial variability of summer and winter average relative humidity).
Fig. 2.
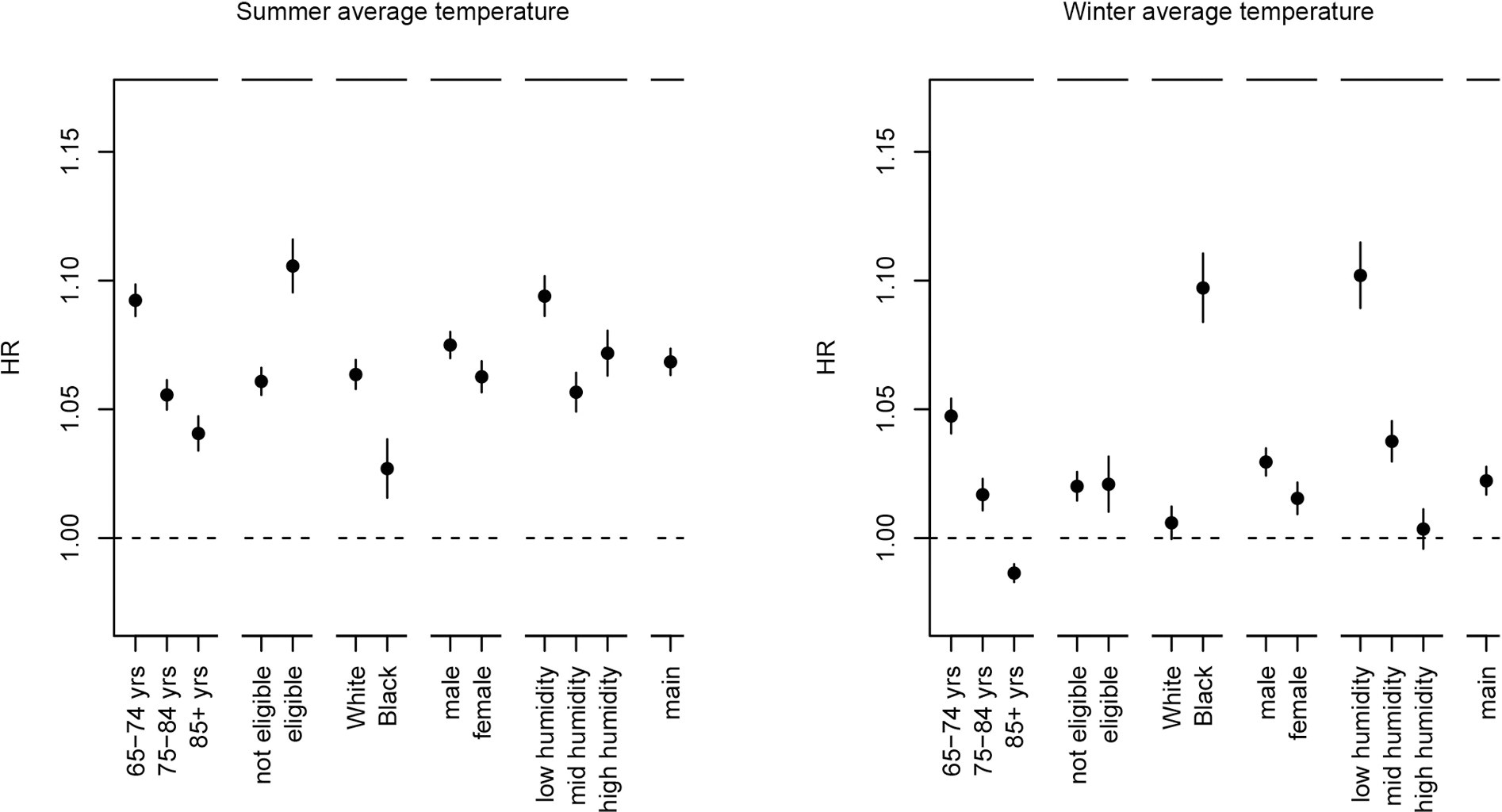
Associations of summer and winter average temperature with CVD hospitalization in stratified analyses.
Note: Associations are expressed per IQR increase (5.2 °C) for summer average temperature and per IQR decrease (11.7 °C) for winter average temperature. Poisson models included summer and winter average temperature and were adjusted for calendar year, median home value, median household income, population density, percent Hispanic, percent Black, percent of the population with less than a high school degree, percent below the poverty level, and percent of owner-occupied housing units, percent population that were ever smokers, mean BMI, summer average relative humidity, winter average relative humidity, an offset for total person-time and strata for all possible combinations of sex, race, Medicaid Eligibility, age at study entry (2-year categories), and follow-up year. To define summer and winter relative humidity strata, we used the following quantiles (q33.3, q66.7) based on the aggregated data: summer relative humidity (%): 86.8, 93.3, winter relative humidity (%): 82.9, 88.8. Person years and CVD hospitalizations are shown in Table S1, HRs are shown in Table S3.
Associations of summer and winter average temperature were generally robust to additional adjustment for precipitation, PM2.5, ozone, heat and cold wave days, exclusion of potential prevalent cases and the use of mean 2000–2016 temperature exposures (Table S4). Positive associations of higher summer and lower winter average temperatures based on daily minimum temperature estimates were slightly stronger than associations of higher summer and lower winter average temperature based on daily maximum temperature estimates. When we adjusted for summer and winter average specific humidity instead of summer and winter average relative humidity, the positive association of higher summer average temperatures attenuated and the positive association of winter average temperatures strengthened. Additional adjustment for summer and winter temperature variability also strengthened the positive association of winter average temperatures.
We observed limited variability in temperature exposures within most climate regions (Fig. S5). Stratified analyses by climate zones showed that in some climate regions associations were in the unexpected direction and/or non-linear (Fig. S5).
Higher annual average temperatures were associated with an increased risk of CVD hospitalization [HR: 1.055, 95%CI: 1.051, 1.060 per IQR (8.0 °C) increase]. We observed that higher summer average temperatures were associated with an increased risk of CHD and CBV hospitalization (Table S5). Lower winter average temperatures were associated with an increased risk of CHD, but not CBV, hospitalization.
4. Discussion
We observed that higher summer average temperatures and lower winter average temperatures were associated with an increased risk of CVD hospitalizations. Positive associations of higher summer average temperatures with CVD hospitalization were stronger for individuals aged <75 years, Medicaid eligible, and White individuals, while positive associations of lower winter average temperatures were stronger for individuals aged <75 years and Black individuals and individuals living in areas with a low winter average relative humidity.
Two reviews reported that long-term exposure to temperature is associated with several health outcomes (Zanobetti and O’Neill, 2018; Zafeiratou et al., 2021). However, because there are relatively few studies examining associations of long-term temperature exposures with health outcomes, it was difficult to make conclusions about specific health outcomes (Zanobetti and O’Neill, 2018; Zafeiratou et al., 2021). Our findings are in line with studies that evaluated associations between long-term seasonal temperature and mortality in the US (Shi et al., 2015, 2016). Previous studies in New-England and the Southeastern US showed that higher summer average temperatures and lower winter average temperatures were associated with increased all-cause mortality (Shi et al., 2015, 2016).
Two recent reviews of long-term and short-term temperature exposure reported more consistent associations of low temperatures with CVD mortality compared to high temperatures (Zafeiratou et al., 2021; Moghadamnia et al., 2017). In this study, positive associations of higher summer average temperatures were stronger in magnitude than associations of lower winter average temperatures. Measurement error might differ between summer and winter temperatures, which could have resulted in less strong associations with winter average temperature. Studies showed stronger relations between indoor and outdoor temperatures during the warm season compared to the cold season (Nguyen et al., 2014; Tamerius et al., 2013; Nguyen and Dockery, 2016). These relations might differ between warmer and cooler areas and SES groups, as prevalence of central heating systems and air conditioning, home insulation and other adaptive measures likely differ between warmer and cooler areas and SES groups.
Positive associations of lower winter average temperatures were strongest for Black individuals, while positive associations of higher summer average temperature were strongest for White individuals. This could be due to differences in regional demographics, relative humidity levels, the impacts of structural racism in the US, or a combination of these factors. The Black population is higher in Southeast of the US where temperatures are generally higher and houses may be less insulated and lack proper heating systems, which could make them more vulnerable to cold. The White population is higher in the North where temperatures are generally lower and air conditioning is less common. The stronger positive associations of summer average temperature for Medicaid eligible individuals could be related to the lack of proper cooling systems. We found slightly stronger positive associations in individuals under 75 years of age, in contrast with other studies that evaluated associations with mortality (Shi et al., 2016; Zanobetti et al., 2012; Rehill et al., 2015b). Associations of temperature might differ between CVD hospitalization and all-cause mortality. The stronger positive associations with individuals aged <75 years could be due to differences in time-activity patterns, including more outside activities than the older population, or because of differences in baseline hazards that may translate into weaker HRs on the multiplicative scale. The stronger positive associations of lower winter average temperatures in low relative humidity areas could be due to irritation of the respiratory system and increased risk of getting a cold and the flu, which could affect CVD hospitalizations. We did not find stronger associations of summer average temperature in high relative humidity areas. This could be due to a higher prevalence of air conditioning in areas with higher temperature and humidity levels.
This study investigated whether living in a zip code with a specific climate (long-term temperature) affects CVD hospitalizations. Our study thus differs from other studies, that used annual series of heat and cold, using a degree-day approach as mean annual degrees above/below minimum mortality temperature, to evaluate harvesting due to acute effects of heat and cold (Rehill et al., 2015a; Armstrong et al., 2017; Goggins et al., 2015). Associations of long-term temperature exposures by definition cannot be short-term displacement of effects (harvesting) as we are looking at yearly averages and displacement of effects by a few weeks will not influence these.
Short-term temperature studies examine health effects attributable to non-optimum daily temperatures variations (Gasparrini et al., 2015; Chen et al., 2018; Yu et al., 2012; Schwartz et al., 2004; Ye et al., 2012). They generally observe that the optimum temperature differs between countries/climate regions (Gasparrini et al., 2015), suggesting potential adaptation effects. However, these studies focus on daily temperature exposure and do not examine the impact of long-term temperature exposures. Our study examines the impact of long-term temperature exposure on CVD hospitalization. Long-term temperature studies require a large-scale geographical dimension and power to separate the effects of temperature from other population characteristics that differ between climate regions (Zafeiratou et al., 2021). We adjusted for several important area-level SES indicators and relative humidity in our main model and sensitivity analyses showed that results were robust to additional adjustment for several meteorological/environmental confounders that vary between climate regions, including precipitation, specific humidity (instead of relative humidity), heat and cold wave days, temperature variability, PM2.5, and ozone.
We did not observe positive associations of an increase in summer average temperature and a decrease in winter average temperature within several climate zones. This may indicate that the associations in the full cohort could be due to regional differences other than temperature. However, the unexpected associations could also be due to limited temperature variability within each climate zone and the moderate to strong correlations between summer and winter average temperature that may have resulted in limited power and unstable effect estimates in climate zone effect modification analyses.
Our study could not disentangle if associations of seasonal temperature exposures with CVD hospitalization are due to cumulative effects of short-term exposures or physiological responses of long-term exposures. Exposure to high temperatures can result in a lowered metabolic rate, increased blood volume and increased sweat production (Dhillon, 2012; Périard et al., 2016). Exposure to low temperatures on the other hand can lead to vasoconstriction, inflammatory responses and elevated resting metabolism and blood viscosity (Tansey and Johnson, 2015; Castellani and Young, 2016; Woodhouse et al., 1993, 1994; Keatinge et al., 1984). These responses may on the long term affect the risk of CVD hospitalization, especially for elderly as cardiovascular functions decline with age.
This study has several strengths. For the contiguous US, for each year, daily temperature data was available on a relatively fine spatial scale (~4 km spatial resolution). We were able to adjust for several important weather exposures, such as relative and specific humidity and number of heat wave days. We included all Medicare FFS beneficiaries aged 65+ years living in the contiguous US and therefore cover a large geographical region with different climates. The inclusion of all FFS Medicare beneficiaries in our cohort also allowed us to have a fairly representative sample of individuals aged 65+ years across the US. However, we note that our cohort did not include all Medicare beneficiaries [Medicare HMO (Health Maintenance Organisations, private plans) beneficiaries were not included]. Medicare FFS beneficiaries may have switched to a Medicare HMO and back during our follow-up period, which could have resulted in some missed cases in our data, as we have no information on Medicare HMO hospitalizations. We also note that the portion of Medicare FFS and HMO beneficiaries differed over time and by region.
Our study also has some limitations. We had no information about individual-level SES (other than Medicaid eligibility), lifestyle factors and use of heating and cooling systems, which may have resulted in an over- or underestimation of the associations. Several zip code-level SES factors that are likely related to individual SES were included, but the potential for residual confounding remains. Further, we used current year temperature exposures, and did not look at previous year exposures or any lagged effects. However, the median correlation between the zip code-level temperature exposures in consecutive years was very strong (Pearson r = 0.94 for summer average temperature, Pearson r = 0.98 for winter average temperature, Table S6), indicating that the spatial variation of the exposures does not change much between years and the impact of potential temporal misalignment would be small. The 4 km resolution of our temperature data may add some measurement error; however, seasonal temperatures do not vary on a small spatial scale and therefore we believe this error is minimal.
5. Conclusions
In conclusion, living in areas with high summer average temperatures or low winter average temperatures could increase the risk of CVD hospitalizations. The magnitude of the associations of summer and winter average temperatures differs by demographics and relative humidity levels.
Supplementary Material
Funding
This study was supported by National Institute of Environmental Health Sciences (R01 ES028033, R01 ES024332, R01 ES026217, 1R01 ES030616, 1R01 ES029950, P30 ES000002) National Institute on Aging (5R01 AG060232-03, R01 AG066793-01, 3R01 AG066793-02S1, 1RF1 AG071024, 1RF1 AG074372-01A1), and the National Heart, Lung and Blood Institute (R01 HL150119), and the National Institute on minority Health and Health Disparities (R01 MD012769). The funders had no role in the design of the study and collection, analysis, and interpretation of data, in writing the manuscript and in the decision to submit the article for publication.
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Francine Laden reports financial support was provided by National Institute of Environmental Health Sciences. Francesca Dominici reports financial support was provided by National Institute of Environmental Health Sciences. Antonella Zanobetti reports financial support was provided by National Institute of Environmental Health Sciences. Peter James reports financial support was provided by National Heart Lung and Blood Institute. Antonella Zanobetti reports financial support was provided by National Institute on Aging. Jaime Hart reports financial support was provided by National Institute of Environmental Health Sciences. Joel Schwartz reports financial support was provided by National Institute of Environmental Health Sciences
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2022.114684.
Ethics approval and consent to participate
This study was approved by the institutional review board at the Harvard T H Chan School of Public Health and was exempt from informed consent requirements as a study of previously collected administrative data.
Data availability
The data that has been used is confidential.
References
- Abatzoglou JT, 2013. Development of gridded surface meteorological data for ecological applications and modelling. Int. J. Climatol. 33 (1), 121–131. Jan 1. [Google Scholar]
- Armstrong B, Bell ML, Coelho M de SZS, Guo YLL, Guo Y, Goodman P, et al. , 2017. Longer-term impact of high and low temperature on mortality: an international study to clarify length of mortality displacement. Environ. Health Perspect. 125 (10). Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett AG, Tong S, Clements ACA, 2010. What measure of temperature is the best predictor of mortality? Environ. Res. 110 (6), 604–611. Aug 1. [DOI] [PubMed] [Google Scholar]
- Brewer LPC, Cooper LA, 2014. STATE of the ART and SCIENCE: Race, Discrimination, and Cardiovascular Disease. Virtual Mentor [Internet]. [PMC free article] [PubMed] [Google Scholar]
- Castellani JW, Young AJ, 2016. Human physiological responses to cold exposure: acute responses and acclimatization to prolonged exposure. Auton. Neurosci. 196, 63–74. Apr 1. [DOI] [PubMed] [Google Scholar]
- Chen R, Yin P, Wang L, Liu C, Niu Y, Wang W, et al. , 2018. Association between ambient temperature and mortality risk and burden: time series study in 272 main Chinese cities. BMJ 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C, Halbleib M, Smith JI, Gibson WP, Doggett MK, Taylor GH, et al. , 2008. Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. Int J Climatol Int J Clim 28 (15), 2031–2064. [Google Scholar]
- de Mestral C, Stringhini S, 2017. Socioeconomic status and cardiovascular disease: an update, 2017 1911 Curr. Cardiol. Rep. 19 (11), 1–12. Sep 30. [DOI] [PubMed] [Google Scholar]
- Dhillon S, 2012. Environmental hazards, hot, cold, altitude, and sun. Infect. Dis. Clin. 26 (3), 707–723. Sep 1. [DOI] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. , 2017. Air pollution and mortality in the Medicare population. N. Engl. J. Med. 376 (26), 2513–2522. Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. , 2019. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ. Int. 130, 104909. Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filice CE, Joynt KE, 2017. Examining race and ethnicity information in Medicare administrative data. Med. Care 55 (12), e170–e176. [DOI] [PubMed] [Google Scholar]
- Froehle AW, 2008. Climate variables as predictors of basal metabolic rate: New equations. Am. J. Hum. Biol. 20 (5), 510–529. Sep 1. [DOI] [PubMed] [Google Scholar]
- Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, et al. , 2015. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 386 (9991), 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggins WB, Yang C, Hokama T, Law LSK, Chan EYY, 2015. Using annual data to estimate the public health impact of extreme temperatures. Am. J. Epidemiol. 182 (1), 80–87. Jul 1. [DOI] [PubMed] [Google Scholar]
- Gorelick N, Hancher M, Dixon M, Ilyushchenko S, Thau D, Moore R, 2017. Google Earth engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 202, 18–27. Dec 1. [Google Scholar]
- IPCC. Global Warming of 1.5°C, 2018. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways. In: The Context of Strengthening the Global Response to the Threat of Climate Change [Internet]. Geneva, Switzerland. https://www.ipcc.ch/sr15/. [Google Scholar]
- Keatinge WR, Coleshaw SRK, Cotter F, Mattock M, Murphy M, Chelliah R, 1984. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. Br. Med. J. 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompmaker JO, Hart JE, James P, Sabath MB, Wu X, Zanobetti A, et al. , 2021. Air pollution and cardiovascular disease hospitalization – are associations modified by greenness, temperature and humidity? Environ. Int. 156, 106715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Lv J, Liu F, Liu P, Yang X, Feng Y, et al. , 2015. Hypertension burden and control in mainland China: analysis of nationwide data 2003–2012. Int. J. Cardiol. 184 (1), 637–644. [DOI] [PubMed] [Google Scholar]
- Metzger KB, Ito K, Matte TD, 2010. Summer heat and mortality in New York City: How hot is too hot? Environ. Health Perspect. 118 (1), 80–86. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KE, Lohmann D, Houser PR, Wood EF, Schaake JC, Robock A, et al. , 2004. The multi-institution North American Land Data Assimilation System (NLDAS): utilizing multiple GCIP products and partners in a continental distributed hydrological. J. Geophys. Res. Atmos. 109 (D7). [Google Scholar]
- Moghadamnia MT, Ardalan A, Mesdaghinia A, Keshtkar A, Naddafi K, Yekaninejad MS, 2017. Ambient temperature and cardiovascular mortality: a systematic review and meta-analysis. PeerJ 2017 (8), 3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufcourt L, Deguen S, Bayat S, Paillard F, Zins M, Grimaud O, 2019. Geographical variations in the prevalence of hypertension in France: Cross-sectional analysis of the CONSTANCES cohort. Eur J Prev Cardiol 26 (12), 1242–1251. [DOI] [PubMed] [Google Scholar]
- Nguyen JL, Dockery DW, 2016. Daily indoor-to-outdoor temperature and humidity relationships: a sample across seasons and diverse climatic regions. Int. J. Biometeorol. 60 (2), 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JL, Schwartz J, Dockery DW, 2014. The relationship between indoor and outdoor temperature, apparent temperature, relative humidity, and absolute humidity. Indoor Air 24 (1), 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périard JD, Travers GJS, Racinais S, Sawka MN, 2016. Cardiovascular adaptations supporting human exercise-heat acclimation. Auton. Neurosci. 196, 52–62. [DOI] [PubMed] [Google Scholar]
- Rehill N, Armstrong B, Wilkinson P, 2015a. Clarifying life lost due to cold and heat: a new approach using annual time series. BMJ Open 5 (4), e005640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehill N, Armstrong B, Wilkinson P, 2015b. Clarifying life lost due to cold and heat: a new approach using annual time series. BMJ Open 5, 5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requia WJ, Di Q, Silvern RF, Kelly JT, Koutrakis P, Mickley LJ, et al. , 2020. An ensemble learning approach for estimating high spatiotemporal resolution of ground-level ozone in the contiguous United States. Environ. Sci. Technol. 54, 11037–11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Samet JM, Patz JA, 2004. Hospital admissions for heart disease. Epidemiology 15 (6), 755–761. [DOI] [PubMed] [Google Scholar]
- Shi L, Kloog I, Zanobetti A, Liu P, Schwartz JD, 2015. Impacts of temperature and its variability on mortality in New England. Nat. Clim. Change 5 (11), 988–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Liu P, Wang Y, Zanobetti A, Kosheleva A, Koutrakis P, et al. , 2016. Chronic effects of temperature on mortality in the Southeastern USA using satellite-based exposure metrics. Sci. Rep. 6 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Wu X, Danesh Yazdi M, Braun D, Abu Awad Y, Wei Y, et al. , 2020. Long-term effects of PM2·5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet. Health 4 (12), e557–e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamerius JD, Perzanowski MS, Acosta LM, Jacobson JS, Goldstein IF, Quinn JW, et al. , 2013. Socioeconomic and outdoor meteorological determinants of indoor temperature and humidity in New York City dwellings. Weather Clim Soc 5 (2), 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey EA, Johnson CD, 2015. Recent advances in thermoregulation. Adv. Physiol. Educ. 39 (1), 139–148. [DOI] [PubMed] [Google Scholar]
- Woodhouse PR, Khaw KT, Plummer M, 1993. Seasonal variation of blood pressure and its relationship to ambient temperature in an elderly population. J. Hypertens. 11 (11), 1267–1274. [PubMed] [Google Scholar]
- Woodhouse P, Khaw K, Plummer M, Meade TW, Foley A, 1994. Seasonal variations of plasma fibrinogen and factor VII activity in the elderly: winter infections and death from cardiovascular disease. Lancet 343 (8895), 435–439. [DOI] [PubMed] [Google Scholar]
- Ye X, Wolff R, Yu W, Vaneckova P, Pan X, Tong S, 2012. Ambient temperature and morbidity: a review of epidemiological evidence. Environ. Health Perspect. 120 (1), 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Mengersen K, Wang X, Ye X, Guo Y, Pan X, et al. , 2012. Daily average temperature and mortality among the elderly: a meta-analysis and systematic review of epidemiological evidence. Int. J. Biometeorol. 56 (4), 569–581. [DOI] [PubMed] [Google Scholar]
- Zafeiratou S, Samoli E, Dimakopoulou K, Rodopoulou S, Analitis A, Gasparrini A, et al. , 2021. A systematic review on the association between total and cardiopulmonary mortality/morbidity or cardiovascular risk factors with long-term exposure to increased or decreased ambient temperature. Sci. Total Environ., 145383. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, O’Neill MS, 2018. Longer-term outdoor temperatures and health effects: a review. Curr Epidemiol Reports 5 (2), 125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, O’Neill MS, Gronlund CJ, Schwartz JD, 2012. Summer temperature variability and long-term survival among elderly people with chronic disease. Proc. Natl. Acad. Sci. U. S. A. 109 (17), 6608–6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.


