Abstract
Background
Oral anticoagulation (OAC) has been considered the standard of care for stroke prophylaxis for patients with nonvalvular atrial fibrillation; however, many individuals are unable or unwilling to take long-term OAC. The safety and efficacy of percutaneous left atrial appendage closure (LAAC) have been controversial, and new trial data have recently emerged. We therefore sought to perform an updated meta-analysis of randomized clinical trials (RCTs) comparing OAC to percutaneous LAAC, focusing on individual clinical endpoints.
Methods
We performed a systematic search of the MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials from January 2000 through December 2021 for all RCTs comparing percutaneous LAAC to OAC in patients with nonvalvular atrial fibrillation. Fixed and random effects meta-analyses of hazard ratios (HRs) were performed using the longest follow-up duration available by intention-to-treat. The prespecified primary endpoint was all-cause mortality.
Results
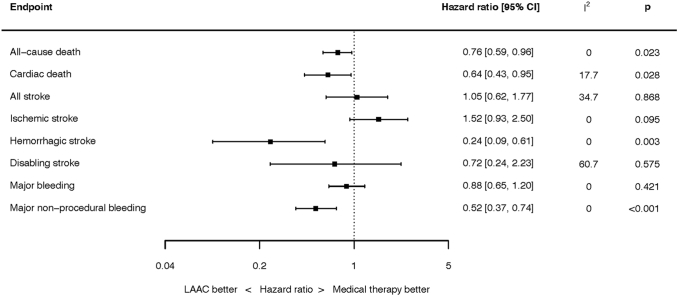
Three RCTs enrolling 1516 patients were identified. The weighted mean follow-up was 54.7 months. LAAC was associated with a reduced risk of all-cause mortality (HR 0.76; 95% confidence interval [CI], 0.59-0.96; p = 0.023), hemorrhagic stroke (HR 0.24; 95% CI, 0.09-0.61; p = 0.003), and major nonprocedural bleeding (HR 0.52; 95% CI, 0.37-0.74; p < 0.001). There was no significant difference between LAAC and OAC for any other endpoints.
Conclusions
The available evidence from RCTs suggests LAAC therapy is associated with reduced long-term risk of death compared with OAC. This may be driven by reductions in hemorrhagic stroke and major nonprocedural bleeding. There were no significant differences in the risk of all stroke. Further large-scale clinical trials are needed to validate these findings.
Keywords: Atrial fibrillation, Left atrial appendage closure, Meta-analysis, Stroke
Graphical abstract
Summary of clinical outcomes.
Introduction
Stroke prophylaxis is the most important consideration in the management of patients with nonvalvular atrial fibrillation (NVAF). Oral anticoagulation (OAC) has traditionally been the standard of care, but many patients are unable or unwilling to take long-term OAC, even with the advent of direct oral anticoagulants (DOACs), which offer several potential benefits over vitamin K antagonists (VKAs).1 Percutaneous left atrial appendage closure (LAAC) emerged as an alternative to OAC and was initially compared with VKAs in randomized clinical trials (RCTs).2, 3, 4, 5, 6 The safety and efficacy of percutaneous LAAC have remained controversial, however, and the number of randomized LAAC trials is limited. With the emergence of new trial data (including the first randomized comparison of LAAC to DOACs7,8), there are now long-term follow-up data from 3 RCTs available. We therefore sought to undertake a meta-analysis of the totality of randomized data focusing on the long-term outcomes of individual clinical endpoints rather than composite outcome measures, adding the long-term results of Left Atrial Appendage Closure vs. Novel Anticoagulation Agents in Atrial Fibrillation (PRAGUE-17) to previously published pooled data from WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation (PROTECT-AF) and Prospective Randomized Evaluation of the Watchman LAA Closure Device In Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy (PREVAIL)6 using appropriate statistical methodology.
Methods
This analysis was conducted in accordance with the published PRISMA guidance9 and was also prospectively registered with the PROSPERO international prospective register of systematic reviews (CRD42020201642). This is a study-level meta-analysis of published data and so ethical approval was not required. YA had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Search Strategy
Two independent authors performed the search (MM and YA) with any disputes resolved by consensus. We performed a systematic search of the MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials from January 2000 through December 2021 for all randomized clinical trials (RCTs) comparing percutaneous LAAC to OAC in patients with NVAF. Our search strings are shown in the Supplemental Table 1. Bibliographies of selected studies and meta-analyses were hand-searched to identify further eligible trials. Conference abstracts from AHA, ACC, ESC, TCT, HRS, EHRA, and EuroPCR were also searched for eligible studies.
Inclusion and Exclusion Criteria
We considered all RCTs comparing LAAC to OAC, including both VKA and DOAC. For inclusion, the trials had to report clinical outcomes after randomization to LAAC or OAC. No observational studies or studies of surgical left atrial appendage occlusion were considered.
Endpoints
The prespecified primary endpoint was all-cause mortality. Additional endpoints were cardiac death, ischemic stroke, hemorrhagic stroke, all stroke, disabling stroke, major bleeding, and nonprocedural major bleeding. Disabling stroke was defined as strokes with a modified Rankin score of greater than 2 after the stroke, or a fatal stroke. Cardiac death was defined as cardiovascular or unexplained death as defined and adjudicated in each individual trial. In PRAGUE-17, major bleeding was defined according to the International Society on Thrombosis and Hemostasis criteria, as clinically overt bleeding accompanied by one or more of the following: a decrease in hemoglobin level ≥2 g/l over a 24-hour period, transfusion of 2 or more units of packed red cells, bleeding at a critical site (intracranial, intraspinal, intraocular, pericardial, intramuscular with compartment syndrome, or retroperitoneal), or fatal bleeding. In PROTECT-AF and PREVAIL, major bleeding was defined as a bleeding event that required at least 2 units of packed red blood cells or surgery to correct.
Data Extraction and Analysis
Three authors (M.B., C.d.N., Y.A.) independently extracted the data. Any disputes were resolved by consensus. To account for time-to-event data and for differing follow-up durations between included trials, the principal investigators of each individual trial were contacted and the results for each individual trial were provided as hazard ratios (HRs) with respective 95% confidence intervals (CIs). A random-effects meta-analysis was performed of the natural logarithm of the HRs and their associated standard errors using the restricted maximum likelihood estimator. The standard error was calculated by dividing the difference between the natural logarithms of the upper and lower 95% CIs by 2× the appropriate normal score (1.96). Where the lower 95% CI approached zero, the standard error was calculated using only the difference between the natural logarithm of the upper 95% CI and the natural logarithm of the point estimate. All outcomes were assessed by the intention-to-treat principle. The latest follow-up data available were used for all trials. The I2 statistic was used to assess heterogeneity.10 Fixed effect analyses were performed as a sensitivity analysis.
Subgroup analyses were performed for sex, age ≥75 years, diabetes, prior stroke, renal dysfunction (estimated glomerular filtration rate ≤60 mL/min/1.73 m2 using the Modification of Diet in Renal Disease formula), CHA2DS2-VASc11 score >4, and HAS-BLED12 score >2. Interactions between subgroups were assessed using a mixed effects meta-analytical model, with both the trial and subgroup as moderators.
Included trials were assessed using the Cochrane risk of bias tool.13 Tests for publication bias would only be performed in the event of at least 10 trials being included for analysis.14 Mean values are expressed as mean ± SD unless otherwise stated. Statistical significance was set at p < 0.05. The statistical programming environment R15 with the metafor package16 was used for all statistical analysis.
Results
Three trials3,4,7 randomizing 1516 patients were eligible; 933 patients were randomized to LAAC and 583 patients to OAC. The source of the included trials is shown in Supplemental Figure 1. The characteristics of the included trials are shown in Supplemental Table 2. The risk of bias assessment is shown in Supplemental Table 3. The PRISMA checklist is shown in Supplemental Table 4. In 2 trials (PROTECT-AF and PREVAIL), the OAC used was VKA, and in one trial (PRAGUE-17), the OAC used was DOAC. In 2 trials, the LAAC device used was the Watchman, while in the PRAGUE-17 trial, 61.3% of LAAC patients received the Amulet device, 35.9% received the Watchman, and 2.8% received the Watchman-FLX. In PROTECT-AF and PREVAIL, postprocedure antithrombotic therapy was VKA for 45 days followed by dual antiplatelet therapy with aspirin and clopidogrel for 6 months and then aspirin monotherapy long-term. In PRAGUE-17, the postprocedure antithrombotic therapy was left to physician discretion, with most (81.8%) receiving dual antiplatelet therapy. The weighted mean follow-up duration was 54.7 months, ranging from 40 months in 1 trial to 60 months in 2 trials.
Mortality
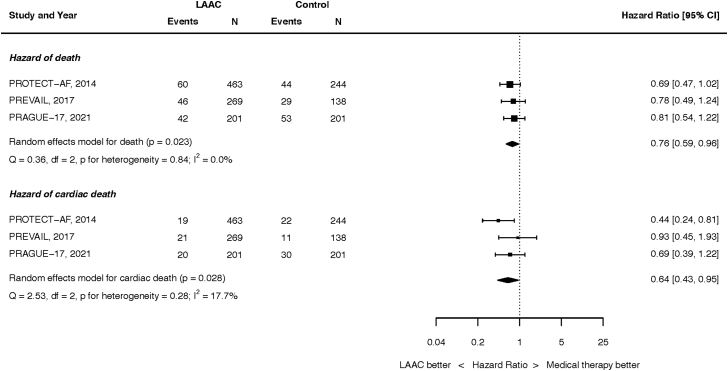
Mortality outcomes are summarized in Figure 1. At the longest follow-up, 148 of the 933 patients randomized to LAAC and 126 of the 583 patients randomized to OAC died. LAAC was associated with a lower hazard of all-cause mortality (HR, 0.76; 95% CI, 0.59-0.96; p = 0.023). There was no heterogeneity between studies (I2 = 0.0%). This result was unchanged in a fixed effect analysis (Supplemental Figure 2).
Figure 1.
Hazard of death. Top panel: all-cause. Bottom panel: cardiac death.
Abbreviations: CI, confidence intervals; LAAC, left atrial appendage closure.
At the longest follow-up, cardiac death occurred in 60 of the 933 patients randomized to LAAC and 63 of the 583 patients randomized to OAC (HR, 0.64; 95% CI, 0.43-0.95; p = 0.028). There was low heterogeneity (I2 = 17.7%). This result was unchanged in a fixed effect analysis (Supplemental Figure 3).
Stroke
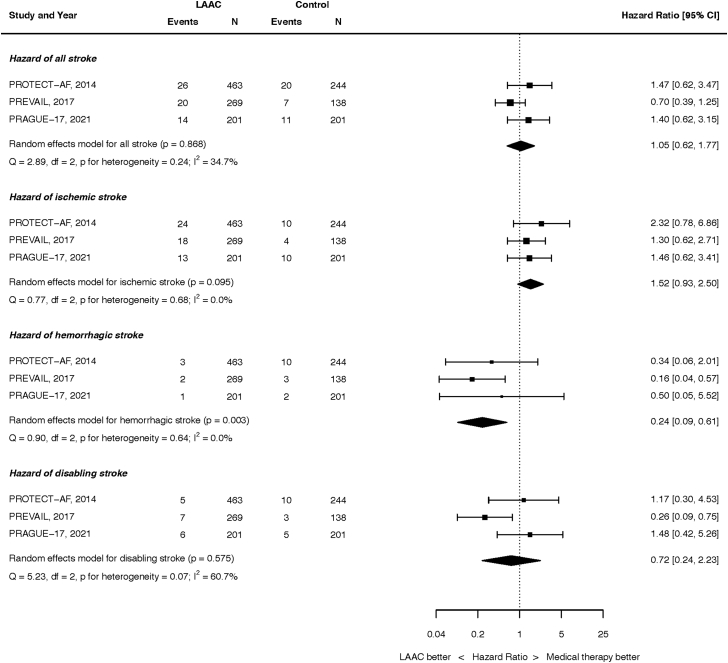
Stroke outcomes are summarized in Figure 2. At the longest follow-up, 60 of the 933 patients randomized to LAAC and 38 of the 583 patients randomized to OAC had experienced a stroke (HR, 1.05; 95% CI, 0.62-1.77; p = 0.868). There was moderate heterogeneity (I2 = 34.7%). This result was unchanged when analyzed by fixed effect (Supplemental Figure 4).
Figure 2.
Hazard of stroke. Top panel: all stroke. Second panel: ischemic stroke. Third panel: hemorrhagic stroke. Bottom panel: disabling stroke.
Abbreviations: CI, confidence intervals; LAAC, left atrial appendage closure.
At the longest follow-up, 55 of the 933 patients randomized to LAAC and 24 of the 583 patients randomized to OAC had an ischemic stroke (HR, 1.52; 95% CI, 0.93-2.50; p = 0.095). There was no heterogeneity (I2 = 0.0%). This result was unchanged when analyzed by fixed effect (Supplemental Figure 5).
At the longest follow-up, 6 of the 933 patients randomized to LAAC and 15 of the 583 patients randomized to OAC had a hemorrhagic stroke (HR, 0.24; 95% CI, 0.09-0.61; p = 0.003). There was no heterogeneity (I2 = 0.0%). This result was unchanged when analyzed by fixed effect (Supplemental Figure 6).
At the longest follow-up, 18 of the 933 patients randomized to LAAC and 18 of the 583 patients randomized to OAC had a disabling or fatal stroke (HR, 0.72; 95% CI, 0.24-2.23; p = 0.575). There was moderate heterogeneity (I2 = 60.7%). This result was unchanged when analyzed by fixed effect (Supplemental Figure 7).
Bleeding
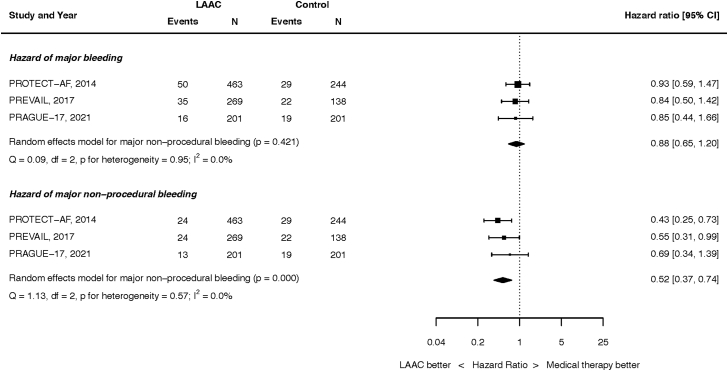
Bleeding outcomes are summarized in Figure 3. At the longest follow-up, 101 of the 933 patients randomized to LAAC and 70 of the 583 patients randomized to OAC had suffered major bleeding (HR, 0.88; 95% CI, 0.65-1.20; p = 0.421). There was no heterogeneity (I2 = 0.0%). This result was unchanged when analyzed by fixed effect (Supplemental Figure 8).
Figure 3.
Hazard of bleeding outcomes. Top panel: all major bleeding. Bottom panel: nonprocedural major bleeding.
Abbreviations: CI, confidence intervals; LAAC, left atrial appendage closure.
At the longest follow-up, 61 of the 933 patients randomized to LAAC and 70 of the 583 patients randomized to OAC had nonprocedural major bleeding (HR, 0.52; 95% CI, 0.37-0.74; p < 0.001). There was no heterogeneity (I2 = 0.0%). This result was unchanged when analyzed by fixed effect (Supplemental Figure 9).
Procedure-Related Adverse Events
In total, 25 of the 933 patients (2.7%) randomized to LAAC had a pericardial effusion, and 6 patients (0.6%) had device embolization. Procedure-related strokes occurred in 6 patients (0.6%) randomized to LAAC. Overall procedure-related complications (including pericardial effusion, device embolization, stroke, vascular complications, and major bleeding) occurred in 64 (6.8%) patients.
Subgroup Analyses
There were no significant interactions between treatment arm and any of the subgroups tested (sex, age ≥75 years, diabetes, prior stroke, renal dysfunction, CHA2DS2-VASc score >4, and HAS-BLED score >2) on the hazard of all-cause mortality, ischemic stroke, or hemorrhagic stroke (P=NS for all subgroups and all outcomes, see Supplemental Tables 5-7).
Discussion
Approximately 40,000 patients have been treated with LAAC within the first 3 years of the commercial availability of the Watchman device in the United States, with increasing year-over-year usage.17 Characterizing the relative risk and effectiveness of LAAC compared with OAC is therefore of paramount importance. The present analysis is, to our knowledge, the most comprehensive synthesis of the available RCT evidence-base for LAAC treatment of NVAF to date, and is the first analysis to include the long-term follow-up of the PRAGUE-17 trial. As summarized in the Graphical Abstract, the principal findings of this study are 1) compared to patients randomized to OAC, patients randomized to LAAC had a 24% lower risk of all-cause mortality; 2) the risk of hemorrhagic stroke was 76% lower with LAAC; 3) the risk of nonprocedural major bleeding was 48% lower with LAAC; 4) there were no significant differences in the risks of all stroke, ischemic stroke, or overall major bleeding; and 5) there was minimal or no heterogeneity between studies for these endpoints, nor among subgroups examined. The statistically significant survival benefit at a weighted mean follow-up of 55 months is of relevance for patients and clinicians when evaluating potential candidacy for LAAC. It may be that further benefits of LAAC accrue over time with even longer-term follow-up, since the procedural risks of LAAC are up-front but the hemorrhagic risks of OAC persist lifelong.
The present study is distinct from prior meta-analytic reports of LAAC in several ways.18 First, the latest follow-up data from all 3 available RCTs have been included; this is the first analysis to include the long-term follow-up data from PRAGUE-17, and therefore is the only analysis to capture the totality of the randomized data with increased accrual of events and provides a true summary of the long-term clinical impact of LAAC compared to OAC. Second, we were able to perform a meta-analysis of HRs, as all PIs of included trials contributed data in the form of HRs and their 95% CIs. This is distinct from prior meta-analyses, and HRs are the most appropriate methodology for analyzing time-to-event data, taking into account the varying follow-up durations of included trials and individual patients within those trials. Furthermore, data on individual endpoints were collected from principal investigators and sponsors of respective trials, allowing for inclusion and presentation of endpoints which were not previously assessed across studies. This includes data on disabling strokes, which has not been previously reported and shows a trend to benefit with LAAC. This is consistent with some other observational data that LAAC reduces stroke severity compared to OAC.19, 20, 21 Potential mechanisms for this include a reduction in hemorrhagic stroke, which is frequently disabling or fatal, and potentially a reduction in the size of thrombi causing ischemic stroke after LAAC. These findings warrant further study. Our analysis also includes detailed subgroup analyses across included trials, which have not been collectively reported previously, and are included in this report.
The recent Left Atrial Appendage Occlusion Study (LAAOS) III trial22 highlighted the importance of the left atrial appendage as a source of stroke in patients with AF. In this trial, patients who were scheduled to undergo cardiac surgery for another indication who also had AF were randomized to undergo or not undergo left atrial appendage occlusion at the time of surgery. The primary outcome was the occurrence of stroke or systemic embolism, and this was significantly reduced in the occlusion group (HR, 0.67; 95% CI, 0.53-0.85; p = 0.001). This finding was despite the fact that at 3 years 76.8% of patients were on OAC, and provides evidence that the benefit of left atrial appendage occlusion is additive to OAC. Whether this benefit would also extend to percutaneous LAAC remains to be seen, and this is a hypothesis that would need to be tested in a randomized trial of percutaneous LAAC vs. no LAAC in patients continuing on background OAC.
The reduction in all-cause mortality noted in the present analysis is broadly consistent with a recent propensity-matched analysis of over 2000 patients,23 and another propensity-matched analysis of 1000 patients comparing LAAC with Amplatzer occluders to OAC.24 One potential mechanism for the mortality benefit of LAAC compared with OAC is reductions in hemorrhagic stroke (HR, 0.24 in this study), an event that is known to be fatal in over 50% of occurrences.25, 26, 27 This reduction in mortality seen in association with reductions in hemorrhagic stroke has also been identified in DOAC trials where a mortality benefit of DOACs over VKAs was observed and was driven by a reduced risk of hemorrhagic stroke.28 Other potential mechanisms underpinning this mortality reduction are reduced nonprocedural major bleeding, which has been consistently shown to be associated with increased mortality in cardiovascular trials,29, 30, 31 either from direct effects of hemorrhage, adverse impact of blood product transfusion, or potentially from cessation of cardioprotective antithrombotic therapies (which could be the result of either patient or physician driven factors).
These major findings were consistent across included trials, with minimal or no heterogeneity present between studies. However, heterogeneity testing may be underpowered, and LAAC was compared to DOACs in only one of the 3 trials in the present meta-analysis. Additional large-scale trials of LAAC vs. DOACs are necessary to address the relative risks and benefits of these alternatives.
Although nonprocedural major bleeding was significantly reduced with LAAC, due to procedural complications, the overall rates of major bleeding were not. Major procedural complications after LAAC were infrequent with only 2.7% of patients suffering a pericardial effusion requiring intervention. The majority of the procedural complications were observed in the earlier PROTECT-AF trial (utilizing the legacy WATCHMAN device), which is consistent with a learning curve phenomenon that has been reported in some observational studies.32 In this regard, a recent large-scale US registry including 38,158 patients demonstrated reassuringly low overall complication (2.16%) and pericardial tamponade (1.39%) rates, as well as high procedural success (98.3%), despite including lower-volume centers and operators.17
Alongside increasing operator experience, there have also been technical advancements in device design. The WATCHMAN FLX device is a next-generation LAAC device with a closed distal end, designed to be atraumatic to the appendage. The Protection Against Embolism for Nonvalvular AF Patients: Investigational Device Evaluation of the Watchman FLX LAA Closure Technology (PINNACLE-FLX) study using this device demonstrated no procedural pericardial effusions and no device embolizations across the 400 studied patients; implantation success was also 98.8%.33 Newer randomized trials will clarify whether this procedural advancement will translate into improved clinical outcomes, and will be inherently more generalizable to contemporary clinical practice.
There were numerically more ischemic strokes with LAAC compared to OAC in this analysis (albeit not reaching statistical significance), which may in part be related to air embolization at the time of implant, incomplete occlusion of the appendage, or device-related thrombosis. Further studies are required to determine whether device enhancements have improved the effectiveness of LAAC and its safety, and whether adjustments to postprocedure antithrombotic regimens can have an impact on reducing device-related thrombosis.
Current American College of Cardiology/American Heart Association/Heart Rhythm Society and European Society of Cardiology guidelines34,35 provide LAAC a Class IIb, Level of Evidence B recommendation, and specify that LAAC should be considered in patients with contraindications to long-term OAC (rather than specifically in the populations studied in the foundational trials). The present meta-analysis should further inform these recommendations. In addition, several ongoing or planned RCTs will inform the relative safety and effectiveness of LAAC in patients with NVAF compared with DOACs or alternative therapies. The WATCHMAN FLX Versus NOAC for Embolic ProtectION in in the Management of Patients With Non-Valvular Atrial Fibrillation (CHAMPION-AF) trial (NCT04394546) is randomizing 3000 patients at high risk for stroke to the newer WATCHMAN FLX device vs. DOAC. Similarly, the Clinical Trial of Atrial Fibrillation Patients Comparing Left Atrial Appendage Occlusion Therapy to Non-vitamin K Antagonist Oral Anticoagulants (CATALYST) trial (NCT04226547) is randomizing the Amulet LAAC device vs. DOACs in 2650 NVAF patients. The Left Atrial Appendage CLOSURE in Patients With Atrial Fibrillation at High Risk of Stroke and Bleeding Compared to Medical Therapy: a Prospective Randomized Clinical Trial (CLOSURE-AF) trial (NCT03463317) is randomizing 1512 NVAF patients at high risk of bleeding to either Watchman or physician-directed best medical therapy. The Assessment of the WATCHMAN™ Device in Patients Unsuitable for Oral Anticoagulation (ASAP-TOO) trial (NCT02928497) was planned to randomize 482 NVAF patients deemed ineligible for OAC to LAAC with Watchman or single antiplatelet therapy (but this trial has now been closed for low enrollment). The Prevention of Stroke by Left Atrial Appendage Closure in Atrial Fibrillation Patients After Intracerebral Hemorrhage: A Multicenter Randomized Clinical Trial (STROKECLOSE) trial (NCT02830152) is randomizing 750 AF patients after intracranial hemorrhage to either Amulet or physician-directed best medical therapy. Until the results of these trials become available, the present meta-analysis represents the most up to date and comprehensive summary of the evidence for LAAC as an alternative to OAC in NVAF patients.
Limitations
This was a study-level meta-analysis with the attendant limitations of the included trials. The OAC in the control arm was VKAs in approximately two-thirds of patients and DOACs in the remainder. DOAC use, rather than VKA, is more reflective of current clinical practice. The postprocedure antithrombotic regimens in the LAAC groups were not uniform across trials, and the definitions of clinical outcomes varied slightly between studies. Despite this, between-study heterogeneity was low or absent for most analyses. Our primary endpoint was all-cause mortality which of all endpoints is the least prone to issues with definitions, bias, or adjudication, and there was no heterogeneity between studies for this outcome. Lack of access to individual patient level data limited the detail of the subgroup analyses and the ability to examine temporal relationships of outcomes. Finally, to avoid bias from measured and unmeasured confounders we limited our study to RCTs which limits generalizability.
Conclusions
The available data from 3 RCTs with long-term follow-up (weighted mean follow-up duration of 55 months) suggest that LAAC is associated with a reduced hazard of death compared with OAC, a difference apparently driven by substantial reductions in hemorrhagic stroke and nonprocedural major bleeding. There were no significant differences in the hazard of ischemic stroke or all-stroke between groups. Further large-scale RCTs are needed to validate these findings (especially compared with DOACs), and to evaluate the role of LAAC in comparison to other control groups.
Ethics Statement
This was a study-level meta-analysis and so ethical approval was not required. The study adhered to all ethical guidelines.
Funding
The authors have no funding to report.
Disclosure statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr Madhavan was supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute to Columbia University Irving Medical Center (T32 HL007854). Dr Makkar has received research grants from Edwards Lifesciences, Abbott, Medtronic, and Boston Scientific; has served as national Principal Investigator for Portico (Abbott) and Acurate (Boston Scientific) U.S. investigation device exemption trials; has received personal proctoring fees from Edwards Lifesciences; and has received travel support from Edwards Lifesciences, Abbott, and Boston Scientific. Dr Osmancik has received occasional speaking honoraria from Bayer and Abbott. Dr Reddy has received grant support from Abbott Inc and Boston Scientific Inc; has consulted for Ablacon, Acutus Medical, Affera, Apama Medical, Aquaheart, Atacor, Autonomix, Axon, Backbeat, BioSig, Biosense-Webster, Biostar Ventures, Biotronik, Cardiofocus, Cardionomic, CardioNXT/AFTx, Circa Scientific, Corvia Medical, Dinova-Hangzhou Nuomao Medtech Co, Ltd, East End Medical, EBR Systems Inc, EPD Solutions, Epix Therapeutics, EpiEP, Eximo, Farapulse, Fire1, Impulse Dynamics, Intershunt, Javelin, Kardium, Keystone Heart, LuxMed, Medlumics, Medtronic, Middlepeak, Nuvera, Philips, Pulse Biosciences, Sirona Medical, Stimda, Thermedical, and Valcare; and owns equity in Ablacon, Acutus Medical, Affera, Apama Medical, Aquaheart, Atacor, Autonomix, Backbeat, BioSig, Biostar Ventures, Circa Scientific, Corvia Medical, Dinova-Hangzhou Nuomao Medtech Co, Ltd, East End Medical, EPD, Epix Therapeutics, EpiEP, Eximo, Farapulse, Fire1, Intershunt, Javelin, Kardium, Keystone Heart, LuxMed, Manual Surgical Sciences, Medlumics, Middlepeak, Newpace, Nuvera, Sirona Medical, Surecor, Valcare, and Vizaramed. Dr Stone has received speaker honoraria from Cook and Terumo; has served as a consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, and Matrizyme; and owns equity/options in Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, the Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, the MedFocus family of funds, and Valfix. Dr Leon has received research support to his institution from Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott; has served on Advisory Boards for Medtronic, Boston Scientific, Gore, Meril Lifescience, and Abbott; and has served as the Co-Principal Investigator of the PARTNER 3 trial (Edwards Lifesciences, no direct compensation). The other authors had no conflicts to declare.
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
Supplementary Material
References
- 1.Salmasi S., Loewen P.S., Tandun R., Andrade J.G., Vera M.A.D. Adherence to oral anticoagulants among patients with atrial fibrillation: a systematic review and meta-analysis of observational studies. BMJ Open. 2020;10(4) doi: 10.1136/bmjopen-2019-034778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes D.R., Reddy V.Y., Turi Z.G., et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 3.Reddy V.Y., Sievert H., Halperin J., et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312(19):1988. doi: 10.1001/jama.2014.15192. [DOI] [PubMed] [Google Scholar]
- 4.Holmes D.R., Kar S., Price M.J., et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy. J Am Coll Cardiol. 2014;64(1):1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Reddy V.Y., Doshi S.K., Kar S., et al. 5-Year outcomes after left atrial appendage closure. J Am Coll Cardiol. 2017;70(24):2964–2975. doi: 10.1016/j.jacc.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Holmes D.R., Doshi S.K., Kar S., et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2015;65(24):2614–2623. doi: 10.1016/j.jacc.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Osmancik P., Herman D., Neuzil P., et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol. 2020;75(25):3122–3135. doi: 10.1016/j.jacc.2020.04.067. [DOI] [PubMed] [Google Scholar]
- 8.Osmancik P., Herman D., Neuzil P., et al. Left atrial appendage closure versus non-warfarin oral anticoagulation in atrial fibrillation: 4-year outcomes of PRAGUE-17. J Am Coll Cardiol. 2021 doi: 10.1016/j.jacc.2021.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 10.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 11.Lip G.Y.H., Halperin J.L. Improving stroke risk stratification in atrial fibrillation. Am J Med. 2010;123(6):484–488. doi: 10.1016/j.amjmed.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Pisters R., Lane D.A., Nieuwlaat R., de Vos C.B., Crijns H.J.G.M., Lip G.Y.H. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J.P.T., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Cochrane Collarobation . The Cochrane Collaboration; 2011. Cochrane Handbook for Systematic Reviews of Interventions - 10.4.3.1 Recommendations on Testing for Funnel Plot Asymmetry. [Google Scholar]
- 15.R Core Team . R Foundation for Statistical Computing; 2016. R: A Language and Environment for Statistical Computing [Internet] [Google Scholar]
- 16.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 17.Freeman J.V., Varosy P., Price M.J., et al. The NCDR left atrial appendage occlusion registry. J Am Coll Cardiol. 2020;75(13):1503–1518. doi: 10.1016/j.jacc.2019.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turagam M.K., Osmancik P., Neuzil P., Dukkipati S.R., Reddy V.Y. Left atrial appendage closure versus oral anticoagulants in atrial fibrillation. J Am Coll Cardiol. 2020;76(23):2795–2797. doi: 10.1016/j.jacc.2020.08.089. [DOI] [PubMed] [Google Scholar]
- 19.Lee O.H., Kim Y.D., Kim J.S., et al. Percutaneous left atrial appendage occlusion yields favorable neurological outcomes in patients with non-valvular atrial fibrillation. Korean Circ J. 2021;51(7):626–638. doi: 10.4070/kcj.2020.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freixa X., Llull L., Gafoor S., et al. Characterization of cerebrovascular events after left atrial appendage occlusion. Am J Cardiol. 2016;118(12):1836–1841. doi: 10.1016/j.amjcard.2016.08.075. [DOI] [PubMed] [Google Scholar]
- 21.Lee O.H., Kim Y.D., Kim J.S., et al. Favorable neurological outcome after ischemic cerebrovascular events in patients treated with percutaneous left atrial appendage occlusion compared with warfarin. Catheter Cardiovasc Interv. 2019;94(1):E23–E29. doi: 10.1002/ccd.27913. [DOI] [PubMed] [Google Scholar]
- 22.Whitlock R.P., Belley-Cote E.P., Paparella D., et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med. 2021;384(22):2081–2091. doi: 10.1056/NEJMoa2101897. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen-Kudsk J.E., Korsholm K., Damgaard D., et al. Clinical outcomes associated with left atrial appendage occlusion versus direct oral anticoagulation in atrial fibrillation. JACC Cardiovasc Interv. 2021;14(1):69–78. doi: 10.1016/j.jcin.2020.09.051. [DOI] [PubMed] [Google Scholar]
- 24.Gloekler S., Fürholz M., de Marchi S., et al. Left atrial appendage closure versus medical therapy in patients with atrial fibrillation: the APPLY study. EuroIntervention. 2020;16(9):e767–774. doi: 10.4244/EIJ-D-20-00201. [DOI] [PubMed] [Google Scholar]
- 25.Flaherty M.L., Woo D., Haverbusch M., et al. Racial variations in location and risk of intracerebral hemorrhage. Stroke. 2005;36(5):934–937. doi: 10.1161/01.STR.0000160756.72109.95. [DOI] [PubMed] [Google Scholar]
- 26.Kojic B., Burina A., Hodzic R., Pasic Z., Sinanovic O. Risk factors impact on the long-term survival after hemorrhagic stroke. Med Arh. 2009;63(4):203–206. [PubMed] [Google Scholar]
- 27.Stefan S., Bo N., Jesper P., Teresa U. Long-term survival and function after stroke. Stroke. 2019;50(1):53–61. doi: 10.1161/STROKEAHA.118.022913. [DOI] [PubMed] [Google Scholar]
- 28.Ruff C.T., Giugliano R.P., Braunwald E., et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 29.Marquis-Gravel G., Dalgaard F., Jones A.D., et al. Post-discharge bleeding and mortality following acute coronary syndromes with or without PCI. J Am Coll Cardiol. 2020;76(2):162–171. doi: 10.1016/j.jacc.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 30.Mehran R., Pocock S., Nikolsky E., et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011;4(6):654–664. doi: 10.1016/j.jcin.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 31.McClure J.D., Ramsay J.C., Berry C. Pooled analysis of bleeding, major adverse cardiovascular events, and all-cause mortality in clinical trials of time-constrained dual-antiplatelet therapy after percutaneous coronary intervention. J Am Heart Assoc. 2020;9(16) doi: 10.1161/JAHA.120.017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boersma L.V.A., Schmidt B., Betts T.R., et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37(31):2465–2474. doi: 10.1093/eurheartj/ehv730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kar S., Doshi S.K., Sadhu A., et al. Primary outcome evaluation of a next generation left atrial appendage closure device: results from the PINNACLE FLX trial. Circulation. 2021;143(18):1754–1762. doi: 10.1161/CIRCULATIONAHA.120.050117. [DOI] [PubMed] [Google Scholar]
- 34.Kirchhof P., Benussi S., Kotecha D., et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 35.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.