Abstract
Background
Optimal timing for intervention remains uncertain in asymptomatic patients with primary mitral regurgitation (MR). We aimed to assess the prognostic value of a new cardiac damage staging classification in patients with asymptomatic moderate or severe primary MR.
Methods
Clinical, Doppler-echocardiographic, and outcome data prospectively collected in 338 asymptomatic patients (64 ± 15 years, 68% men) with at least moderate primary MR were retrospectively analyzed. Patients were hierarchically classified as per the following staging classification: no cardiac damage (stage 0), mild left ventricular or left atrial damage (stage 1), moderate or severe left ventricular or left atrial damage (stage 2), pulmonary vasculature or tricuspid valve damage (stage 3), or right ventricular damage (stage 4).
Results
There was a stepwise increase in 10-year mortality rates as per cardiac damage stage: 20.0% in stage 0, 25.6% in stage 1, 31.5% in stage 2, and 61.3% in stage 3-4 (p < 0.001). The staging classification was significantly associated with increased risk of mortality (hazard ratio = 1.41 per one-stage increase, 95% confidence interval: 1.07-1.85, p = 0.015) and the composite of cardiovascular mortality or hospitalization (hazard ratio = 1.51 per one-stage increase, 95% confidence interval: 1.07-2.15, p = 0.020) in multivariable analysis adjusted for EuroSCORE II, mitral valve intervention as a time-dependent variable, and other risk factors. The proposed scheme showed incremental value over several clinical variables (net reclassification index = 0.40, p = 0.03).
Conclusions
The new staging classification provides independent and incremental prognostic value in patients with asymptomatic moderate or severe MR.
Keywords: Asymptomatic, Cardiac damage staging, Echocardiography, Mitral valve intervention, Primary mitral regurgitation, Risk stratification
Introduction
Mitral regurgitation (MR) is one of the most prevalent valvular heart diseases worldwide and has important prognostic and therapeutic implications.1 The therapeutic management of asymptomatic patients with chronic primary MR remains challenging and controversial.2, 3, 4, 5 In patients with asymptomatic severe MR, the clinical dilemma is indeed to decide between a “watchful waiting” vs. an early surgical mitral valve (MV) repair strategy. On the one hand, current and previous guidelines recommend MV intervention (i) in patients with severe primary MR, (ii) in patients with symptoms and/or left ventricular (LV) systolic dysfunction defined by a LV ejection fraction (LVEF) <60%, LV end-systolic diameter >40 mm in the 2020 American College of Cardiology/American Heart Association (ACC/AHA) and in the 2021 European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) guidelines, or a new-onset of atrial fibrillation (AF) and/or pulmonary hypertension defined as a systolic pulmonary arterial pressure (SPAP) >50 mmHg, and (iii) if a high likelihood of MV reparability and low surgical risk is anticipated.6, 7, 8, 9 On the other hand, several nonrandomized studies reported better outcomes with early MV intervention than with watchful waiting in asymptomatic severe primary MR.10, 11, 12, 13 It is likely that neither of these 2 strategies would be optimal for each asymptomatic patient, and there is thus an important need to individualize the therapeutic management strategy and timing of intervention as per the MR severity and the patient’s risk profile. The prognosis in asymptomatic moderate/severe valvular heart disease is essentially determined by the extent of cardiac damage associated with the valve disease. To this effect, Généreux et al.14 have proposed a new classification scheme to stage cardiac damage in patients with symptomatic severe aortic stenosis, and this scheme has subsequently been validated15 and adapted for patients with asymptomatic moderate or severe aortic stenosis.16 We hypothesized that a cardiac damage staging classification may also help to enhance risk stratification and better determine optimal timing for MV intervention in asymptomatic patients with primary MR. Hence, the objective of this study was to assess the prognostic value of a new cardiac damage staging scheme in asymptomatic patients with moderate or severe primary MR.
Materials and Methods
Study Population
The study cohort included 338 consecutive asymptomatic patients with moderate or severe primary MR who were prospectively recruited and followed at 2 heart valve clinics (Institut Universitaire de Cardiologie et de Pneumologie de Québec, Canada, and Groupement des Hôpitaux de l’Institut Catholique de Lille, France), between January 2002 and December 2019. Exclusion criteria were a history of rheumatic valve disease, endocarditis, pericarditis, more than mild concomitant left-sided valvular heart diseases (i.e., > mild mitral stenosis, aortic regurgitation, or aortic stenosis), secondary MR, patients with class I indication for MV surgery (i.e., severe MR with symptoms and/or LV systolic dysfunction) as per practice guidelines (European or American) of the location (Europe or North America) of the referral center, and pregnant or breast-feeding women (Supplemental Figure 1). Patients presenting with symptoms at baseline, including exertional dyspnea, angina, presyncope, or syncope, were excluded. However, patients presenting with mild symptoms (New York Heart Association [NYHA] functional class II) not considered by their treating cardiologist to be related to MR were included. Moreover, patients with equivocal clinical history or symptoms underwent exercise testing to confirm asymptomatic status. The clinical and echocardiographic data were collected prospectively in the context of heart valve clinics, and the analyses were performed retrospectively. The methods for collection of clinical and echocardiographic data are described in detail in the Supplemental Material. Briefly, fasting blood samples were measured using automated techniques standardized with the Canadian and France reference laboratory, MR severity was evaluated by echocardiography as per an integrative multiparameter approach, and all other echocardiographic data were measured or remeasured in the research echo laboratories of each institution, as recommended by current American Society of Echocardiography guidelines.17,18
Cardiac Damage Staging Classification
Patients were hierarchically classified into the following: stage 0: no extra-MV damage; stage 1: mild LV or left atrial (LA) damage as defined by an LV end-diastolic diameter >55 mm, an LV end-systolic diameter >35 mm, a forward LVEF ≤60%, and/or an indexed LA volume ≥40 ml/m2; stage 2: moderate or severe LV or LA damage as defined by an LV end-diastolic diameter >60 mm, an LV end-systolic diameter >40 mm, a forward LVEF ≤50%, an indexed LA volume ≥60 ml/m2 and/or AF; stage 3: pulmonary vasculature and/or tricuspid valve damage as defined by the presence of systolic pulmonary hypertension (SPAP ≥50 mmHg) and/or the presence of moderate or greater tricuspid regurgitation; and stage 4: right ventricular (RV) damage as defined by the presence of moderate or greater RV systolic dysfunction. RV dysfunction was determined by a multiparameter approach including the following quantitative criteria: tricuspid annulus systolic velocity S’ <9.5 cm/s and tricuspid annular plane systolic excursion <17 mm.19 Patients were hierarchically classified into a given stage (worst stage) if at least one of the proposed criteria was met in that stage. The echocardiographic parameters and criteria proposed in this new staging scheme were selected because they are easily obtainable in the context of the echocardiographic examination and because they have been previously validated and/or used in clinical practice.6,7,20, 21, 22, 23, 24, 25, 26, 27 The LVEF that is included in the staging classification is the forward LVEF, calculated by dividing the forward aortic stroke volume (SV) by the LV end-diastolic volume.28 In patients with MR, the forward LVEF is lower than the standard (i.e., total) LVEF measured by the biplane Simpson method.
Study Endpoints
The primary endpoint for this study was all-cause mortality during the entire follow-up regardless of whether the patient underwent MV intervention. Valve intervention was however entered as a time-dependent variable in the multivariable analysis. Referral for valve intervention was left at the discretion of the patient’s treating physician. The secondary endpoint was the composite of cardiac death during the entire follow-up or hospitalization for cardiovascular causes under medical management. Information on date and cause of death was obtained from review of medical records and/or the death certificate.
Statistical Analysis
Continuous data are expressed as mean ± SD or median (interquartile range [IQR]) and tested for the normality of distribution and homogeneity of variances with the Shapiro-Wilk and Levene tests, respectively. Comparison of continuous data was performed using one-way analysis of variance or Kruskal-Wallis test, as appropriate. Categorical data were expressed as percentage and compared with the χ2 test or Fisher’s exact test, as appropriate. Kaplan-Meier curves and log-rank tests of the time-to-event data were used to illustrate and compare the survival function as per overall staging. Multivariable Cox proportional hazards models adjusted for EuroSCORE II, systemic arterial hypertension, coronary artery disease (CAD), history of stroke, baseline MR severity, and MV intervention (included as a time-dependent variable) were used to determine the independent association between the staging classification and the different endpoints. Results of the Cox models were presented as hazard ratio (HR) and 95% confidence intervals (CIs). The staging classification was tested as an ordinal (increase of one stage) and as a dichotomic (≥stage 3) variable. The proportional hazard assumption was assessed by Schoenfeld residuals. The selection of the variables for the multivariable analysis was based on their clinical relevance (i.e., known risk factors) and/or because of their significant association with mortality in univariable analysis. Subgroup analyses were performed to determine the association between staging classification and mortality in each subgroup of MR severity (i.e., moderate and severe). The continuous net reclassification index (NRI) was used to determine the incremental value of the staging classification in predicting 5-year risk of mortality. Patients lost to follow-up before 5-year were censored. A p value of <0.05 was considered statistically significant. Statistical analyses were performed with SPSS software (V.26, IBM, Chicago, IL) and Stata software (V.14.2, StataCorp, College Station, Texas).
Results
Study Population and Clinical Characteristics
Clinical and echocardiographic characteristics of the study population are presented in Supplemental Table 1. Among the 338 patients (mean age = 64 ± 15 years), 68% were male, 47% had systemic arterial hypertension, 21% were in chronic AF, 73% were in NYHA class I, and 27% were in NYHA class II. At baseline, the mean effective regurgitant orifice area was 0.42 ± 0.20 cm2, the mean regurgitant volume was 63 ± 27 ml, and 45% (n = 151) of the patients had severe MR (Supplemental Table 1).
The prevalence of the cardiac damage stages and the distribution of their individual components are presented in Supplemental Tables 2 and 3. As per the staging scheme, 37 (10.9%) patients were in stage 0, 43 (12.7%) patients were in stage 1, 199 (58.9%) patients were in stage 2, 38 (11.2%) patients were in stage 3, and 21 (6.2%) patients were in stage 4. The clinical and echocardiographic characteristics of the study population as per each stage are presented in Tables 1 and 2. The proportion of patients with mild symptoms (NYHA class II) was similar between all stages (22, 34, 25, and 22% in stages 1, 2, 3, and 4, respectively). A total of 44 (13%) patients who had equivocal symptomatic status following physical examination and questionnaire underwent exercise testing to confirm the absence of symptoms. Patients in the most advanced stages of cardiac damage were older and had higher percentage of renal disease, CAD, higher EuroSCORE II, higher prevalence of severe MR, and lower LV SV and E/A ratio (all p < 0.04; Tables 1 and 2). Among patients in cardiac damage stage ≥2 (n = 258), none had class I indication (by study design) and 71% (184 patients) had class IIa (i.e., new-onset AF, SPAP at rest >50 mmHg, indexed LA volume ≥60 mL/m2, or LA diameter ≥55 mm) indications for MV intervention as per the 2021 European guidelines (Supplemental Table 4).
Table 1.
Baseline clinical characteristics of the study population as per cardiac damage staging classification
| Variables | Stage 0 (n = 37; 10.9%) | Stage 1 (n = 43; 12.7%) | Stage 2 (n = 199; 58.9%) | Stage 3 (n = 38; 11.2%) | Stage 4 (n = 21; 6.2%) | p value |
|---|---|---|---|---|---|---|
| Clinical | ||||||
| Age, y | 63 ± 15 | 63 ± 16 | 62 ± 14 | 75 ± 11 | 64 ± 15 | <0.001 |
| Male, n (%) | 25 (68) | 27 (63) | 144 (72) | 18 (47) | 15 (71) | 0.04 |
| BSA, m2 | 1.81 ± 0.20 | 1.79 ± 0.19 | 1.87 ± 0.22 | 1.67 ± 0.20 | 1.87 ± 0.22 | <0.001 |
| BMI, kg/m2 | 24.6 ± 3.8 | 24.3 ± 3.7 | 24.6 ± 4.0 | 23.0 ± 2.9 | 24.8 ± 3.6 | 0.19 |
| HR, beat/min | 72 ± 14 | 66 ± 13 | 72 ± 14 | 72 ± 14 | 72 ± 22 | 0.14 |
| SBP, mm Hg (n = 277) | 136 ± 14 | 133 ± 20 | 134 ± 19 | 126 ± 21 | 128 ± 20 | 0.08 |
| DBP, mm Hg (n = 277) | 79 ± 10 | 75 ± 9 | 77 ± 12 | 72 ± 13 | 78 ± 27 | 0.10 |
| Hypertension, n (%) | 19 (53) | 20 (48) | 83 (43) | 22 (60) | 11 (52) | 0.33 |
| Obesity, n (%) | 4 (11) | 4 (9) | 18 (9) | 1 (3) | 1 (5) | 0.70 |
| Dyslipidemia, n (%) | 8 (22) | 11 (26) | 59 (30) | 13 (35) | 7 (33) | 0.74 |
| Diabetes, n (%) | 1 (3) | 3 (7) | 14 (7) | 2 (5) | 1 (5) | 0.96 |
| Renal disease, n (%) | 1 (3) | 2 (5) | 5 (3) | 5 (14) | 1 (5) | 0.04 |
| Smoking∗, n (%) | 13 (36) | 13 (31) | 53 (28) | 17 (49) | 8 (38) | 0.16 |
| COPD, n (%) | 4 (11) | 1 (2) | 14 (7) | 7 (20) | 1 (5) | 0.07 |
| History of MI, n (%) | 4 (11) | 1 (2) | 12 (6) | 5 (13) | 0 (0) | 0.17 |
| CAD, n (%) | 3 (8) | 3 (7) | 9 (5) | 7 (20) | 1 (5) | 0.03 |
| History of stroke/TIA, n (%) | 1 (3) | 2 (5) | 10 (5) | 3 (9) | 3 (14) | 0.36 |
| Atrial fibrillation, n (%) | 0 (0) | 0 (0) | 48 (25) | 12 (32) | 8 (38) | - |
| NYHA, n (%) | 0.002 | |||||
| I | 26 (100) | 25 (78) | 88 (66) | 9 (75) | 14 (78) | |
| II | 0 (0) | 7 (22) | 45 (34) | 3 (25) | 4 (22) | |
| EuroSCORE II, % | 1.1 (0.7-1.9) | 1.1 (0.9-1.8) | 0.9 (0.6-1.4) | 2 (1.1-3.3) | 1.1 (0.8-1.7) | <0.001 |
| Medication | ||||||
| Beta-blockers, n (%) | 7 (19) | 9 (21) | 50 (26) | 10 (26) | 7 (33) | 0.79 |
| ACE/ARA II, n (%) | 19 (53) | 19 (45) | 84 (43) | 22 (60) | 9 (43) | 0.41 |
| Laboratory data | ||||||
| Creatinine, μmol/L (n = 217) | 79 (69-88) | 79 (61-86) | 81 (74-94) | 87 (70-112) | 94 (72-103) | 0.10 |
| Renal clearance, ml/min (n = 217) | 80 (49-96) | 84 (62-105) | 80 (63-99) | 43 (32-66) | 80 (58-90) | <0.001 |
Notes. Continuous data are mean ± SD or median (interquartile range). Categorical data are n (%). Bold indicates statistical significance.
ACE = angiotensin-converting-enzyme inhibitor, ARA II = angiotensin II receptor blockers, BMI = body mass index, BSA = body surface area, CAD = coronary artery disease, COPD = chronic obstructive pulmonary disease, DBP = diastolic blood pressure, EuroSCORE II = European System of Cardiac Operative Risk Evaluation, HR = heart rate, MI = myocardial infarction, NYHA = New York Heart Association functional class, SBP = systolic blood pressure, TIA = transient ischemic attack.
Smoking = current or history of smoking.
Table 2.
Baseline echocardiographic characteristics of the study population as per cardiac damage staging classification
| Variables | Stage 0 (n = 37; 10.9%) | Stage 1 (n = 43; 12.7%) | Stage 2 (n = 199; 58.9%) | Stage 3 (n = 38; 11.2%) | Stage 4 (n = 21; 6.2%) | p value |
|---|---|---|---|---|---|---|
| Prolapse, n (%) | 35 (95) | 35 (81) | 186 (94) | 34 (90) | 18 (86) | 0.09 |
| Flail, n (%) | 5 (14) | 8 (19) | 76 (38) | 11 (29) | 6 (29) | 0.01 |
| Affected leaflet, n (%) (n = 295) | 0.03 | |||||
| Anterior leaflet | 3 (9) | 9 (28) | 19 (10) | 2 (6) | 3 (19) | |
| Posterior leaflet | 21 (66) | 19 (59) | 138 (76) | 28 (82) | 8 (50) | |
| Bileaflet | 7 (22) | 4 (13) | 24 (13) | 4 (12) | 5 (31) | |
| MR severity | <0.001 | |||||
| Moderate, n (%) | 33 (89) | 32 (74) | 96 (48) | 17 (45) | 9 (43) | |
| Severe, n (%) | 4 (11) | 11 (26) | 103 (52) | 21 (55) | 12 (57) | |
| PISA EROA, cm2 (n = 253) | 0.28 ± 0.14 | 0.28 ± 0.14 | 0.45 ± 0.24 | 0.38 ± 0.23 | 0.45 ± 0.26 | <0.001 |
| Doppler EROA, cm2 (n = 239) | 0.29 ± 0.14 | 0.26 ± 0.13 | 0.51 ± 0.18 | 0.48 ± 0.25 | 0.41 ± 0.23 | <0.001 |
| Mean EROA, cm2 (n = 257) | 0.29 ± 0.13 | 0.27 ± 0.12 | 0.48 ± 0.18 | 0.45 ± 0.24 | 0.43 ± 0.21 | <0.001 |
| PISA RV, mL (n = 253) | 46 ± 21 | 48 ± 24 | 66 ± 28 | 62 ± 35 | 68 ± 38 | <0.001 |
| Doppler RV, mL (n = 253) | 48 ± 19 | 48 ± 30 | 69 ± 32 | 75 ± 41 | 57 ± 37 | <0.001 |
| Mean RV, mL (n = 264) | 47 ± 16 | 49 ± 26 | 67 ± 24 | 74 ± 37 | 64 ± 29 | <0.001 |
| LV cardiac output, L/min | 4.8 ± 1.0 | 4.7 ± 1.2 | 4.3 ± 1.2 | 3.8 ± 1.0 | 3.7 ± 1.3 | <0.001 |
| LVEDD, mm | 46 ± 4 | 50 ± 4 | 57 ± 6 | 51 ± 6 | 54 ± 9 | - |
| LVEDD index, mm/m2 | 26 ± 3 | 28 ± 3 | 31 ± 4 | 30 ± 4 | 29 ± 5 | - |
| LVESD, mm | 27 ± 4 | 30 ± 5 | 34 ± 7 | 31 ± 6 | 36 ± 11 | - |
| LVESD index, mm/m2 | 15 ± 3 | 17 ± 3 | 19 ± 4 | 18 ± 3 | 19 ± 6 | - |
| LVSV, mL | 68 ± 12 | 72 ± 14 | 62 ± 15 | 54 ± 14 | 52 ± 15 | <0.001 |
| LVSV index, mL/m2 | 38 ± 6 | 40 ± 9 | 33 ± 7 | 32 ± 8 | 28 ± 8 | <0.001 |
| Simpson LVEF, % (n = 295) | 65 ± 7 | 67 ± 6 | 66 ± 6 | 65 ± 6 | 63 ± 10 | 0.58 |
| Forward LVEF, % | 69 ± 7 | 63 ± 14 | 38 ± 9 | 47 ± 19 | 39 ± 15 | - |
| LA volume index, mL/m2 (n = 283) | 31 ± 5 | 37 ± 9 | 53 ± 21 | 47 ± 14 | 60 ± 46 | - |
| SPAP, mmHg (n = 259) | 32 ± 7 | 33 ± 7 | 33 ± 7 | 48 ± 15 | 31 ± 8 | - |
| TAPSE, mm (n = 233) | 24 ± 3 | 25 ± 4 | 26 ± 5 | 24 ± 3 | 17 ± 5 | - |
| E/A ratio (n = 262) | 1.3 ± 0.5 | 1.3 ± 0.5 | 1.8 ± 0.7 | 1.5 ± 0.5 | 1.4 ± 0.6 | <0.001 |
| E/e’ ratio (n = 258) | 10.3 ± 3.1 | 10.5 ± 3.6 | 11.5 ± 4.8 | 13.3 ± 4.8 | 10.5 ± 4.6 | 0.17 |
| S′, cm/s (n = 215) | 14.3 ± 3.1 | 14.8 ± 3.4 | 15.2 ± 3.0 | 15.8 ± 3.5 | 11.2 ± 4.1 | - |
| TR ≥ moderate, n (%) | 0 (0) | 0 (0) | 0 (0) | 28 (74) | 2 (10) | - |
Notes. Continuous data are mean ± SD or median (interquartile range). Categorical data are n (%). Bold indicates statistical significance.
EROA = effective regurgitant orifice area, LA = left atrium, LV = left ventricular, LVEDD = LV end-diastolic diameter, LVEF = LV ejection fraction, LVESD = LV end-systolic diameter, LVSV = LV stroke volume, MR = mitral regurgitation, PISA = proximal isovelocity surface area, RV = regurgitant volume, S’ = tricuspid annulus systolic velocity, SPAP = systolic pulmonary artery pressure, TAPSE = tricuspid annular plane systolic excursion, TR = tricuspid regurgitation.
Association Between Cardiac Damage Staging And Outcomes
During a median follow-up time of 5.6 years (IQR: 2.7-9.6), there were 86 (25%) deaths, and 27 (31%) deaths were of cardiovascular cause. One hundred eighty-five (55%) patients were referred for MV intervention at a median time of 6.8 years (IQR: 4.1-12.0) during follow-up, and 41 (12%) patients were hospitalized for cardiovascular causes prior to surgery or death. The composite of cardiovascular mortality or hospitalization for cardiac causes occurred in 57 (17%) patients. The proportion of patients who underwent MV intervention was as follows: 13 (35.1%) patients in stage 0 (median [IQR] time-to-intervention: 3.7 [2.8-5.4] years), 22 (51.2%) patients in stage 1 (4.6 [1.3-9.0] years), 126 (63.3%) patients in stage 2 (0.8 [0.3-2.8] years), 13 (34.2%) patients in stage 3 (1.7 years [0.7-4.0]), and 11 (52.4%) patients in stage 4 (0.9 years [0.3-3.7]) (p = 0.001).
Staging Classification and Long-Term Survival
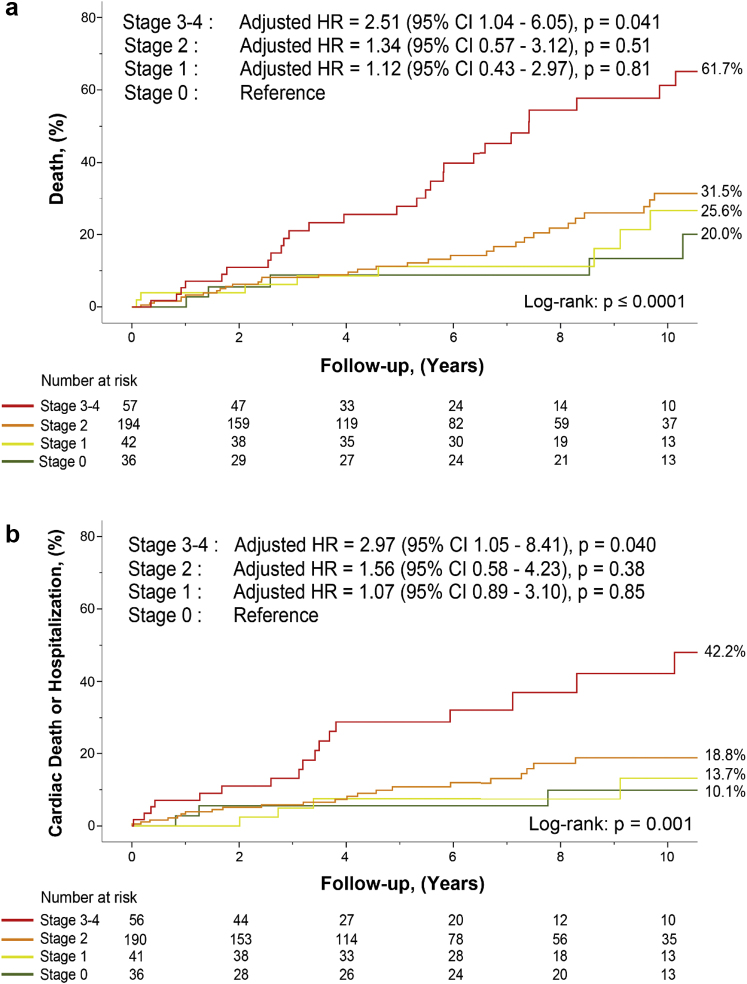
Given that there was a small number of patients classified into stage 4 (n = 21) and that patients in stages 3 and 4 presented similar baseline risk profiles and outcomes (Table 1 and Supplemental Table 3), stages 3 and 4 were merged together into a single group (i.e., stage 3-4) for subsequent analyses. Figure 1 shows all-cause mortality and the composite of cardiovascular mortality or hospitalization as per the staging classification (Figure 1a and b, respectively). There was a stepwise increase in the risk of all-cause mortality and the composite of cardiovascular death or hospitalization for each increase in the cardiac damage stage, and the increase in risk was more pronounced and occurred earlier during follow-up for stage 3-4 (all long-rank p ≤ 0.001; Figure 1).
Figure 1.
Analysis of mortality and hospitalization as per the cardiac damage staging classification. Kaplan-Meier curves of all-cause mortality (a) and the composite of cardiovascular mortality or hospital admission (b) as per the staging classification. Percentage (%) is mortality rates after 10 years of follow-up. The number at the bottom of the graphs is numbers of patients at risk at each time interval.
Abbreviations: CI, confidence interval; HR, hazard ratio.
After multivariable adjustment for several risk factors and MV intervention in Cox proportional hazard models, the staging classification remained significantly associated with an increased risk of all-cause (HR [95% CI] per one-stage increase = 1.41 [1.05-1.58], p = 0.015; Table 3) and the composite of cardiovascular mortality or hospitalization (HR [95% CI] per one-stage increase = 1.51 [1.07-2.15], p = 0.020; Table 3). Multivariable Cox analyses using cardiac damage staging in dichotomous format (stage ≥ vs. < 3) showed a strong and independent association with all-cause mortality (HR [95% CI] = 2.04 [1.22-3.37], p = 0.006; Supplemental Table 5) and the composite of cardiovascular death or hospitalization (2.24 [1.18-4.25], p = 0.014; Supplemental Table 5). In Cox models exclusively for cardiovascular mortality, trends are observed for association with staging classification and stage ≥3 (Supplemental Figure 2 and Supplemental Tables 6 and 7).
Table 3.
Association of staging classification with increased risk of mortality and hospitalization
| Variables | All-cause mortality (86 events) |
Hospitalization for cardiovascular cause or cardiac mortality (57 events) |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis |
Multivariable analysis |
Univariable analysis |
Multivariable analysis |
|||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Stage of cardiac damage (per one-stage increase) | 1.68 (1.26-2.23) | <0.001 | 1.41 (1.07-1.85) | 0.015 | 1.65 (1.13-2.40) | 0.009 | 1.51 (1.07-2.15) | 0.020 |
| EuroSCORE II | 1.53 (1.34-1.75) | <0.001 | 1.36 (1.18-1.58) | <0.001 | 1.53 (1.29-1.84) | <0.001 | 1.36 (1.11-1.65) | 0.002 |
| Hypertension | 1.73 (1.10-2.70) | 0.02 | 1.45 (0.90-2.34) | 0.12 | 1.77 (0.99-3.14) | 0.053 | 1.47 (0.79-2.74) | 0.22 |
| Obesity | 1.22 (0.49-3.00) | 0.67 | - | - | 4.00 (0.55-29.02) | 0.17 | - | - |
| Coronary artery disease | 1.08 (0.47-2.48) | 0.86 | - | - | 1.62 (0.64-4.08) | 0.31 | - | - |
| History of stroke | 3.42 (1.75-6.66) | <0.001 | 1.99 (0.98-4.05) | 0.06 | 3.23 (1.36-7.66) | 0.008 | 1.73 (0.69-4.37) | 0.24 |
| MR severity | 2.01 (1.30-3.10) | 0.002 | 2.05 (1.25-3.38) | 0.005 | 1.34 (0.76-2.37) | 0.32 | 1.40 (0.72-2.72) | 0.32 |
| Mitral valve intervention (as time-dependent variable) | 0.40 (0.24-0.67) | <0.0001 | 0.41 (0.24-0.71) | 0.002 | 0.22 (0.10-0.50) | <0.001 | 0.21 (0.09-0.50) | <0.001 |
Staging Classification and Outcomes as Per MR Severity
In the subset of patients with moderate MR (n = 187; 55%), the distribution as per staging classification was as follows: 17.6% in stage 0, 17.1% in stage 1, 51.3% in stage 2, 9.1% in stage 3, and 4.8% in stage 4 (Supplemental Figure 3a).
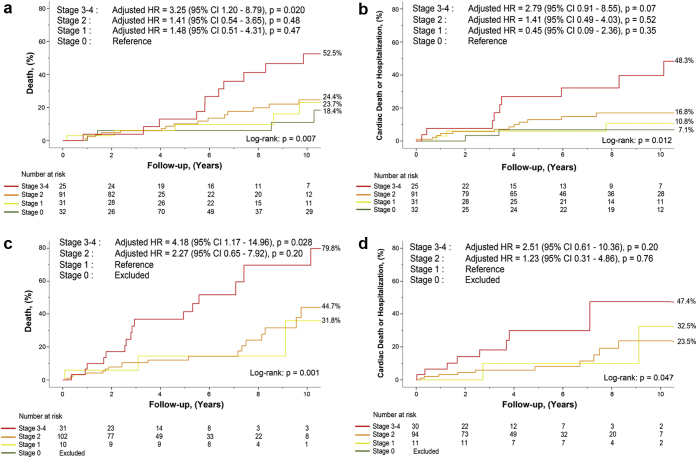
In the subset of patients with severe MR (n = 151, 45%), the distribution was as follows: 2.6% in stage 0, 7.3% in stage 1, 68.2% in stage 2, 13.9% in stage 3, and 7.9% in stage 4 (Supplemental Figure 3b). In both moderate and severe MR subgroups, stage ≥3 was significantly associated with an increased risk of all-cause mortality and of the composite of cardiovascular mortality or hospitalization (Figure 2, panel a to d; Supplemental Tables 8 and 9). In the latter, severe MR subgroup presented a robust trend (p = 0.09) as opposed to significant association in the moderate MR subgroup (Supplemental Tables 8 and 9).
Figure 2.
Analysis of mortality and hospitalization as per the cardiac damage staging classification in the subsets of patients with moderate vs. severe primary MR. All-cause mortality (a and c) and the composite of cardiovascular mortality or hospital admission (b and d) as per the staging classification in the subsets of patients with moderate MR (n = 187; panels a and b) and patients with severe MR (n = 151; panels c and d).
Abbreviations as in Figure 1. MR, mitral regurgitation.
Incremental Prognostic Value of Staging Classification for All-Cause Mortality
The cardiac damage staging provided significant incremental value to predict the 5-year risk of mortality (40 events) over clinical risk factors including EuroSCORE II, hypertension, history of stroke, and MR severity (NRI = 0.3977, p = 0.034; Table 4). Similar results were found if the cardiac damage stage 3-4 was taken alone instead of staging as an ordinal variable (NRI = 0.4915, p = 0.009; Table 4).
Table 4.
Incremental prognostic value of staging classification
| NRI for 5-y mortality (40 events) | NRI | p value |
|---|---|---|
| Multivariable model including: | ||
| EuroSCORE II, hypertension, history of stroke, and MR severity | Referent | |
| Stage of cardiac damage (per one-stage increase) | 0.3977 | 0.034 |
| Multivariable model including: | ||
| EuroSCORE II, hypertension, history of stroke, and MR severity | Referent | |
| ≥ Stage 3 | 0.4915 | 0.009 |
Interaction Between Staging Classification and Subgroups of Patients With Primary MR
The interaction between staging classification and several subgroups of patients with primary MR with regard to association with all-cause mortality is presented in Supplemental Figure 4. There was no significant interaction between subgroups and staging classification.
Discussion
This study is the first to propose and validate the prognostic value of a new staging classification to assess the extent of cardiac damage in a cohort of asymptomatic patients with moderate or severe primary MR. The main findings of this study are as follows: (i) There was a significant and stepwise increase in the risk of all-cause mortality and cardiovascular death or hospitalization for each increment in the cardiac damage stage; (ii) The increase in mortality risk was more pronounced and occurred earlier with stages 3 and 4, representing pulmonary, tricuspid, and RV damage; (iii) The staging classification provided significant incremental prognostic value beyond the traditional risk factors and clinical risk score to predict 5-year mortality; (iv) Among patients with stage ≥2 (n = 258), 29% (74 patients) had no indication (I, IIa, or IIb) for early intervention as per the recent European guidelines. These findings suggest that this new staging system could be an additive clinical tool to enhance risk stratification and therapeutic decision-making in asymptomatic moderate or severe primary MR (Figure 3).
Figure 3.
Association between cardiac damage staging classification and risk of mortality. The figure shows the cardiac damage staging classification and the prevalence of cardiac damage stages in the study population (a), and the association with increased risk of all-cause mortality for each increment in the stage of cardiac damage (b) by adjusted Cox curves. ∗HR is the hazard ratio for all-cause mortality, per one-stage increase, obtained by Cox multivariable analysis adjusted for EuroSCORE II, hypertension, history of stroke, MR severity, mitral valve intervention as a time-dependent variable, and stage of damage as a continuous variable expressed by one-step increase. †HR is the hazard ratio for all-cause mortality, for stage ≥3, obtained by Cox multivariable analysis adjusted for the same variables.
Abbreviations as in Figures 1 and 2. LA, left atrial; LV, left ventricular; LVEF, LV ejection fraction; RV, right ventricular.
Clinical Importance and Usefulness of the Cardiac Damage Staging System in Asymptomatic Primary MR
The strategy for optimal timing of MV intervention in the individual patient with asymptomatic severe primary MR remains uncertain and controversial. The current guidelines4,6,7 generally recommend a more conservative approach with consideration of valve intervention only in the presence of some specific criteria (i.e., LV dysfunction, AF, LA dilation, pulmonary hypertension, flail leaflet, etc.), whereas the results of several previous studies10, 11, 12, 13 provide support to early surgery in all asymptomatic patients with severe primary MR. Other studies29, 30, 31 also report an increased risk of mortality and adverse events in asymptomatic patients with moderate MR, raising the importance of close follow-up and risk stratification in these patients. It is unlikely that a given strategy (i.e., early intervention or watchful waiting) will be optimal for all asymptomatic patients with moderate or severe MR. Hence, it is of upmost importance to develop and validate clinical tools to enable individualized risk stratification and decision-making for timing of intervention. Grigioni et al.32 previously developed a mortality risk score (MIDA score), which demonstrated good prognostic value in patients with primary MR. However, this previous study predominantly included patients with flail leaflets and did not include parameters of tricuspid regurgitation or RV function in the risk score.33 In the present study, patients with evidence of significant tricuspid regurgitation or RV dysfunction (stages 3 or 4) were at much higher risk for adverse events than patients in stages 0-2. One of the advantages of the cardiac damage staging classification proposed in the present study is that it can easily be implemented in clinical practice with the use of parameters that are routinely measurable in the context of the echocardiogram assessment of MR. Furthermore, this classification scheme is based on a multiparameter approach, which provides a comprehensive assessment of all cardiac chambers.34 It should also be noted that patients do not necessarily progress through each stage. Hence, a patient in stage 3 may not necessarily meet the criteria for stage 1 or 2. However, the prognosis is essentially determined by the most advanced stage, regardless of whether the lower stage(s) is (are) fulfilled or not.
One striking result of the present study is that 76% of the patients with moderate or severe MR were in stage ≥2 and were thus at an increased risk of adverse events, despite the absence of symptoms. This finding underlines the fact that the absence of symptoms is not necessarily reliable to rule out the presence of advanced cardiac damage and thus the risk of poor prognosis. One potential criticism of this staging scheme is that the cardiac damage may not be directly related to the MR, per se. However, even if the cardiac damage is in part related to another concomitant disease (e.g., CAD, hypertension, etc.), the patient with an advanced cardiac stage is still at a higher risk for adverse events and may nonetheless be more vulnerable to the effect of a moderate or severe MR. To this effect, subgroup analyses revealed that the association of stage ≥3 with adverse outcomes is similar in patients with vs. without concomitant CAD or hypertension.
Parameters and Criteria Included in the Cardiac Damage Scheme
In the staging scheme previously proposed and validated for patients with aortic stenosis, stage 1 consisted in evidence of damage at the LV level, whereas stage 2 consisted in damage at the LA level. However, in the context of MR, we rather elected to format stages 1 and 2 so that they both assess the damage at the LV and LA levels but with different degrees of severity: stage 1: mild damage of LV and/or LA and stage 2: moderate or severe damage of LV and/or LA. The vast majority of the parameters and thresholds that we included in stages 1 and 2 as well as in stages 3 and 4 are supported by previous studies.23, 24, 25, 26, 27,35 It is important to note that the LVEF included in stages 1 and 2 is the forward LVEF (i.e., the forward aortic SV divided by the LV end-diastolic volume) instead of the standard total LVEF included in the guidelines. We used the forward LVEF in this new staging scheme because this parameter was previously reported to be superior to the total LVEF to detect subclinical LV systolic dysfunction and predict outcomes in patients with primary MR.21 Because of the presence of MR, the forward SV and thus the forward LVEF are substantially lower than the total SV and LVEF, respectively (Table 2). Hence, the majority of cases having a forward LVEF <60% and thus being in stage 1 or more still had a total LVEF >60% and thus no indication for intervention as per the guidelines.
Clinical Implications
In this asymptomatic MR population, only 11% of patients were in stage 0, and yet 17% were in stages ≥3 and hitherto do not report any symptom. These findings are consistent with previous studies reporting that a substantial proportion of patients with MR with echocardiographic evidence of subclinical cardiac dysfunction and heart failure are asymptomatic.36,37 They also provide support to the current guidelines,6,7 which state that irreversible consequences of severe MR may primarily affect the functional status of the ventricles and may occur despite the absence of symptoms. However, the main advantages of the new staging scheme proposed in this study vs. the approach proposed in the guidelines to trigger intervention in severe MR are that (i) this scheme assesses the presence of extravalvular damage not only at the LV level (as in the guidelines) but also at the level of the other cardiac chambers; (ii) it is based on a multiparameter approach (vs. a few parameters of LV function in the guidelines). Consequently, this staging scheme may be more sensitive than the guideline criteria to identify asymptomatic patients with severe MR who are at a higher risk of adverse outcomes in the short term and who may thus benefit from earlier intervention. As a matter of fact, 29% of the patients with advanced cardiac damage staging (≥2) had no indication (I, IIa, or IIb) as per the guidelines.
The findings of this study demonstrate the importance of using a cardiac damage staging approach based on Doppler-echocardiographic parameters to guide therapeutic management in patients with asymptomatic MR. In asymptomatic patients with moderate or severe aortic stenosis, patients with stage ≥2 had an increased risk of mortality in the short term, and these results thus provide support to the consideration of early aortic valve intervention in the presence of stage 2 and beyond.16 In the context of the present study in asymptomatic patients with moderate or severe primary MR, the level at which the mortality risk increases substantially in the short/midterm appears to be at stage 3 and beyond. These results suggest that a watchful waiting strategy would potentially be reasonable for asymptomatic patients with moderate or severe MR harboring stage 0, 1, or 2. However, damage at the level of the right-side unit (i.e., stages 3 or 4) was associated with an increased risk of mortality. Hence, patients in stages 3 or 4 (17% of the patients in the present study) may potentially benefit from earlier intervention. This hypothesis needs, however, to be investigated in future studies.
Study Limitations and Strengths
Although the clinical and echocardiographic data were prospectively collected, the present analysis is of retrospective nature and is, thus, subject to inherent limitations related to such design. The small sample size of the present study is a limitation, but this study includes a “real-life” population comprehensively followed, in which the symptomatic status was carefully assessed and monitored in the context of heart valve clinics. We elected to include patients with mild symptoms (i.e., NYHA class II), which were considered not related to the MR by the treating cardiologist, because the therapeutic management and the determination of the optimal timing for intervention are also challenging in these patients. We also included patients with moderate MR at baseline because a substantial proportion of these patients may exhibit rapid progression to symptoms or LV dysfunction and be at an increased risk for mortality and cardiovascular hospitalization. As a matter of fact, the presence of cardiac damage stage 3 or 4 was associated with marked increase in the risk of mortality not only in patients with severe MR but also in those with moderate MR. Nonetheless, a comprehensive risk stratification assessment should be accomplished before surgical referral based on the cardiac stage.
Finally, to ease the implementation and generalization of the cardiac damage staging scheme in the clinical setting, we included in the staging scheme parameters that can be measured in the context of the routine echocardiogram. However, each of these parameters is subject to measurement errors and variability. One of the strengths of the proposed grading scheme in this context is that the definition of each stage is rather based on a multiparameter approach, which attenuates the limitations associated with each single parameter. Further studies are needed to determine if the addition of other parameters obtained by rest (e.g., global LV longitudinal strain) or exercise stress (e.g., exercise SPAP) echocardiography or by other modalities (cardiac magnetic resonance, blood biomarkers, etc.) could further improve the prognostic value of the proposed staging scheme in the asymptomatic primary MR population.
Conclusion
The new and simple cardiac damage staging classification proposed in this study provides incremental prognostic value over traditional clinical risk factors to predict survival and hospital admission for cardiovascular causes in asymptomatic patients with moderate or severe primary MR. This staging classification may be helpful to identify patients at a higher risk of adverse events and who might thus benefit from early elective MV intervention. The findings of this study suggest that a stage ≥3, which was identified in 17% of the present series, could be considered as a potential trigger for early elective valve intervention in these patients, but this hypothesis needs to be tested in further studies.
Ethics Statement
This study was conducted in accordance to the Declaration of Helsinki standards. The study protocol was approved by each institutional review board, and written informed consent was waived for this retrospective analysis.
Funding
This work was supported by a research grant (FDN-143225) from the Canadian Institutes of Health Research (CIHR), Ottawa, Ontario, Canada. J.B. is supported by a doctoral scholarship from the CIHR. L.T. is supported by a doctoral scholarship from Fonds de Recherche en Santé-Québec(FRSQ), Montréal, Québec, Canada. M.A.C. holds a New National Investigator award from the Heart and Stroke Foundation of Canada and an Early Career Investigator award from CIHR. P.P. holds the Canada Research Chair in Valvular Heart Diseases from CIHR, Ottawa, Ontario, Canada.
Disclosure statement
Dr Pibarot received funding from Edwards Lifesciences and Medtronic for echocardiography core laboratory analyses in the field of transcatheter aortic valve replacement with no direct personal compensation. Dr Clavel received funding from Edwards Lifesciences for computed tomography core laboratory analyses in the field of surgical aortic valve prosthesis with no direct personal compensation and research grant from Medtronic. All other authors reported no conflict of interest to disclose.
Acknowledgments
We thank all patients for participation and all collaborators of the 2 institutions for their time and efforts.
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
Contributor Information
Sylvestre Maréchaux, Email: marechaux.sylvestre@ghicl.net.
Philippe Pibarot, Email: philippe.pibarot@med.ulaval.ca.
Supplementary Material
References
- 1.Coffey S., Roberts-Thomson R., Brown A., et al. Global epidemiology of valvular heart disease. Nat Rev Cardiol. 2021;18(12):853–864. doi: 10.1038/s41569-021-00570-z. [DOI] [PubMed] [Google Scholar]
- 2.Carabello B.A. Timing of surgery for primary MR: we’re not there yet but we’re getting closer. J Am Coll Cardiol Img. 2020;13(2 Pt 2):586–588. doi: 10.1016/j.jcmg.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura R.A., Vahanian A., Eleid M.F., Mack M.J. Mitral valve disease--current management and future challenges. Lancet. 2016;387(10025):1324–1334. doi: 10.1016/S0140-6736(16)00558-4. [DOI] [PubMed] [Google Scholar]
- 4.Bonow R.O., O’Gara P.T., Adams D.H., et al. 2020 focused update of the 2017 ACC expert consensus decision pathway on the management of mitral regurgitation: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;75(17):2236–2270. doi: 10.1016/j.jacc.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Tietge W.J., de Heer L.M., van Hessen M.W., et al. Early mitral valve repair versus watchful waiting in patients with severe asymptomatic organic mitral regurgitation; rationale and design of the Dutch AMR trial, a multicenter, randomised trial. Neth Heart J. 2012;20(3):94–101. doi: 10.1007/s12471-012-0249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2021;77(4):450–500. doi: 10.1016/j.jacc.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Baumgartner H., Falk V., Bax J.J., et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease: the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2017;38(36):2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura R.A., Otto C.M., Bonow R.O., et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;70(2):252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 10.Suri R.M., Vanoverschelde J.L., Grigioni F., et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA. 2013;310(6):609–616. doi: 10.1001/jama.2013.8643. [DOI] [PubMed] [Google Scholar]
- 11.Kang D.H., Kim J.H., Rim J.H., et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation. 2009;119(6):797–804. doi: 10.1161/CIRCULATIONAHA.108.802314. [DOI] [PubMed] [Google Scholar]
- 12.Kang D.H., Park S.J., Sun B.J., et al. Early surgery versus conventional treatment for asymptomatic severe mitral regurgitation: a propensity analysis. J Am Coll Cardiol. 2014;63(22):2398–2407. doi: 10.1016/j.jacc.2014.02.577. [DOI] [PubMed] [Google Scholar]
- 13.Montant P., Chenot F., Robert A., et al. Long-term survival in asymptomatic patients with severe degenerative mitral regurgitation: a propensity score-based comparison between an early surgical strategy and a conservative treatment approach. J Thorac Cardiovasc Surg. 2009;138(6):1339–1348. doi: 10.1016/j.jtcvs.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 14.Genereux P., Pibarot P., Redfors B., et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J. 2017;38(45):3351–3358. doi: 10.1093/eurheartj/ehx381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vollema E.M., Amanullah M.R., Ng A.C.T., et al. Staging cardiac damage in patients with symptomatic aortic valve stenosis. J Am Coll Cardiol. 2019;74(4):538–549. doi: 10.1016/j.jacc.2019.05.048. [DOI] [PubMed] [Google Scholar]
- 16.Tastet L., Tribouilloy C., Maréchaux S., et al. Staging cardiac damage in patients with asymptomatic aortic valve stenosis. J Am Coll Cardiol. 2019;74(4):550–563. doi: 10.1016/j.jacc.2019.04.065. [DOI] [PubMed] [Google Scholar]
- 17.Zoghbi W.A., Adams D., Bonow R.O., et al. Recommendations for non invasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Rudski L.G., Lai W.W., Afilalo J., et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Tribouilloy C., Rusinaru D., Szymanski C., et al. Predicting left ventricular dysfunction after valve repair for mitral regurgitation due to leaflet prolapse: additive value of left ventricular end-systolic dimension to ejection fraction. Eur J Echocardiogr. 2011;12(9):702–710. doi: 10.1093/ejechocard/jer128. [DOI] [PubMed] [Google Scholar]
- 21.Dupuis M., Mahjoub H., Clavel M.A., et al. Forward left ventricular ejection fraction: a simple risk marker in patients with primary mitral regurgitation. J Am Heart Assoc. 2017;6(11):e006309. doi: 10.1161/JAHA.117.006309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rusinaru D., Tribouilloy C., Grigioni F., et al. Left atrial size is a potent predictor of mortality in mitral regurgitation due to flail leaflets: results from a large international multicenter study. Circ Cardiovasc Imaging. 2011;4(5):473–481. doi: 10.1161/CIRCIMAGING.110.961011. [DOI] [PubMed] [Google Scholar]
- 23.Barbieri A., Bursi F., Grigioni F., et al. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: a multicenter long-term international study. Eur Heart J. 2011;32(6):751–759. doi: 10.1093/eurheartj/ehq294. [DOI] [PubMed] [Google Scholar]
- 24.Grigioni F., Benfari G., Vanoverschelde J.L., et al. Long-term implications of atrial fibrillation in patients with degenerative mitral regurgitation. J Am Coll Cardiol. 2019;73(3):264–274. doi: 10.1016/j.jacc.2018.10.067. [DOI] [PubMed] [Google Scholar]
- 25.Ghoreishi M., Evans C.F., deFilippi C.R., et al. Pulmonary hypertension adversely affects short-and long-term survival after mitral valve operation for mitral regurgitation: implications for timing of surgery. J Thorac Cardiovasc Surg. 2011;142(6):1439–1452. doi: 10.1016/j.jtcvs.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 26.Le Tourneau T., Messika-Zeitoun D., Russo A., et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol. 2010;56(7):570–578. doi: 10.1016/j.jacc.2010.02.059. [DOI] [PubMed] [Google Scholar]
- 27.Tribouilloy C., Grigioni F., Avierinos J.F., et al. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. J Am Coll Cardiol. 2009;54(21):1961–1968. doi: 10.1016/j.jacc.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 28.Dumesnil J.G., Dion D., Yvorchuk K., Davies R.A., Chan K. A new, simple and accurate method for determining ejection fraction by Doppler echocardiography. Can J Cardiol. 1995;11(11):1007–1014. [PubMed] [Google Scholar]
- 29.Dziadzko V., Clavel M.A., Dziadzko M., et al. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet. 2018;391(10124):960–969. doi: 10.1016/S0140-6736(18)30473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samad Z., Shaw L.K., Phelan M., et al. Long-term outcomes of mitral regurgitation by type and severity. Am Heart J. 2018;203:39–48. doi: 10.1016/j.ahj.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Dziadzko V., Dziadzko M., Medina-Inojosa J.R., et al. Causes and mechanisms of isolated mitral regurgitation in the community: clinical context and outcome. Eur Heart J. 2019;40(27):2194–2202. doi: 10.1093/eurheartj/ehz314. [DOI] [PubMed] [Google Scholar]
- 32.Grigioni F., Clavel M.A., Vanoverschelde J.L., et al. The MIDA Mortality Risk Score: development and external validation of a prognostic model for early and late death in degenerative mitral regurgitation. Eur Heart J. 2018;39(15):1281–1291. doi: 10.1093/eurheartj/ehx465. [DOI] [PubMed] [Google Scholar]
- 33.Vahanian A., Iung B. Predicting the outcome of degenerative mitral regurgitation: a step forward but still a long way to go. Eur Heart J. 2018;39(15):1292–1294. doi: 10.1093/eurheartj/ehx583. [DOI] [PubMed] [Google Scholar]
- 34.Tastet L., Généreux P., Bernard J., Pibarot P. The role of extra-valvular cardiac damage staging in aortic valve disease management. Can J Cardiol. 2021;37(7):1004–1015. doi: 10.1016/j.cjca.2021.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura T., Ohtaki E., Tanaka K., et al. Echocardiographic prediction of left ventricular dysfunction after mitral valve repair for mitral regurgitation as an indicator to decide the optimal timing of repair. J Am Coll Cardiol. 2003;42(3):458–463. doi: 10.1016/s0735-1097(03)00649-1. [DOI] [PubMed] [Google Scholar]
- 36.Yang H., Negishi K., Wang Y., Nolan M., Marwick T.H. Imaging-guided cardioprotective treatment in a community elderly population of stage B heart failure. J Am Coll Cardiol Img. 2017;10(3):217–226. doi: 10.1016/j.jcmg.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Hiemstra Y.L., Tomsic A., van Wijngaarden S.E., et al. Prognostic value of global longitudinal strain and etiology after surgery for primary mitral regurgitation. J Am Coll Cardiol Img. 2020;13(2 Pt 2):577–585. doi: 10.1016/j.jcmg.2019.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.