Abstract
The incidence of diabetic kidney disease (DKD) is sharply increasing worldwide. Microalbuminuria is the primary clinical marker used to identify DKD, and its initiating step in diabetes is glomerular endothelial cell dysfunction, particularly glycocalyx impairment. The glycocalyx found on the surface of glomerular endothelial cells, is a dynamic hydrated layer structure composed of pro-teoglycans, glycoproteins, and some adsorbed soluble components. It reinforces the negative charge barrier, transduces the shear stress, and mediates the interaction of blood corpuscles and podocytes with endothelial cells. In the high-glucose environment of diabetes, excessive reactive oxygen species and proinflammatory cytokines can damage the endothelial glycocalyx (EG) both directly and indirectly, which induces the production of microalbuminuria. Further research is required to elucidate the role of the podocyte glycocalyx, which may, together with endothelial cells, form a line of defense against albumin filtration. Interestingly, recent research has confirmed that the negative charge barrier function of the glycocalyx found in the glomerular basement membrane and its repulsion effect on albumin is limited. Therefore, to improve the early diagnosis and treatment of DKD, the potential mechanisms of EG degradation must be analyzed and more responsive and controllable targets must be explored. The content of this review will provide insights for future research.
Keywords: Glycocalyx, Diabetic kidney disease, Endothelial cells, Reactive oxygen species, Microalbuminuria, Enzyme
Core Tip: In the diabetic microenvironment, various harmful factors, such as oxidative stress and inflammation, contribute to endothelial glycocalyx (EG) disruption through direct damage to the glycocalyx or indirect degradation due to the upregulation of related sheddases. Shedding one or more components after damage to the EG is an early sign of numerous pathological states, including diabetes. The loss of filtration barrier integrity can lead to microalbuminuria, which is predictive of diabetic kidney disease (DKD). Identifying and targeting the key molecules involved in glycocalyx damage thus represent current hot topics in DKD research.
INTRODUCTION
Over the past 30 years, the number of people with diabetes mellitus has quadrupled globally, and approximately 1 in 11 adults currently have diabetes (mainly type 2)[1]. Moreover, most patients with diabetes also have complications, which seriously affect their quality of life and life expectancy. Diabetic complications are categorized as macrovascular (e.g., cardiovascular disorders) and microvascular (e.g., renal, retinal, and neurologic disease). In recent decades, cohort studies from high-income countries have shown that the relative risk of microvascular complications is at least 10 times higher in patients with diabetes than in patients without diabetes, while the relative risk of macrovascular complications is 2-4 times higher[2]. In developing countries, patients with diabetes have a higher risk of renal complications but a lower risk of coronary heart disease[3], which further reveals the increasing incidence of diabetic microvascular disease complications, especially diabetic kidney disease (DKD). However, the pathogenesis of DKD is incredibly complicated and remains poorly understood, and current treatments have limited efficacy. In the last ten years, DKD has replaced glomerulonephritis as the primary reason for chronic kidney disease in China[4] and it has also become the leading global cause of end-stage renal disease[5]. Understanding the pathogenesis of early DKD is, thus, of profound significance because it could aid in delaying, preventing, or reversing the progression of this disease and improving the prognosis of patients.
The glomerular filtration barrier (GFB) comprises three distinct layers: Endothelial cells, glomerular basement membrane (GBM), and podocytes. The pathological changes that occur with DKD include glomerular capillary hypertrophy, GBM thickening, podocyte foot process disappearance, and mesangial expansion. Microalbuminuria occurs prior to these changes and is denoted by a slight increase in the urinary excretion of albumin (20-200 μg/min in humans) prior to overt DKD, and is the first predictor that a patient has a high risk of developing DKD, with both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM)[6]. It is noted that renal tubules have powerful reab-sorption, and a 50% increase in the filtration rate increases urinary albumin in the sub-microalbumin range[7]. Thus, to facilitate albumin flux increases that are sufficient to produce microalbuminuria, normal renal tubule reuptake requires structural alterations to the GFB[8]. Furthermore, endothelial dysfunction has been found to precede the onset of microalbuminuria[9]. The trigger for endothelial dysfunction is based on the permselectivity of GFB to molecules of different sizes and charges. The albumin filtration increase can be estimated, and it depends on the size or charge selectivity of the defect[10]. Studies have found that the occurrence of microalbuminuria is tied to charge selectivity in diabetic animal models and patients with T1DM and T2DM[10-12]. The lack of charge selectivity is observed earlier than the depletion of size selectivity, and the size selectivity defect only appears after the transition to the macroalbuminuria stage[12]. Consequently, the pre-emptive advantage of the charge selective defect suggests that the damage to the endothelial glycocalyx (EG) with a negative charge most likely represents the first step in the progression of microalbumin in patients with DKD. Thus, the structure and function of the EG, the mechanism of EG damage, and potential therapeutic strategies must be further explored to curb the rapid spread of DKD.
GLYCOCALYX STRUCTURAL AND FUNCTIONAL ALTERATIONS IN DKD
EG
Glomerular endothelial cells are highly differentiated with cytoplasmic decay zones dotted with many fenestrae, which are round transcellular pores at 60-80 nm in diameter[13]. The fenestrae were previously considered empty, which means that they are a weak barrier against albumin filtration[14]. Although fenestrated capillaries are much more permeable to water and small solutes than non-fenestrated capillaries, there is little albumin in the GBM and adjacent podocytes under physiological conditions[15,16]. Albumin is a polar protein with a total net charge ranging from -12 to -18 at physiological pH[17]. Studies using dextran with different charges found that polycationic DEAE dextran was cleared more at a certain molecular radius than neutral dextran, which was filtered more freely than negatively charged sulfate dextran[18]. These phenomena can only be explained by the negatively charged glycocalyx. In addition, the Starling hypothesis indicates the primary method of fluid exchange between plasma and tissue in most capillaries. On this basis, the revised Starling hypothesis states that at a steady state, colloidal osmotic pressure differences, which are resistant to hydrostatic pressure, exist across the EG rather than the entire vessel wall, effectively preventing albumin from leaving the vessel[19]. Observing the glycocalyx on the surface of the endothelial cells requires specific fixation and staining techniques. The immunofluorescence confocal technique is now widely used with lectin to fluorescently label glycocalyx components, which can be directly observed in the 200-400 nm thick glycocalyx covering the luminal surface of the glomerular endothelial cells in the fenestral and inter-fenestral domains. The EG is a complex layer on the glomerular endothelial cells composed of proteoglycans (PGs), glycoproteins, and glycolipids. It integrates components, such as plasma proteins, α-acid glycoproteins, antithrombin III, extracellular superoxide dismutase (SOD), lipase, growth factors, and chemokines, to form a looser layer known as the endothelial surface layer (ESL)[20]. The PGs of the EG consist of core proteins and glycosaminoglycan (GAG) side chains. To the best of our knowledge, the main core proteins are syndecans and glypicans. The GAGs include heparin sulfate (HS), hyaluronic acid (HA), chondroitin sulfate (CS), and keratan sulfate, which are all negatively charged due to their carboxyl and/or sulfate groups[21]. Short exposure to glucose levels > 15 mmol/L resulted in a 50% loss of the glycocalyx in healthy individuals[22]. In C57BL/6 mice, acute hyperglycemia increased EG permeability[23]. EG shedding increases the concentration of several types of EG in the blood or plasma, such as HA, HS, and syndecans. Thus, the plasma levels of these molecules can be regarded as a responsive indicator for EG degradation. Glycocalyx hydrolysis is clo-sely related to sheddases, such as heparinase (HPSE), matrix metalloproteinase (MMP), hyaluronidase (HYAL), and neuraminidase (NEU)[24]. In patients with T1DM, the loss of approximately half of the body’s glycocalyx was accompanied by elevated plasma HA and HYAL levels. More importantly, the glycocalyx volume was decreased in T1DM patients with microalbuminuria when compared with those without[25], and similar results were reported in patients with T2DM[26]. These findings suggest that the decrease in EG correlates strongly with microalbuminuria. Swärd and Rippe[27] proposed a more precise exposure time for hyperglycemia. Short-term (lasting minutes to hours) exposure to hyperglycemia produced microproteinuria via protein kinase Cα and downstream Rho-associated coiled-coil protein kinase pathways mediating F-actin cytoskeleton rearrangements, while long-term (lasting two weeks) exposure induced the permeability of glomerular endothelial cells to albumin associated with EG disruption. It should be noted that besides serving as a filter barrier, EG ensures vessel patency (through its antithrombotic and antiadhesive properties), transduces shear stress, regulates the vascular tone (by sensing fluid shear forces), and protects endothelial cells from oxidative stress (via combining free radical scavengers)[28].
Core proteins and MMPs: Syndecans and glypicans are the main core proteins in EG. Other core proteins, such as mimecans and perlecans, are soluble and secreted in both the EG and blood[29]. Syndecans are transmembrane proteins that mainly bind HS or CS chains[28,30]. There are six significant subtypes of syndecans[31], and syndecan-1 and 4 are particularly prominent in nephrons[32]. The former is connected to three HS chains[33], while the latter can carry 3-5 HS chains[32] and is most abundant in the syndecans family in human glomerular endothelial cells[34]. Glypicans are anchored to glycosylphosphatidylinositol and have four main isomers[30,31], of which glypican-1 binds almost exclusively to the HS chain[35], but close to the cell membrane, glypican-1 binds to 3-4 HS chains[33]. Heparan sulfate PGs (HSPGs), composed of syndecan and HS, are most abundant on the cell surface[36], followed by phosphatidyl inositol PGs, composed of glypicans linked to HS[37]. An essential function of the syndecan core proteins is to put the highly bioactive GAGs in the right place at the right time[38]. MMPs are a kind of zinc-reliant endopeptidase that are mainly synthesized by inflammatory cells, although MMPs can also be synthesized by endothelial cells and vascular smooth muscle cells when stimulated by macrophages[39]. MMP-2 and MMP-9 can be activated by MMP14 (also known as membrane type 1)[32]. MMP-2, MMP-9, and MMP-14 can cleave syndecans at different sites to produce various sizes of proteolytic fragments[40]. Typically, MMP-9 degrades syndecan-1 and MMP-2 cracks syndecan-4, allowing syndecans and HS to be released into the blood[39]. Diabetic conditions promote the overexpression of endothelial MMP-9[34], MMP-2[26], and urinary MMP-14[41], and the activity of these MMPs is elevated in the kidneys of diabetic humans[41,42] and mice[43].
HS and HPSE: HS is the most common GAG in the glycocalyx and accounts for approximately 50%-90% of its amount[44]. It is comprised of 300 alternate N-acetyl-glucosamine a1 to 4 glucuronic acid b1 to 4 residues[45]. HS biosynthesis exists in the Golgi apparatus, and its characteristics include chain initiation, polymerization, and modification[46]. HS can be extensively modified, including N-deacetylation/N-sulfation of N-acetylglucosamine, isomerization of C5 glucuronic acid to iduronic acid, and 2-O-, 3-O-, and 6-O-sulfation[45]. Different combinations of these modifications produce structurally diverse HS chains, which dictate the binding and modulation of specific proteins and regulate the activity of various biological molecules, such as cytokines and growth factors on the cell surface[45,47]. In vitro studies have found that high levels of glucose reduced HS synthesis and increased the monolayer albumin flux in glomerular endothelial cells, implying that the presence of HS in EG limits proteinuria[48]. The structure of the HS chain may be edited by HS modification enzymes, including HPSE, β (1-4)-endoglucuronidase, which clears HS at specific sites, and HS 6-O-endosulfatase, which explicitly removes 6-O-sulfonate[49]. HPSE is the most well characterized of these enzymes, and it is the sole mammalian endoglycosidase that cuts HS[50]. The nascent HPSE is inactive and requires activation by cathepsin L[46]. Active HPSE cleaves glycosidic bonds within the HS chain to yield HS fragments that are 5-7 kDa in size, and this cleavage requires the N-and 6-0-sulfated moieties to have specific sequences, such as the trisaccharide sequence GlcNS60S-α(1-4)-GlcA-β(1-4)-GlcNS6OS[51]. Intracellular HPSE has a variety of biological functions, including the regulation of cellular autophagy, communication, and survival. Conversely, extracellular HPSE is related to inflammation, vascular instability, and fibrosis and is a crucial contributor to renal damage in patients with DKD and glomerulonephritis[47]. The first study to reveal a role for HPSE in the development of proteinuria was performed on rats with puromycin aminoglycoside nephropathy, and it showed that HPSE overexpression was an essential contributing factor to HS loss in proteinuria[52]. In Zucker fatty rats proteinuria was associated with a significant glycocalyx reduction, and this was at least in part related to elevated HPSE levels[53]. However, specific HPSE inhibitors PI-88[54] or polyclonal anti-HPSE antibodies 226[55] reduced proteinuria levels and alleviated renal damage. At the same time, the over-expression of HPSE in transgenic over-expressing mice (HPSE-TG) resulted in early proteinuria and renal failure[56]. According to a previous study, the transcription factor early growth response 1 is responsible for activating the HPSE promoter under hyperglycemic conditions[50]. Compared with healthy volunteers, patients with DKD show increased urinary and renal HPSE activity[57]. Furthermore, HPSE was upregulated in response to a high-glucose environment and DKD mediators, such as advanced glycation end products (AGEs), in mouse DKD models[58] and renal-derived cell lines (endothelial cells, renal epithelial cells, and proximal tubular cells)[59-61]. In addition, Schmidt et al[62] proposed that there was a relationship between urinary HS and renal function. It was believed that urinary HS could predict the progression of renal dysfunction. However, the increase in permeability caused by glycocalyx injury was not enough to reduce the glomerular filtration rate (GFR), and the obstruction of the secondary capillary lumen, caused by leukocyte or platelet interactions with the endothelial cells, was the cause of GFR reduction.
HA and HYAL: HA is a non-protein-bound, non-sulfate, negatively charged GAG, a linear polysaccharide held together by the repeat units of D-glucuronic acid and N-acetyl-D-glucosamine by glycosidic bonds repeated thousands of times[39,63]. HA is synthesized from three isoforms of hyaluronic acid synthase (HAS). HAS-2 is the major synthetase of HA and is expressed in most cells and essential for life[63]. Van den Berg et al[63] found that the HAS-2 deletion in the endothelial cells of adult mice (selective inactivation of the HAS-2 gene in endothelial cells of adult mice carrying the floxed HAS-2 allele) substantially reduced the glycocalyx structure. Importantly, HA is a specific binding site for angiopoietin-1 (Ang-1) and a key regulator of endothelial cell quiescence and maintenance of endothelial barrier function. HA deficiency triggers Ang-1-Tie2 receptor signaling disorder, which is characterized by vascular instability, mesangial dissolution, telangiectasia, and proteinuria, and gradually progresses to glomerular capillary rarefication and glomerulosclerosis, which produces the human DKD phenotype. They observed endothelial HA in renal biopsies from patients with different severities of diabetes and found that endothelial HA in glomerular capillaries gradually disappeared with the formation of DKD lesions. In patients with acute hyperglycemia or T1DM, the EG volume decreased by 50%-80% and the serum HA concentrations increased by 30%-80%[20]. Degradation of the EG and increased serum HA concentrations were also observed in T2DM patients[26] and rodent models of T1DM[64]. This shedding of HA resulted in increased vascular permeability with albumin escape. Despite the prominent role of HA in EG, its method of binding to the cell membrane and integrating with EG is unknown. Although HA may bind to the cell surface receptor CD44, covalent bonding is not observed, and it may also attach to the extracellular part of HAS, interact with CS on syndecan-1, connect with EG through HA-binding proteins, or even independently assemble into a fibrous network[20]. Increased glycosylation of CD44 was reported to weaken its ability to bind HA in a high-glucose environment, thereby decreasing HA binding to EG[65]. The human body contains six kinds of HYAL to degrade HA. HYAL-1 and HYAL-2 are common in mammalian tissues and cooperate to complete HA degradation[66]. HYAL-2 is a glycosylphosphatidylinositol anchoring enzyme that attaches to the outside of the cell membrane[67] and is accountable for the extracellular degradation of high molecular weight HA into an intermediate fragment that is approximately 20 kDa[39]. The intermediate HA fragment is then endocytosed into the cell via endocytic vesicles and degraded into a small fragment by HYAL-1[68]. Interestingly, there are large differences between high and low molecular weight HA. The former can enhance endothelial barrier function, but the latter can damage endothelial cells in various ways. For instance, low molecular weight HA induces endothelial cell inflammation through Toll-like receptors 2 and 4, stimulates the expression of vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1), leading to macrophage infiltration, cell inflammation, and injury, and it activates phagocytes to generate reactive oxygen species (ROS) in a size-dependent manner[39]. A study by Dane et al[69] found that 4 wk after injecting HYAL into C57BL/6 mice, the glomerular albumin permeability increased by 90% and the EG returned to integrity 4 wk after performing the injection. However, no significant proteinuria was observed during the experience, and this was possibly due to the protective effects of the normal apolipoprotein-E (apo-E) levels in these animals. Similar to the findings in apo-E absence mice, HYAL infusion results in EG disorder and proteinuria[70]. Several studies have also shown that the increase in HA concentration and HYAL activity, resulted in a thinning of the glycocalyx due to HA degradation, and this increased the transcapillary escape rate of albumin in mice with diabetes[71,72] and humans with T1DM[25] and T2DM[26]. Furthermore, Dogné et al[73] demonstrated that increasing the EG depth and maintaining the HA content during early DKD in HYAL-1-deficient mice contributed to preserving endothelial function and the functional barrier. Similarly, supplementation of HA analogs could compensate for glycocalyx loss. Thus, HA shedding could be utilized as a valuable observation for the pathogenesis of diabetic renal complications, and the presence of HA may prevent the emergence of early DKD.
Sialic acid and NEU: Sialic acid (SA), otherwise known as N-acetylneuraminic acid, is a constituent of cell membrane glycoproteins and glycolipids[74]. SA occurs at the glycocalyx surface and participates in signal recognition and the binding of sugars to proteins[39]. NEUs, tagged as sialidases, are a family of enzymes that regulate cell surface SA expression by removing SA from the glycocalyx[75]. Puerta-Guardo et al[76] found that nonstructural protein 1 induced NEUs expression, causing SA shedding and EG degradation. It could also activate cathepsin L in endothelial cells and affect HPSE activity[77]. What counts is that NEU could remove most of the glycocalyx and influenced the water, small solutes (as measured by transendothelial electrical resistance), and albumin fluxes, whereas HPSE (using HPSE III or recombinant HPSE-1), which removed HS GAGs alone, only had a remarkable impact on albumin filtration without changing water and small solute passages[78]. The results indicate that NEU is the most efficient enzyme with which to remove glycocalyx residues. Other studies have found that SA may directly regulate ESL permeability by steric hindrance and/or inducing secondary changes in ESL, such as the disruption of the albumin binding to EG[79]. Whether they really participate in the filtration barrier is currently unknown and this will require further research.
Cell adhesion molecules: Cell adhesion molecules include selectin, integrin, and the immunoglobulin superfamily[80]. Two major kinds of selectins are observed: P-selectin and E-selectin. P-selectin is produced and stored in the Weibel-Palade bodies found in endothelial cells and secreted in response to thrombin and histamine stimulation[81]. E-selectin is de novo synthesized in response to the stimulation of cytokines, such as interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), and lipopolysaccharide[82]. Integrins are heterodimeric membrane proteins made up of noncovalently bound α and β subunits. The luminal membrane of endothelial cells expresses integrin ανβ3, which influences the interaction between platelets and endothelial cells[83]. The immunoglobulin superfamily glycoproteins include ICAM-1 and ICAM-2, VCAM-1, and platelet endothelial adhesion molecule-1, which promotes the adhesion of leukocytes and platelets to endothelial cells[84]. The Ib-IX-V complex, another well-defined glycoprotein consisting of four different proteins (Ibα, Ibβ, IX, and V), binds to the von Willebrand factor and P-selectin to accelerate hemostasis[85]. GAG chains cover adhesion molecules in the physiological state, which sterically prevents leukocytes or platelets from binding to the cell adhesion molecule receptors. However, EG degradation activates and exposes adhesion molecules, and this contributes to increased leukocyte adhesion and thrombosis[86].
Shear stress changes and proteinuria: The source of proteinuria in DKD may arise from damage to the glycocalyx caused by alterations in shear stress. Shear stress is the mechanical force exerted by blood flow on the vessel wall. It significantly influences the structure and function of endothelial cells. The specific location and composition of the EG determine its unique function as a mechanosensor. The GAGs that extend into the extracellular region, which may deform, transmit the shear stress of the perceived blood flow to the core protein components, triggering core protein displacement. The cytoplasmic domain of the core proteins is linked to signaling elements, such as G-protein receptors, including those associated with endothelial nitric oxide synthase (eNOS) formation and cytoskeletal elements, such as actin[87], and this regulates transcription in the nucleus[20]. Florian et al[87] identified HS-GAG as a mechanosensor for the NO response, which is involved in mechanosensing and mediates NO production under shear stress. The depletion of syndecan-1 or 4 altered the mechanosensing and cell alignment[88,89]. In addition, reports suggested that degradation of EG by NEU and HYAL reduced flow-induced NO production[90], indirectly confirming the indispensable role of HA and SA in mechanical transduction. Furthermore, the glycocalyx participates in the scattering of concentrations of the agonists, and flow changes affect agonists’ distribution, thus transferring flow conditions to the cells. This results in variations in magnitude and the temporal and spatial distributions of the shear stress will modulate vascular tone and induce alterations in endothelial permeability and hydraulic conductivity, cytoskeletal structure, surface adhesion molecule expression, and gene expression[87].
The glycocalyx is undoubtedly present in podocytes and GBM, but there are differences in its composition and structure. The GBM glycocalyx was once considered the charge-selective barrier of the glomerular filtration layer. In recent years, an increasing number of studies have broken this traditional concept and suggested that the glycocalyx does not function as a major negative charge barrier in the GBM. The loss of its anion site does not lead to proteinuria. By comparison, the podocyte glycocalyx may, together with EG, constitute a “defensive line” that restricts albumin filtration.
Glycocalyx in the GBM
The primary components of the GBM include type IV collagens with α3, 4, and 5 chains, laminin β2, negatively charged GAGs, and core proteins, such as agrin, perlecan, nidogen, and collagen XVIII[24,91]. Laminin and collagen networks are the main determinants of the penetrative and selective barrier functions of the GBM[24]; however, their roles are beyond the scope of this article. Agrin is mostly produced by podocytes, has a molecular mass of 212 kDa, and carries at least two HS chains, and it constitutes the staple PGs of the GBM in all adult species studied[92]. Perlecan, with a molecular weight of 467 kDa, is mainly produced by glomerular endothelial cells and is attached to three HS side chains by the N-terminal domain I attachment sites. It occurs in the GBM during development but after this stage it is predominantly found in the mesangial matrix and Bowman’s capsule, as is collagen XVIII[93,94]. The HS in the GBM consists of alternating glucosamine and D-glucuronic acid/L-aduronic acid residues, which are negatively charged due to the presence of multiple carboxyl and N-, 2-O-, 6-O-, and 3-O- sulfate groups[95]. Additionally, the carbohydrate side chain SA is involved in the formation of the negative charge for GBM[96]. Previous studies believed that GAGs in the GBM, including HS, could repel negative charges, including albumin, and prevent their filtration. For example, ferritin and bovine serum albumin filtration occurred by inculcating bacterial GAGs degrading enzymes to remove GAGs at the original site of the GBM[97,98]. Injection of the anti-HS antibody JM403 causes hematuria and albuminuria in rats[99]. In many renal diseases, such as DKD, lupus nephritis, minimal change disease, and membranous nephropathy, the content of HS in the GBM was reduced and inversely correlated with urinary protein excretion levels[51,100].
However, the primary negative charge-dependent barrier function of the HS in the GBM does not stand up to scrutiny. One key component of HS assembly is the Ext1 gene product-the HS co-polymerase subunit. Using podocyte-specific Ext1 knockout (PEXTKO) mice to stop the polymerization of HS secreted by podocytes, Chen et al[101] found that the glomerulus foot process disappeared and mild and non-significant proteinuria occurred. To verify the presence or absence of anion sites in the GBM, they used GBM-specific HS-GAGs monoclonal antibody and polyethyleneimine staining to show a significant and sustained reduction in glomerular capillary wall HS-GAGs. Nevertheless, it is important to note that the staining of the HS-GAGs in the glomeruli was not wholly eliminated as mesangial and endothelial cells could still assemble HS-GAGs. Hence, HSPG secreted by podocytes is not necessary to limit proteinuria, and other mechanisms may exist. In this study, it cannot be ignored that the HSPG secreted by podocytes appears to have the ability to control podocyte behavior. However, Harvey et al[102] reported that GBM-specific agrin knockout mice did not develop podocyte foot process effacement. This suggests that neither HS-GAGs on agrin nor the agrin core proteins are critical in mediating foot process morphology. In immortalized podocytes, the loss of EXT1 results in the absence of not only ECM-related HS-GAGs but also cell membrane-associated HS-GAGs. From a cell-matrix interaction perspective, the podocyte phenotype of PEXTKO may be due to the podocyte surface HSPG’s inability to interact with HS-GAG-binding proteins such as laminin in the GBM[101]. Research investigating perlecan-HS and perlecan/agrin-HS dual mutated mice showed that anionic sites were significantly decreased within the GBM. However, the glomerular structures and renal functions were not altered overall and measurable proteinuria was not observed[103]. This indicates that the major role of HS in the GBM as a charge-selective barrier of capillaries can be ruled out. However, further research into the function of the HPSE and HS of the GBM in proteinuria production under pathological conditions, such as the Streptozotocin (STZ)-induced albuminuria in HPSE-TG mice or PEXTKO mice, is still required[51].
Podocyte glycocalyx
Several studies have been performed on the glycocalyx of podocytes. According to the different structures, podocytes can be separated into four areas: Apical membrane area of foot processes, cytoskeleton area, hiatus membrane between foot processes, and GBM junction area (bottom of foot processes of podocytes). The apical membrane region of the foot process is rich in SA and sulfate PGs, which provide the surface layer of the foot process with an anion charge barrier. The podocyte skeleton region, comprised of microtubules and filaments, maintains the typical morphology of podocytes and foot processes. The 25-60 nm hiatus between adjacent foot processes is connected by the slit diaphragm (SD), a specialized tight junction of proteins, including nephrin and podocin. This has been considered the central area for size selectivity in the filter barrier. However, Lawrence et al[104] recently stated that size-selective penetration into the lamina densa of the GBM and the podocyte glycocalyx, coupled with saturable tubular trapping, determines the macromolecules that enter the urine without direct size selection through the SD. Unlike the glycocalyx on the apical membrane region of the podocyte, the GBM junction area is covered by a unique glycocalyx[105]. It is presumed that the glycocalyx between the podocytes and GBM could be responsible for a portion of the charge selectivity of the GFB[24]. Dystroglycan, a highly glycosylated protein, is mainly localized in the basolateral and apical membranes of the cell. It can act as a receptor for laminin and agrin in the GBM and maintain SD structures by charge repulsion[91]. Significantly, the major salivary protein of the podocyte glycocalyx is podocalyxin, and this is mainly expressed in the apical membrane area and is highly glycosylated by 20% hexose, 4.5% SA, and N-acetylglucosamine[106]. In the human minimal change disease model simulated by puromycin aminoglycosides, the podocalyxin SA content in podocytes decreased, and foot processes fused, suggesting that the foot process fusion in puromycin aminoglycoside nephropathy was related to the reduction in SA[107]. Likewise, perfusion of the isolated rat kidney with polycations (e.g., protamine sulfate) to neutralize the polyanionic surface led to podocyte foot process retraction, SD displacement, and tight junction and gap junction formation between foot processes[108]. These phenomena considered that protamine sulfate neutralizes the negative charges of sulfate and SA residues on the PG membranes, which in turn altered podocyte morphology and intercellular connections through the attachment of ezrin protein and Na+/H+-exchanger regulatory factor 2 to the actin cytoskeleton, increasing albumin filtration through the podocyte[109]. In addition, it was also reported earlier that decreased podocyte-associated sulfate carbohydrates in DKD contribute to abnormally elevated urinary albumin excretion rates[110]. Thus, the loss of the podocyte glycocalyx charge and the secondary changes in podocyte morphology may have caused abnormal albuminuria.
However, the role of the podocyte glycocalyx in DKD is still in dispute. Garsen et al[111] used podocyte-specific endothelin receptor type A (ETRA or Ednra)/ETRB or Ednrb deficient (podETRKO) mice to induce diabetes. They found that diabetic wild-type (WT) mice displayed increased cortical HPSE mRNA, glomerular HPSE protein expression, and glomerular HPSE activity, whereas glomerular HS expression was decreased. The glycocalyx thickness of endothelial cells and podocytes was reduced by approximately 50%-60%, with significant proteinuria. In contrast, in diabetic podETRKO mice, HPSE and HS expression was normal, only the podocyte glycocalyx decreased by approximately 25%, and the proteinuria decreased significantly. The reduced podocyte glycocalyx thickness in the podETRKO mice appeared to be insufficient to produce albuminuria. Nevertheless, they did not rule out the possibility that proteinuria in diabetic WT mice required a combined reduction in the endothelial and podocyte glycocalyx. Furthermore, the critical role of growth factors in podocyte-endothelial crosstalk involves maintaining glycocalyx integrity, and this will be discussed in the following section.
MECHANISMS OF GLYCOCALYX DAMAGE
The specific mechanisms of glycocalyx damage in the early stages of DKD that were associated with oxidative stress, proinflammatory cytokines, growth factors, transcription factors, and other factors were reviewed. It is of note that all these mechanisms will require further refinement beyond existing reports in future studies.
Oxidative stress
ROS overproduction is vital for the pathogenesis of DKD. Specifically, the generation of ROS with DKD results from mitochondrial production, NAD(P)H oxidase, and xanthine oxidase (XO), among others[112]. The damage to the glycocalyx caused by ROS can be summarized as follows: (1) ROS can degrade HA, HS, and CS, which will directly destroy the glycocalyx. ROS cleave HS chains primarily from the glomerular EG, disrupting the EG via a direct mode of action without affecting the GAGs biosynthesis pathway[61]; (2) ROS can upregulate the expression of related sheddases to degrade the glycocalyx. For example, ROS activates MMPs and dissolves syndecan domains to induce glycocalyx proteolysis, which causes the glycocalyx to fall off[113]; and (3) ROS are capable of inactivating endogenous inhibitors of neutrophil elastase, and the neutrophil elastase then binds the HS chains of the syndecans, leading to their degradation[114].
In DKD research, ET and ET-related receptors have always been a research focus. Recently, the specific involvement of mitochondrial ROS in endothelial injury in early diabetic mice was well documented. Mitochondrial DNA damage in the glomerular endothelial cells of DKD-susceptible mice and DKD patients was associated with increased glomerular Ednra expression[115]. Higher plasma ET-1 levels were also observed in diabetic patients and DKD animal models[116,117]. Ebefors et al[118] reported that podocyte-derived ET-1 increased the expression of HPSE and HYAL in glomerular endothelial cells through Ednra, thereby mediating ESL loss. In mice, endothelial damage (including glycocalyx damage), proteinuria, podocyte loss, and glomerulosclerosis induced by diabetes were mitigated by mitochondrial ROS scavenging or a specific Ednra blockade. Therefore, Qi et al[115] proposed that the upregulation of endothelial Ednra and the activation of circulating ET-1 characterize DKD susceptibility in mice and humans. Combined with previous control studies by Garsen et al[111] that involved diabetic podETRKO mice and WT mice, ETRA/ETRB deficiency was found to protect the endothelial and podocyte glycocalyx from HPSE degradation and reduce the production of proteinuria. It can thus be concluded that the overactivation of the ET-1 signaling path in the endotheliocyte and/or podocytes is a detrimental factor associated with glycocalyx damage and proteinuria in DKD. AGEs are also involved in mitochondrial ROS production under high-glucose conditions. AGEs can act on the receptor of AGEs (RAGE) on podocytes and activate the nuclear factor kappa-B (NF-κB) pathway, leading to increased HPSE synthesis[119].
NAD(P)H oxidase appears to be an essential mediator of ROS generation in glomerular endothelial cells. Human glomerular endothelial cells treated in a high-glucose environment showed increased ROS production, and this could be blocked entirely by NADPH oxidase inhibitors[120]. NAD(P)H oxidase 2 (NOX2) and NOX4 have substantial roles in glycocalyx injury related to DKD. In the early stage of diabetes in Akita mice, NAD(P)H oxidase was activated in endothelial cells, and the ROS level was increased. Excessive ROS activated the transcription of HPSE via the nuclear translocation of the E-26 transcription factor[121]. To further validate that NAD(P)H oxidase activation initiated and worsened DKD progression, Nagasu et al[122] bred endothelium-targeted Akita mice overexpressing NOX2 (NOX2-TG-Akit mice), which exhibited reduced ESL and had further increases in their capillary permeability. When NOX2-TG-Akit mice were treated with gp91TAT, a NOX2-specific inhibitor, at 6-8 wk of age, glomerular tomato lectin staining was restored and similar to that in the WT mice. Besides the NOX2 subunits, NOX4 expression was also increased in diabetic kidneys[123] and was interconnected with inflammation. The ROS produced by NOX4 increased the damage to the macromolecules and led to the generation of advanced oxidation protein products, advanced lipid oxidation end products, and AGEs[124]. In the glomerulus, AGEs acted through RAGE to stimulate the release of proinflammatory cytokines and the expression of DKD-related molecules, such as vascular endothelial growth factor (VEGF), connective tissue growth factor, transforming growth factor-β, insulin-like growth factor-I, platelet-derived growth factor, TNF, IL-1β, and IL-6[125,126]. Overall, endothelial injury is clearly related to increased endothelial ROS production by NAD(P)H oxidase, represents a critical step in the pathogenesis of DKD, and may be a potential therapeutic target for its onset.
XO is mainly expressed in the liver and intestine[127]. In STZ-induced diabetic rats, XO expression was increased in the liver and was taken up by glomerular endothelial cells via blood circulation, where it could then bind with sulfated GAGs on the endothelial surface[128-130]. There was no difference in xanthine oxidoreductase activity in liver tissues between the WT and Akita mice, but the Akita mice showed higher xanthine oxidoreductase activity in renal tissues. Renal XO produced excessive ROS in endothelial cells, which led to a disturbance of endothelial homeostasis, a reduction of the glycocalyx, and proteinuria. Topi, a non-purine selective XO inhibitor, could reduce albuminuria by mitigating endothelial damage induced by glomerular oxidative stress from XO activation[131]. It was thus inferred that ROS causing glycocalyx damage in DKD originates, at least in part, from the XO system.
Proinflammatory cytokines
Inflammation is viewed as a vital mechanism in the development and progression of diabetes mellitus, and it persists for a long period before the onset of DKD[132]. TNF-α is a proinflammatory cytokine that can directly destroy the glycocalyx[133] but also increase the permeability of endothelial cells by activating MMP-9, mediating the destruction of the EG caused by syndecan-4 and HS shedding[34]. The clinical use of a TNF inhibitor (enalapril) attenuated glycocalyx loss in an experimental endotoxin model[134]. High levels of glucose could cause the abnormal regulation of TNF-α mediators, producing microalbuminuria[135]. In patients with type 2 DKD, the increase in serum IL-1β preceded the increase in serum HS, suggesting that abnormal inflammasomes predate and may contribute to the impairment of EG[132]. Reine et al[136] showed that IL-1β, via MMP-9, induced syndecan-4 shedding in con-ditionally immortalized human glomerular endothelial cells in a dose-dependent manner. The NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasome is one of the most comprehensively studied inflammasomes involved in the emergence and development of various inflammation-related diseases, and diabetes is no exception[137]. The NLRP3 inflammasome activates IL-1β and IL-18, which can both subsequently activate the intracellular signaling molecule MyD88 by binding to the cell surface receptor, and this activates the NF-κB signaling pathway. The activated NF-κB signaling pathway can increase the secretion of proinflammatory mediators, such as cytokines and chemokines, and ultimately destroy the EG[138]. The damage to the vascular EG exposes ICAM-1 and VCAM-1. Circulating leukocytes are, thus, more likely to adhere to the endothelial cells, further contributing to inflammation and endothelial dysfunction. Furthermore, fragments produced by glycocalyx degradation induce the polarization of T helper 1 cells, which subsequently induces the upregulation of CD44 and Toll-like receptors 2 and 4. This results in the adhesion and rolling of macrophages and monocytes, activation of the NF-κB pathway, and upregulation of the expression of HPSE, MMPs, HYAL, HAS, and NEU[39]. Diabetic inflammatory conditions and glycocalyx shedding cause a vicious cycle, and thus, the integrity of the glycocalyx must be protected under inflammatory conditions.
Monocyte chemoattractant protein-1 (MCP-1) is involved in the recruitment of monocytes, the migration of monocytes and macrophages, and the regulation of macrophage differentiation. Once chemokines are induced, chemical ligand gradients or chemokine gradients are formed for the directed migration of cells expressing appropriate chemokine receptors. Moreover, these gradients are formed along extracellular structures, such as the HS GAGs of the glycocalyx[139]. In patients with DKD, MCP-1 was increased in renal tissues and urine[140]. Even in the early period of DKD, macrophages can be identified in the glomeruli[141]. Infiltrating macrophages could secrete cathepsin L, activate HPSE, and disrupt EG. The C-C chemokine receptor type 2 (CCR2) is an MCP-1 cognate receptor. The blockade of CCR2 with the small molecule CCX140-B was found to reduce proteinuria in patients with DKD[142]. An animal study by Boels et al[143] showed that the treatment of diabetic apo-E knockout mice with an MCP-1 inhibitor, the Spiegelmer emapticap pegol (NOX-E36), for 4 wk, resulted in the polarization of tissue macrophages to an anti-inflammatory phenotype, restoration of glomerular EG, and the reduction of albuminuria, despite the persistent loss of podocyte function. Meanwhile, in a double-blinded, randomized, multicenter pilot study, NOX-E36 was also observed to be safe and well tolerated and to have beneficial effects on the urinary albumin/creatinine ratio and hemoglobin A1c in patients with T2DM and albuminuria in five European countries[144]. The cellular mechanisms involved in EG degradation remain obscure. It has been reported that proinflammatory factors such as TNF-α activate mast cells to generate HYAL, HPSE, and MMP-9/2[39]. Mast cells can also activate adipose tissue cells to release HPSE, which can then degrade HS chains[145].
Growth factors
The crosstalk between glomerular endothelial cells and podocytes is a major event in the progression of DKD, in which growth factors play a pivotal role[146]. There are five VEGF variants in humans, VEGF-A, -B, -C, -D, and the placenta growth factor[147]. VEGF-A165, a VEGF-A splice variant, is the most abundant isoform in the human body. It mainly forms and maintains endothelial fenestration through VEGF receptor 2 (VEGFR-2)[148]. VEGF-A165 was upregulated during the early stages of DKD in both humans[149] and in experimental models[150]. Moreover, VEGFR-2 was also upregulated in early DKD and associated with enhanced glomerular endothelial VEGF-A165-VEGFR-2 signaling[151]. VEGF-A165 boosted the production of MMP-9, A disintegrin, and metalloproteinase domain 17, and increased the removal of sulfate GAGs from the glycocalyx[152,153]. In contrast, VEGF-A165b protected the EG, as demonstrated in an early mouse model of T1DM. The application of human recombinant VEGF-A165b restored glomerular EG thickness, possibly via delayed downstream signaling by VEGF-A165b-induced VEGFR-2, thus indicating VEGFR-2/VEGFR-1 heterodimer formation[154]. Aside from VEGF-A165b, VEGF-C can also antagonize VEGF-A/VEGFR-2 signaling and reduce macromolecular protein passage[78]. The VEGF-C treatment blocked the VEGF-A-induced increase in glomerular permeability in vitro and rescued the elevated albumin permeability in the glomeruli of type 2 diabetic mice with proteinuria. Glomerular albumin permeability was increased in mice when administered either acutely (30 min) or chronically (2 wk) with shedding enzymes, but VEGF-C blocked this effect while maintaining the EG depth and/or coverage[155]. Most importantly, VEGF-C could also induce HA and CS synthesis and significantly increased the expression of N-deacetylase/N-sulfotransferase-2, which is responsible for adding a sulfate group to GAGs to increase the negative charge of the glycocalyx[153]. Angiopoietins are another type of endothelial cell growth factor that interact with VEGF to regulate endothelial cell permeability[31]. The two paramount members of the Angiopoietins family are Ang-1 and Ang-2[156]. For normal endothelial function, the receptor Tie2 must interact with VEGFR-2[157]. In normal physiological conditions, the phosphorylation of the receptor is mainly induced by Ang-1. However, in diabetic pathological conditions, the balance between these two isoforms is disrupted, and Ang-2 prevails, which results in increased HPSE-dependent glycocalyx degradation[158]. Furthermore, Ang-2 increases VEGF-A expression, which in turn reinjures the glycocalyx by upregulating MMP-9[152]. It is safe to conclude that VEGF-A165b, VEGF-C, and Ang-1 can inhibit increases in the glomerular VEGF-A165 signal, rebalance the related sheddase, restore the EG layer, and reduce proteinuria in patients with diabetes.
Others
Krüppel-like factor 2 (KLF2), an essential member of the KLFs, is highly expressed in vascular endothelial cells and participates in the regulation of vascular tone, anti-inflammation, anti-thrombosis, angiogenesis, and other essential processes that are required to maintain vascular homeostasis[159-161]. According to research, KLF2 expression was reduced in STZ-induced diabetic rats. Compared with diabetic WT mice, diabetic KLF2 knockout mice showed increased glomerular expression of VEGF-A, VEGFR-2, and Ang-2 and decreased expression of VEGFR-1, Tie2, and Ang-1, as well as decreased expression of the zonula occludens-1 (ZO-1), glycocalyx, and eNOS. These data suggest that KLF2 down-regulation may contribute to glomerular endothelial cell damage in early DKD. The potential gene regulated by KLF2, NOS-3, reportedly encodes eNOS. In diabetic kidneys, eNOS expression may be inhibited by high levels of glucose, and KLF2 is required as a compensatory mechanism to maintain its expression. However, the specific mechanism by which KLF2 reduces endothelial damage in a diabetic environment will require further investigation. KLF2 may attenuate DKD by activating anti-oxidative stress and anti-inflammatory pathways[161]. Long non-coding RNA H19 was obviously increased in diabetic glomeruli and high glucose-stimulated rat glomerular endothelial cells. Deficiency or silencing of the H19 gene could significantly relieve endothelial structural damage in diabetic rats by upregulating the expression of ZO-1, occludin, syndecan-1, and endothelial cell activation markers sVCAM-1 and sICAM-1 via the Akt/eNOS signaling pathway[162]. The antiaging gene Klotho encodes a single-channel transmembrane protein expressed in the kidney[163]. Klotho protein expression was diminished in the kidneys of patients with early DKD, Akita mice, and diabetic models like STZ-induced or db/db mice[164-167]. Moreover, Klotho gene deficiency aggravated glomerular injury in diabetic models[166]. To date, the molecular mechanisms underlying Klotho loss and its contributions to diabetic glomerular injury have not yet been confirmed. Oxidative stress or the extracellular signal-regulated kinase, NF-κB, induces low-density lipoprotein oxidation and may suppress Klotho expression in Akita mice[167]. Kadoya et al[167] used lectin staining to measure glomerular ESL and discovered that Akita mice had distinctly smaller areas of positive staining than WT mice while KLTG Akita mice (obtained by crossing Klotho transgenic mice with Akita mice) had decidedly restored areas of positive staining and reduced albuminuria. As Klotho induces the expression of manganese SOD (MnSOD), which is a major superoxide scavenger and is resistant to oxidative stress, it was hypothesized that Klotho protects against glycocalyx damage by inducing MnSOD. Therefore, the recombinant Klotho protein may be a new target for future DKD treatments.
THERAPIES TARGETING GLYCOCALYX DAMAGE
At present, two targeted treatment strategies are available for glycocalyx damage: Replace the lost glycocalyx components directly and weaken or enhance the specific targets of the glycocalyx damage process to prevent further damage.
Glycocalyx replacement therapy
Attempts to supplement charge loss via GAGs have focused on sulodexide, a compound of small molecular mass GAGs (80% HS and 20% CS), which has been used to treat microvascular complications in patients with diabetes[168]. Initially, a few small studies were conducted which demonstrated its effectiveness in DKD patients with microalbuminuria[26,169]. Subsequently, however, two more extensive randomized, double-blinded, placebo-controlled studies were conducted, and they confirmed that treatment with sulodexide did not decrease proteinuria[168,170]. It is important to emphasize that many researchers believed that the role of sulodexide was underestimated in these later studies[171]. Furthermore, research is also required to determine whether sulodexide is absorbed through the gastrointestinal tract[31]. Using a transplantation-induced ischemia/reperfusion model, Jacob et al[172] discovered that albumin supplementation reduced glycocalyx shedding and leukocyte adhesion to the endothelial cells.
Glycocalyx degradation-blocking therapy
In addition to the HPSE antibodies or specific HPSE inhibitors that could prevent the degradation of GAGs, heparin (analogs) was also found to have a protective effect on DKD because it effectively inhibited HPSE activity. If heparin components with the maximum HPSE inhibitory effect and minimum anticoagulant activity are selected, then these heparin derivatives could function as inhibitors to protect the glycocalyx. Other potential targets may be the transcription, transport, and processing levels of HPSE[51]. A previous study showed that the steroid hormone vitamin D can reduce the expression of HPSE in damaged cells, both in vivo and in vitro, and its mechanism may be to directly bind to the HPSE promoter through its receptor, affecting the activity of the HPSE promoter[173]. In addition, RAAS blockers like angiotensin-converting enzyme inhibitors could inhibit HPSE activity[174]. Piperazine ferulate has been widely used in the treatment of various kidney diseases. It was recently reported that piperazine ferulate downregulates the expression of HPSE-1 and increases the expression of syndecan-1 by regulating the expression of AMP-activated protein kinase (AMPK), thereby reducing the degradation of the glomerular glycocalyx and alleviating the damage to the glomerular endothelial cell filtration barrier that was induced by high levels of glucose[175]. Manipulating the glycocalyx by inhibiting MMPs provides an attractive therapeutic target for DKD. MMP inhibitor therapy has become a reality in clinical settings, as, for example, tetracycline, an antibiotic agent, can inhibit MMPs at subantibiotic doses[176]. The development of more specific MMP inhibitors is expected to reduce some of the adverse reactions associated with the broad-spectrum MMP inhibitors currently involved in clinical trials[32].
Enhanced oxidative stress damages the glycocalyx in DKD, both directly and indirectly. The selection of targeted antioxidants is thus also essential. AdipoRon is an oral, synthetic adiponectin receptor agonist that activates the AMPK/peroxisome proliferation-activated receptor-α pathway, reducing high glucose-induced oxidative stress and apoptosis in endothelial cells and thus improves endothelial dysfunction[177]. The RAAS blocker telmisartan reduced proteinuria[178] and NAD(P)H-dependent oxidase activity[179] in T2DM patients. Pyrazolopyridine compounds, GKT136901 and GKT137831, were dual inhibitors of the NOX1 and NOX4 subtypes that reduced ROS formation in db/db mice[180]. Recent studies have found that Cyclocarya paliurus triterpenoids mitigate oxidative stress in endothelial cells through the ROCK pathway, reduce VCAM-1 and ICAM-1 levels, block glycocalyx damage, and ultimately improve renal endothelial function[181]. Most importantly, the Ednra receptor antagonist could reduce glomerular vasodilation, promote the binding of ET-1 to Ednrb, enhance NO synthesis, decrease the production of ROS, reduce glycocalyx damage, and change the glomerular permeability of albumin[182,183]. The first use of Bosentan was found to have little effect on reducing proteinuria in recent studies, while the use of Avosentan was also discontinued early due to the high incidence of heart failure[184]. Although Endra has been reported to cause sodium retention (Ednra blocking reduces the constriction of efferent arterioles and hyperfiltration), most studies indicate that fluid retention results from Ednrb blocking because Ednrb activation in the renal collecting ducts promotes sodium and water excretion through sodium channels[185]. Based on pharmacological actions, it is believed that the selectivity of Bosentan (Ednra: Ednrb block = 20:1) and Avosentan (Ednra: Ednrb block = 50-300:1) for Ednra is reduced at high doses, resulting in sodium and fluid retention due to the Ednrb blockade[184]. If so, low-dose and highly selective ET receptor antagonists may be the way forward to improve the effective clinical use of this class of drugs. Atrasentan (Ednra: Ednrb block = 1200:1) is a selective receptor blocker[185]. Boels et al[183] found that Atrasentan therapy restored EG, reduced glomerular HPSE expression, increased the renal NO concentration, and significantly altered the glomerular macrophage M1 and M2 balance, eventually reducing the urinary protein creatinine ratio in diabetic apo-E deficient mice. This result was also verified in vitro in the co-culture of endothelial cells and pericytes exposed to laminar flow. Nevertheless, even with highly selective antagonists, increasing the dose may lead to fluid retention and heart failure. It underscores the necessity of drug combinations so that the benefits from Atrasentan treatments for nephropathy can be achieved while also reducing the incidence of adverse cardiovascular events. Ultimately, 0.75 mg/d Atrasentan as an adjunct to RAAS inhibition was identified as the optimal dose for renal protection, as this could minimize proteinuria but also had the lowest indicator for salt retention in patients with T2DM and DKD[186]. Another approach to avoiding heart failure is to use a combination of Ednra inhibitors with sodium-glucose cotransporter 2 (SGLT2) inhibitors. The ZENITH trial tested this hypothesis by randomizing chronic kidney disease patients with and without T2DM to receive Zibotentan in combination with the SGLT2 inhibitor dagliazine. The trial results are expected to be available in 2023[187]. The Ednra inhibitors are thus a welcome pharmacological addition that could help to further reduce the risk of renal outcomes in patients already treated with RAAS and SGLT2 inhibitors[188]. Moreover, the hypoglycemic agents SGLT2 inhibitors had beneficial effects on the endothelium, primarily through their anti-inflammatory and antioxidant effects[189]. The glucagon-like peptide-1 receptor agonist can lower the harmful effects of oxidative stress and inflammation in endothelial cell mitochondria by activating glucagon-like peptide-1 receptor[190].
Inflammation and oxidative stress are always inextricably intertwined. Inhibiting the NLRP3 inflammasome and IL-1β could reduce mitochondrial ROS production[191], and some NLRP3 inhibitory molecules, for example, MCC950, CY-09, OLT1177, and FT011, have been developed for in vitro and animal experiments[192-195]. Pentoxifylline (PTF), a methylxanthine-derived phosphodiesterase inhibitor, had powerful antioxidant properties when used alone[196] or in combination with angiotensin-converting enzyme inhibitor in small studies of DKD[197]. A meta-analysis reported that pentoxifyllines had a significant antiproteinuric effect in all patients with DKD, which might be attributed to a decrease in pro-inflammatory cytokines[198]. The transforming growth factor-β inhibitor pirfenidone[199] has also been found to improve oxidative stress in chronic hyperglycemic renal lesions in rats. However, the studies on this medicine are in their preliminary stages and further evaluations are required[200].
CONCLUSION
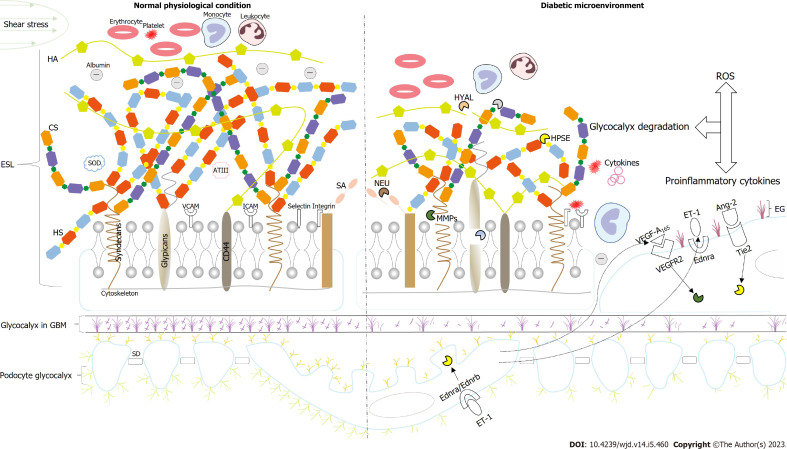
Approximately half of all patients with DKD may eventually develop end-stage renal disease and face dialysis treatment, creating serious health and economic burdens for countries, societies, and individuals. It is thus imperative that methods are developed to delay, prevent, or reverse DKD progression at an early stage. Microalbuminuria is the best predictor of high DKD risk. Endothelial dysfunction with glycocalyx damage has been identified as the first step in developing microalbuminuria in early DKD. The EG, or ESL, is a complex dynamic hydrated structure that is integral to the formation of the glomerular negative charge barrier. Under normal physiological conditions, the degradation and remodeling of the EG can maintain a balance that effectively prevents albumin filtration. However, in the diabetic microenvironment, excessive oxidative stress, inflammation, and other harmful factors combined with the presence of related degrading enzymes promote the increased shedding of glycocalyx components and homeostasis imbalance, leading to endothelial dysfunction and eventual proteinuria. In addition, the interaction between podocytes and endothelial cells and the joint shedding of the EG and podocyte glycocalyx are also one of the important causes of proteinuria. Therefore, targeting the key molecules in the glycocalyx damage mechanism to prevent the continuous loss of the glycocalyx or replace the lost glycocalyx components is thus a promising therapeutic strategy. Furthermore, this strategy also highlights the importance precision medicine will have in the future (Figure 1).
Figure 1.
The glycocalyx in the physiological state and the diabetic microenvironment. Under normal physiological conditions, endothelial glycocalyx shedding and recovery are in a state of equilibrium, which can form an albumin exclusion barrier on the endothelial surface. However, in the diabetic microenvironment, inflammation, oxidative stress, and other harmful factors can not only directly destroy the glycocalyx but also hydrolyze the glycocalyx by activating the related sheddases, such as heparinase (HPSE), hyaluronidase, matrix metalloproteinases (MMPs), and neuraminidase, resulting in the shedding of a large number of glycocalyx components, leukocyte and platelet adhesion, macrophage infiltration, and microalbuminuria. In addition, the interaction between podocytes and endothelial cells plays a vital role in glycocalyx degradation. For example, the production of vascular endothelial growth factor A165 (VEGF-A165) by podocytes acts on VEGF receptor 2 (VEGER2) in endothelial cells to induce the production of MMPs. Angiopoietin-2 (Ang-2) acts on Tie2 to increase the expression of HPSE, and Ang-2 also upregulates VEGF-A165 to degrade the glycocalyx further. In addition, there is an interaction between Tie2 and VEGER2. Endothelin-1 acts on endothelial cells Ednra to produce reactive oxygen species; it also acts on Ednra/Ednrb in podocytes to induce the production of HPSE to degrade the glycocalyx. HA: Hyaluronic acid; CS: Chondroitin sulfate; HS: Heparin sulfate; SA: Sialic acids; HPSE: Heparinase; MMPs: Matrix metalloproteinases; HYAL: Hyaluronidase; NEU: Neuraminidase; ROS: Reactive oxygen species; VEGF-A165: Vascular endothelial growth factor A165; VEGFR-2: Vascular endothelial growth factor receptor 2; ET-1: Endothelin-1; Ednra: Endothelin receptor type A; Ednrb: Endothelin receptor type B; Ang-2: Angiopoietin-2; SOD: Superoxide dismutase; AT III: Antithrombin III; ICAM: Intercellular adhesion molecule; VCAM: Vascular cell adhesion molecule; EG: Endothelial glycocalyx; GBM: Glomerular basement membrane; ESL: Endothelial surface layer; SD: Slit diaphragm.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 7, 2022
First decision: February 28, 2023
Article in press: April 12, 2023
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng TH, Taiwan; Jabbarpour Z, United Kingdom S-Editor: Wang JJ L-Editor: A P-Editor: Chen YX
Contributor Information
Hui Yu, Department of Nephrology, Qilu Hospital of Shandong University, Jinan 250012, Shandong Province, China.
Yi-Yun Song, Department of Nephrology, Qilu Hospital of Shandong University, Jinan 250012, Shandong Province, China.
Xian-Hua Li, Department of Nephrology, Qilu Hospital of Shandong University, Jinan 250012, Shandong Province, China. lixianhua7075@sina.com.
References
- 1.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 2.Gregg EW, Sattar N, Ali MK. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016;4:537–547. doi: 10.1016/S2213-8587(16)30010-9. [DOI] [PubMed] [Google Scholar]
- 3.Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2:56–64. doi: 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, Li Y, Yeung RO, Wang J, Matsushita K, Coresh J, Zhao MH, Wang H. Trends in Chronic Kidney Disease in China. N Engl J Med. 2016;375:905–906. doi: 10.1056/NEJMc1602469. [DOI] [PubMed] [Google Scholar]
- 5.Barutta F, Bellini S, Canepa S, Durazzo M, Gruden G. Novel biomarkers of diabetic kidney disease: current status and potential clinical application. Acta Diabetol. 2021;58:819–830. doi: 10.1007/s00592-020-01656-9. [DOI] [PubMed] [Google Scholar]
- 6.Parving HH, Chaturvedi N, Viberti G, Mogensen CE. Does microalbuminuria predict diabetic nephropathy? Diabetes Care. 2002;25:406–407. doi: 10.2337/diacare.25.2.406. [DOI] [PubMed] [Google Scholar]
- 7.Lazzara MJ, Deen WM. Model of albumin reabsorption in the proximal tubule. Am J Physiol Renal Physiol. 2007;292:F430–F439. doi: 10.1152/ajprenal.00010.2006. [DOI] [PubMed] [Google Scholar]
- 8.Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol. 2001;281:F579–F596. doi: 10.1152/ajprenal.2001.281.4.F579. [DOI] [PubMed] [Google Scholar]
- 9.Stehouwer CD. Endothelial dysfunction in diabetic nephropathy: state of the art and potential significance for non-diabetic renal disease. Nephrol Dial Transplant. 2004;19:778–781. doi: 10.1093/ndt/gfh015. [DOI] [PubMed] [Google Scholar]
- 10.Deckert T, Kofoed-Enevoldsen A, Vidal P, Nørgaard K, Andreasen HB, Feldt-Rasmussen B. Size- and charge selectivity of glomerular filtration in Type 1 (insulin-dependent) diabetic patients with and without albuminuria. Diabetologia. 1993;36:244–251. doi: 10.1007/BF00399958. [DOI] [PubMed] [Google Scholar]
- 11.Jeansson M, Granqvist AB, Nyström JS, Haraldsson B. Functional and molecular alterations of the glomerular barrier in long-term diabetes in mice. Diabetologia. 2006;49:2200–2209. doi: 10.1007/s00125-006-0319-z. [DOI] [PubMed] [Google Scholar]
- 12.Lemley KV, Blouch K, Abdullah I, Boothroyd DB, Bennett PH, Myers BD, Nelson RG. Glomerular permselectivity at the onset of nephropathy in type 2 diabetes mellitus. J Am Soc Nephrol. 2000;11:2095–2105. doi: 10.1681/ASN.V11112095. [DOI] [PubMed] [Google Scholar]
- 13.Ballermann BJ. Contribution of the endothelium to the glomerular permselectivity barrier in health and disease. Nephron Physiol. 2007;106:p19–p25. doi: 10.1159/000101796. [DOI] [PubMed] [Google Scholar]
- 14.Satchell SC, Tooke JE. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium? Diabetologia. 2008;51:714–725. doi: 10.1007/s00125-008-0961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levick JR, Smaje LH. An analysis of the permeability of a fenestra. Microvasc Res. 1987;33:233–256. doi: 10.1016/0026-2862(87)90020-3. [DOI] [PubMed] [Google Scholar]
- 16.Ryan GB, Karnovsky MJ. Distribution of endogenous albumin in the rat glomerulus: role of hemodynamic factors in glomerular barrier function. Kidney Int. 1976;9:36–45. doi: 10.1038/ki.1976.5. [DOI] [PubMed] [Google Scholar]
- 17.Peters T Jr. Serum albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- 18.Bohrer MP, Deen WM, Robertson CR, Troy JL, Brenner BM. Influence of molecular configuration on the passage of macromolecules across the glomerular capillary wall. J Gen Physiol. 1979;74:583–593. doi: 10.1085/jgp.74.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arkill KP. A Reinterpretation of Evidence for the Endothelial Glycocalyx Filtration Structure. Front Cell Dev Biol. 2021;9:734661. doi: 10.3389/fcell.2021.734661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dogné S, Flamion B, Caron N. Endothelial Glycocalyx as a Shield Against Diabetic Vascular Complications: Involvement of Hyaluronan and Hyaluronidases. Arterioscler Thromb Vasc Biol. 2018;38:1427–1439. doi: 10.1161/ATVBAHA.118.310839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandhi NS, Mancera RL. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des. 2008;72:455–482. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 22.Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, Vink H, Kastelein JJ, Stroes ES. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55:480–486. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- 23.Zuurbier CJ, Demirci C, Koeman A, Vink H, Ince C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol (1985) 2005;99:1471–1476. doi: 10.1152/japplphysiol.00436.2005. [DOI] [PubMed] [Google Scholar]
- 24.Schlöndorff D, Wyatt CM, Campbell KN. Revisiting the determinants of the glomerular filtration barrier: what goes round must come round. Kidney Int. 2017;92:533–536. doi: 10.1016/j.kint.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, Kastelein JJ, Stroes ES, Vink H. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55:1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 26.Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M, Stroes ES, Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:2646–2655. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swärd P, Rippe B. Acute and sustained actions of hyperglycaemia on endothelial and glomerular barrier permeability. Acta Physiol (Oxf) 2012;204:294–307. doi: 10.1111/j.1748-1716.2011.02343.x. [DOI] [PubMed] [Google Scholar]
- 28.Aldecoa C, Llau JV, Nuvials X, Artigas A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: a review. Ann Intensive Care. 2020;10:85. doi: 10.1186/s13613-020-00697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinsella MG, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr. 2004;14:203–234. doi: 10.1615/critreveukaryotgeneexpr.v14.i3.40. [DOI] [PubMed] [Google Scholar]
- 30.Zeng Y, Tarbell JM. The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS One. 2014;9:e86249. doi: 10.1371/journal.pone.0086249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korakas E, Ikonomidis I, Markakis K, Raptis A, Dimitriadis G, Lambadiari V. The Endothelial Glycocalyx as a Key Mediator of Albumin Handling and the Development of Diabetic Nephropathy. Curr Vasc Pharmacol. 2020;18:619–631. doi: 10.2174/1570161118666191224120242. [DOI] [PubMed] [Google Scholar]
- 32.Ramnath RD, Butler MJ, Newman G, Desideri S, Russell A, Lay AC, Neal CR, Qiu Y, Fawaz S, Onions KL, Gamez M, Crompton M, Michie C, Finch N, Coward RJ, Welsh GI, Foster RR, Satchell SC. Blocking matrix metalloproteinase-mediated syndecan-4 shedding restores the endothelial glycocalyx and glomerular filtration barrier function in early diabetic kidney disease. Kidney Int. 2020;97:951–965. doi: 10.1016/j.kint.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amirpour-Najafabadi B, Hosseini SS, Sam-Sani P, Rezaei E, Ramezani M, Changizi-Ashtiyani S. The glycocalyx, a novel key in understanding of mechanism of diabetic nephropathy: a commentary. J Diabetes Metab Disord. 2021;20:2049–2053. doi: 10.1007/s40200-021-00826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramnath R, Foster RR, Qiu Y, Cope G, Butler MJ, Salmon AH, Mathieson PW, Coward RJ, Welsh GI, Satchell SC. Matrix metalloproteinase 9-mediated shedding of syndecan 4 in response to tumor necrosis factor α: a contributor to endothelial cell glycocalyx dysfunction. FASEB J. 2014;28:4686–4699. doi: 10.1096/fj.14-252221. [DOI] [PubMed] [Google Scholar]
- 35.Fransson LA, Belting M, Cheng F, Jönsson M, Mani K, Sandgren S. Novel aspects of glypican glycobiology. Cell Mol Life Sci. 2004;61:1016–1024. doi: 10.1007/s00018-004-3445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 37.Weksberg R, Squire JA, Templeton DM. Glypicans: a growing trend. Nat Genet. 1996;12:225–227. doi: 10.1038/ng0396-225. [DOI] [PubMed] [Google Scholar]
- 38.McCarthy KJ. Syndecan-4: major player or innocent bystander of the endothelial glycocalyx? Kidney Int. 2020;97:858–860. doi: 10.1016/j.kint.2020.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Z, Wu N, Wang J, Zhang Q. Roles of Endovascular Calyx Related Enzymes in Endothelial Dysfunction and Diabetic Vascular Complications. Front Pharmacol. 2020;11:590614. doi: 10.3389/fphar.2020.590614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manon-Jensen T, Multhaupt HA, Couchman JR. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J. 2013;280:2320–2331. doi: 10.1111/febs.12174. [DOI] [PubMed] [Google Scholar]
- 41.Lauhio A, Sorsa T, Srinivas R, Stenman M, Tervahartiala T, Stenman UH, Grönhagen-Riska C, Honkanen E. Urinary matrix metalloproteinase -8, -9, -14 and their regulators (TRY-1, TRY-2, TATI) in patients with diabetic nephropathy. Ann Med. 2008;40:312–320. doi: 10.1080/07853890801923746. [DOI] [PubMed] [Google Scholar]
- 42.Romanic AM, Burns-Kurtis CL, Ao Z, Arleth AJ, Ohlstein EH. Upregulated expression of human membrane type-5 matrix metalloproteinase in kidneys from diabetic patients. Am J Physiol Renal Physiol. 2001;281:F309–F317. doi: 10.1152/ajprenal.2001.281.2.F309. [DOI] [PubMed] [Google Scholar]
- 43.Qing-Hua G, Ju-Ming L, Chang-Yu P, Zhao-Hui L, Xiao-Man Z, Yi-Ming M. The kidney expression of matrix metalloproteinase-9 in the diabetic nephropathy of Kkay mice. J Diabetes Complications. 2008;22:408–412. doi: 10.1016/j.jdiacomp.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Götte M. Syndecans in inflammation. FASEB J. 2003;17:575–591. doi: 10.1096/fj.02-0739rev. [DOI] [PubMed] [Google Scholar]
- 45.Rops AL, van der Vlag J, Lensen JF, Wijnhoven TJ, van den Heuvel LP, van Kuppevelt TH, Berden JH. Heparan sulfate proteoglycans in glomerular inflammation. Kidney Int. 2004;65:768–785. doi: 10.1111/j.1523-1755.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 46.Garsen M, Rops AL, Rabelink TJ, Berden JH, van der Vlag J. The role of heparanase and the endothelial glycocalyx in the development of proteinuria. Nephrol Dial Transplant. 2014;29:49–55. doi: 10.1093/ndt/gft410. [DOI] [PubMed] [Google Scholar]
- 47.Rabelink TJ, van den Berg BM, Garsen M, Wang G, Elkin M, van der Vlag J. Heparanase: roles in cell survival, extracellular matrix remodelling and the development of kidney disease. Nat Rev Nephrol. 2017;13:201–212. doi: 10.1038/nrneph.2017.6. [DOI] [PubMed] [Google Scholar]
- 48.Singh A, Fridén V, Dasgupta I, Foster RR, Welsh GI, Tooke JE, Haraldsson B, Mathieson PW, Satchell SC. High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am J Physiol Renal Physiol. 2011;300:F40–F48. doi: 10.1152/ajprenal.00103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Hoven MJ, Wijnhoven TJ, Li JP, Zcharia E, Dijkman HB, Wismans RG, Rops AL, Lensen JF, van den Heuvel LP, van Kuppevelt TH, Vlodavsky I, Berden JH, van der Vlag J. Reduction of anionic sites in the glomerular basement membrane by heparanase does not lead to proteinuria. Kidney Int. 2008;73:278–287. doi: 10.1038/sj.ki.5002706. [DOI] [PubMed] [Google Scholar]
- 50.Gil N, Goldberg R, Neuman T, Garsen M, Zcharia E, Rubinstein AM, van Kuppevelt T, Meirovitz A, Pisano C, Li JP, van der Vlag J, Vlodavsky I, Elkin M. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes. 2012;61:208–216. doi: 10.2337/db11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Hoven MJ, Rops AL, Vlodavsky I, Levidiotis V, Berden JH, van der Vlag J. Heparanase in glomerular diseases. Kidney Int. 2007;72:543–548. doi: 10.1038/sj.ki.5002337. [DOI] [PubMed] [Google Scholar]
- 52.Levidiotis V, Kanellis J, Ierino FL, Power DA. Increased expression of heparanase in puromycin aminonucleoside nephrosis. Kidney Int. 2001;60:1287–1296. doi: 10.1046/j.1523-1755.2001.00934.x. [DOI] [PubMed] [Google Scholar]
- 53.Kuwabara A, Satoh M, Tomita N, Sasaki T, Kashihara N. Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia. 2010;53:2056–2065. doi: 10.1007/s00125-010-1810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levidiotis V, Freeman C, Punler M, Martinello P, Creese B, Ferro V, van der Vlag J, Berden JH, Parish CR, Power DA. A synthetic heparanase inhibitor reduces proteinuria in passive Heymann nephritis. J Am Soc Nephrol. 2004;15:2882–2892. doi: 10.1097/01.ASN.0000142426.55612.6D. [DOI] [PubMed] [Google Scholar]
- 55.Levidiotis V, Freeman C, Tikellis C, Cooper ME, Power DA. Heparanase is involved in the pathogenesis of proteinuria as a result of glomerulonephritis. J Am Soc Nephrol. 2004;15:68–78. doi: 10.1097/01.asn.0000103229.25389.40. [DOI] [PubMed] [Google Scholar]
- 56.Zcharia E, Metzger S, Chajek-Shaul T, Aingorn H, Elkin M, Friedmann Y, Weinstein T, Li JP, Lindahl U, Vlodavsky I. Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. FASEB J. 2004;18:252–263. doi: 10.1096/fj.03-0572com. [DOI] [PubMed] [Google Scholar]
- 57.Katz A, Van-Dijk DJ, Aingorn H, Erman A, Davies M, Darmon D, Hurvitz H, Vlodavsky I. Involvement of human heparanase in the pathogenesis of diabetic nephropathy. Isr Med Assoc J. 2002;4:996–1002. [PubMed] [Google Scholar]
- 58.van den Hoven MJ, Rops AL, Bakker MA, Aten J, Rutjes N, Roestenberg P, Goldschmeding R, Zcharia E, Vlodavsky I, van der Vlag J, Berden JH. Increased expression of heparanase in overt diabetic nephropathy. Kidney Int. 2006;70:2100–2108. doi: 10.1038/sj.ki.5001985. [DOI] [PubMed] [Google Scholar]
- 59.Maxhimer JB, Somenek M, Rao G, Pesce CE, Baldwin D Jr, Gattuso P, Schwartz MM, Lewis EJ, Prinz RA, Xu X. Heparanase-1 gene expression and regulation by high glucose in renal epithelial cells: a potential role in the pathogenesis of proteinuria in diabetic patients. Diabetes. 2005;54:2172–2178. doi: 10.2337/diabetes.54.7.2172. [DOI] [PubMed] [Google Scholar]
- 60.Masola V, Gambaro G, Tibaldi E, Onisto M, Abaterusso C, Lupo A. Regulation of heparanase by albumin and advanced glycation end products in proximal tubular cells. Biochim Biophys Acta. 2011;1813:1475–1482. doi: 10.1016/j.bbamcr.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Singh A, Ramnath RD, Foster RR, Wylie EC, Fridén V, Dasgupta I, Haraldsson B, Welsh GI, Mathieson PW, Satchell SC. Reactive oxygen species modulate the barrier function of the human glomerular endothelial glycocalyx. PLoS One. 2013;8:e55852. doi: 10.1371/journal.pone.0055852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmidt EP, Overdier KH, Sun X, Lin L, Liu X, Yang Y, Ammons LA, Hiller TD, Suflita MA, Yu Y, Chen Y, Zhang F, Cothren Burlew C, Edelstein CL, Douglas IS, Linhardt RJ. Urinary Glycosaminoglycans Predict Outcomes in Septic Shock and Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2016;194:439–449. doi: 10.1164/rccm.201511-2281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Berg BM, Wang G, Boels MGS, Avramut MC, Jansen E, Sol WMPJ, Lebrin F, van Zonneveld AJ, de Koning EJP, Vink H, Gröne HJ, Carmeliet P, van der Vlag J, Rabelink TJ. Glomerular Function and Structural Integrity Depend on Hyaluronan Synthesis by Glomerular Endothelium. J Am Soc Nephrol. 2019;30:1886–1897. doi: 10.1681/ASN.2019020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ikegami-Kawai M, Okuda R, Nemoto T, Inada N, Takahashi T. Enhanced activity of serum and urinary hyaluronidases in streptozotocin-induced diabetic Wistar and GK rats. Glycobiology. 2004;14:65–72. doi: 10.1093/glycob/cwh011. [DOI] [PubMed] [Google Scholar]
- 65.Shakya S, Wang Y, Mack JA, Maytin EV. Hyperglycemia-Induced Changes in Hyaluronan Contribute to Impaired Skin Wound Healing in Diabetes: Review and Perspective. Int J Cell Biol. 2015;2015:701738. doi: 10.1155/2015/701738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai Z, Zhang Y, Miao X, Li S, Yang H, Ling Q, Hoffmann PR, Huang Z. Use of a Mouse Model and Human Umbilical Vein Endothelial Cells to Investigate the Effect of Arsenic Exposure on Vascular Endothelial Function and the Associated Role of Calpains. Environ Health Perspect. 2019;127:77003. doi: 10.1289/EHP4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kong X, Chen L, Ye P, Wang Z, Zhang J, Ye F, Chen S. The role of HYAL2 in LSS-induced glycocalyx impairment and the PKA-mediated decrease in eNOS-Ser-633 phosphorylation and nitric oxide production. Mol Biol Cell. 2016;27:3972–3979. doi: 10.1091/mbc.E16-04-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel H, Chen J, Das KC, Kavdia M. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc Diabetol. 2013;12:142. doi: 10.1186/1475-2840-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dane MJ, van den Berg BM, Avramut MC, Faas FG, van der Vlag J, Rops AL, Ravelli RB, Koster BJ, van Zonneveld AJ, Vink H, Rabelink TJ. Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am J Pathol. 2013;182:1532–1540. doi: 10.1016/j.ajpath.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 70.Meuwese MC, Broekhuizen LN, Kuikhoven M, Heeneman S, Lutgens E, Gijbels MJ, Nieuwdorp M, Peutz CJ, Stroes ES, Vink H, van den Berg BM. Endothelial surface layer degradation by chronic hyaluronidase infusion induces proteinuria in apolipoprotein E-deficient mice. PLoS One. 2010;5:e14262. doi: 10.1371/journal.pone.0014262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ikegami-Kawai M, Suzuki A, Karita I, Takahashi T. Increased hyaluronidase activity in the kidney of streptozotocin-induced diabetic rats. J Biochem. 2003;134:875–880. doi: 10.1093/jb/mvg214. [DOI] [PubMed] [Google Scholar]
- 72.Leskova W, Pickett H, Eshaq RS, Shrestha B, Pattillo CB, Harris NR. Effect of diabetes and hyaluronidase on the retinal endothelial glycocalyx in mice. Exp Eye Res. 2019;179:125–131. doi: 10.1016/j.exer.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dogné S, Rath G, Jouret F, Caron N, Dessy C, Flamion B. Hyaluronidase 1 Deficiency Preserves Endothelial Function and Glycocalyx Integrity in Early Streptozotocin-Induced Diabetes. Diabetes. 2016;65:2742–2753. doi: 10.2337/db15-1662. [DOI] [PubMed] [Google Scholar]
- 74.Avasthi PS, Koshy V. The anionic matrix at the rat glomerular endothelial surface. Anat Rec. 1988;220:258–266. doi: 10.1002/ar.1092200306. [DOI] [PubMed] [Google Scholar]
- 75.Xiao H, Woods EC, Vukojicic P, Bertozzi CR. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc Natl Acad Sci U S A. 2016;113:10304–10309. doi: 10.1073/pnas.1608069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puerta-Guardo H, Glasner DR, Espinosa DA, Biering SB, Patana M, Ratnasiri K, Wang C, Beatty PR, Harris E. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Rep. 2019;26:1598–1613.e8. doi: 10.1016/j.celrep.2019.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Puerta-Guardo H, Glasner DR, Harris E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016;12:e1005738. doi: 10.1371/journal.ppat.1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Desideri S, Onions KL, Baker SL, Gamez M, El Hegni E Hussien H, Russell A, Satchell SC, Foster RR. Endothelial glycocalyx restoration by growth factors in diabetic nephropathy. Biorheology. 2019;56:163–179. doi: 10.3233/BIR-180199. [DOI] [PubMed] [Google Scholar]
- 79.Betteridge KB, Arkill KP, Neal CR, Harper SJ, Foster RR, Satchell SC, Bates DO, Salmon AHJ. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J Physiol. 2017;595:5015–5035. doi: 10.1113/JP274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 81.Dole VS, Bergmeier W, Mitchell HA, Eichenberger SC, Wagner DD. Activated platelets induce Weibel-Palade-body secretion and leukocyte rolling in vivo: role of P-selectin. Blood. 2005;106:2334–2339. doi: 10.1182/blood-2005-04-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okada Y, Copeland BR, Mori E, Tung MM, Thomas WS, del Zoppo GJ. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke. 1994;25:202–211. doi: 10.1161/01.str.25.1.202. [DOI] [PubMed] [Google Scholar]
- 83.Xiong JP, Stehle T, Goodman SL, Arnaout MA. Integrins, cations and ligands: making the connection. J Thromb Haemost. 2003;1:1642–1654. doi: 10.1046/j.1538-7836.2003.00277.x. [DOI] [PubMed] [Google Scholar]
- 84.Müller AM, Hermanns MI, Cronen C, Kirkpatrick CJ. Comparative study of adhesion molecule expression in cultured human macro- and microvascular endothelial cells. Exp Mol Pathol. 2002;73:171–180. doi: 10.1006/exmp.2002.2446. [DOI] [PubMed] [Google Scholar]
- 85.López JA. The platelet glycoprotein Ib-IX complex. Blood Coagul Fibrinolysis. 1994;5:97–119. [PubMed] [Google Scholar]
- 86.Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol. 2003;23:1541–1547. doi: 10.1161/01.ATV.0000085630.24353.3D. [DOI] [PubMed] [Google Scholar]
- 87.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 88.Ebong EE, Lopez-Quintero SV, Rizzo V, Spray DC, Tarbell JM. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr Biol (Camb) 2014;6:338–347. doi: 10.1039/c3ib40199e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baeyens N, Mulligan-Kehoe MJ, Corti F, Simon DD, Ross TD, Rhodes JM, Wang TZ, Mejean CO, Simons M, Humphrey J, Schwartz MA. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc Natl Acad Sci U S A. 2014;111:17308–17313. doi: 10.1073/pnas.1413725111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun. 2007;355:228–233. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harvey SJ, Miner JH. Revisiting the glomerular charge barrier in the molecular era. Curr Opin Nephrol Hypertens. 2008;17:393–398. doi: 10.1097/MNH.0b013e32830464de. [DOI] [PubMed] [Google Scholar]
- 92.Groffen AJ, Buskens CA, van Kuppevelt TH, Veerkamp JH, Monnens LA, van den Heuvel LP. Primary structure and high expression of human agrin in basement membranes of adult lung and kidney. Eur J Biochem. 1998;254:123–128. doi: 10.1046/j.1432-1327.1998.2540123.x. [DOI] [PubMed] [Google Scholar]
- 93.Groffen AJ, Veerkamp JH, Monnens LA, van den Heuvel LP. Recent insights into the structure and functions of heparan sulfate proteoglycans in the human glomerular basement membrane. Nephrol Dial Transplant. 1999;14:2119–2129. doi: 10.1093/ndt/14.9.2119. [DOI] [PubMed] [Google Scholar]
- 94.Saarela J, Rehn M, Oikarinen A, Autio-Harmainen H, Pihlajaniemi T. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am J Pathol. 1998;153:611–626. doi: 10.1016/S0002-9440(10)65603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Esko JD, Lindahl U. Molecular diversity of heparan sulfate. J Clin Invest. 2001;108:169–173. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nayak BR, Spiro RG. Localization and structure of the asparagine-linked oligosaccharides of type IV collagen from glomerular basement membrane and lens capsule. J Biol Chem. 1991;266:13978–13987. [PubMed] [Google Scholar]
- 97.Kanwar YS, Linker A, Farquhar MG. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980;86:688–693. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosenzweig LJ, Kanwar YS. Removal of sulfated (heparan sulfate) or nonsulfated (hyaluronic acid) glycosaminoglycans results in increased permeability of the glomerular basement membrane to 125I-bovine serum albumin. Lab Invest. 1982;47:177–184. [PubMed] [Google Scholar]
- 99.van den Born J, van den Heuvel LP, Bakker MA, Veerkamp JH, Assmann KJ, Berden JH. A monoclonal antibody against GBM heparan sulfate induces an acute selective proteinuria in rats. Kidney Int. 1992;41:115–123. doi: 10.1038/ki.1992.15. [DOI] [PubMed] [Google Scholar]
- 100.van den Born J, van den Heuvel LP, Bakker MA, Veerkamp JH, Assmann KJ, Weening JJ, Berden JH. Distribution of GBM heparan sulfate proteoglycan core protein and side chains in human glomerular diseases. Kidney Int. 1993;43:454–463. doi: 10.1038/ki.1993.67. [DOI] [PubMed] [Google Scholar]
- 101.Chen S, Wassenhove-McCarthy DJ, Yamaguchi Y, Holzman LB, van Kuppevelt TH, Jenniskens GJ, Wijnhoven TJ, Woods AC, McCarthy KJ. Loss of heparan sulfate glycosaminoglycan assembly in podocytes does not lead to proteinuria. Kidney Int. 2008;74:289–299. doi: 10.1038/ki.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, Berden JH, Moeller MJ, Holzman LB, Burgess RW, Miner JH. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol. 2007;171:139–152. doi: 10.2353/ajpath.2007.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goldberg S, Harvey SJ, Cunningham J, Tryggvason K, Miner JH. Glomerular filtration is normal in the absence of both agrin and perlecan-heparan sulfate from the glomerular basement membrane. Nephrol Dial Transplant. 2009;24:2044–2051. doi: 10.1093/ndt/gfn758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lawrence MG, Altenburg MK, Sanford R, Willett JD, Bleasdale B, Ballou B, Wilder J, Li F, Miner JH, Berg UB, Smithies O. Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc Natl Acad Sci U S A. 2017;114:2958–2963. doi: 10.1073/pnas.1616457114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roth J, Brown D, Orci L. Regional distribution of N-acetyl-D-galactosamine residues in the glycocalyx of glomerular podocytes. J Cell Biol. 1983;96:1189–1196. doi: 10.1083/jcb.96.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schnabel E, Dekan G, Miettinen A, Farquhar MG. Biogenesis of podocalyxin--the major glomerular sialoglycoprotein--in the newborn rat kidney. Eur J Cell Biol. 1989;48:313–326. [PubMed] [Google Scholar]
- 107.Kerjaschki D, Vernillo AT, Farquhar MG. Reduced sialylation of podocalyxin--the major sialoprotein of the rat kidney glomerulus--in aminonucleoside nephrosis. Am J Pathol. 1985;118:343–349. [PMC free article] [PubMed] [Google Scholar]
- 108.Pavenstädt H. The charge for going by foot: modifying the surface of podocytes. Exp Nephrol. 1998;6:98–103. doi: 10.1159/000020511. [DOI] [PubMed] [Google Scholar]
- 109.Takeda T, McQuistan T, Orlando RA, Farquhar MG. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest. 2001;108:289–301. doi: 10.1172/JCI12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ina K, Kitamura H, Nakamura M, Ono J, Takaki R. Loss of sulfated carbohydrate from the glomerular podocyte as a cause of albuminuria in experimental diabetic rats: ultrastructural histochemical study. J Diabet Complications. 1991;5:173–175. doi: 10.1016/0891-6632(91)90063-u. [DOI] [PubMed] [Google Scholar]
- 111.Garsen M, Lenoir O, Rops AL, Dijkman HB, Willemsen B, van Kuppevelt TH, Rabelink TJ, Berden JH, Tharaux PL, van der Vlag J. Endothelin-1 Induces Proteinuria by Heparanase-Mediated Disruption of the Glomerular Glycocalyx. J Am Soc Nephrol. 2016;27:3545–3551. doi: 10.1681/ASN.2015091070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Østergaard JA, Cooper ME, Jandeleit-Dahm KAM. Targeting oxidative stress and anti-oxidant defence in diabetic kidney disease. J Nephrol. 2020;33:917–929. doi: 10.1007/s40620-020-00749-6. [DOI] [PubMed] [Google Scholar]
- 113.Lipowsky HH, Lescanic A. The effect of doxycycline on shedding of the glycocalyx due to reactive oxygen species. Microvasc Res. 2013;90:80–85. doi: 10.1016/j.mvr.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van Golen RF, van Gulik TM, Heger M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic Biol Med. 2012;52:1382–1402. doi: 10.1016/j.freeradbiomed.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 115.Qi H, Casalena G, Shi S, Yu L, Ebefors K, Sun Y, Zhang W, D'Agati V, Schlondorff D, Haraldsson B, Böttinger E, Daehn I. Glomerular Endothelial Mitochondrial Dysfunction Is Essential and Characteristic of Diabetic Kidney Disease Susceptibility. Diabetes. 2017;66:763–778. doi: 10.2337/db16-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hargrove GM, Dufresne J, Whiteside C, Muruve DA, Wong NC. Diabetes mellitus increases endothelin-1 gene transcription in rat kidney. Kidney Int. 2000;58:1534–1545. doi: 10.1046/j.1523-1755.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 117.Zanatta CM, Veronese FV, Loreto Mda S, Sortica DA, Carpio VN, Eldeweiss MI, da Silva VD, Lopes TG, Gross JL, Canani LH. Endothelin-1 and endothelin a receptor immunoreactivity is increased in patients with diabetic nephropathy. Ren Fail. 2012;34:308–315. doi: 10.3109/0886022X.2011.647301. [DOI] [PubMed] [Google Scholar]
- 118.Ebefors K, Wiener RJ, Yu L, Azeloglu EU, Yi Z, Jia F, Zhang W, Baron MH, He JC, Haraldsson B, Daehn I. Endothelin receptor-A mediates degradation of the glomerular endothelial surface layer via pathologic crosstalk between activated podocytes and glomerular endothelial cells. Kidney Int. 2019;96:957–970. doi: 10.1016/j.kint.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.An X, Zhang L, Yao Q, Li L, Wang B, Zhang J, He M. The receptor for advanced glycation endproducts mediates podocyte heparanase expression through NF-κB signaling pathway. Mol Cell Endocrinol. 2018;470:14–25. doi: 10.1016/j.mce.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 120.Jaimes EA, Hua P, Tian RX, Raij L. Human glomerular endothelium: interplay among glucose, free fatty acids, angiotensin II, and oxidative stress. Am J Physiol Renal Physiol. 2010;298:F125–F132. doi: 10.1152/ajprenal.00248.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu WC, Liu YN, Kang BB, Chen JH. Trans-activation of heparanase promoter by ETS transcription factors. Oncogene. 2003;22:919–923. doi: 10.1038/sj.onc.1206201. [DOI] [PubMed] [Google Scholar]
- 122.Nagasu H, Satoh M, Kiyokage E, Kidokoro K, Toida K, Channon KM, Kanwar YS, Sasaki T, Kashihara N. Activation of endothelial NAD(P)H oxidase accelerates early glomerular injury in diabetic mice. Lab Invest. 2016;96:25–36. doi: 10.1038/labinvest.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 124.Rutledge JC, Ng KF, Aung HH, Wilson DW. Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nat Rev Nephrol. 2010;6:361–370. doi: 10.1038/nrneph.2010.59. [DOI] [PubMed] [Google Scholar]
- 125.Forbes JM, Cooper ME, Oldfield MD, Thomas MC. Role of advanced glycation end products in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S254–S258. doi: 10.1097/01.asn.0000077413.41276.17. [DOI] [PubMed] [Google Scholar]
- 126.D'Agati V, Schmidt AM. RAGE and the pathogenesis of chronic kidney disease. Nat Rev Nephrol. 2010;6:352–360. doi: 10.1038/nrneph.2010.54. [DOI] [PubMed] [Google Scholar]
- 127.Moriwaki Y, Yamamoto T, Suda M, Nasako Y, Takahashi S, Agbedana OE, Hada T, Higashino K. Purification and immunohistochemical tissue localization of human xanthine oxidase. Biochim Biophys Acta. 1993;1164:327–330. doi: 10.1016/0167-4838(93)90266-t. [DOI] [PubMed] [Google Scholar]
- 128.Desco MC, Asensi M, Márquez R, Martínez-Valls J, Vento M, Pallardó FV, Sastre J, Viña J. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes. 2002;51:1118–1124. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 129.Adachi T, Fukushima T, Usami Y, Hirano K. Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial-cell surface. Biochem J. 1993;289:523–527. doi: 10.1042/bj2890523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Battelli MG, Bolognesi A, Polito L. Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Biochim Biophys Acta. 2014;1842:1502–1517. doi: 10.1016/j.bbadis.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 131.Itano S, Kadoya H, Satoh M, Nakamura T, Murase T, Sasaki T, Kanwar YS, Kashihara N. Non-purine selective xanthine oxidase inhibitor ameliorates glomerular endothelial injury in Ins(Akita) diabetic mice. Am J Physiol Renal Physiol. 2020;319:F765–F772. doi: 10.1152/ajprenal.00236.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiang L, Zhou J, Zhang L, Du Y, Jiang M, Xie L, Ma Z, Chen F. The association between serum interleukin-1 beta and heparin sulphate in diabetic nephropathy patients. Glycoconj J. 2021;38:697–707. doi: 10.1007/s10719-021-10035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Henry CB, Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000;279:H2815–H2823. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- 134.Nieuwdorp M, Meuwese MC, Mooij HL, van Lieshout MH, Hayden A, Levi M, Meijers JC, Ince C, Kastelein JJ, Vink H, Stroes ES. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis. 2009;202:296–303. doi: 10.1016/j.atherosclerosis.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 135.Moriwaki Y, Yamamoto T, Shibutani Y, Aoki E, Tsutsumi Z, Takahashi S, Okamura H, Koga M, Fukuchi M, Hada T. Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism. 2003;52:605–608. doi: 10.1053/meta.2003.50096. [DOI] [PubMed] [Google Scholar]
- 136.Reine TM, Lanzalaco F, Kristiansen O, Enget AR, Satchell S, Jenssen TG, Kolset SO. Matrix metalloproteinase-9 mediated shedding of syndecan-4 in glomerular endothelial cells. Microcirculation. 2019:e12534. doi: 10.1111/micc.12534. [DOI] [PubMed] [Google Scholar]
- 137.Danielski LG, Giustina AD, Bonfante S, Barichello T, Petronilho F. The NLRP3 Inflammasome and Its Role in Sepsis Development. Inflammation. 2020;43:24–31. doi: 10.1007/s10753-019-01124-9. [DOI] [PubMed] [Google Scholar]
- 138.Qu J, Cheng Y, Wu W, Yuan L, Liu X. Glycocalyx Impairment in Vascular Disease: Focus on Inflammation. Front Cell Dev Biol. 2021;9:730621. doi: 10.3389/fcell.2021.730621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Haller H, Bertram A, Nadrowitz F, Menne J. Monocyte chemoattractant protein-1 and the kidney. Curr Opin Nephrol Hypertens. 2016;25:42–49. doi: 10.1097/MNH.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 140.Tashiro K, Koyanagi I, Saitoh A, Shimizu A, Shike T, Ishiguro C, Koizumi M, Funabiki K, Horikoshi S, Shirato I, Tomino Y. Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathy. J Clin Lab Anal. 2002;16:1–4. doi: 10.1002/jcla.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton) 2006;11:226–231. doi: 10.1111/j.1440-1797.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 142.de Zeeuw D, Bekker P, Henkel E, Hasslacher C, Gouni-Berthold I, Mehling H, Potarca A, Tesar V, Heerspink HJ, Schall TJ CCX140-B Diabetic Nephropathy Study Group. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 2015;3:687–696. doi: 10.1016/S2213-8587(15)00261-2. [DOI] [PubMed] [Google Scholar]
- 143.Boels MGS, Koudijs A, Avramut MC, Sol WMPJ, Wang G, van Oeveren-Rietdijk AM, van Zonneveld AJ, de Boer HC, van der Vlag J, van Kooten C, Eulberg D, van den Berg BM, IJpelaar DHT, Rabelink TJ. Systemic Monocyte Chemotactic Protein-1 Inhibition Modifies Renal Macrophages and Restores Glomerular Endothelial Glycocalyx and Barrier Function in Diabetic Nephropathy. Am J Pathol. 2017;187:2430–2440. doi: 10.1016/j.ajpath.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 144.Menne J, Eulberg D, Beyer D, Baumann M, Saudek F, Valkusz Z, Więcek A, Haller H Emapticap Study Group. C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol Dial Transplant. 2017;32:307–315. doi: 10.1093/ndt/gfv459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol. 2007;95:167–255. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- 146.Mahtal N, Lenoir O, Tharaux PL. Glomerular Endothelial Cell Crosstalk With Podocytes in Diabetic Kidney Disease. Front Med (Lausanne) 2021;8:659013. doi: 10.3389/fmed.2021.659013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 148.Ostendorf T, Kunter U, Eitner F, Loos A, Regele H, Kerjaschki D, Henninger DD, Janjic N, Floege J. VEGF(165) mediates glomerular endothelial repair. J Clin Invest. 1999;104:913–923. doi: 10.1172/JCI6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hovind P, Tarnow L, Oestergaard PB, Parving HH. Elevated vascular endothelial growth factor in type 1 diabetic patients with diabetic nephropathy. Kidney Int Suppl. 2000;75:S56–S61. [PubMed] [Google Scholar]
- 150.Tsuchida K, Makita Z, Yamagishi S, Atsumi T, Miyoshi H, Obara S, Ishida M, Ishikawa S, Yasumura K, Koike T. Suppression of transforming growth factor beta and vascular endothelial growth factor in diabetic nephropathy in rats by a novel advanced glycation end product inhibitor, OPB-9195. Diabetologia. 1999;42:579–588. doi: 10.1007/s001250051198. [DOI] [PubMed] [Google Scholar]
- 151.Cooper ME, Vranes D, Youssef S, Stacker SA, Cox AJ, Rizkalla B, Casley DJ, Bach LA, Kelly DJ, Gilbert RE. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999;48:2229–2239. doi: 10.2337/diabetes.48.11.2229. [DOI] [PubMed] [Google Scholar]
- 152.Veron D, Reidy KJ, Bertuccio C, Teichman J, Villegas G, Jimenez J, Shen W, Kopp JB, Thomas DB, Tufro A. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int. 2010;77:989–999. doi: 10.1038/ki.2010.64. [DOI] [PubMed] [Google Scholar]
- 153.Foster RR, Armstrong L, Baker S, Wong DW, Wylie EC, Ramnath R, Jenkins R, Singh A, Steadman R, Welsh GI, Mathieson PW, Satchell SC. Glycosaminoglycan regulation by VEGFA and VEGFC of the glomerular microvascular endothelial cell glycocalyx in vitro. Am J Pathol. 2013;183:604–616. doi: 10.1016/j.ajpath.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cébe Suarez S, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, Manlius C, Wood J, Ballmer-Hofer K. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci. 2006;63:2067–2077. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Onions KL, Gamez M, Buckner NR, Baker SL, Betteridge KB, Desideri S, Dallyn BP, Ramnath RD, Neal CR, Farmer LK, Mathieson PW, Gnudi L, Alitalo K, Bates DO, Salmon AHJ, Welsh GI, Satchell SC, Foster RR. VEGFC Reduces Glomerular Albumin Permeability and Protects Against Alterations in VEGF Receptor Expression in Diabetic Nephropathy. Diabetes. 2019;68:172–187. doi: 10.2337/db18-0045. [DOI] [PubMed] [Google Scholar]
- 156.Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs. 2003;12:933–941. doi: 10.1517/13543784.12.6.933. [DOI] [PubMed] [Google Scholar]
- 157.Satchell SC, Harper SJ, Tooke JE, Kerjaschki D, Saleem MA, Mathieson PW. Human podocytes express angiopoietin 1, a potential regulator of glomerular vascular endothelial growth factor. J Am Soc Nephrol. 2002;13:544–550. doi: 10.1681/ASN.V132544. [DOI] [PubMed] [Google Scholar]
- 158.Dessapt-Baradez C, Woolf AS, White KE, Pan J, Huang JL, Hayward AA, Price KL, Kolatsi-Joannou M, Locatelli M, Diennet M, Webster Z, Smillie SJ, Nair V, Kretzler M, Cohen CD, Long DA, Gnudi L. Targeted glomerular angiopoietin-1 therapy for early diabetic kidney disease. J Am Soc Nephrol. 2014;25:33–42. doi: 10.1681/ASN.2012121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lin Z, Natesan V, Shi H, Dong F, Kawanami D, Mahabeleshwar GH, Atkins GB, Nayak L, Cui Y, Finigan JH, Jain MK. Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2010;30:1952–1959. doi: 10.1161/ATVBAHA.110.211474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chiplunkar AR, Curtis BC, Eades GL, Kane MS, Fox SJ, Haar JL, Lloyd JA. The Krüppel-like factor 2 and Krüppel-like factor 4 genes interact to maintain endothelial integrity in mouse embryonic vasculogenesis. BMC Dev Biol. 2013;13:40. doi: 10.1186/1471-213X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhong F, Chen H, Wei C, Zhang W, Li Z, Jain MK, Chuang PY, Wang Y, Mallipattu SK, He JC. Reduced Krüppel-like factor 2 expression may aggravate the endothelial injury of diabetic nephropathy. Kidney Int. 2015;87:382–395. doi: 10.1038/ki.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu X, Li MH, Zhao YY, Xie YL, Yu X, Chen YJ, Li P, Zhang WF, Zhu TT. LncRNA H19 deficiency protects against the structural damage of glomerular endothelium in diabetic nephropathy via Akt/eNOS pathway. Arch Physiol Biochem. 2022:1–10. doi: 10.1080/13813455.2022.2102655. [DOI] [PubMed] [Google Scholar]
- 163.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 164.Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, Samejima K, Yamaguchi Y, Matsui M, Akai Y, Konishi N, Iwano M, Nabeshima Y, Saito Y. Decreased renal α-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012;81:539–547. doi: 10.1038/ki.2011.423. [DOI] [PubMed] [Google Scholar]
- 165.Cheng MF, Chen LJ, Cheng JT. Decrease of Klotho in the kidney of streptozotocin-induced diabetic rats. J Biomed Biotechnol. 2010;2010:513853. doi: 10.1155/2010/513853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG, Choudhary S. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes. 2011;60:1907–1916. doi: 10.2337/db10-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kadoya H, Satoh M, Haruna Y, Sasaki T, Kashihara N. Klotho attenuates renal hypertrophy and glomerular injury in Ins2Akita diabetic mice. Clin Exp Nephrol. 2016;20:671–678. doi: 10.1007/s10157-015-1202-3. [DOI] [PubMed] [Google Scholar]
- 168.Lewis EJ, Lewis JB, Greene T, Hunsicker LG, Berl T, Pohl MA, de Zeeuw D, Heerspink HL, Rohde RD, Atkins RC, Reutens AT, Packham DK, Raz I Collaborative Study Group. Sulodexide for kidney protection in type 2 diabetes patients with microalbuminuria: a randomized controlled trial. Am J Kidney Dis. 2011;58:729–736. doi: 10.1053/j.ajkd.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 169.Gambaro G, Kinalska I, Oksa A, Pont'uch P, Hertlová M, Olsovsky J, Manitius J, Fedele D, Czekalski S, Perusicová J, Skrha J, Taton J, Grzeszczak W, Crepaldi G. Oral sulodexide reduces albuminuria in microalbuminuric and macroalbuminuric type 1 and type 2 diabetic patients: the Di.N.A.S. randomized trial. J Am Soc Nephrol. 2002;13:1615–1625. doi: 10.1097/01.asn.0000014254.87188.e5. [DOI] [PubMed] [Google Scholar]
- 170.Packham DK, Wolfe R, Reutens AT, Berl T, Heerspink HL, Rohde R, Ivory S, Lewis J, Raz I, Wiegmann TB, Chan JC, de Zeeuw D, Lewis EJ, Atkins RC Collaborative Study Group. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23:123–130. doi: 10.1681/ASN.2011040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gambaro G. Discounting the efficacy of sulodexide in diabetic nephropathy is premature. Am J Kidney Dis. 2012;60:169–170. doi: 10.1053/j.ajkd.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 172.Jacob M, Paul O, Mehringer L, Chappell D, Rehm M, Welsch U, Kaczmarek I, Conzen P, Becker BF. Albumin augmentation improves condition of guinea pig hearts after 4 hr of cold ischemia. Transplantation. 2009;87:956–965. doi: 10.1097/TP.0b013e31819c83b5. [DOI] [PubMed] [Google Scholar]
- 173.Garsen M, Sonneveld R, Rops AL, Huntink S, van Kuppevelt TH, Rabelink TJ, Hoenderop JG, Berden JH, Nijenhuis T, van der Vlag J. Vitamin D attenuates proteinuria by inhibition of heparanase expression in the podocyte. J Pathol. 2015;237:472–481. doi: 10.1002/path.4593. [DOI] [PubMed] [Google Scholar]
- 174.van den Hoven MJ, Waanders F, Rops AL, Kramer AB, van Goor H, Berden JH, Navis G, van der Vlag J. Regulation of glomerular heparanase expression by aldosterone, angiotensin II and reactive oxygen species. Nephrol Dial Transplant. 2009;24:2637–2645. doi: 10.1093/ndt/gfp182. [DOI] [PubMed] [Google Scholar]
- 175.Yang YY, Chen Z, Yang XD, Deng RR, Shi LX, Yao LY, Xiang DX. Piperazine ferulate prevents high-glucose-induced filtration barrier injury of glomerular endothelial cells. Exp Ther Med. 2021;22:1175. doi: 10.3892/etm.2021.10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chang JJ, Kim-Tenser M, Emanuel BA, Jones GM, Chapple K, Alikhani A, Sanossian N, Mack WJ, Tsivgoulis G, Alexandrov AV, Pourmotabbed T. Minocycline and matrix metalloproteinase inhibition in acute intracerebral hemorrhage: a pilot study. Eur J Neurol. 2017;24:1384–1391. doi: 10.1111/ene.13403. [DOI] [PubMed] [Google Scholar]
- 177.Kim Y, Lim JH, Kim MY, Kim EN, Yoon HE, Shin SJ, Choi BS, Kim YS, Chang YS, Park CW. The Adiponectin Receptor Agonist AdipoRon Ameliorates Diabetic Nephropathy in a Model of Type 2 Diabetes. J Am Soc Nephrol. 2018;29:1108–1127. doi: 10.1681/ASN.2017060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Galle J, Schwedhelm E, Pinnetti S, Böger RH, Wanner C VIVALDI investigators. Antiproteinuric effects of angiotensin receptor blockers: telmisartan versus valsartan in hypertensive patients with type 2 diabetes mellitus and overt nephropathy. Nephrol Dial Transplant. 2008;23:3174–3183. doi: 10.1093/ndt/gfn230. [DOI] [PubMed] [Google Scholar]
- 179.Takaya T, Kawashima S, Shinohara M, Yamashita T, Toh R, Sasaki N, Inoue N, Hirata K, Yokoyama M. Angiotensin II type 1 receptor blocker telmisartan suppresses superoxide production and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. Atherosclerosis. 2006;186:402–410. doi: 10.1016/j.atherosclerosis.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 180.Jha JC, Banal C, Chow BS, Cooper ME, Jandeleit-Dahm K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxid Redox Signal. 2016;25:657–684. doi: 10.1089/ars.2016.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang R, Xu S, Zhang X, Zheng X, Liu Y, Jiang C, Liu J, Shang X, Fang S, Zhang J, Yin Z, Pan K. Cyclocarya paliurus triterpenoids attenuate glomerular endothelial injury in the diabetic rats via ROCK pathway. J Ethnopharmacol. 2022;291:115127. doi: 10.1016/j.jep.2022.115127. [DOI] [PubMed] [Google Scholar]
- 182.Verhaar MC, Strachan FE, Newby DE, Cruden NL, Koomans HA, Rabelink TJ, Webb DJ. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation. 1998;97:752–756. doi: 10.1161/01.cir.97.8.752. [DOI] [PubMed] [Google Scholar]
- 183.Boels MG, Avramut MC, Koudijs A, Dane MJ, Lee DH, van der Vlag J, Koster AJ, van Zonneveld AJ, van Faassen E, Gröne HJ, van den Berg BM, Rabelink TJ. Atrasentan Reduces Albuminuria by Restoring the Glomerular Endothelial Glycocalyx Barrier in Diabetic Nephropathy. Diabetes. 2016;65:2429–2439. doi: 10.2337/db15-1413. [DOI] [PubMed] [Google Scholar]
- 184.Zhou Y, Chi J, Huang Y, Dong B, Lv W, Wang YG. Efficacy and safety of endothelin receptor antagonists in type 2 diabetic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Diabet Med. 2021;38:e14411. doi: 10.1111/dme.14411. [DOI] [PubMed] [Google Scholar]
- 185.de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, Correa-Rotter R, Kohan D, Lambers Heerspink HJ, Makino H, Perkovic V, Pritchett Y, Remuzzi G, Tobe SW, Toto R, Viberti G, Parving HH. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol. 2014;25:1083–1093. doi: 10.1681/ASN.2013080830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Koomen JV, Stevens J, Mostafa NM, Parving HH, de Zeeuw D, Heerspink HJL. Determining the optimal dose of atrasentan by evaluating the exposure-response relationships of albuminuria and bodyweight. Diabetes Obes Metab. 2018;20:2019–2022. doi: 10.1111/dom.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Heerspink HJL, Xie D, Bakris G, Correa-Rotter R, Hou FF, Kitzman DW, Kohan D, Makino H, McMurray JJV, Perkovic V, Rossing P, Parving HH, de Zeeuw D on behalf on the SONAR Investigators. Early Response in Albuminuria and Long-Term Kidney Protection during Treatment with an Endothelin Receptor Antagonist: A Prespecified Analysis from the SONAR Trial. J Am Soc Nephrol. 2021;32:2900–2911. doi: 10.1681/ASN.2021030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Heerspink HJL, de Zeeuw D. Endothelin Receptor Antagonists for Kidney Protection: Lessons from the SONAR Trial. Clin J Am Soc Nephrol. 2022;17:908–910. doi: 10.2215/CJN.00560122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kimura Y, Kuno A, Tanno M, Sato T, Ohno K, Shibata S, Nakata K, Sugawara H, Abe K, Igaki Y, Yano T, Miki T, Miura T. Canagliflozin, a sodium-glucose cotransporter 2 inhibitor, normalizes renal susceptibility to type 1 cardiorenal syndrome through reduction of renal oxidative stress in diabetic rats. J Diabetes Investig. 2019;10:933–946. doi: 10.1111/jdi.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M, Tsukiyama K, Narita T, Takahashi T, Drucker DJ, Seino Y, Yamada Y. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 2014;85:579–589. doi: 10.1038/ki.2013.427. [DOI] [PubMed] [Google Scholar]
- 191.Shahzad K, Bock F, Dong W, Wang H, Kopf S, Kohli S, Al-Dabet MM, Ranjan S, Wolter J, Wacker C, Biemann R, Stoyanov S, Reymann K, Söderkvist P, Groß O, Schwenger V, Pahernik S, Nawroth PP, Gröne HJ, Madhusudhan T, Isermann B. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015;87:74–84. doi: 10.1038/ki.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O'Neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jiang H, He H, Chen Y, Huang W, Cheng J, Ye J, Wang A, Tao J, Wang C, Liu Q, Jin T, Jiang W, Deng X, Zhou R. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med. 2017;214:3219–3238. doi: 10.1084/jem.20171419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tan SM, Zhang Y, Cox AJ, Kelly DJ, Qi W. Tranilast attenuates the up-regulation of thioredoxin-interacting protein and oxidative stress in an experimental model of diabetic nephropathy. Nephrol Dial Transplant. 2011;26:100–110. doi: 10.1093/ndt/gfq355. [DOI] [PubMed] [Google Scholar]
- 195.Marchetti C, Swartzwelter B, Gamboni F, Neff CP, Richter K, Azam T, Carta S, Tengesdal I, Nemkov T, D'Alessandro A, Henry C, Jones GS, Goodrich SA, St Laurent JP, Jones TM, Scribner CL, Barrow RB, Altman RD, Skouras DB, Gattorno M, Grau V, Janciauskiene S, Rubartelli A, Joosten LAB, Dinarello CA. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc Natl Acad Sci U S A. 2018;115:E1530–E1539. doi: 10.1073/pnas.1716095115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Navarro JF, Mora C, Rivero A, Gallego E, Chahin J, Macía M, Méndez ML, García J. Urinary protein excretion and serum tumor necrosis factor in diabetic patients with advanced renal failure: effects of pentoxifylline administration. Am J Kidney Dis. 1999;33:458–463. doi: 10.1016/s0272-6386(99)70182-4. [DOI] [PubMed] [Google Scholar]
- 197.Harmankaya O, Seber S, Yilmaz M. Combination of pentoxifylline with angiotensin converting enzyme inhibitors produces an additional reduction in microalbuminuria in hypertensive type 2 diabetic patients. Ren Fail. 2003;25:465–470. doi: 10.1081/jdi-120021159. [DOI] [PubMed] [Google Scholar]
- 198.McCormick BB, Sydor A, Akbari A, Fergusson D, Doucette S, Knoll G. The effect of pentoxifylline on proteinuria in diabetic kidney disease: a meta-analysis. Am J Kidney Dis. 2008;52:454–463. doi: 10.1053/j.ajkd.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 199.Miric G, Dallemagne C, Endre Z, Margolin S, Taylor SM, Brown L. Reversal of cardiac and renal fibrosis by pirfenidone and spironolactone in streptozotocin-diabetic rats. Br J Pharmacol. 2001;133:687–694. doi: 10.1038/sj.bjp.0704131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Singh DK, Winocour P, Farrington K. Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol. 2011;7:176–184. doi: 10.1038/nrendo.2010.212. [DOI] [PubMed] [Google Scholar]