Abstract
Aging is characterized by the gradual deterioration of function at the molecular, cellular, tissue, and organism levels in humans. The typical diseases caused by changes in body composition, as well as functional decline in the human body’s organs due to aging include sarcopenia and metabolic disorders. The accumulation of dysfunctional aging β cells with age can cause decreased glucose tolerance and diabetes. Muscle decline has a multifactorial origin, involving lifestyle habits, disease triggers, and age-dependent biological changes. The reduced function of β cells in elderly people lowers insulin sensitivity, which affects protein synthesis and interferes with muscle synthesis. The functional decrease and aggravation of disease in elderly people with less regular exercise or physical activity causes imbalances in food intake and a continuous, vicious cycle. In contrast, resistance exercise increases the function of β cells and protein synthesis in elderly people. In this review, we discuss regular physical activities or exercises to prevent and improve health, which is sarcopenia as decreased muscle mass and metabolic disorders as diabetes in the elderly.
Keywords: Elderly, Diabetes, Sarcopenia, Resistance exercise, Aging, Muscle
Core Tip: Exercise or physical activity should be regularly performed even before aging begins, and muscle mass should be increased through resistance exercise. The protein intake necessary for protein synthesis during resistance exercise should also be maintained in elderly people and those with diabetes or/and sarcopenia.
INTRODUCTION
Aging is characterized by the gradual deterioration of function at the molecular, cellular, tissue, and organism levels, and human age is a major risk factor for diseases, including cardiovascular disease, diabetes, osteoporosis, and cancer[1]. Also, gradual decreases in muscle mass, especially in the lower extremities, and increases in fat volume, especially visceral and intermuscular fat, are general body composition changes associated with aging[2]. The typical diseases caused by changes in body composition (decreased muscle mass and increased fat mass), as well as functional decline in the human body’s organs due to aging, include sarcopenia and metabolic disorders. Moreover, according to a recent estimate by the International Diabetes Federation, 8.8% (425 million people) of the world’s 20-79-year-old population suffered from diabetes in 2017, and the number is expected to rise to 9.9% (629 million people) in 2045[3].
Elderly has complex diseases, not single diseases. Most review studies focus only on a single disease. In addition, it has been suggested that sarcopenia in the elderly plays a pivotal role in the pathogenesis of the frailty and functional disorders in diabetes. Through this review, we discuss regular physical activities or resistance exercises to prevent and improve health, which are sarcopenia as decrease muscle mass and metabolic disorders as diabetes in the elderly.
CAUSES OF DIABETES DUE TO AGING
Several factors are involved in the high prevalence of type 2 diabetes (T2D) in elderly people: (1) In relation to aging, T2D is associated with the decreased function of β cells that secrete insulin and decreased insulin sensitivity[4]; and (2) changes in the body composition related to aging lead to changes in insulin sensitivity due to a decrease in the amount of lean body mass and an increase in the amount of body fat[5].
The pancreas is an essential organ with both endocrine and exocrine tissues and plays an essential function in maintaining nutrient metabolism homeostasis in the body[6]. The accumulation of dysfunctional aging β cells with age can cause decreased glucose tolerance and diabetes[7]. Telomeres shortened by aging were reported to impair β cell function and participate in β cell destruction in the late stage of T2D[8]. The deletion of aging β cells in mouse models of type 1 diabetes showed increased insulin secretion and preserved insulin secretion ability, providing a link between cell aging and severe insulin deficiency[9].
In addition, considering that pancreatic weight, total insulin content, island size, and average insulin levels do not change, impaired signal transmission due to glucose stimulation during the aging process could be a decisive cause[10]. Some evidence suggested that the activation of inflammatory pathways contributed to insulin resistance in elderly people[11]. For example, aging is associated with inflammatory conditions in metabolic tissues and the upregulation of inflammatory cytokines, such as tumor necrosis factor-alpha, interleukin-6 (IL-6), and IL-1 family members, which can directly interfere with insulin signaling pathways and cause metabolic dysfunction[12-14]. Aging toll-like receptor-4 deficient mice with reduced inflammatory responses showed decreased expressions of inflammatory markers and p16Ink4a (also known as CDKN2A) in adipose tissue and improved glucose tolerance compared to aging mice with intact inflammatory responses[15].
CAUSES OF SARCOPENIA DUE TO AGING
Muscles are the most necessary body components and play a pivotal role in maintaining a healthy life. Muscles are directly or indirectly related to muscle strength, energy, balance, and immunity. However, aging is a powerful vehicle for promoting sarcopenia[16,17]. It is known that basal metabolic rate decreases during the normal aging process. After the age of 30, it decreases at a rate of 3%-8% per decade due to involuntary muscle loss. After the age of 50, approximately 1%-2% of muscle mass is lost per year. This rate increases to 3% per year after the age of 60, along with a decrease in strength of 1.5% annually[18,19].
Muscle loss has multiple factors, including lifestyle habits, disease triggers, and age-dependent biological changes. It is dealt with in the geriatric literature. However, it is starting to be studies into other areas dealing with the complexity of frail older persons. Testosterone levels gradually decrease with aging, and muscle protein synthesis and muscle mass can be reduced[20]. Growth hormone and insulin-like growth factor levels are also gradually and progressively decreased during normal aging. Such decreases are associated with decrease in muscle mass, not muscle strength[21,22].
The term sarcopenia was coined by Rosenberg[23] to describe the an age-related reduction in muscle mass that occurred with advancing age. However, muscle quality and structure are very important for each individual. V, and valid measurements are needed to establish the power of muscle mass[24]. Thus, sarcopenia that appears in elderly people and can be defined as the pathological loss of skeletal muscle[25]. It is characterized by structural changes in muscles along with that accompany dysfunction of muscles or decreased muscle strength. Sarcopenia should be considered a geriatric syndrome since multiple contributing factors (the aging process, diet, bed rest, sedentary lifestyle, chronic diseases, and drug treatment[26-28]) can cause the loss of muscle mass and that leads to an impaired state of health[29,30].
Sarcopenia has a multiple factorial origin[31]. Lifestyle habits, including physical inactivity, rest, and malnutrition, are known to can play an important role in most cases. In elderly people, changes in the endocrine system are, which is typical during the of aging process. They, can cause an imbalance between the anabolic process and the catabolic process[32], and a decreases of in anabolic hormones (testosterone, estrogens, growth hormone, insulin-like growth factor-1)[33], changes alterations of in the renin-angiotensin system[34], and vitamin D deficiency[35]. Low-grade systemic inflammation associated with, typical of aging and chronic disease, also plays an important role in increasing inflammatory cytokines.
RELATIONSHIP BETWEEN GLUCOSE METABOLIC AND EXERCISE
Glucose absorption by skeletal muscle contraction is caused by the presence of glucose transporter type 4 on the surface membrane and by accelerated diffusion according to the internal diffusion gradient for glucose[36]. Thus, the main step in controlling glucose absorption in skeletal muscles is the transport of glucose through cell membranes, and insulin and contractions induced in vivo by acute exercise or electrical stimulation can mediate glucose absorption in muscles[37].
Both aerobic exercise training and resistance exercise training are well known for their ability to restore systemic glucose homeostasis in people with metabolic T2D disease[38]. The relationship between glucose metabolism control and aerobic or resistance or combined exercise for both male and female pre-diabetes or diabetes patients are as follows. Twelve weeks of aerobic physical activity (60 min/d, 3 d/wk at 55%-65% HRR of rhythmic physical activity) and 12 wk of resistance physical activity (60 min/d, 3 d/wk at 55%-65% of 1 RM of machine weight) significantly decreased glycated hemoglobin (HbA1c) levels in pre-diabetes elderly people[39]; 12 wk of aquatic exercise (50 min/d, 3 d/wk at a rating on the perceived exertion scale of 10-16) improved glycemic control and decreased HbA1c in type 2 diabetes mellitus (T2DM) elderly people[40]; 6 mo of combined exercise (30 min of moderate aerobic exercise and 10 min of resistance exercise at 50%-70% of 1RM) significantly decreased HbA1c levels in T2DM elderly people[41]; 14 wk of resistance exercise (45 min/d, 3 d/wk at 60%-80% of 1RM for 1-8 wk and 70%-80% of 1RM for 10-14 wk) reduced plasma HbA1c levels and increased muscle glycogen stores in elderly people[42]; 2 years of aerobic exercise (60 min/d, 3 d/wk at 60%-70% of the HRmax) and resistance exercise (50 min/d, 3 d/wk of 13 types of resistance training protocols) HbA1C levels and β cell function were exercise responses in elderly patients with pre-diabetes[43]; 6 mo of resistance exercise (55 min/d, 3 d/wk at 75%-85% of 1 RM) was effective in improving glycemic control as shown by greater decreases in HbA1c levels[44]; 6 wk of high-intensity exercise training (3 d/wk supervised program at over 85% HRmax) increased insulin sensitivity in patients with T2DM[45]; 12 wk of 3 types of physical training (resistance, aerobic, and combined; 60 min/d, 3 d/wk) increased insulin receptor substrate (IRS)-1 expression by 65% in the resistance group and 90% in the combined group of patients with T2DM[46]; 8 wk of resistance and aerobic exercise (50 min/d, 2-3 d/wk at 65%-70% of 1RM and 65%-70% HRmax) significantly decreased HbA1c levels in both exercise groups[47], and 16 wk of low-intensity resistance training (2 d/wk at using body weight) significantly decreased HbA1c levels[48]. Nine studies contained elderly with T2DM are summarized the latest resistance exercises from traditional resistance exercises in Table 1.
Table 1.
Resistance exercise and diabetes
|
Ref.
|
Study population and intervention
|
Study outcome
|
| Kim et al[39], 2022 | 36 elderly people with pre-diabetics; 12 wk of resistance physical activity (60 min/d, 3 d/wk at 55%-65% of 1RM of machine weight) | Decreased glycated HbA1c levels |
| Nuttamonwarakul et al[40], 2012 | 20 elderly people with T2D; 12 wk of aquatic exercise (50 min/d, 3 d/wk at a perceived exertion (RPE) rating of 10-16) | Improved glycemic control and decreased HbA1c |
| Tan et al[41], 2012 | 25 elderly people with T2D; 6 mo of combined exercise (30 min of moderate aerobic exercise and 10 min of resistance exercise at 50%-70% of 1RM) | Decreased HbA1c levels |
| Castaneda et al[42], 2002 | 62 elderly patients with T2D; 14 wk of resistance exercise (45 min/d, 3 d/wk at 60%-80% of 1RM for 1-8 wk and 70%-80% of 1RM for 10-14 wk) | Reduced plasma glycosylated hemoglobin levels and increased muscle glycogen stores |
| He et al[43], 2022 | 82 elderly people with pre-diabetes; 2 years of resistance exercise (50 min/d, 3 d/wk of 13 types of resistance training protocols) | HbA1C levels and β cell function were resistance exercise response |
| Dunstan et al[44], 2002 | 36 elderly people with T2D; 6 mo of resistance exercise (55 min/d, 3 d/wk at 75%-85% of 1RM) | Improving glycemic control and decreases HbA1c levels |
| Jorge et al[46], 2011 | 48 middle-aged adults with T2D; 4 groups: Aerobic (n = 12), resistance (n = 12), combined (n = 12), and control (n = 12); 12 wk of training (60 min/d, 3 d/wk) | IRS-1 expression increased by 65% in the resistance group and by 90% in the combined group in T2DM |
| Ng et al[47], 2010 | 25 elderly people with T2D; 8 wk of resistance (50 min/d, 2-3 d/wk at 65%-70% of 1RM) | Decreased HbA1c levels |
| Takenami et al[48], 2019 | 10 elderly patients with T2D; 16 wk of low-intensity resistance training (2 d/wk at using body weight) | Decreased glycated hemoglobin |
HbA1c: Hemoglobin; IRS: Insulin receptor substrate; T2DM: Type 2 diabetes mellitus.
EXERCISE FOR THE TREATMENT OF SARCOPENIA AND DIABETES
Sarcopenia is the age-related loss of skeletal muscle mass and strength that develops slowly over decades and becomes an important factor in disability in the elderly population[49]. Insulin resistance in muscle protein metabolism with aging appears to be responsible for insensitivity to mixed supplements, and the presence of insulin resistance in muscle protein metabolism with aging independent of glucose tolerance has been demonstrated in healthy elderly subjects without diabetes[50]. Thus, the higher prevalence of sarcoidosis in T2DM individuals may be explained by other mechanisms, and the anabolic action of insulin in skeletal muscle is well known and may be progressively lost in T2DM due to decreased insulin sensitivity associated with the disease[51]. The decrease in muscle strength in elderly diabetes patients may be due, in part, to the intrinsic impairment of muscle strength generation, and a decrease in insulin signaling leads to a decrease in protein synthesis and an increase in proteolysis, which may ultimately lead to a decrease in muscle mass[52].
Resistance exercise is traditionally performed to increase muscle mass. Resistance exercise has a beneficial effect on sarcopenia in the general elderly population and is effective in coping with muscle mass reductions and performance deterioration in elderly patients with T2D[53,54]. Importantly, resistance exercise has also been found to have a beneficial effect on blood sugar profiles and insulin sensitivity[55]. In particular, in the case of elderly people, exercise is essential for preventing and managing sarcopenia because it counteracts the decline in both aging and muscle weakness caused by diabetes[56].
Compared to females who reported performing no strength training, females who performed strength training showed a 30% reduction in T2D (hazard ratio = 0.70, 95% confidence interval: 0.61-0.80)[57]. Short-term acute (2 d) moderate-intensity resistance exercise (50% of 1 RM) effectively reduced blood glucose levels and blood glucose fluctuations in elderly patients with T2M and sarcopenia[58]. Table 2 summarizes the benefit of resistance exercise in elderly people with sarcopenia.
Table 2.
Resistance exercise and sarcopenia
|
Ref.
|
Study population and intervention
|
Study outcome
|
| Zhao et al[58], 2022 | 24 elderly patients with T2D and sarcopenia; short-term acute resistance exercise (40 min/d, 3 d at 50% of 1RM) | Decreased blood glucose levels, blood glucose fluctuations and the risk of hypoglycemia |
| Seo et al[62], 2021 | 12 elderly females with sarcopenia; 16 wk of resistance training (60 min/d, 3 d/wk at 4-8 on the OMNI scale) | Improved functional fitness and muscle quality |
| Dong et al[63], 2019 | 21 elderly patients on maintenance hemodialysis with sarcopenia; 12 wk of resistance exercise (3 d/wk at their own body weight and elastic balls) | Improved physical activity status (maximum grip strength, daily pace, and physical activity level), and Inflammatory factors (IL-6, IL-10, and TNF-α) |
| Liao et al[64], 2018 | 56 elderly females with sarcopenia obesity; 12 wk of elastic band resistance training (3 training sessions every week for 12 wk, each training session was performed for 55 min) | Significant beneficial effect on muscle mass, muscle quality, and physical function |
| Hamaguchi et al[65], 2017 | 7 elderly females with sarcopenia; 6 wk of progressive power training (2 sessions per week for 6 wk; when the subject was capable of completing all 8 sets, the weight was increased by 380-760 g in the next session) | BMD and knee extensor strength were significantly greater in the training group than in the control group |
| Vasconcelos et al[66], 2016 | 14 elderly females with sarcopenia; 10 wk of resistance exercise (60 min/d, 2 d/wk; 1-2 wk at 50% of 1RM, 3-4 wk at 75% of 1RM, 5-6 wk at 40% of new 1RM, and 7-10 wk at 60% of new 1RM) | Knee extensor power was significantly higher in the training group than in the control group |
| Stoever et al[67], 2018 | 28 elderly people with sarcopenia obesity; 16 wk of progressive resistance training (2 d/wk, increasing to 80%-85% of maximum strength with 3 sets of 8 to 12 repetitions) | Increase performance in hand-grip strength, gait speed, SPPB score, and modified PPT score |
T2D: Type 2 diabetes; IL: Interleukin; TNF: Tumor necrosis factor; SPPB: Short Physical Performance Battery; PPT: Physical performance test.
Aging can accelerate the loss of muscle mass and function, and the loss of muscle mass and function may impair glucose metabolism and aggravate diabetes[59]. For this reason, elderly people especially, need to increase muscle mass, and the only way to increase muscle mass is to perform resistance exercises. The inclusion of gradual resistance exercise in lifestyle modification programs should be considered for elderly patients with sarcopenia and T2D or both[58,60]. There is also a general consensus that a moderate increase in daily protein intake to 0.8 g/kg/d or more in elderly people may enhance the metabolism of muscle proteins and reduce the progressive loss of muscle mass with aging[61].
CONCLUSION
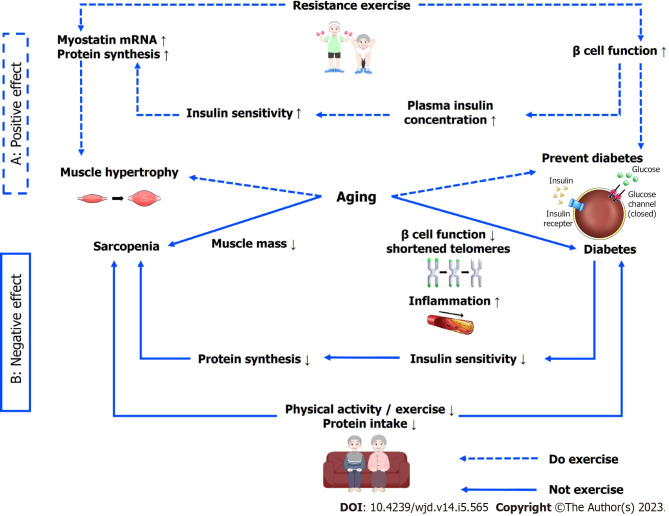
Among the various diseases caused by aging, diabetes and sarcopenia appear in elderly people. Reduced β cell function in elderly people lowers insulin sensitivity, which affects protein synthesis and interferes with muscle synthesis. The functional decrease and aggravation of disease in elderly people with less regular exercise or physical activity causes imbalances in food intake and a continuous, vicious cycle. In contrast, resistance exercise increases β cell function and protein synthesis in elderly people. A summary of our conclusions is shown in Figure 1. Regular physical activity and/or resistance exercise in the elderly is effective in preventing and promoting sarcopenia and diabetes. On the contrary, aging increases the risk of exposure to sarcopenia and diabetes. Therefore, exercise or physical activity should be regularly performed even before aging begins, and muscle mass should be increased through resistance exercise. The protein intake necessary for protein synthesis during resistance exercise should also be maintained in elderly people and those with diabetes or/and sarcopenia.
Figure 1.
The summary of the factors that cause diabetes and sarcopenia due to the aging and benefits of resistance exercise in the elderly is as follows.
Footnotes
Conflict-of-interest statement: The authors declare that they have no competing interests.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 19, 2022
First decision: February 8, 2023
Article in press: April 10, 2023
Specialty type: Health care sciences and services
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, India; Cabo C, Portugal; Cai L, United States S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
Contributor Information
Seung-Taek Lim, Institute of Sports and Arts Convergence (ISAC), Inha University, Incheon 22212, South Korea; Waseda Institute for Sport Sciences, Waseda University, Saitama 341-0018, Japan.
Sunghwun Kang, Laboratory of Exercise Physiology, College of Art, Culture and Engineering, Kangwon National University, Chuncheon-si 24341, South Korea; Interdisciplinary Program in Biohealth-machinery convergence engineering, Kangwon National University, Chuncheon-si 24341, South Korea. 94psycho@kangwon.ac.kr.
References
- 1.Booth LN, Brunet A. The Aging Epigenome. Mol Cell . 2016;62:728–744. doi: 10.1016/j.molcel.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Nevitt M, Harris TB. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr . 2005;82:872–8; quiz 915. doi: 10.1093/ajcn/82.4.872. [DOI] [PubMed] [Google Scholar]
- 3.Park S, Kim SK, Kim JY, Lee K, Choi JR, Chang SJ, Chung CH, Park KS, Oh SS, Koh SB. Exposure to pesticides and the prevalence of diabetes in a rural population in Korea. Neurotoxicology . 2019;70:12–18. doi: 10.1016/j.neuro.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhu M, Liu X, Liu W, Lu Y, Cheng J, Chen Y. β cell aging and age-related diabetes. Aging (Albany NY) . 2021;13:7691–7706. doi: 10.18632/aging.202593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Sofiani ME, Ganji SS, Kalyani RR. Body composition changes in diabetes and aging. J Diabetes Complications . 2019;33:451–459. doi: 10.1016/j.jdiacomp.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shih HP, Wang A, Sander M. Pancreas organogenesis: from lineage determination to morphogenesis. Annu Rev Cell Dev Biol . 2013;29:81–105. doi: 10.1146/annurev-cellbio-101512-122405. [DOI] [PubMed] [Google Scholar]
- 7.Aguayo-Mazzucato C, Andle J, Lee TB Jr, Midha A, Talemal L, Chipashvili V, Hollister-Lock J, van Deursen J, Weir G, Bonner-Weir S. Acceleration of β Cell Aging Determines Diabetes and Senolysis Improves Disease Outcomes. Cell Metab . 2019;30:129–142.e4. doi: 10.1016/j.cmet.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo N, Parry EM, Li LS, Kembou F, Lauder N, Hussain MA, Berggren PO, Armanios M. Short telomeres compromise β-cell signaling and survival. PLoS One . 2011;6:e17858. doi: 10.1371/journal.pone.0017858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson PJ, Shah A, Ntranos V, Van Gool F, Atkinson M, Bhushan A. Targeted Elimination of Senescent Beta Cells Prevents Type 1 Diabetes. Cell Metab . 2019;29:1045–1060.e10. doi: 10.1016/j.cmet.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Liu F, Yang P, Xiong F, Yu Q, Li J, Zhou Z, Zhang S, Wang CY. Aging and stress induced β cell senescence and its implication in diabetes development. Aging (Albany NY) . 2019;11:9947–9959. doi: 10.18632/aging.102432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer AK, Gustafson B, Kirkland JL, Smith U. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia . 2019;62:1835–1841. doi: 10.1007/s00125-019-4934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballak DB, Stienstra R, Tack CJ, Dinarello CA, van Diepen JA. IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine . 2015;75:280–290. doi: 10.1016/j.cyto.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes . 2003;52:2784–2789. doi: 10.2337/diabetes.52.11.2784. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzo M, Fernández-Veledo S, Vila-Bedmar R, Garcia-Guerra L, De Alvaro C, Nieto-Vazquez I. Insulin resistance induced by tumor necrosis factor-alpha in myocytes and brown adipocytes. J Anim Sci . 2008;86:E94–104. doi: 10.2527/jas.2007-0462. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh AK, O'Brien M, Mau T, Yung R. Toll-like receptor 4 (TLR4) deficient mice are protected from adipose tissue inflammation in aging. Aging (Albany NY) . 2017;9:1971–1982. doi: 10.18632/aging.101288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried LP, Hall WJ. Editorial: Leading on behalf of an aging society. J Am Geriatr Soc . 2008;56:1791–1795. doi: 10.1111/j.1532-5415.2008.01939.x. [DOI] [PubMed] [Google Scholar]
- 17.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the "Silver Tsunami": Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev . 2016;25:1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr . 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr . 2002;76:473–481. doi: 10.1093/ajcn/76.2.473. [DOI] [PubMed] [Google Scholar]
- 20.Tenover JS, Matsumoto AM, Clifton DK, Bremner WJ. Age-related alterations in the circadian rhythms of pulsatile luteinizing hormone and testosterone secretion in healthy men. J Gerontol . 1988;43:M163–M169. doi: 10.1093/geronj/43.6.m163. [DOI] [PubMed] [Google Scholar]
- 21.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science . 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 22.Papadakis MA, Grady D, Black D, Tierney MJ, Gooding GA, Schambelan M, Grunfeld C. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med . 1996;124:708–716. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 23.Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1988. Am J Clin Nutr . 1989;50:1121–1235. [PubMed] [Google Scholar]
- 24.McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan . 2014;3:9. doi: 10.1186/2046-2395-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc . 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 26.Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr . 2008;87:1562S–1566S. doi: 10.1093/ajcn/87.5.1562S. [DOI] [PubMed] [Google Scholar]
- 27.Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging . 2008;12:427–432. doi: 10.1007/BF02982703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson DD. Aging and sarcopenia. J Musculoskelet Neuronal Interact . 2007;7:344–345. [PubMed] [Google Scholar]
- 29.Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett-Connor E, Orwoll ES Osteoporotic Fractures in Men Research Group. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc . 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 30.Rolland Y, Czerwinski S, Abellan Van Kan G, Morley JE, Cesari M, Onder G, Woo J, Baumgartner R, Pillard F, Boirie Y, Chumlea WM, Vellas B. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging . 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc . 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzetti E, Lees HA, Wohlgemuth SE, Leeuwenburgh C. Sarcopenia of aging: underlying cellular mechanisms and protection by calorie restriction. Biofactors . 2009;35:28–35. doi: 10.1002/biof.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakuma K, Yamaguchi A. Sarcopenia and cachexia: the adaptations of negative regulators of skeletal muscle mass. J Cachexia Sarcopenia Muscle . 2012;3:77–94. doi: 10.1007/s13539-011-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter CS, Onder G, Kritchevsky SB, Pahor M. Angiotensin-converting enzyme inhibition intervention in elderly persons: effects on body composition and physical performance. J Gerontol A Biol Sci Med Sci . 2005;60:1437–1446. doi: 10.1093/gerona/60.11.1437. [DOI] [PubMed] [Google Scholar]
- 35.Cesari M, Incalzi RA, Zamboni V, Pahor M. Vitamin D hormone: a multitude of actions potentially influencing the physical function decline in older persons. Geriatr Gerontol Int . 2011;11:133–142. doi: 10.1111/j.1447-0594.2010.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev . 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 37.Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci U S A . 1995;92:5817–5821. doi: 10.1073/pnas.92.13.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans PL, McMillin SL, Weyrauch LA, Witczak CA. Regulation of Skeletal Muscle Glucose Transport and Glucose Metabolism by Exercise Training. Nutrients . 2019;11 doi: 10.3390/nu11102432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim BR, Lim ST. Effects of Leisure-Time Physical Activity on Cognitive Reserve Biomarkers and Leisure Motivation in the Pre-Diabetes Elderly. Healthcare (Basel) . 2022;10 doi: 10.3390/healthcare10040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nuttamonwarakul A, Amatyakul S, Suksom D. Twelve Weeks of Aqua-Aerobic Exercise Improve Health-Related Physical Fitness and Glycemic Control in Elderly Patients with Type 2 Diabetes. J Exerc Physiol Online . 2012;15:64–70. [Google Scholar]
- 41.Tan S, Li W, Wang J. Effects of six months of combined aerobic and resistance training for elderly patients with a long history of type 2 diabetes. J Sports Sci Med . 2012;11:495–501. [PMC free article] [PubMed] [Google Scholar]
- 42.Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, Roubenoff R, Tucker KL, Nelson ME. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care . 2002;25:2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 43.He Y, Feng Y, Shi J, Tang H, Chen L, Lou Q. β-Cell function and body mass index are predictors of exercise response in elderly patients with prediabetes. J Diabetes Investig . 2022;13:1253–1261. doi: 10.1111/jdi.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J, Zimmet P. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care . 2002;25:1729–1736. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- 45.Fealy CE, Nieuwoudt S, Foucher JA, Scelsi AR, Malin SK, Pagadala M, Cruz LA, Li M, Rocco M, Burguera B, Kirwan JP. Functional high-intensity exercise training ameliorates insulin resistance and cardiometabolic risk factors in type 2 diabetes. Exp Physiol . 2018;103:985–994. doi: 10.1113/EP086844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jorge ML, de Oliveira VN, Resende NM, Paraiso LF, Calixto A, Diniz AL, Resende ES, Ropelle ER, Carvalheira JB, Espindola FS, Jorge PT, Geloneze B. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism . 2011;60:1244–1252. doi: 10.1016/j.metabol.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Ng CL, Goh SY, Malhotra R, Østbye T, Tai ES. Minimal difference between aerobic and progressive resistance exercise on metabolic profile and fitness in older adults with diabetes mellitus: a randomised trial. J Physiother . 2010;56:163–170. doi: 10.1016/s1836-9553(10)70021-7. [DOI] [PubMed] [Google Scholar]
- 48.Takenami E, Iwamoto S, Shiraishi N, Kato A, Watanabe Y, Yamada Y, Yamada S, Ishii N. Effects of low-intensity resistance training on muscular function and glycemic control in older adults with type 2 diabetes. J Diabetes Investig . 2019;10:331–338. doi: 10.1111/jdi.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makanae Y, Fujita S. Role of Exercise and Nutrition in the Prevention of Sarcopenia. J Nutr Sci Vitaminol (Tokyo) . 2015;61 Suppl:S125–S127. doi: 10.3177/jnsv.61.S125. [DOI] [PubMed] [Google Scholar]
- 50.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J . 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Izzo A, Massimino E, Riccardi G, Della Pepa G. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients . 2021;13 doi: 10.3390/nu13010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Umegaki H. Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr Gerontol Int . 2016;16:293–299. doi: 10.1111/ggi.12688. [DOI] [PubMed] [Google Scholar]
- 53.Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev . 2010;9:226–237. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cadore EL, Izquierdo M. Exercise interventions in polypathological aging patients that coexist with diabetes mellitus: improving functional status and quality of life. Age (Dordr) . 2015;37:64. doi: 10.1007/s11357-015-9800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kadoglou NP, Fotiadis G, Kapelouzou A, Kostakis A, Liapis CD, Vrabas IS. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabet Med . 2013;30:e41–e50. doi: 10.1111/dme.12055. [DOI] [PubMed] [Google Scholar]
- 56.Nomura T, Kawae T, Kataoka H, Ikeda Y. Assessment of lower extremity muscle mass, muscle strength, and exercise therapy in elderly patients with diabetes mellitus. Environ Health Prev Med . 2018;23:20. doi: 10.1186/s12199-018-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shiroma EJ, Cook NR, Manson JE, Moorthy MV, Buring JE, Rimm EB, Lee IM. Strength Training and the Risk of Type 2 Diabetes and Cardiovascular Disease. Med Sci Sports Exerc . 2017;49:40–46. doi: 10.1249/MSS.0000000000001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao D, Shi W, Bi L, Qi Y, Hu S, Li C, Zhang Y, Zheng X. Effect of short-term acute moderate-intensity resistance exercise on blood glucose in older patients with type 2 diabetes mellitus and sarcopenia. Geriatr Gerontol Int . 2022;22:653–659. doi: 10.1111/ggi.14437. [DOI] [PubMed] [Google Scholar]
- 59.Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes . 2019;12:1057–1072. doi: 10.2147/DMSO.S186600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scott D, de Courten B, Ebeling PR. Sarcopenia: a potential cause and consequence of type 2 diabetes in Australia's ageing population? Med J Aust . 2016;205:329–333. doi: 10.5694/mja16.00446. [DOI] [PubMed] [Google Scholar]
- 61.Genaro Pde S, Martini LA. Effect of protein intake on bone and muscle mass in the elderly. Nutr Rev . 2010;68:616–623. doi: 10.1111/j.1753-4887.2010.00321.x. [DOI] [PubMed] [Google Scholar]
- 62.Seo MW, Jung SW, Kim SW, Lee JM, Jung HC, Song JK. Effects of 16 Weeks of Resistance Training on Muscle Quality and Muscle Growth Factors in Older Adult Women with Sarcopenia: A Randomized Controlled Trial. Int J Environ Res Public Health . 2021;18 doi: 10.3390/ijerph18136762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong ZJ, Zhang HL, Yin LX. Effects of intradialytic resistance exercise on systemic inflammation in maintenance hemodialysis patients with sarcopenia: a randomized controlled trial. Int Urol Nephrol . 2019;51:1415–1424. doi: 10.1007/s11255-019-02200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liao CD, Tsauo JY, Huang SW, Ku JW, Hsiao DJ, Liou TH. Effects of elastic band exercise on lean mass and physical capacity in older women with sarcopenic obesity: A randomized controlled trial. Sci Rep . 2018;8:2317. doi: 10.1038/s41598-018-20677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamaguchi K, Kurihara T, Fujimoto M, Iemitsu M, Sato K, Hamaoka T, Sanada K. The effects of low-repetition and light-load power training on bone mineral density in postmenopausal women with sarcopenia: a pilot study. BMC Geriatr . 2017;17:102. doi: 10.1186/s12877-017-0490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vasconcelos KS, Dias JM, Araújo MC, Pinheiro AC, Moreira BS, Dias RC. Effects of a progressive resistance exercise program with high-speed component on the physical function of older women with sarcopenic obesity: a randomized controlled trial. Braz J Phys Ther . 2016;20:432–440. doi: 10.1590/bjpt-rbf.2014.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stoever K, Heber A, Eichberg S, Brixius K. Influences of Resistance Training on Physical Function in Older, Obese Men and Women With Sarcopenia. J Geriatr Phys Ther . 2018;41:20–27. doi: 10.1519/JPT.0000000000000105. [DOI] [PubMed] [Google Scholar]