Abstract
Background
Given the reduced immune response to vaccines in older populations, this study aimed to evaluate the efficacy of COVID-19 vaccinations and its impact on breakthrough infection, hospital admission, and mortality in the elderly.
Methods
We carried out a systemic review and meta-analysis where MEDLINE, Web of Science, EMBASE, ClinicalTrials.gov, and Cochrane Central Register for Controlled Trials were queried to identify relevant literature. We included randomized controlled trials (RCTs), non-randomized trials, prospective, observational cohort, and case–control studies assessing breakthrough infection, hospital admission, and mortality after coronavirus 2 (SARS-CoV-2) vaccination in the elderly (≥ 60 years old).
Results
Overall, 26 studies were included in this meta-analysis. Compared with the unvaccinated group, the vaccinated group showed a decreased risk of SARS-CoV-2 infection after 28–34 (relative risk [RR] = 0.42, 95% confidence interval [CI] 0.37–0.49) and 35–60 days (RR = 0.49, 95% CI 0.37–0.62). There was a step-wise increase in efficacy with additional doses with the two-dose group experiencing decreased risk of breakthrough infection (RR = 0.37, 95% CI 0.32–0.42), hospital admissions (RR = 0.25, 95% CI 0.14–0.45), disease severity (RR = 0.38, 95% CI 0.20–0.70), and mortality (RR = 0.21, 95% CI 0.14–0.32) compared with those receiving one or no doses. Similarly three-dose and four-dose vaccine groups also showed a decreased risk of breakthrough infection (3-dose: RR = 0.14, 95% CI 0.10–0.20; 4-dose RR = 0.46, 95% CI 0.4–0.53), hospital admissions (3-dose: RR = 0.11, 95% CI 0.07–0.17; 4-dose: RR = 0.42, 95% CI 0.32–0.55), and all-cause mortality (3-dose: RR = 0.10, 95% CI 0.02–0.48; 4-dose: RR = 0.48, 95% CI 0.28–0.84) Subgroup analysis found that protection against mortality for vaccinated vs. unvaccinated groups was similar by age (60–79 years: RR = 0.59; 95% CI, 0.47–0.74; ≥ 80 years: RR = 0.76; 95% CI, 0.59–0.98) and gender (female: RR = 0.66; 95% CI, 0.50–0.87, male: (RR = 0.58; 95% CI, 0.44–0.76), and comorbid cardiovascular disease (CVD) (RR = 0.69; 95% CI, 0.52–0.92) or diabetes (DM) (RR = 0.59; 95% CI, 0.39–0.89.
Conclusions
Our pooled results showed that SARS-CoV-2 vaccines administered to the elderly is effective in preventing prevent breakthrough infection, hospitalization, severity, and death. What’s more, increasing number of vaccine doses is becoming increasingly effective.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-023-08254-9.
KEY WORDS: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), The elderly, Breakthrough infection, Hospital admission, Mortality, Meta-analysis
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections have posed a serious threat to global human health over the past three years. Preventive strategies for SARS-CoV-2 transmission and infection include effective isolation, the use of vaccinations and the use of antiviral drugs. Specific mRNA, viral-vector-based, and inactivated SARS-CoV-2 vaccines are being used worldwide. While efficacy of vaccines have been confirmed in adults 1–3, there is a debate regarding the outcomes of vaccination in the elderly 4,5. Several factors affect vaccine efficacy including age, prior antigen exposure, vaccine schedule, and vaccine dose 6. Older populations tend to have poorer immune responses to vaccines with a decline in immune function with age.7. In some countries older adults have lower vaccination rates and higher vaccine hesitancy than younger adults 8–10. Comorbidities, vaccine hesitancy, or lower vaccination rates, are important causes for increased infection and death in the elderly. Moreover, the size and proportion of the elderly population has been increasing worldwide.
Our study purpose was to assess the effectiveness of the SARS-CoV-2 vaccine to reduce the risks of breakthrough SARS-CoV-2 infection, virial-associated hospital admission, and mortality among adults greater than 60 years of age.
METHODS
We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This study is registered with PROSPERO (number CRD42022372066).
Search strategy and study selection
We queried MEDLINE (PubMed, January 1, 2020, to December 31, 2022), Web of Science, EMBASE (January 1, 2020, to December 31, 2022), ClinicalTrials.gov, and Cochrane Central Register of Controlled Trials. The search strategy was shown in the Supplemental Table 1. Manual searches of references cited by the identified original studies and relevant review articles were also performed, and the selected papers were evaluated. We did not use a reference librarian in creating or conducting these searchers.
Inclusion and exclusion criteria
Studies that met the following criteria were included in our meta-analysis: 1) Age ≥ 60 years old; 2) randomized controlled trials (RCTs), non-randomized trials, and observational studies; 3) vaccinated groups compared with unvaccinated groups; and 4) availability of an outcome (breakthrough infection, hospital admissions, severity of disease, or all-cause mortality).
Studies were excluded if they met any of the following criteria: 1) the study was not in English; 2) different publications analyzing the same population or duplicates.
We defined a breakthrough infection as a SARS-CoV-2 infection confirmed by positive laboratory nucleic acid after 14 days of any SARS-CoV-2 vaccination. 11
Data collection
Three researchers performed the searches and reviewed the results. Data were independently extracted by all three researchers. From each study, we extracted data on author, year, country, study design, sample size, age, sex, vaccine type, homologous or heterologous vaccinations, prior SARS-CoV-2 infection, vaccine dose, follow-up, virus type and outcomes. The primary outcome was the occurrence of breakthrough SARS-CoV-2 infection. Secondary outcomes included hospital admissions, disease severity, and all-cause mortality. We defined a severe disease as respiratory failure requiring mechanical ventilation or shock or other organ failures requiring Intensive Care Unit (ICU) care.
Relative risks (RRs) (and 95% CI) were either calculated or extracted from individual studies. Any disagreement in data extraction was resolved through discussion among the researchers in consultation with other authors and a consensus was reached.
Risk of bias assessment
The risk of bias in non-randomized studies of interventions (ROBINS-I) tool was also used to assess the quality of the included non-RCTs. Studies were ranked as low, moderate, serious, or critical risk of bias in the seven domains (confounding, selection of participants in to the study, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported result). Low risk: the study is evaluated to be at low risk of bias for all domains. Moderate risk: the study is evaluated to be at low or moderate risk of bias for all domains. Serious risk: the study is evaluated to be at serious risk of bias in at least one domain, but not at critical risk of bias in any domain. Critical risk: the study is evaluated to be at critical risk of bias in at least one domain. Any discrepancies were resolved through discussion with a third author (Jin HM) and consensus was reached.
Statistical analyses
Data were analyzed using a random effects model (STATA,version 14.0; StataCorp LLC, TX, USA, metan command). According to the follow-up time (14–20 days, 28–34 days and 35–60 days), we used subgroup analyses to evaluate breakthrough infection comparisons of different levels of vaccination (none, one, two, three or four doses). Heterogeneity was assessed with the I2 statistic, while subgroup analysis and meta-regression (restricted maximum likelihood (REML) heterogeneity estimator) were performed to identify sources of heterogeneity. Sensitivity analyses were done to evaluate the effects of age, gender and different disease conditions on the risk of hospital admissions, the severity of disease, or mortality. We also assessed whether any study was overly influential by stepwise elimination. The Egger’s test was used to investigate the presence of publication bias. If there was any publication bias, it was adjusted using the trim-and-fill method with the “meta trim” command. Statistical significance for all analyses was set at P < 0.05.
RESULTS
Study flow and characteristics
The PRISMA chart is presented in Figure S1. Overall, 26 studies were included in this meta-analysis 11–36. Overall, there were 26 studies involving 8,968,085 participants that were included (Table 1). Sample sizes ranged from 98 to 2,413,356 participants with age ≥ 60 years old and the proportions of men in study populations ranged from 30 to 95%. Of the 26 included studies, 21 studies were prospective or retrospective cohort studies, 5 case–control studies and no randomized controlled trials (RCTs). Included studies came from the US, UK, Qatar, Israel, Spain, Italy, Pakistan, France, Denmark, China, Portugal, Hungary, and Sweden.
Table 1.
Characteristics of 26 studies
| Study | Country | Study design |
Sample size |
Age (years) |
Men, % |
Vaccine type | homologous or heterologous vaccinations | Prior SARS-CoV-2 infection | Dose | follow-up | Virus type | outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lopez Bernal J et al. 11 | UK | case–control study | 44,590 | ≥ 60 | NA |
BNT162b2 or ChAdOx1 |
homologous | mixed | 2 | ≥ 35 days | B.1.1.7 |
Breakthrough infection Hospitalization All-cause mortality |
| Butt AA et al. 12 | Qatar | retrospective cohort study | 98 | ≥ 60 | 60.7 |
BNT162b2 or mRNA-1273 |
homologous | No | 2 | 3 months | NA |
Hospitalization Severity of disease |
| Butt AA et al. 13 | Qatar | retrospective cohort study | 171 | ≥ 60 | 95.31 | BNT162b2 | homologous | No | 2 | 3.5 months | NA |
Hospitalization Severity of disease |
| Haas EJ et al. 14 | Israel | retrospective cohort study | 1,127,965 | ≥ 65 | 72.2 | BNT162b2 | homologous | No | 2 | 69 days | B.1.1.7 | Hospitalization |
| Hyams C et al. 15 | UK | case–control study | 466 | ≥ 80 | 50 |
BNT162b2 or ChAdOx1 |
/ | No | 1 | 60 days | B.1.1.7 | Breakthrough infection |
| Martínez-Baz I et al. 16 | Spain | prospective cohort study | 4539 | ≥ 60 | 49.1 |
BNT162b2 or ChAdOx1 |
homologous | No | 2 | 3 months | B.1.1.7 | Breakthrough infection |
| Naleway AL et al. 17 | US | retrospective cohort study | 90,971 | ≥ 65 | 55.6 |
BNT162b2 or ChAdOx1 |
homologous | No | 2 | ≥ 14 days | Delta |
Breakthrough infection Hospitalization |
| Rivasi G et al. 18 | Italy | retrospective cohort study | 3730 | 84 | 31 | BNT162b2 | homologous | mixed | 2 | 6 months | NA |
Breakthrough infection Hospitalization Severity of disease |
| Shrotri M et al. 19 | UK | prospective cohort study | 10,412 | ≥ 80 | 30.4 |
BNT162b2 or ChAdOx1 |
/ | No | 1 | 49 days | B.1.1.7 | Breakthrough infection |
| Tenforde MW et al. 20 | US | retrospective cohort study | 417 | ≥ 65 | 52 |
BNT162b2 or mRNA-1273 |
homologous | No | 2 | 3 months | NA | Hospitalization |
| Vasileiou E et al. 21 | UK | prospective cohort study | 861,033 | ≥ 65 | 40 |
BNT162b2 or ChAdOx1 |
/ | No | 1 | 42 days | NA | Hospitalization |
| Aslam J et al. 22 | Pakistan | prospective cohort study | 85 | ≥ 66 | 57.7 |
mRNA-1273 or BNT162b2 or ChAdOx1 |
/ | No | 1 | 4 months | NA | All-cause mortality |
| Bar-On YM et al. 23 | Israel | retrospective cohort study | 1,252,331 | ≥ 60 | 48.2 | BNT162b2 | homologous | No | 4 | 56 days | omicron |
Hospitalization Severity of disease |
| Suarez Castillo M et al. 24 | France | case–control study | 2,413,356 | ≥ 60 | 48 |
mRNA-1273 or BNT162b2 or ChAdOx1 |
homologous | No | 2 | ≥ 14 days | omicron | Breakthrough infection |
| Gazit S et al. 25 | Israel | case–control study | 97,499 | ≥ 60 | 45.7 | BNT162b2 | homologous | mixed | 4 | 2 months | omicron |
Breakthrough infection Hospitalization Severity of disease All-cause mortality |
| Goldin S et al. 26 | US | Nationwide Cohort study | 43,596 | ≥ 65 | NA | BNT162b2 | homologous | No | 2 | 28 days | NA |
Breakthrough infection All-cause mortality |
| Gram MA et al. 27 | Denmark | Nationwide Cohort study | 30,237 | ≥ 60 | 46.7 |
BNT162b2 or mRNA-1273 |
homologous | No | 3 | 3 months |
alpha/delta/ omicron |
Breakthrough infection |
| Kelly JD et al. 28 | US | retrospective nationwide Cohort study | 1,100,280 | ≥ 65 | 91.8 |
BNT162b2 or mRNA-1273 |
heterologous | No | 3 | 5 months | delta/omicron |
Hospitalization All-cause mortality |
| Lu G et al. 29 | China | retrospective cohort study | 1377 | ≥ 60 | 46.62 | inactived | homologous | No | 2 | 1 months | omicron |
Hospitalization Severity of disease |
| Machado A et al. 30 | Portugal | retrospective cohort study | 1,884,932 | ≥ 65 | 44.6 | mRNA-1273 | homologous | No | 2 | 98 days | NA |
Breakthrough infection Hospitalization All-cause mortality |
| Magen O et al. 31 | Israel | retrospective cohort study | 258,994 | ≥ 60 | 49 | BNT162b2 | homologous | No | 4 | 46 days | omicron |
Breakthrough infection Hospitalization Severity of disease All-cause mortality |
| Muhsen K et al. 32 | Israel | retrospective cohort study | 18,611 | ≥ 60 | 31.7 | BNT162b2 | homologous | No | 3 | 63 days | NA |
Breakthrough infection Hospitalization All-cause mortality |
| Muhsen K et al. 33 | Israel | prospective cohort study | 43,775 | ≥ 60 | 32.2 | BNT162b2 | homologous | No | 4 | 73 days | omicron |
Breakthrough infection Hospitalization Severity of disease All-cause mortality |
| Müller V et al. 34 | Hungary | nationwide, retrospective cohort study | 1,984,176 | ≥ 65 | 38.8 |
mRNA-1273 or BNT162b2 or ChAdOx1 |
heterologous | mixed | 3 | 3.25 months | delta |
Breakthrough infection Hospitalization All-cause mortality |
| Nordström P et al. 35 | Sweden | nationwide, retrospective cohort study | 24,524 | ≥ 80 | 32.2 |
BNT162b2 or mRNA-1273 |
heterologous | No | 4 | 2 months | omicron | All-cause mortality |
| Tenforde MW et al. 36 | US | test-negtive case–control study | 1653 | ≥ 65 | 56.1 |
BNT162b2 or mRNA-1273 |
homologous | No | 2 | 28 days | alpha/delta | Hospitalization |
Homologous vaccinations, the same immunogen vaccination regimen; Heterologous vaccinations, the different immunogen vaccination regimen
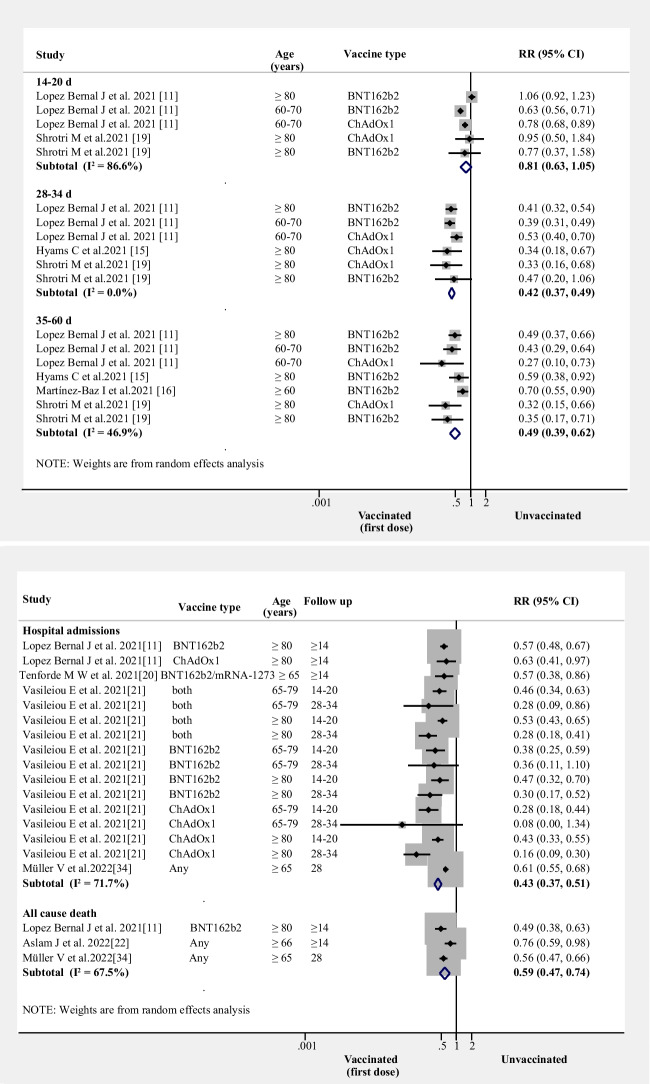
Relative risks for breakthrough SARS-CoV-2 infection, hospital admissions, and all-cause mortality after one dose of vaccine in the elderly
Compared to unvaccinated elders, one dose did not reduce risk of breakthrough SARS-CoV-2 infection in the first 14–20 days (RR = 0.81, 95% CI 0.63–1.05, I2 = 87%, n = 2 studies). However, by 28–34 days (RR = 0.42, 95% CI 0.37–0.49, I2 = 0%, n = 3 studies) and 35–60 days (RR = 0.49, 95% CI: 0.37–0.62, I2 = 47%, n = 4 studies), one dose decreased risk of breakthrough SARS-CoV-2 infection (Fig. 1A).
Figure 1.
RRs for breakthrough SARS-CoV-2 infection, hospital admissions, and all-cause mortality after the first dose of vaccine (A) RRs for breakthrough SARS-CoV-2 infection associated with the first dose of vaccine (B) RRs for hospital admissions and all-cause mortality associated with first dose of vaccine. (Both: BNT162b2 and mRNA-1273; Any: BNT162b2 or ChAdOx1 or mRNA-1273, et al.)
One dose also reduced the risk of hospital admissions (RR = 0.43, 95% CI 0.37–0.51, I2 = 72%, n = 4 studies; (Fig. 1B)) and all-cause mortality (RR = 0.59, 95% CI 0.47–0.74, I2 = 68%, n = 3 studies; (Fig. 1B)).
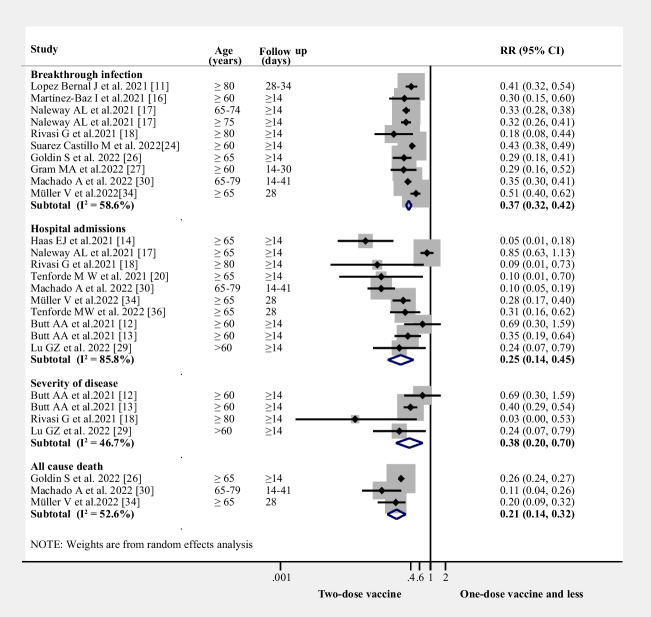
Relative risks for breakthrough SARS-CoV-2 infection, hospital admissions, the severity of disease and all-cause mortality after two doses of vaccine in the elderly
Those receiving two doses had decreased breakthrough infections compared with the one or no doses (RR = 0.37, 95% CI 0.32–0.42, I2 = 59%, n = 9 studies; (Fig. 2)). Compared to the first-dose vaccine or unvaccinated group, the two-dose vaccine group also decreased hospital admissions (RR = 0.25, 95% CI 0.14–0.45, I2 = 86%, n = 10 studies; (Fig. 2)), disease severity (RR = 0.38, 95% CI 0.20–0.70, I2 = 47%, n = 4 studies; (Fig. 2) and mortality(RR = 0.21, 95% CI 0.14–0.32, I2 = 53%, n = 3 studies; (Fig. 2).
Figure 2.
RRs for breakthrough SARS-CoV-2 infection, hospital admissions, the severity of disease, and all-cause mortality after two-dose of the vaccine
Relative risks of breakthrough SARS-CoV-2 infection, hospital admissions, and all-cause mortality after three doses of the vaccine in the elderly
Similarly, the three-dose vaccine group showed a decreased risk of breakthrough infection (RR = 0.14, 95% CI 0.10–0.20, I2 = 82%, n = 3 studies), hospital admissions (RR = 0.11, 95% CI 0.07–0.17, I2 = 76%, n = 4 studies), and all-cause mortality (RR = 0.10, 95% CI 0.02–0.48, I2 = 74%, n = 3 studies) compared to the two-dose vaccine or less group in the elderly (Fig. 3).
Figure 3.
RRs for breakthrough SARS-CoV-2 infection, hospital admissions, and all-cause mortality after three-dose of the vaccine
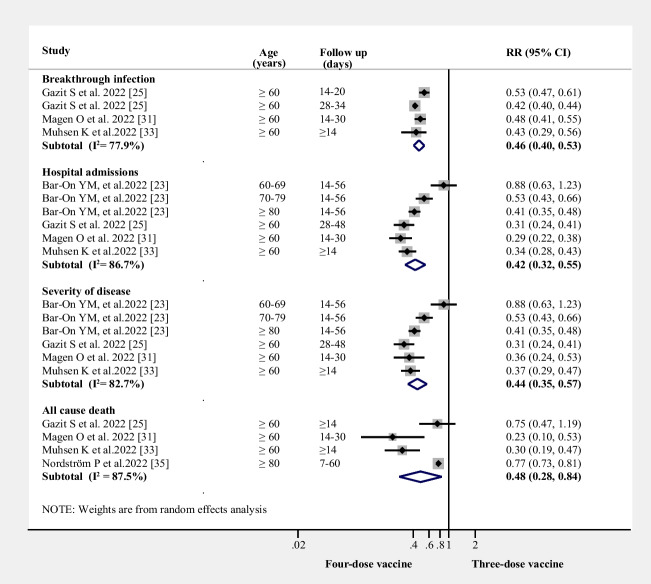
Relative risks for breakthrough SARS-CoV-2 infection, hospital admissions, the severity of disease, and all-cause mortality after four vaccine doses in the elderly
The four-dose vaccine group was associated with decreased breakthrough infections when compared with the three-dose vaccine group (RR = 0.46, 95% CI 0.40–0.53, I2 = 78%, n = 4 studies; (Fig. 4)). Compared with the three-dose vaccine group, the four-dose vaccine group also showed a decreased risk of hospital admissions (RR = 0.42, 95% CI 0.32–0.55, I2 = 87%, n = 4 studies; (Fig. 4)) and disease severity (RR = 0.44, 95% CI 0.35–0.57, I2 = 83%, n = 4 studies; (Fig. 4)). Similarly, pooled results from four studies indicated that a four-dose vaccine was associated with a decreased risk of mortality compared with the three-dose or fewer vaccine group (RR = 0.48, 95% CI 0.28–0.84, I2 = 88%, n = 4 studies; (Fig. 4)).
Figure 4.
RRs for breakthrough SARS-CoV-2 infection, hospital admissions, the severity of disease, and all-cause mortality after four-dose of the vaccine
Subgroup and meta-regression analysis of relative risks for hospital admissions, the severity of disease and all-cause mortality
Age, sex, comorbid conditions, and vaccination type were potential confounders related to hospital admission, disease severity and all-cause mortality. As shown in Figure S2, the estimated RR indicated that age groups 60–74 years and ≥ 75 years experienced a decreased risk of hospital admissions in the vaccinated group (RR = 0.42, 95% CI 0.33–0.53; RR = 0.33, 95% CI 0.16–0.65, respectively) compared to the unvaccinated group. Similarly, the estimated RR indicated that age groups 60–69 years and 70–79 years were also associated with a decreased risk of the severity of disease (RR = 0.45, 95% CI 0.30–0.68; RR = 0.53, 95% CI 0.36–0.79, respectively; Figure S3). Furthermore, in the vaccinated groups, it was found that age groups 60–79 years (RR = 0.59; 95% CI, 0.47–0.74), age groups ≥ 80 years (RR = 0.76; 95% CI, 0.59–0.98), female (RR = 0.66; 95% CI, 0.50–0.87), male (RR = 0.58; 95% CI, 0.44–0.76), and coexistence with CVD (RR = 0.69; 95% CI, 0.52–0.92) and DM (RR = 0.59; 95% CI, 0.39–0.89) showed a significantly lower risk of all-cause mortality in comparison with the unvaccinated group (Figure S4). The meta-regression demonstrated that sample size and comorbid conditions influenced the hospital admissions, vaccine dose and comorbid conditions influenced the severity of disease, and study design and comorbid conditions influenced all-cause mortality. The summary of the meta-regression of the hospital admissions, the severity of disease and all-cause mortality among vaccinated groups can be found in the Supplemental Table 2.
Comparison of efficiency of homologous and heterologous vaccination
All two dose studies were homologous vaccinations. Only three trials reported on heterologous vaccination after three or four doses of vaccination. Both of the homologous and heterologous vaccination had similar effects in the prevention of hospital admission and mortality due to SARS-CoV-2 infection (Figure S5).
Sensitivity analysis and publication bias
No study was overly influential, excluding any single study did not change our results. We also found no evidence of publication bias was found in the pooled studies on breakthrough infection, breakthrough infection (p = 0.17), disease severity (p = 0.77) or all-cause mortality (p = 0.53). However, there was a significant publication bias in hospital admissions (P = 0.03). After adjustment for potentially missing studies, hospital admissions was reduced among those who were vaccinated (RR: 0.346, 95% CI 0.285–0.420).
Risk of bias assessment
The detailed risk assessment for the included studies using the ROBINS-I tool is shown in the Supplemental Table 3. Eight studies were assessed as low risk of overall bias; 18 studies were graded as of moderate risk of overall bias. No studies were assessed to have severe or critical bias.
DISCUSSION
Our meta-analysis of 26 trials showed that the SARS-CoV-2 vaccine is effective in preventing SARS-CoV-2 breakthrough infections, hospital admissions, and mortality in the elderly, regardless of whether they receive one, two, three, or four doses. We also found that each additional dose of vaccine increased the benefit.
Increasing age is the most significant risk factor for SARS-CoV-2-related death among the non-vaccinated population 37. Several studies have reported weaker vaccine-induced immune responses in older adults, including lower concentrations of neutralizing antibodies, than in younger adults 38,39. While immunological studies suggest that these vaccinations would be less effective in the elderly, our pooled results support vaccine effectiveness against SARS-CoV-2, with increasing effectiveness with additional doses.
There are concerns whether excessive injection of vaccine boosters may lead to damage to the body's immune system and a low immune response. Studies in mice suggest that there is a reduction of the overall immune responses in both the titer of RBD-specific antibodies and the serum neutralizing potency against SARS-CoV-2 pseudo-viruses, with three boosters, indicating that repetitive administration of RBD vaccine boosters might induce humoral immune tolerance, rather than promote immunity 40,41. Our study suggests that four doses of vaccination leads to improved protection. At present, the optimum number of vaccination boosters is uncertain, but the potential immune suppression by continuous use of SARS-CoV-2 vaccine boosters should raise concern and studies are needed to confirm whether this occurs in humans, especially in the older population due to age-related changes of the immune system.
There are several limitations to this meta-analysis. First, our results are based on relatively few trials for each outcome. Secondly, some of our analyses had high levels of heterogeneity. This may be due to different age stratification and variable follow-up intervals in several of the included studies. Third, this meta-analysis only studied the main outcomes at 14–20 days, 28–34 days, and 35–60 days. Studies on long-term vaccine protection are lacking, and future studies are needed to further investigate whether more boosters are needed in vulnerable older people and the timing of such boosters. Fourth, we cannot assess the side effects of the vaccines, because trials did not describe vaccine side effects. Future research should report side effects of vaccines in the elderly.
In summary, we found that SARS-CoV-2 vaccine is effective in preventing breakthrough infections, infection severity, hospitalization and mortality. Increasing number of boosters was associated with greater benefit.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Study selection process (PDF 54 KB)
Supplementary file2 Subgroup analysis of the vaccinated group for the risk of hospital admissions. (PDF 145 KB)
Supplementary file3 Subgroup analysis of the vaccinated group for the risk of severity of the disease (PDF 145 KB)
Supplementary file4 Subgroup analysis of the vaccinated group for the risk of all-cause mortality (PDF 157 KB)
Supplementary file5 Comparison of efficiency of homologous and heterologous vaccination (PDF 121 KB)
Supplementary file6 Supplement Table 1-3 (DOCX 41 KB)
Acknowledgements
All authors have approved the final version of the manuscript and have agreed to submit it to this journal. Yang XH, Bao WJ and Zhang H contributed equally to this paper.
Authors' contributions
HM Jin and SK Fu conceived and designed the study. XH Yang, H Zhang and WJ Bao selected the articles, extracted, and analyzed the data. XH Yang, H Zhang and WJ Bao wrote the first draft of the manuscript. XH Yang and WJ Bao interpreted the data and contributed to the writing of the final version of the manuscript. All authors agreed with the results and conclusions of this Article.
Funding
This study was supported by Key Specialty of Plasma Purification in Shanghai Pudong Hospital (Zdzk2020-12), Talent Project of Shanghai Pudong Hospital (YJYJRC202110) and National Key Research and Development Plan Project (2020YFC2005002).
Data Availability
The data used of this meta-analysis are available from the corresponding author.
Declarations
Conflicts of interest
None of the authors had any conflicts of interest.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang XH, Bao WJ and Zhang H contributed equally to this paper.
References
- 1.Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahmani K, Shavaleh R, Forouhi M, et al. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Front Public Health. 2022;10:873596. doi: 10.3389/fpubh.2022.873596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CJ, Woo W, Kim AY, et al. Clinical manifestations of COVID-19 breakthrough infections: A systematic review and meta-analysis. J Med Virol. 2022;94(9):4234–4245. doi: 10.1002/jmv.27871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Liu S, Li F, et al. Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: a systematic review and meta-analysis. Front Immunol. 2022;13:965971. doi: 10.3389/fimmu.2022.965971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobczak M, Pawliczak R. COVID-19 vaccination efficacy in numbers including SARS-CoV-2 variants and age comparison: a meta-analysis of randomized clinical trials. Ann Clin Microbiol Antimicrob. 2022;21(1):32. doi: 10.1186/s12941-022-00525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21(2):83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crooke SN, Ovsyannikova IG, Poland GA, et al. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaitán-Rossi P, Mendez-Rosenzweig M, García-Alberto E, et al. Barriers to COVID-19 vaccination among older adults in Mexico City. Int J Equity Health. 2022;21(1):85. doi: 10.1186/s12939-022-01685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cristea D, Ilie DG, Constantinescu C, et al. Acceptance, Hesitancy, and Refusal in Anti-COVID-19 Vaccination: A Cluster Analysis Aiming at the Typology behind These Three Concepts. Vaccines (Basel) 2022;10(9):1496. doi: 10.3390/vaccines10091496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhanani LY, Franz B. A meta-analysis of COVID-19 vaccine attitudes and demographic characteristics in the United States. Public Health. 2022;207:31–38. doi: 10.1016/j.puhe.2022.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butt AA, Yan P, Shaikh OS, et al. Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination in a high-risk national population. EClinMed. 2021;40:101117. doi: 10.1016/j.eclinm.2021.101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butt AA, Nafady-Hego H, Chemaitelly H, et al. Outcomes Among Patients with Breakthrough SARS-CoV-2 Infection After Vaccination. Int J Infect Dis. 2021;110:353–358. doi: 10.1016/j.ijid.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyams C, Marlow R, Maseko Z, et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis. 2021;21(11):1539–1548. doi: 10.1016/S1473-3099(21)00330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-Baz I, Miqueleiz A, Casado I, et al. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Euro Surveill. 2021;26(21):2100438. doi: 10.2807/1560-7917.ES.2021.26.21.2100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naleway AL, Groom HC, Crawford PM, et al. Incidence of SARS-CoV-2 Infection, Emergency Department Visits, and Hospitalizations Because of COVID-19 Among Persons Aged ≥12 Years, by COVID-19 Vaccination Status - Oregon and Washington, July 4-September 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(46):1608–1612. doi: 10.15585/mmwr.mm7046a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivasi G, Bulgaresi M, Mossello E, et al. Course and Lethality of SARS-CoV-2 Epidemic in Nursing Homes after Vaccination in Florence, Italy. Vaccines (Basel) 2021;9(10):1174. doi: 10.3390/vaccines9101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrotri M, Krutikov M, Palmer T, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021;21(11):1529–1538. doi: 10.1016/S1473-3099(21)00289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenforde MW, Olson SM, Self WH, et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years - United States, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):674–679. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aslam J, Rauf Ul Hassan M, et al. Association of disease severity and death outcome with vaccination status of admitted COVID-19 patients in delta period of SARS-COV-2 in mixed variety of vaccine background. Saudi J Biol Sci. 2022;29(7):103329. doi: 10.1016/j.sjbs.2022.103329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N Engl J Med. 2022;386(18):1712–1720. doi: 10.1056/NEJMoa2201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suarez Castillo M, Khaoua H, et al. Vaccine effectiveness and duration of protection against symptomatic infections and severe Covid-19 outcomes in adults aged 50 years and over, France, January to mid-December 2021. Glob Epidemiol. 2022;4:100076. doi: 10.1016/j.gloepi.2022.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gazit S, Saciuk Y, Perez G, et al. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. BMJ. 2022;377:e071113. doi: 10.1136/bmj-2022-071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldin S, Adler L, Azuri J, et al. BNT162b2 mRNA COVID-19 (Comirnaty) vaccine effectiveness in elderly patients who live in long-term care facilities: a nationwide cohort. Gerontology. 2022;2022:1–8. doi: 10.1159/000521899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gram MA, Emborg HD, Schelde AB, et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or Omicron SARS-CoV-2 variant: A nationwide Danish cohort study. PLoS Med. 2022;19(9):e1003992. doi: 10.1371/journal.pmed.1003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly JD, Leonard S, Hoggatt KJ, et al. Incidence of severe COVID-19 illness following vaccination and booster with BNT162b2, mRNA-1273, and Ad26.COV2.S vaccines. JAMA. 2022;328(14):1427–1437. doi: 10.1001/jama.2022.17985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu G, Zhang Y, Zhang H, et al. Geriatric risk and protective factors for serious COVID-19 outcomes among older adults in Shanghai Omicron wave. Emerg Microbes Infect. 2022;11(1):2045–2054. doi: 10.1080/22221751.2022.2109517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machado A, Kislaya I, Rodrigues AP, et al. COVID-19 vaccine effectiveness against symptomatic SARS-CoV-2 infections, COVID-19 related hospitalizations and deaths, among individuals aged ≥65 years in Portugal: A cohort study based on data-linkage of national registries February-September 2021. PLoS One. 2022;17(9):e0274008. doi: 10.1371/journal.pone.0274008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magen O, Waxman JG, Makov-Assif M, et al. Fourth Dose of BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2022;386(17):1603–1614. doi: 10.1056/NEJMoa2201688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muhsen K, Maimon N, Mizrahi AY, et al. Association of BNT162b2 Vaccine Third Dose Receipt With Incidence of SARS-CoV-2 Infection, COVID-19-Related Hospitalization, and Death Among Residents of Long-term Care Facilities, August to October 2021. JAMA Netw Open. 2022;5(7):e2219940. doi: 10.1001/jamanetworkopen.2022.19940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muhsen K, Maimon N, Mizrahi AY, et al. Association of Receipt of the Fourth BNT162b2 Dose With Omicron Infection and COVID-19 Hospitalizations Among Residents of Long-term Care Facilities. JAMA Intern Med. 2022;182(8):859–867. doi: 10.1001/jamainternmed.2022.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller V, Polivka L, Valyi-Nagy I, et al. Booster Vaccination Decreases 28-Day All-Cause Mortality of the Elderly Hospitalized Due to SARS-CoV-2 Delta Variant. Vaccines (Basel) 2022;10(7):986. doi: 10.3390/vaccines10070986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nordström P, Ballin M, Nordström A. Effectiveness of a fourth dose of mRNA COVID-19 vaccine against all-cause mortality in long-term care facility residents and in the oldest old: A nationwide, retrospective cohort study in Sweden. Lancet Reg Health Eur. 2022;2022:100466. doi: 10.1016/j.lanepe.2022.100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tenforde MW, Self WH, Adams K, et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA. 2021;326(20):2043–2054. doi: 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widge AT, Rouphael NG, Jackson LA, et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N Engl J Med. 2021;384(1):80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collier DA, Ferreira IATM, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(7872):417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao FX, Wu RX, Shen MY, et al. Extended SARS-CoV-2 RBD booster vaccination induces humoral and cellular immune tolerance in mice. iScience. 2022;25(12):105479. doi: 10.1016/j.isci.2022.105479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filardi BA, Monteiro VS, Schwartzmann PV, et al. Age-dependent impairment in antibody responses elicited by a homologous CoronaVac booster dose. Sci Transl Med. 2023;15(683):eade6023. doi: 10.1126/scitranslmed.ade6023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Study selection process (PDF 54 KB)
Supplementary file2 Subgroup analysis of the vaccinated group for the risk of hospital admissions. (PDF 145 KB)
Supplementary file3 Subgroup analysis of the vaccinated group for the risk of severity of the disease (PDF 145 KB)
Supplementary file4 Subgroup analysis of the vaccinated group for the risk of all-cause mortality (PDF 157 KB)
Supplementary file5 Comparison of efficiency of homologous and heterologous vaccination (PDF 121 KB)
Supplementary file6 Supplement Table 1-3 (DOCX 41 KB)
Data Availability Statement
The data used of this meta-analysis are available from the corresponding author.