Abstract
BACKGROUND
Acute cholangitis (AC) constitutes an infection with increased mortality rates in the past. Due to new diagnostic tools and therapeutic methods, the mortality of AC has been significantly reduced nowadays. The initial antibiotic treatment of AC has been oriented to the most common pathogens connected to this infection. However, the optimal duration of the antibiotic treatment of AC is still debatable.
AIM
To investigate if shorter-course antibiotic treatments could be similarly effective to long-course treatments in adults with AC.
METHODS
This study constitutes a systematic review and meta-analysis of the existing literature concerning the duration of antibiotic therapy of AC and an assessment of the quality of the evidence. The study was conducted in accordance with the recommendations of the Preferred Reporting Items for Systematic Review and Meta-Analyses. Fifteen studies were included in the systematic review, and eight were eligible for meta-analysis. Due to heterogeneous duration cutoffs, three study-analysis groups were formed, with a cutoff of 2-3, 6-7, and 14 d.
RESULTS
A total of 2763 patients were included in the systematic review, and 1313 were accounted for the meta-analysis. The mean age was 73.66 ± 14.67 years, and the male and female ratio was 1:08. No significant differences were observed in the mortality rates of antibiotic treatment of 2-3 d, compared to longer treatments (odds ratio = 0.78, 95% confidence interval: 0.23-2.67, I2 = 9%) and the recurrence rates and hospitalization length were also not different in all study groups.
CONCLUSION
Short- and long-course antibiotic treatments may be similarly effective concerning the mortality and recurrence rates of AC. Safe conclusions cannot be extracted concerning the hospitalization duration.
Keywords: Acute cholangitis, Antibiotic, Short-course, Long-course, Antimicrobial, Treatment duration
Core Tip: The exact duration of antibiotic therapy for acute cholangitis in adult patients remains a controversial subject in the field of gastroenterology. A total antibiotic treatment of 4-7 d is recommended by the Tokyo Guidelines of 2018. However, recent studies present that schemata of shorter-course therapies could promise similar efficacy and safety. In our study, we systematically reviewed the existing literature in order to compare the death and recurrence rates and the length of hospitalization between patients with antibiotic treatments of shorter and longer durations. Our findings showed no significant differences between the study groups in all outcomes.
INTRODUCTION
The mortality rates of acute cholangitis (AC) have significantly decreased by comparing patients’ data before 1980 and after 2000, from 10%-30% to 2.7%-10%[1]. Multiple factors, such as the development of modern diagnostic techniques, therapeutic methods for bile duct decompression, and new antibiotics adapted to microbiological studies, have significantly contributed to this improvement in the prognosis of AC[2].
The bacterial species commonly detected in AC differ in relationship with the severity of AC[1,3]. Because of this fact, the empirical antibiotic therapy provided should be oriented to the severity grade as defined in the Tokyo Guidelines (TG)[4]. Previous operations, the origin of the infection, possible allergies, pharmacodynamics, pharmacokinetics, local antibiogram and liver or renal dysfunction should also determine the choice of antimicrobial therapy in patients with AC[5].
A major issue concerning the therapy of AC is the exact duration of the definite antibiotic treatment. The TG 2018 (TG18) suggest that the antimicrobial therapy should last 4-7 d, and if gram-positive cocci are present, a minimum therapy of two weeks should be administrated[5]. This interval between the recommended treatment days is relatively wide, and the recommendation provided is not based on a high level of evidence (level C). Recent studies suggest that even a therapy of two or three days could be equally effective and safe, and it could reduce the length of in-hospital stay of patients, with a consequent reduction in the economic burden on the health systems[6-8]. A randomized controlled trial also showed similar clinical outcomes for patients with intraabdominal infections who received antibiotic therapy for two days after the resolution of fever, compared to those who received therapy for a maximum of 10 d[9].
Our purpose in conducting this systematic review and meta-analysis is to collect all available data existing in the current bibliography in order to ascertain if short-course antibiotic therapies could promise lower mortality, lower rates of recurrent AC, and shorter hospitalizations in adults with AC, compared to long-course antibiotic therapies and, if possible, to attempt to clarify the optimal duration of the antimicrobial treatment of the AC.
MATERIALS AND METHODS
Review protocol
Our systematic review was conducted according to the guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA)[10]. Two independent reviewers (Kasparian K and Christou CD) performed research in Medline-PubMed and Cochrane databases by applying a predefined research algorithm. The last search date was the 5th of October, 2022. The studies were initially controlled according to their title and abstract and followingly according to their full texts and the extractability of their content. We included studies concerning adults who received antibiotic therapy against AC, and whose therapy duration or mean/median duration was documented. We included only original studies in our systematic review (observational studies and randomized controlled trials). The exclusion criteria for the articles in our review, were studies concerning infantile population, animal studies, non-English studies, case reports or case series, comments or editorials, book chapters, studies with non-extractable data, abstract publications, protocols of incomplete clinical trials, irrelevant articles, surveys, and articles whose full-texts could not be retrieved. Conflicts during the eligibility process were resolved after a discussion between the two reviewers.
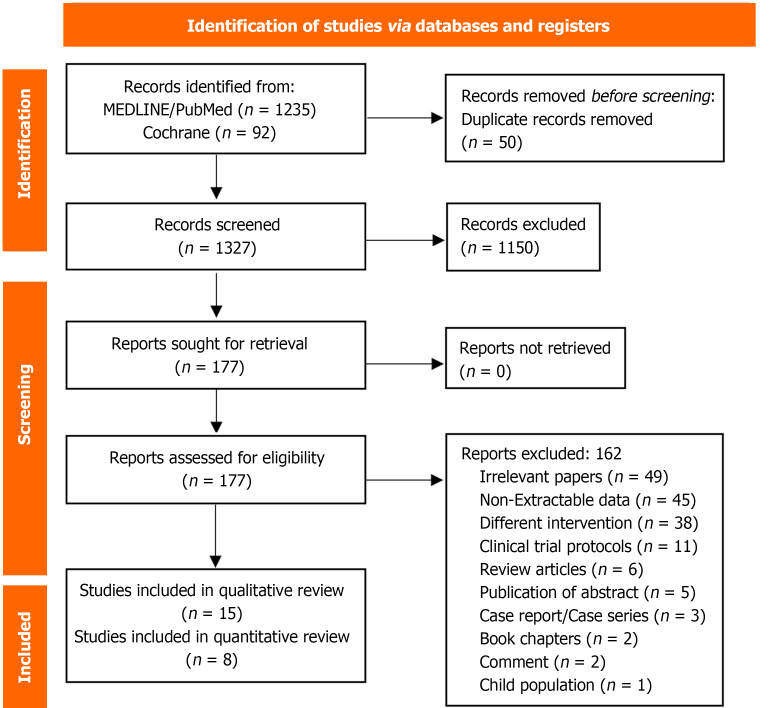
From the 1380 articles retrieved from the research, 50 were removed as duplicates and 1150 were excluded according to their titles and abstracts. From the 177 remaining studies, 15 were included in our systematic review[6,8,11-23], and the quantitative analysis of our research question was based on eight studies[8,11,12,16,17,21-23]. Forty-five papers contained data that was not extractable for our review, one study concerned a population of non-adult patients, 49 studies were irrelevant to our research questions, and the remaining excluded papers concerned non-original studies. One of the final papers constitutes a randomized-controlled trial, while the other fourteen concern retrospective observational studies. The details of the reasons for the exclusion of the papers are summarized in the PRISMA flowchart in Figure 1.
Figure 1.
The Preferred Reporting Items for Systematic Review and Meta-Analyses flow chart of the systematic review and meta-analysis.
Data extraction
The two reviewers (Kasparian K and Christou CD) collected and tabulated independently the data of the final papers chosen for this review. Each reviewer gathered data concerning the study protocol, the baseline characteristics of the participants, and data concerning the outcomes and their definitions. The baseline characteristics to be collected for our review were predefined: Mean age and standard deviations (SD), gender, comorbidities, severity grade of AC, antibiotic therapy, and biliary drainage method. Heterogenous numerical data were transformed into the same predefined measurement units for statistical analysis. The two reviewers compared the data that each one collected, and conflicts were resolved between them.
Outcomes
The primary outcome of our study was the mortality of the patients. Secondary outcomes were the recurrence rate and the length of hospitalization of AC, measured with days. All outcomes were predefined. As mortality, we define the total, all-cause mortality of the participants until the end of the follow-up period of each study. In-hospital mortality due to AC was not examined.
Due to the heterogeneity in the time cutoff points between the intervention groups in each study, our quantitative analysis was divided into three subgroups. More specifically, an analysis was conducted among the studies which compared a therapy of 2-3 d to longer antibiotic treatments[6,8,11,21], studies with a cutoff of 6-7 d were separately analyzed[22,23], and the two papers which assessed clinical outcomes in AC-patients who received antibiotic treatment of for 14 d or less were summarized together[12,16].
Statistical analysis
All continuous variables are presented with the means and SD. For numerical variables which were not normally distributed and for which their medians and interquartile ranges or ranges were provided, we used the Hozo equation to calculate their means and SD[24]. For the data synthesis of the numerical variables, standardized mean differences (SMD) and 95% confidence intervals (95%CI) were calculated, with the use of Hedge´s method, for bias to be minimized[25]. For the categorical variables, we collected the absolute values and calculated the odds ratios (OR) and their 95%CIs. The data synthesis of the qualitative variables was based on the random effects model. In cases where no events were presented concerning a qualitative variable in a study group, the proper changes were conducted, in order for the statistical analysis to be feasible[26]. For the evaluation of the study heterogeneity, the I2 value was calculated for each outcome. We presume I2 > 70% as an indicator of heterogeneity among the studies. For all other measurements, we selected a P value of 0.05 as the cutoff point for the presence of statistical significance. All calculations and Forest plots were conducted through the program R Studio, version 1.4.1103.
Quality assessment
To assess the quality of the observational studies included in our systematic review and meta-analysis, the Newcastle-Ottawa Scale was used[27]. The randomized clinical trials were evaluated for quality according to the Risk-of-Bias tool version 2 (RoB 2) provided by Cochrane[28]. Two reviewers (Kasparian K and Christou CD) independently assessed the final studies using the methods mentioned above, and possible conflicts were resolved afterward.
RESULTS
Baseline characteristics
As mentioned, 15 studies were included in our systematic review, and eight were considered eligible for the meta-analysis. The included studies were primarily conducted in Japan (8/15), two in the Netherlands, and the remaining studies originated from medical centers in France, Germany, Pakistan, South Korea, and Thailand. In 11/15 studies, the diagnosis of AC was based on the TG18/TG13 guidelines[6,8,12-14,16-18,20,21,23], two studies defined AC, depending on the TG07[15,19], Doi et al[22] controlled the presence the proper code of the 10th Edition of the International Classification of Diseases in hospital registries and positive bile or blood cultures for the selection of the patients, while van Lent et al[11] based the diagnosis of cholangitis on clinical, laboratory and imaging criteria.
The overall number of patients included in the systematic review was n = 2763, which corresponded to 2812 cases of AC. In the eight studies selected for the quantitative analysis, a total of 1219 patients and 1313 cases received antibiotic treatment against AC. The pooled mean age of the participants in the meta-analysis was 73.66 ± 14.67 years, and 721 (54.9%) of them were males, with a male to female ratio of 1:0.8. According to the TG18 severity grading system, 400/972 (41.1%) patients were classified as grade I, 525/972 (54.1%) as grade II while 45/972 (4.6%) of the patients suffered from grade III AC. Positive blood culture was detected in 383/742 (51.6%) participants. Escherichia coli (188/383, 49.1%) and Klebsiella spp. (75/383, 19.6%) were the most frequently identified pathogens, while 26/278 (9.3%) patients presented infection with gram-positive bacteria. A total of 244/1155 (21.1%) patients suffered from diabetes mellitus, 126/779 (16.2%) had a history of chronic kidney disease, and 107/779 (13.7%) participants presented a cardiac comorbidity, either chronic heart failure or coronary artery disease. Most patients received Cephalosporines (229/540, 42.4%) as antibiotic treatment and based on the available data, 275/326 (84.3%) patients underwent papillotomy during endoscopic retrograde cholangiopancreatography. For the last characteristic, only two studies included available data. Details for the baseline characteristics of the studies are summarized in Table 1.
Table 1.
Baseline characteristics of the studies
|
Ref.
|
Country
|
Study period
|
Study design
|
Definition of AC
|
Population
|
Intervention
|
Outcomes
|
| Ferstl et al[23], 2022 | Germany | 2008-2019 | Retrospective observational study | TG18/TG13 | Grade I and grade II AC after ERCP | Antibiotic therapy of 6 d | Recurrent cholangitis within 28 d |
| Kihara and Yokomizo[20], 2022 | Japan | January 2009 to August 2018 | Retrospective observational study | TG18/TG13 | Postoperative cholangitis after pancreaticoduodenectomy | Antibiotic therapy and pancreaticoduoedenectomy | Clinical characteristics and outcomes in patients with acute cholangitis |
| Masuda et al[6], 2022 | Japan | January 2018 to July 2020 | Retrospective observational study | TG18/TG13 | Grade I and grade II AC after successful ERCP | Antibiotic therapy of 3 d | 30-d-mortality, recurrent cholangitis within 3 mo, length of hospitalization, in-hospital mortality |
| Sokal et al[14], 2022 | France | 2016-2018 | Retrospective observational study | TG18/TG13 | Patients with AC with and without malignant etiology | Cancer-associated AC | Duration of antibiotic therapy, 28-d-mortality, liver abscess |
| Masuda et al[17], 2021 | Japan | April 2018 to March 2020 | Retrospective observational study | TG18/TG13 | AC patients with positive blood or bile culture and early ERCP | AC due to antibiotic resistant bacteria | Duration of antibiotic therapy, duration of hospitalization, in-hospital mortality, increased disease severity |
| Akhtar et al[18], 2020 | Pakistan | June 2012 to June 2017 | Cross-sectional observational study | TG18/TG13 | AC patients without liver metastases or other reason for deranged liver function test. 70% of patients received ERCP | 3-mo-mortality | Duration of antibiotic therapy, clinical severity, bacteremia |
| Haal et al[21], 2020 | Netherlands | January 2012 to January 2017 | Retrospective observational study | TG18/TG13 | AC only due to stone in the common bile duct, without prior antibiotic therapy after ERCP | Antibiotic therapy of ≤ 3 d | 3-mo-mortality, length of hospitalization, recurrent cholangitis, other complications |
| Satake et al[8], 2020 | Japan | April 2014 to March 2019 | Retrospective observational study | TG18/TG13 | Grade I and grade II AC only due to choledocholithiasis who underwent ERCP | Antibiotic therapy of ≤ 3 d | 30-d-mortality, length of hospitalization, recurrent cholangitis within 3 mo |
| Netinatsunton et al[16], 2019 | Thailand | August 2017 to August 2018 | Randomized controlled trial | TG18/TG13 | AC only due to choledocholithiasis without presence of the Reynold´s pentad. Time to ERCP same between the study groups | Antibiotic therapy of ≤ 14 d | Recurrent cholangitis, length of hospitalization |
| Doi et al[22], 2018 | Japan | January 2012 to February 2017 | Retrospective observational study | ICD-10 and positive blood culture | AC and positive blood culture | Antibiotic therapy of ≤ 7 d | 30-d-mortality, recurrent cholangitis within 3 mo (recurrence of symptoms) |
| Tagashira et al[13], 2017 | Japan | January 2009 to December 2015 | Retrospective observational study | TG18/TG13 | Bacteriemic AC and ERCP where indicated | Adequate initial antibiotic therapy | Duration of antibiotic treatment, 30-d mortality |
| Uno et al[12], 2017 | Japan | July 2012 to March 2014 | Retrospective observational study | TG18/TG13 | AC patients with gram-negative bacteriemia and after ERCP | Antibiotic therapy of ≤ 14 d | 30-d mortality, recurrent cholangitis within 3 mo, antimicrobial treatment duration |
| Park et al[15], 2014 | South Korea | September 2010 to November 2012 | Randomized controlled trial | TG07 | AC with bacteremia and ERCP within 24 h after admission | Intravenous antibiotic therapy of 6 d plus 8 d oral antibiotic therapy | 30-d mortality, length of hospitalization, eradication of bacteria after 30 d |
| Kogure et al[19], 2011 | Japan | September 2007 to August 2009 | Retrospective observational study | TG07 | Moderate and severe AC with ERCP | Antibiotic therapy of 3 d | Recurrent cholangitis |
| Van Lent et al[11], 2002 | Netherlands | February 1999 to September 1999 | Retrospective observational study | Fever > 38 °C and elevated bilirubin levels or bile duct dilatation in ultrasound | AC after successful ERCP. Exclusion of patients with primary sclerosing cholangitis, liver transplant recipients, bile duct atresia, inflammatory bowel disease | Antibiotic therapy of ≤ 3 d | 6-mo mortality and recurrent cholangitis |
AC: Acute cholangitis; TG18: Tokyo Guidelines 2018; TG13: Tokyo Guidelines 2013; TG07: Tokyo Guidelines 2007; ICD-10: The 10th Edition of the International Classification of Diseases; ERCP: Endoscopic retrograde cholangiopancreatography.
Mortality
Among the eight studies which were included in the quantitative analysis, six of them included mortality as an outcome[6,8,11,12,21,22], while five of the studies that were not suitable for the meta-analysis provided information for the participants’ death[13-15,17,18]. Six studies calculated a 30-d mortality rate[6,8,12,13,15,22], Sokal et al[14] presented data concerning 28-d mortality, and Haal et al[21] conducted a follow-up of 3 mo concerning the death rates of the participants. Van Lent et al[11] observed the mortality of the patients for the following six months after the intervention.
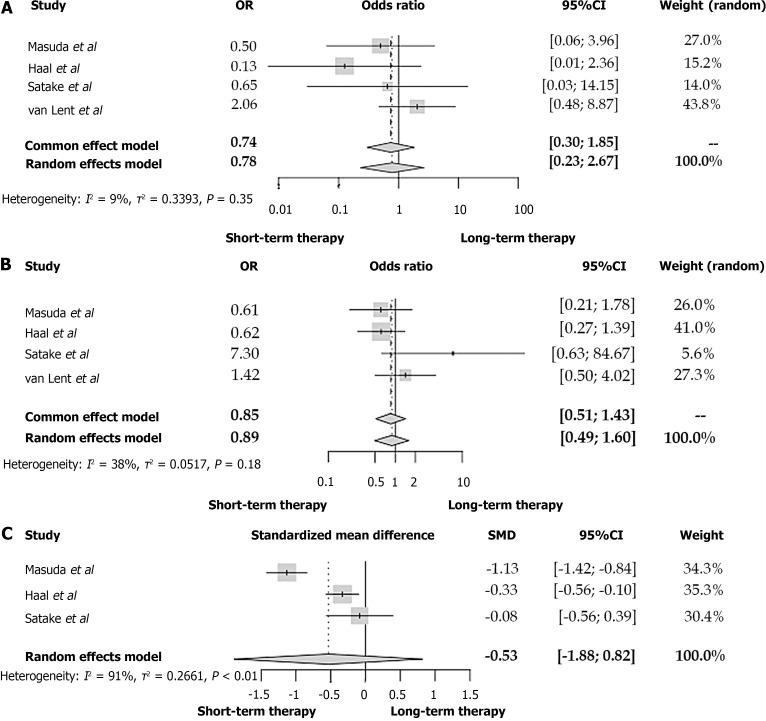
After summarizing the data of the four studies with a duration of 2-3 of antibiotic treatment as cutoff point[6,8,11,21], no significant difference in the mortality between the two patient groups is present (OR = 0.78, 95%CI: 0.23-2.67, I2 = 9%) (Figure 2A). No heterogeneity among these studies concerning our primary outcome is noted (I2 = 9%).
Figure 2.
Forest plot between patients with acute cholangitis who received an antibiotic therapy of 2-3 d and acute cholangitis-patients with longer antibiotic treatment. A: Mortality; B: Recurrent cholangitis; C: Duration of hospitalization. OR: Odds ratio; CI: Confidence interval; SMD: Standardized mean difference.
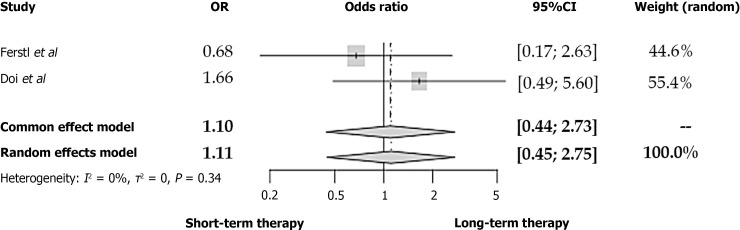
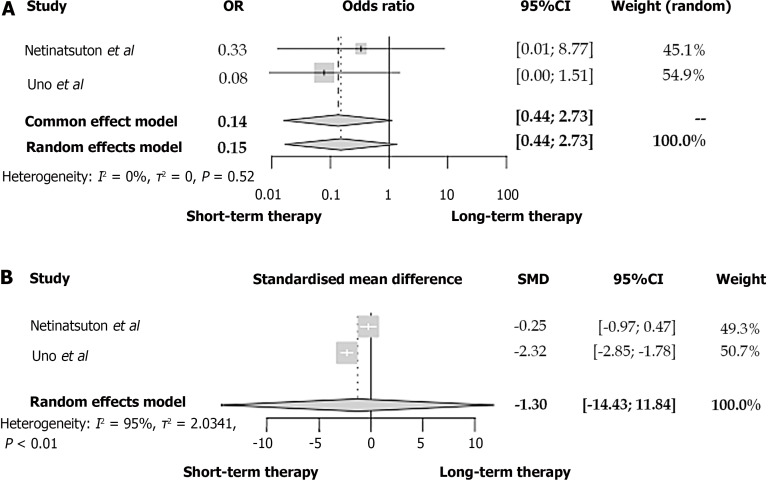
In the study of Doi et al[22], patients who received an antibiotic treatment of fewer than seven days presented similar death rates compared to those with longer therapeutic schemata (OR = 0.82, 95%CI: 0.18-2.95, I2 = 9%). The 30-d mortality rates of AC patients did not seem to differ between antibiotic therapies, of 14 d or shorter, according to Uno et al[12] (2 vs 0, P = 0.79, Fisher´s exact test).
Similar findings are also observable in the rest of the studies, which were not included in the meta-analysis. Sokal et al[14] presented that although patients with cancer-related-AC took an antibiotic treatment of mean duration with no significant difference compared to those that suffered from non-cancer-related-AC (10.6 ± 9.7 vs 7.8 ± 7.5 d, P = 0.13), the patients who belonged in the first group had significantly higher mortality rates (17 vs 0 deaths, P = 0.0002). In the rest of the studies, no differences concerning mortality were to be noted[13,15,17,18].
Recurrent cholangitis
Five of the included papers calculated a recurrence rate up to a follow-up period of 3 mo[6,8,12,21,22], van Lent et al[11] estimated a 28-d recurrence rate, Ferstl et al[23] recorded the shortest follow-up period among the studies (28 d) and two studies did not clarify the exact duration of follow-up for this outcome[16,19].
According to the studies retrieved from our review, a two to three-day antibiotic therapy was not associated with a significantly more frequent appearance of recurrent cholangitis (OR = 0.89, 95%CI: 0.49-1.60) (Figure 2B)[6,8,11,21]. No differences were also noted in the rates of recurrent AC neither in the 6-7-d-cutoff (OR = 1.11, 95%CI: 0.45-2.75) nor the 14-d-cutoff study groups (OR = 0.15, 95%CI: 0.02-1.35) (Figures 3 and 4A respectively). In all these comparisons, no significant study heterogeneity was observed (I2 = 38%, 0%, and 0%, respectively). In the study of Kogure et al[19], no recurrent cholangitis was observed in both study groups, with and without withdrawal of the antibiotic therapy on the third day of treatment.
Figure 3.
Forest plot of recurrent cholangitis between patients with acute cholangitis who received an antibiotic therapy of 6-7 d and acute cholangitis-patients with longer antibiotic treatment. OR: Odds ratio; CI: Confidence interval.
Figure 4.
Forest plot between patients with acute cholangitis who received an antibiotic therapy of 14 d and acute cholangitis-patients with shorter antibiotic treatment. A: Recurrent cholangitis; B: Duration of hospitalization. OR: Odds ratio; CI: Confidence interval; SMD: Standardized mean difference.
Length of hospitalization
Although in the studies of Masuda et al[6] and Haal et al[21], the patients who belong to the two-day and three-day antibiotic therapy have a significantly shorter hospitalization duration, the SMD of all three studies with a cutoff of 2-3 d is not significantly lower in the short-term antibiotic group (SMD = -0.53, 95%CI: -1.88 to 0.82, I2 = 91%) (Figure 2C). Similarly, no difference was found between the short- and long-term treatment groups concerning the length of in-hospital stay (SMD = -1.30, 95%CI: -14.43 to 11.84, I2 = 95%) (Figure 4B). However, the considerable study heterogeneity (I2 = 91% and 95%, respectively) does not allow us to extract strong conclusions. On the contrary, Park et al[15] estimated that a switch from intravenous to oral antibiotic therapy after six days from the treatment initiation led to a significantly lower length of hospitalization in comparison with a switch at ten days of treatment.
Quality assessment
Based on the Newcastlte-Ottawa Scale, six of the observational studies included in the systematic review were considered of good quality[6,8,13,17,21,23], while the remaining seven were graded as low-quality studies, concerning our outcomes of interest, mostly due to lack of comparability between the cohort participants[11,13,14,18-20,22] (Table 2). In the study of Netinatsunton et al[16], as far as the randomization process is concerned, although the patients were randomized with the use of a computer-generated process and the baseline characteristics of the patients did not significantly differ, the results of the randomization were stated to be concealed in envelopes. However, the status and the accessibility of the envelopes are not provided, which raises concerns regarding the absolute transparency of the randomization. Additionally, no information is provided to manage the patients who did not adhere to the trial. Furthermore, the fact that no more information is provided concerning the construction of the study, as the trial protocol is provided neither in the clinicaltrials.gov website nor in the text in its complete form, leads us to the conclusion that the risk of bias in this study is high.
Table 2.
Quality assessment of the observational studies
|
|
Ferstl et al[23], n = 115
|
Kihara and Yokomizo[20], n = 112
|
Masuda et al[6], n = 11
|
Sokal et al[14], n = 107
|
Masuda et al[17], n = 110
|
Akhtar et al[18], n = 55
|
Haal et al[21], n = 113
|
Satake et al[8], n = 101
|
Doi et al[22], n = 114
|
Tagashira et al[13], n = 106
|
Uno et al[12], n = 105
|
Kogure et al[19], n = 111
|
Van Lent et al[11], n = 104
|
| Selection | |||||||||||||
| Representativeness of exposed cohort | * | - | * | - | * | * | * | * | * | * | * | * | * |
| Selection of non-exposed cohort | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Ascertainment of exposure | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Demonstration that outcome of interest was not present at start of study | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Comparability | |||||||||||||
| Comparability of the cohorts | ** | - | ** | - | ** | - | ** | ** | - | - | ** | - | - |
| Outcome | |||||||||||||
| Assessment of outcome | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Was follow-up long enough for outcomes to occur? | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Adequacy of follow-up cohorts | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Overall | Good | Low | Good | Low | Good | Low | Good | Good | Low | Low | Good | Low | Low |
*Represents study meets a criterion in each section of the Newcastle-Ottawa scale; -Represents study meets no criterion in each section of the Newcastle-Ottawa scale.
The trial of Park et al[15] has been based on a computer-generated block randomization model. However, no allocation concealment was possible due to the different application ways of the different interventions (oral vs intravenous). The authors followed an intention-to-treat model, but the lack of blinding may have led to a bias in measuring the outcome. Due to these facts, we conclude that the trial of Park et al[15] may also be connected to a high risk of bias.
DISCUSSION
To our knowledge, this is the first complete meta-analysis examining the duration of antibiotic therapy against the AC. In previously conducted systematic reviews with similar thematology, the limited data and the heterogenous outcomes and populations did not allow the authors to perform a meta-analysis[7,29]. Our data synthesis showed that antibiotic treatment of less than 2 or 3 d is not associated with significantly higher mortality rates, according to the random effects model (OR = 0.82, 95%CI: 0.18-2.95, I2 = 9%). Our findings agree with the conclusions of the systematic review of Haal et al[7]. The fact that van Lent et al[11] did not base their diagnosis on the TG07 or TG18/13, possibly because the study was conducted before the establishment of the diagnostic criteria, may weaken the weight of this outcome. Although the combination of the diagnostic criteria of van Lent (fever > 38°C + elevated bilirubin levels or dilated bile duct by ultrasound) theoretically covers the criteria needed for the diagnosis of AC[30], the authors did not provide the exact cutoff, above which the bilirubin levels are considered elevated and the sensitivity of ultrasound on the diagnosis of AC is limited[31,32]. This could lead to a false estimation of the actual patients suffering from AC. In the studies of Doi et al[22] and Uno et al[12], who set higher cutoffs for the definition of short- and long-term treatments, significance in the OR of the mortality rates was also not reached[12,22]. The findings of Sokal et al[33] that cancer-related AC patients presented a higher 28-d mortality compared to patients with AC not related to malignancies, in combination with the fact that the patients in the two groups received an antibiotic therapy of no different duration, may imply that the antibiotic therapy in cancer-related AC-patients should be longer. This study alone can provide no evidence for this assumption.
Concerning the rates of recurrent cholangitis, no significant differences were found between the study groups in all three duration cutoffs we set for our analysis. Although Uno et al[12] estimated a slightly higher recurrence rate in the group with > 14 d of antibiotic treatment, our synthesis with the study of Netinatsunton et al[16] resulted in no significantly different rates compared with the longer-course group. Haal et al[7] and Tinusz et al[29] enforce our results that the duration of antibiotic treatment of AC does not seem to affect the possibility of the appearance of cholangitis recurrence. The increasing resistance of the usual bacteria causing AC, such as Escherichia coli, the higher rates of gram-positive pathogens in recurrent cholangitis, and the possible lack of coverage against those bacteria in the initial empiric scheme provided against AC may constitute the major factors for the appearance of episodes of cholangitis recurrence[3,34,35]. As Tagashira et al[13] stated, inadequate initial antibiotic treatment could increase mortality and adverse events in bacteremic patients with AC.
Among the studies with a cutoff of 2-3 d of antibiotic treatment, Masuda et al[6] and Haal et al[21] presented a significantly shorter in-hospital stay for patients receiving short-term treatment compared to the control group of the study. However, when summarizing those two studies with the observational study of Satake et al[8], the SMD in the length of hospitalization was not significant (SMD = -0.6, 95%CI: -2.27 to 1.07, I2 = 0%). Antibiotic treatment of less than 14 did also not seem to lead to a shorter hospitalization of patients compared to the control group, according to our study (SMD = -1.3, 95%CI: -14.49 to 11.89, I2 = 96%), even though the short-course antibiotic treatment in Uno et al[12], presented a significantly lower hospitalization duration. However, the high heterogeneity of the studies included in these tests concerning this outcome (I2 = 91% and 96%, respectively) may have affected our results. Park et al[15] reinforced the claim that shorter antibiotic treatment may benefit the patients concerning their length of stay in the hospital, as no mortality cases were recorded. In every case, more studies examining the hospitalization length should be conducted, as the extraction of evidence that proves that a short-course antibiotic treatment leads to shorter hospitalizations would protect patients from exposure to several dangers, such as thromboembolic episodes and unwanted infections[36,37].
Our study presents several limitations which should be accounted for. First of all, as many studies were conducted before 2013, the choice of the participants as patients suffering from AC was not based on the updated TG18/13, especially in the study of van Lent et al[11]. This may alter the actual population we wish to study. The difference in patients’ inclusion and exclusion criteria and the variable severity grades of the patients included in the studies also constitute an important limitation. Additionally, small discrepancies are also noticed in the follow-up periods concerning the mortality and recurrent cholangitis in each study. These inequalities in the follow-up of the patients may lead to lost data which may have altered our results. Finally, significant statistical heterogeneity was detected among the studies selected for the quantitative analysis of the length of hospitalizations, which may lead to invalid conclusions.
CONCLUSION
Depending on our findings, the duration of antibiotic therapy may not significantly affect the mortality rate, the rate of recurrent cholangitis, and the length of hospitalization of patients suffering from AC. More specifically, a 2 to 3-d antibiotic treatment could be similarly effective in preventing mortality and recurrent cholangitis, as the 4 to 7-d therapy proposed by the TG18. However, these results are based on a small number of heterogeneous studies. It is vital that more primary and secondary studies are conducted for new recommendations with high-level evidence to be established.
ARTICLE HIGHLIGHTS
Research background
The mortality rates of acute cholangitis (AC) have significantly decreased in the last decades. The development of new diagnostic and therapeutic tools has contributed to this result.
Research motivation
The Tokyo Guidelines of 2018 suggest an antibiotic treatment of four to seven days in AC cases without gram-positive cocci. This interval between the recommended treatment days is relatively wide, and the recommendation provided is not based on a high level of evidence (level C).
Research objectives
The aim of this study is to investigate if shorter-course antibiotic treatments could be similarly effective to long-course treatments in adults with AC.
Research methods
We conducted a systematic review and meta-analysis of the existing literature based on the recommendations of the Preferred Reporting Items for Systematic Review and Meta-Analyses. Two reviewers (Kasparian K and Christou CD) conducted the literature research, study selection, and data collection. The inclusion and exclusion criteria were predefined. The data synthesis, statistical analysis, and Forest plot creation were conducted through the program R Studio version 1.4.1103.
Research results
Fifteen studies were included in the systematic review and eight in the final meta-analysis. Most of the patients were classified as Grade I (41,1%) or Grade II (54,1%), while only 4,6% of the participants suffered from Grade III AC. No significant differences were observed between patients receiving a 2-3 d antibiotic therapy and those who were treated with longer antibiotic schemata concerning the mortality (odds ratio = 0.78, 95% confidence interval: 0.23-2.67, I2 = 9%). In all calculations conducted, no differences could be detected among patients receiving shorter and longer antibiotic treatments concerning the rates of recurrent AC and the length of hospitalization.
Research conclusions
Short- and long-course antibiotic treatments may be similarly effective concerning the mortality and recurrence rates of AC.
Research perspectives
This study could constitute the occasion for the conduction of more primary and secondary studies for new robust recommendations with a high level of evidence to be established.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 4, 2023
First decision: April 10, 2023
Article in press: April 23, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jain N, Latvia; Kimura Y, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
Contributor Information
Karampet Kasparian, Clinic of Oncology, Gastroenterology and Hematology, Alfried Krupp Hospital, Essen 45131, Germany; Second Propedeutic Department of Internal Medicine, Hippokration General Hospital, Aristotle University of Thessaloniki, Thessaloniki 54642, Greece. kar.kasparian@gmail.com.
Chrysanthos D Christou, Department of Transplantation Surgery, Hippokration General Hospital, Aristotle University of Thessaloniki, Thessaloniki 54642, Greece.
Konstantinos Petidis, Second Propedeutic Department of Internal Medicine, Hippokration General Hospital, Aristotle University of Thessaloniki, Thessaloniki 54642, Greece.
Michail Doumas, Second Propedeutic Department of Internal Medicine, Hippokration General Hospital, Aristotle University of Thessaloniki, Thessaloniki 54642, Greece.
Olga Giouleme, Second Propedeutic Department of Internal Medicine, Hippokration General Hospital, Aristotle University of Thessaloniki, Thessaloniki 54642, Greece.
References
- 1.Kimura Y, Takada T, Strasberg SM, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Windsor JA, Mayumi T, Yoshida M, Miura F, Higuchi R, Gabata T, Hata J, Gomi H, Dervenis C, Lau WY, Belli G, Kim MH, Hilvano SC, Yamashita Y. TG13 current terminology, etiology, and epidemiology of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:8–23. doi: 10.1007/s00534-012-0564-0. [DOI] [PubMed] [Google Scholar]
- 2.Zimmer V, Lammert F. Acute Bacterial Cholangitis. Viszeralmedizin. 2015;31:166–172. doi: 10.1159/000430965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomi H, Takada T, Hwang TL, Akazawa K, Mori R, Endo I, Miura F, Kiriyama S, Matsunaga N, Itoi T, Yokoe M, Chen MF, Jan YY, Ker CG, Wang HP, Wada K, Yamaue H, Miyazaki M, Yamamoto M. Updated comprehensive epidemiology, microbiology, and outcomes among patients with acute cholangitis. J Hepatobiliary Pancreat Sci. 2017;24:310–318. doi: 10.1002/jhbp.452. [DOI] [PubMed] [Google Scholar]
- 4.Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, Liu KH, Su CH, Wada K, Jagannath P, Itoi T, Gouma DJ, Mori Y, Mukai S, Giménez ME, Huang WS, Kim MH, Okamoto K, Belli G, Dervenis C, Chan ACW, Lau WY, Endo I, Gomi H, Yoshida M, Mayumi T, Baron TH, de Santibañes E, Teoh AYB, Hwang TL, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Higuchi R, Kitano S, Inomata M, Deziel DJ, Jonas E, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos) J Hepatobiliary Pancreat Sci. 2018;25:17–30. doi: 10.1002/jhbp.512. [DOI] [PubMed] [Google Scholar]
- 5.Gomi H, Solomkin JS, Schlossberg D, Okamoto K, Takada T, Strasberg SM, Ukai T, Endo I, Iwashita Y, Hibi T, Pitt HA, Matsunaga N, Takamori Y, Umezawa A, Asai K, Suzuki K, Han HS, Hwang TL, Mori Y, Yoon YS, Huang WS, Belli G, Dervenis C, Yokoe M, Kiriyama S, Itoi T, Jagannath P, Garden OJ, Miura F, de Santibañes E, Shikata S, Noguchi Y, Wada K, Honda G, Supe AN, Yoshida M, Mayumi T, Gouma DJ, Deziel DJ, Liau KH, Chen MF, Liu KH, Su CH, Chan ACW, Yoon DS, Choi IS, Jonas E, Chen XP, Fan ST, Ker CG, Giménez ME, Kitano S, Inomata M, Mukai S, Higuchi R, Hirata K, Inui K, Sumiyama Y, Yamamoto M. Tokyo Guidelines 2018: antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25:3–16. doi: 10.1002/jhbp.518. [DOI] [PubMed] [Google Scholar]
- 6.Masuda S, Koizumi K, Makazu M, Uojima H, Kubota J, Kimura K, Nishino T, Sumida C, Ichita C, Sasaki A, Shionoya K. Antibiotic Administration within Two Days after Successful Endoscopic Retrograde Cholangiopancreatography Is Sufficient for Mild and Moderate Acute Cholangitis. J Clin Med. 2022;11 doi: 10.3390/jcm11102697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haal S, Wielenga MCB, Fockens P, Leseman CA, Ponsioen CY, van Soest EJ, van Wanrooij RLJ, Sieswerda E, Voermans RP. Antibiotic Therapy of 3 Days May Be Sufficient After Biliary Drainage for Acute Cholangitis: A Systematic Review. Dig Dis Sci. 2021;66:4128–4139. doi: 10.1007/s10620-020-06820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satake M, Yamaguchi Y. Three-day antibiotic treatment for acute cholangitis due to choledocholithiasis with successful biliary duct drainage: A single-center retrospective cohort study. Int J Infect Dis. 2020;96:343–347. doi: 10.1016/j.ijid.2020.04.074. [DOI] [PubMed] [Google Scholar]
- 9.Sawyer RG, Claridge JA, Nathens AB, Rotstein OD, Duane TM, Evans HL, Cook CH, O'Neill PJ, Mazuski JE, Askari R, Wilson MA, Napolitano LM, Namias N, Miller PR, Dellinger EP, Watson CM, Coimbra R, Dent DL, Lowry SF, Cocanour CS, West MA, Banton KL, Cheadle WG, Lipsett PA, Guidry CA, Popovsky K STOP-IT Trial Investigators. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015;372:1996–2005. doi: 10.1056/NEJMoa1411162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Lent AU, Bartelsman JF, Tytgat GN, Speelman P, Prins JM. Duration of antibiotic therapy for cholangitis after successful endoscopic drainage of the biliary tract. Gastrointest Endosc. 2002;55:518–522. doi: 10.1067/mge.2002.122334. [DOI] [PubMed] [Google Scholar]
- 12.Uno S, Hase R, Kobayashi M, Shiratori T, Nakaji S, Hirata N, Hosokawa N. Short-course antimicrobial treatment for acute cholangitis with Gram-negative bacillary bacteremia. Int J Infect Dis. 2017;55:81–85. doi: 10.1016/j.ijid.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Tagashira Y, Sakamoto N, Isogai T, Hikone M, Kosaka A, Chino R, Higuchi M, Uehara Y, Honda H. Impact of inadequate initial antimicrobial therapy on mortality in patients with bacteraemic cholangitis: a retrospective cohort study. Clin Microbiol Infect. 2017;23:740–747. doi: 10.1016/j.cmi.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Sokal A, Chawki S, Nguyen Y, Sauvanet A, Ponsot P, Maire F, Fantin B, de Lastours V. Specificities of acute cholangitis in patients with cancer: a retrospective comparative study of 130 episodes. Eur J Clin Microbiol Infect Dis. 2022;41:143–146. doi: 10.1007/s10096-021-04289-0. [DOI] [PubMed] [Google Scholar]
- 15.Park TY, Choi JS, Song TJ, Do JH, Choi SH, Oh HC. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59:2790–2796. doi: 10.1007/s10620-014-3233-0. [DOI] [PubMed] [Google Scholar]
- 16.Netinatsunton N, Limmathurotsakul D, Attasaranya S, Sottisuporn J, Pattarapuntakul T, Ovartlarnporn B. Short Duration versus 14-day Antibiotic Treatment in Acute Cholangitis due to Bile Duct Stone: A randomized study. J Gastroenterol Hepatol Res. 2019;8:3020–3024. [Google Scholar]
- 17.Masuda S, Koizumi K, Uojima H, Kimura K, Nishino T, Tasaki J, Ichita C, Sasaki A. Effect of Antibiotic Resistance of Pathogens on Initial Antibiotic Therapy for Patients With Cholangitis. Cureus. 2021;13:e18449. doi: 10.7759/cureus.18449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akhtar F, Siddique MZ, Raza A, Mehmood S, Yusuf MA, Sultan F. Microbiology and clinical characteristics of acute cholangitis with their impact on mortality; a retrospective cross sectional study. J Pak Med Assoc. 2020;70:607–612. doi: 10.5455/JPMA.29747. [DOI] [PubMed] [Google Scholar]
- 19.Kogure H, Tsujino T, Yamamoto K, Mizuno S, Yashima Y, Yagioka H, Kawakubo K, Sasaki T, Nakai Y, Hirano K, Sasahira N, Isayama H, Tada M, Kawabe T, Omata M, Harada S, Ota Y, Koike K. Fever-based antibiotic therapy for acute cholangitis following successful endoscopic biliary drainage. J Gastroenterol. 2011;46:1411–1417. doi: 10.1007/s00535-011-0451-5. [DOI] [PubMed] [Google Scholar]
- 20.Kihara Y, Yokomizo H. The clinical features of late postoperative cholangitis following pancreaticoduodenectomy brought on by conditions other than cancer recurrence: a single-center retrospective study. BMC Surg. 2022;22:301. doi: 10.1186/s12893-022-01752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haal S, Ten Böhmer B, Balkema S, Depla AC, Fockens P, Jansen JM, Kuiken SD, Liberov BI, van Soest E, van Hooft JE, Sieswerda E, Voermans RP. Antimicrobial therapy of 3 days or less is sufficient after successful ERCP for acute cholangitis. United European Gastroenterol J. 2020;8:481–488. doi: 10.1177/2050640620915016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doi A, Morimoto T, Iwata K. Shorter duration of antibiotic treatment for acute bacteraemic cholangitis with successful biliary drainage: a retrospective cohort study. Clin Microbiol Infect. 2018;24:1184–1189. doi: 10.1016/j.cmi.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Ferstl PG, Queck A, Bremer K, Filmann N, Weiler N, Welker MW, Waidmann O, Knabe M, Bechstein WO, Hogardt M, Kempf VAJ, Zeuzem S, Trebicka J, Friedrich-Rust M, Walter D. Comparison of short-course antibiotic therapy of 6 or less days with a longer treatment in patients with cholangitis after liver transplantation. Transpl Infect Dis. 2022;24:e13868. doi: 10.1111/tid.13868. [DOI] [PubMed] [Google Scholar]
- 24.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin L, Aloe AM. Evaluation of various estimators for standardized mean difference in meta-analysis. Stat Med. 2021;40:403–426. doi: 10.1002/sim.8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 27.Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067–1076. doi: 10.1097/ACM.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3, 2022. British: Cochrane Collaboration, 2022. [Google Scholar]
- 29.Tinusz B, Szapáry L, Paládi B, Tenk J, Rumbus Z, Pécsi D, Szakács Z, Varga G, Rakonczay Z Jr, Szepes Z, Czimmer J, Vincze Á, Hegyi P, Erőss B. Short-Course Antibiotic Treatment Is Not Inferior to a Long-Course One in Acute Cholangitis: A Systematic Review. Dig Dis Sci. 2019;64:307–315. doi: 10.1007/s10620-018-5327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiriyama S, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Yokoe M, Kimura Y, Tsuyuguchi T, Itoi T, Yoshida M, Miura F, Yamashita Y, Okamoto K, Gabata T, Hata J, Higuchi R, Windsor JA, Bornman PC, Fan ST, Singh H, de Santibanes E, Gomi H, Kusachi S, Murata A, Chen XP, Jagannath P, Lee S, Padbury R, Chen MF Tokyo Guidelines Revision Committee. New diagnostic criteria and severity assessment of acute cholangitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat Sci. 2012;19:548–556. doi: 10.1007/s00534-012-0537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walas MK, Skoczylas K, Gierbliński I. Errors and mistakes in the ultrasound diagnostics of the liver, gallbladder and bile ducts. J Ultrason. 2012;12:446–462. doi: 10.15557/JoU.2012.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majeed AW, Ross B, Johnson AG, Reed MW. Common duct diameter as an independent predictor of choledocholithiasis: is it useful? Clin Radiol. 1999;54:170–172. doi: 10.1016/s0009-9260(99)91008-5. [DOI] [PubMed] [Google Scholar]
- 33.Sokal A, Sauvanet A, Fantin B, de Lastours V. Acute cholangitis: Diagnosis and management. J Visc Surg. 2019;156:515–525. doi: 10.1016/j.jviscsurg.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Hara T, Taniguchi M, Hattori C, Sakai H, Oka K, Iwai N, Tsuji T, Harada T, Okuda T, Komaki T, Sakagami J, Kagawa K. Microbiological analysis of patients with first and recurrent episodes of acute cholangitis in a middle-sized hospital: A single-center retrospective study in rural North Kyoto, Japan. J Infect Chemother. 2022;28:413–419. doi: 10.1016/j.jiac.2021.11.025. [DOI] [PubMed] [Google Scholar]
- 35.van den Hazel SJ, Speelman P, Tytgat GN, Dankert J, van Leeuwen DJ. Role of antibiotics in the treatment and prevention of acute and recurrent cholangitis. Clin Infect Dis. 1994;19:279–286. doi: 10.1093/clinids/19.2.279. [DOI] [PubMed] [Google Scholar]
- 36.Welch C, K Hassan-Smith Z, A Greig C, M Lord J, A Jackson T. Acute Sarcopenia Secondary to Hospitalisation - An Emerging Condition Affecting Older Adults. Aging Dis. 2018;9:151–164. doi: 10.14336/AD.2017.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heit JA, Melton LJ 3rd, Lohse CM, Petterson TM, Silverstein MD, Mohr DN, O'Fallon WM. Incidence of venous thromboembolism in hospitalized patients vs community residents. Mayo Clin Proc. 2001;76:1102–1110. doi: 10.4065/76.11.1102. [DOI] [PubMed] [Google Scholar]